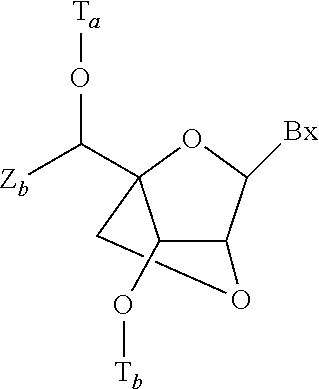
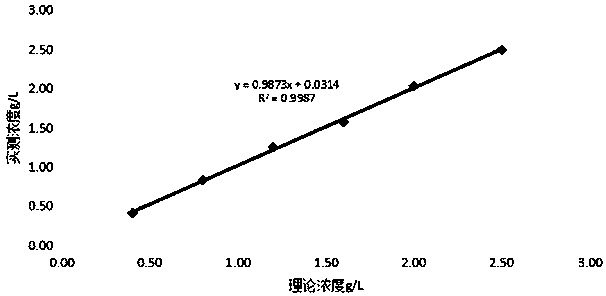
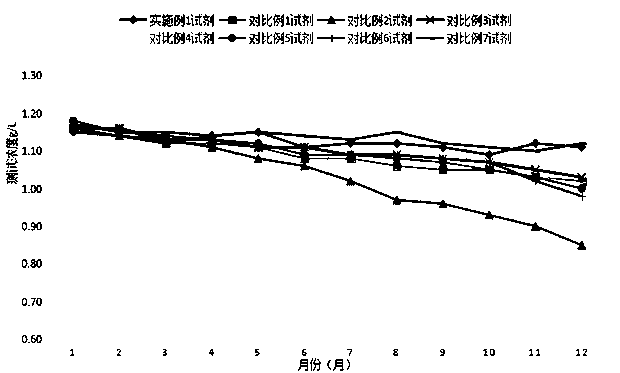
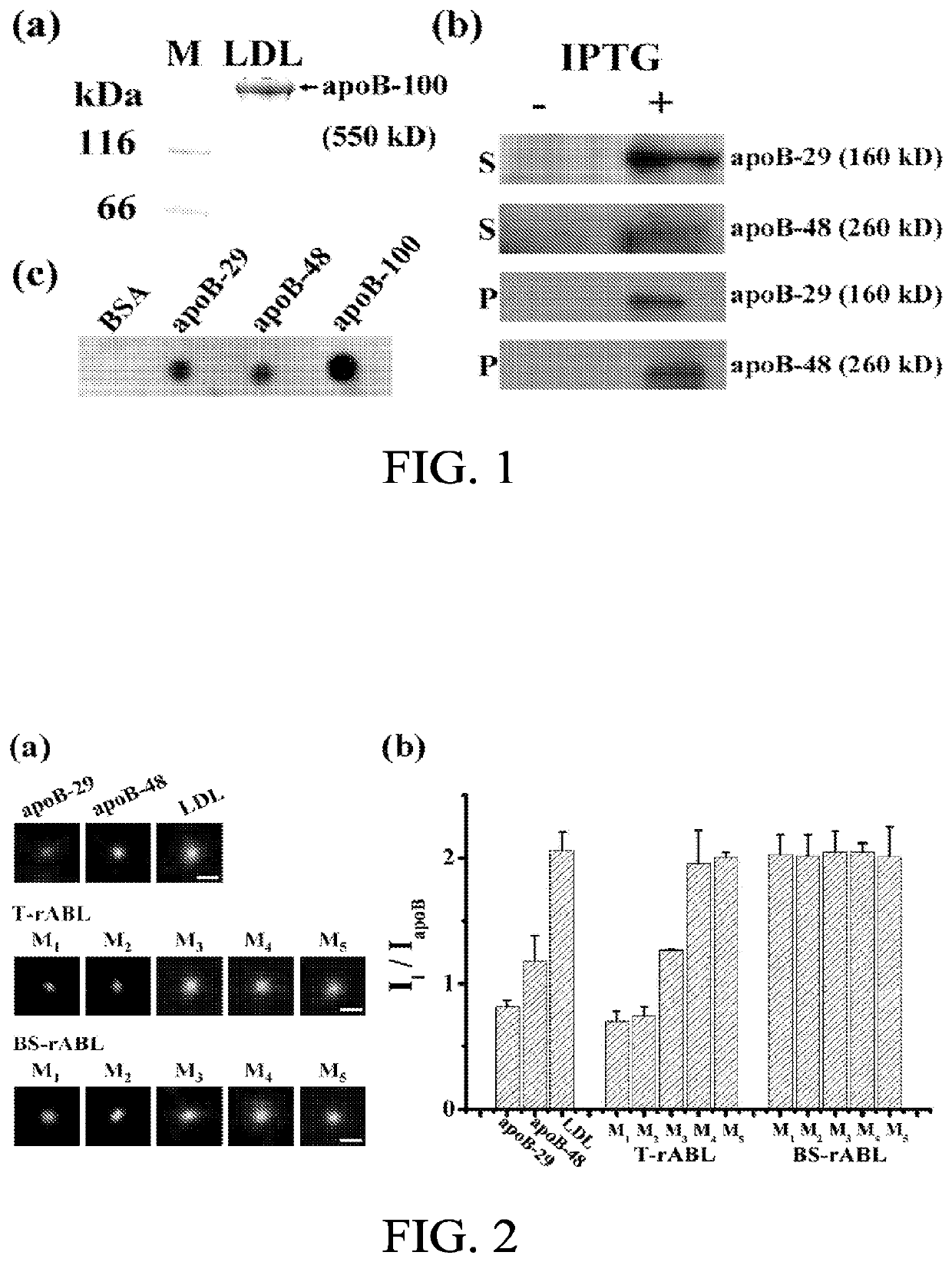
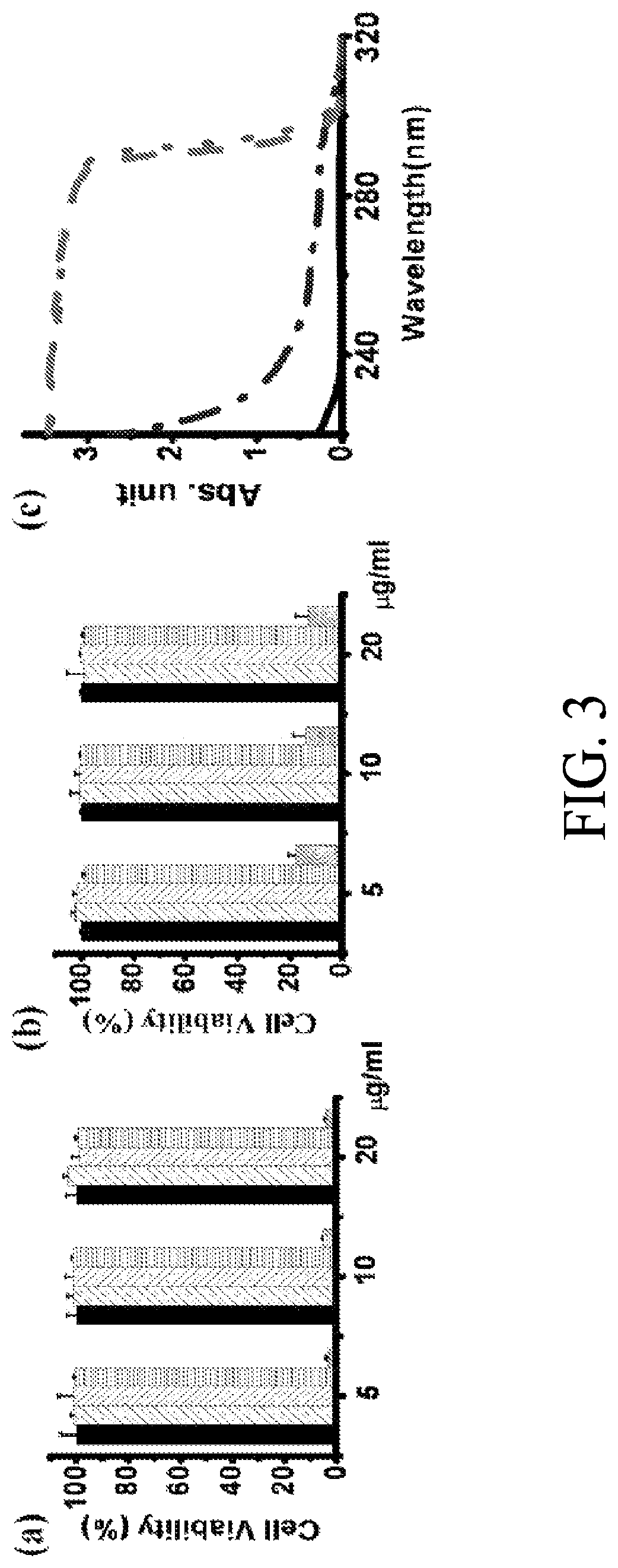
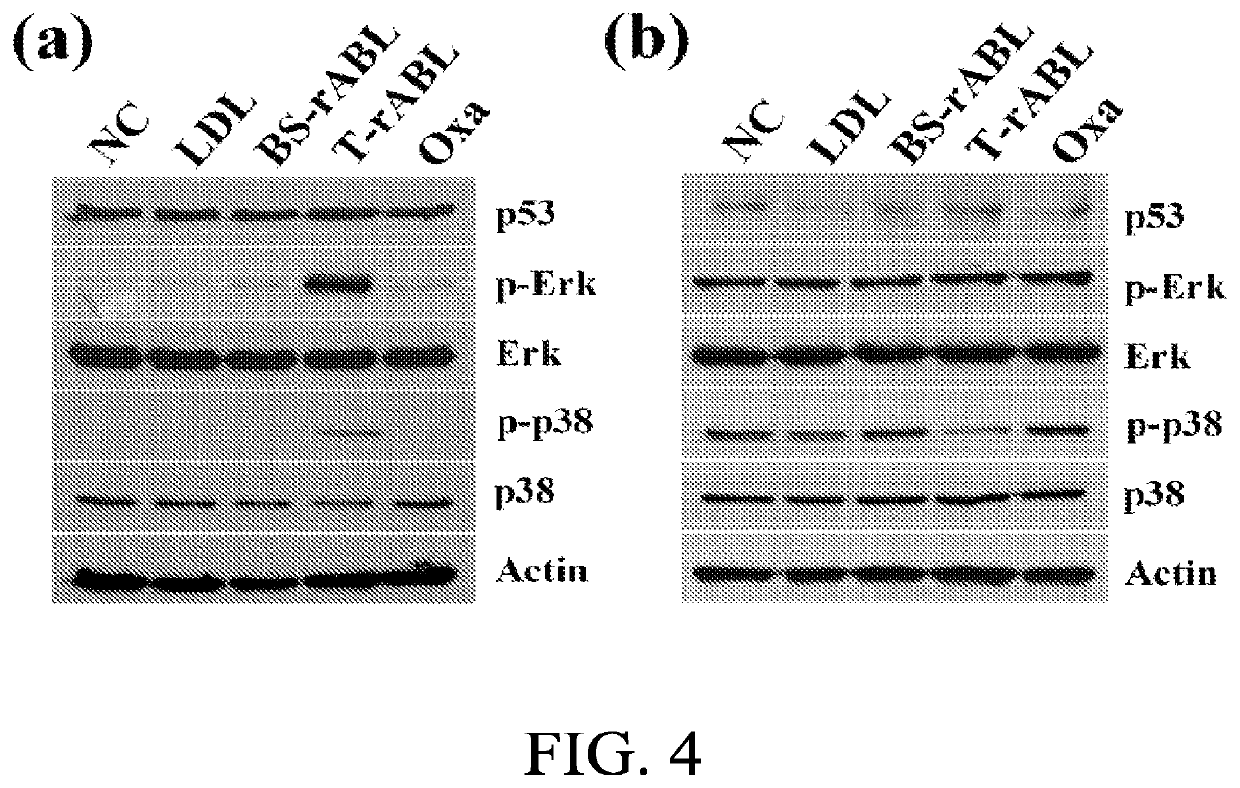
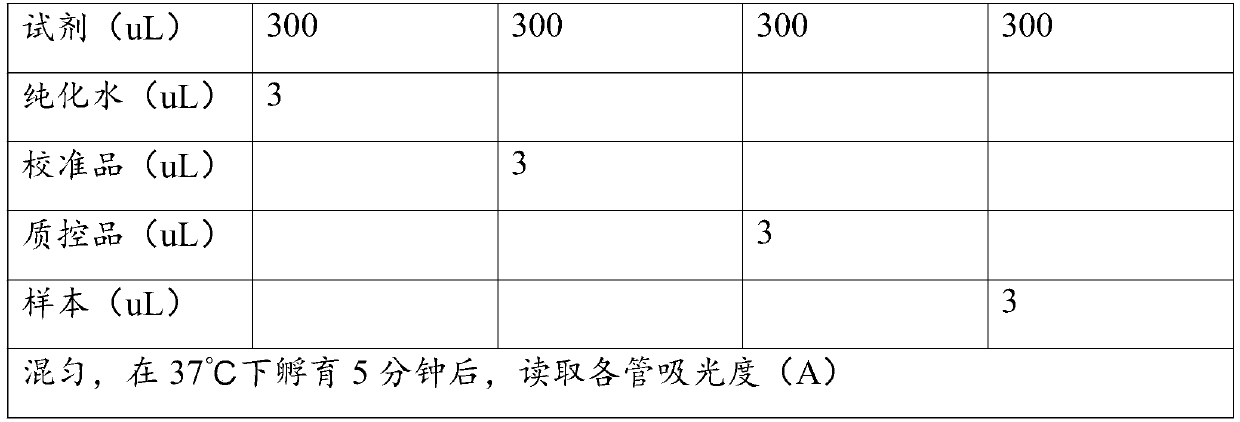
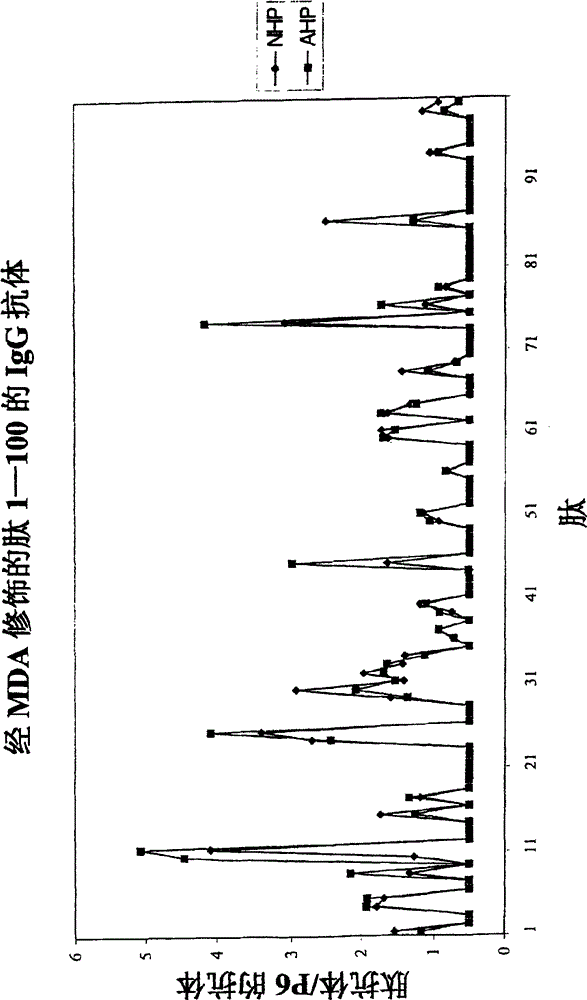
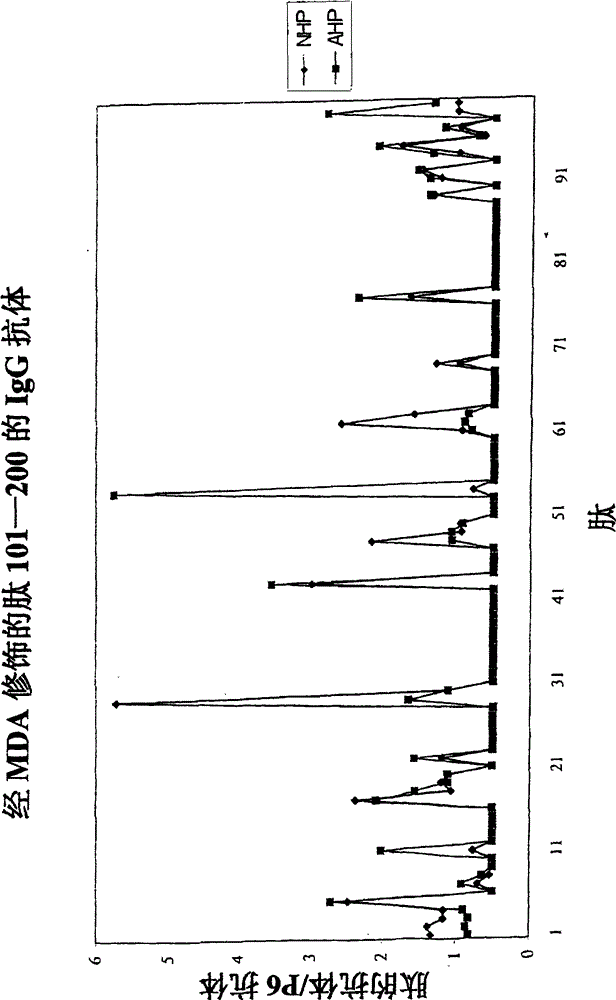
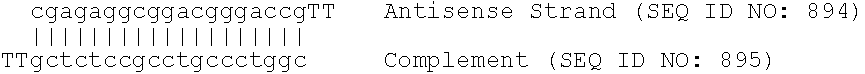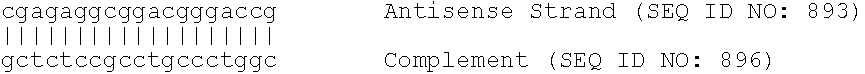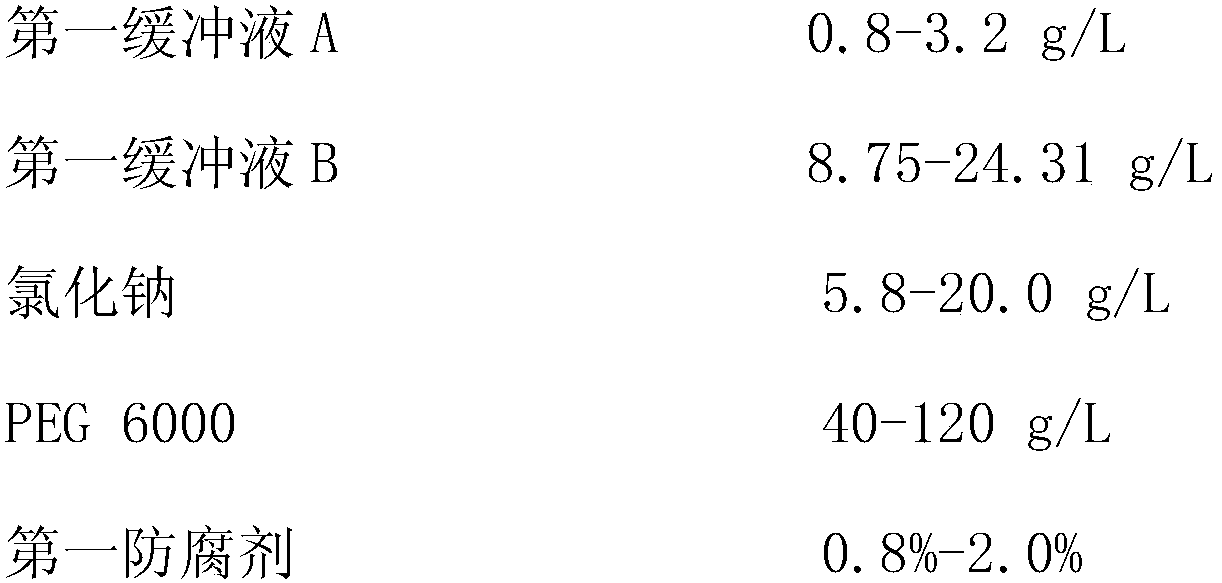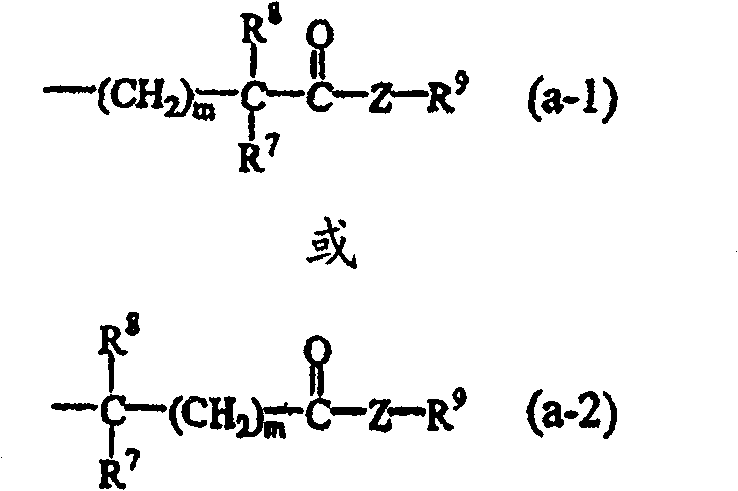Patents
Literature
52 results about "Apolipoprotein B" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Apolipoprotein B (ApoB) is a protein that in humans is encoded by the APOB gene.
Antisense modulation of apolipoprotein B-expression
ActiveUS7803930B2Reduce riskInhibit expressionBiocidePeptide/protein ingredientsDiseaseApolipoproteins b
Antisense compounds, compositions and methods are provided for modulating the expression of apolipoprotein B. The compositions comprise antisense compounds, particularly antisense oligonucleotides, targeted to nucleic acids encoding apolipoprotein B. Methods of using these compounds for modulation of apolipoprotein B expression and for treatment of diseases associated with expression of apolipoprotein B are provided.
