A kind of atorvastatin calcium composition, preparation and preparation method thereof
A technology of atorvastatin calcium and composition, applied in the field of medicine, can solve the problems of reduced clinical safety, lack of any lipid-lowering activity, aggravated gastrointestinal irritation, etc., to reduce the incidence of adverse reactions and avoid the dissolution rate The difference is too large, the effect of reducing gastrointestinal adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] Secondly, the present invention provides a kind of preparation method of the composition of atorvastatin calcium, and this preparation method comprises following preparation steps:
[0049] (1) Suspend or dissolve atorvastatin calcium, micronized basic auxiliary materials, Tween and optional hydroxypropyl cellulose in a composite solvent of an appropriate amount of methanol and purified water, spray dry, and sieve to obtain mixture;
[0050] (2) Prepared by uniformly mixing the above atorvastatin calcium mixture with other remaining auxiliary materials.
[0051] In one embodiment, the basic auxiliary material is the basic auxiliary material obtained after the micronization step, and the sieving treatment is to pass through a 100-mesh sieve.
[0052] In one embodiment, the optional hydroxypropyl cellulose in step (1) means that hydroxypropyl cellulose can be added or not added in this step, and methanol and purified The volume ratio of water is 1:1-5, preferably 1:3. ...
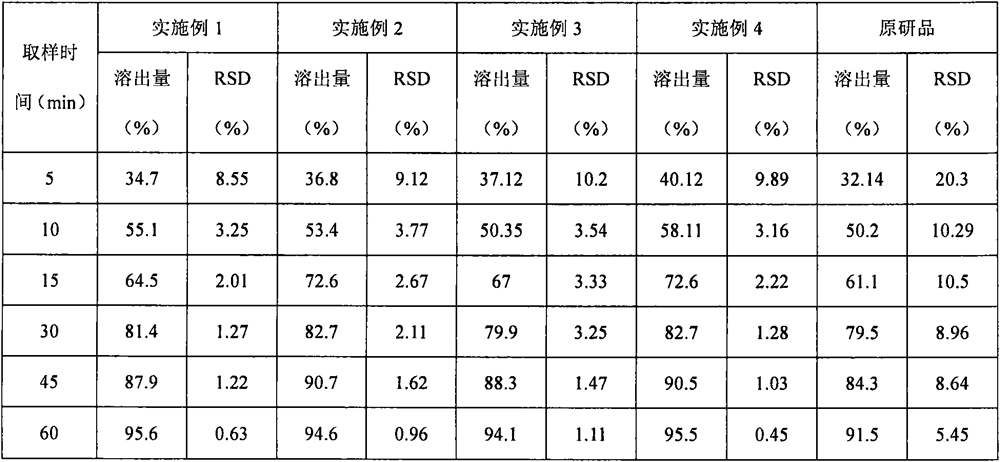
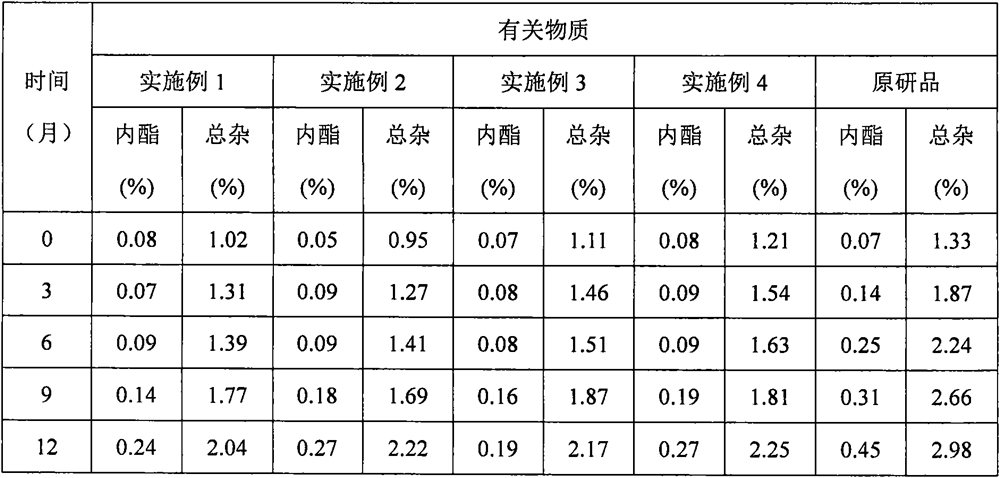
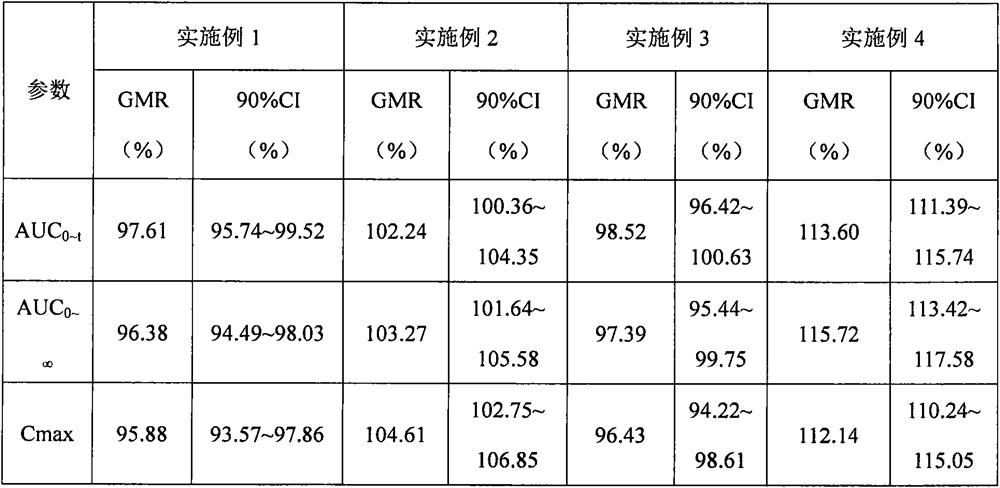
Embodiment 1
[0064] prescription:
