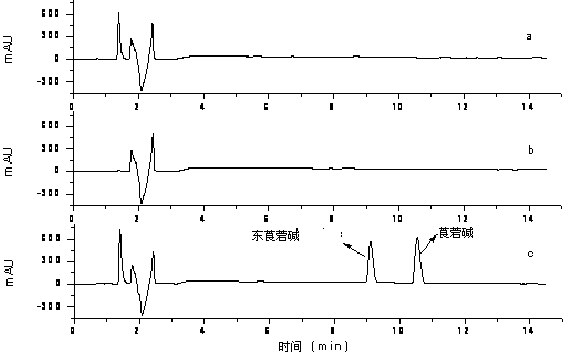
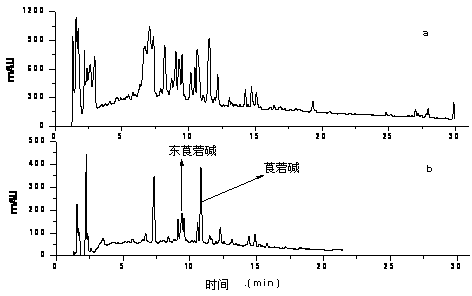
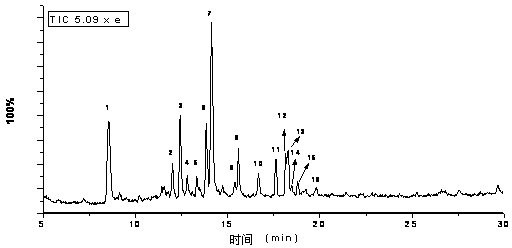
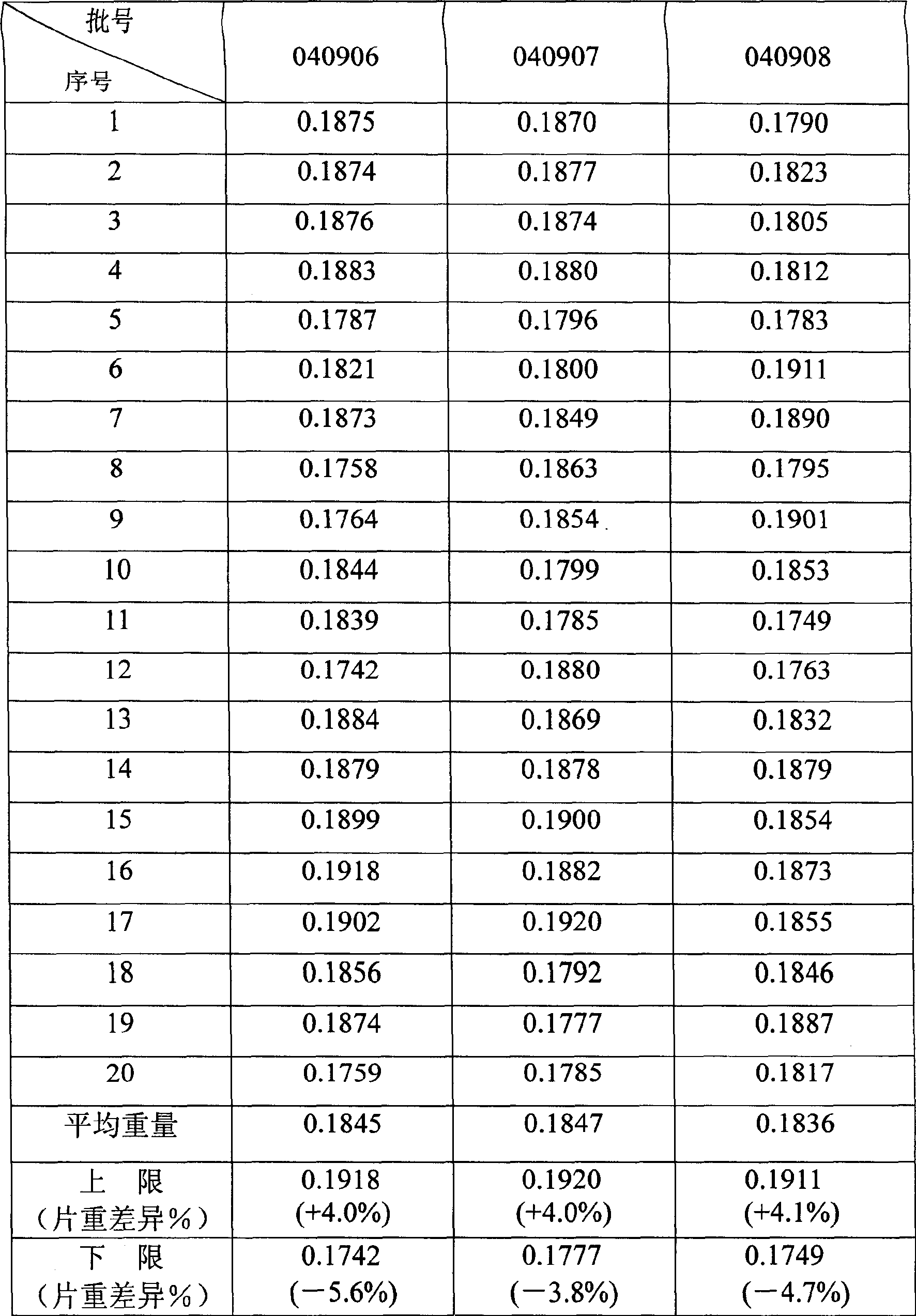
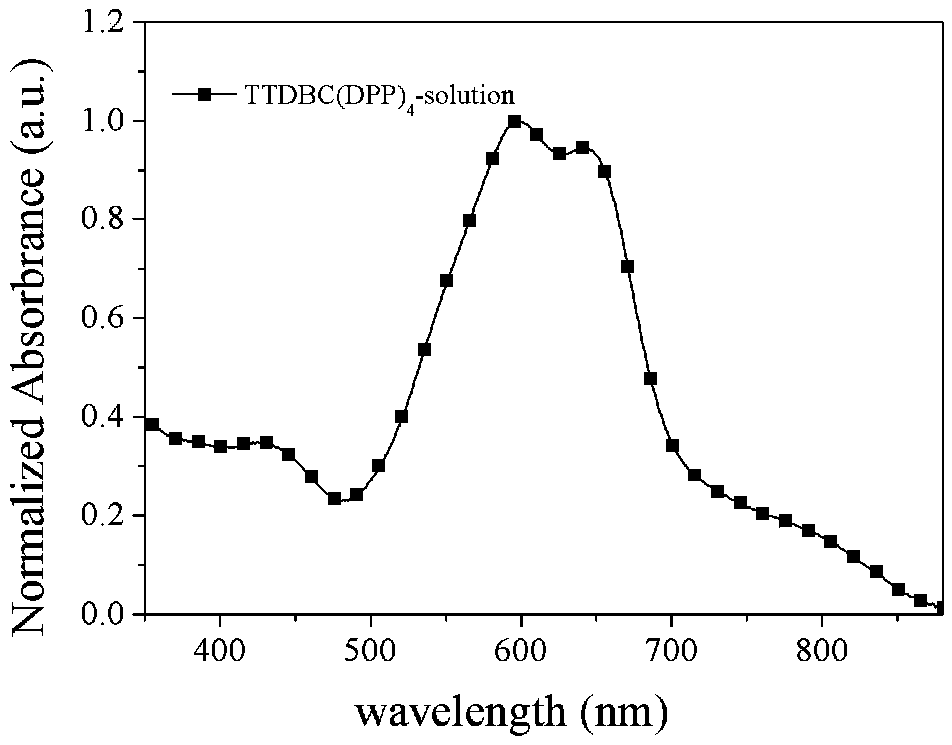
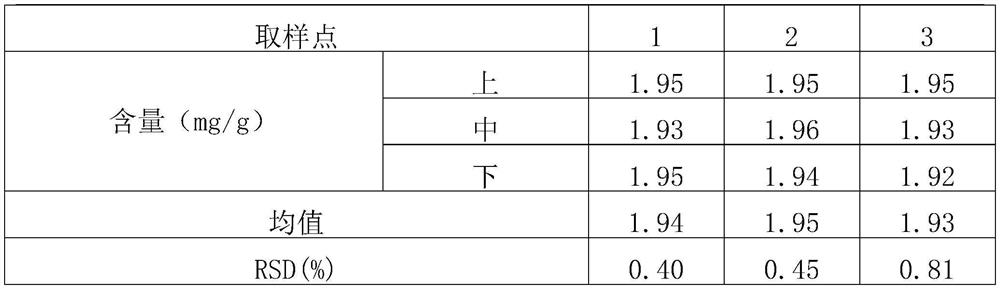
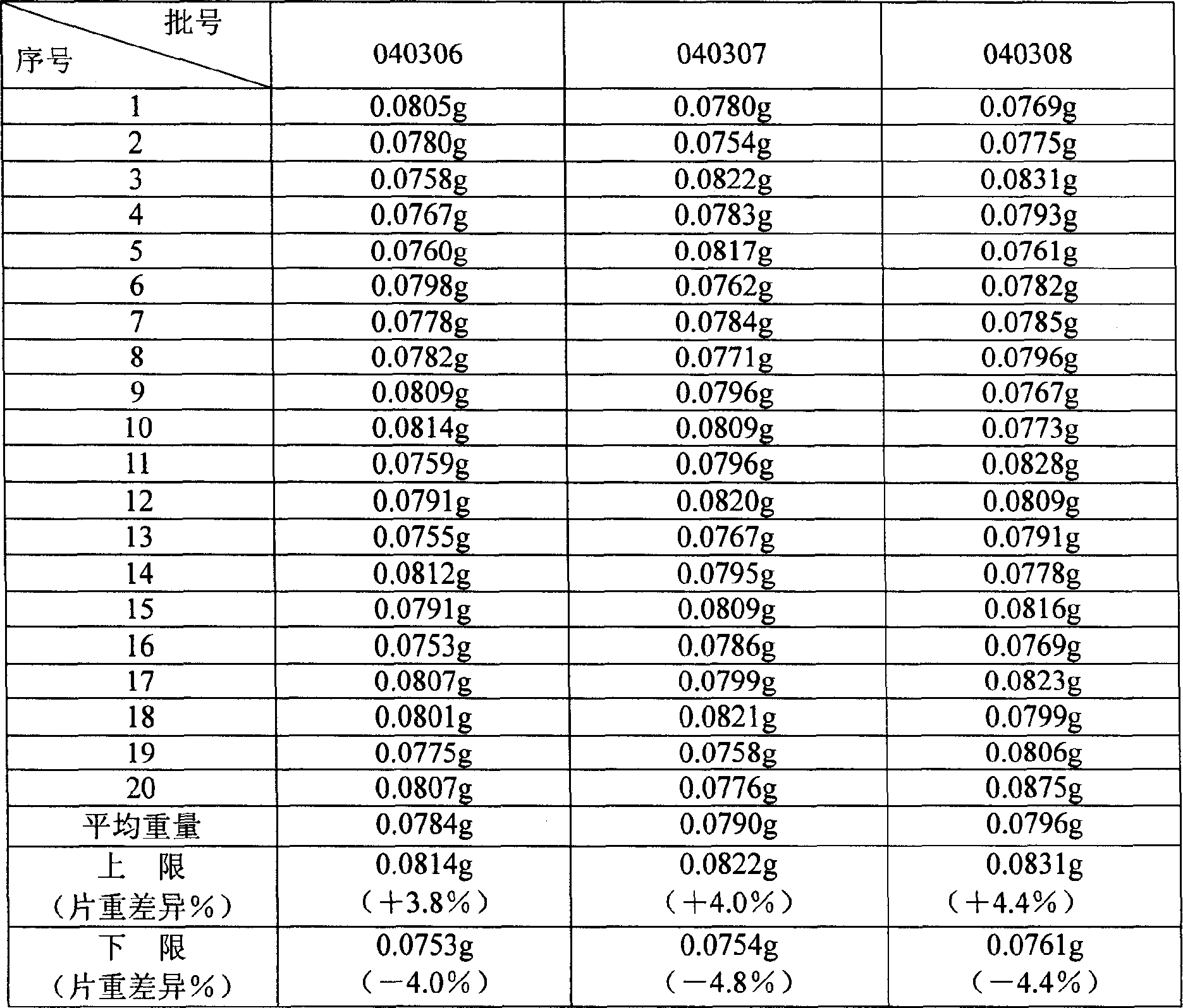
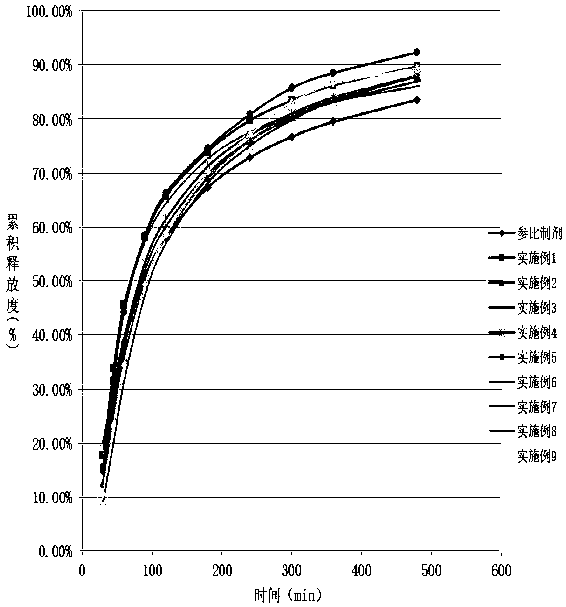
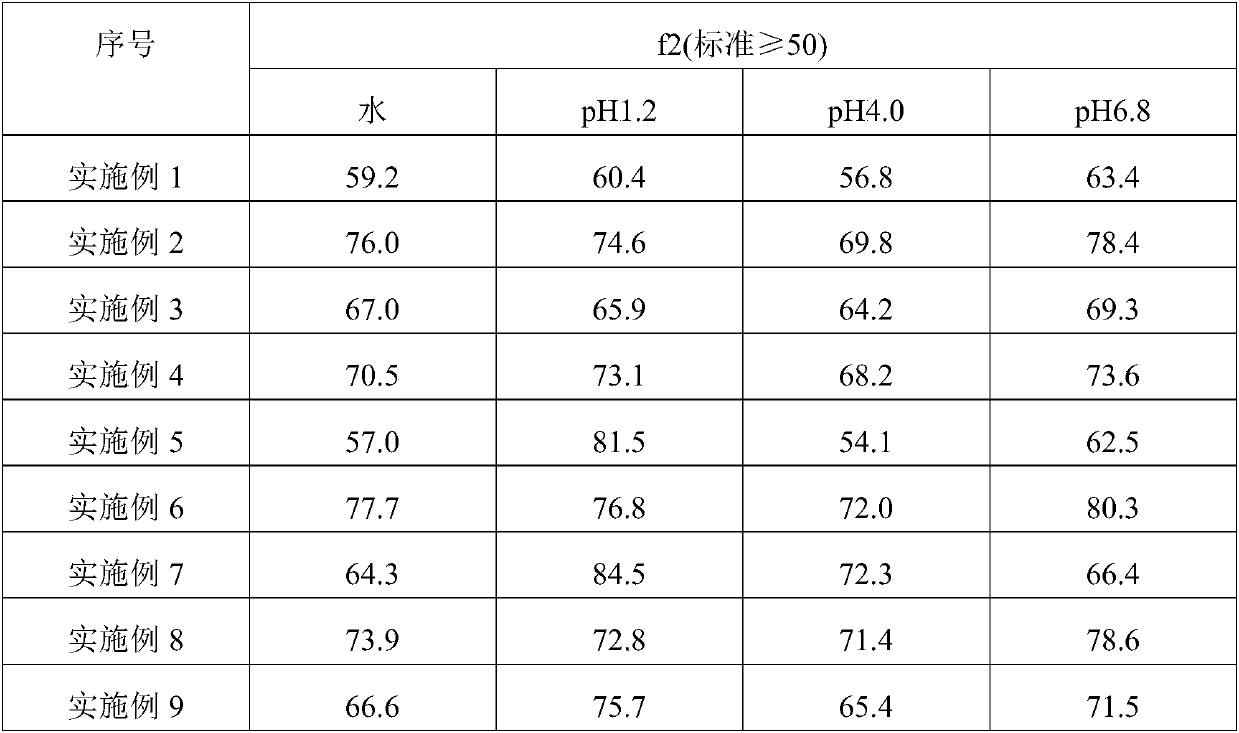
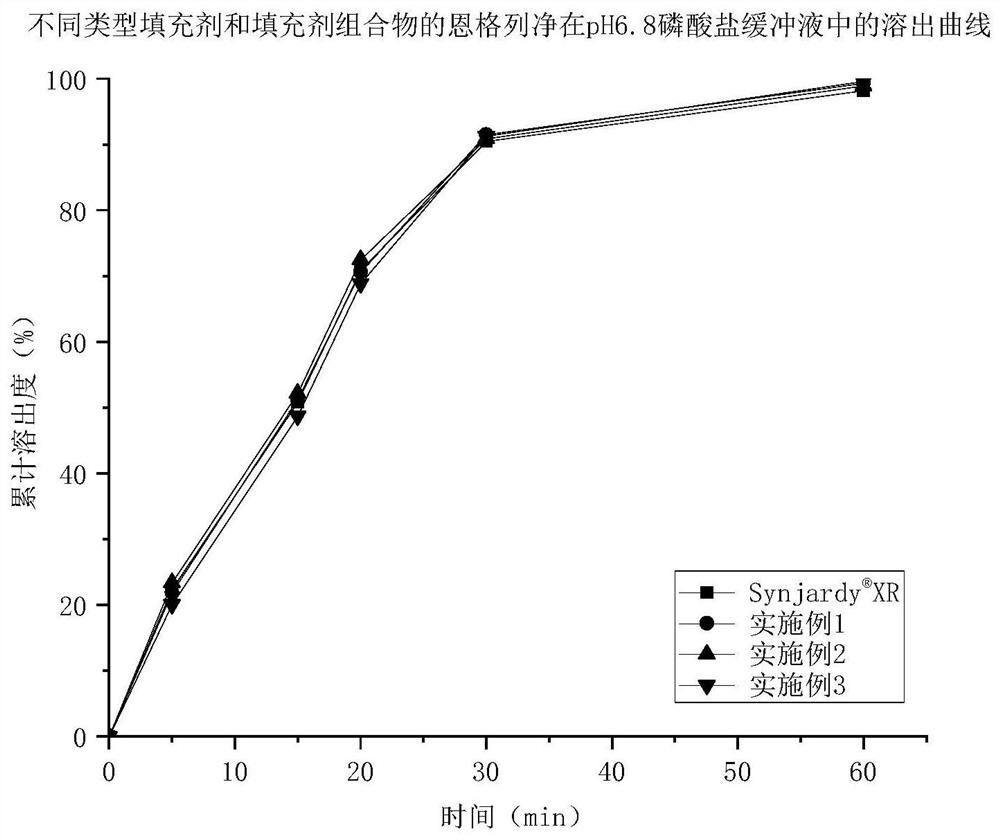
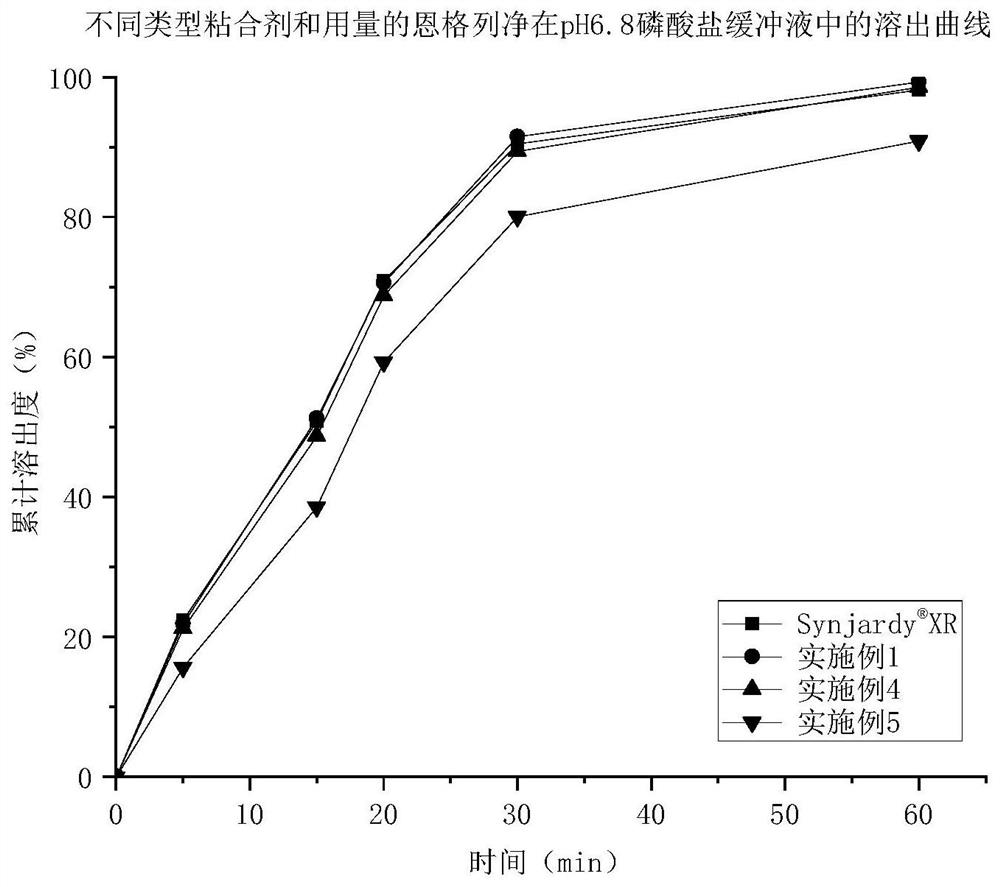
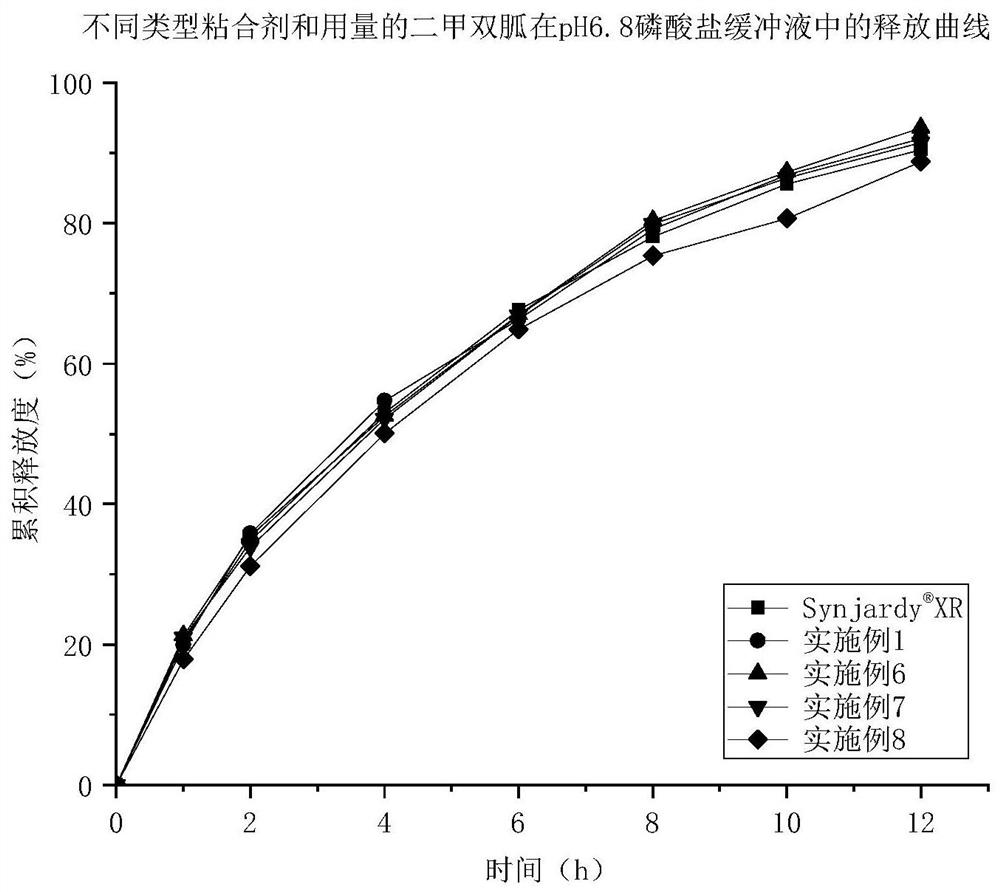
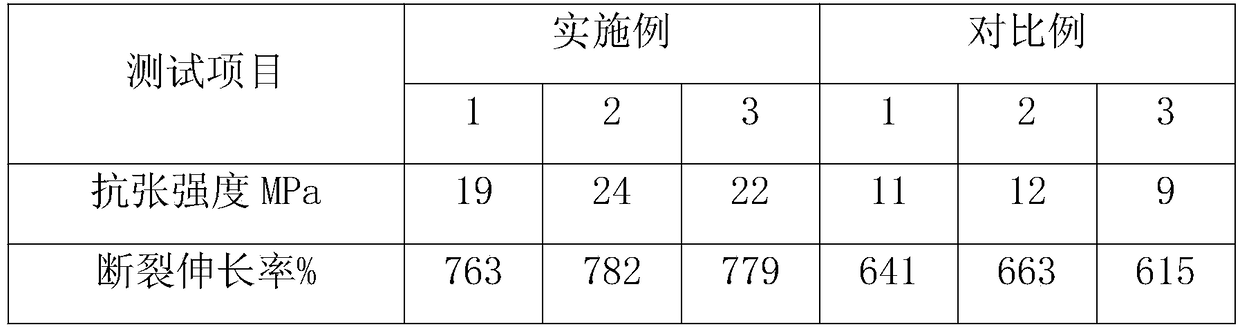
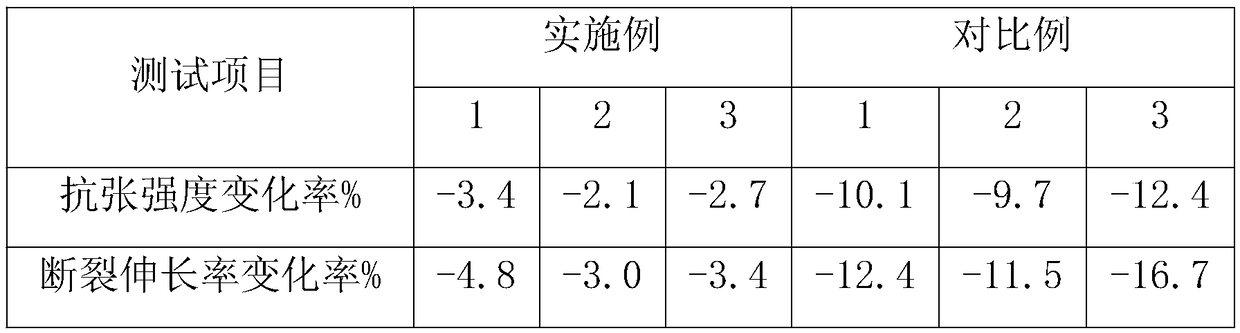
Patents
Literature
48results about How to "Good batch-to-batch reproducibility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
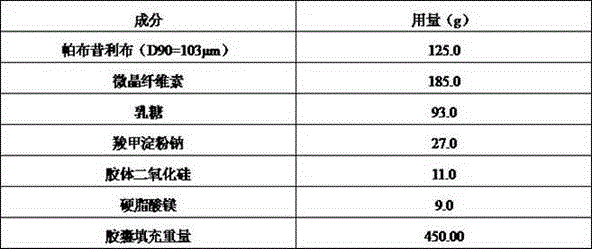
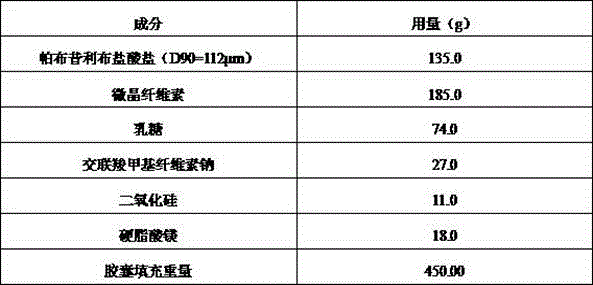
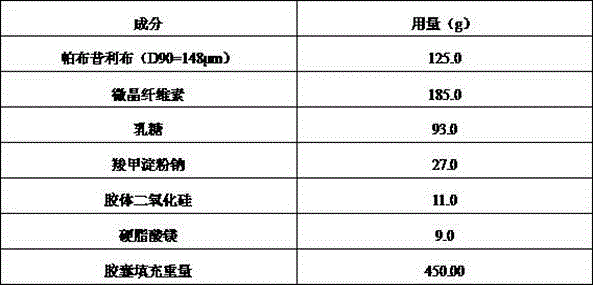
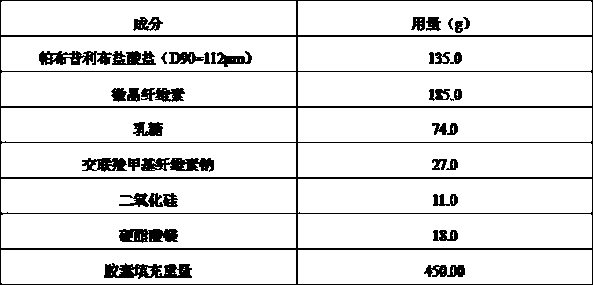
Palbociclib pharmaceutical composition and preparation method thereof
ActiveCN105748435AShorten absorption timeGuaranteed validityOrganic active ingredientsCapsule deliveryMedicineBiological availability
The invention discloses a palbociclib pharmaceutical composition, comprising palbociclib or its medicinal salt and a pharmaceutically acceptable auxiliary material, wherein the palbociclib or its medicinal salt is 50-150 Mum, and the medicinal salt includes isethionates, hydrochlorides, sulfates or benzene sulfonates.Direct mixing and capsuling process of raw and auxiliary materials is used for the pharmaceutical composition provided herein, the biological availability is high, product quality stability is good, the technical process is easy to control, inter-batch reproducibility is good, and the composition is easy to industrialize.