Owner:KASTLE THERAPEUTICS LLC
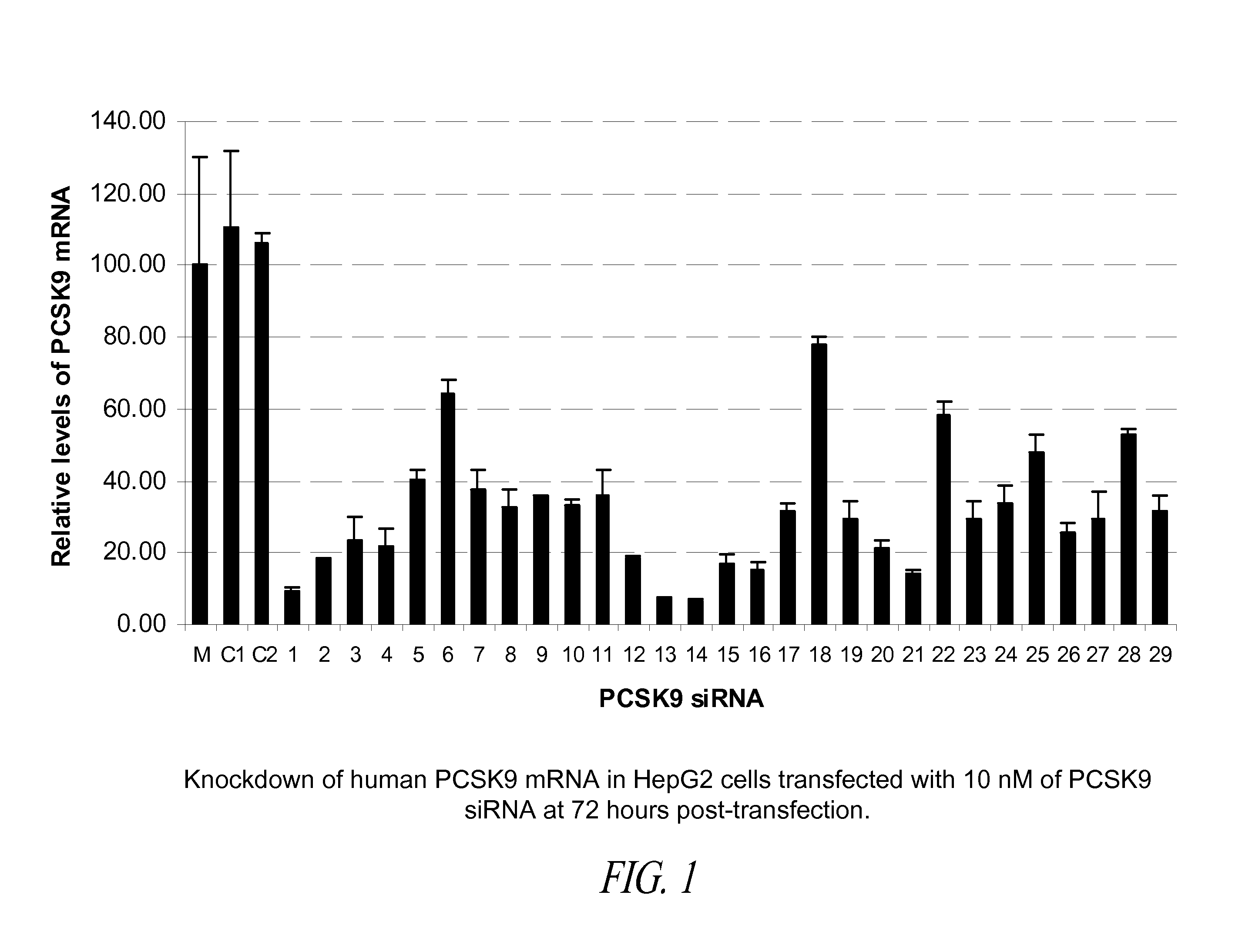
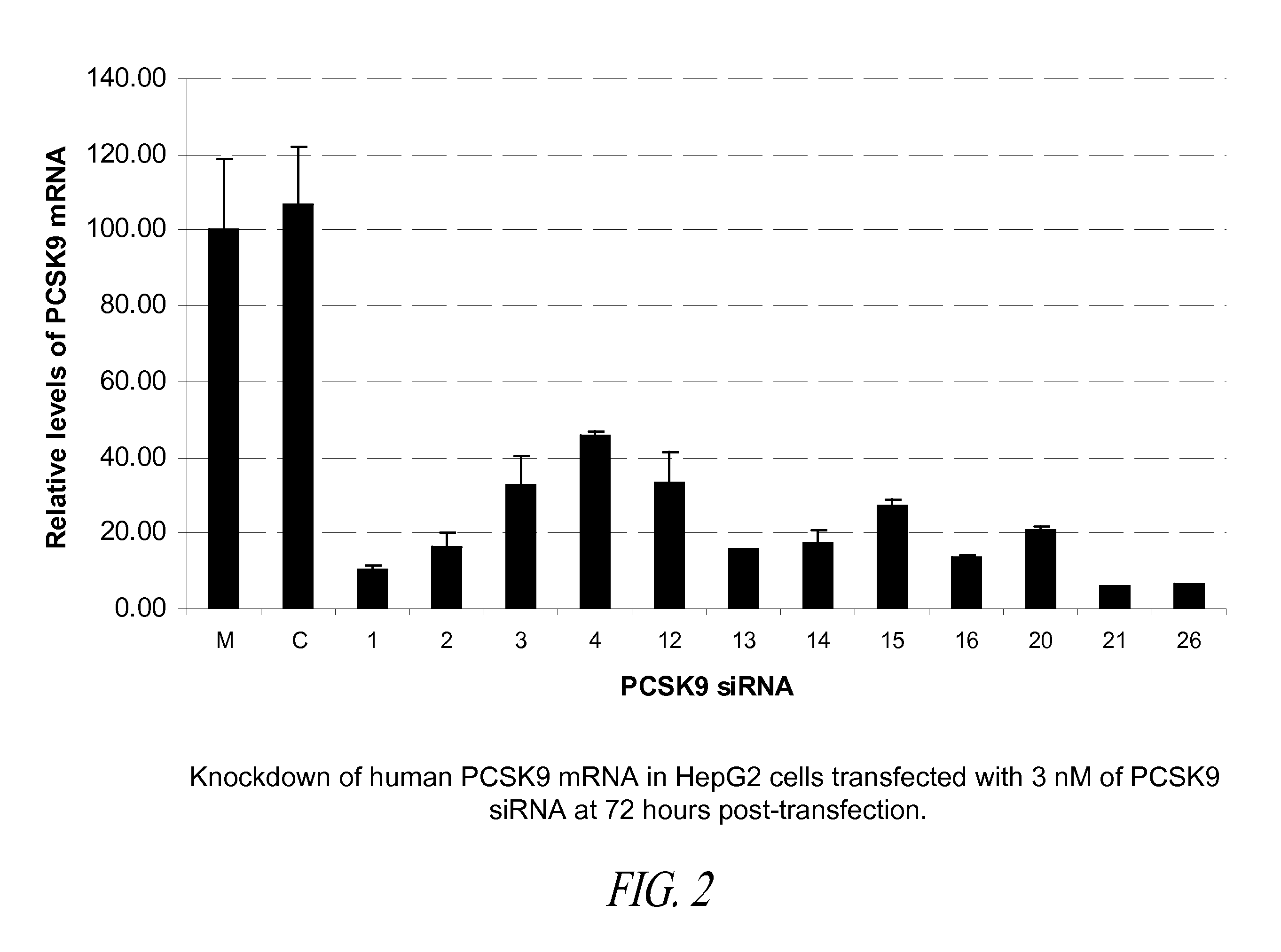
Compositions comprising human pcsk9 and apolipoprotein b sirna and methods of use
InactiveUS20110065644A1Additional stabilityImprove stabilityOrganic active ingredientsSugar derivativesPCSK9Nucleic acid molecule
The present invention provides siRNA nucleic acid molecules that inhibit PCSK9 or apolipoprotein B expression. Methods of using the nucleic acid molecules are also provided.
Owner:INTRADIGM CORP
Therapeutic uses of beta-casein a2 and dietary supplement containing beta-casein a2
InactiveUS20060280802A1Improve performanceLow serum levelsPeptide/protein ingredientsMetabolism disorderVery low-density lipoproteinDietary supplement
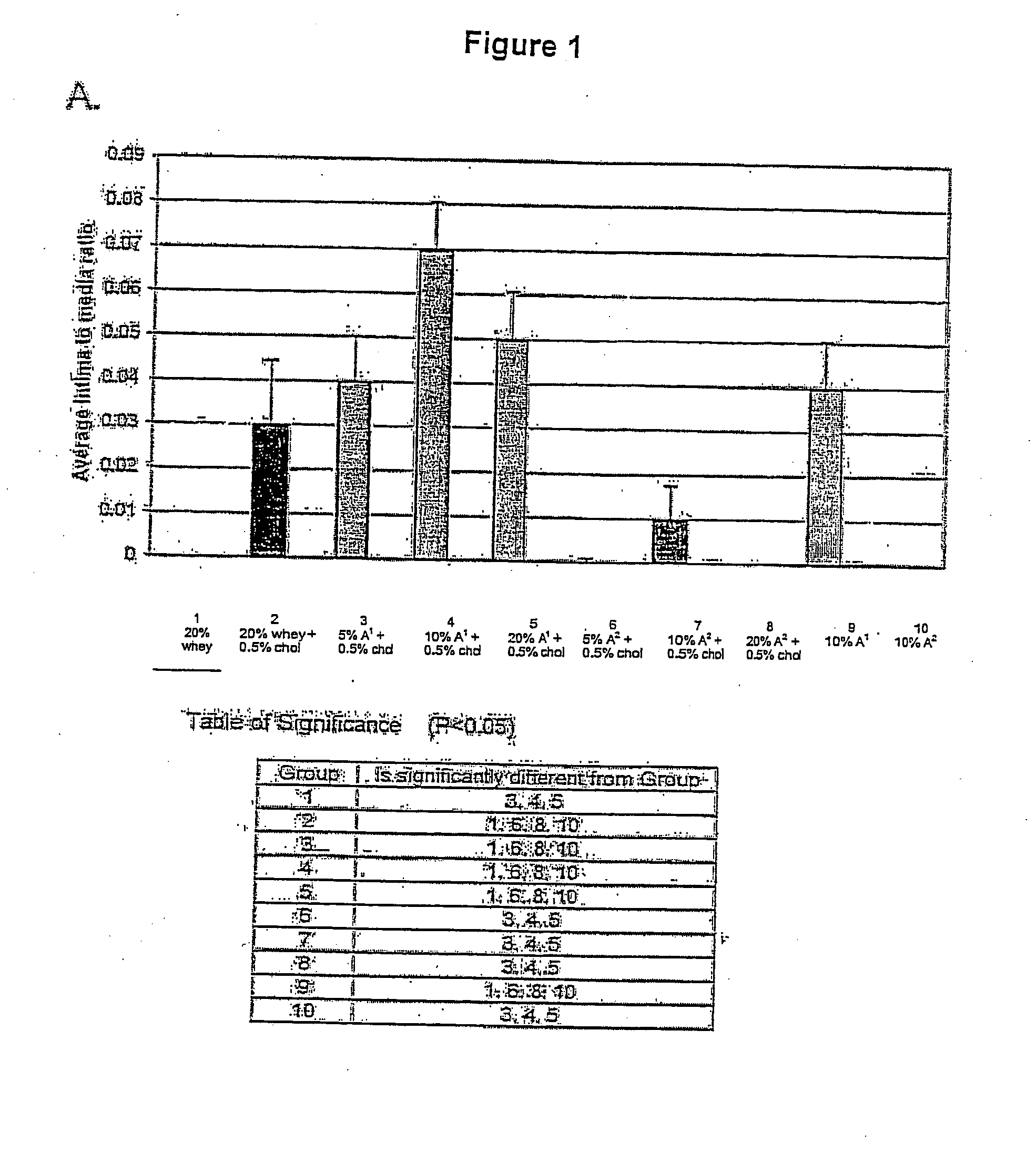
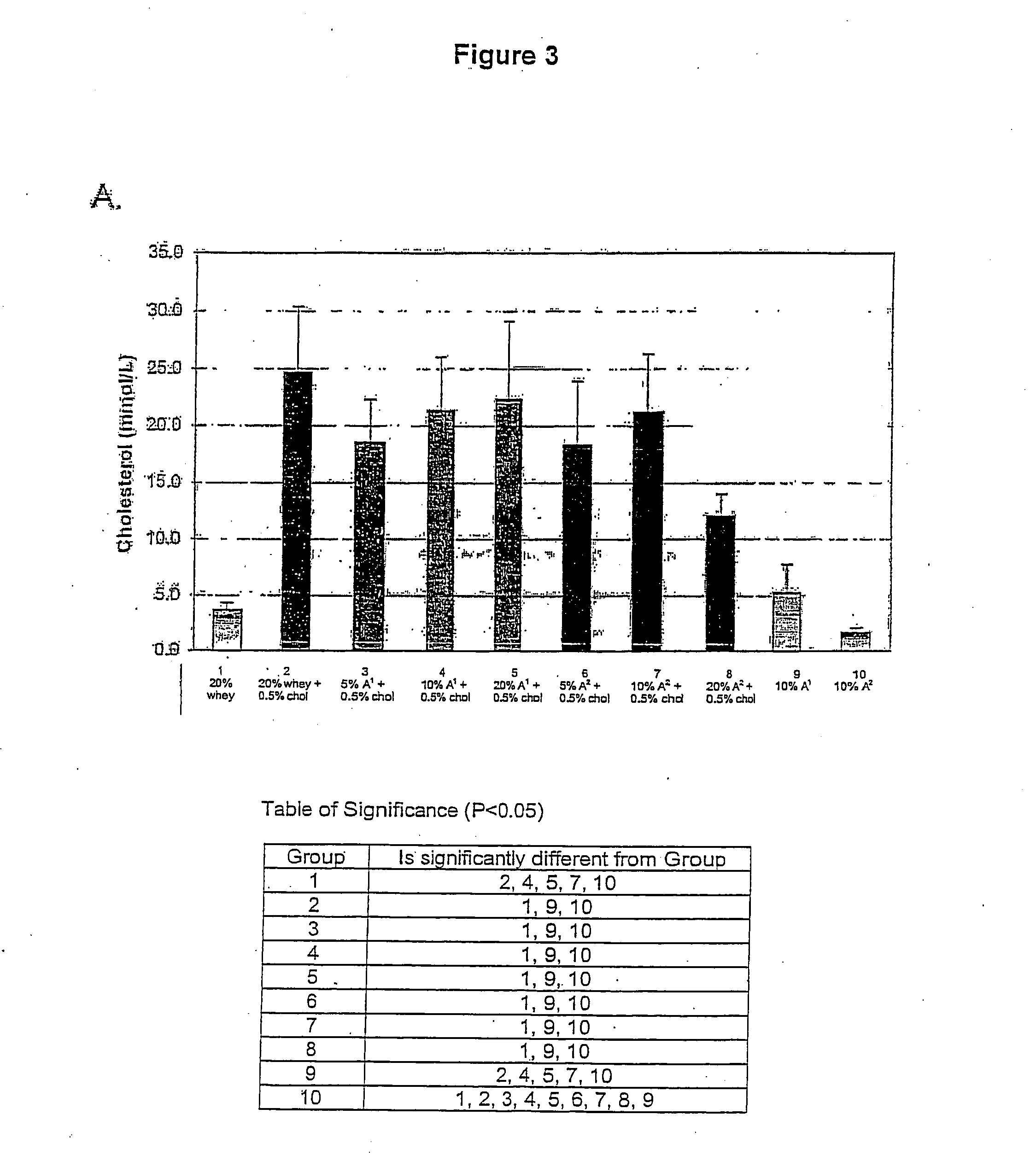
A method of reducing the serum level in a mammal of any one more cholesterol, low density lipoprotein (LDL) cholesterol relative to high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, very low density lipoprotein (VLDL) cholesterol, apolipoprotein B, and triglycerides by the ingestion of a composition containing β-casein A2. A dietary supplement formed by the addition of β-casein to a food or drink for human consumption where the β-casein is comprised of at least 95% β-casein A2, preferably solely β-casein A2.
