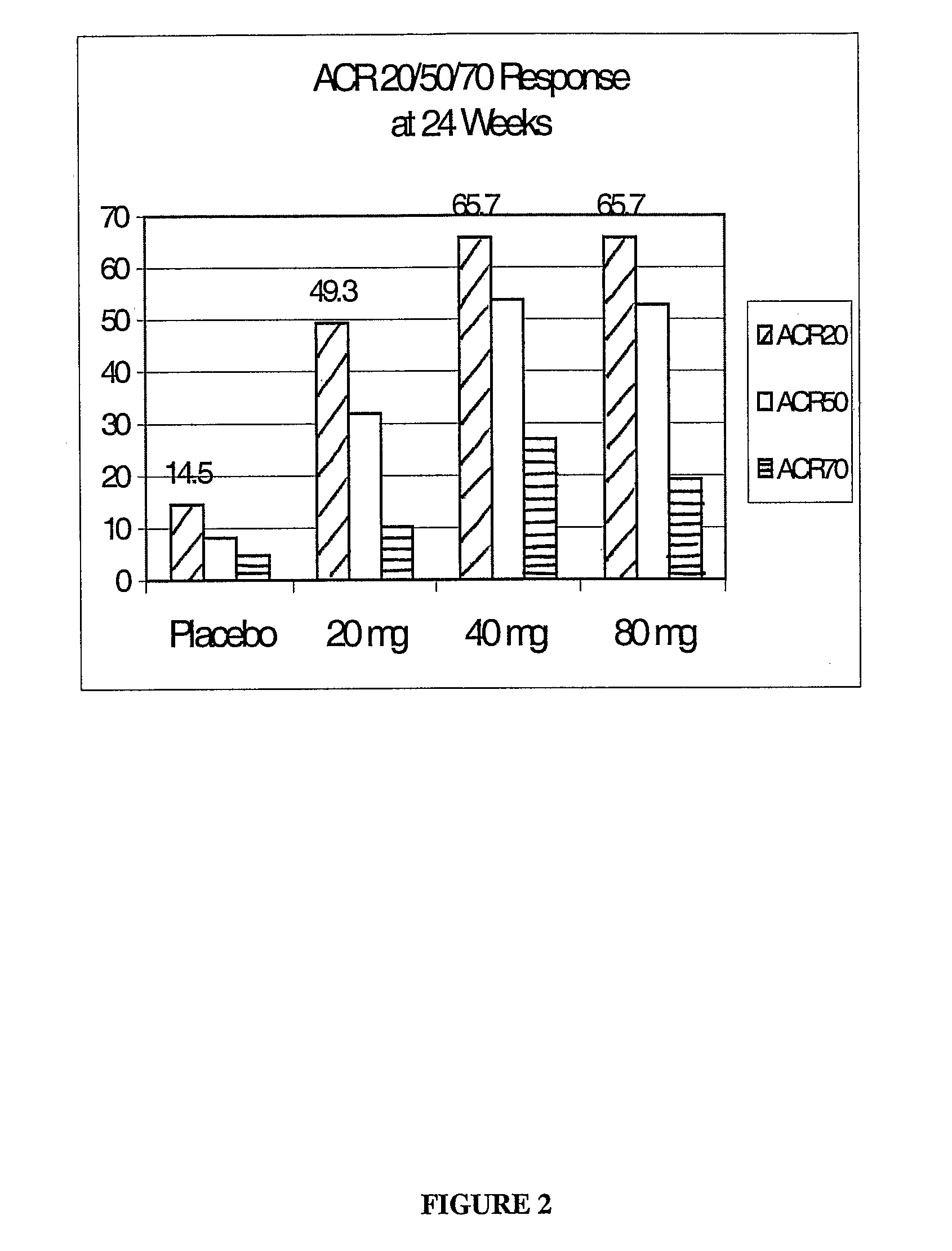
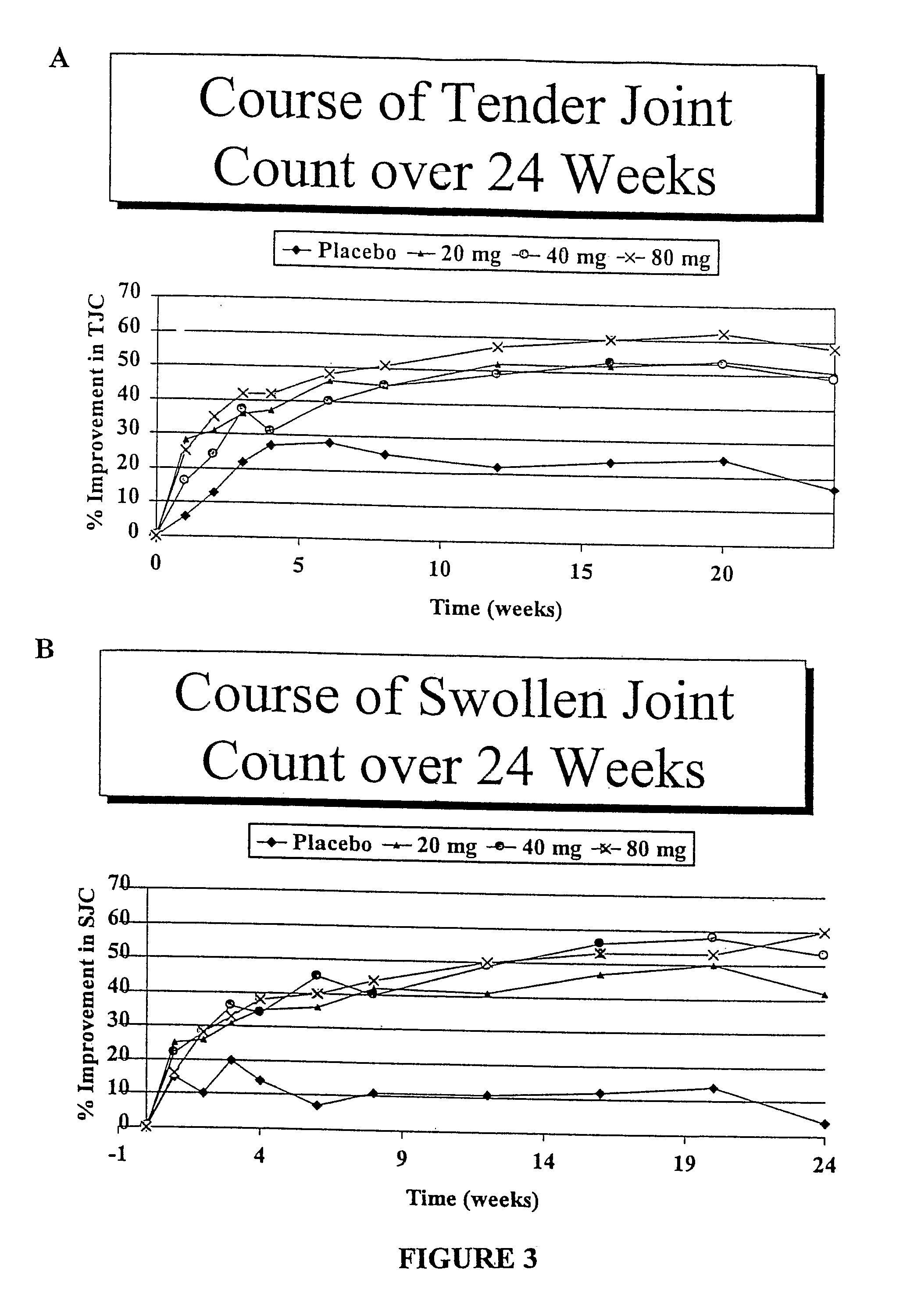
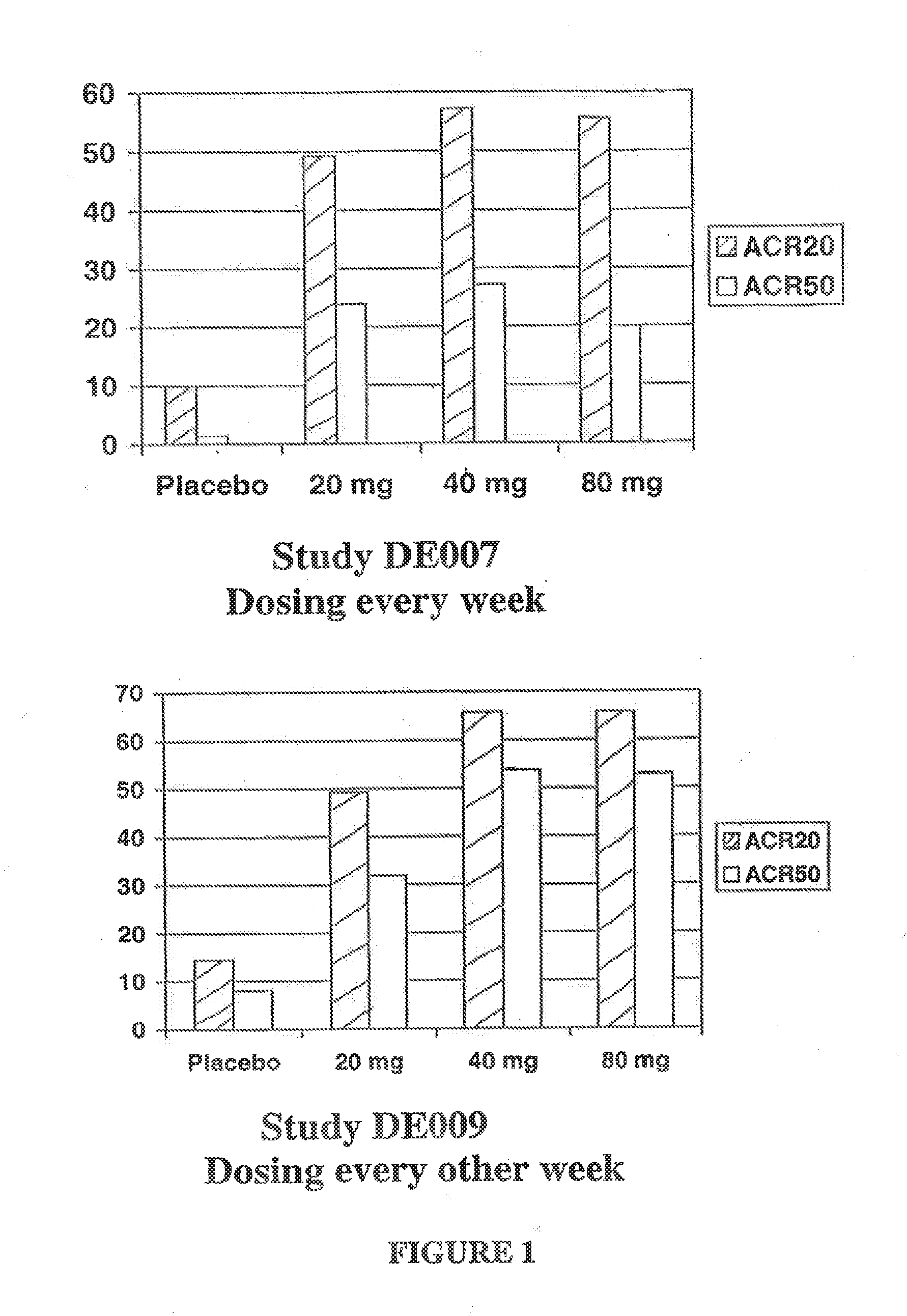
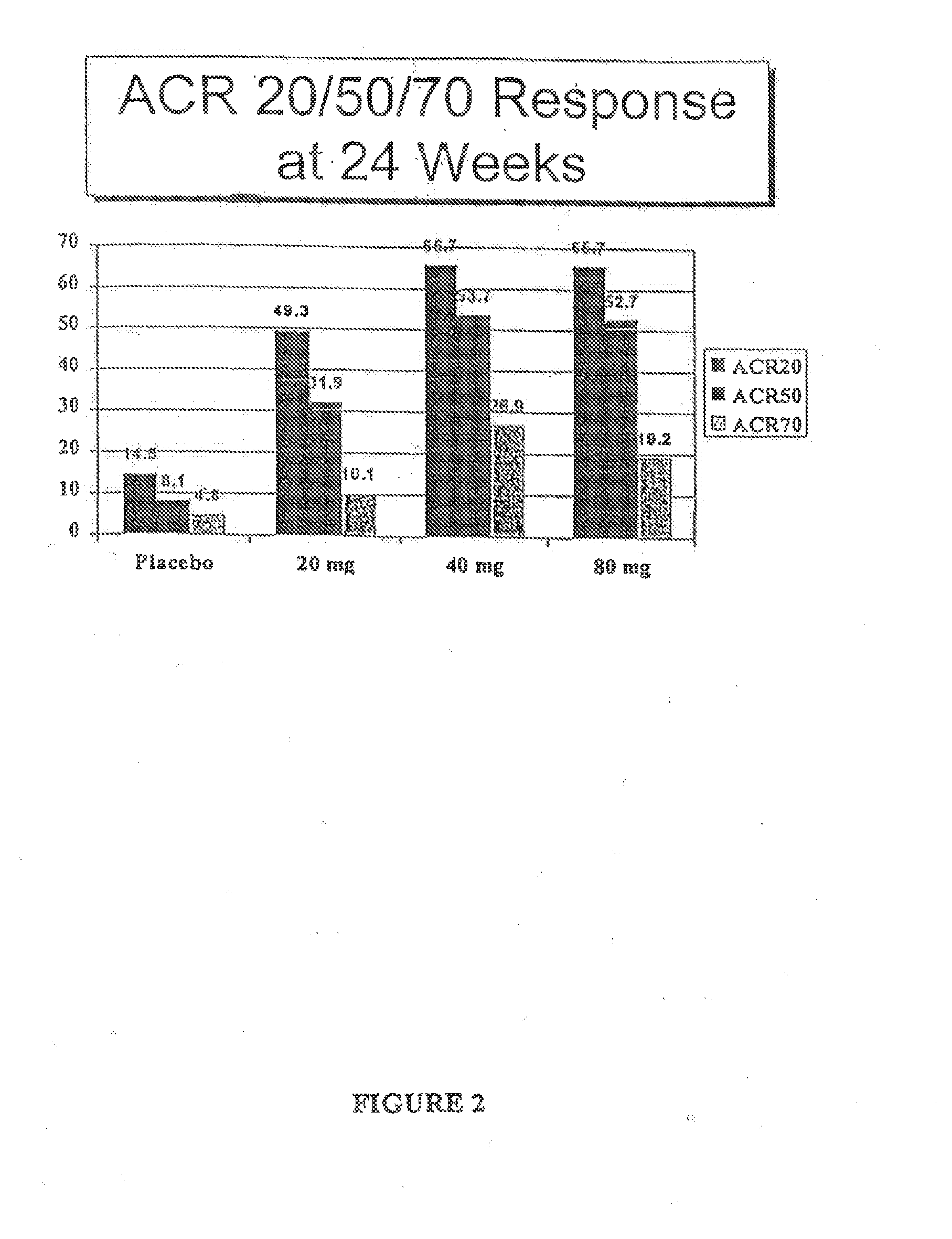
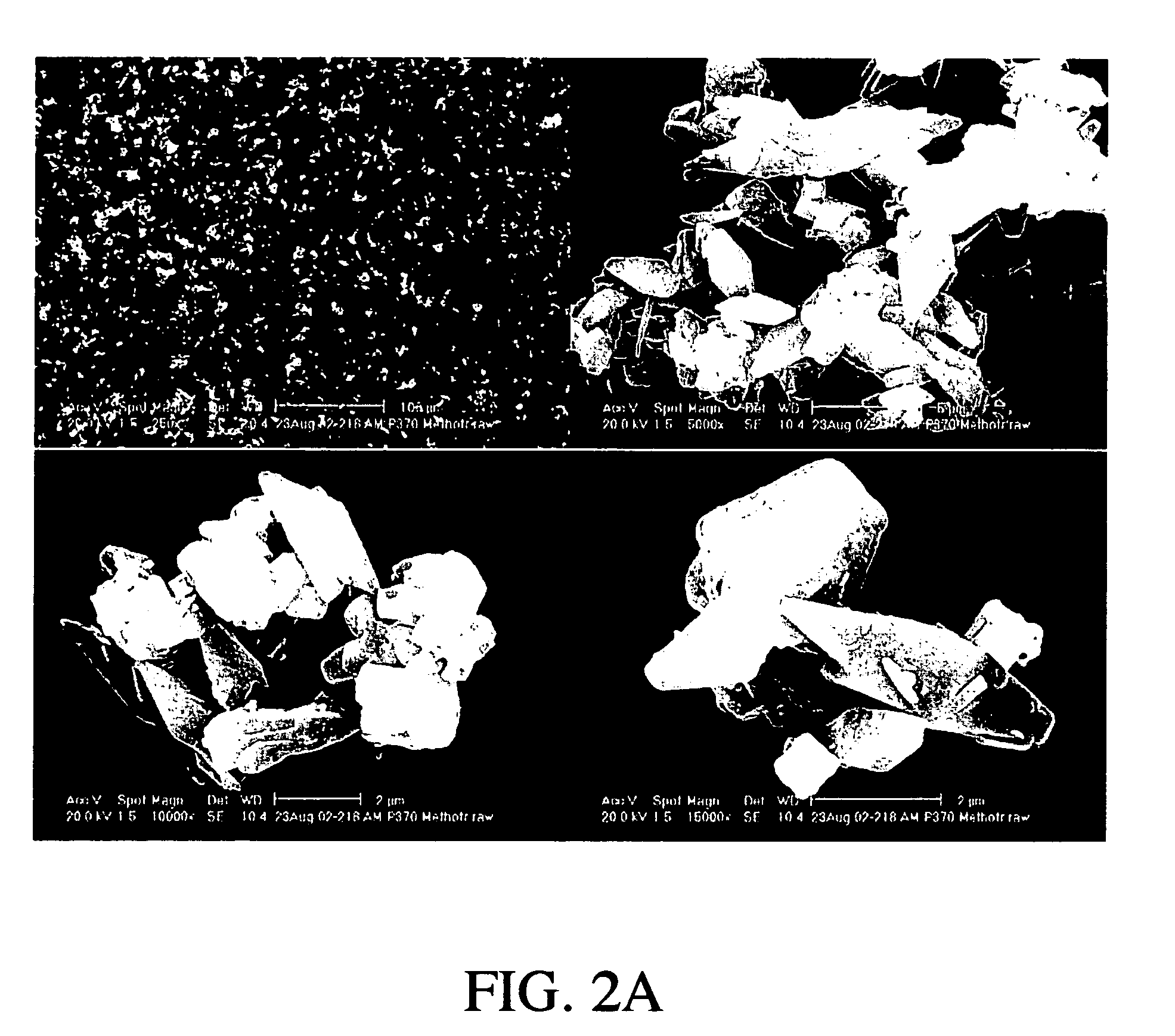
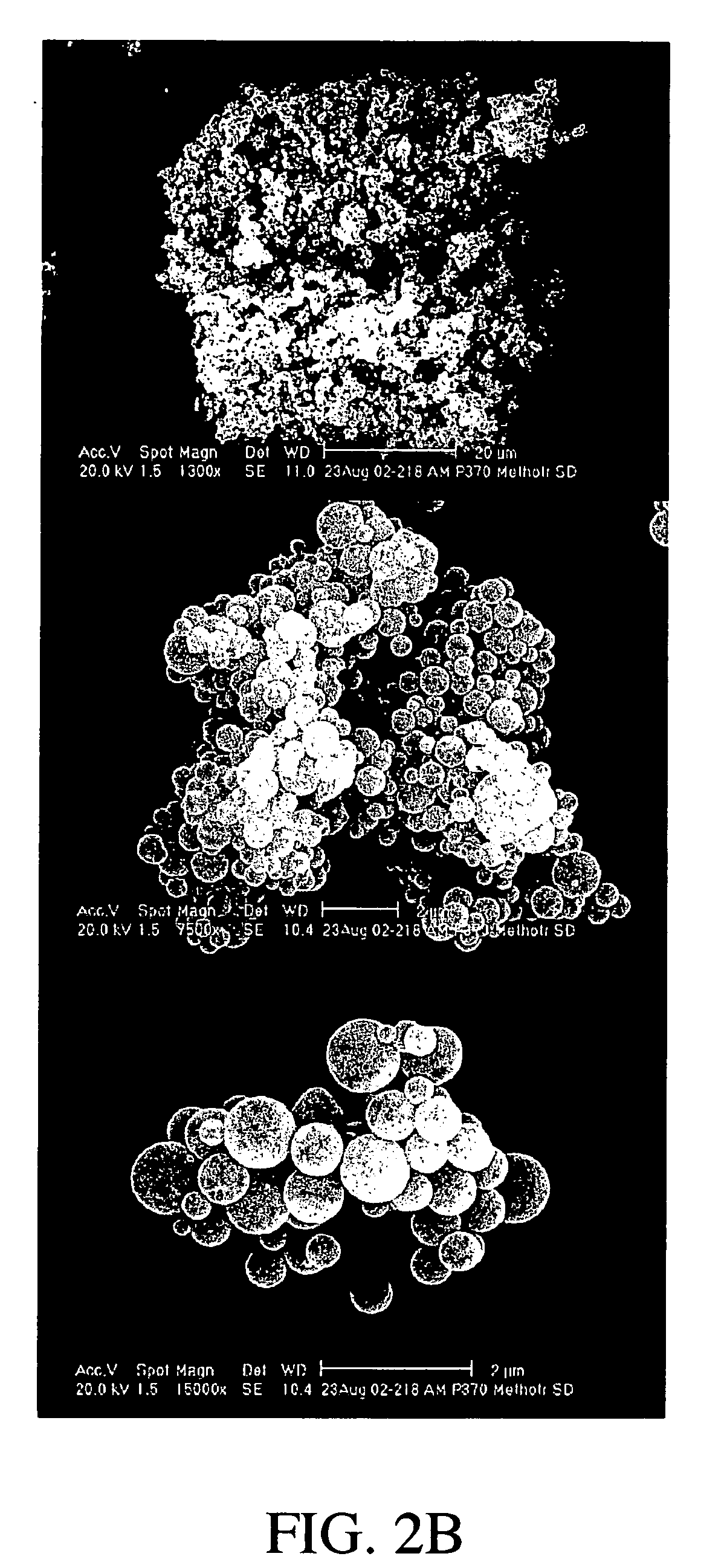
Patents
Literature
228 results about "MTX - Methotrexate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methotrexate (MTX), formerly known as amethopterin, is a chemotherapy agent and immune system suppressant. It is used to treat cancer, autoimmune diseases, ectopic pregnancy, and for medical abortions. Types of cancers it is used for include breast cancer, leukemia, lung cancer, lymphoma, and osteosarcoma.