Owner:河北泽运生物医药科技有限公司
Method for synthesizing doping lithium manganic acid
InactiveCN101152963ALow priceFair priceCell electrodesManganates/permanganatesElectrolysisPhysical chemistry
The present invention provides a synthesizing method of doping lithium manganese oxide, which is essentially used for lithium ion battery anode active material. A doping material is mixed into Li2CO3 and electrolysis MnO 2 material. The doping material comprises any two of Al, Co, Cr, Fe, Y trivalent cations and any one of F, Cl, I anions. The raw material is mechanically mixed, high-speed milled and sieved to prepare a mixture. The mixture is roasted according to a set program in a rotary resistance furnace. After cooling, the mixture is milled and sieved again to prepare the product. The method is simple in reaction process, low in cost and even in mixture, which effectively improves the electrochemical property and recycling property of material and causes no pollution to the environment.
Owner:JIANGSU SHUANGDENG GROUP
Nanocrystalline of hydrophobic drug, as well as preparation and application methods of nanocrystalline
InactiveCN104546728ANarrow particle size distributionHigh drug loadingPowder deliveryKetone active ingredientsTumor tissueEmulsion
The invention relates to nanocrystalline of a hydrophobic drug. The nanocrystalline of the hydrophobic drug is characterized by comprising the hydrophobic drug and amphipathic molecule of wrapping the outside of the hydrophobic drug, wherein the nanocrystalline is obtained by removing solvent molecules of an O / W type emulsion containing the hydrophobic drug. The nanocrystalline of the