Owner:A2 MILK CO LTD
Immune turbidimetry for detection of apoliprotein M
In the method, reaction between polyclonal antibody of anti apolipoprotein M and apolipoprotein M of sample to be tested forms compound, which causes change of cloudiness. The change is expressed by intensity of light transmission or intensity of light scattering. Content of apolipoprotein M of sample to be tested is looked out from curve between concentrations of standard apolipoprotein M and relevant intensity of light transmission or intensity of light. Titer of polyclonal antibody is at least 1:128 containing multi antigenic determinant. Sample to be tested is human blood plasma or serum in empty stomach diluted. Polyclonal antibody of anti apolipoprotein M is mixed with diluted sample. Features of thee method are: convenience, good repeatability, and high adaptability.
Owner:张晓膺
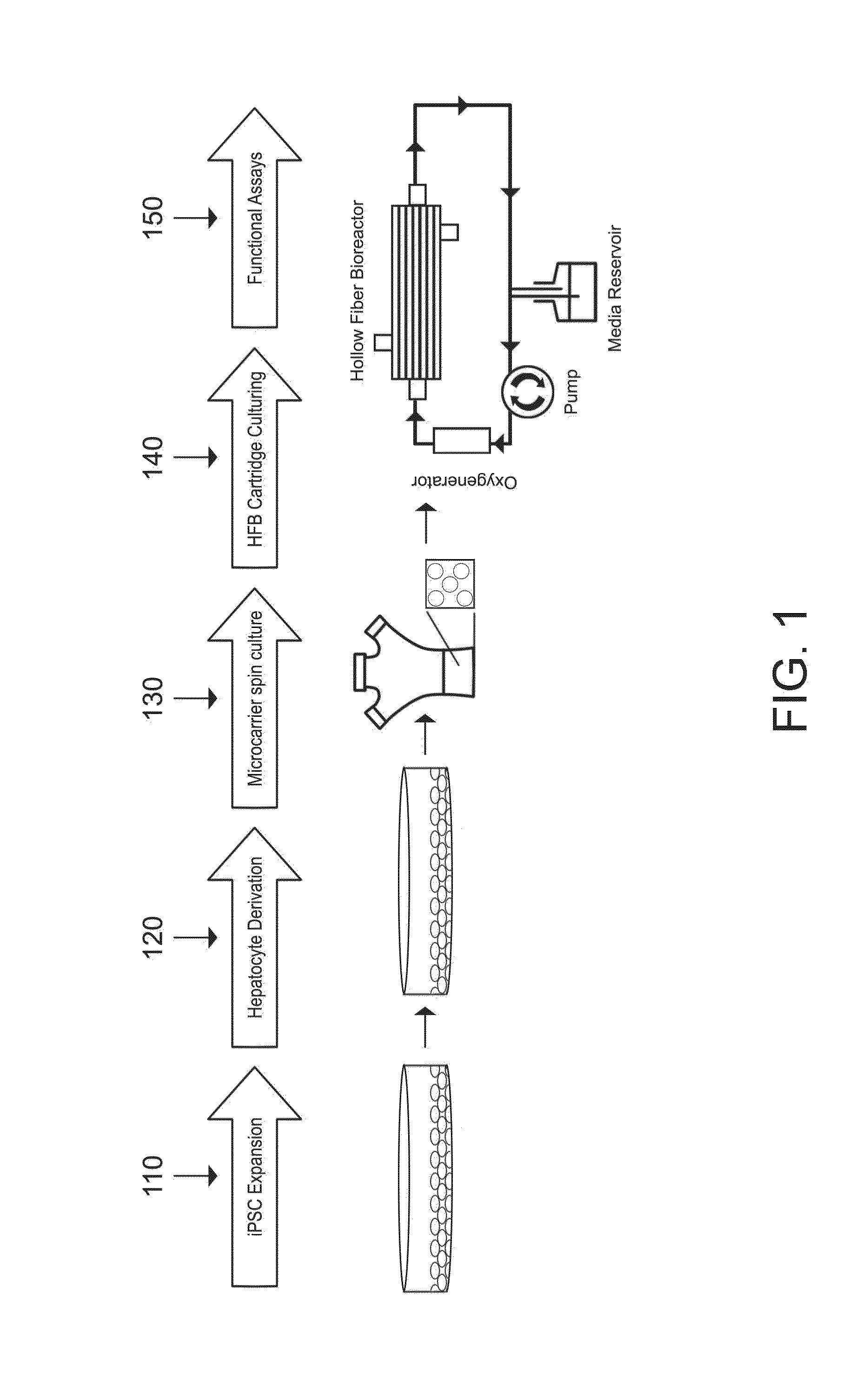
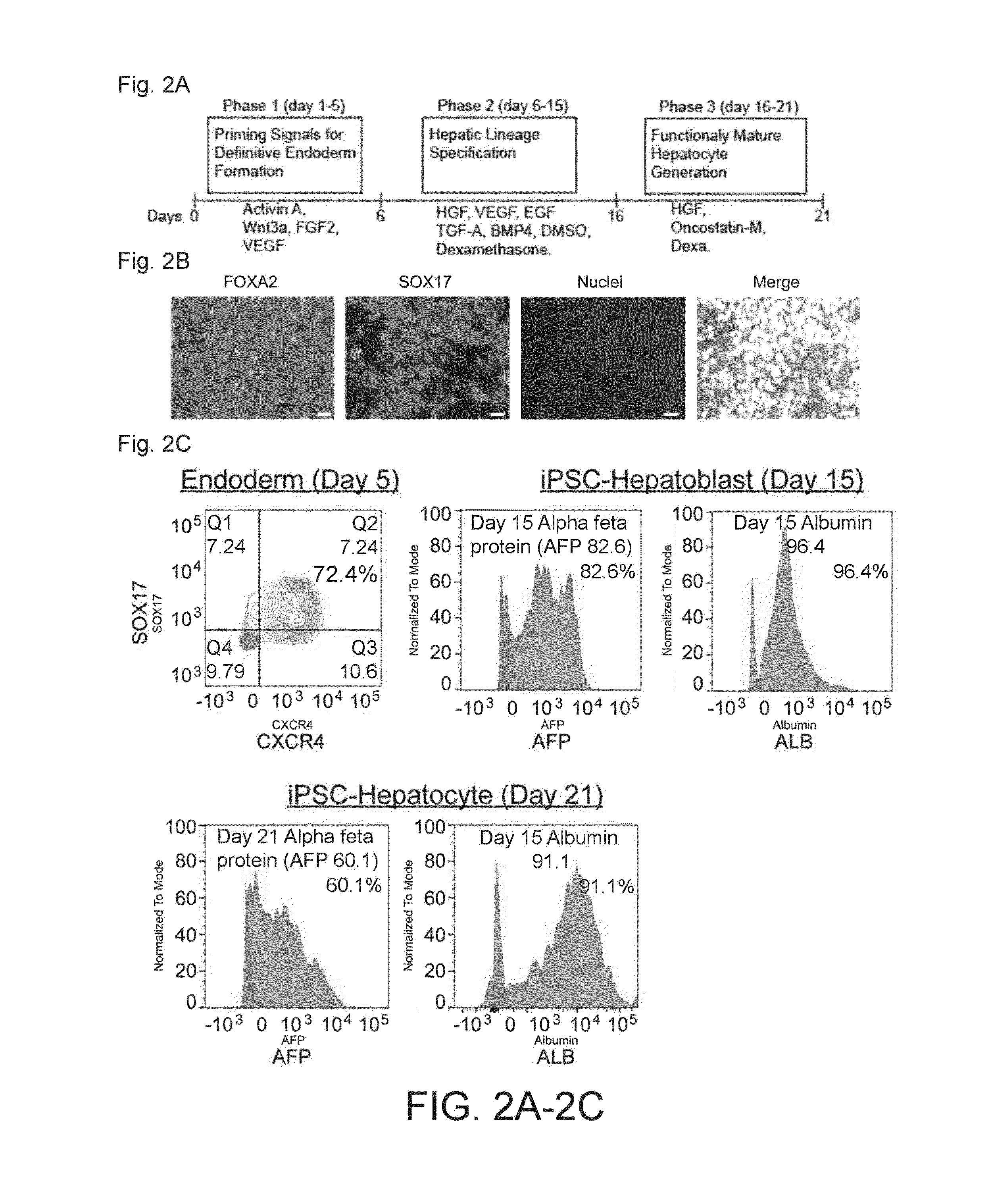
Induced pluripotent stem cell-derived hepatocyte based bioartificial liver device
InactiveUS20160256672A1Reduce toxic buildupQuality andCulture processDialysis systemsBioartificial liver deviceHuman albumin
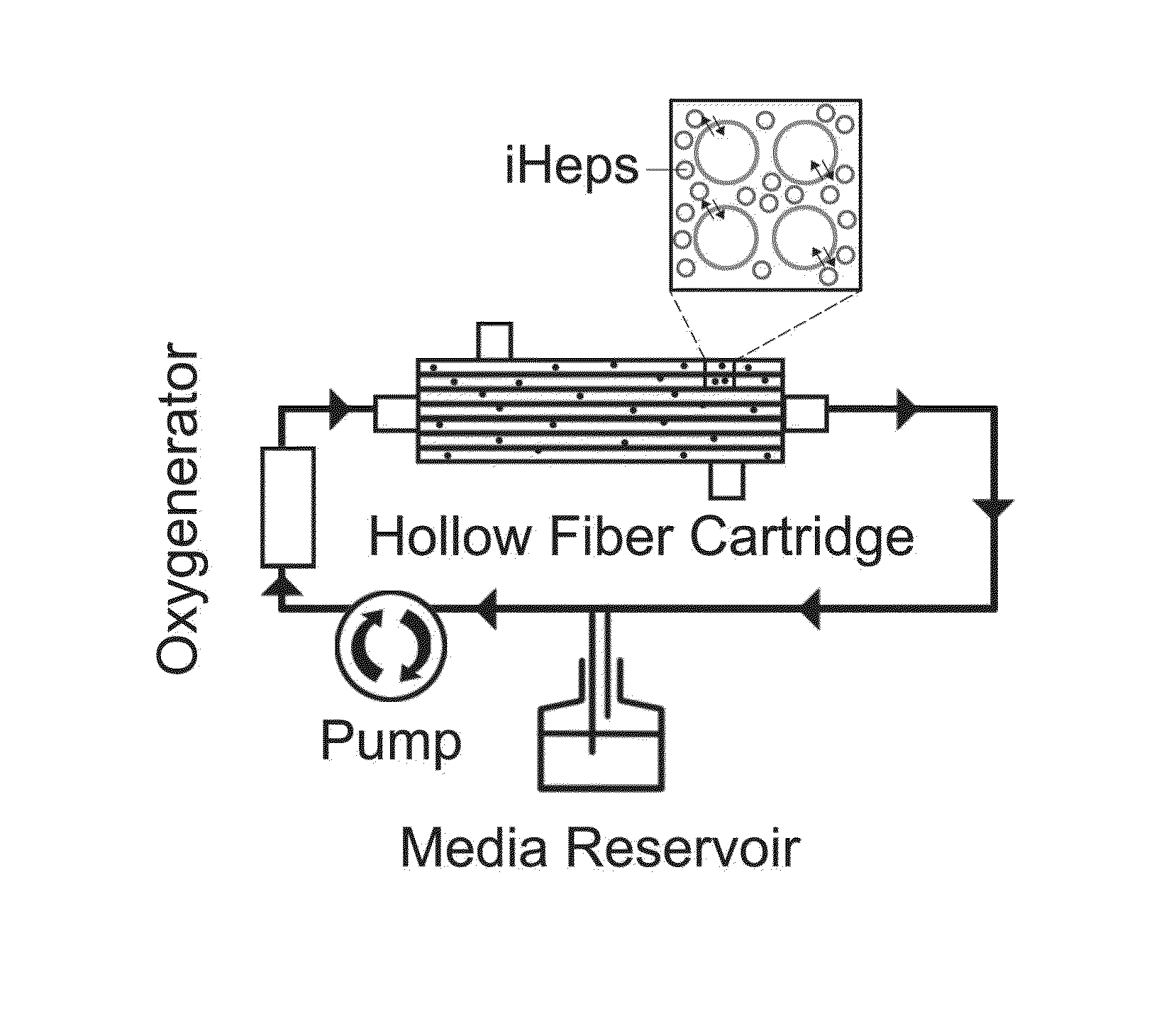
Human induced pluripotent stem cell (iPSC) technology combined with a hollow fiber based bioartificial liver (BAL) device can benefit patients with liver failure. Defined iPSC lines can provide unlimited supply of functional hepatocytes by developing iPSC derived hepatocytes (iHeps). Disclosed herein is a protocol for deriving metabolically active hepatocytes from iPSCs. In some embodiments, iHeps were cultured on microcarrier beads in spinner flasks. Subsequently, the iHep-microcarrier complexes were loaded into the extracapillary space of a hollow fiber bioreactor cartridge and cultured using closed circuit continuous flow system. The iHeps secreted human albumin, prothrombin and apolipoprotein B into the hollow fiber intracapillary space media which indicated the maintenance of plasma protein secretory function. In addition, the continuous flow system improved the maturation of iHeps. Thus, the iPSC hepatocytes in the bioartificial liver device maintained the secretory function and exhibited cell maturation.