Intrathecal administration of rituximab for treatment of central nervous system lymphomas
InactiveUS20020009444A1Prevent intermolecular disulfide formationPromote recoveryBiocidePeptide/protein ingredientsMeningesImmunocompromised patient
This invention describes methods of using anti-B cell antibodies, preferably anti-CD20 antibodies, and most preferably Rituximab, to treat B cell lymphomas of the brain, especially primary central nervous system lymphomas (PCNSLs), and to prevent meningeal relapse. The antibodies can be administered intrathecally alone, or in combination with other chemotherapeutics, such as methotrexate, or other anti-B cell antibodies to treat PCNSL in both immunocompromised and non-immunocompromised patients. These antibodies can also be used to diagnose patients with CNS lymphoma, especially in immunocompromised patients.
Owner:BIOGEN INC
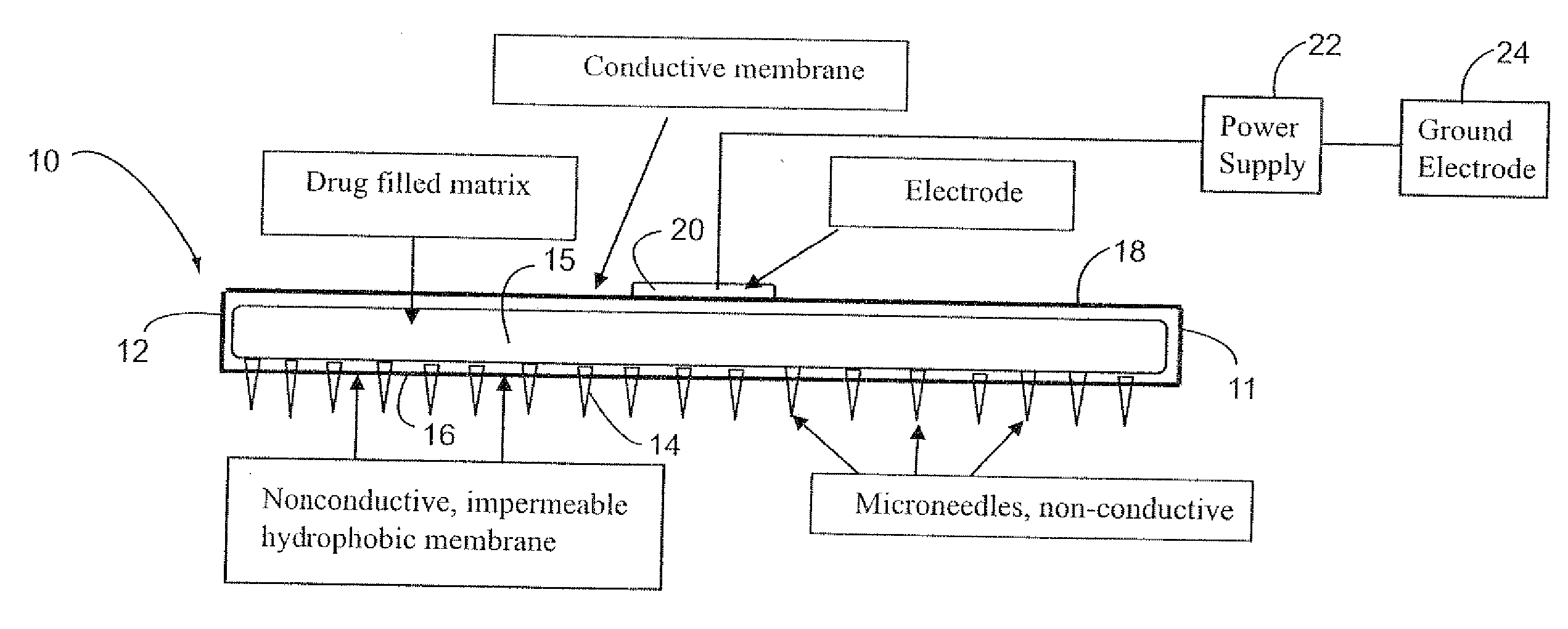
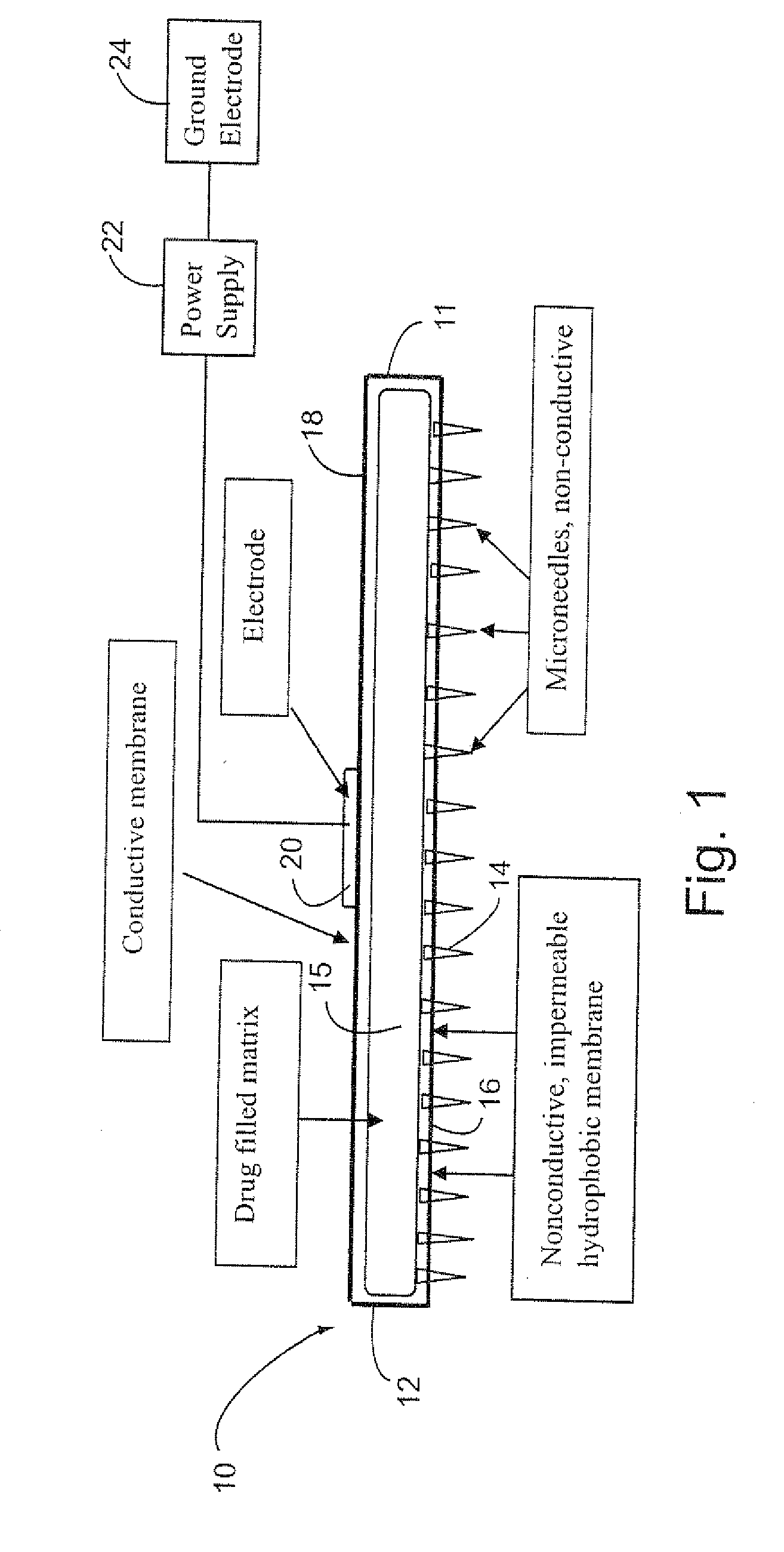
Electrokinetic system and method for delivering methotrexate
InactiveUS20070185432A1Improve consistencyIncrease the number ofElectrotherapySurgeryEngineeringConductive materials
The electrokinetic methotrexate delivery system includes at least one applicator having a multiplicity of non-conductive micro-needles carried on a non-conductive surface of the applicator. The opposite surface is formed of electrically conductive material for contact with an active electrode. The applicator includes a matrix containing a medicament, e.g., methotrexate, or a carrier therefor between the opposite surfaces. When the applicator is applied to the individual's skin with the micro-needles penetrating the skin, an electrical current is completed through the power source, the active electrode, methotrexate, or electrically conductive carrier therefor, the targeted treatment site, the individual's body, a ground electrode and the power supply, thereby electokinetically driving the medicament through the non-conductive micro-needles into the targeted treatment site.
Owner:NITRIC BIOTHERAPEUTICS INC
METHODS OF ADMINISTERING ANTI-TNFalpha ANTIBODIES
InactiveUS20120177596A1Reduce in quantityPatient compliance is goodSenses disorderNervous disorderHuman tumorFactor ii
Methods of treating disorders in which TFNα activity is detrimental via biweekly, subcutaneous administration of human antibodies, preferably recombinant human antibodies, that specifically bind to human tumor necrosis factor α (hTNFα) are disclosed. The antibody may be administered with or without methotrexate. These antibodies have high affinity for hTNFα (e.g., Kd=10−8 M or less), a slow off rate for hTNFα dissociation (e.g., Koff=10−3 sec−1 or less) and neutralize hTNFα activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. Kits containing a pharmaceutical composition and instructions for dosing, and preloaded syringes containing pharmaceutical compositions are also encompassed by the invention.
Owner:ABBVIE BIOTECHNOLOGY LTD
METHODS OF ADMINISTERING ANTI-TNFalpha ANTIBODIES
InactiveUS20130004507A1Reduce in quantityLess frequentSenses disorderNervous disorderHuman tumorAntigen binding
Methods of treating disorders in which TFNα activity is detrimental via biweekly, subcutaneous administration of human antibodies, preferably recombinant human antibodies, that specifically bind to human tumor necrosis factor α (hTNFα) are disclosed. The antibody may be administered with or without methotrexate. These antibodies have high affinity for hTNFα (e.g., Kd=10−8 M or less), a slow off rate for hTNFα dissociation (e.g., Koff=10−3 sec−1 or less) and neutralize hTNFα activity in vitro and in vivo. An antibody of the invention can be a full-length antibody or an antigen-binding portion thereof. Kits containing a pharmaceutical composition and instructions for dosing, and preloaded syringes containing pharmaceutical compositions are also encompassed by the invention.
Owner:ABBOTT BIOTECHNOLOGY LTD
Antineoplastic conjugates of transferrin, albumin and polyethylene glycol
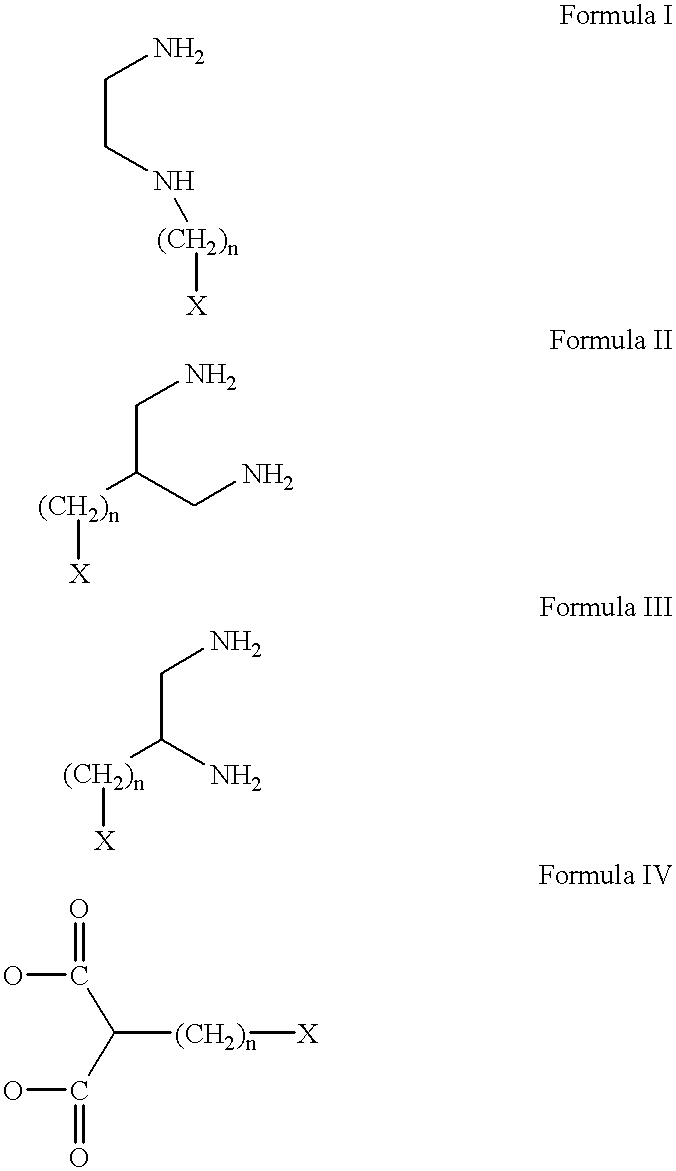
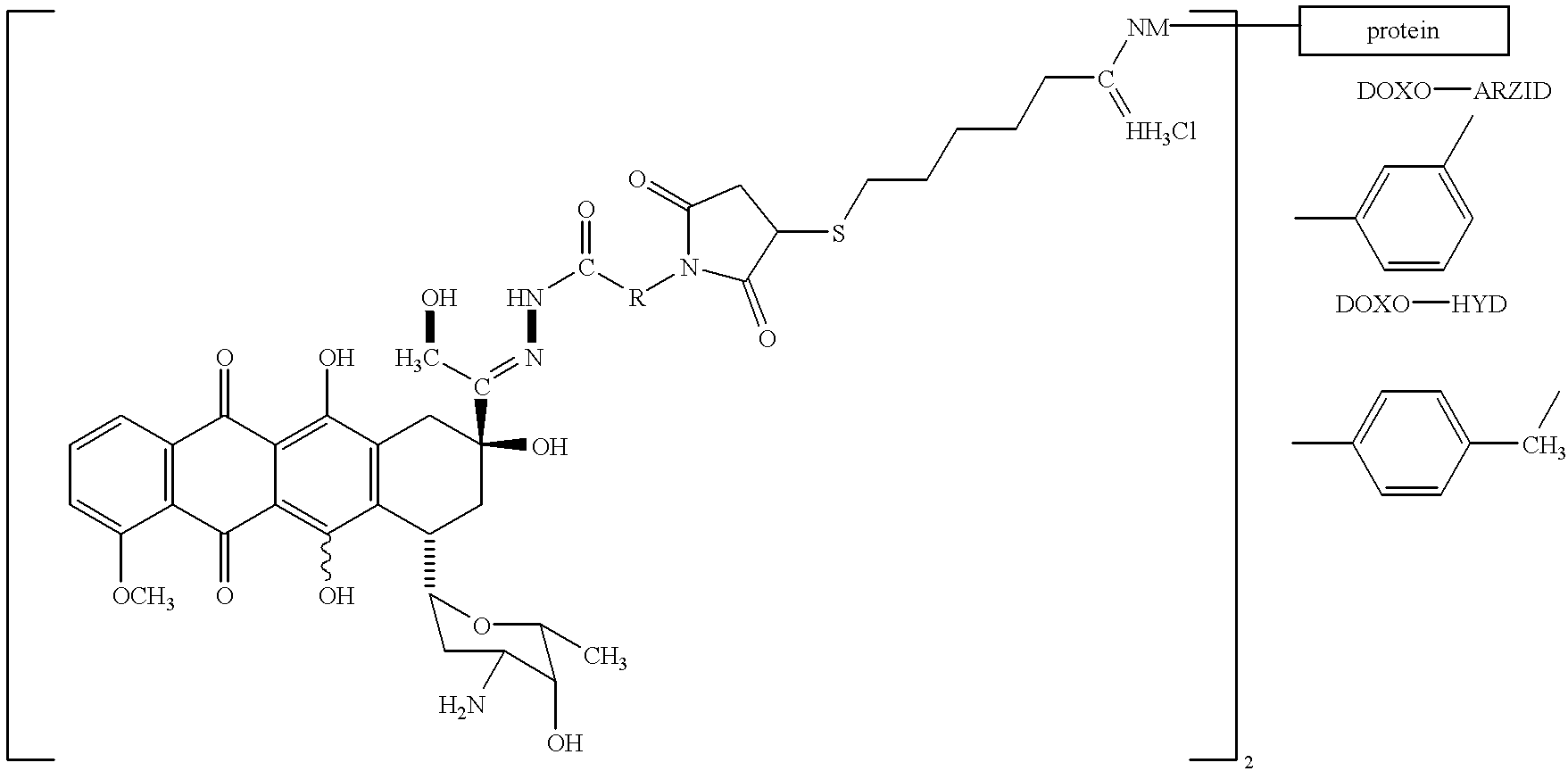
Conjugates of transferrin, albumin and polyethylence glycol consisting of native or thiolated transferrin or albumin or of polyethylene glycol (MW between approximately 5,000 and 20,0000) with at least one HS-, HO- or H2N group and cytostatic compounds derived through maleinimide or N-hydroxysuccinimide ester compounds, such as doxorubicin, daunorubicin, epirubicin, idarubicin, mitoxandrone, chloroambucil, melphalan, 5-fluorouracyl, 5'-desoxy-5-fluorouridine, thioguanine, methotrexate, paclitaxel, docetaxel, topotecan, 9-aminocamptothecin, etoposide, teniposide, mitopodoside, vinblastine, vincristine, vindesine, vinorelbine or a compound of general formula A, B, C or D, where n=0-6, X=-NH2, -OH, -COOH, -O-CO-R-COR*, -NH-CO-R-COR*, where R is an aliphatic carbon chain with 1-6 carbon atoms or a substituted or unsubstituted phenylene group and R* H, phenyl, alkyl with 1-6 carbon atoms.
Owner:KRATZ FELIX
Nanochanneled device and related methods
ActiveUS20120095443A1Amenability to selectHigh mechanical strengthMedical devicesNanomedicineIn vivoBiomedical engineering
A capsule configured for in vivo refilling of a thereapeutic agent. In certain embodiments, the capsule may contain methotrexate.
