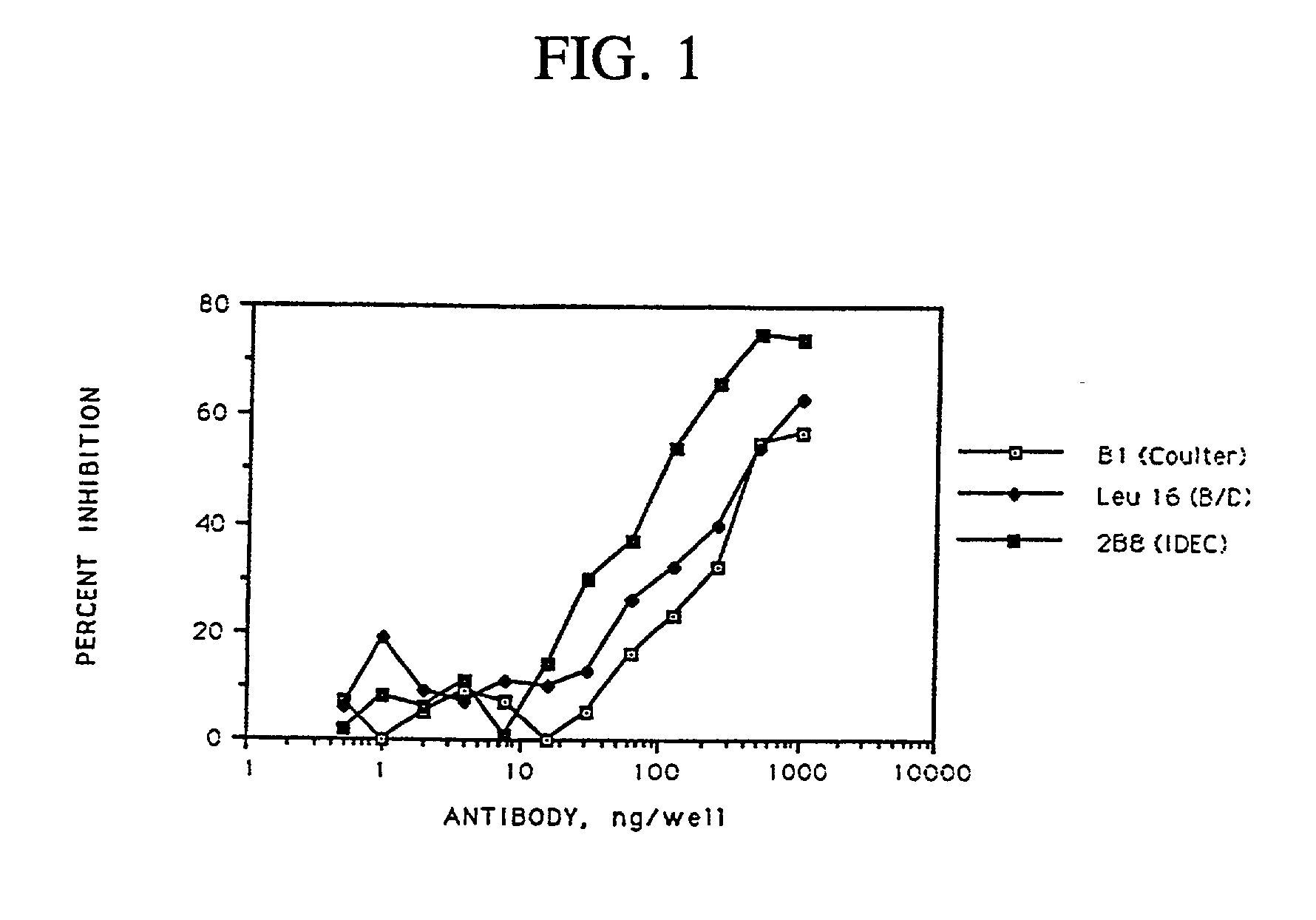
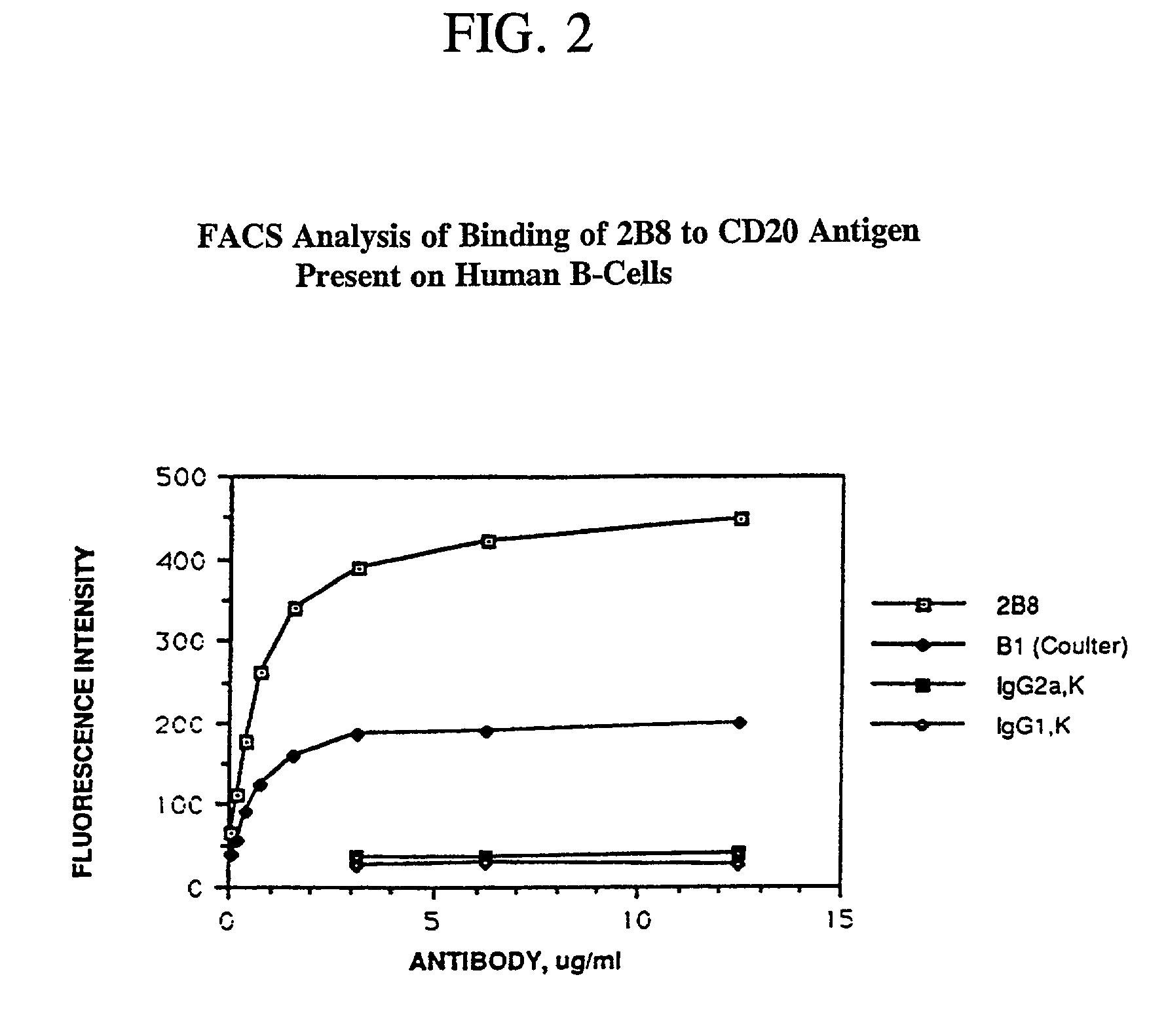
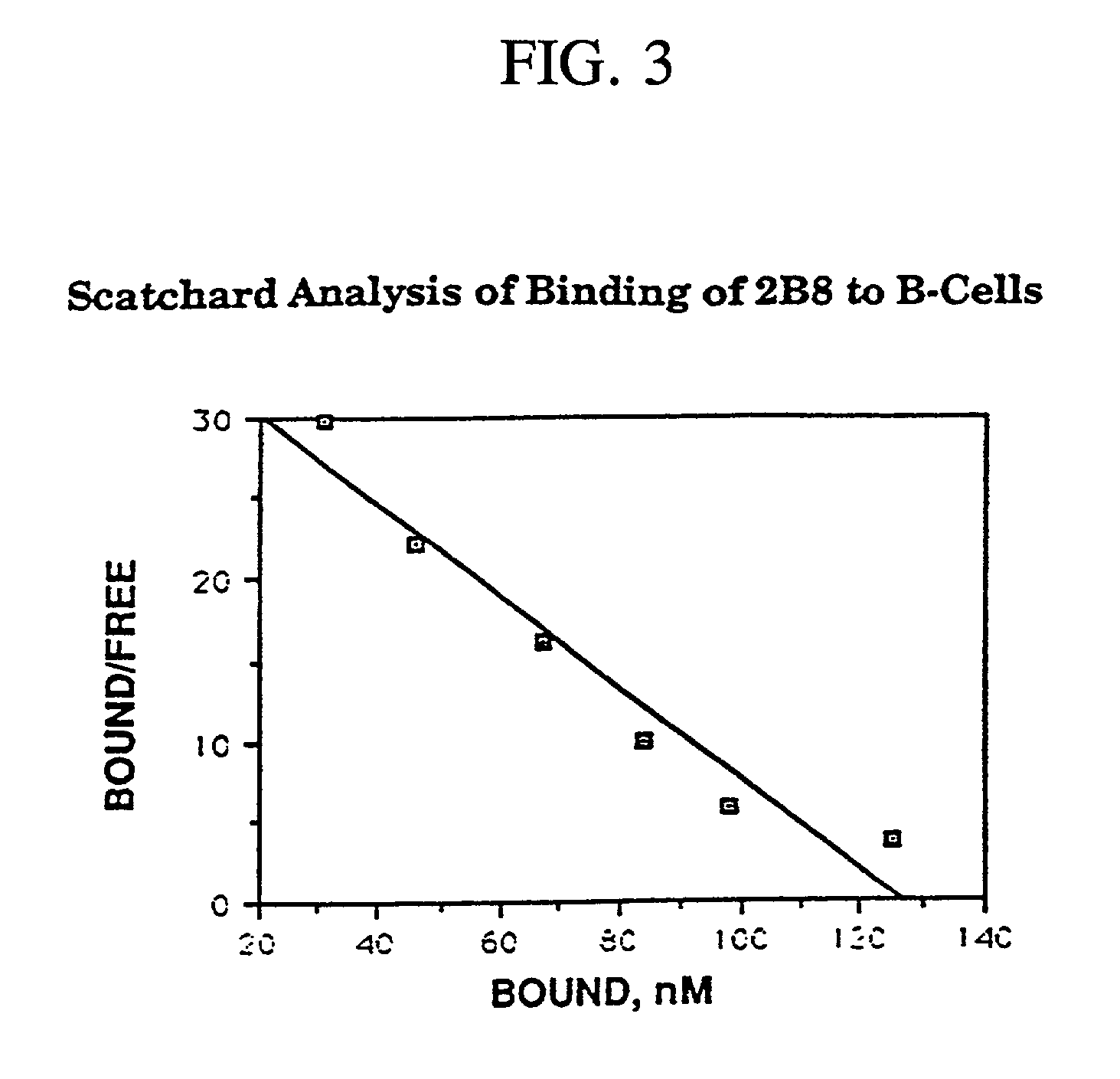