Owner:CEDARS SINAI MEDICAL CENT
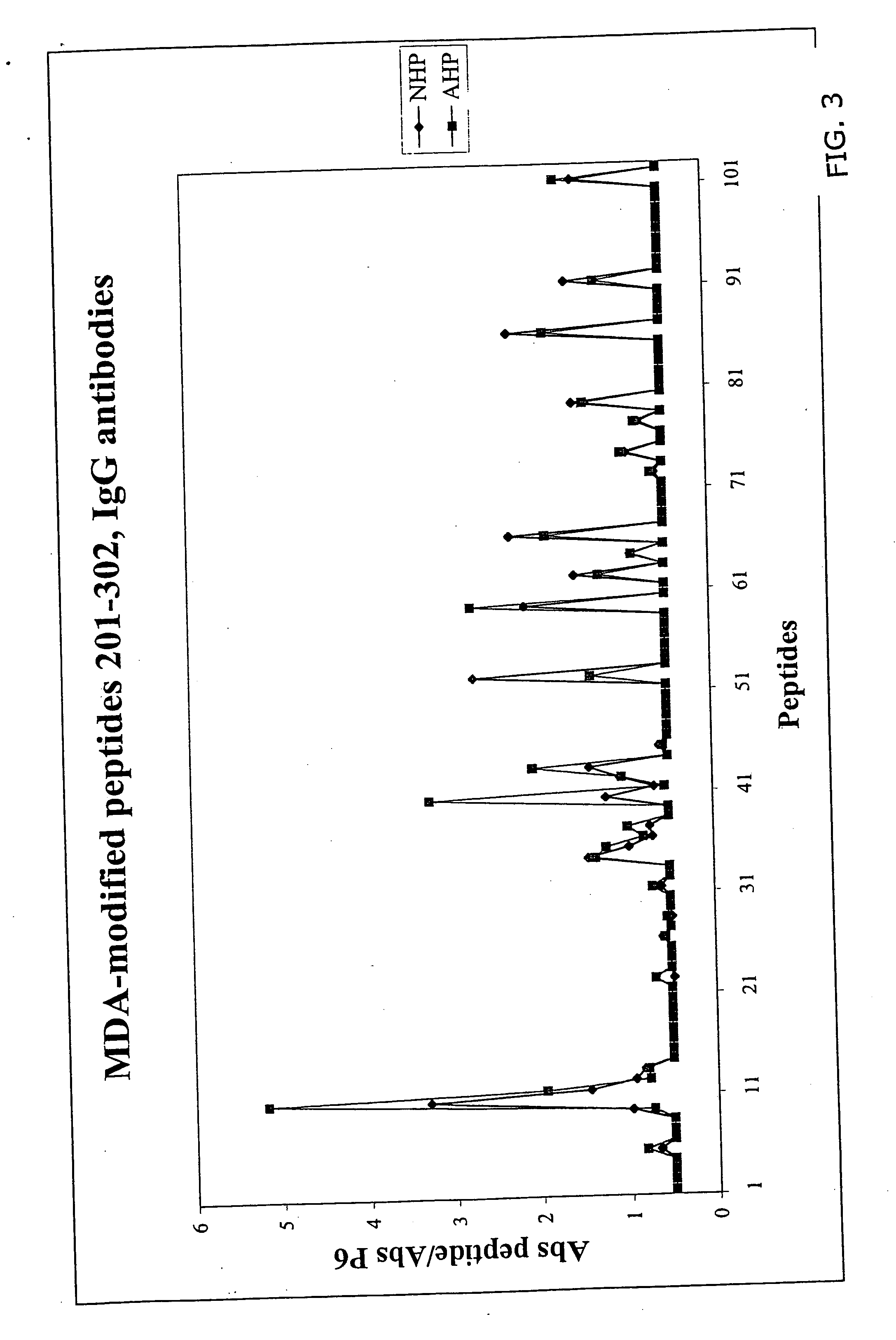
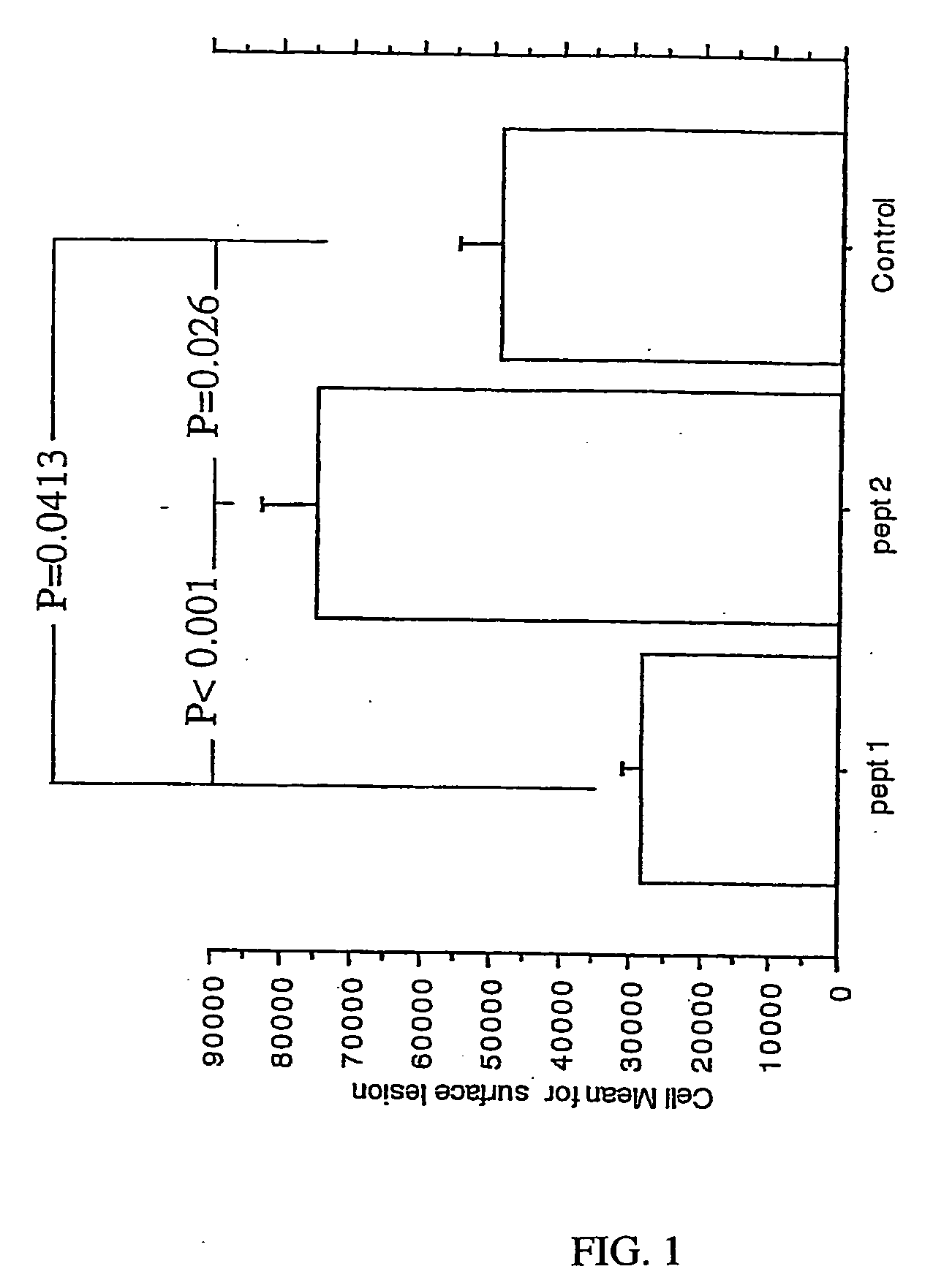
Peptide-based immunization therapy for treatment of atherosclerosis
The present invention relates to antibodies raised against fragments of apolipoprotein B, in particular defined peptides thereof, for immunization or therapeutic treatment of mammals, including humans, against ischemic cardiovascular diseases, using one or more of said antibodies.
Owner:CARDIOVAX
Peptide-based immunization therapy for treatment of atherosclerosis
InactiveUS20060233817A1Strong immune responseGood effectPeptide/protein ingredientsDisease diagnosisMammalTherapeutic treatment
The present invention relates to a fragment of apolipoprotein B, for immunization for prophylactic or therapeutic treatment of mammals, including humans, against ischemic cardiovascular diseases, in particular myocardial infarction or stroke, as well as diagnosing the presence or absence of antibodies related to increased or decreased risk of developing ischemic cardiovascular diseases including stroke, using said peptide in an assay, pharmaceutical compositions comprising the peptide. The invention further encompasses a particular peptide sequence aggravating disease, which sequence then can be used for diagnostic assays.
Owner:CARDIOVAX
Modulation of apolipoprotein C-III (ApoCIII) expression in lipoprotein lipase deficient (LPLD) populations
ActiveUS9593333B2Lower triglyceride levelsAvoid delayOrganic active ingredientsMetabolism disorderDyslipidemiaApolipoprotein C-III
Provided are methods, compounds, and compositions for reducing expression of ApoCIII mRNA and protein for treating, preventing, delaying, or ameliorating Fredrickson Type I dyslipidemia / FCS / LPLD, in a patient. Such methods, compounds, and compositions increase HDL levels and / or improving the ratio of TG to HDL and reducing plasma lipids and plasma glucose in the patient, and are useful to treat, prevent, delay, or ameliorate any one or more of pancreatitis, cardiovascular disease, metabolic disorder, and associated symptoms.
Owner:IONIS PHARMA INC
Use of prion conversion modulating agents
The use of Apolipoprotein B, Apolipoprotein E, fragments and mimetics thereof is provided for diagnostic, detection, prognostic and therapeutic applications In prion diseases. More specifically, the invention provides the use of Apolipoprotein B or fragments thereof for modulating or identifying modulators of the prion protein replication which are implicated in the pathogenesis of transmissible spongiform encephalopathics and other prion diseases.