hydrophobic drug provided by the invention is uniform in particle size and good in dispersity in water and has universality; the effective components of the drug are kept well; the surface-wrapped amphipathic molecule can be doped with a targeting molecule; after the amphipathic molecule is doped with the targeting molecule, the targeting functionalization of a nanocrystalline preparation can be achieved; distribution of the drug in a tumor tissue is increased; and the toxic and side effects of chemotherapy drugs are reduced. The preparation method of the nanocrystalline of the hydrophobic drug provided by the invention is simple to operate and good in repeatability; the controllability of the particle size of the hydrophobic drug can be achieved; the product quality is easy to control; and the batch reproducibility of the drug is good.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
A composite bergenin dispersible tablet and preparation method thereof
InactiveCN1864679AHigh dissolution rateGood dissolution uniformityOrganic active ingredientsPill deliveryCarboxymethyl starchChlorphenamine maleate
The compound bergenin disperser tablet consists of bergenin in 82.0-90.0 wt%, chlorphenamine maleate 1.0-2.0 wt%, carboxymethyl starch sodium 5.0-10.0 wt%, magnesium stearate 0.5-2.0 wt%, superfine silica gel powder 0.5-1.5 wt%, sodium dodecyl sulfate 0.1-0.5 wt%, polyvinyl pyrrolidone 1.0-3.0 wt% and aspartame 0.1-0.5 wt%. The compound bergenin disperser tablet is prepared through micronizing the components, mixing, wet pelletizing and tabletting. It may be disintegrated fast within 2 min and has high bergenin and chlorphenamine maleate leaching speed.