Owner:THE OHIO STATE UNIV RES FOUND +1
Jak1 selective inhibitor and uses thereof
InactiveUS20150118229A1High selectivityLow potencyBiocideSenses disorderInflammatory bowel diseaseDosage schedule
Owner:ABBVIE INC
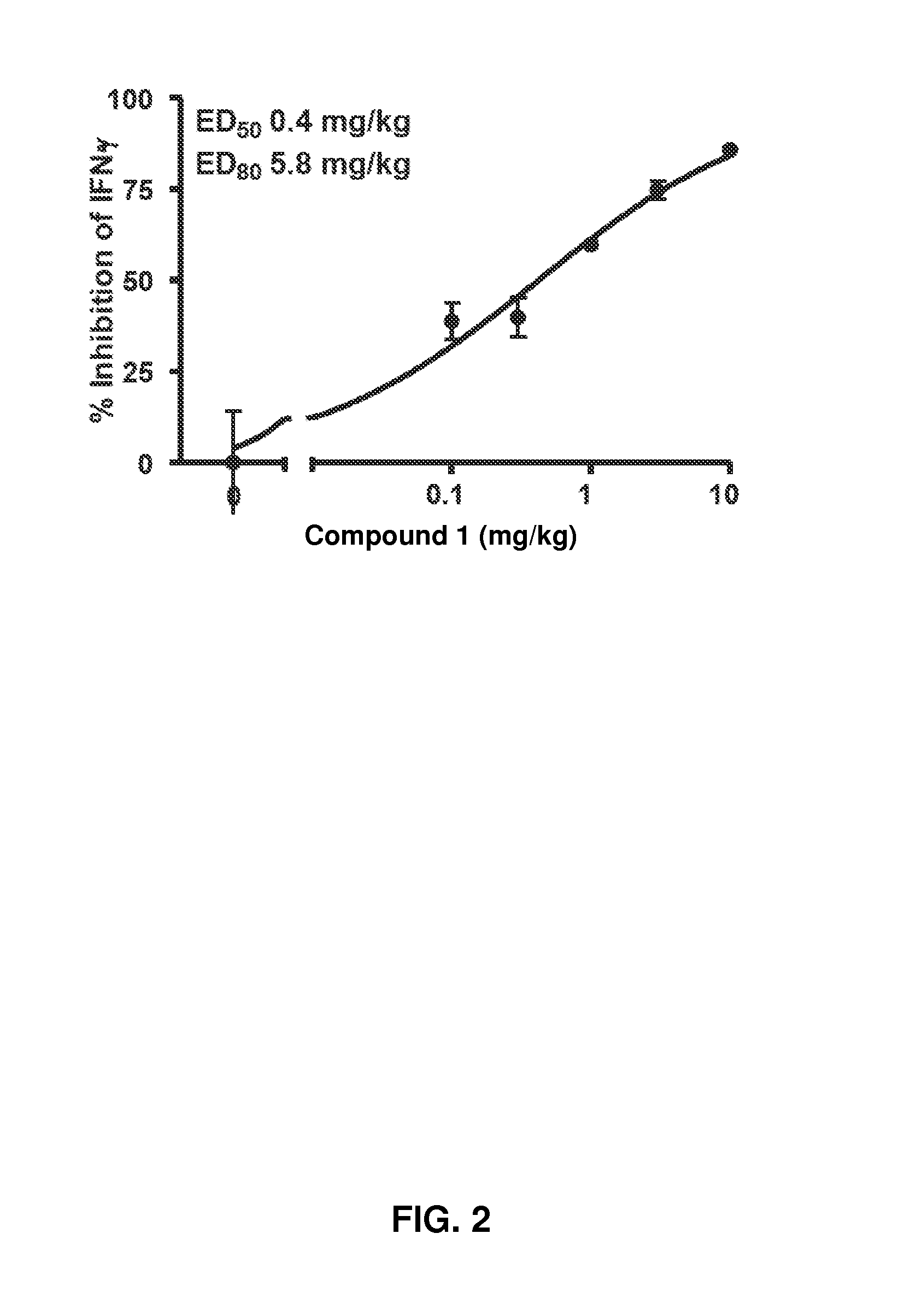
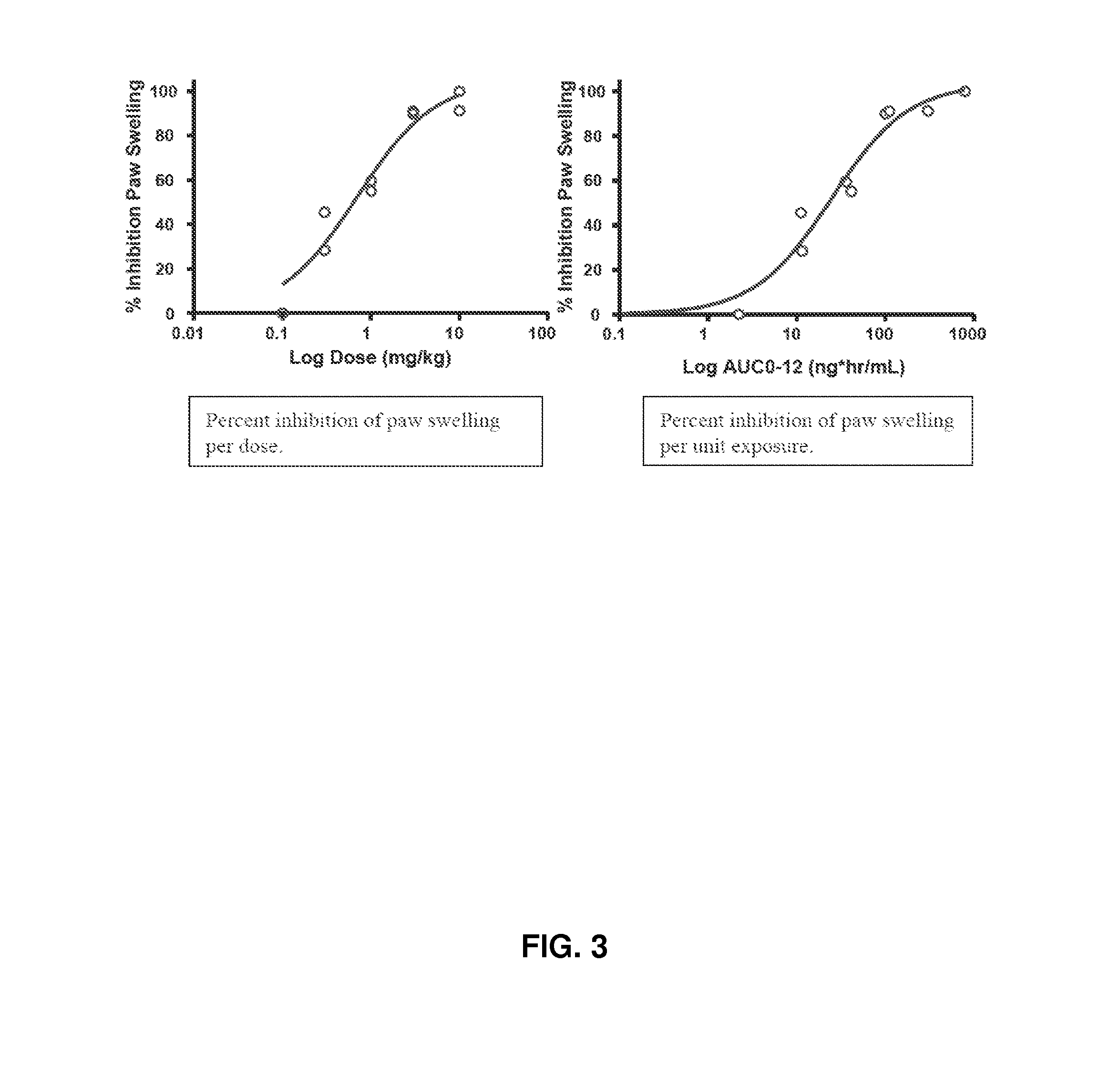
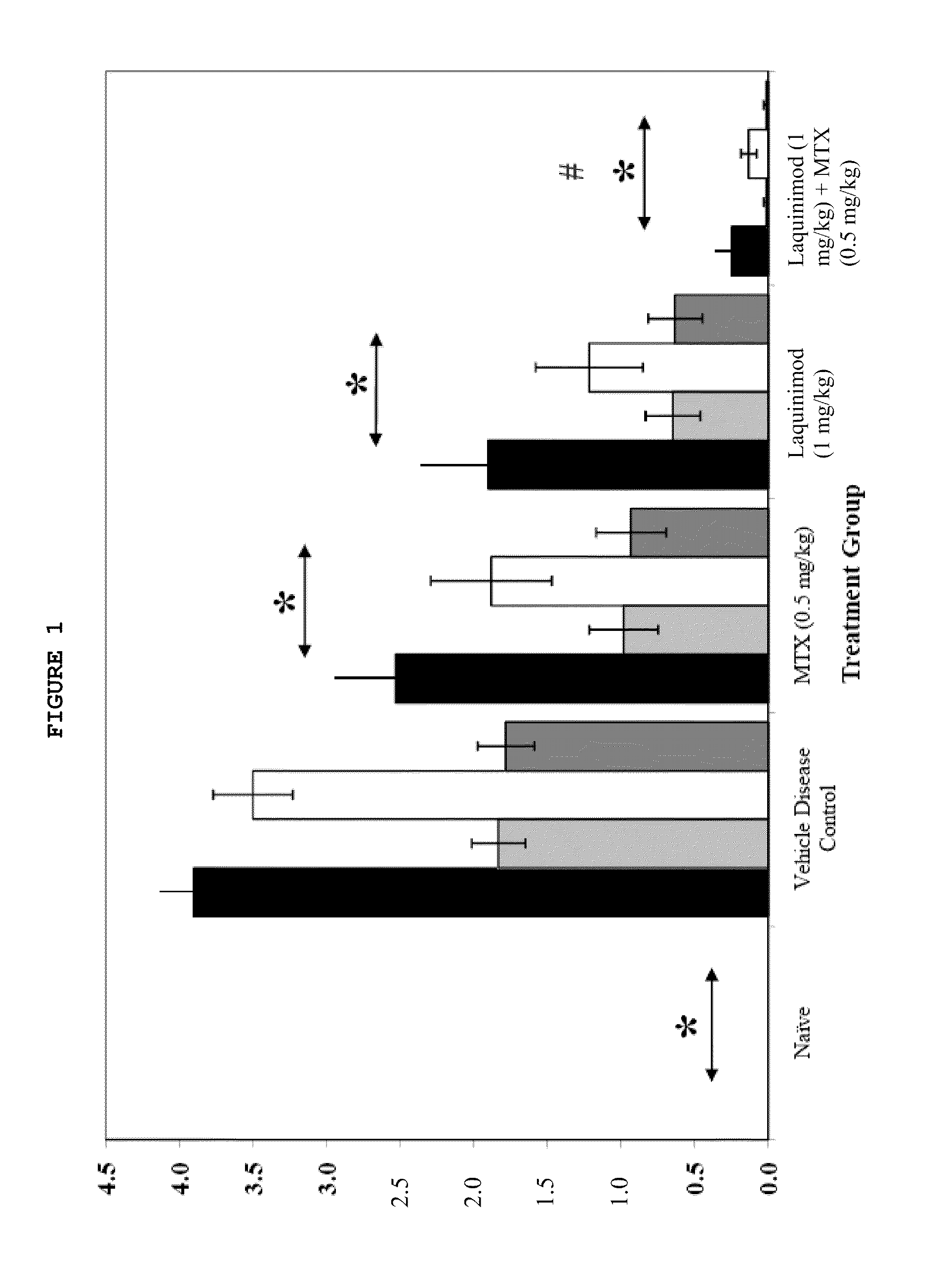
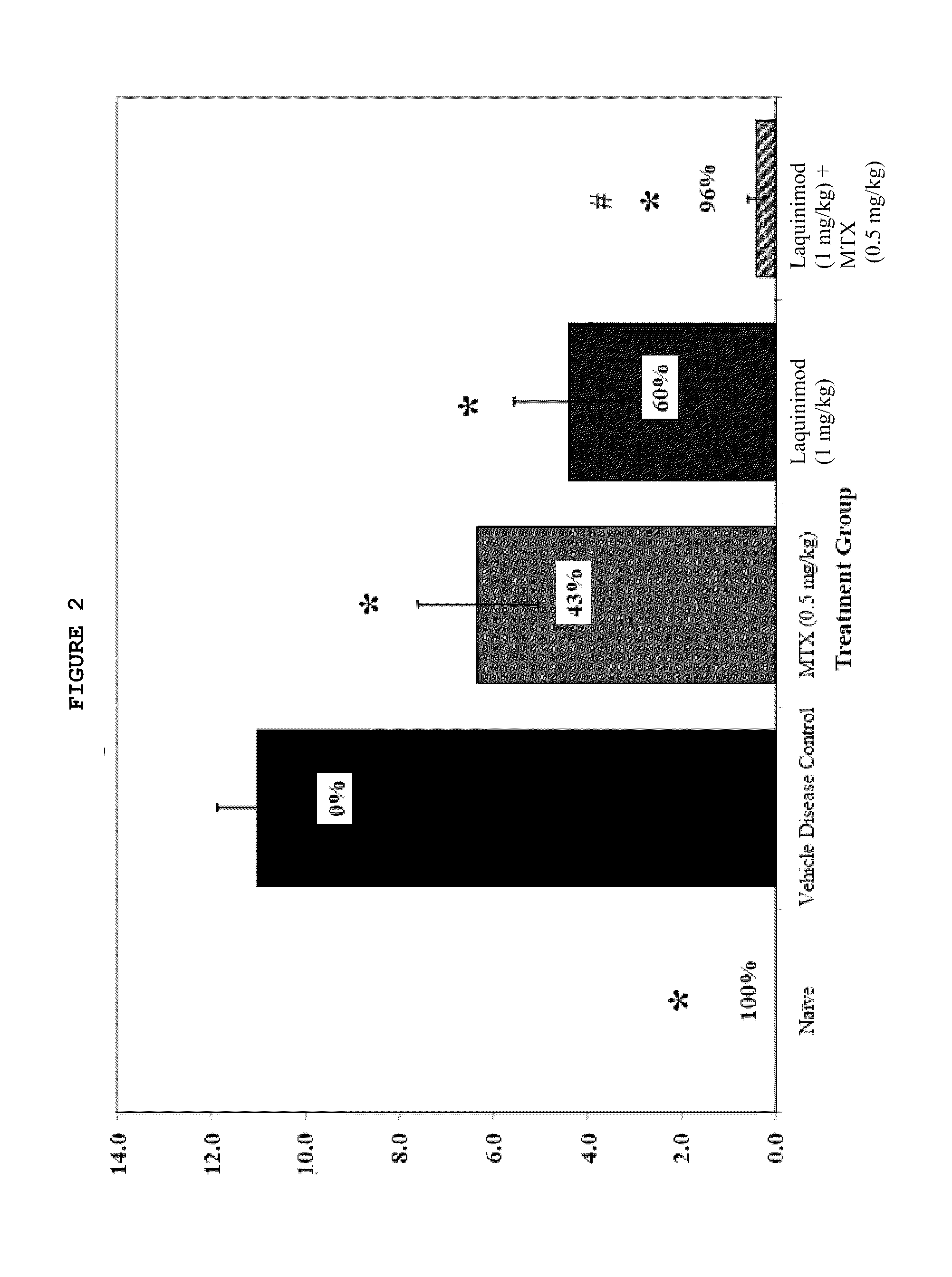
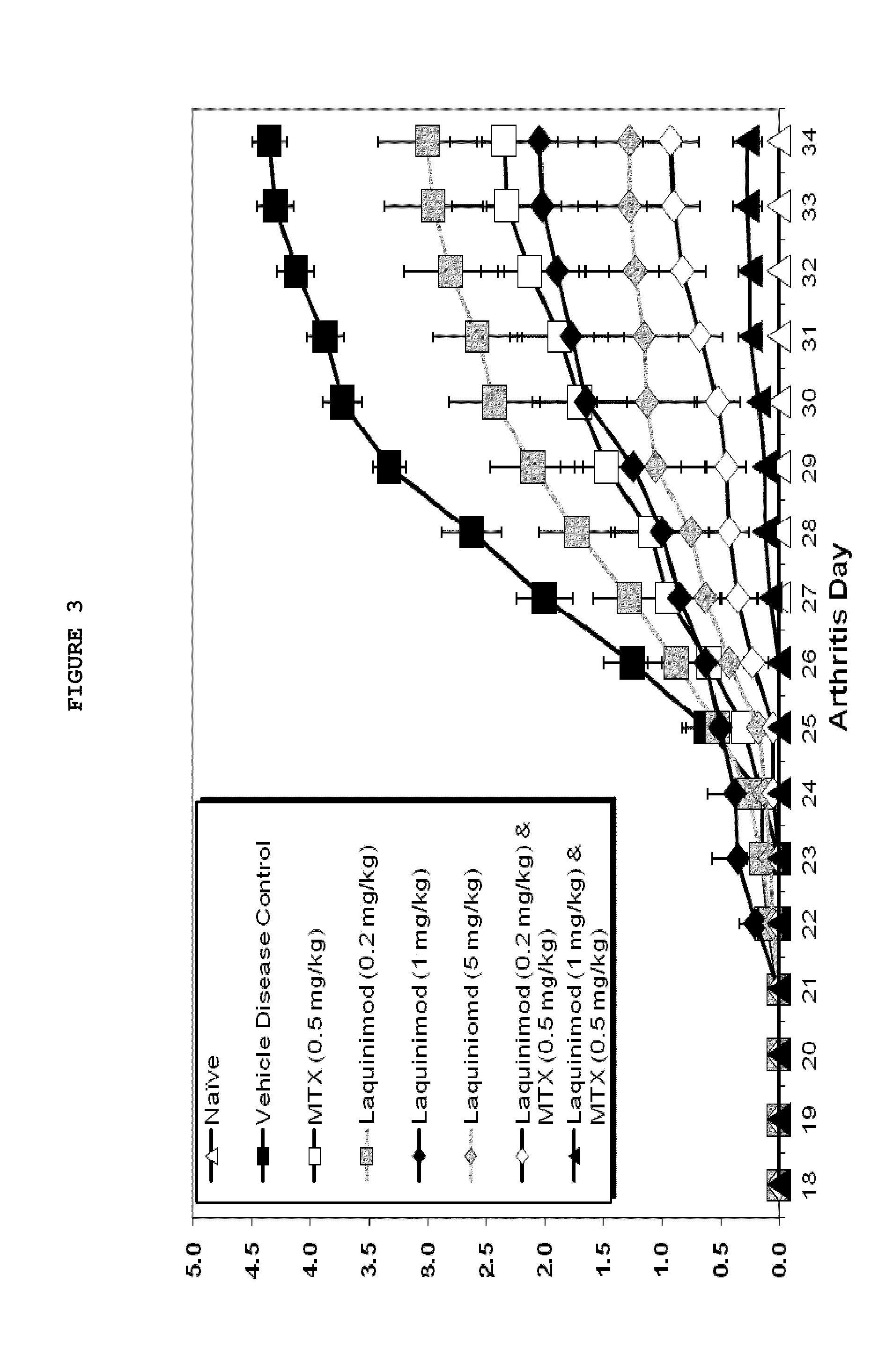
Treatment of rheumatoid arthritis with a combination of laquinimod and methotrexate
This invention provides a method of treating a subject afflicted with rheumatoid arthritis comprising periodically administering to the subject an amount of laquinimod or pharmaceutically acceptable salt thereof and an amount of methotrexate, wherein the amounts when taken together are effective to treat the subject. This invention also provides laquinimod or pharmaceutically acceptable salt thereof for use in combination with methotrexate in treating a subject afflicted with rheumatoid arthritis. This invention also provides a pharmaceutical composition comprising an amount of laquinimod or pharmaceutically acceptable salt thereof and an amount of methotrexate for use in treating a subject afflicted with rheumatoid arthritis.
Owner:TEVA PHARMA IND LTD
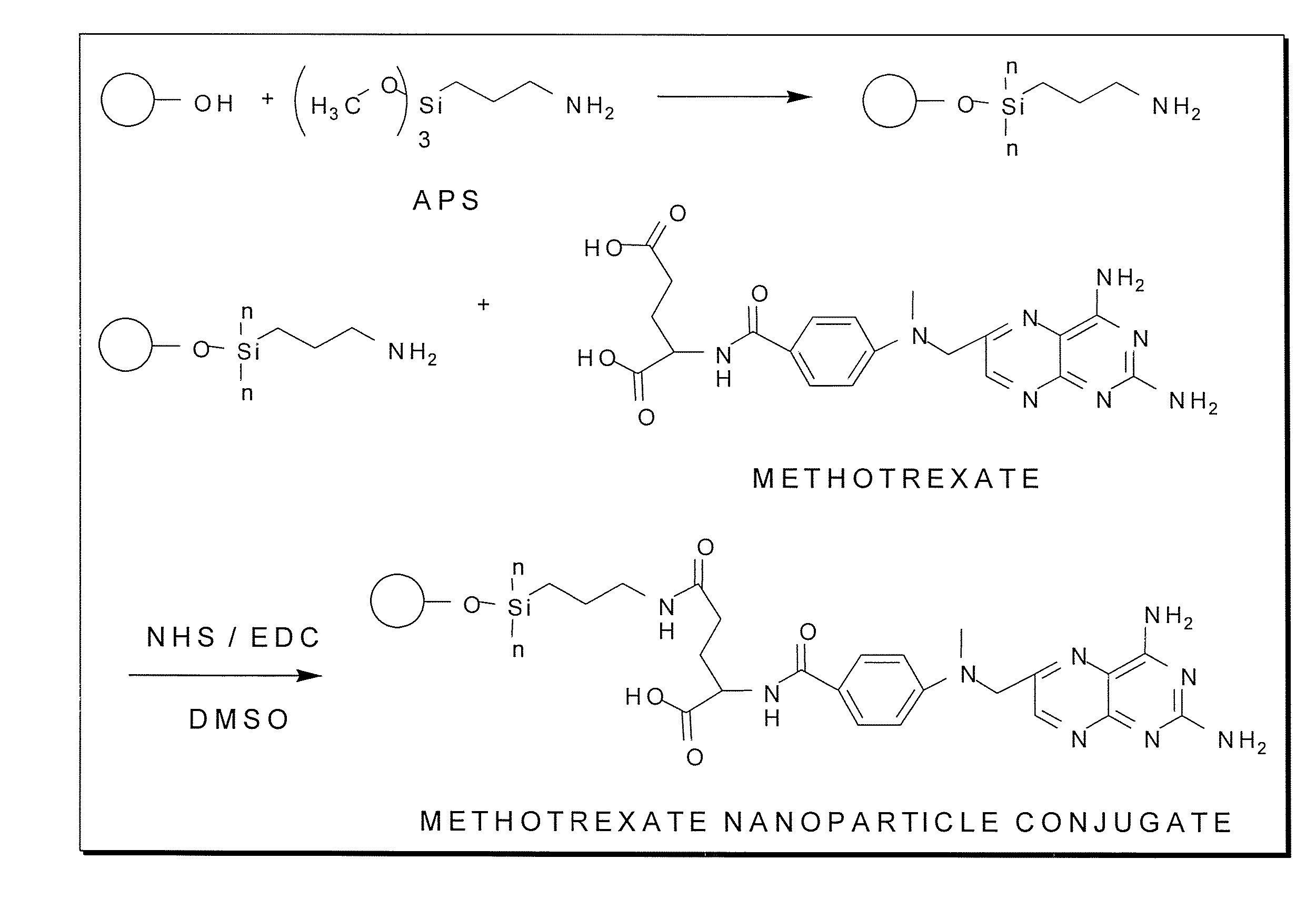
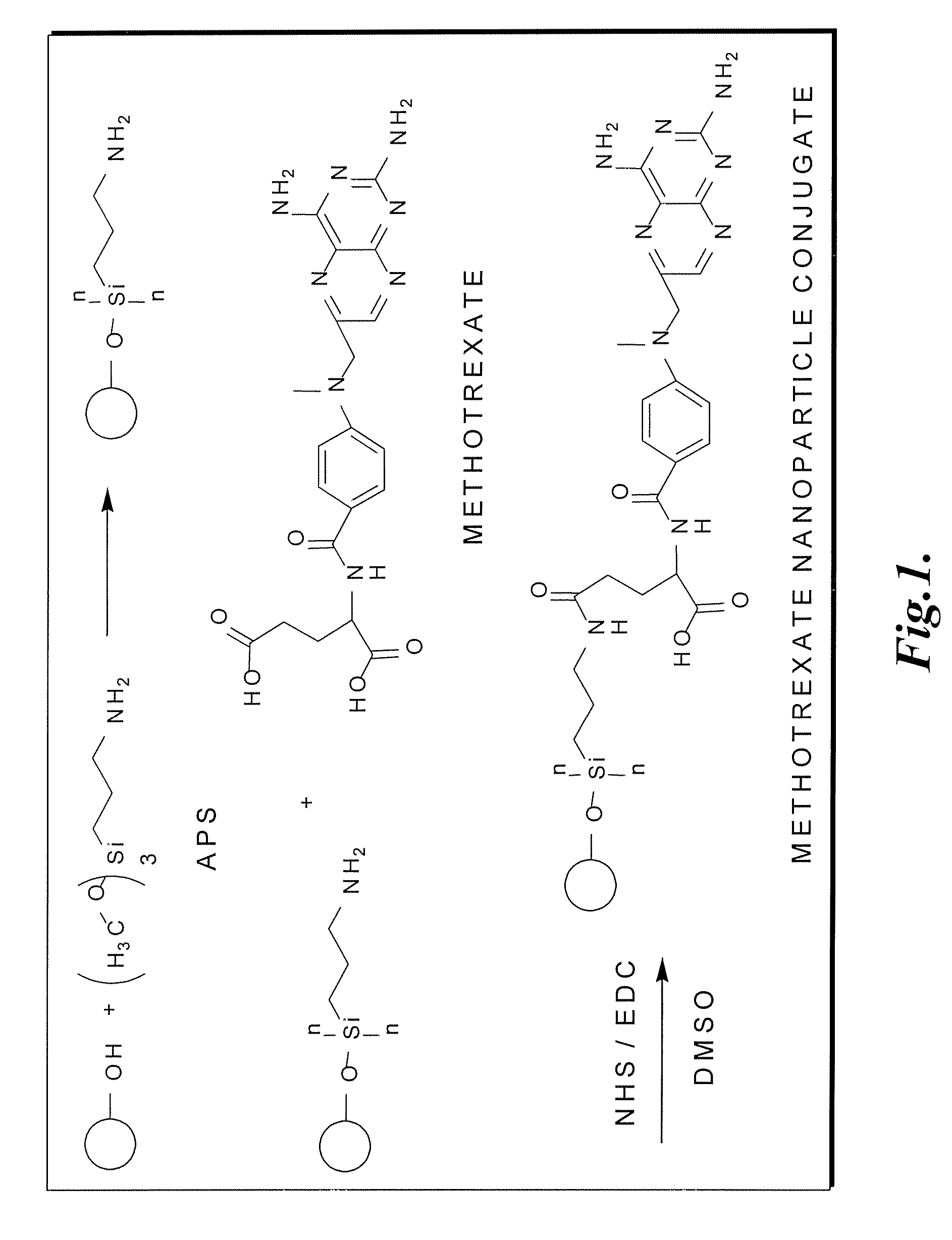
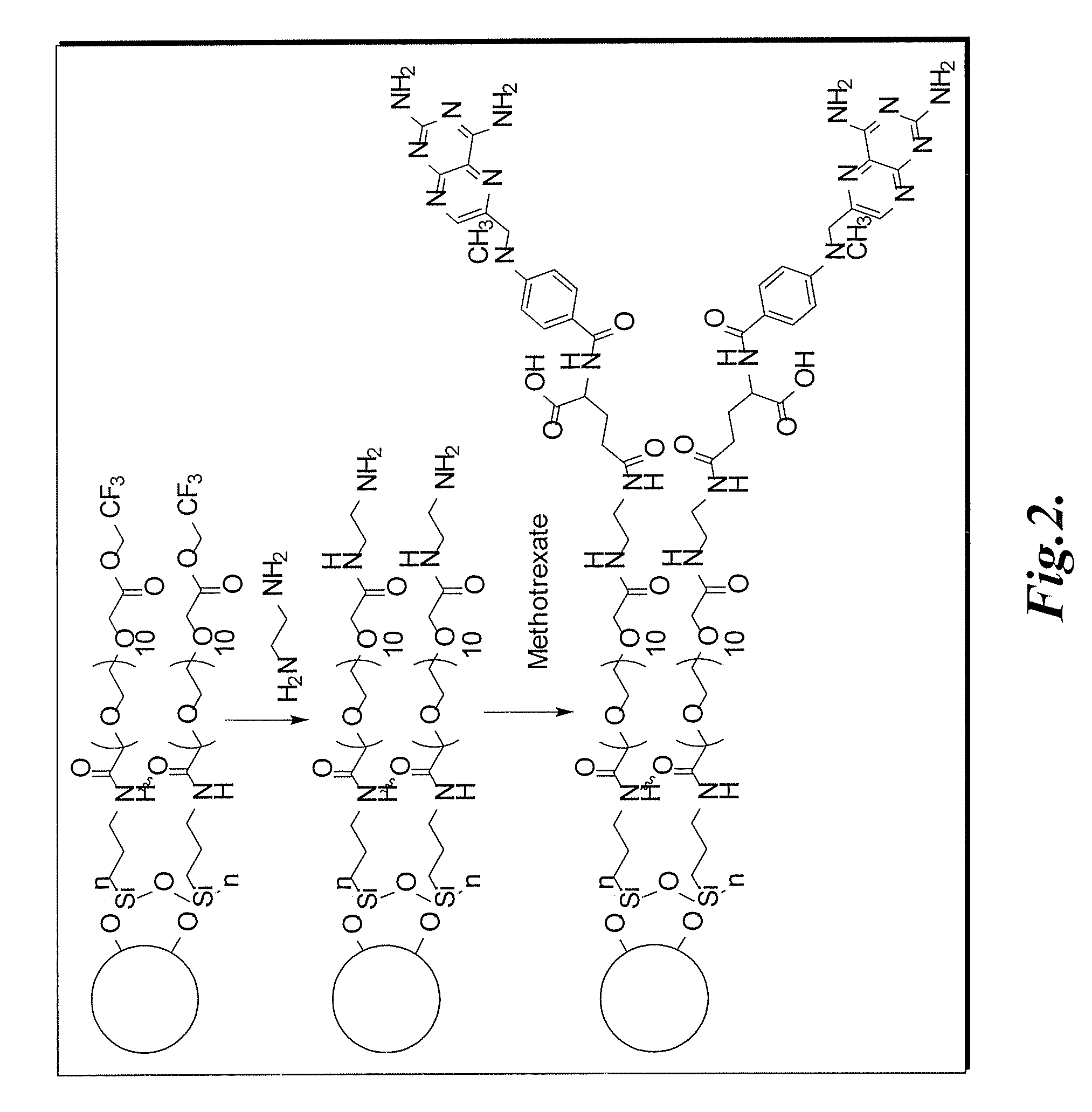
Methotrexate-modified nanoparticles and related methods
Methotrexate-modified nanoparticles that target tumors, compositions that include the nanoparticles, methods of imaging tissues using the nanoparticles, and methods for treating tissues using the nanoparticles.
