Peptide-based immunization therapy for treatment of atherosclerosis
A technology for atherosclerosis and mammals, applied in the direction of anti-animal/human immunoglobulin, chemical instruments and methods, peptides, etc., can solve problems such as adverse immune reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
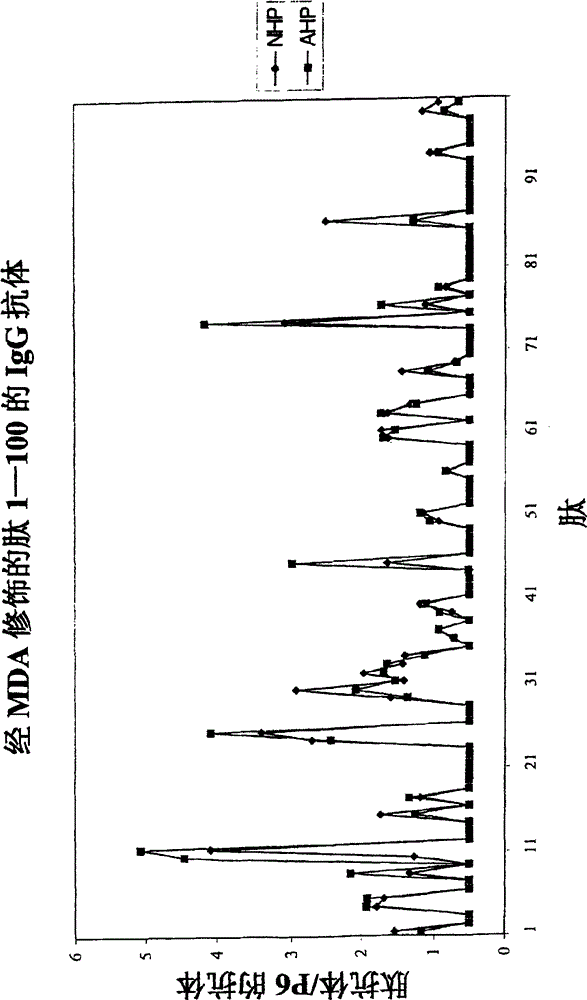
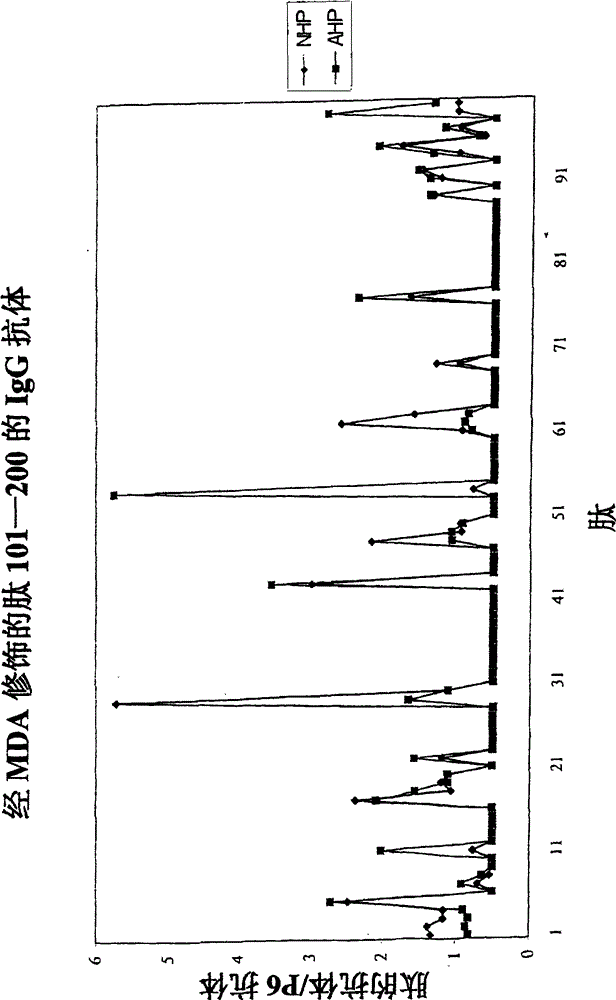
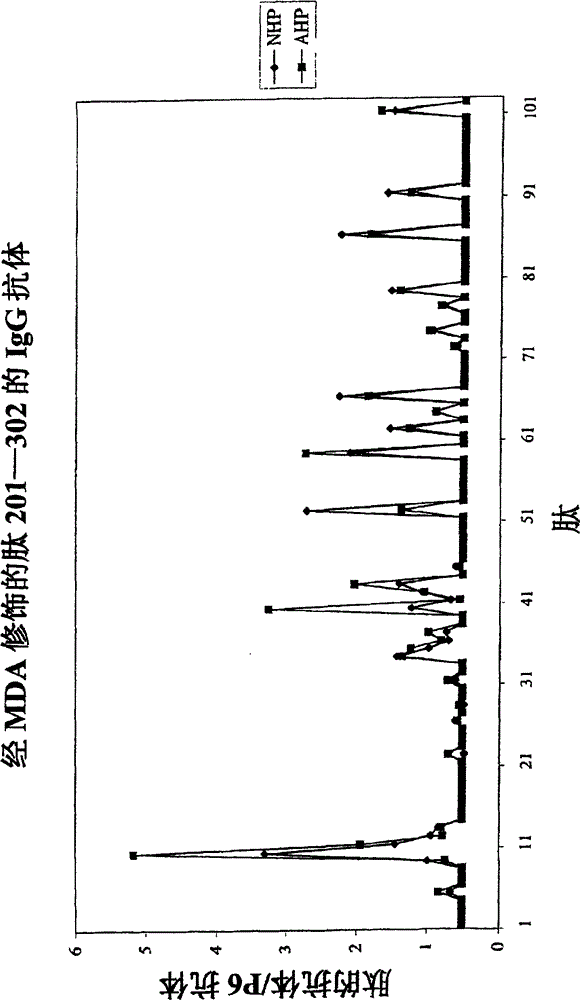
[0033] The molecular characterization of epitopes of oxidized LDL that lead to antibody-dependent immune responses in humans was investigated. The method used takes advantage of the fact that immune responses are almost always directed against peptide sequences with a length of 5 to 6 amino acids. LDL contains only one protein, apolipoprotein B, which is 4563 amino acids in length. During oxidation, apolipoprotein B is cleaved and aldehyde adducts are attached to positively charged amino acids, particularly lysine. This means that immune cells have access to peptide sequences that are not normally exposed to the immune system due to the three-dimensional structure of apolipoprotein B, and / or that normally exposed peptide sequences bind aldehydes to haptenize them, thus possessing immunogenicity.
[0034] Therefore, the following peptides, whether in their native form or as MDA derivatives, should be present in sufficient quantities to generate an immune response are:
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com