Owner:马晶
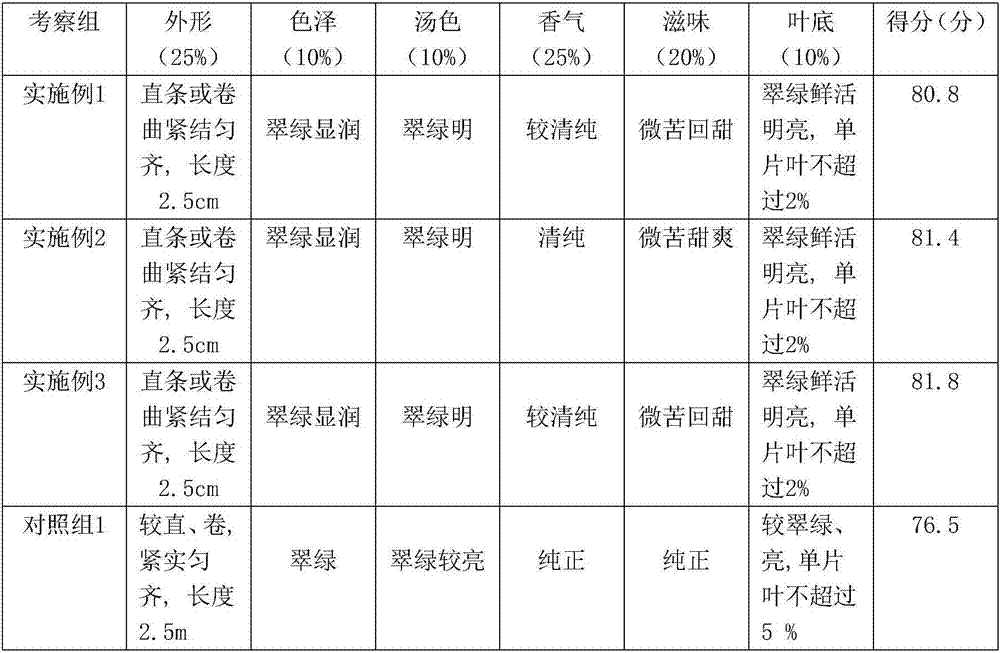
Deep processing method of small-leaf Kuding tea
InactiveCN107094958AGood batch-to-batch reproducibilitySoup color emerald greenTea substituesDeep processingTea leaf
The invention provides a deep processing method of small-leaf Kuding tea. The method takes local small-leaf tea in Guizhou as tea raw material, and performs steps of picking, rinsing, soaking, withering, twisting, fermenting, drying and others; the method is creatively improved on the basis of a traditional tea manufacturing technique; the prepared tea leaf is good in batch reproducibility; the tea leaf is bright green in soup, clear and bright in color, pure in fragrance, slightly bitter and sweet in taste; the leaf back is green, fresh and soft; therefore, the small-leaf Kuding tea meets the requirement of the first-grade tea.
Owner:黔西南州天麒绿色产业开发有限公司
Methotrexate oral disintegrating tablet and its preparation method
InactiveCN1754538AHigh dissolution ratePromote absorptionOrganic active ingredientsPill deliveryDrugMTX - Methotrexate
The invention provides a methotrexate oral disintegrating tablet for rapid disintegrating in oral cavity without water, which is prepared by comminuting methotrexate, sodium carboxymethylstarch, crystalline cellulose, mannitol, magnesium stearate, passing through 100 mesh sieves, mixing homogeneously, and pelleting directly.
Owner:马晶
Pretreatment method for alkaloid in Anisodus tanguticus
InactiveCN102850343AGood selective enrichment effectHigh selectivityOrganic chemistryPretreatment methodSolid phase extraction
The invention provides a pretreatment method for alkaloid in Anisodus tanguticus. The method is suitable for sample pretreatment in separation, preparation and qualitative and quantitative operation of alkaloid in Anisodus tanguticus. The method mainly includes pulverizing Anisodus tanguticus medicinal material, adding ethanol for extraction, collecting extracting solution, concentrating to obtain extract, tanking the extract, performing selective enrichment to the alkaloid in crude alkaloid by silica gel-based strong cation exchange (SCX) solid phase extraction (SPE) material to obtain alkaloid enriched segment for use in separation analysis and separation preparation of alkaloid in Anisodus tanguticus. The invention has good selectivity and high alkaloid content in fraction, and can greatly improve separation analysis and separation preparation efficiency of alkaloid in Anisodus tanguticus. The pretreatment method has high repeatability and good operability in operating process, is easy for realizing standardization and industrialization, and has a certain directing significance for selectively obtaining alkaloid enriched fraction from Anisodus tanguticus.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Leponex orally disintegrating tablet and preparation method thereof
InactiveCN1806805AGood dissolution uniformityMedication convenienceOrganic active ingredientsNervous disorderCarboxymethyl starchCellulose
The invention discloses a clozapine oral disintegrating tablet, which is prepared from 50.0-60.0 wt% of clozapine, 5.0-10.0.0wt% of sodium carboxymethylstarch, 30.0-40.0.0wt% of crystalline cellulose, 2.0-5.0wt% of miropowdered silica gel, 0.1-2.0wt% of magnesium stearate and 0.1-0.5 wt% of Aspartame through disintegrating and passing through 100 mesh sieve, mixing homogeneously, and carrying out powder direct pelleting method to obtain the composition.
Owner:马晶
Preparation method of SERS (Surface-Enhanced Raman Scattering) chip
ActiveCN108872185ARealize high density stackingHigh activityMaterial nanotechnologyRaman scatteringHigh fluxSpray coating
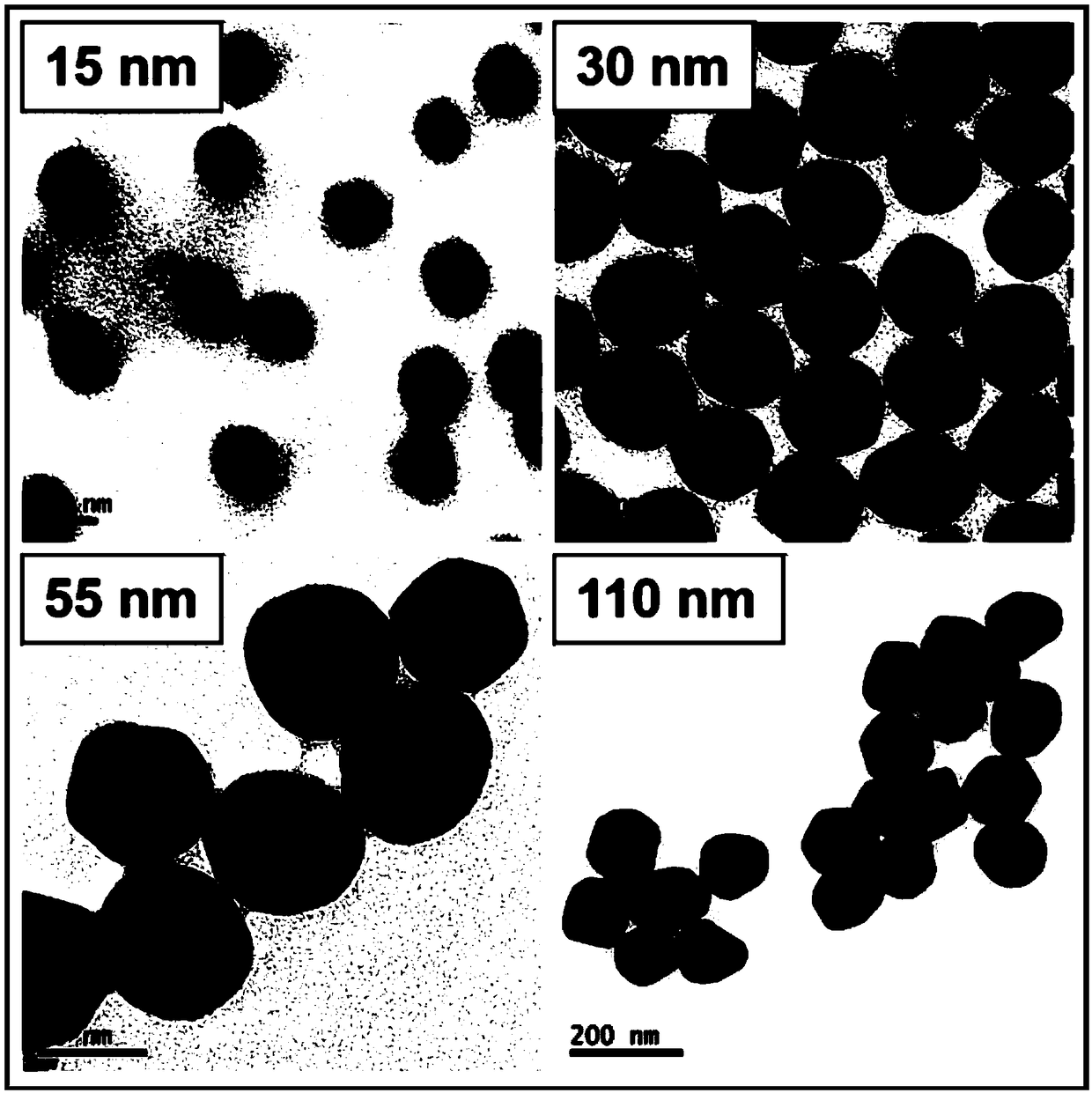
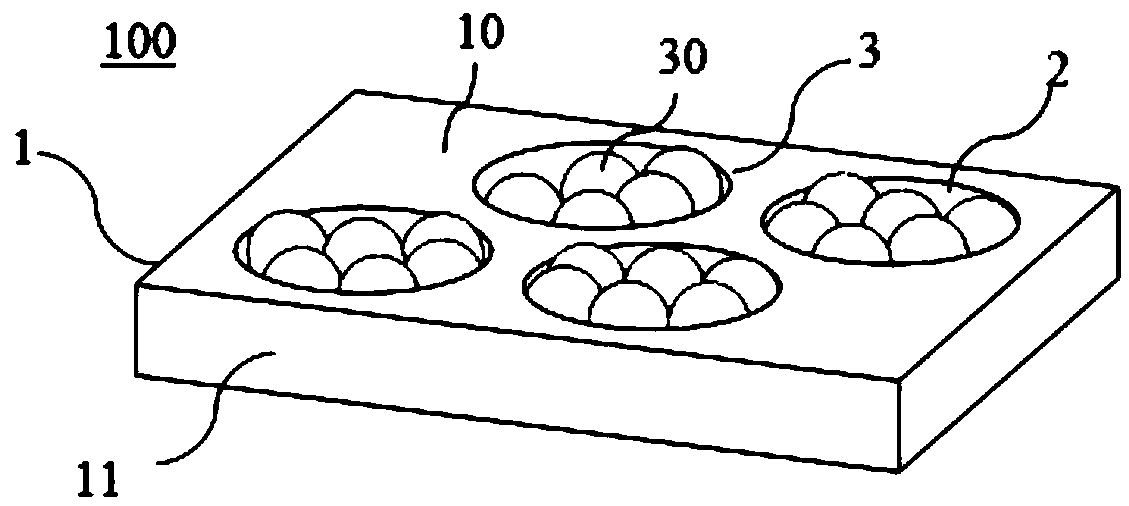
The invention provides a preparation method of an SERS (Surface-Enhanced Raman Scattering) chip. By an ultrasonic spray coating method, a dispersion solution containing metal nanometer particles is sprayed and coated onto the surface of a substrate with a plurality of concave pits on the surface; solvents of the dispersion solution are removed through volatilization, so that the metal nanometer particles are self-assembled in the concave pits of the substrate. The ultrasonic spray coating is used for preparing the SERS chip; the cost is low; the high-quality SERS chip can be prepared at high flux; the SERS chip prepared by the method has good SERS activity, ultrahigh uniformity, batch reproducibility and ultrahigh stability, and can be used for trace substance detection.