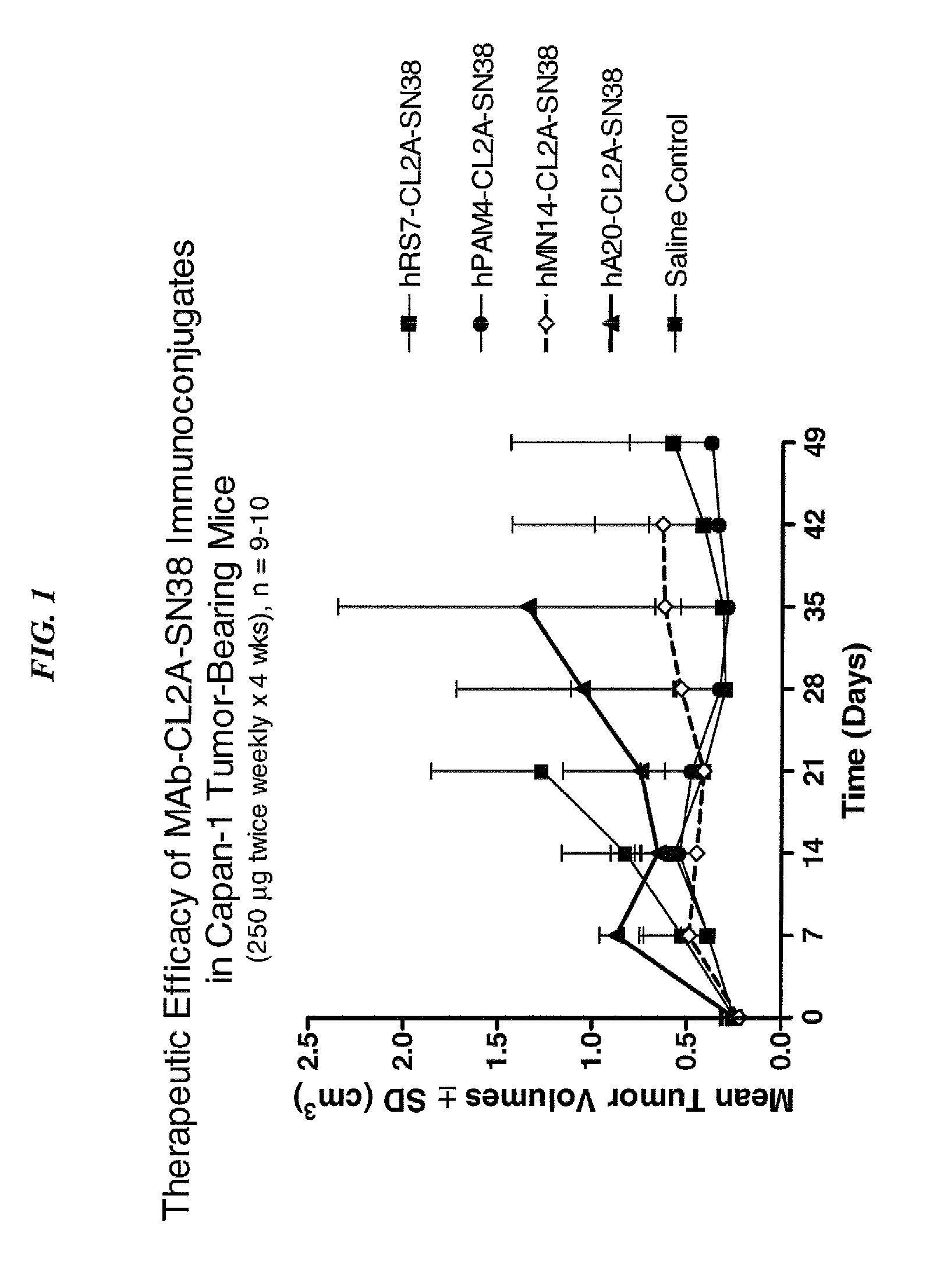
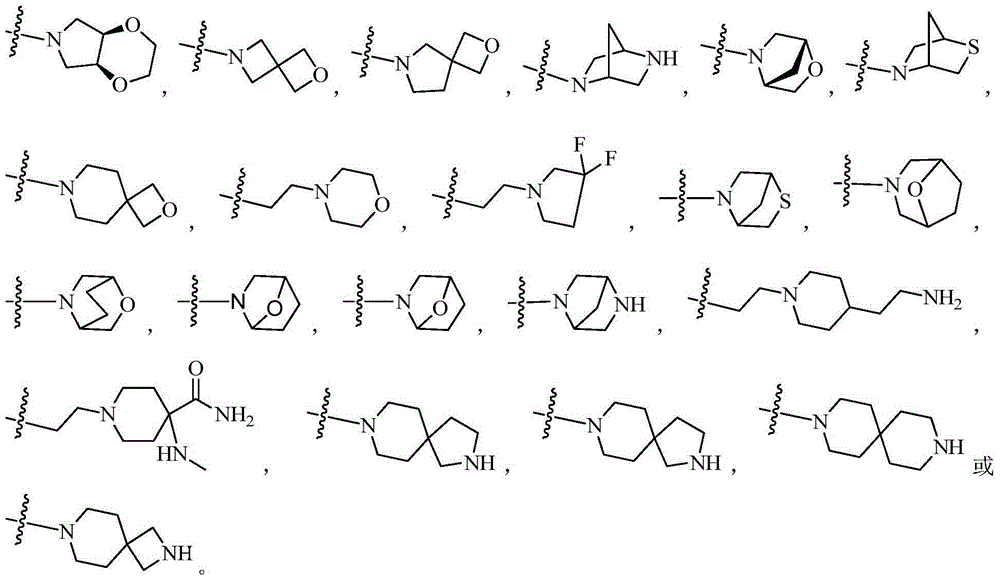Patents
Literature
279 results about "Cell lymphoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Lymphoma is a type of cancer involving cells of the immune system, primarily cells involved in the lymphatic system of the body.