Owner:MERCK SERONO SA
Monoclonal antibody against slightly oxidized low-density lipoprotein and hybridoma for producing the same
ActiveUS20130253174A1Simple methodGood effectImmunoglobulins against animals/humansBiological testingMonoclonal antibodyLow-density lipoprotein
Owner:HOKKAIDO UNIVERSITY
Apolipoprotein B nano antibody and coding sequence and application thereof
InactiveCN103342750AEfficient expressionBacteriaImmunoglobulins against animals/humansEscherichia coliAntiendomysial antibodies
The invention discloses a VHH chain of a nano antibody of apolipoprotein B, which comprises a framework region (FR) and a complementary determining region (CDR). The invention discloses an amino acid sequence of the FR selected from the lower group and an amino acid sequence of the CDR. The invention also discloses an apolipoprotein B nano antibody and a DNA molecule for coding the VHH chain of the nano antibody of apolipoprotein B or the apolipoprotein B nano antibody, and discloses a host cell capable of expressing the nano antibody of apolipoprotein B. Moreover, the invention discloses an application of the apolipoprotein B nano antibody for detecting the apolipoprotein B. Through the nano antibody gene sequence and host cell disclosed by the invention, the nano antibody can be efficiently expressed in Escherichia coli and is used for developing an apolipoprotein B detection reagent.
Owner:SOUTHEAST UNIV
Kit for determining concentration of apolipoprotein C-III and preparation method
InactiveCN109187996AGood repeatabilityHigh sensitivityBiological testingBiotin-streptavidin complexStreptolydigin
The invention relates to the technical field of biology, and particularly to a kit for determining a concentration of an apolipoprotein C-III and a preparation method. The invention adopts the following technical scheme: the kit for determining the concentration of the apolipoprotein C-III is characterized by comprising a reagent R1 and a reagent R2; biotin and streptavidin are added into the reagent R2; the ratio of the biotin to the apolipoprotein C-III antibody is 2:1, and the ratio of the streptavidin to the biotin-apolipoprotein C-III antibody is 1:1. The kit for determining the concentration of the apolipoprotein C-III and the preparation method disclosed by the invention have the advantages that a cascaded amplification reaction is adopted in which the apolipoprotein C-III antibodyis connected with the biotin at the radio of 1:2, and is mixed and reacted with the streptavidin at the radio of 1:1, thereby significantly improving the repeatability and sensitivity, and the linearrange can reach 2-100 mg / L.
Owner:苏州普瑞斯生物科技有限公司
A method for constructing a gene detection library for familial hypercholesterolemia and its kit
ActiveCN109517884BHigh precisionEasy to operateMicrobiological testing/measurementLibrary creationGenes mutationDimer
Owner:ANNGEEN BIOTECHNOLOGY CO LTD
Rapid purification method of apolipoprotein B
ActiveCN102766205AReduce purification costsHigh purityApolipeptidesPeptide preparation methodsActive agentBlood plasma
The invention provides a rapid purification method of apolipoprotein B, comprising the following steps of: A) taking human plasma and adding a buffering solution to be diluted and uniformly mixed; B) placing an obtained diluted plasma sample into a first water bath with the temperature of 65-75 DEG C to be heated; centrifuging and taking liquid supernatant; C) adding a surfactant with the volume percentage of 0.5-3% into the liquid supernatant and uniformly mixing to obtain processed liquid supernatant; D) placing the treated liquid supernatant into a second water bath with the temperature of50-60 DEG C to be heated; centrifuging, removing the liquid supernatant and collecting sediments; and E) washing the sediments by using a Tris-HCl buffering solution with the concentration of 0.01-0.1 mol / L for two times, and filtering to obtain purified apolipoprotein B. The rapid purification method of the apolipoprotein B, disclosed by the invention, has the advantages of being simple in operation steps, simple in needed equipment and high in purity of a purified product, and effectively reduces the purification cost.
Owner:广东派特埃尔生物科技有限公司
DNA encoding human monocyte-macrophage apolipoprotein B receptor gene and protein
InactiveUS6194558B1Cell receptors/surface-antigens/surface-determinantsSugar derivativesChylomicronTG - Triglyceride
The present invention provides an isolated DNA molecule that codes for a cell-surface binding protein in human monocytes and macrophages. In addition, an amino acid sequence derived from the nucleotide sequence is provided. The newly-identified cell-surface binding protein described herein is instrumental in the apoB-mediated cellular uptake of plasma chylomicrons and remnants and hypertriglyceridemic tryglyceride-rich lipoproteins in an ApoE- and lipoprotein lipase- and heparin sulfate proteoglycan-independent pathway. The present invention also provides evidence that the ligand for the receptor is within the N-terminal region of apoB-48 or B-100 at or near the lipoprotein lipase binding domain and not in a heparin binding domain.
Owner:UAB RES FOUND
Apolipoprotein C-II or its active fractions for the prevention or treatment of obesity and related metabolic disorders
InactiveUS20110230398A1Prevention and treatment of obesityAvoid damagePeptide/protein ingredientsMetabolism disorderPhysiologyDietary supplement
Apoliporotein C-II or any active fraction of its amino acid sequence that activates lipoprotein lipase (LPL) in mammals. Increase in activity of LPL can increase expenditure of energy, and therefore an activator of LPL can be an effective agent for the prevention or treatment of obesity or related metabolic disorders, including cardiovascular disease, diabetes and hypertension in mammals including domestic pets and humans. Apolipoprotein C-II or its active fractions can be used as a diet supplement in the form of fortification to a natural product such as milk, cereal, beverage; as a nutritional supplement; or as a therapeutic agent in conventional forms of administration.
Owner:GUPTA SURENDRA K
Assay for diabetes
An assay for testing a subject for diabetes or a predisposition to diabetes including analyzing a biological fluid from a subject for the presence of one or more proteins selected from the group consisting of Alpha 2 macroglobulin, Apolipoprotein AII, Immunoglobulin alpha heavy chain constant region, Immunoglobulin mu chain C region, Chain A of Human IgA1, Inter-alpha-trypsin inhibitor heavy chain H4 precursor, and Apolipoprotein B11; wherein detection of the protein is indicative of diabetes or a predisposition to diabetes in the subject.
Owner:MINOMIC
Monoclonal antibody against oxidized low-density lipoprotein
ActiveUS8729240B2Simple methodGood effectImmunoglobulins against animals/humansBiological testingMonoclonal antibodyOxidized low density lipoprotein
Provided is a monoclonal antibody against slightly oxidized LDL, which can play a role as an important tool in the research and development of oxidized LDL. Also provided are a kit for the simple detection of slightly oxidized LDL and a method for the simple detection of slightly oxidized LDL from the biological sample of a subject to be tested which use the monoclonal antibody. By means of ELISA (Enzyme-Linked Immunosorbent Assay) using the monoclonal antibody as the solid phase antibody and an anti-apolipoprotein B antibody as the detection antibody, the degree of reaction between a severely oxidized low-density lipoprotein and the monoclonal antibody is low in comparison to the degree of reaction between a slightly oxidized low-density lipoprotein and the monoclonal antibody, and the monoclonal antibody specifically reacts with an oxidized low-density lipoprotein.
Owner:HOKKAIDO UNIVERSITY
Anti-obese immunogenic hybrid polypeptides and Anti-obese vaccine composition comprising the same
InactiveUS20110002955A1Process stabilityStable anti-obesityPeptide/protein ingredientsImmunoglobulinsNucleotideHepatitis B virus surface Antigen
Disclosed is an immunogenic hybrid polypeptide for the prevention and treatment of obesity, in which a mimetic peptide of a B cell epitope of apolipoprotein B-IOO; a rabies virus helper T cell epitope or hepatitis B virus surface antigen helper T cell epitope and a C-terminal peptide fragment of mouse apolipoprotein Cu or a mimetic peptide of a B cell epitope of apolipoprotein B-100 are fused to each other in that order in the direction from the N terminus to the C terminus thereof. Also, a vaccine composition for the prevention and treatment of obesity, comprising the immunogenic hybrid polypeptide is disclosed, along with a polynucleotide encoding the immunogenic hybrid polypeptide, a recombinant expression vector carrying the polynucleotide, a host cell anchoring the recombinant expression vector, and a method for producing the immunogenic hybrid polypeptide by culturing the host cell transformed with the recombinant expression vector.
Owner:SJ BIOMEDIC INC
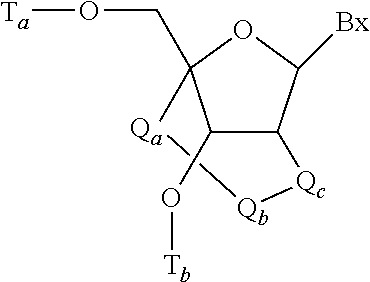
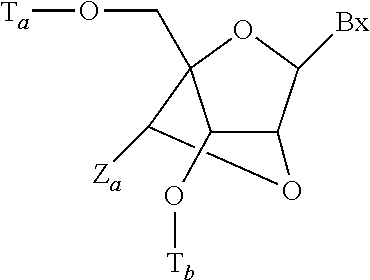
Reconstituted apolipoprotein b lipoparticle, a preparation method and uses thereof
The present invention provides a method for preparing a reconstituted apolipoprotein B lipoparticle and the method comprises steps of (a) dissolving an apolipoprotein B and a lipid in a first buffer containing 2 M to 8 M urea and 1 wt % to 15 wt % amphiphilic compounds to form a mixture; and (b) dialyzing the mixture against a second buffer containing 0 M to 2M urea and 0 wt % to 0.5 wt % amphiphilic compounds for 1 to several times for lowering concentrations of the urea and the amphiphilic compounds in the mixture. The present invention further provides an apolipoprotein B lipoparticle and a use for the production of an apolipoprotein B lipoparticle used for delivering a hydrophobic substance.
Owner:NAT CHIAO TUNG UNIV
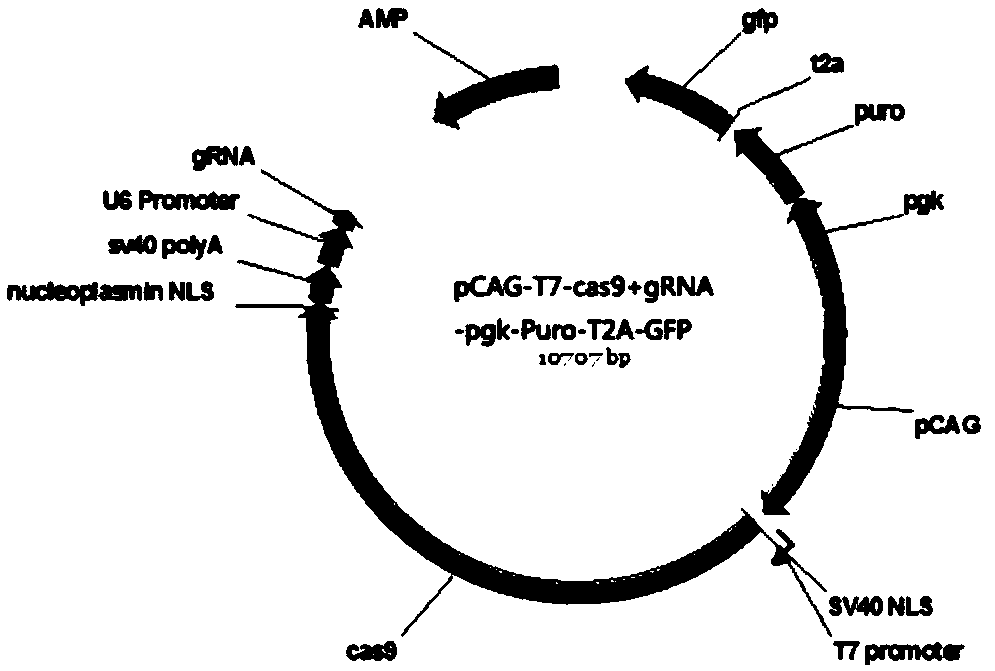
SgRNA targeting APOBEC3G (apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like protein 3 protein G) gene and method for knocking out macaca fascicularis APOBEC3G gene
The invention belongs to the technical field of biology, and particularly relates to SgRNA targeting an APOBEC3G (apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like protein 3 protein G) gene and a method for knocking out the macaca fascicularis APOBEC3G gene. In allusion to mRNA of the macaca fascicularis APOBEC3G gene, a target sequence is established and the corresponding sgRNA is synthesized, and then a knockout vector is constructed for co-transfecting macaca fascicularis embryo fibroblasts to obtain APOBEC3G gene-knocked macaca fascicularis embryo fibroblasts, or the sgRNA and Cas9 mRNA are mixed and injected into a macaca fascicularis embryo to obtain the APOBEC3G gene-knocked macaca fascicularis embryo. Detection and identification of a knockout effect show that the gene knockout of the APOBEC3G gene on macaca fascicularis cells and the macaca fascicularis embryo can be effectively achieved, so that a solid foundation is laid for establishment of an AIDS (acquired immunodeficiency syndrome) animal model.