Owner:苏州英菲尼纳米科技有限公司
SERS (Surface Enhanced Raman Scattering) unit, and preparation method and application thereof
PendingCN108844943AHigh SERS activityAchieve self-assemblyMaterial nanotechnologyAnodisationRaman scatteringSelf assembled
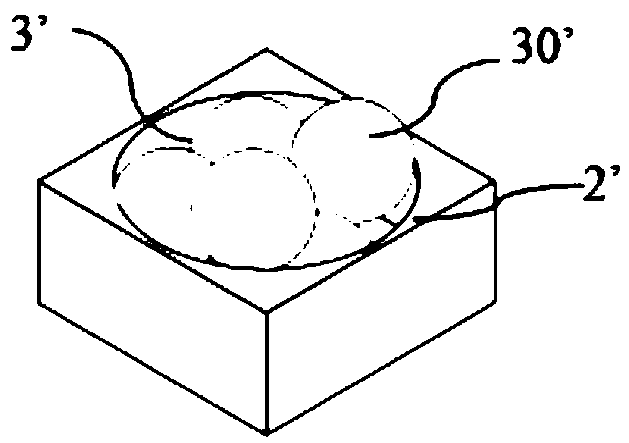
The invention discloses an SERS (Surface Enhanced Raman Scattering) unit which comprises a substrate having a plurality of nano-recesses scattered on the surface thereof, and a plurality of nano-particle aggregates, each nano-particle aggregate is formed by aggregating a plurality of nano-particles, and each nano-particle aggregate is limited by a corresponding nano-recess. The invention also discloses a preparation method of the SERS unit. The SERS unit can be obtained by impregnating the substrate with nano-recesses into a dispersion containing nano-particles, and then self-assembling the nano-particles. The SERS unit can be directly applied as an SERS chip, has the advantages of high SERS activity, high uniformity, excellent stability, high batch reproducibility and the like, is easy toprepare, can be produced in a large area and on a large scale, and has a broad business prospect.
Owner:苏州英菲尼纳米科技有限公司
Montelukast tablet composition and preparation method thereof
InactiveCN103040784AGood practicalityLow content of related substancesRespiratory disorderCoatingsChemistryFilm coating
The invention discloses a Montelukast tablet composition. The composition comprises Montelukast, porous filling agent, disintegrating agent, lubricant, opadry film coating premixing agent and purified water; and the stability of the Montelukast can be remarkably improved. The invention further discloses a preparation method for the Montelukast tablet composition; the powder is adopted to directly compress tablets, so that the operation is convenient, the influence to the product quality from the drying process and the hot and humid condition is avoided, the content of related substances is low, and the stability is remarkably improved.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Drug-film affinity measuring method based on polydiacetylene sensor
InactiveCN102435572AGood reproducibilityReduce testing costsMaterial analysis by observing effect on chemical indicatorColor/spectral properties measurementsHigh-Throughput Screening MethodsDiacetylene
The invention discloses a drug-film affinity measuring method based on a polydiacetylene sensor, which comprises the following steps of: inserting phospholipid into a diacetylene vesicle sensor so as to form biofilm like polydiacetylene / phospholipid color change vesicles; and measuring color change levels before and after the color change vesicles interact with drug molecules, and calculating affinity of the drug molecules and the color change vesicles by using a certain model and a mathematical treatment method. The method is simple, convenient, quick, good in repeatability, and the like; the requirements on the analysis conditions are not high, so that the method can be realized by utilizing a general visible spectrophotometer only in a general test lab; and the method can be compatiblewith a high-throughput micropore plate mode, so that the method is suitable for high-throughput screening of the drug-film affinity.
Owner:CHINA PHARM UNIV
Preparation method and application of solid-phase microextraction fiber of self-assembly multi-layer porphyrin organic frame compound
ActiveCN109589937AEfficient determinationImprove adsorption capacityIon-exchange process apparatusComponent separationFiberSolid-phase microextraction
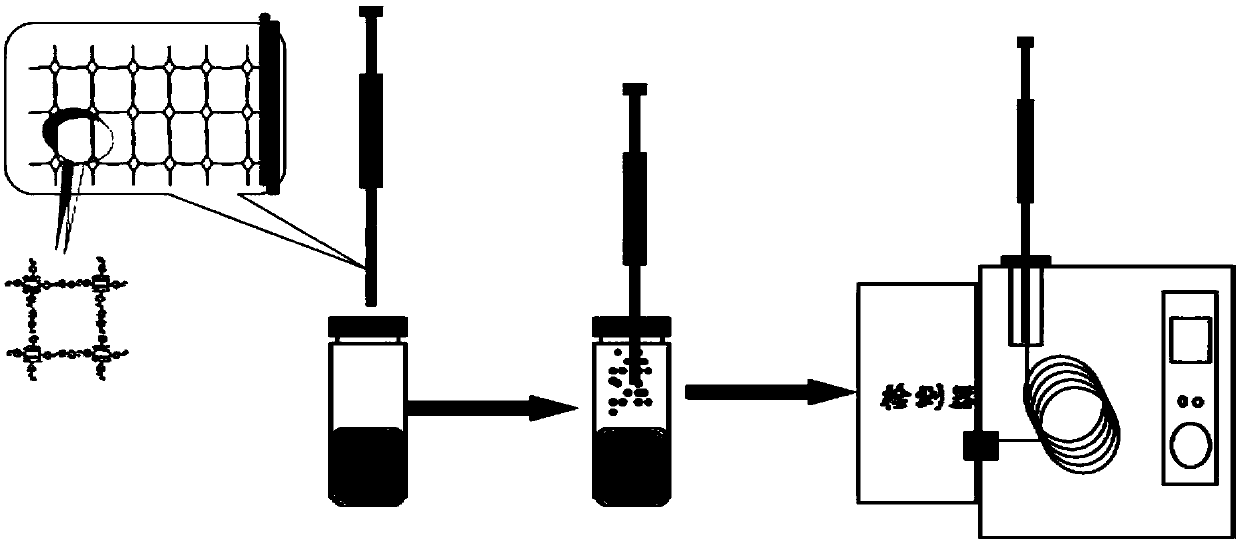
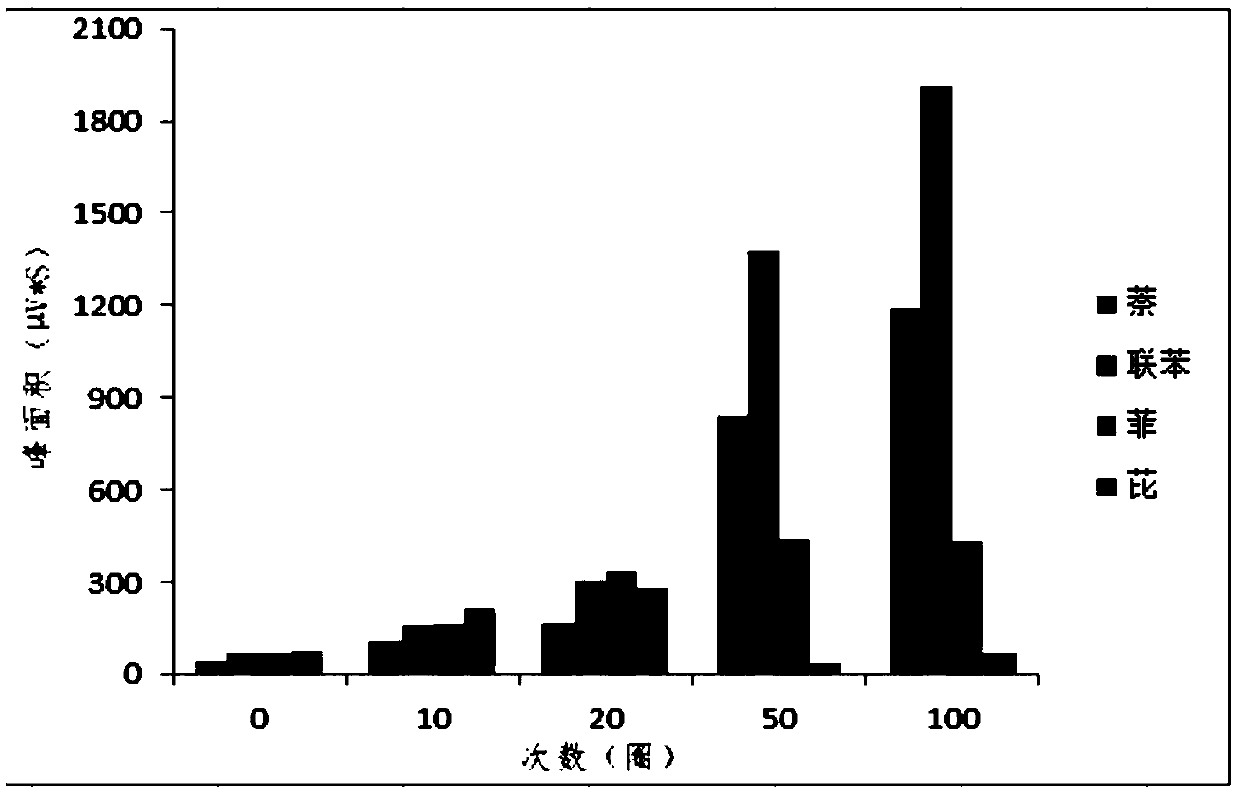
The invention discloses a preparation method and application of a solid-phase microextraction fiber of a self-assembly multi-layer porphyrin organic frame compound, and belongs to the field of analytical chemistry and environmental chemistry. A stainless steel wire pretreated with aqua regia is sequentially modified with silicon hydroxyl and amino, and the porphyrin / porphyrin copper organic covalent frame compound modified solid-phase microextraction fiber is obtained by adopting a layer by layer self-assembly growth mode. A method for detecting the content of trace polycyclic aromatic hydrocarbon in water by the SPME-GC coupling technique is established, and an urban actual water sample is detected by the SPME-GC combined analysis, which discovers that the actual water sample can be effectively detected, and the home-made COFs material modified solid-phase microextraction fiber has a relatively good actual operation value.
Owner:HENAN INST OF SCI & TECH
Leponex dispersible tablet and preparation method thereof
InactiveCN1806806AMedication convenienceImprove complianceOrganic active ingredientsNervous disorderCelluloseSilica gel
The invention discloses a clozapine dispersion tablet, which is prepared from 50.0-65.0 wt% of clozapine, 1.0-3.0.0wt% of sodium carboxymethylstarch, 25.0-40.0.0wt% of crystalline cellulose, 0.1-0.5.0wt% of Aspartame, 0.2-1.0.0wt% of magnesium stearate, 0.1-0.5wt% of sodium dodecyl sulfate, 2.0-4.0% of polyvinylpyrrolidone and 1.0-3.0% of miropowdered silica gel through disintegrating and passing through 100 mesh sieve, mixing homogenously, adjusting sheet weight, tabletting so as to obtain the dispersible tablets.
