Leponex orally disintegrating tablet and preparation method thereof
A technology of orally disintegrating tablets and clozapine, which is applied in the technical field of improving oral preparations of clozapine, can solve problems such as low dissolution rate, slow disintegration speed, and non-recording
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Clozapine 100.0g
[0043] Sodium carboxymethyl starch 14.0g
[0044] Microcrystalline Cellulose 60.0g
[0045] Micronized silica gel 5.0g
[0046] Aspartame 0.5g
[0047] Magnesium stearate 0.9g
[0048] Made into 1000 pieces
[0049] Clozapine, microcrystalline cellulose, sodium carboxymethyl starch, micropowder silica gel, and magnesium stearate were all passed through a 100-mesh sieve.
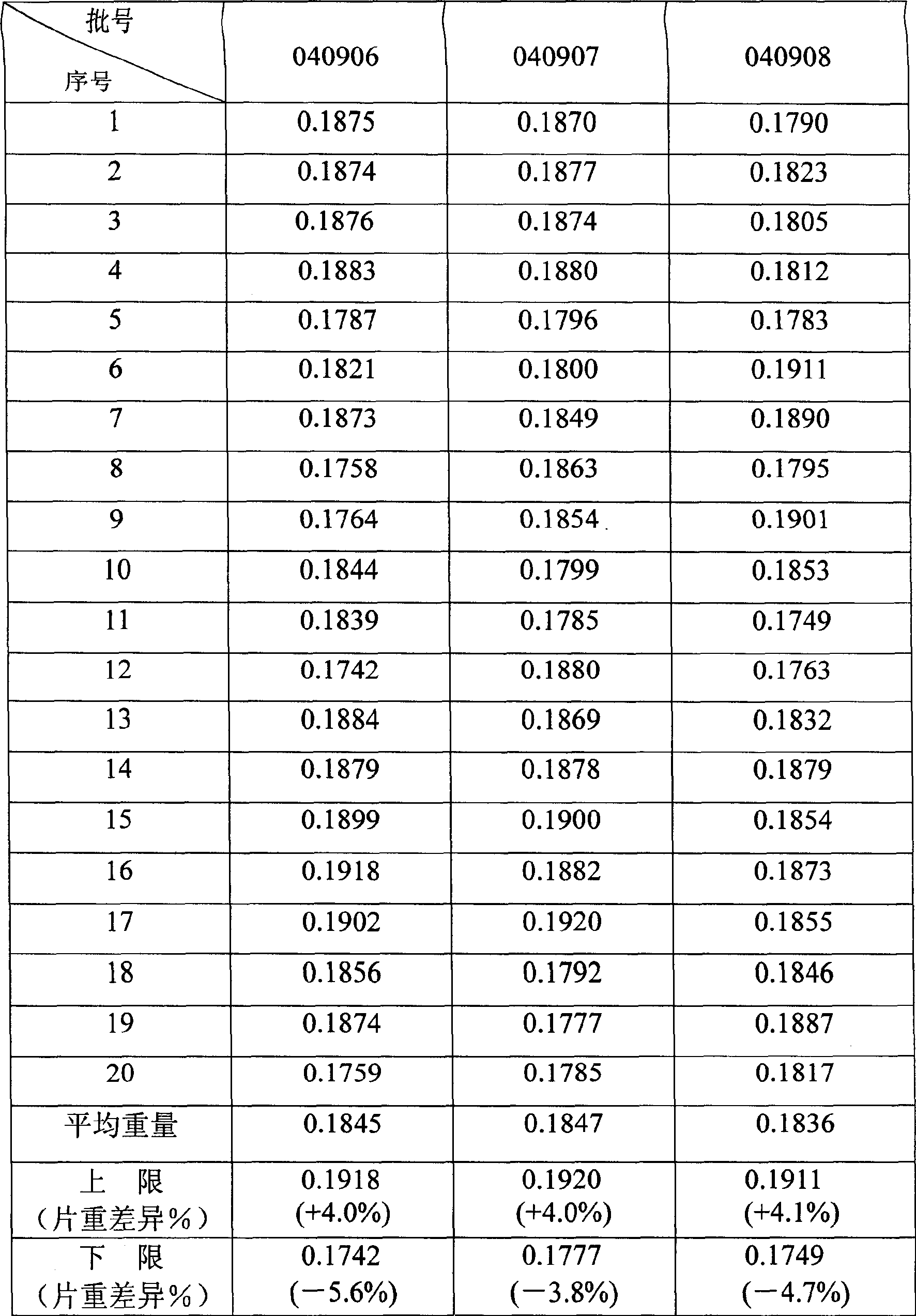
[0050] Weigh 100.0 grams of clozapine, first mix with 14.0 grams of sodium carboxymethyl starch in equal increments, then add 60.0 grams of microcrystalline cellulose and mix thoroughly, then mix with 5.0 grams of micropowder silica gel, 0.5 grams of aspartame Sweetened and mixed with 0.9 g of magnesium stearate. Detect the percentage content of clozapine in the mixed powder, calculate the weight of the tablet, and press it into a tablet. In this way, 1000 orally disintegrating tablets of clozapine can be compressed.
[0051] Its disintegration t...
Embodiment 2
[0053] Clozapine 85.0g
[0054] Sodium carboxymethyl starch 10.3g
[0055] Microcrystalline Cellulose 55.8g
[0056] Micronized silica gel 3.9g
[0057] Aspartame 0.3g
[0058] Magnesium Stearate 2.7g
[0059] Made into 1000 pieces
[0060] Clozapine, microcrystalline cellulose, sodium carboxymethyl starch, micropowder silica gel, and magnesium stearate were all passed through a 100-mesh sieve.
[0061] Weigh 85.0 grams of clozapine, first mix it with 10.3 grams of sodium carboxymethyl starch in equal increments, then add 55.8 grams of microcrystalline cellulose and mix thoroughly, then add 3.9 grams of micropowder silica gel, 0.3 aspartame Mix with 2.7 grams of magnesium stearate. Detect the percentage content of clozapine in the mixed powder, calculate the weight of the tablet, and press it into a tablet. In this way, 1000 orally disintegrating tablets of clozapine can be compressed.
[0062] Its disintegration time is 32 seconds, ...
Embodiment 3
[0064] Clozapine 87.0g
[0065] Sodium carboxymethyl starch 7.3g
[0066] Microcrystalline Cellulose 47.6g
[0067] Micronized silica gel 2.9g
[0068] Aspartame 0.1g
[0069] Magnesium stearate 0.1g
[0070] Made into 1000 pieces
[0071] Clozapine, microcrystalline cellulose, sodium carboxymethyl starch, magnesium stearate, and micropowder silica gel were all passed through a 100-mesh sieve.
[0072] Weigh 87.0 grams of clozapine, first mix with 7.3 grams of sodium carboxymethyl starch in an equal amount, then add 47.6 grams of microcrystalline cellulose and mix thoroughly, then successively 2.9 grams of micropowder silica gel, 0.1 grams of aspartame Mix with 0.1 gram of magnesium stearate. Detect the percentage content of clozapine in the mixed powder, calculate the weight of the tablet, and press it into a tablet. In this way, 1000 orally disintegrating tablets of clozapine can be compressed.
[0073] Its disintegration time ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com