Owner:IND TECH RES INST +1
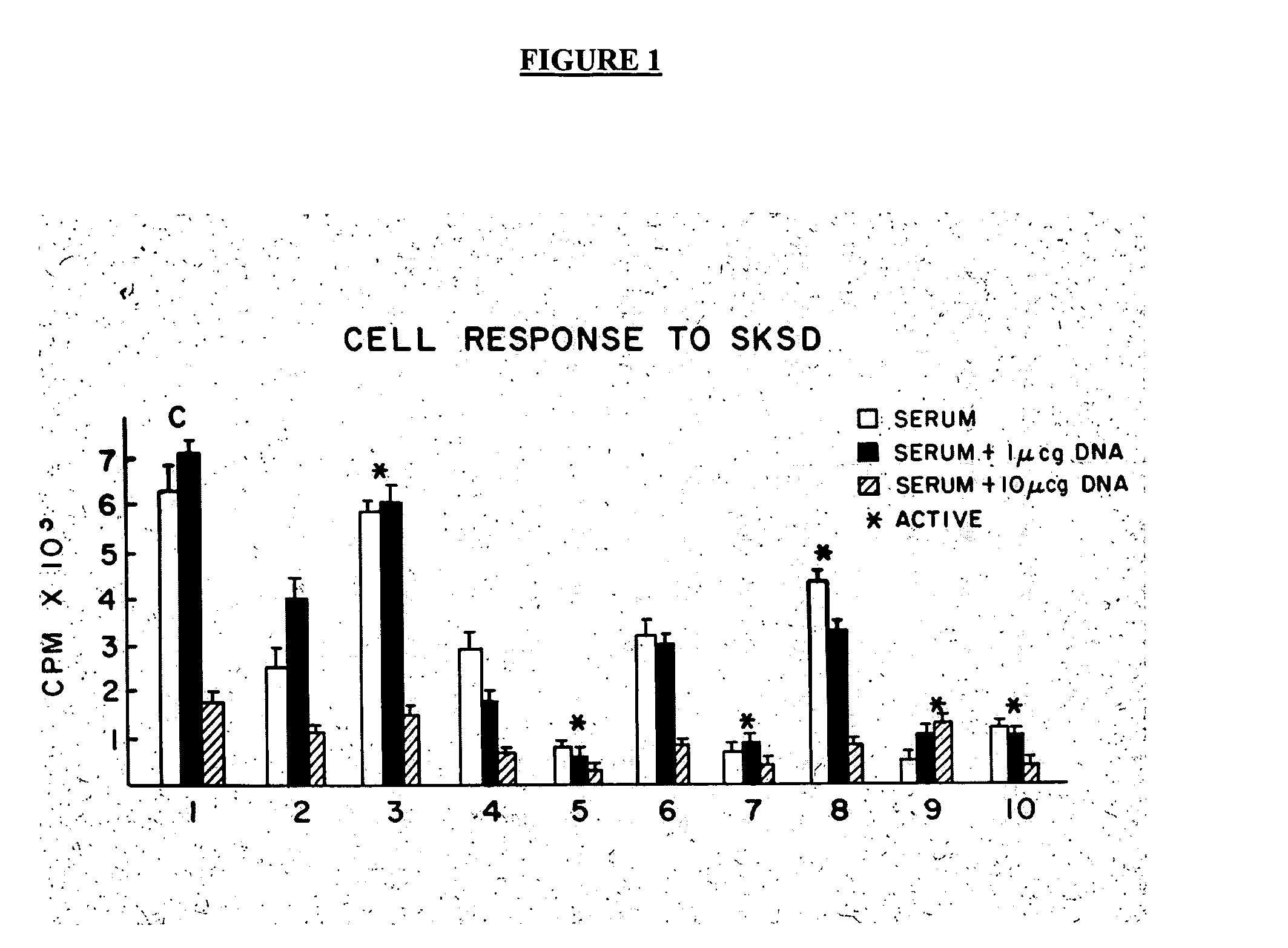
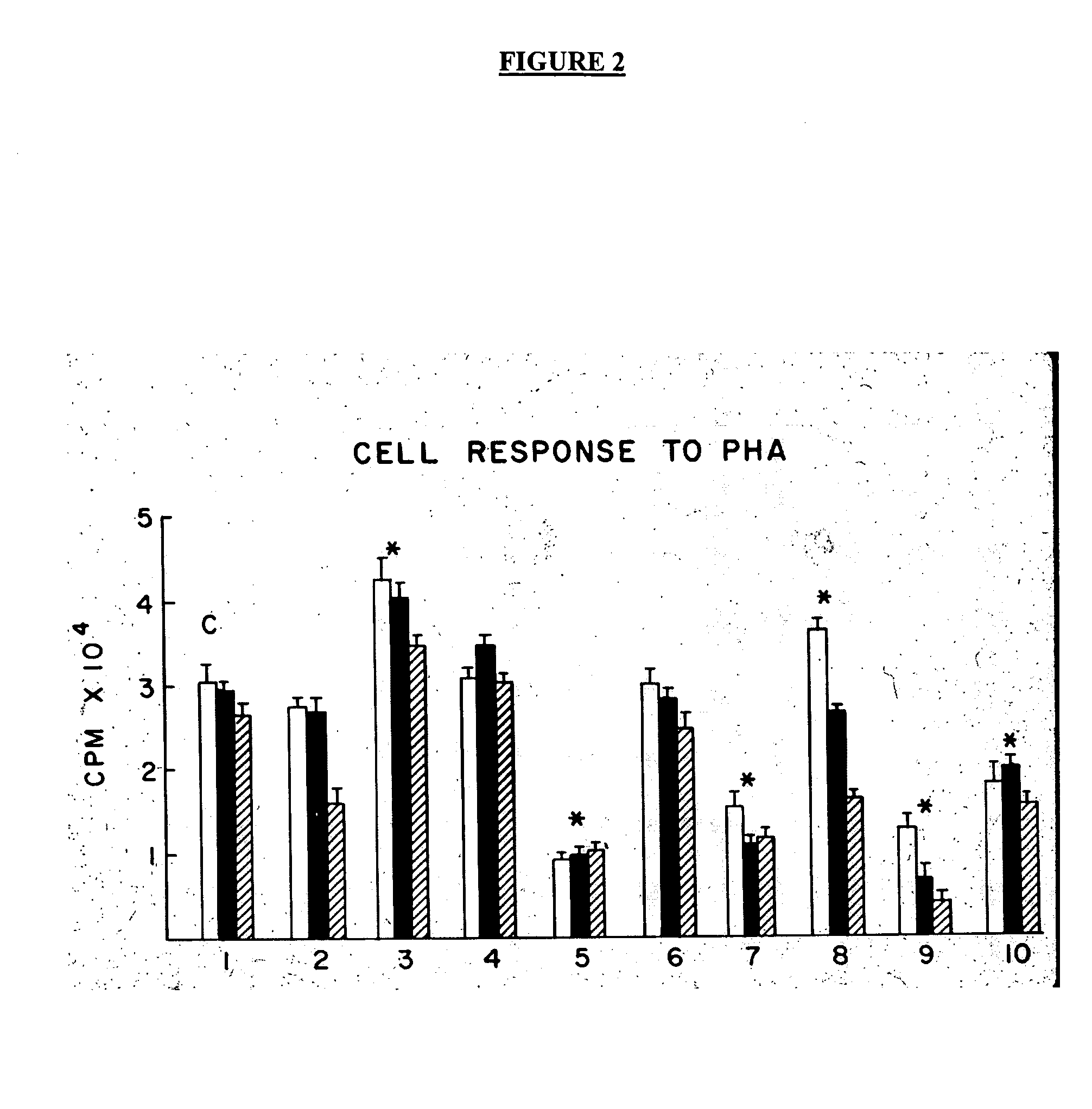
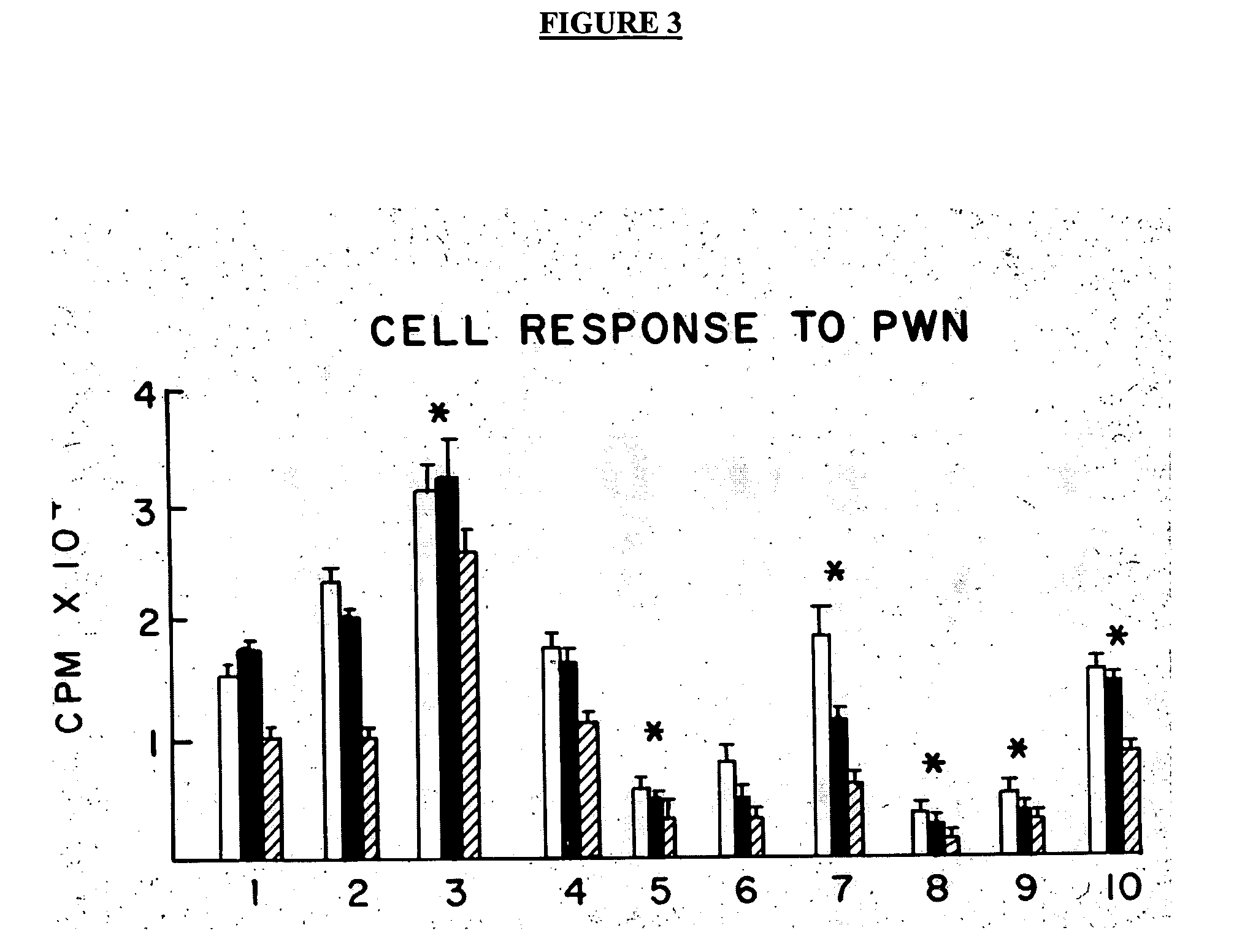
Treatment of inflammatory, non-infectious, autoimmune, vasculitic, degenerative vascular, host-v-graft diseases, Alzheimers disease, and amyloidosis using mammalian, dsDNA vaccination
The present invention relates generally to compositions and methods using mammalian, dsDNA (Double Stranded Deoxyribonucleic Acid) vaccination for the induction and maintenance of regulator suppressor T cells resulting in suppression of non infectious, and post infectious, inflammatory, allergic, auto-immune, vasculitic, certain degenerative vascular, and graft versus host diseases, with or without the use of IL-10, and with or without the use or TGFβ, with or without the use of anti-IL 6 receptor antibody, anti TNF antibody and or Plasmapheresis, IVIG, Corticosteroids, Methotrexate, Bromocriptine, and or vitamin D analogues.
Owner:LAWLESS OLIVER J
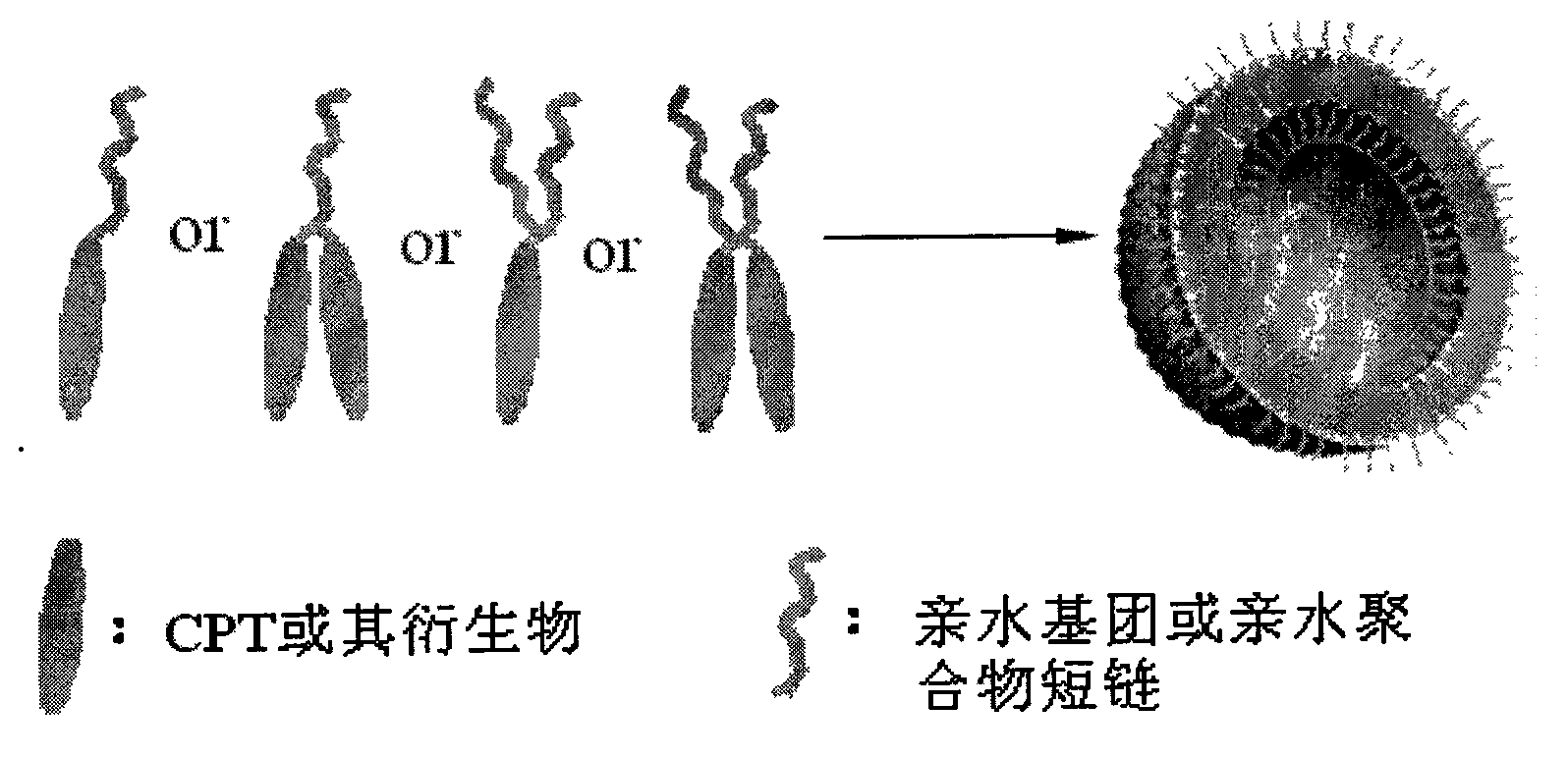
Camptothecin, self-emulsifying medicine precursor of derivative thereof and application thereof
InactiveCN101628919AImprove the delivery effectGood biocompatibilitySugar derivativesGroup 5/15 element organic compoundsAlcoholMatrine
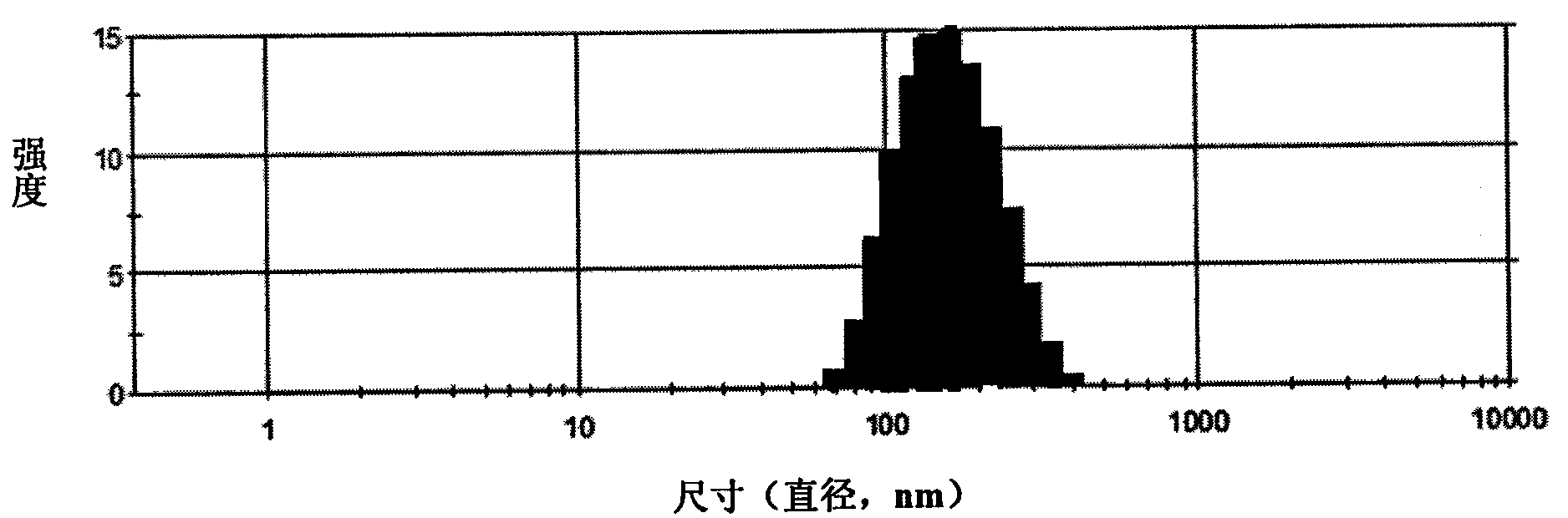
The invention discloses camptothecin and a self-emulsifying medicine precursor of a derivative thereof. The self-emulsifying medicine precursor is prepared by the covalent union of medicine molecules and hydrophilic radicals, wherein the medicine molecules are camptothecin molecules or camptothecin derivative molecules, and the medicine carrying quantity of the precursor is as high as more than 50%. The invention also discloses application of the self-emulsifying medicine precursor. The self-emulsifying medicine precursor can form micelles or vesicles with nano size in water, can be used as a medicine carrier used for loading one or a plurality of other anticancer medicines, such as CPT derivatives, yew alcohol, turmeric essence, methotrexate, irinotecan, danshinolic acid, matrine, doxorubicine and the like, can form a nano medicine carrying a plurality of medicines and can realize the synergic treatment of the medicines.