[0065] Raw materials parts by weight Atorvastatin Calcium 1 calcium carbonate 2.75 Microcrystalline Cellulose PH101 5.50 lactose 2.85 Croscarmellose sodium (additional) 0.41 twain 0.04 Hypromellose EF 0.311 Croscarmellose sodium (additional) 0.41 Magnesium stearate 0.04
[0066] Preparation Process:
[0067] (1) Micronize the calcium carbonate in the prescription to less than 20 μm.
[0068] (2) Suspend atorvastatin calcium and micronized calcium carbonate, hydroxypropyl cellulose EF, and Tween in a mixed solvent of an appropriate amount of methanol and purified water, wherein the volume ratio of methanol to purified water is 1:3 , stirred evenly, and spray-dried to obtain a mixture. The spray-drying parameters were 70° C. of inlet air temperature, 40° C. of outlet air temperature, 35 Hz of induced air frequency, 40 Hz of blast air frequency, and 300 Hz of atomization frequency. The o...
Embodiment 2
[0072] prescription:
[0073] Raw materials parts by weight Atorvastatin Calcium 1 calcium silicate 3.03 Microcrystalline Cellulose PH101 6.34 Lactose 312 3.02 Crospovidone (internal addition) 0.40 twain 0.05 Hypromellose ELF 0.312 Crospovidone (additional) 0.33 Magnesium stearate 0.05
[0074] Preparation Process:
[0075] (1) Micronize the calcium carbonate in the prescription to less than 20 μm.
[0076] (2) Suspend atorvastatin calcium and micronized calcium silicate, hydroxypropyl cellulose ELF, and Tween in a mixed solvent of an appropriate amount of methanol and purified water, wherein the volume ratio of methanol to purified water is 1 : 3, stir evenly, spray dry to obtain mixture, spray dry parameters are 70 ℃ of air inlet temperature, 40 ℃ of air outlet temperature, 35 Hz of induced wind frequency, 40 Hz of blast frequency, 300 Hz of atomization frequency, gained mixture passes 100 mesh sieves.
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com