Intrathecal administration of rituximab for treatment of central nervous system lymphomas
InactiveUS20020009444A1Prevent intermolecular disulfide formationPromote recoveryBiocidePeptide/protein ingredientsMeningesImmunocompromised patient
This invention describes methods of using anti-B cell antibodies, preferably anti-CD20 antibodies, and most preferably Rituximab, to treat B cell lymphomas of the brain, especially primary central nervous system lymphomas (PCNSLs), and to prevent meningeal relapse. The antibodies can be administered intrathecally alone, or in combination with other chemotherapeutics, such as methotrexate, or other anti-B cell antibodies to treat PCNSL in both immunocompromised and non-immunocompromised patients. These antibodies can also be used to diagnose patients with CNS lymphoma, especially in immunocompromised patients.
Owner:BIOGEN INC
Combined use of anti-cytokine antibodies or antagonists and anti-CD20 for treatment of B cell lymphoma
InactiveUS20020012665A1Avoiding and decreasing and resistanceOrganic active ingredientsIn-vivo radioactive preparationsFactor iiBiological activation
The present invention discloses combined therapies for treating hematologic malignancies, including B cell lymphomas and leukemias or solid non-hematologic tumors, comprising administration of anti-cytokine antibodies or antagonists to inhibit the activity of cytokines which play a role in perpetuating the activation of B cells. The administration of such antibodies and antagonists, particularly anti-IL10 antibodies and antagonists, is particularly useful for avoiding or decreasing the resistance of hematologic malignant cells or solid tumor cells to chemotherapeutic agents and anti-CD20 or anti-CD22 antibodies. The invention also provides combination therapies for solid tumors having B cell involvement comprising the administration of an anti-cytokine antibody and a B cell depleting antibody such as RITUXAN(R).
Owner:BIOGEN INC
Radiolabeling kit and binding assay
InactiveUS20020102208A1No reduction in immunoreactivityNegligible lossIn-vivo radioactive preparationsDepsipeptidesTherapeutic antibodyAssay
Antibody binding assays and radiolabeling kits are disclosed for radiolabeling and testing therapeutic antibodies in the commercial setting. In particular, the kits are designed for making and evaluating radiolabeled anti-CD20 conjugates to be used for the treatment and imaging of B cell lymphoma tumors. All kit reagents are sterile and are designed to achieve a high level of antibody radiolabeling and product stability with results which are highly reproducible.
Owner:BIOGEN INC
Camptothecin Conjugates of Anti-CD22 Antibodies for Treatment of B Cell Diseases
ActiveUS20110305631A1Increase the number ofNervous disorderPeptide/protein ingredientsCD20Autoimmune condition
Disclosed herein are compositions and methods of use comprising combinations of anti-CD22 antibodies with a therapeutic agent. The therapeutic agent may be attached to the anti-CD22 antibody or may be separately administered, either before, simultaneously with or after the anti-CD22 antibody. In preferred embodiments, the therapeutic agent is an antibody or fragment thereof that binds to an antigen different from CD22, such as CD19, CD20, CD21, CD22, CD23, CD37, CD40, CD40L, CD52, CD80 and HLA-DR. However, the therapeutic agent may an immunomodulator, a cytokine, a toxin or other therapeutic agent known in the art. More preferably, the anti-CD22 antibody is part of a DNL complex, such as a hexavalent DNL complex. Most preferably, combination therapy with the anti-CD22 antibody or fragment and the therapeutic agent is more effective than the antibody alone, the therapeutic agent alone, or the combination of anti-CD22 antibody and therapeutic agent that are not conjugated to each other. Administration of the anti-CD22 antibody and therapeutic agent induces apoptosis and cell death of target cells in diseases such as B-cell lymphomas or leukemias, autoimmune disease or immune dysfunction disease.
