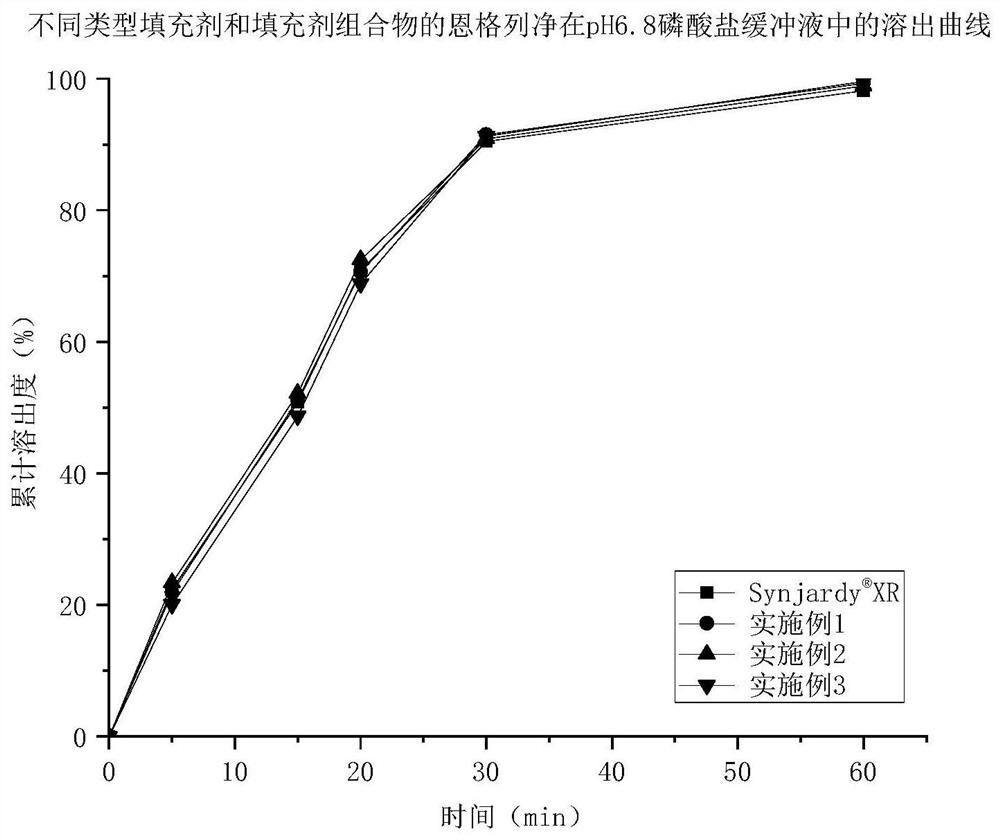
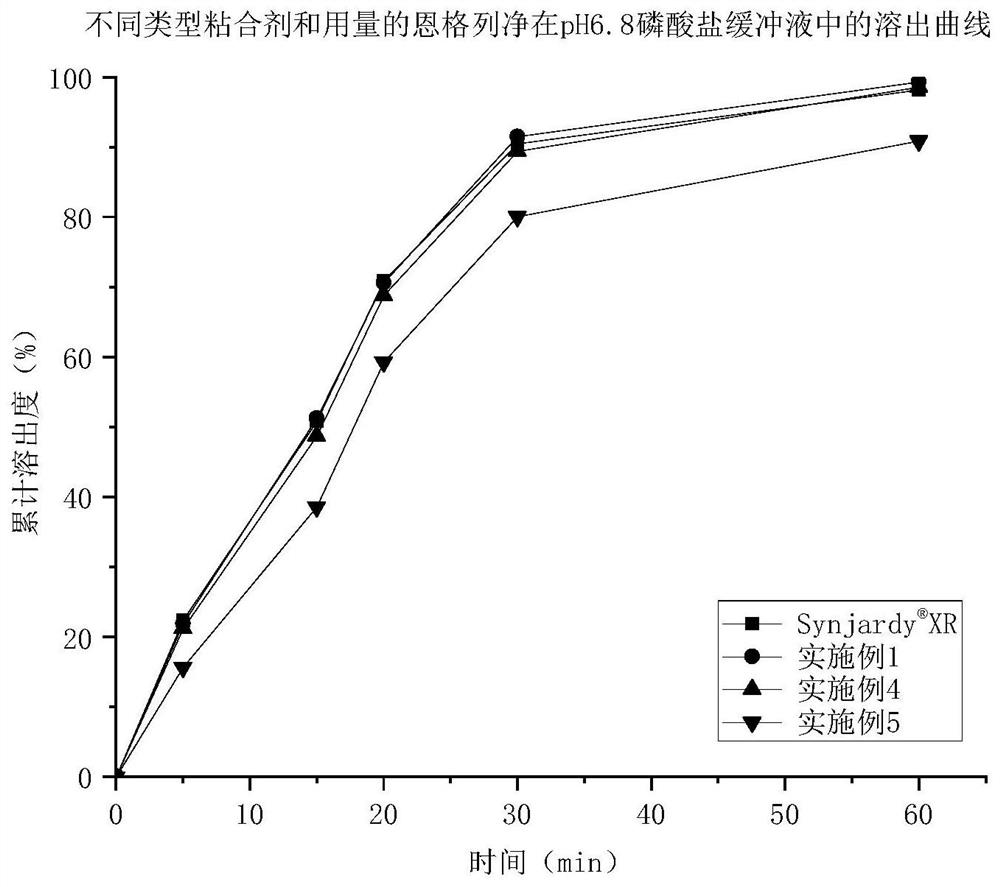
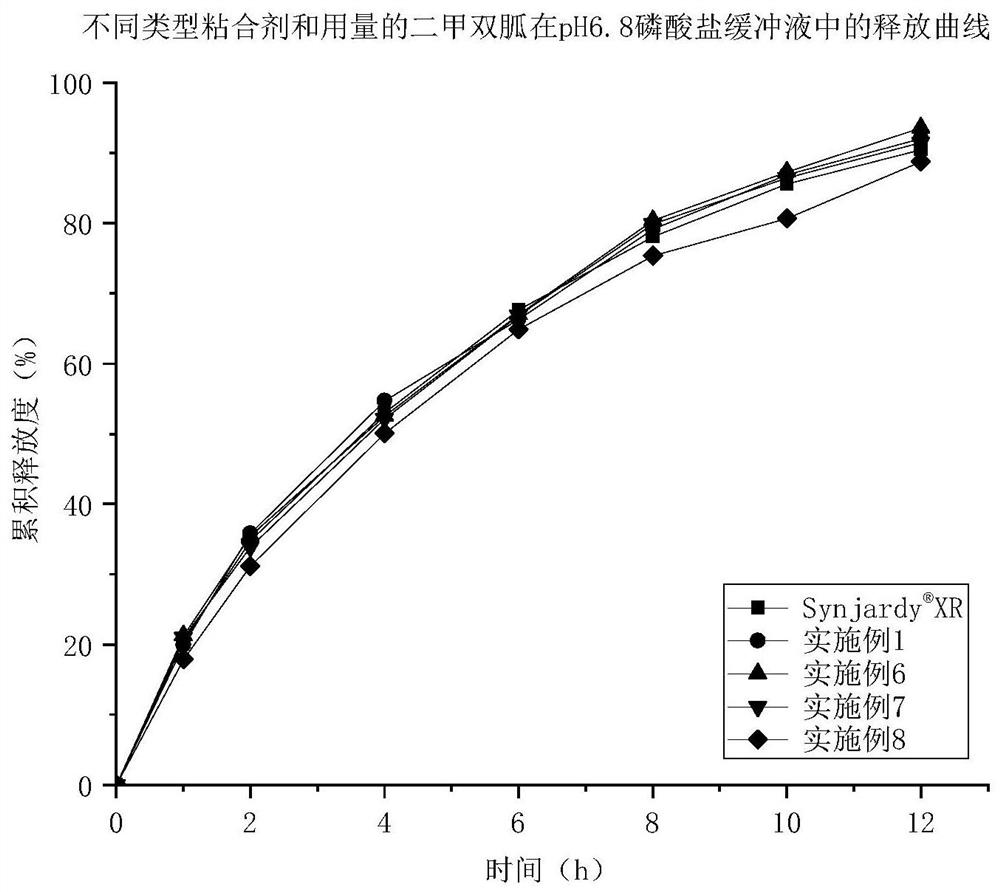
Empagliflozin metformin sustained release preparation and preparation method thereof
A technology of empagliflozin and metformin hydrochloride, which is applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of high particle fluidity and compressibility, and double-layer tablets There are cracks in the connection, high setting requirements, etc., to achieve the effect of facilitating drug absorption, reducing the number of medications, and good reproducibility between batches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
[0041] Preparation Process:
[0042] a. Metformin hydrochloride sustained-release pellets: Mix metformin hydrochloride, microcrystalline cellulose, sodium carboxymethylcellulose and hypromellose K100M evenly, and use water as a wetting agent to evenly spray in the mixed powder, Made of soft material with moderate humidity. The soft material is extruded into strips through the sieve plate of the extruder, adjust the appropriate rounding speed and rounding time, and then put the extruded product in the rounding barrel, take it out after it has a certain hardness and roundness, and put it in the oven Dry at 50°C for 4 hours;
[0043] b, Empagliflozin immediate-release pellets: mix microcrystalline cellulose and lactose evenly, use a binder containing Empagliflozin (preparation of carboxymethylcellulose sodium aqueous solution as a binder, and then add Englie Evenly dispersed in the binder solution) and evenly sprayed in the mixed powder to make a soft material with ...
Embodiment 2
[0047]
[0048] Empagliflozin immediate-release pellets are filled with microcrystalline cellulose and pregelatinized starch, and other formulation compositions and preparation processes are the same as in Example 1.
Embodiment 3
[0050]
[0051] The filler of the empagliflozin immediate-release pellet core is microcrystalline cellulose, and the composition and preparation process of other prescriptions are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com