Owner:ZHEJIANG UNIV
Synthesis of methotrexate-containing heterodimeric molecules
InactiveUS7230101B1Improve membrane permeabilityEffective isolationBiocideOrganic chemistryProtein targetCombinatorial chemistry
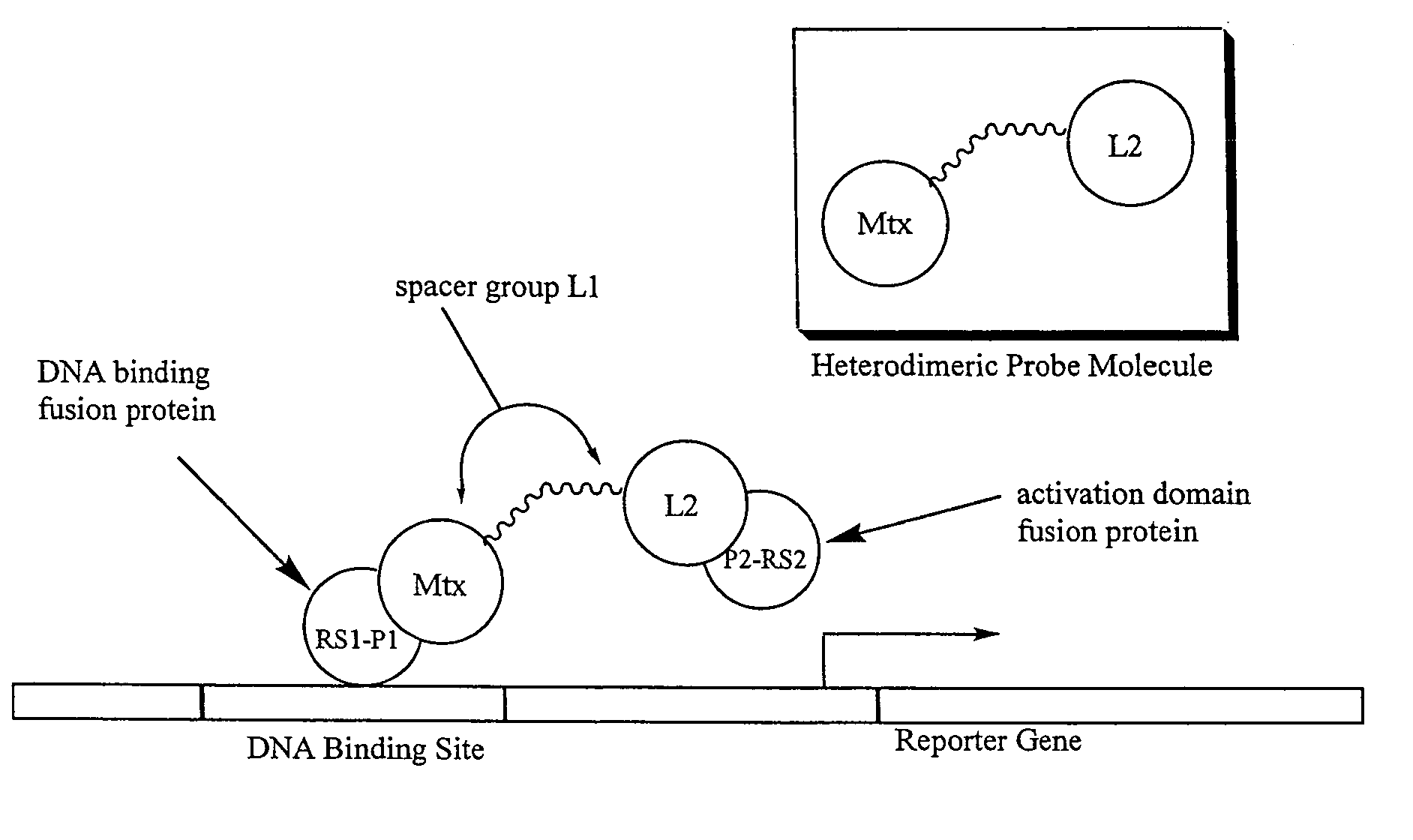
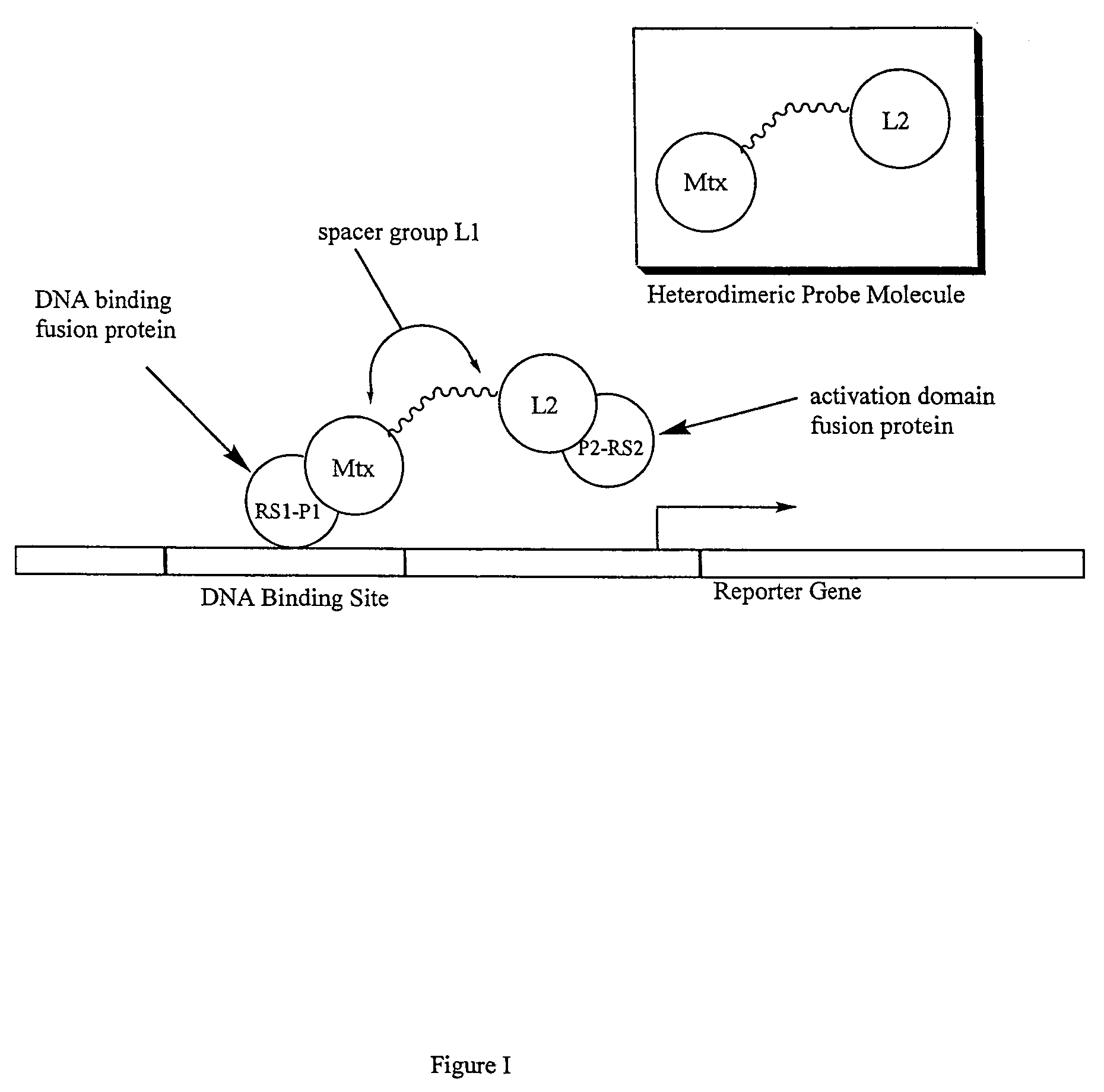
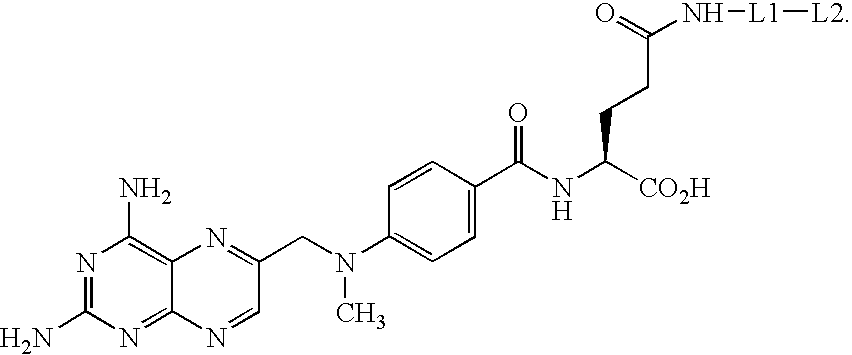
The present invention relates to novel compositions of methotrexate-containing heterodimeric probe molecules, also known as chemical inducers of dimerization (CID), useful in three-hybrid assays. The invention further relates to synthesis of said compositions and their intermediates. Another aspect of the invention is a method for using the heterodimeric probe molecules described herein in drug screens to identify potential protein targets to a given ligand, optimize protein-ligand interactions, or identify potential ligands for a given protein target. In certain embodiments, the invention contemplates the synthesis of the following methotrexate-containing heterodimeric probe:
Owner:AGGENIX
Methods and Devices for Preventing or Delaying Posterior Capsule Opacification
InactiveUS20110082543A1Prevents and minimizes and delays formationMinimize formationPharmaceutical delivery mechanismEye treatmentChemical MoietyBovine serum albumin
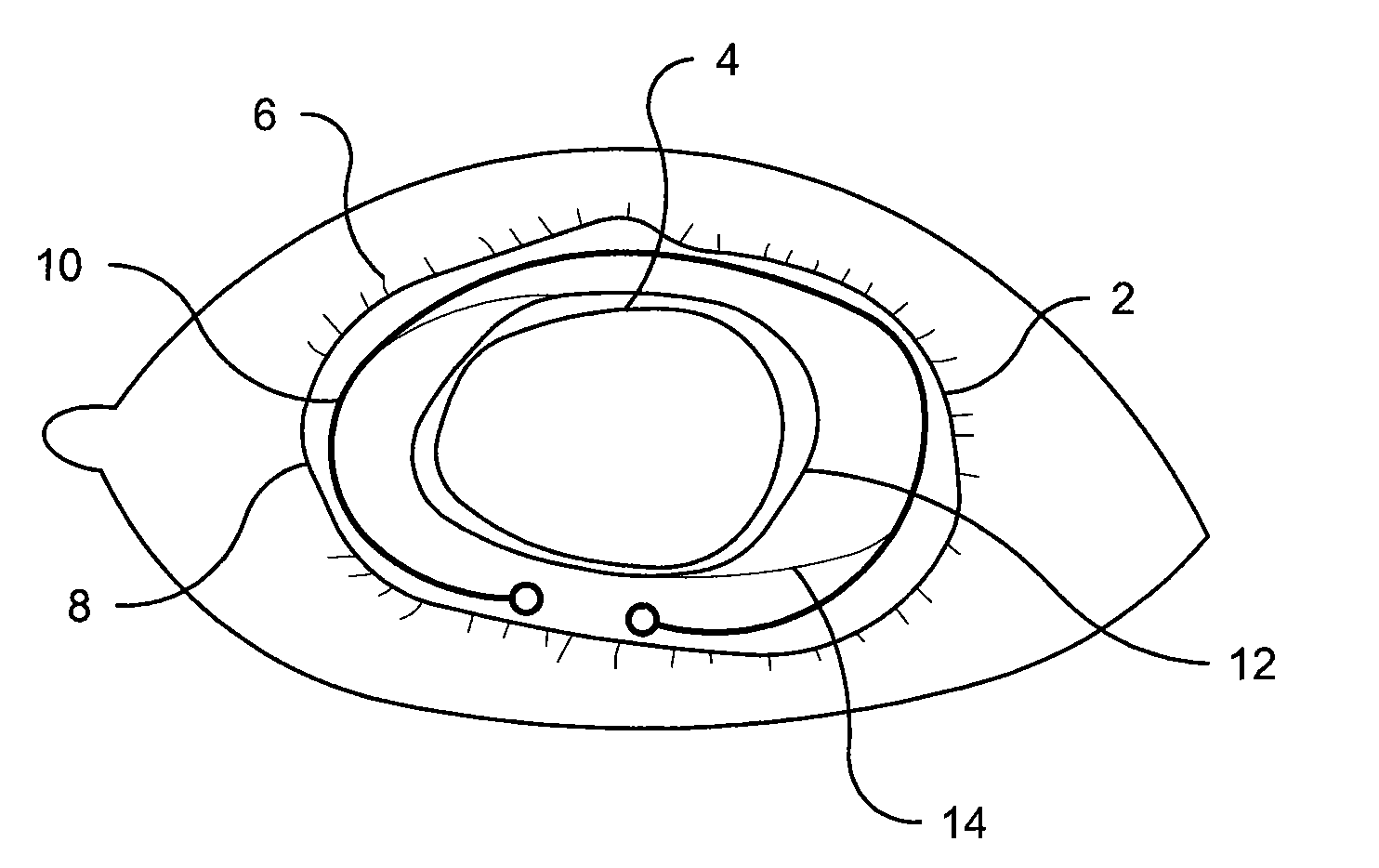
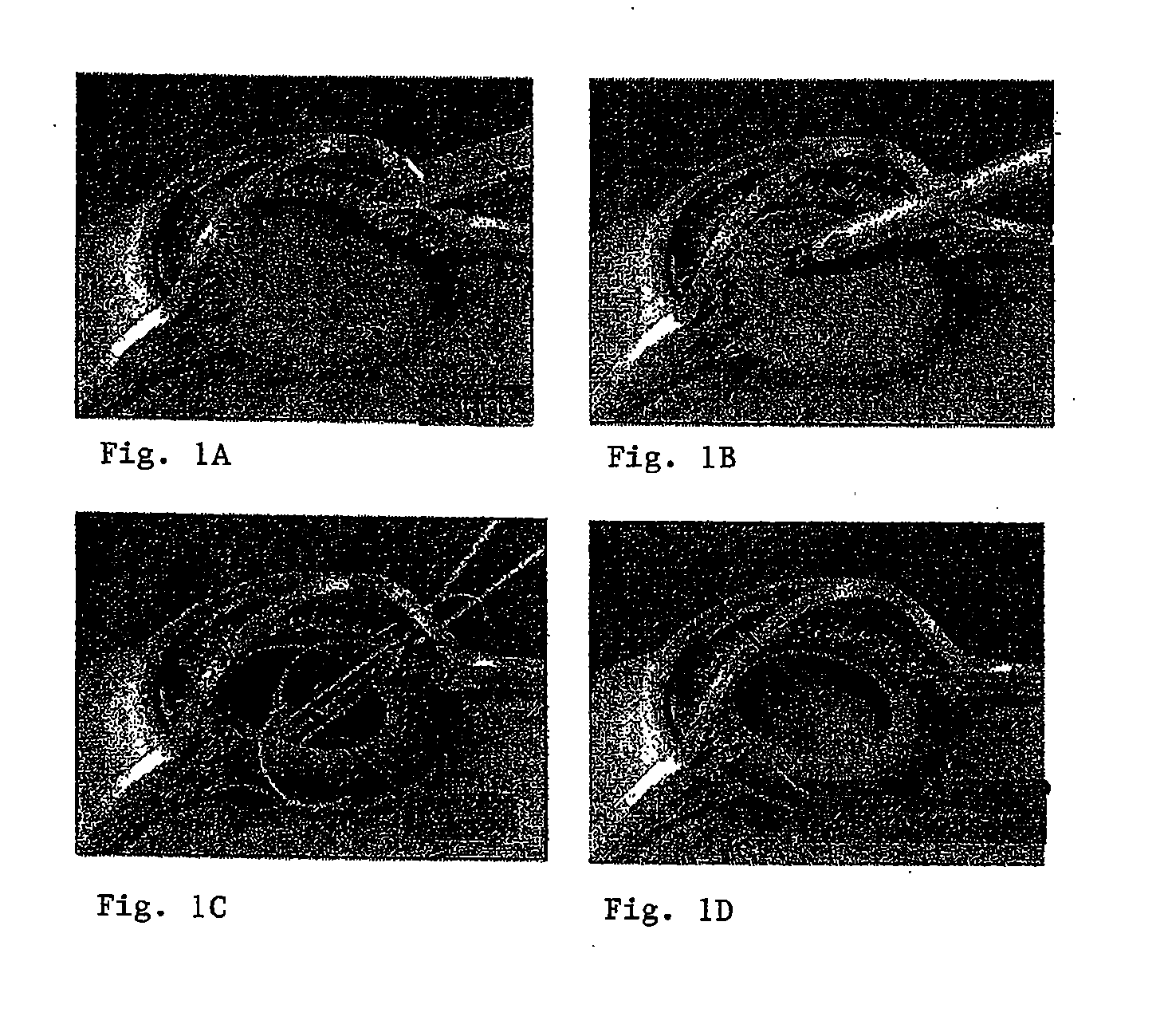
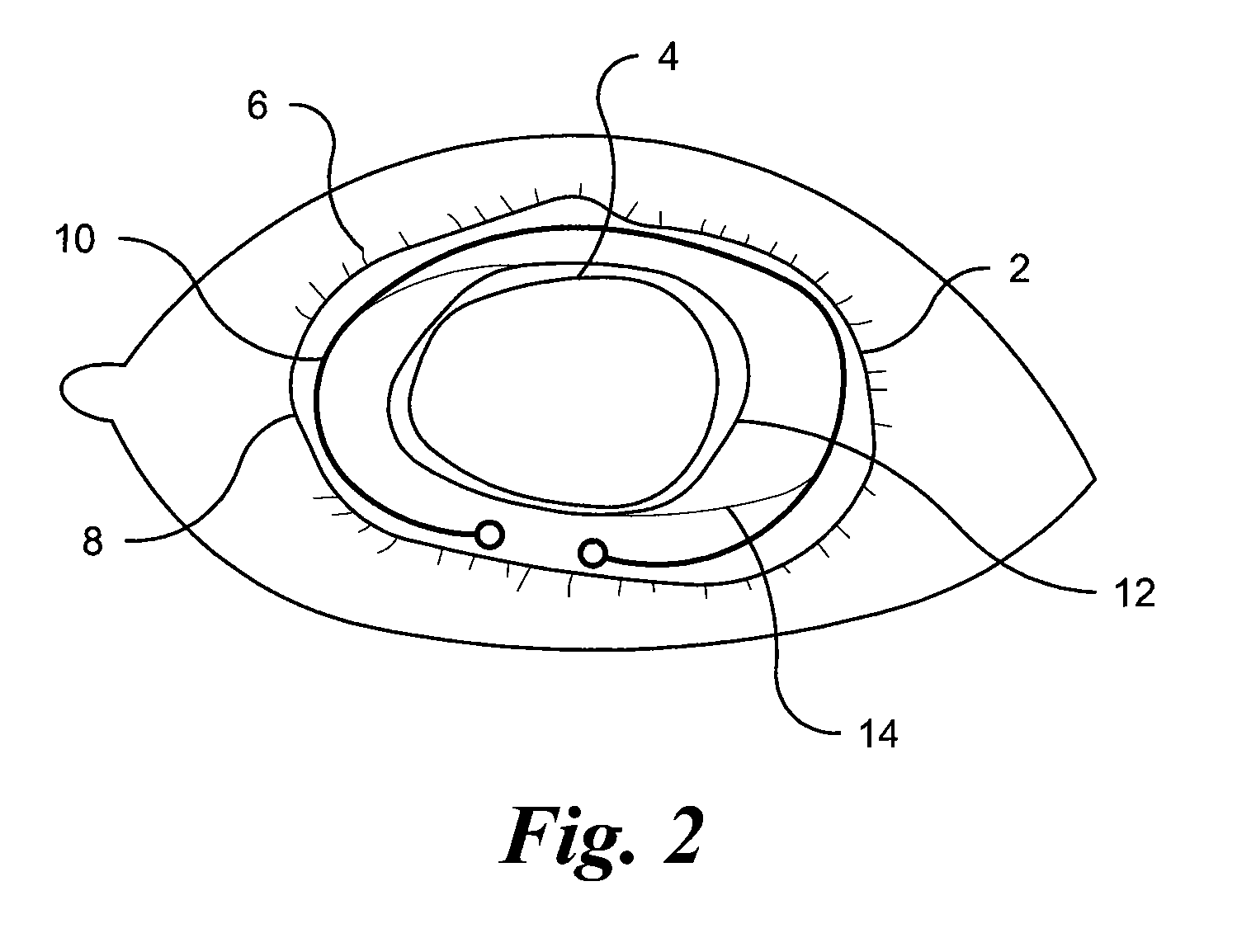
Several methods for preventing, minimizing, or delaying the incidence of posterior capsule opacification are provided. A first method involves chemically activating the surface of an implantable ocular device, such as an intraocular lens or a capsular tension ring, by grafting a chemical moiety onto the surface of the device, covalently attaching a non-cytotoxic inhibitor compound to the chemical moiety to produce an inhibitor implantable ocular device, and implanting this inhibitor implantable ocular device into the capsular bag of an eye of a patient during extracapsular cataract surgery. Appropriate inhibitor compounds include RGD mimetics, RGD peptides, and flavonoids. A second method involves surface modifying the exterior surface of a capsular tension ring by covalently attaching a mitotic inhibitor, preferably a conjugate of methotrexate and a bovine serum albumin, and implanting this inhibitor tension ring into the capsular bag of an eye of a patient during extracapsular cataract surgery. A third method involves surface modifying the exterior surface of a capsular tension ring by coating or grafting the exterior surface with a charged polyethylamine and implanting this inhibitor tension ring into the capsular bag of an eye of a patient during extracapsular cataract surgery. An implantable ocular device according to the invention, such as an intraocular lens or a capsular tension ring, contains a substrate with a chemical moiety grafted thereon and a non-cytotoxic inhibitor compound covalently bonded to the chemical moiety or contains a substrate modified with a mitotic inhibitor or charged polyethylamine. The inhibitor devices inhibits proliferation and migration of lens epithelial cells on the posterior capsule of the eye of the patient, thereby preventing, minimizing, or delaying the onset of posterior capsule opacification.