Owner:SOUTH CHINA AGRI UNIV
Methods and Compositions for Modifying Apolipoprotein B mRNA Editing
InactiveUS20100297219A1Increase heightTreat and prevent atherogenic diseasePowder deliveryFungiLipofectamineDelivery vehicle
Products and methods for modifying apolipoprotein B mRNA editing in vivo, reducing serum LDL levels, and treating or preventing an atherogenic disease or disorder are disclosed. Such methods involve the use of a protein including APOBEC-1 or fragments thereof which can edit mRNA encoding apolipoprotein B. The protein including APOBEC-1 can be taken up by cells in the form of a delivery vehicle, such as a liposome or niosome, or directly as a chimeric protein which includes a first polypeptide that includes a protein transduction domain and a second polypeptide that includes APOBEC-1 or a fragment thereof which can edit mRNA encoding apolipoprotein B.
Owner:UNIVERSITY OF ROCHESTER
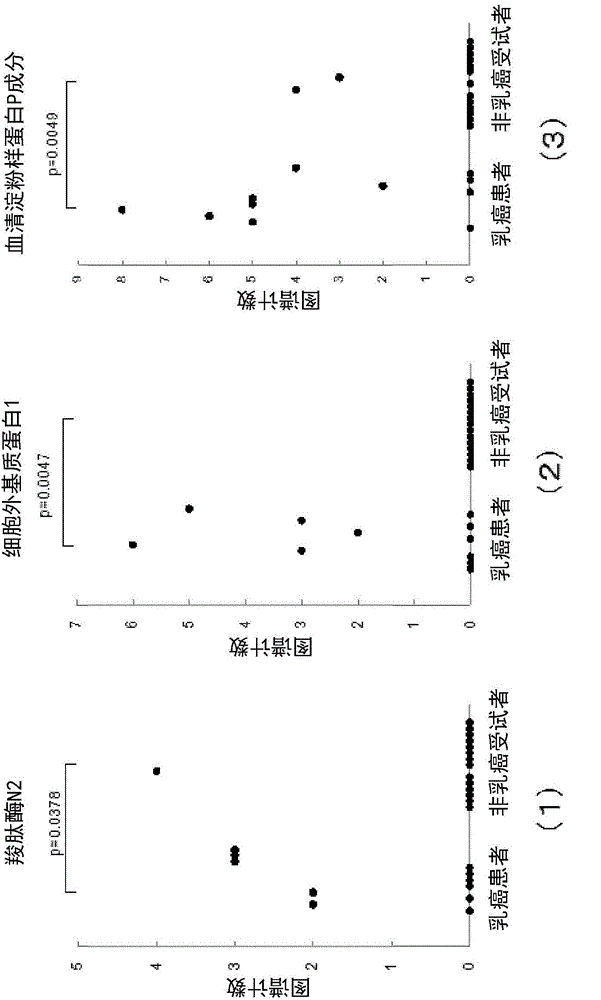
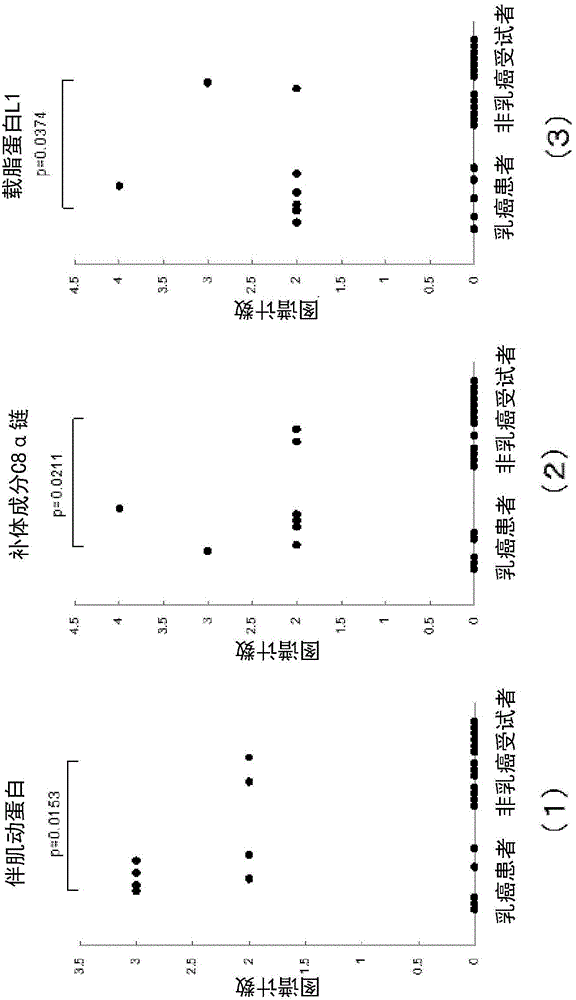
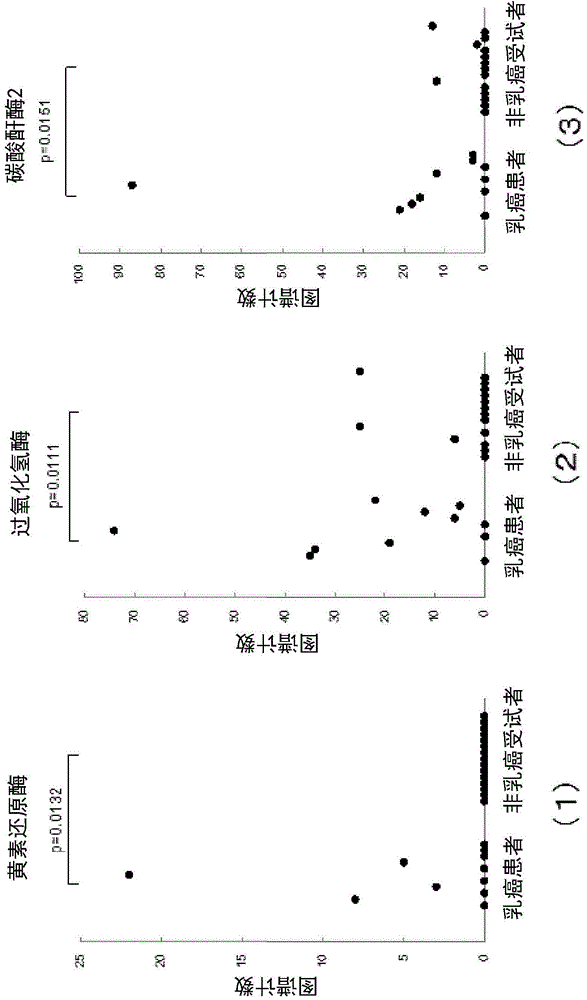
Method for determining breast cancer
InactiveCN104583777AHigh precisionMicrobiological testing/measurementDisease diagnosisExtracellular matrix protein 1Flavin reductase
The present invention relates to: a breast cancer marker which is selected from the group consisting of carboxypeptidase N subunit 2, extracellular matrix protein 1, serum amyloid P component, nebulin, complement component C8 alpha chain, apolipoprotein L1, flavin reductase, catalase, carbonic anhydrase 2, apolipoprotein C-I, nuclear pore glycoprotein 210, superoxide dismutase [Cu-Zn], bisphosphoglycerate mutase, carbonic anhydrase 1 and peroxiredoxin 2; a method for determining breast cancer, which comprises detecting the breast cancer marker in a sample and determining breast cancer on the basis of the results of the detection; and a kit for use in the method.
Owner:WAKO PURE CHEMICAL INDUSTRIES +1
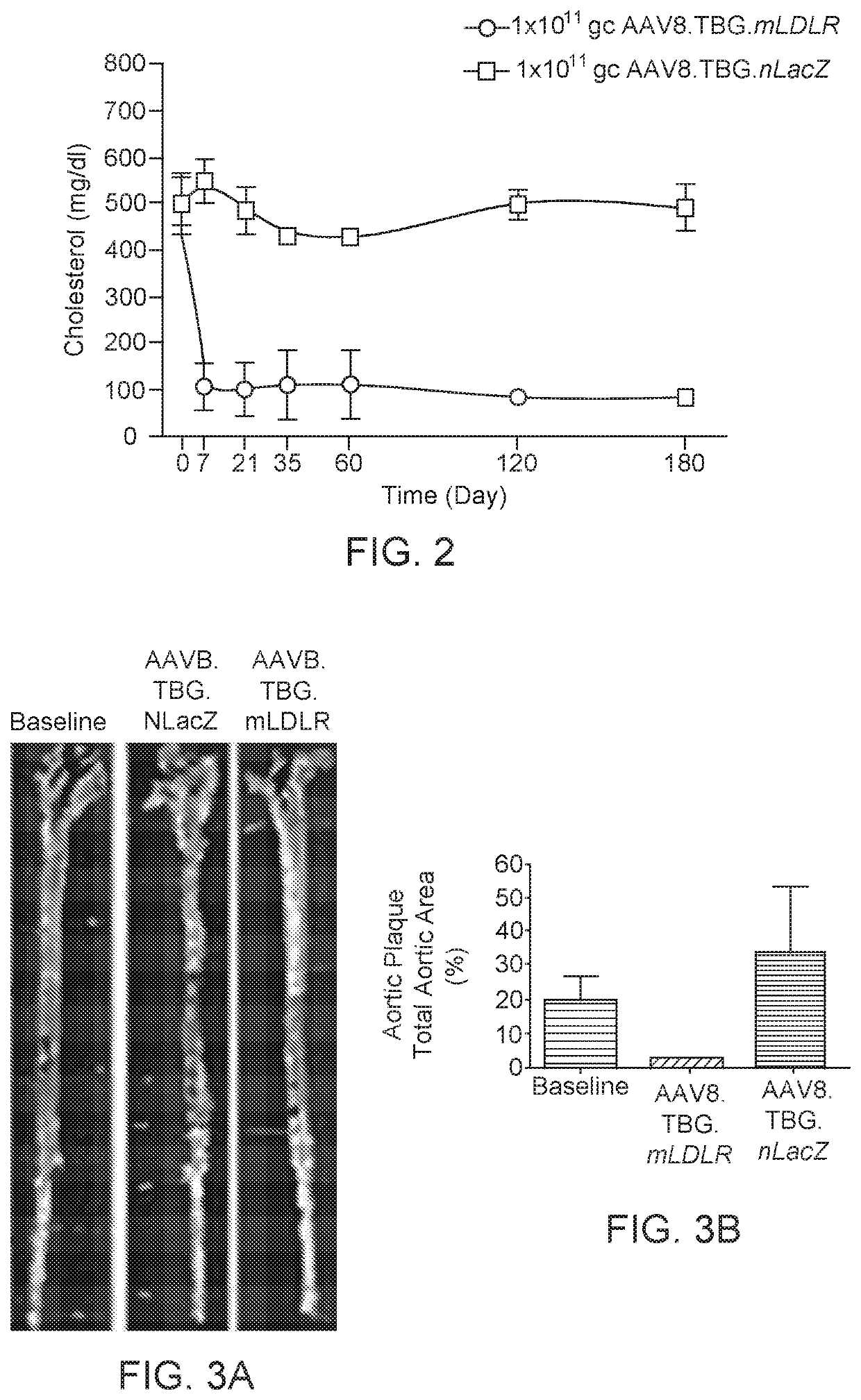
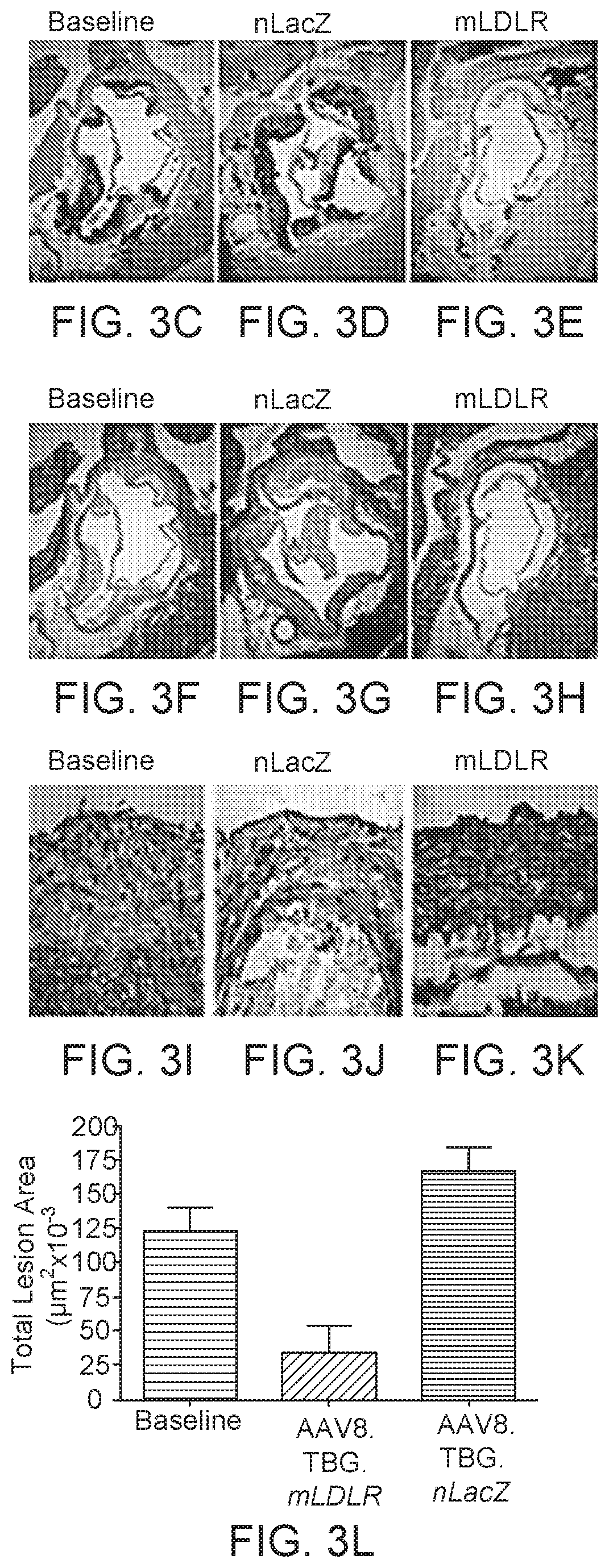
Gene therapy for treating familial hypercholesterolemia
ActiveUS10889832B2Improve responseSafe deliveryCell receptors/surface-antigens/surface-determinantsVectorsFamilial hypercholesteremiaTG - Triglyceride
Compositions and regimens useful in reducing one or more of: LDL-cholesterol, total cholesterol, and / or fasting triglycerides in a subject, and / or modifying fractional catabolic rate (FCR) of LDL apolipoprotein B (apoB) from baseline to a selected time point after rAAV administration are provided. The method involves administering to the human subject via a peripheral vein by infusion of a suspension of replication deficient recombinant adeno-associated virus (rAAV).