Owner:马晶
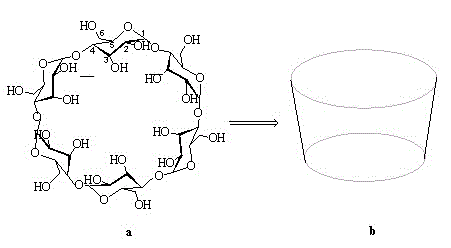
Beta-cyclodextrin derivative and preparation method thereof, and polyurea-bond cyclodextrin chiral stationary phase prepared from beta-cyclodextrin derivative
ActiveCN103910812AWide variety of sourcesMild reaction conditionsOther chemical processesBulk chemical productionSimulated moving bedCyclodextrin Derivatives
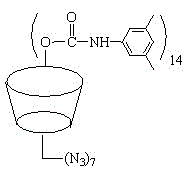
The invention discloses a beta-cyclodextrin derivative and a preparation method thereof and a polyurea-bond cyclodextrin chiral stationary phase prepared from the beta-cyclodextrin derivative. The beta-cyclodextrin derivative has a molecular formula of [((CH3)2C6H3NHCO)14C42H49O28)(N3)7] and has a structural formula as shown in a formula (I) which is described in the specification. The beta-cyclodextrin derivative is used as a chiral selector and undergoes a Staudinger reaction with ammoniated silica gel so as to obtain the novel poly-bonded cyclodextrin chiral stationary phase. The chiral stationary phase provided by the invention has good stability in a mobile phase with strong polarity and high water content, has good selectivity with regard to chiral compounds and can be applied in high performance liquid chromatography (HPLC), simulated moving bed chromatography (SMB), supercutical fluid chromatography (SFC), etc.
Owner:广州研创生物技术发展有限公司
Carbazole nine-element condensed ring central core-containing D(A-Ar)4 type organic photoelectric material, and preparation method and applications thereof
InactiveCN109180706AClear molecular structureMolecular weight determinationOrganic chemistrySolid-state devicesOrganic solar cellCarbazole
The invention belongs to the technical of organic photoelectric material, and more specifically relates to a carbazole nine-element condensed ring central core-containing D(A-Ar)4 type organic photoelectric material, and a preparation method and applications thereof. According to the preparation method, a three-element carbazole central ring unit and a thiophene derivative are subjected to coupling, ring closure reaction and coupling reaction are adopted so as to obtain an aromatic compound derivative containing a carbazole based nine-element condensed ring coplanar structure, and a D(A-Ar)4 butterfly type organic small molecular photovoltaic material containing a carbazole nine-element condensed ring center donor core. The aromatic compound derivative containing a carbazole based nine-element condensed ring coplanar structure, and the D(A-Ar)4 butterfly type organic small molecular photovoltaic material possesses high heat stability and excellent plane performance. The structure witha wide spectrum absorption range and an appropriate energy level is promising to be taken as organic solar energy cell donors or receptor materials.
Owner:CHANGZHOU UNIV
Printed electrostatic flocking material and digital transfer printing process thereof
InactiveCN109334150AHigh color fastnessHigh color yieldLayered productsDuplicating/marking methodsProduct patternEngineering
The invention provides a digital transfer printing process for a printed electrostatic flocking material. The digital transfer printing process comprises a step of preparing a transfer printing base material, wherein the step of preparing the transfer printing base material comprises the following steps: printing dye color paste onto a transfer printing base material by using a digital ink-jet printer, and forming the transfer printing base material with patterns and decorative designs; the adopted transfer printing base material is transfer printing paper or a transfer printing film. The printing process provided by the invention has the characteristics of being high in color yield, high in fixation rate, vivid in product pattern effect and excellent in batch reproducibility.
Owner:山东领潮新材料有限公司 +2
Methotrexate oral disintegrating tablet and its preparation method
InactiveCN1320887CHigh dissolution rateFast absorptionOrganic active ingredientsPill deliveryCelluloseMANNITOL/SORBITOL
The invention provides a methotrexate oral disintegrating tablet for rapid disintegrating in oral cavity without water, which is prepared by comminuting methotrexate, sodium carboxymethylstarch, crystalline cellulose, mannitol, magnesium stearate, passing through 100 mesh sieves, mixing homogeneously, and pelleting directly.
Owner:马晶
Perphenazine orally disintegrating tablet and preparation method thereof
InactiveCN1806803APromote absorptionHigh dissolution rateOrganic active ingredientsNervous disorderCellulosePerphenazine
The invention discloses a Perphenazine oral disintegrating tablet, which is prepared from 2.0-10.0 wt% of Perphenazine, 20.0-30.0wt% of sodium carboxymethylstarch, 50.0-70.0wt% of crystalline cellulose, 5.0-10.0wt% of miropowdered silica gel, 0.5-2.0wt% of magnesium stearate, and 0.1-0.5 wt% of polysorbate through passing through 100 mesh sieves, mixing homogenously, and carrying out powder direct pelleting method to obtain the composition.
Owner:马晶
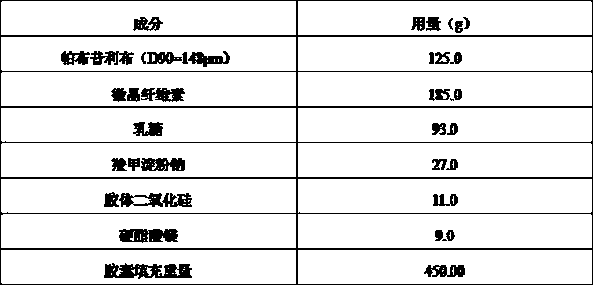
A kind of palbociclib pharmaceutical composition and preparation method thereof
ActiveCN105748435BShorten absorption timeGuaranteed validityOrganic active ingredientsCapsule deliveryMedicineBiological availability
Owner:河北泽运生物医药科技有限公司
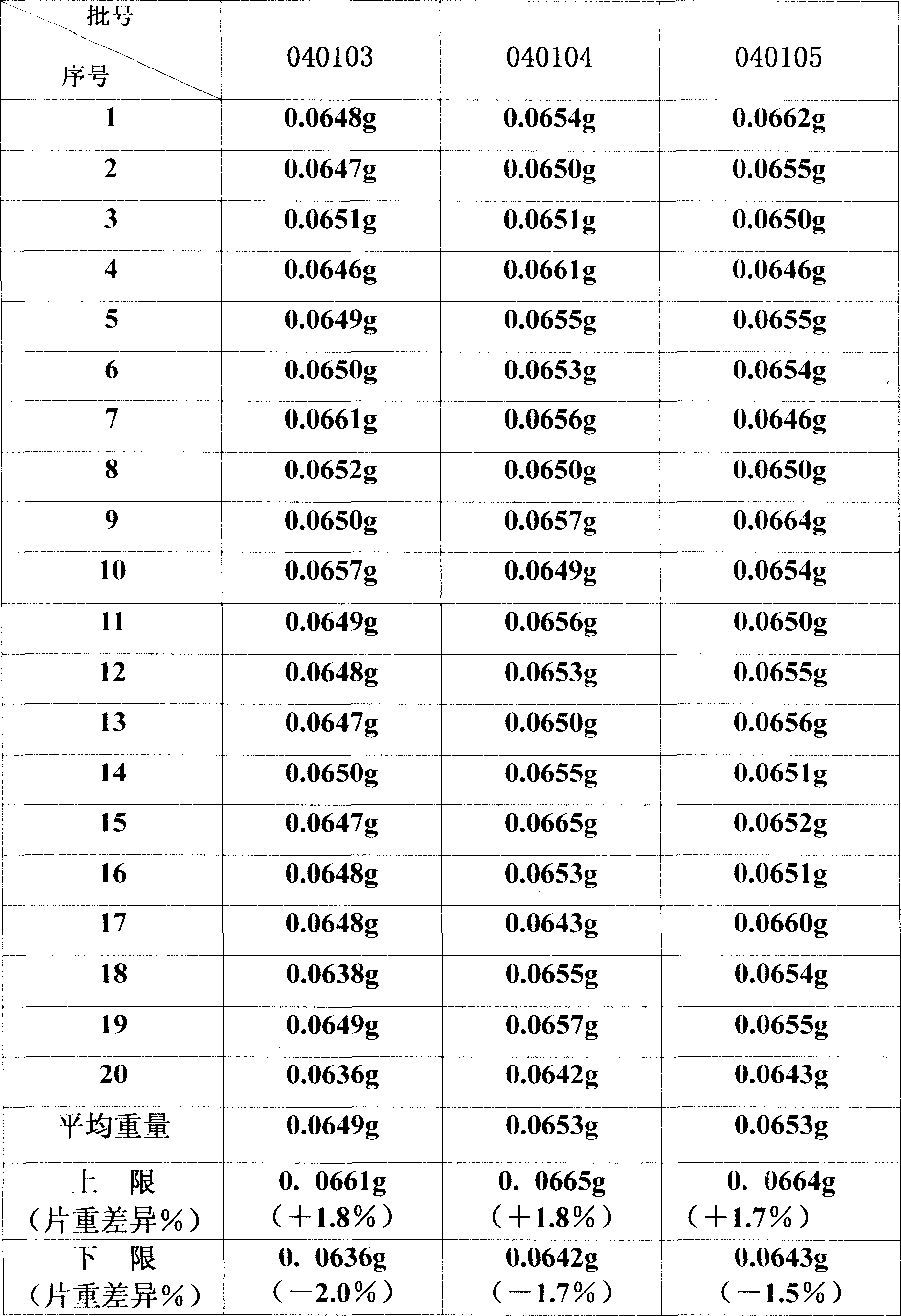
Preparation technology of roflumilast film-coated tablets
InactiveCN111643470AFriability meets requirementsGood granulationPharmaceutical non-active ingredientsRespiratory disorderAdhesiveMagnesium stearate
The invention relates to the technical field of tablet preparation, in particular to a preparation technology of roflumilast film-coated tablets. The preparation technology comprises the following steps of S1, micronizing roflumilast, and filtering starch, lactose and magnesium stearate through an 80-mesh sieve for later use; S2, dissolving hydroxypropyl methylcellulose into water to prepare aqueous solutions of different concentrations for later use; S3, mixing a mixture obtained by multiple equal incremental blending of 1 g of roflumilast and 15 g of starch with 398 g of lactose and remaining starch in the step S1; S4, adding an adhesive for granulation after mixing is ended; S5, preparing wet granules from prepared soft materials; S6, drying the wet granules obtained in the step S5; S7,carrying out granulation on the dried granules through a 20-mesh sieve; and S8, carrying out tableting by using a shallow concave mold with the diameter of 9 mm. When granulation is carried out by using the method, the granulation condition is good, the granules are uniform, the tablets are bright and clean, and the friability of tablet cores meets the requirements. Samples meeting the standard can be prepared through the method, the interbatch reproducibility is good, the samples are equivalent to originally commercially available products in quality, and production can be smoothly implemented through the preparation technology of a prescription.