Owner:CLEO COSMETIC & PHARMA
Methods of Treating Chronic Inflammatory Diseases Using a GM-CSF Antagonist
InactiveUS20080206241A1Improve stabilityBiocideSenses disorderChronic pelvic inflammatory diseaseRheumatoid arthritis
The invention is based on the discovery that GM-CSF antagonists can be used for the treatment of chronic inflammatory disease, such as rheumatoid arthritis. Accordingly, the invention provides methods of administering a GM-CSF antagonist, e.g., a GM-CSF antibody, and an anti-folate compounds, e.g., methotrexate, to a patient that has RA and pharmaceutical compositions comprising such antagonists.
Owner:KALOBIOS PHARMA
Use of A3 Adenosine Receptor Agonist in Osteoarthritis Treatment
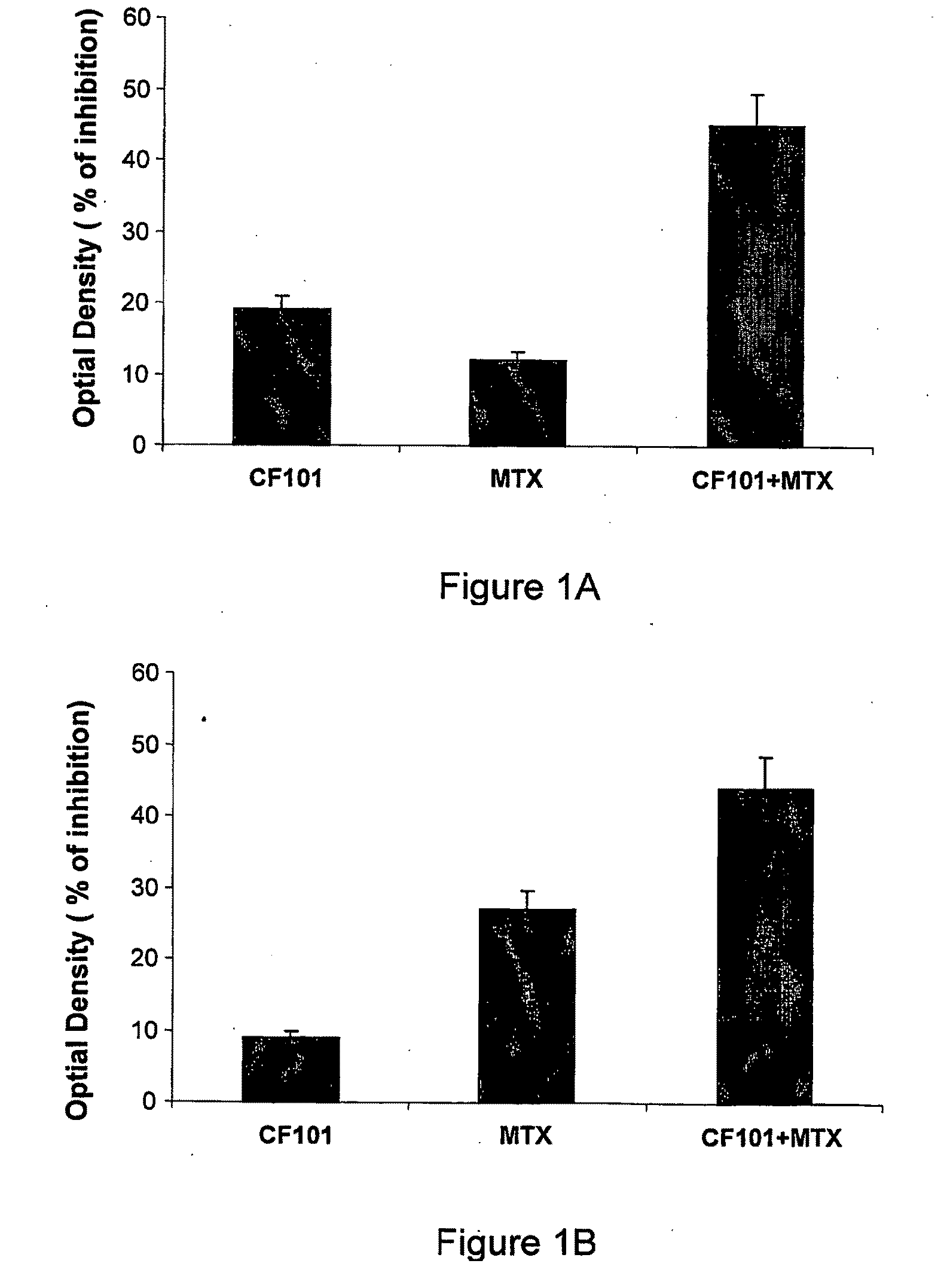
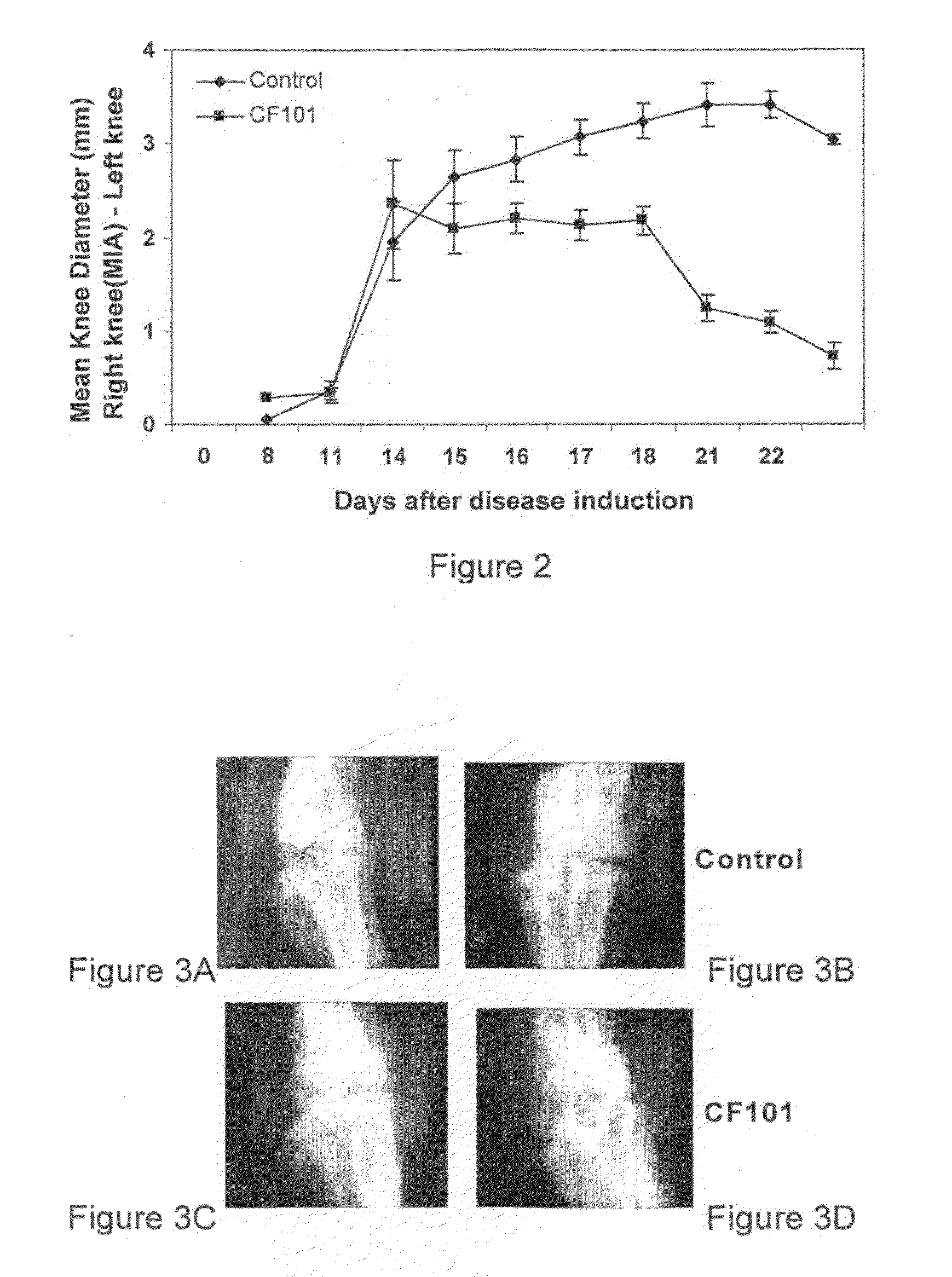
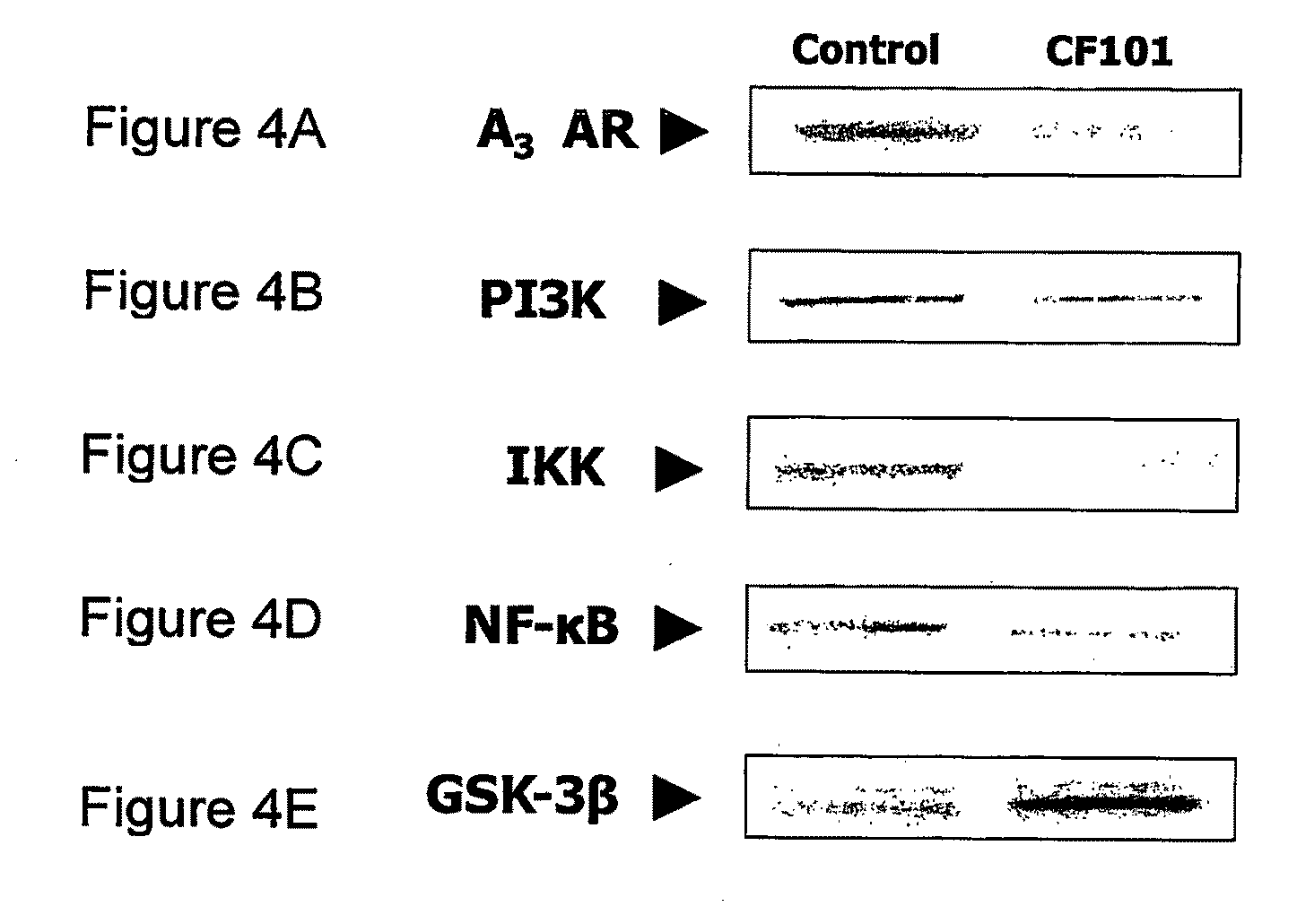
The present invention provides the use of an A3 adenosine receptor agonist (A3AR agonist) for the preparation of a pharmaceutical composition for the treatment of a mammal subject having osteoarthritis (OA), as well as to a method for the treatment of OA in a mammal subject, the method comprises administering to said subject in need of said treatment an amount of an A3AR agonist, the amount being effective to treat or prevent the development of OA. Preferred but not exclusive A3AR agonists in accordance with the invention are IB-MECA and Cl—IB-MECA. The A3AR agonist may be administered in combination with another drug, such as, Methotrexate (MTX). The invention also provides pharmaceutical compositions for treatment of osteoarthritis comprising an amount of an A3AR agonist.
Owner:CAN-FITE BIOPHARMA LTD
GnRH polypeptide-methotrexate conjugate, and preparation method and application thereof
ActiveCN104371009AOrganic active ingredientsLuteinising hormone-releasing hormoneTumor targetTumor targeting
The invention discloses a GnRH polypeptide-methotrexate conjugate shown as a formula in the specification, and the GnRH polypeptide-methotrexate conjugate can be prepared from GnRH polypeptide or analogs thereof and methotrexate or derivatives thereof. The invention also provides a preparation method of the GnRH polypeptide-methotrexate conjugate and application of the GnRH polypeptide-methotrexate conjugate to prepare antitumor medicines. The GnRH polypeptide-methotrexate conjugate is simple in preparation technology, high in yield and easy to purify, has good tumor targeting property on the aspect of resisting tumors, and is capable of centralizing a medicine at the tumor position.
Owner:上海市生物医药技术研究院
Application of DNA (Deoxyribonucleic Acid)-PKcs in preparation of medicine for changing drug resistance of tumor cells on MTX (Methotrexate)
ActiveCN103301447AReduce the degree of amplificationDouble minute reductionOrganic active ingredientsPeptide/protein ingredientsHigh concentrationResearch Object
Owner:HARBIN MEDICAL UNIVERSITY
Methods and compositions for optimizing blood and tissue stability of camptothecin and other albumin-binding therapeutic compounds
InactiveUS20100240602A1Improve stabilityHigh affinitySalicyclic acid active ingredientsBiocideNitrocamptothecinHIV positives
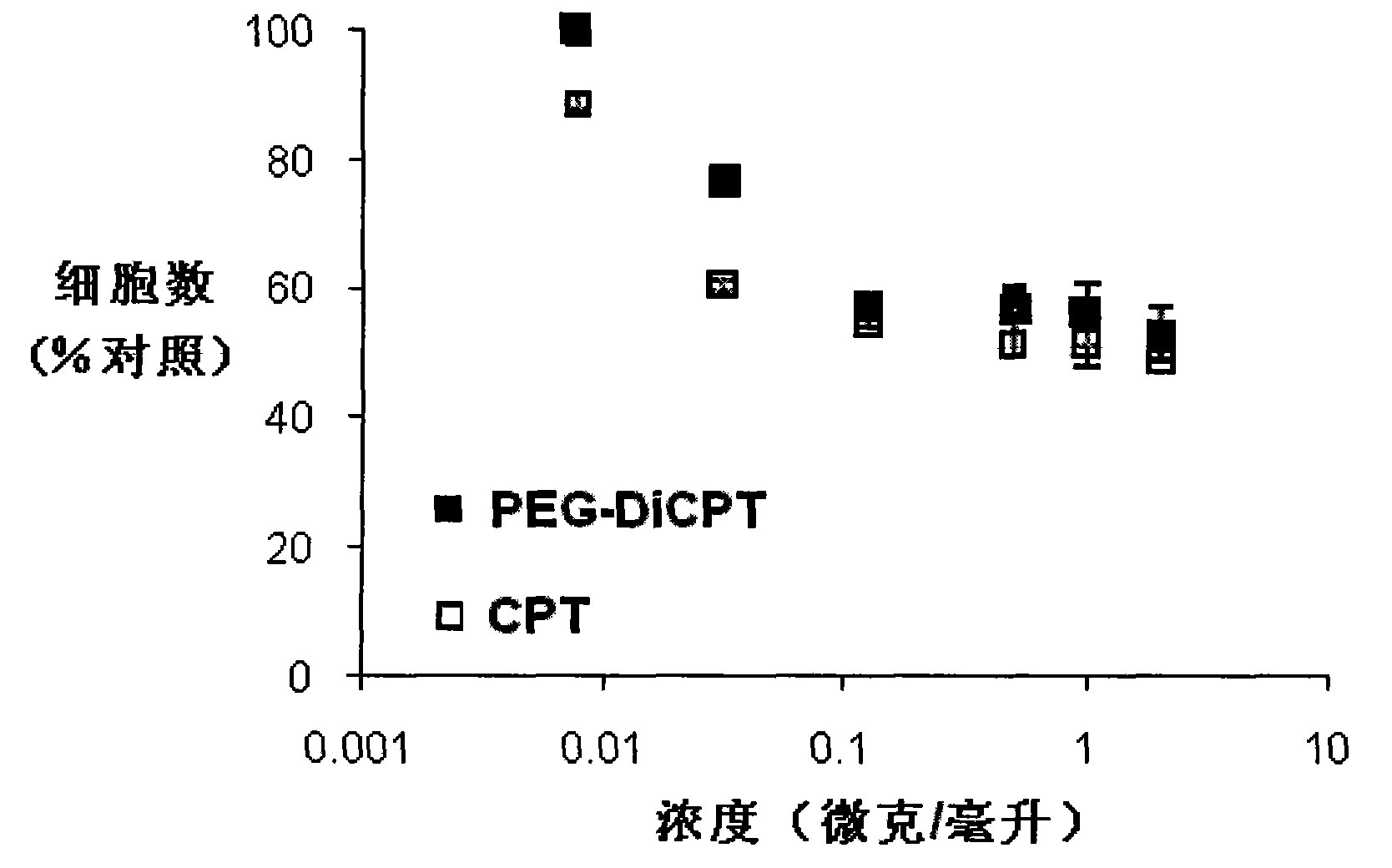
The present invention provides methods and formulations for optimizing the anti-cancer and anti-HIV activities of a camptothecin drug, including camptothecin and its related analogs including 9-aminocamptothecin and 9-nitrocamptothecin. The invention involves methodologies and formulations that limit human serum albumin-mediated reduction of the anti-cancer and anti-HIV effects of the camptothecins, and the methods and formulations provide combination therapies in which binding of the camptothecin agent to human serum albumin can be modulated by the administration of a competing agent such as ibuprofen, clofibrate or clofibric acid that also binds human serum albumin. Reduced camptothecin drug binding to human serum albumin can result in elevated camptothecin free drug levels and thus improve the effectiveness of treatment regimens involving these drugs. Further agents such as methotrexate and AZT can also be used in cancer and HIV-positive patients employing camptothecin drugs.