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Serum apolipoprotein B assay kit, preparation method and application thereof
ActiveCN111426845ANo reconstitution requiredImprove stabilityBiological material analysisBiological testingActive agentA lipoprotein
The invention provides an apolipoprotein B (ApoB) assay kit. The kit contains a reagent R1 and a reagent R2, wherein the reagent R1 is prepared from the following components: a 4-hydroxyethylpiperazine ethanesulfonic acid (HEPES) buffer solution, NaCl, guanidine hydrochloride, sodium decyl sulfate, tetramethylurea, polyethylene glycol 6000, a surfactant and a preservative; and the reagent R2 is prepared from the 4-hydroxyethylpiperazine ethanesulfonic acid (HEPES) buffer solution, goat anti-human ApoB antibody coated latex particles, the surfactant, a stabilizer and the preservative. The invention also provides a preparation method and an application of the kit, and the kit is beneficial to exposure of antigen sites in lipoprotein and promotion of an antigen-antibody reaction due to mutualsynergy of multiple components, and is a liquid kit with strong stability, high sensitivity, good repeatability and low cost.
Owner:中拓生物有限公司 +2
Reconstituted apolipoprotein B lipoparticle, a preparation method and uses thereof
ActiveUS10792255B2Reduce concentrationEther/acetal active ingredientsDiagnostic recording/measuringBiochemistryOrganic chemistry
The present invention provides a method for preparing a reconstituted apolipoprotein B lipoparticle and the method comprises steps of (a) dissolving an apolipoprotein B and a lipid in a first buffer containing 2 M to 8 M urea and 1 wt % to 15 wt % amphiphilic compounds to form a mixture; and (b) dialyzing the mixture against a second buffer containing 0 M to 2M urea and 0 wt % to 0.5 wt % amphiphilic compounds for 1 to several times for lowering concentrations of the urea and the amphiphilic compounds in the mixture. The present invention further provides an apolipoprotein B lipoparticle and a use for the production of an apolipoprotein B lipoparticle used for delivering a hydrophobic substance.
Owner:NAT CHIAO TUNG UNIV
Immunoturbidimetry apolipoprotein B single reagent with stable liquid and freezing resistance
PendingCN111024963AAffect performanceImprove stabilityBiological material analysisBiological testingAntiendomysial antibodiesMagnesium salt
The invention discloses an immunoturbidimetry apolipoprotein B single reagent with stable liquid and freezing resistance. The single reagent comprises 20-200mmol / L Buffer solution with a PH value ranging from 7.0 to 8.0 , 40.0-200.0 g / L of ApoB antibody subjected to antibody treatment liquid pre-precipitation and centrifugal treatment, 5.0-50.0 g / L of polyethylene glycol 2,000-10,000, 20.0-200.0 g / L of glycerol, 0.5-5.0 g / L of surfactant, and 0.2-2.0 g / L of preservative, wherein the antibody treating fluid comprises 20-200 mmol / L of buffer solution with the pH value ranging from 7.0 to 8.0, 30.0-80.0 g / L of polyethylene glycol 2,000 to 10,000, 5.0-30.0 g / L of magnesium salt and 0.2-2.0 g / L of preservative. Under low temperature conditions, under the promotion action of polyethylene glycoland magnesium salt with a certain concentration, protein easy to precipitate in the antibody is precipitated in an accelerated mode, then high-speed centrifugation is conducted, precipitate is removed, stability is improved when the antibody and the non-specific polyethylene glycol sensitizer coexist in the single reagent, protein precipitation after the reagent is frozen is prevented, and therebyperformance of the reagent is prevented from being affected.
Owner:桂林英美特生物技术研究所
Peptide-based immunization therapy for treatment of atherosclerosis
InactiveCN103122031BAvoid it happening againApolipeptidesPeptide/protein ingredientsApolipoproteins bTherapeutic treatment
The present invention relates to antibodies raised against fragments of apolipoprotein B, in particular defined peptides thereof, for immunization or therapeutic treatment of mammals, including humans, against ischemic cardiovascular diseases, using one or more of said antibodies.
Owner:CARDIOVAX
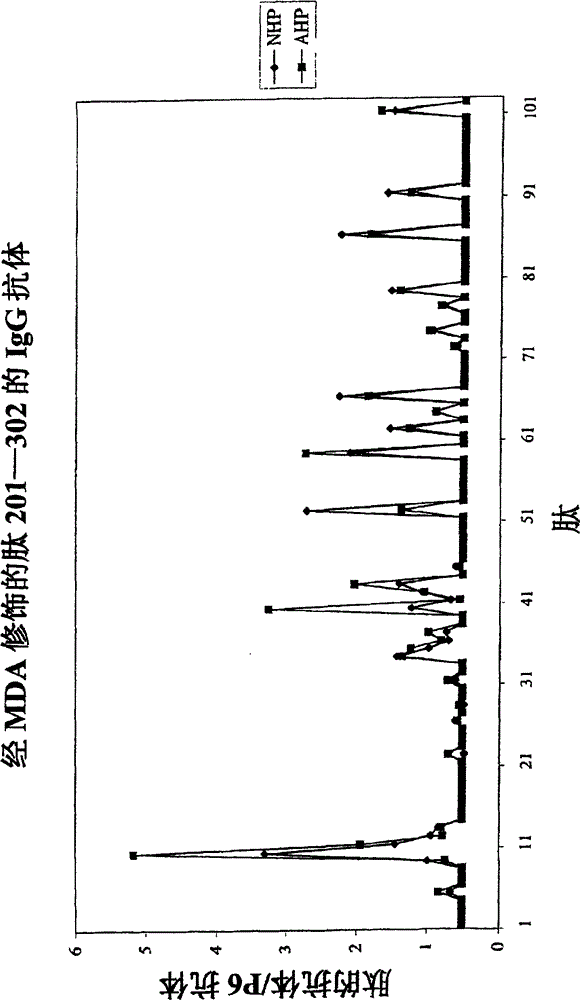
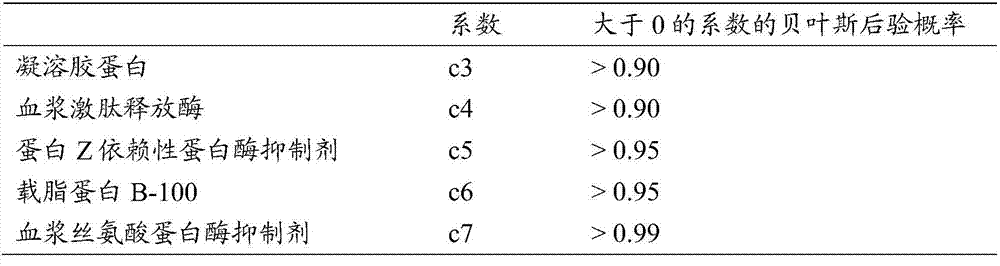
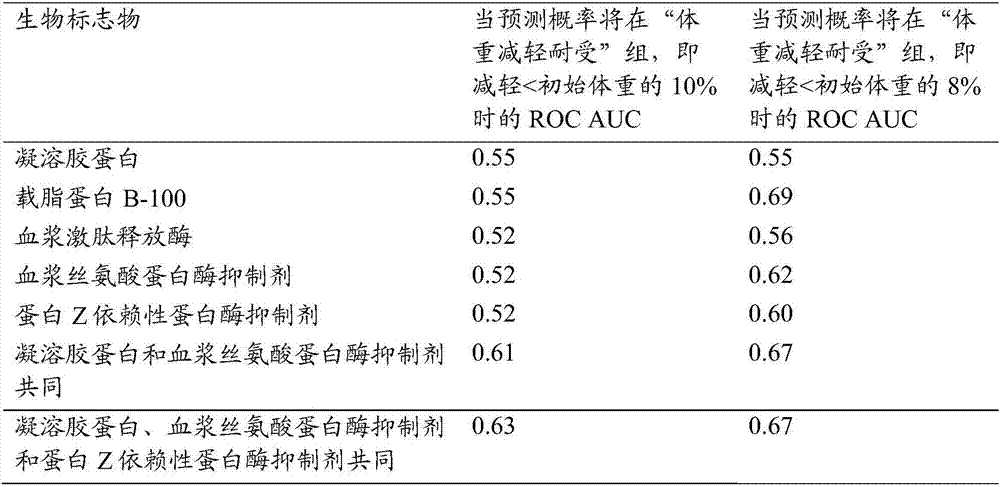
Biomarkers for predicting degree of weight loss in female subjects
A method for predicting the degree of weight loss in a female subject attainable by applying one or more dietary interventions to a subject, said method comprising; determining the level of one or more biomarkers in one or more samples obtained from the subject, wherein the biomarkers are selected from gelsolin, apolipoprotein B-100, plasma kallikrein, protein Z- dependent protease inhibitor and plasma serine protease inhibitor.
Owner:SOC DES PROD NESTLE SA
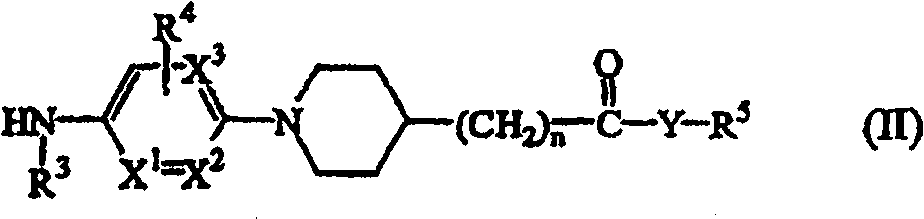
N-aryl piperidine substituted biphenylcarboxamides as inhibitors of apolipoprotein B
InactiveCN100548985CDecrease or increase effective daily doseOrganic chemistryMetabolism disorderDrugType ii diabetes
N-aryl piperidine substituted biphenylcarboxamides compounds of formula (I) methods for preparing such compounds, pharmaceutical compositions comprising said compounds as well as the use of said compounds as a medicine for the treatment of hyperlipidemia, obesity and type II diabetes.
Owner:JANSSEN PHARMA NV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com