Owner:山东希尔康泰药业有限公司
Forming process for net corrugated board structure of cloth dyeing machine
ActiveCN105057982AImprove efficiencyReduce surface abrasionShaping toolsTextile treatment machine partsBoard structureForming processes
The invention relates to the technical field of bending, in particular to a forming process for a net corrugated board structure of a cloth dyeing machine. The process includes the following steps that firstly, a board is cut into flat plates according to the calculated corrugated board unfolding size; secondly, the flat plates are polished, and protection films are attached to the flat plates; thirdly, the flat plates are placed on a working table of a bending machine and are pressed and bent through the bending machine and a pressing and bending die so that a plurality of arc-shaped protruding strips (1) can be formed in the middles of the flat plates; fourthly, the two sides of the flat plates are bent inwards through the bending machine and an edge bending die; and fifthly, greasy dirt processing is conducted on the surfaces of the flat plates. The process has the beneficial effects that the bending machine and the dies are adopted, continuous hydraulic forming is conducted on the size-fixed board, efficiency is high, surface scratches are fewer, the batch repeatability is high, and products are stable.
Owner:HANGZHOU DONGLIN DYEING & FINISHING MACHINERY
Breviscapine orally disintegrating tablet and preparation method thereof
InactiveCN1806808AHigh dissolution rateGreat tasteOrganic active ingredientsNervous disorderCelluloseOrally disintegrating tablet
The invention discloses a Breviscapine oral disintegrating tablet, which comprises 21.0-35.0 wt% of Breviscapine, 11.0-15.0.0wt% of sodium carboxymethylstarch, 45.0-60.0 wt% of crystalline cellulose, 1.0-2.0 wt% of magnesium stearate, 5.0-10.0 wt% of miropowdered silica gel, and 0.1-0.5wt% of Aspartame. the relative weight ratio of Breviscapine and sodium carboxymethylstarch is 1:0.3-1:0.7.
Owner:马晶
Roxatidine acetate hydrochloride slow-release capsules and preparation method thereof
PendingCN110403918AHas a long-acting sustained-release effectImprove bioavailabilityOrganic active ingredientsDigestive systemMedicineSustained Release Capsule
The invention discloses roxatidine acetate hydrochloride slow-release capsules and a preparation method thereof. A main medicine is roxatidine acetate hydrochloride, auxiliary materials consist of blank pill cores, slow release materials, a plastifier and a pore-forming agent, wherein the weight ratio of the roxatidine acetate hydrochloride to the blank pill cores to the slow release materials tothe plastifier to the pore-forming agent is 1.00 to (0.90-1.00) to (0.10-0.20) to (0.01-0.02) to (0.01-0.02). A preparation method of the roxatidine acetate hydrochloride slow-release capsules disclosed by the invention is simple and convenient to operate, good in inter-batch repeatability and easy in amplified production. The coating effects are good, during coating, good film forming characteristics of a coating solution are maintained, the coating uniformity is good, and the loss of the coating solution can be reduced. The dissolving-out behaviors of the roxatidine acetate hydrochloride slow-release capsules in four kinds of dissolving-out mediums of water, a dissolving-out medium of which the pH is 1.2, a dissolving-out medium of which the pH is 4.0 and a dissolving-out medium of whichthe pH is 6.8 are consistent in those of an original developing agent.
Owner:FUZHOU MINHAI PHARMA
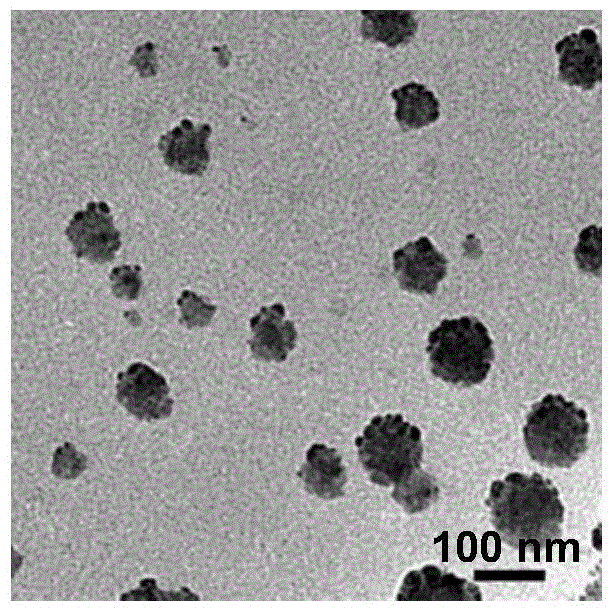
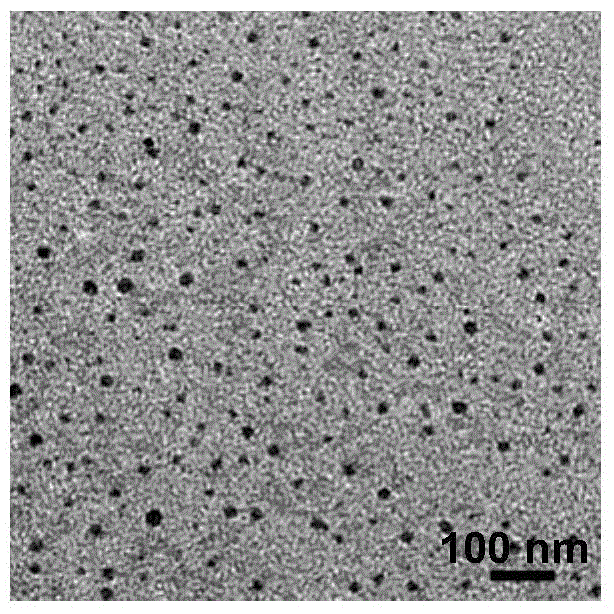
Preparation method for nanocrystalline palladium-carbon catalyst with high activity
InactiveCN105771973ALarge particle sizeWide distributionMetal/metal-oxides/metal-hydroxide catalystsDispersityUltrasonic assisted
The invention discloses a preparation method for a nanocrystalline palladium-carbon catalyst with high activity. The preparation method comprises the following steps: preparation of a palladium complex; adsorption and adhesion of the palladium complex; preparation of a reducing agent; ultrasonic-assisted reduction and drying; etc. The nanocrystalline palladium-carbon catalyst with high activity prepared in the invention mainly overcomes the problems of difficulty in effective control of granularity and dispersity of palladium in palladium-carbon catalysts produced by traditional precipitation methods and impregnation methods, poor repeatability of batch production of the catalysts, large particle sizes, wide distribution range, low dispersity and poor stability of prepared active palladium particles, contamination of products caused by shedding of nanometer palladium particles, low repeated usage frequencies, etc. The nanocrystalline palladium-carbon catalyst with high activity prepared in the invention has the advantages of small sizes and smaller dispersity of active palladium particles, and better catalytic activity, better selectivity and higher repeated usage frequencies in catalytic reactions, etc.
Owner:SHAANXI KENUOHUA CHEM TECH CO LTD
Method for judging quality uniformity of leaf type tobacco flakes
InactiveCN112526080AEasy to operateEasy to testMaterial weighingChemical compositionAnalytical chemistry
The invention discloses a method for judging quality uniformity of leaf type tobacco flakes, wherein the method comprises the steps: taking down eggplant core tobacco flakes from equipment to obtain acertain number of samples, measuring the moisture content and chemical components of the samples by adopting an industrial standard method, and calculating a stability coefficient according to a moisture content value and a nicotine value; and meanwhile, screening the samples by screening equipment, enabling each screen to represent a sample with a structure of one type, distinguishing the samples with different structures, calculating the stability coefficient of the mass of the sample on each layer of screen, and calculating the proportion of the mass of each layer of screen in all the samples to the mass of the total samples, so as to calculate the comprehensive variation coefficient and the stability coefficient of the tobacco flake structure. The closer the stability coefficient is to 100%, the better the uniformity of the quality of the tobacco flakes is, the higher the stability level is, the method is easy to operate, multiple indexes are combined to evaluate the tobacco flakes, and the method has the advantage of being reliable in evaluation numerical value.
Owner:HUBEI CHINA TOBACCO IND
Empagliflozin metformin sustained release preparation and preparation method thereof
ActiveCN113616624ANice appearanceImprove liquidityOrganic active ingredientsMetabolism disorderMetformin hclImmediate release
The invention belongs to the field of pharmaceutical preparations, and particularly relates to an empagliflozin metformin sustained release preparation and a preparation method thereof. An empagliflozin pellet core is a quick release part, a metformin hydrochloride pellet core is a sustained release part, the two pellet cores are coated with isolation layers respectively, and then the two drug-loaded pellets are mixed and filled into capsules. The invention further discloses the preparation method of the empagliflozin metformin sustained release preparation. The preparation method comprises the steps that (1), the metformin hydrochloride pellet core is prepared through an extrusion and spheronization process; (2), the empagliflozin quick release pellet core is prepared through the extrusion and spheronization process; (3), the two drug-loaded pellet cores are coated with the isolation layers respectively; and (4), the two drug-loaded pellets are filled into the capsules according to a certain proportion. The preparation process is good in stability, simple, high in production efficiency and suitable for industrial mass production.
Owner:南京康川济医药科技有限公司
Low temperature-resistant cable sheath material and preparation method thereof
InactiveCN108623895AReasonable formulaEasy to manufacturePlastic/resin/waxes insulatorsFiberAging resistance
The invention provides a low temperature-resistant cable sheath material and a preparation method thereof. The low temperature-resistant cable sheath material comprises the following raw materials inparts by weight: 90-110 parts of an ethylene-vinyl acetate copolymer, 20-40 parts of bisphenol A-type polyarylate, 1-5 parts of polyphenylene sulfide fiber, 5-15 parts of calcium oxide, 5-15 parts ofparaffin oil, 0.2-0.6 part of diisononyl phthalate, 0.1-0.3 part of terephthalate and 0.05-0.15 part of a light stabilizer. The preparation method comprises the following steps: S1, preparing raw materials; S2, premixing the polyphenylene sulfide fiber, the calcium oxide and the paraffin oil; S3, mixing; S4, extruding, moulding and drying. The cable sheath material provided by the invention is reasonable in formula, low in temperature resistance and wear resistance, good in aging resistance and long in service life, the raw materials and the preparation process are environment-friendly, the preparation method is simple, reproducibility between batches is good, and the suitability for industrial production is achieved.