Owner:BURKE THOMAS G +1
Methotrexate compositions
Owner:NOVARTIS FARMA
Preventive and/or Therapeutic Medicine for Rheumatoid Arthritis
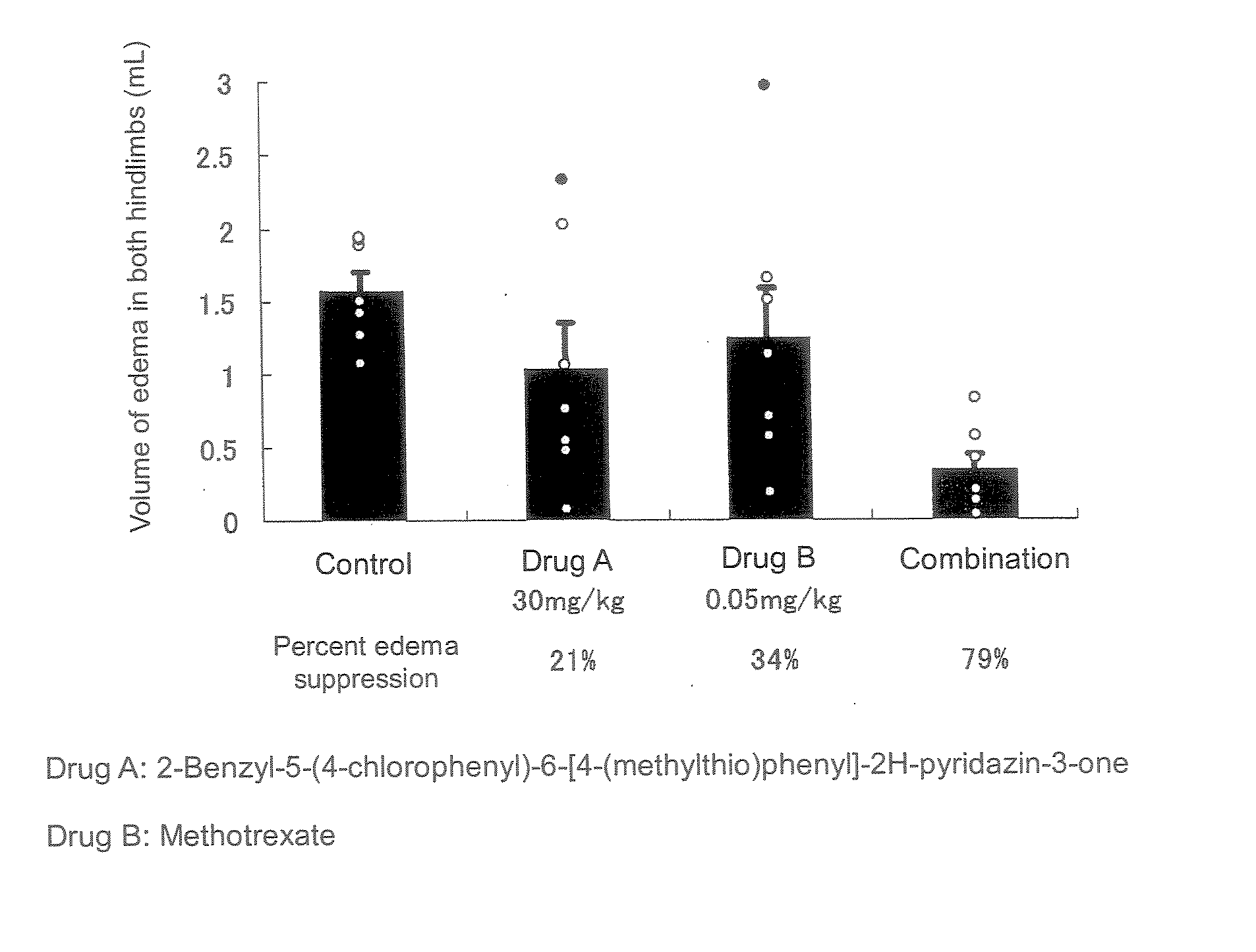
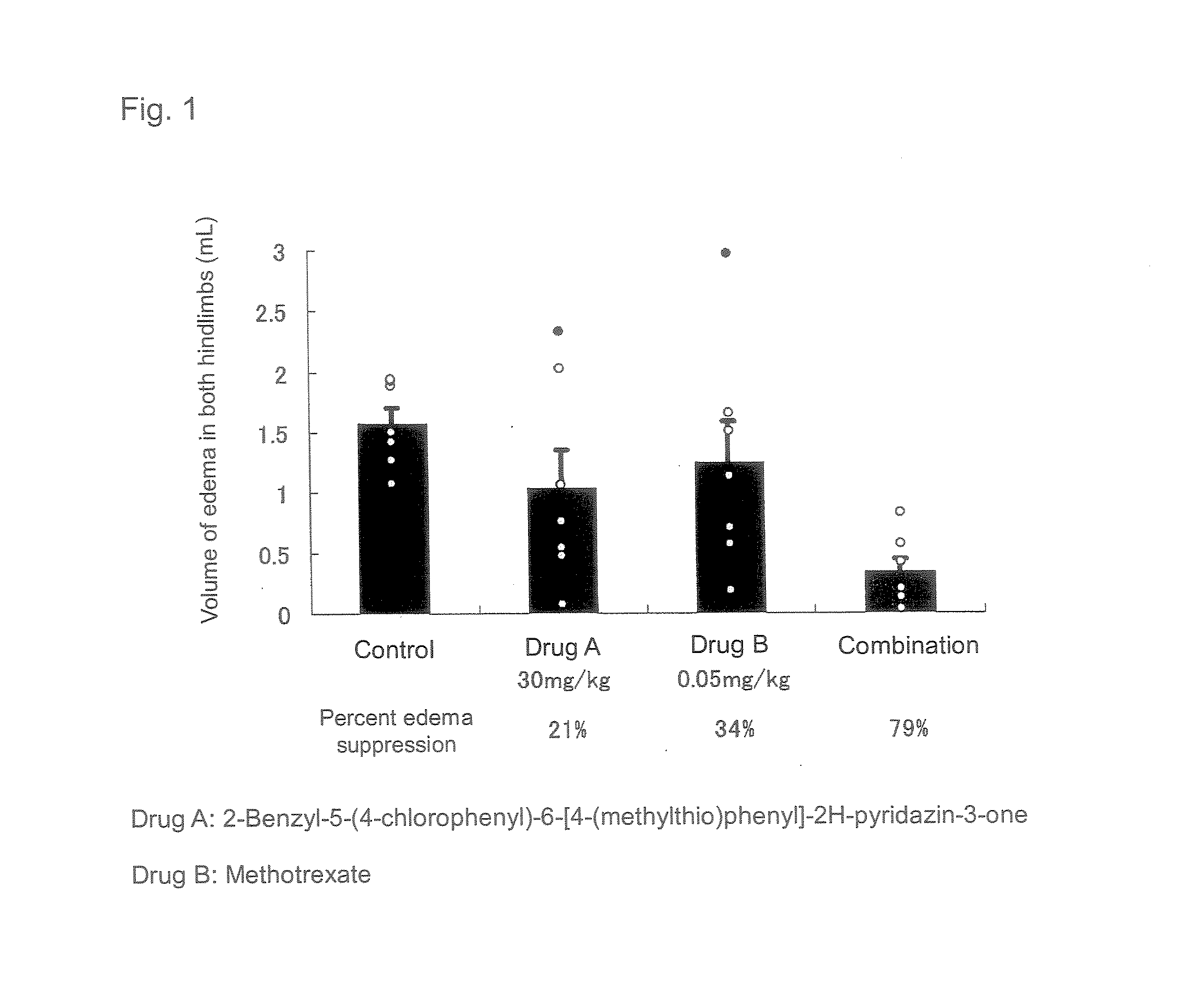
The present invention relates to a preventive and / or therapeutic medicine for rheumatoid arthritis containing 2-benzyl-5-(4-chlorophenyl-6-[4-(methylthio)phenyl]-2H-pyridazin-3-one and methotrexate. The medicine of the present invention can be administered orally and exhibits suppressed side effects and excellent potency for suppression of arthritis.
Owner:KOWA CO LTD
Slow-released injection containing methotrexate and its synergist
The slow released anticancer injection containing methotrexate and its synergist consists of slow released microballoon and solvent. The slow released microballoon includes effective anticancer component and slow releasing supplementary material, and the solvent is common solvent or special solvent containing suspending agent. The effective anticancer component is methotrexate synergist or the composition of methotrexate and its synergist; the methotrexate synergist is selected from phosphorinositide 3-kinase (PI3K) inhibitor, pyrimidine analogue and DNA repair enzyme inhibitor; the slow releasing supplementary material is PLA, PLGA, EVAc, etc or their composition; and the suspending agent is sodium carboxymethyl cellulose, etc. The slow released microballoon may be also prepared into slow released implantation preparation. Implanting or injecting the slow released preparation to local tumor part can lower the systematic toxic reaction of the medicine and raise the medicine concentration of local tumor part selectively to raise the treating effect.
Owner:JINAN SHUAIHUA PHARMA TECH
Methods for direct detection of individual methotrexate metabolites
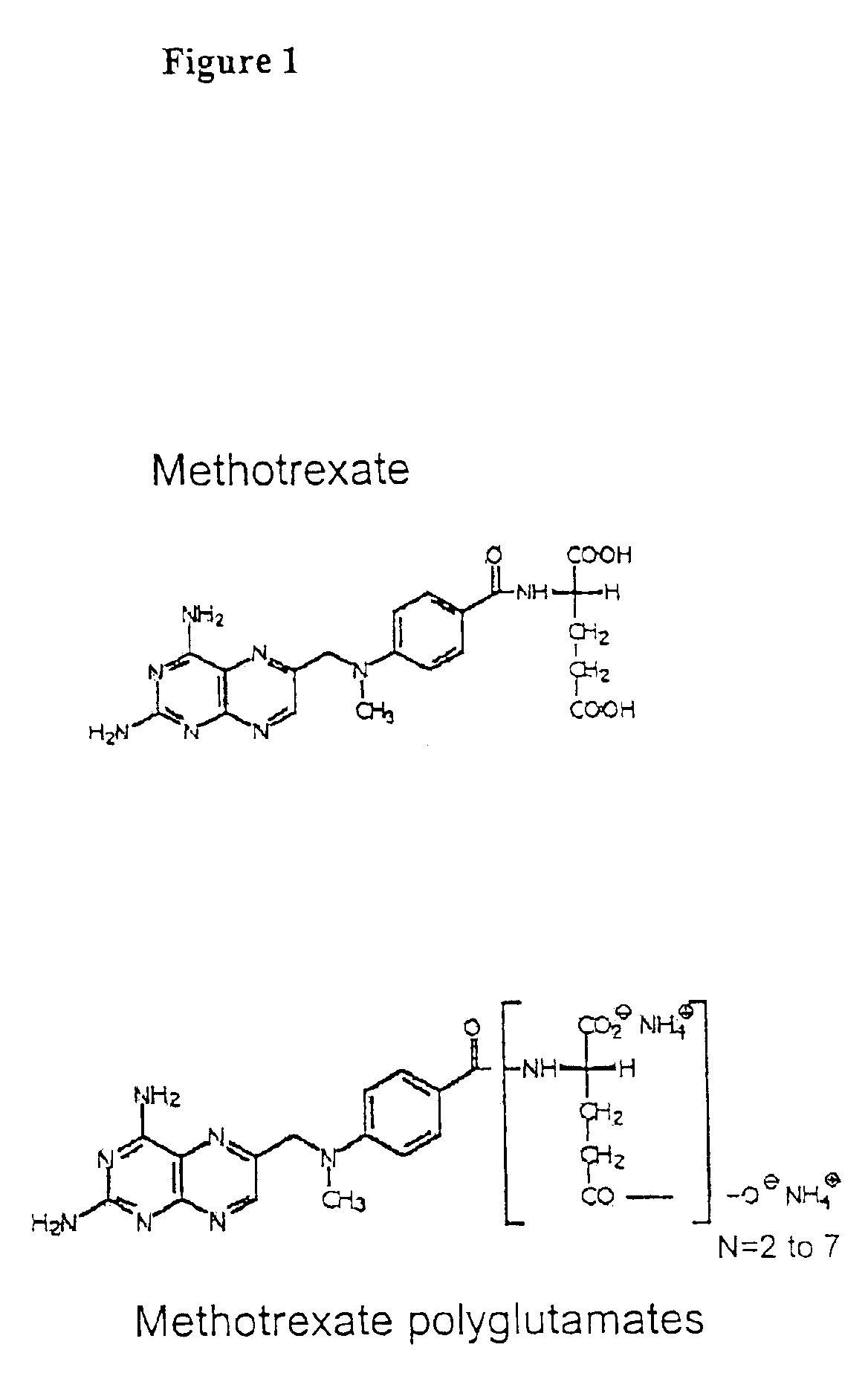
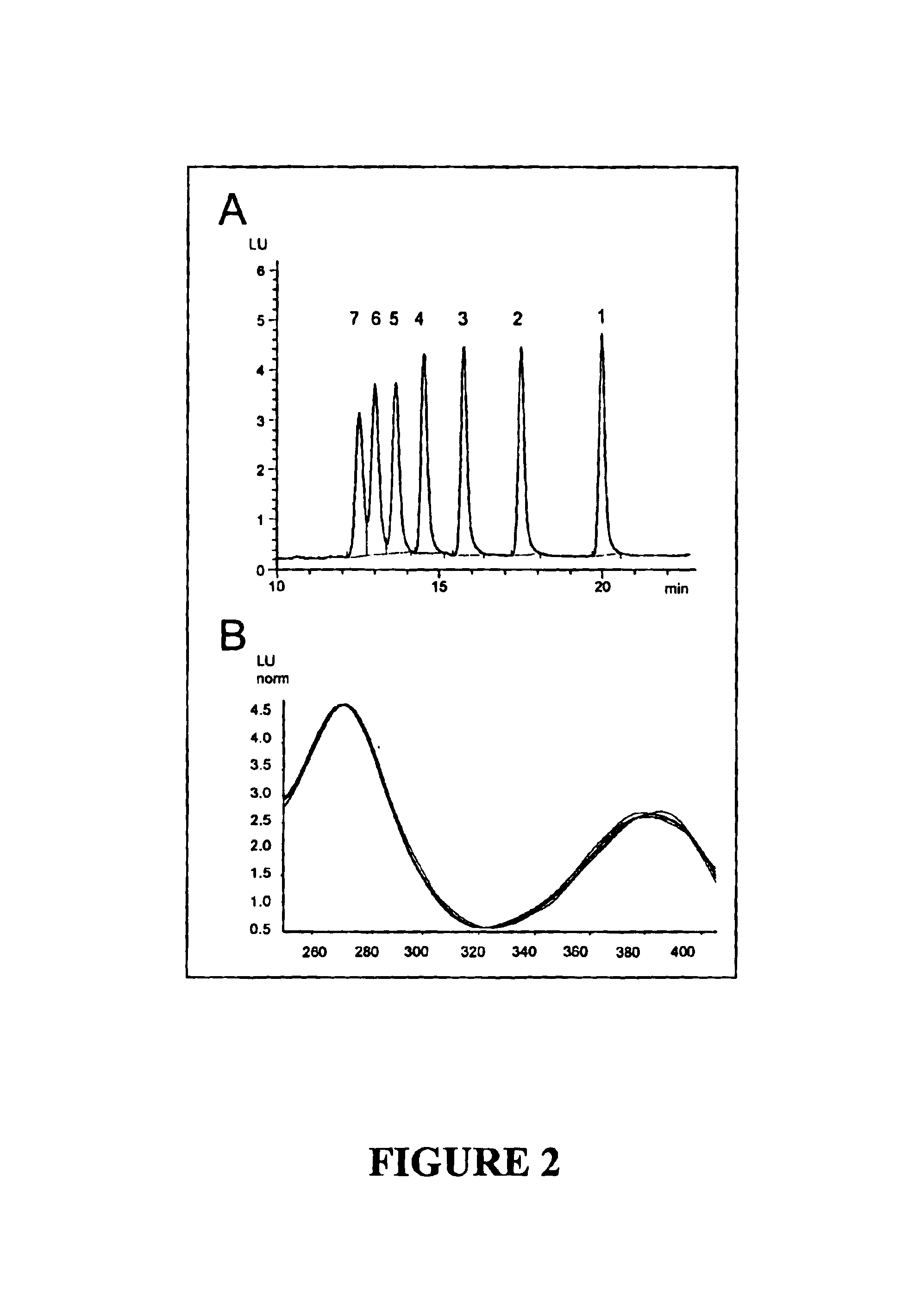
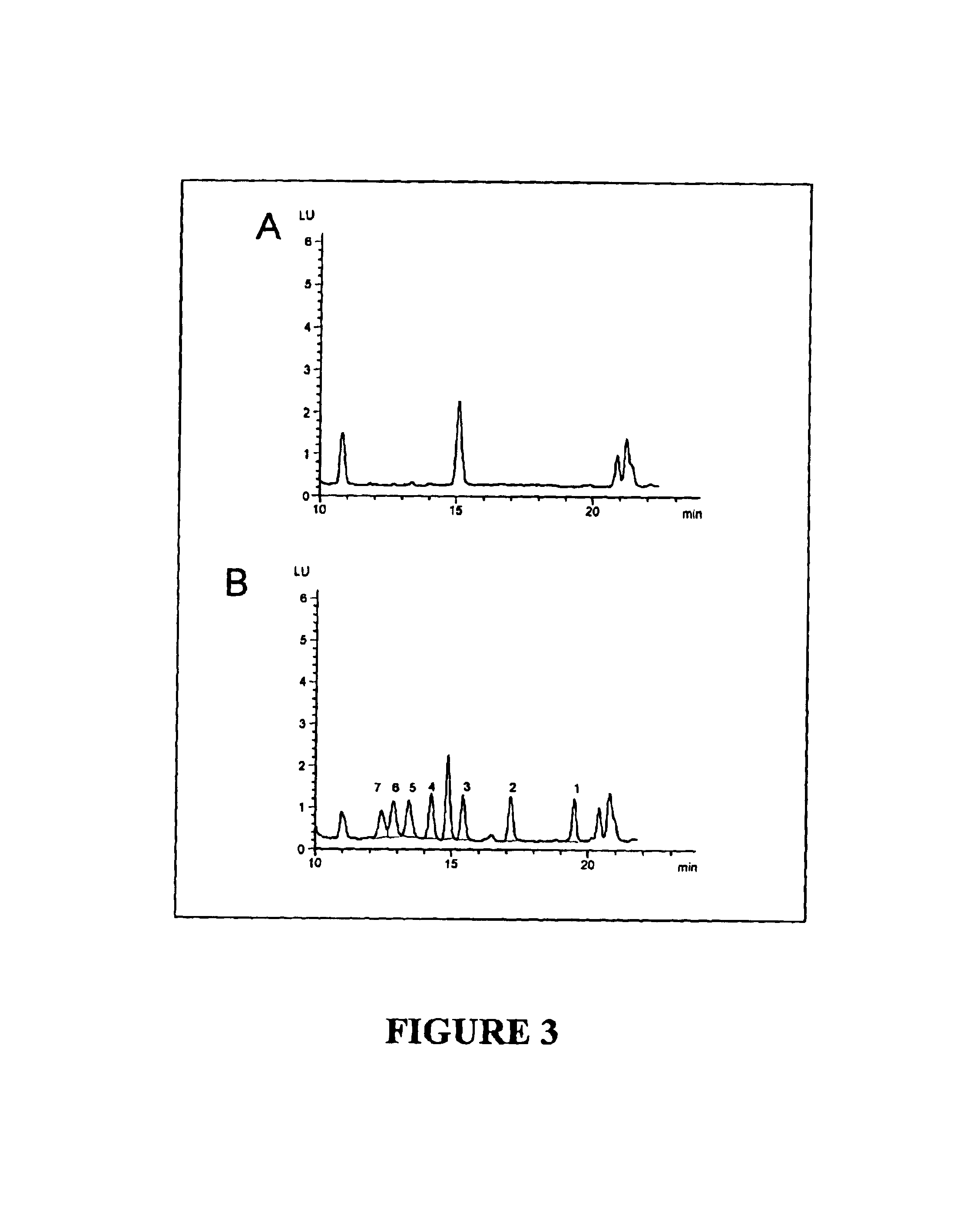
The present invention provides a method for determining a level of a methotrexate polyglutamate (MTXPG) in a cellular extract by resolving at least one MTXPG in a cellular extract obtained from a human undergoing methotrexate therapy; and detecting at least one resolved MTXPG, thereby determining a level of the resolved MTXPG. A method of the invention can be useful, for example, for determining a level of MTXPG3, MTXPG4 or MTXPG5 or for determining a level of each methotrexate polyglutamate species (MTXPG1 to MTXPG7) present in the cellular extract.
Owner:EXAGEN INC
Sustained release formulation of methotrexate as a disease-modifying antirheumatic drug (DMARD) and an Anti-cancer agent
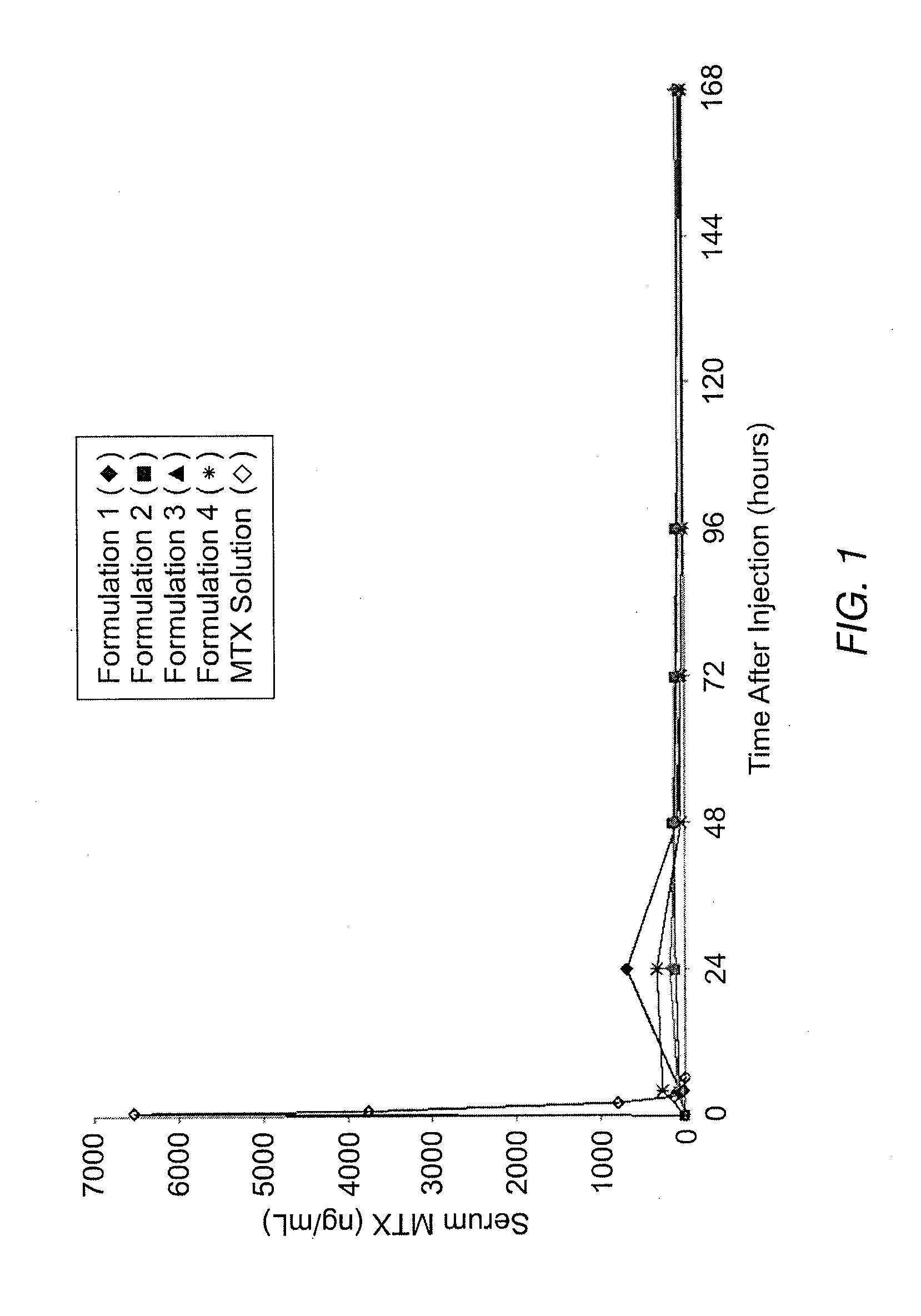
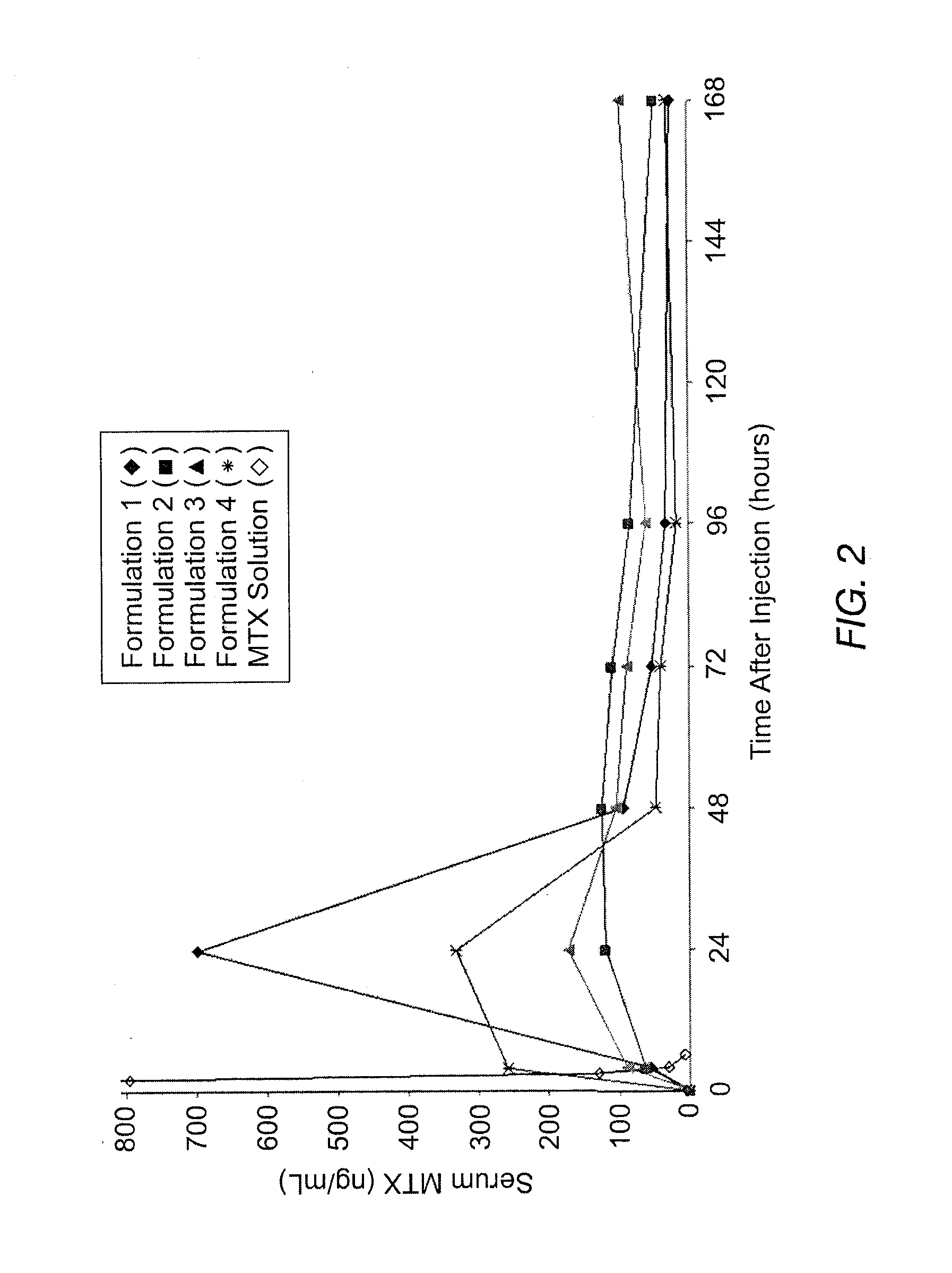
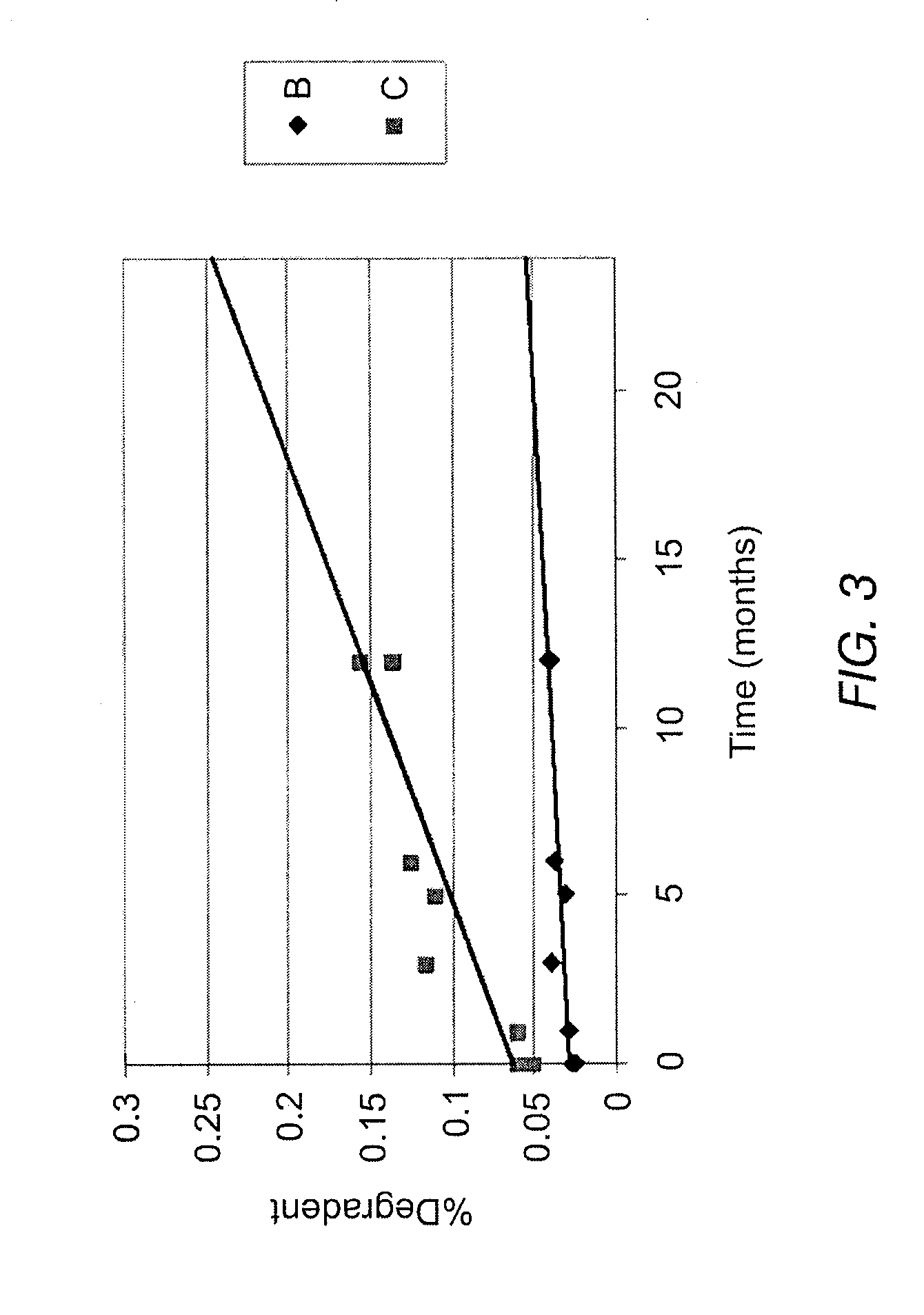
Disclosed is are formulations comprising a multivesicular liposome and MTX, the administration of which results in a Cmax of MTX between 5% and 50% of the Cmax of an immediate release dosage form of MTX, the duration of which lasts from about 1 to about 30 days. Also disclosed are methods of treating autoimmune diseases and cancer by administering these formulations of MTX.
Owner:PACIRA PHARMA INC
Drug eluting stent coating with extended duration of drug release
A stent having a drug eluting formulation has three components: 1) Anti-neointimal hyperplasia or anti-restenosis agent 2) Main polymer 3) Additive polymer The anti-neointimal hyperplasia or anti-restenosis agent includes, but not limited to, Paclitaxel, Taxol, Rapamycin, Tacrolimus, Actinomycin D, Methotrexate, Doxorubicin, cyclophosphamide, and 5-fluorouracil, 6-mercapatopurine, 6-thioguanine, cytoxan, cyclosporine, cytarabinoside, cis-platin, chlorambucil, busulfan, and any other drug that can inhibit cell proliferation, and combinations thereof. The main polymer includes, but not limited to, polystyrene, parylene and polyurethane. The additive polymer includes, but not limited to, polyethylene glycol capped with diisocyanate moiety (NCO-PEG). TABLERatio between three components without solvent%ComponentformulationAgent1-10%Main polymer80-98% Additive1-19%polymer9.0 g of parylene, 0.6 g of tacrolimus, 0.4 g of NCO-PEG and 0.01 g of triethylene amine were dissolved in 90 g of tetrahydrofuran. The resulting mixture was heated at 40° C. for 30 minutes and cooled to room temperature. To the solution was added 0.1 g of pH 8.0 aqueous solution and mixed thoroughly. The resulting solution is applied to bare metal stents for coating.
Owner:HAHN SOONKAP
Use of regularly scheduled high dose intravenous methotrexate therapy, with interim administration of immunomodulatory agents, to treat multiple sclerosis and other diseases of the central nervous system
InactiveUS6903100B2Halted deteriorationImprove scoreBiocidePeptide/protein ingredientsCytotoxicityHigh doses
The present invention is directed to the treatment of multiple sclerosis by periodically administering a high dose of methotrexate at a level sufficiently high to cross the blood brain barrier. The methotrexate administration is accompanied by leucovorin rescue of the periphery. The high dose methotrexate is preferably administered at 1 to 4 month intervals. The periodic high dose methotrexate treatment may be used in conjunction with interim treatments using a therapeutic agent that is effective in treating MS, but does not cross the BBB in cytotoxic amounts. It is contemplated that the method of the present invention may be employed to treat other non-infectious, non-neoplastic inflammatory conditions of the CNS.
Owner:MIDAMERICA NEUROSCI RES FOUND
Application of LincRNA (large intergenic non-coding RNA) in preparation of medicine for controlling tumor treatment drug resistance of methotrexate
ActiveCN103623429ARegulation of drug resistanceImprove toleranceGenetic material ingredientsAntineoplastic agentsTreatment effectNon-coding RNA
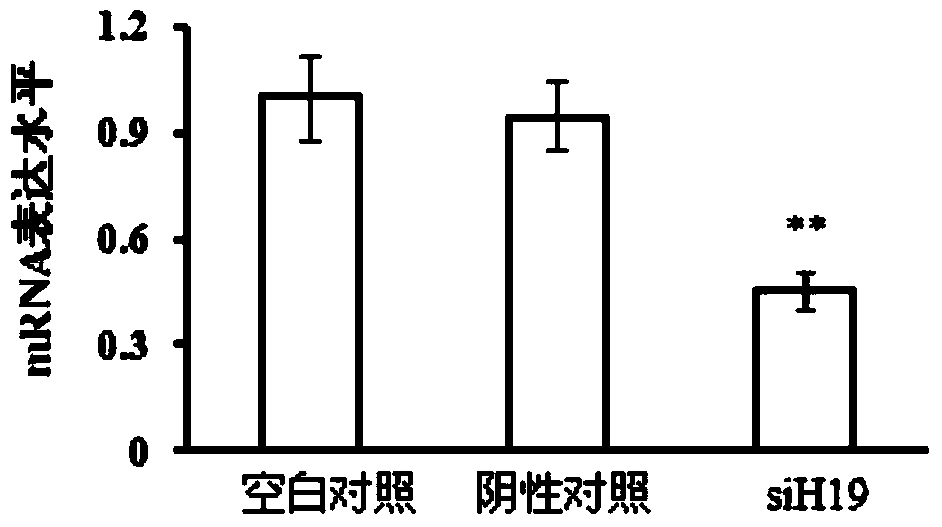
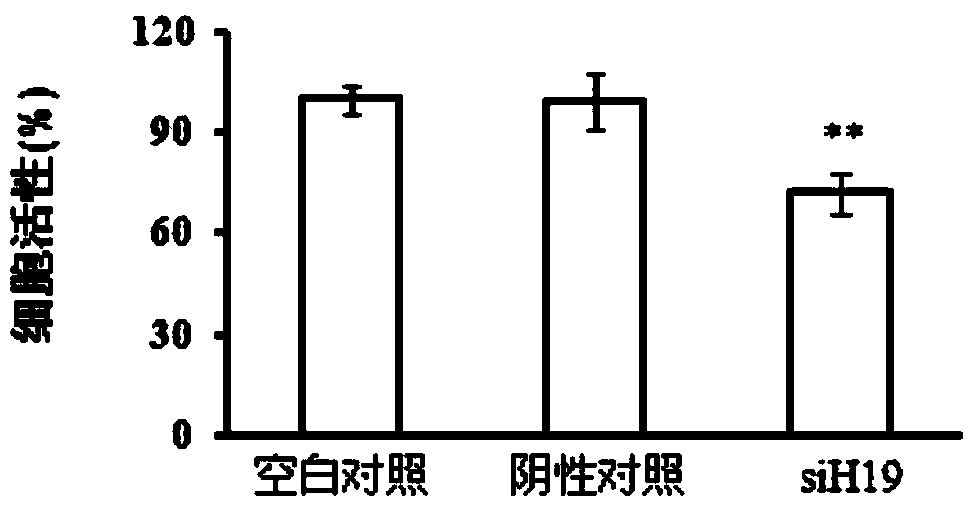
The invention discloses application of LincRNA (large intergenic non-coding RNA) in preparation of a medicine for controlling tumor treatment drug resistance of methotrexate. A coding sequence of LincRNA is shown in SEQ ID No:1. A non-coding RNA H19 (with a sequence shown in SEQ ID No:1) serves as a miRNA sponge or miRNA competitor and can be combined with miRNAs and the miRNAs can be combined with 3'-UTR of ABCC2 / ABCG2 genes, so that the genetic expression of ABCC2 / ABCG2 is inhibited, the tolerance of methotrexate in tumor cells is further improved, the non-coding RNA H19 possibly serves as a diagnostic marker or a molecular target of the methotrexate in tumor treatment, and the tumor treatment effect is improved.
Owner:GUANGZHOU INST OF ADVANCED TECH CHINESE ACAD OF SCI
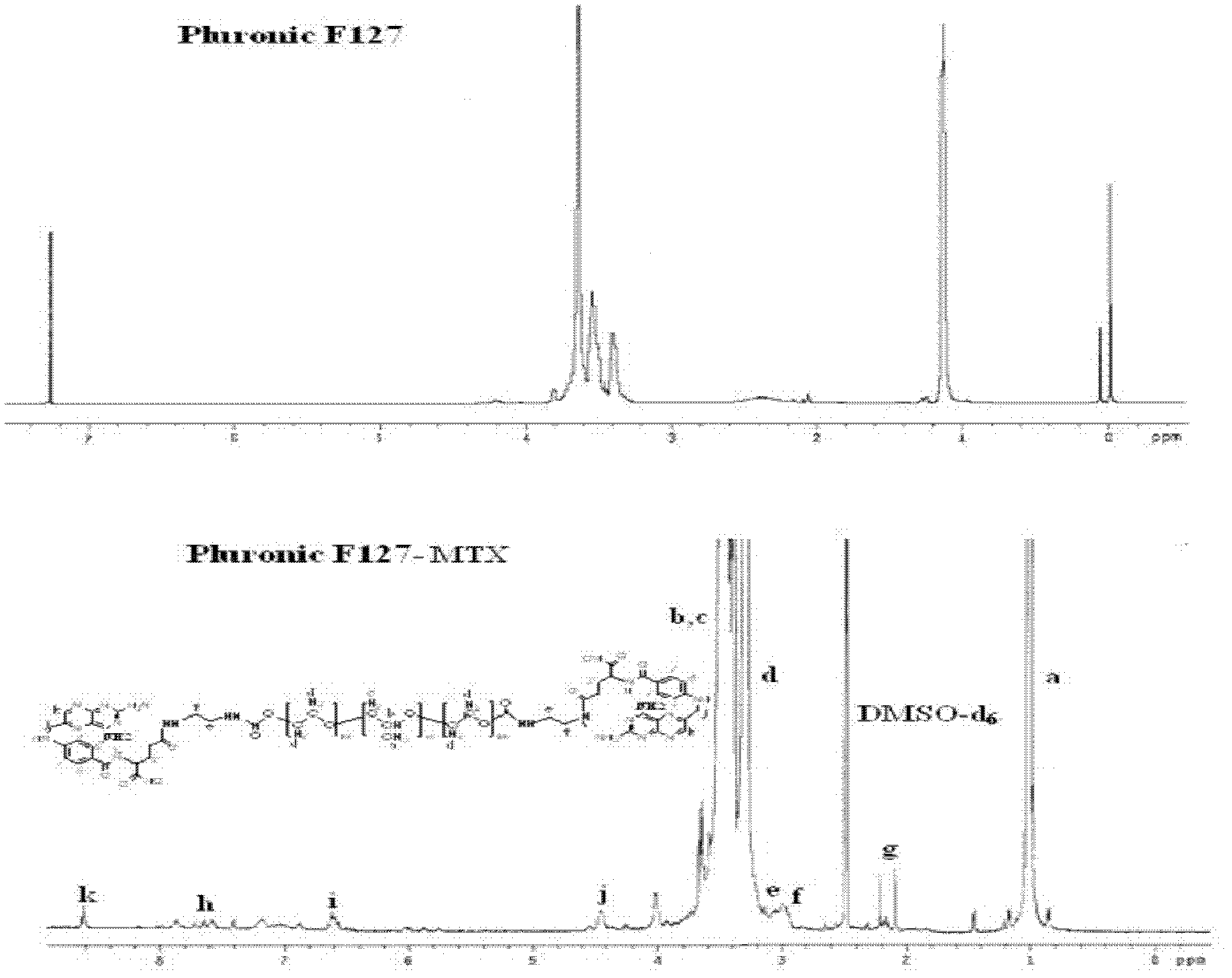
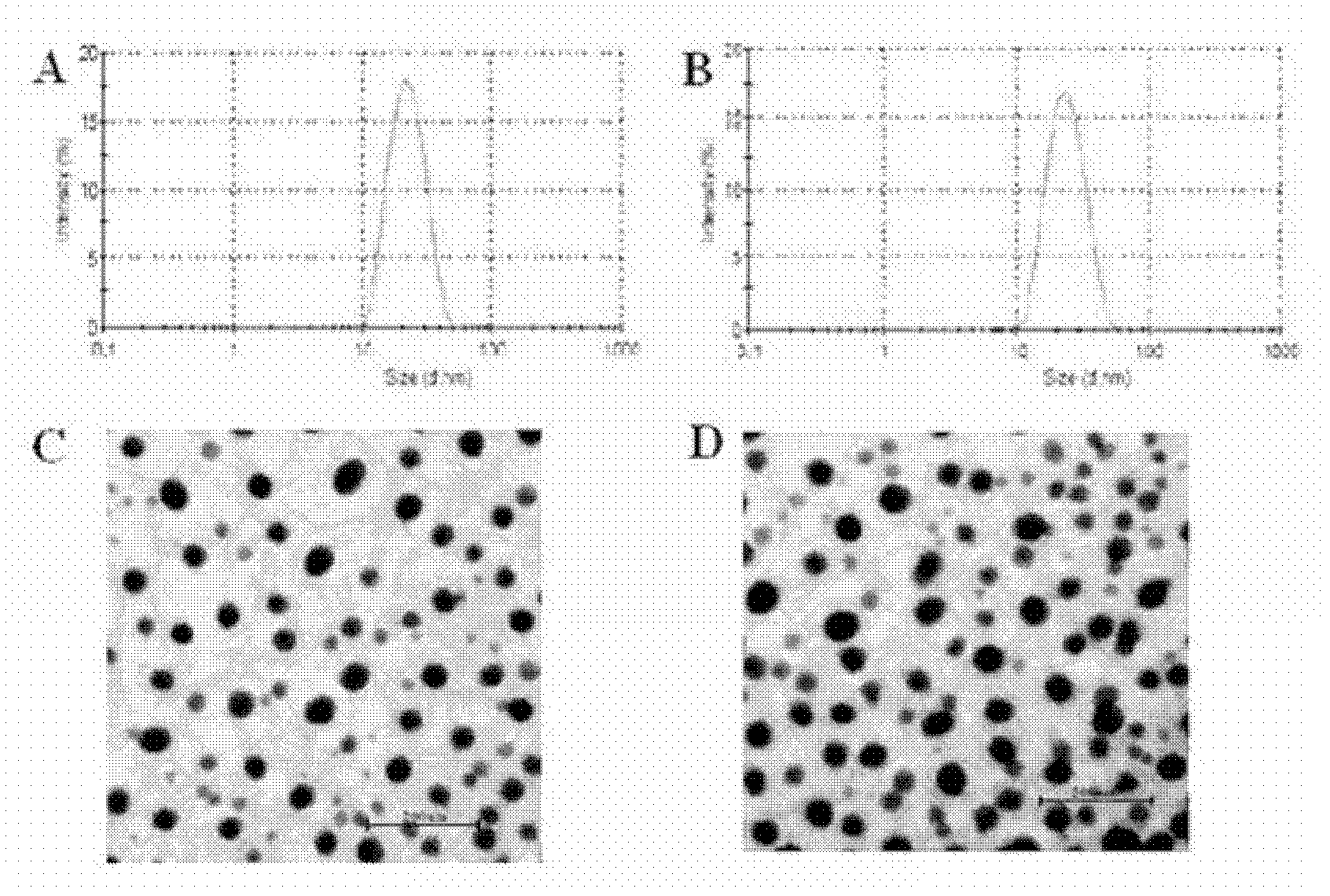
Methotrexate (MTX)-Pluronic copolymer mixed micelle carrying indissoluble medicament and preparation method thereof
InactiveCN103006535AHigh drug loadingReverse drug resistanceOrganic active ingredientsPharmaceutical delivery mechanismChemical ligationMixed micelle
The invention belongs to the technical field of biological medicine and relates to a novel methotrexate (MTX)-Pluronic copolymer mixed micelle carrying an indissoluble medicament and a preparation method thereof. The method comprises the following steps of: performing chemical connection and copolymerization on Pluronic and MTX to obtain an MTX-Pluronic copolymer; and mixing the MTX-Pluronic copolymer with Pluronic-kind matters to prepare an MTX-Pluronic copolymer mixed micelle system, wherein a hydrophobic core of the mixed micelle carries the indissoluble medicament. Tests prove that the mixed micelle can improve the medicament-loading capacity of the indissoluble medicament, effectively reverses the medicament tolerance of tumors and has the medicament-delivery characteristic targeting folate receptors.
Owner:FUDAN UNIV
Combination cancer therapy with an hsp90 inhibitor and an antimetabolite
InactiveUS20140296176A1Increasing side effect profileSurprising biological activityBiocideCarbohydrate active ingredientsCytarabineHsp Inhibitor
The invention provides a method of treating a subject with cancer, particularly leukemia, lymphoma, solid cancer such as colorectal cancer, gastric cancer, bladder cancer, non-small cell lung cancer, and breast cancer, comprising administering to the subject a compound of formulae (I) 40 or (Ia) in combination with an antimetabolite such as methotrexate, pemetrexed, cytarabine or nelarabine, or 5-fluorouracil, or capecitabine or their derivatives.
Owner:SYNTA PHARMA CORP
Composition for treating disease
InactiveUS20110229465A1Reduce in quantityLevel therapeutic effectBiocideAntipyreticDiseaseRegulatory T cell
The present invention provides pharmaceutical compositions and kits comprising an agent capable of activating CD4+CD25+ regulatory T cells and methotrexate, and methods of treatment and medical uses utilising the same.
Owner:BIOTEST SERUM INST GMBH
Methotrexate adjuvants to reduce toxicity and methods for using the same
Methods are provided for using methotrexate (MTX) in which reduced host toxicity is observed. Aspects of the methods include administering to a subject an effective amount of MTX in conjunction with a MTX toxicity-reducing adjuvant, such as a 2,2′-anhydropyrimidine, a derivative thereof or a uridine phosphorylase inhibitor. Also provided are compositions that find use in practicing embodiments of the invention. The methods and compositions find use in a variety of applications, including the treatment of a variety of different disease conditions.
Owner:TOSK INC +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com