Owner:ANHUI NIKOLA ELECTRONICS TECH CO LTD
Application of CCA-CD co-assembly in preparation of macromolecular toxin detoxification drug
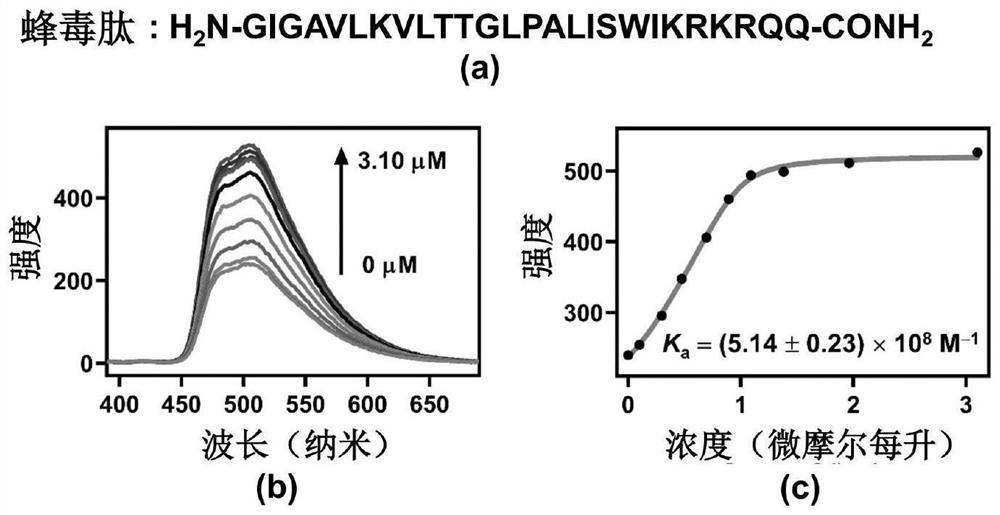
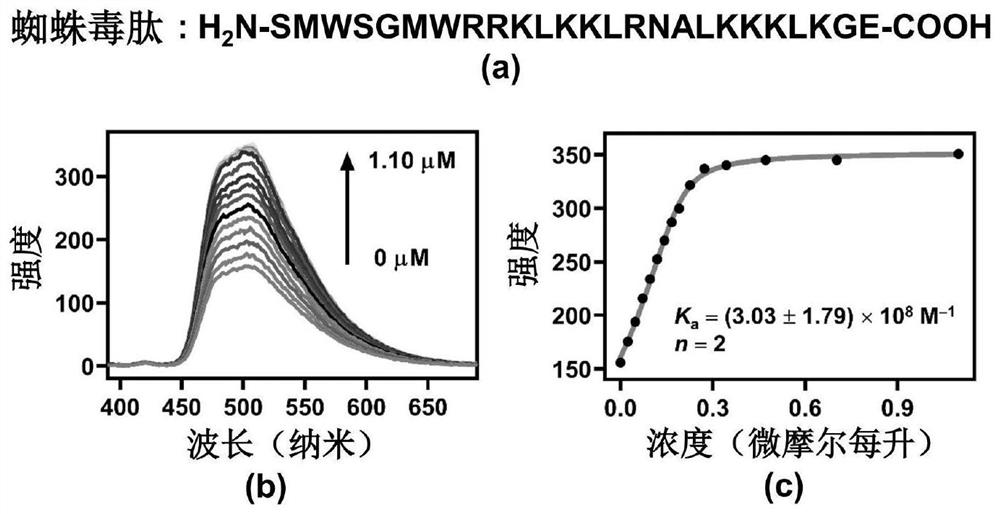
ActiveCN113509486AClear chemical structureGood batch-to-batch reproducibilityOrganic active ingredientsNanomedicineCytotoxicityMelittin
The invention relates to an application of a CCA-CD co-assembly in the preparation of a macromolecular toxin detoxification drug. The co-assembly CCA-CD can be used for effectively complexing melittin, spider venom and snake venom and relieving the cytotoxicity of melittin, spider venom and snake venom, and has the potential of treating poisoning of toxic macromolecules. Each component of the CCA-CD is clear in chemical structure and good in batch reproducibility, and the CCA-CD has very high chemical stability and thermal stability, does not influence the activity of important proteins in a living body, and has good biocompatibility.
Owner:NANKAI CANGZHOU BOHAI NEW AREA GREENING CHEM RES CO LTD
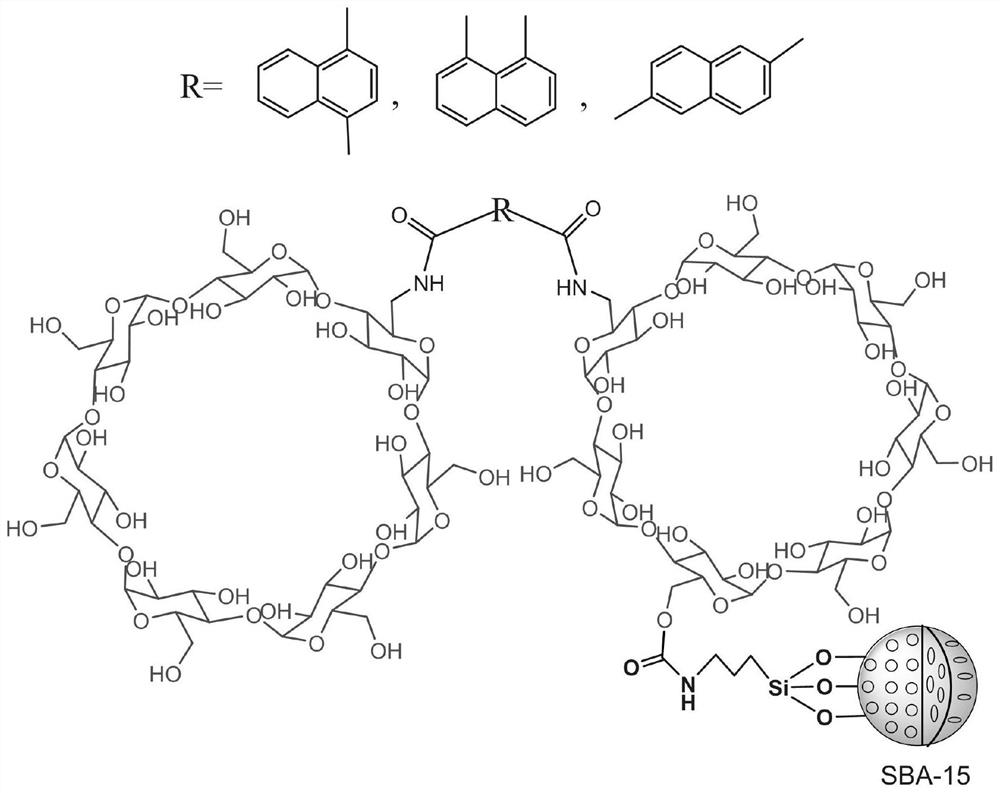
Preparation method and application of a naphthaloyl bridged double β-cyclodextrin bonded chiral stationary phase
ActiveCN110918076BGood separation of enantiomersSynergistic recognitionOther chemical processesStationary phaseCyclodextrin
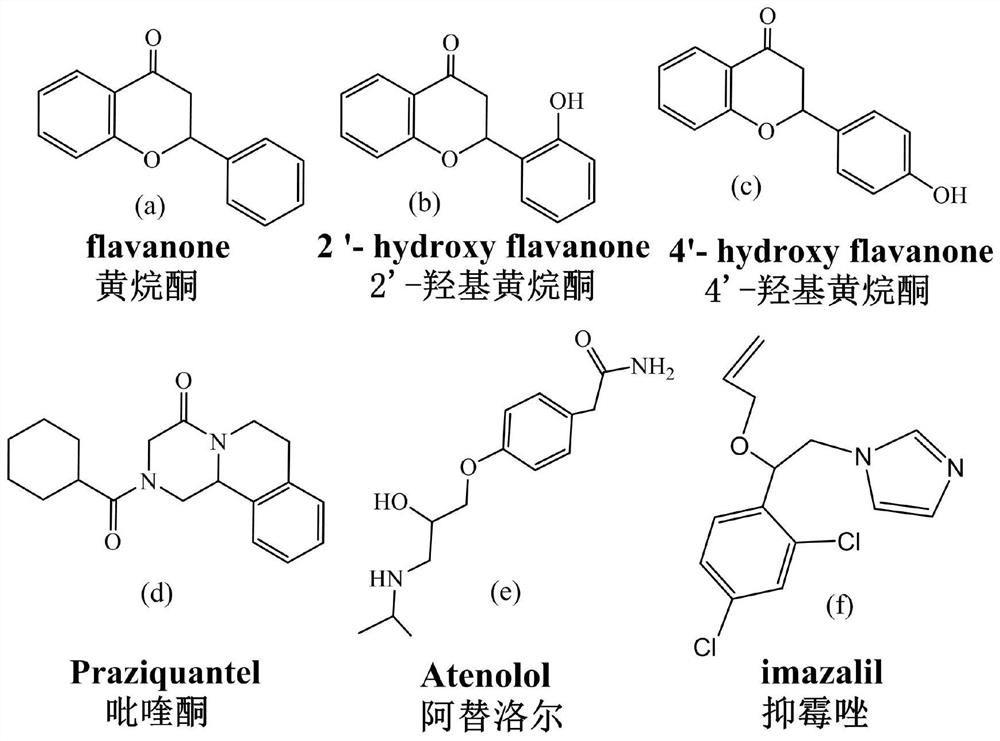
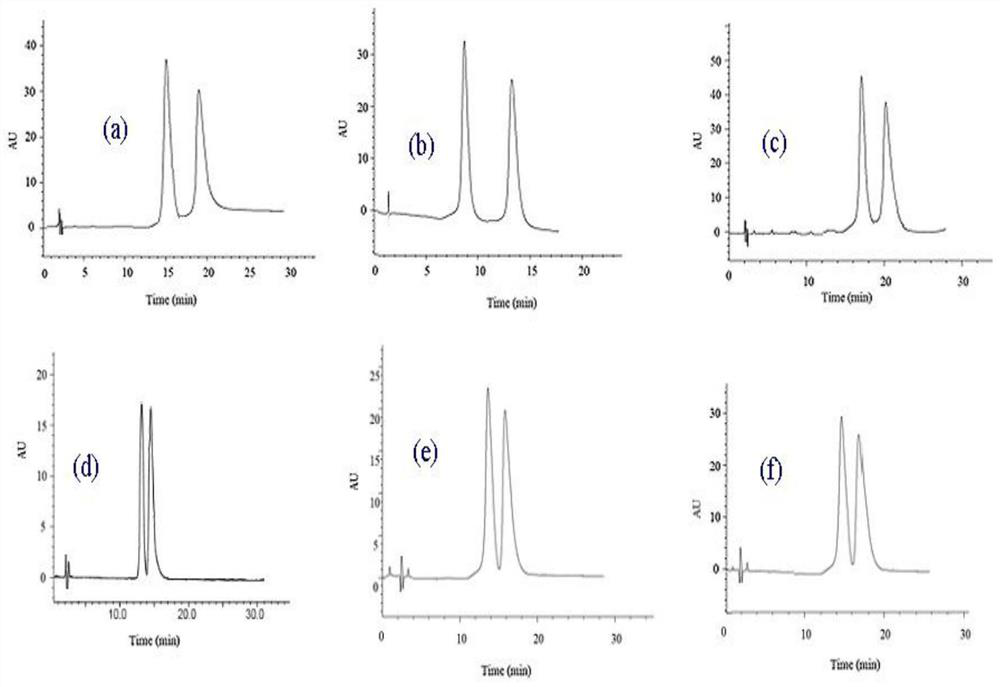
The invention discloses a preparation method of a naphthaloyl-bridged bis-β-cyclodextrin bonded chiral stationary phase, using naphthaloyl-bridged bis-β-cyclodextrin as a chiral stationary phase ligand, SBA-15 The naphthaloyl bridged bis-cyclodextrin bonded chiral separation material is a silica gel substrate, and the naphthaloyl bridged biscyclodextrin stationary phase of the present invention has obvious advantages in chiral separation. The traditional single β-cyclodextrin stationary phase cannot resolve substances such as flavanone (a), 4’-hydroxyflavanone (c), praziquantel (d), and imazalil (f). The naphthaloyl bridged double β-cyclodextrin stationary phase prepared by the invention can be completely resolved.
Owner:NANCHANG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com