Owner:IMMUNOMEDICS INC
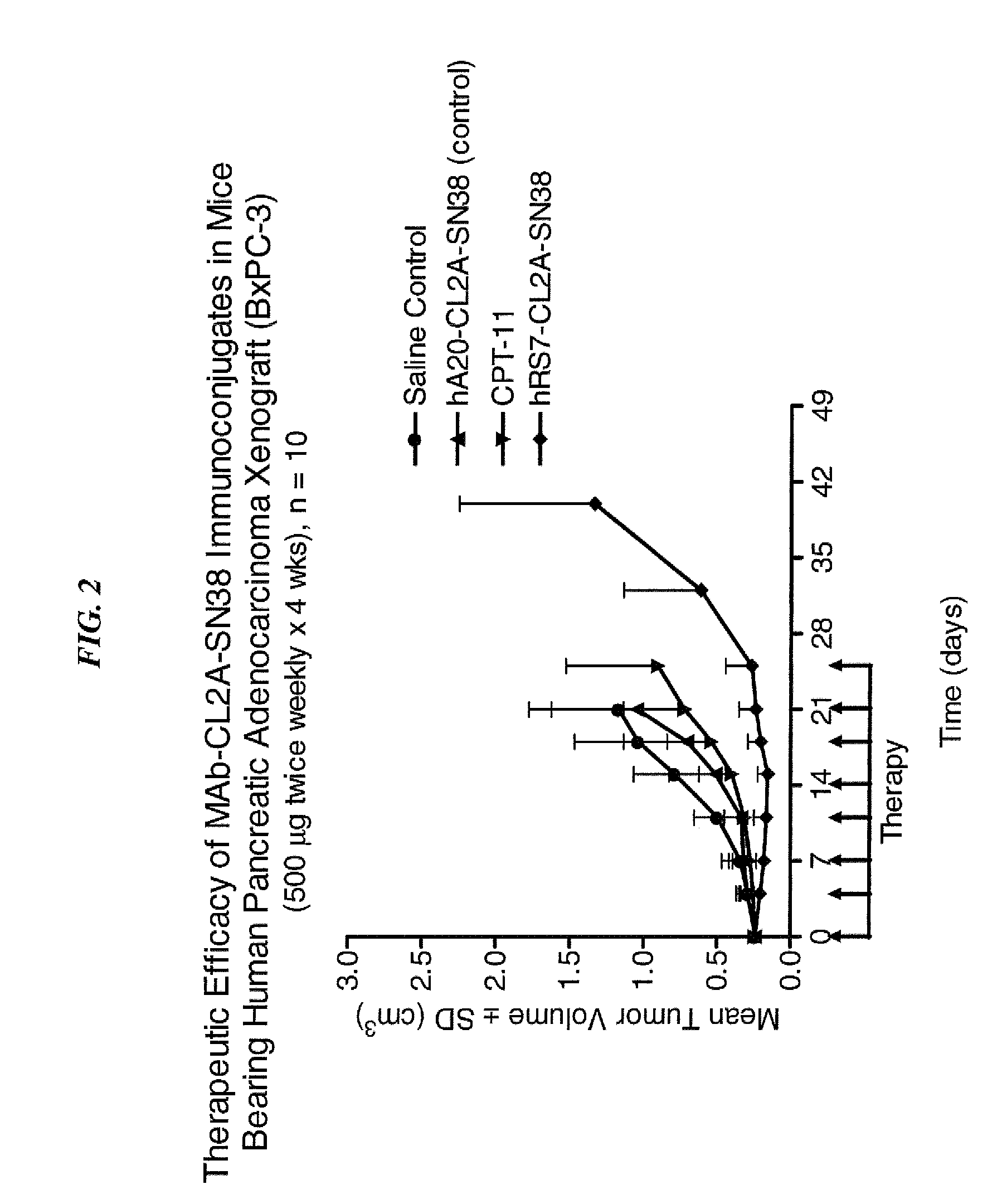
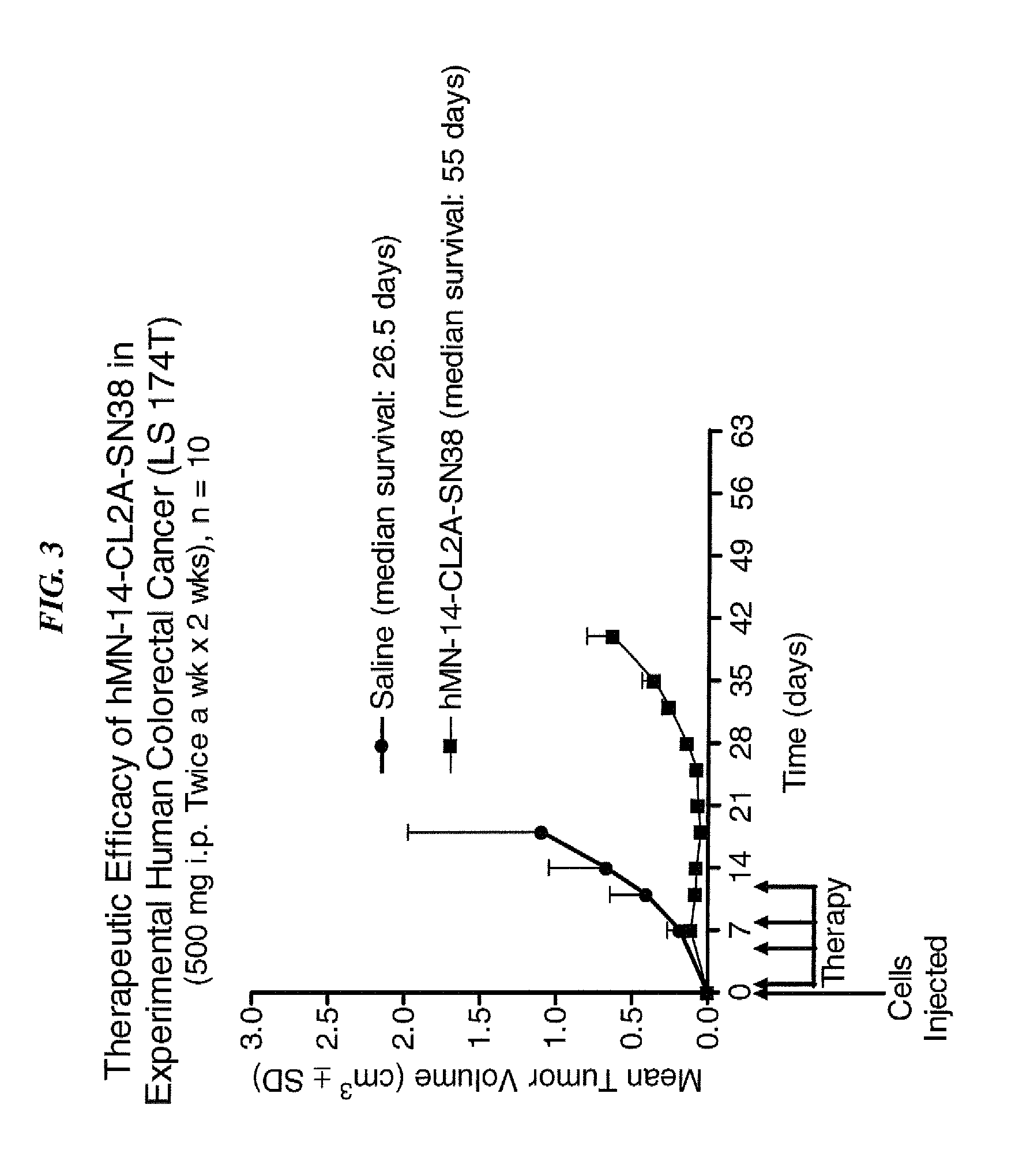
Immunoconjugates and humanized antibodies specific for B-cell lymphoma and leukemia cells
InactiveUS20050106108A1Lowered HAMA reactionReduced responsePeptide/protein ingredientsAntibody mimetics/scaffoldsHuman antimouse AntibodyComplementarity determining region
A chimeric LL2 monoclonal antibody is described in which the complementarity determining regions (CDRs) of the light and heavy chains of the murine LL2 anti-B-lymphoma, anti-leukemia cell monoclona lantibody has been recombinantly joined to the human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-cell lymphoma and leukemia cell internalization capacity of the parental murine LL2 monoclonal antibody, and which has the potential of exhibiting reduced human anti-mouse antibody production activity. A humanized LL2 monoclonal antibody is described in which the CDRs of the light and heavy chains have been recombinantly joined to a framework sequence of human light and heavy chains variable regions, respectively, and subsequently linked to human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-lymphoma and leukemia cell internalization capacities of the parental murine and chimeric LL2 monoclonal antibodies, and which has the potential for exhibiting reduced human anti-mouse antibody production activity. Vectors for producing recombinant chimeric and humanized chimeric monoclonal antibodies are provided. Isolated DNAs encoding the amino acid sequences of the LL2 variable light and heavy chain and CDR framework regions are described. Conjugates of chimeric and humanized chimeric LL2 antibodies with cytotoxic agents or labels find use in therapy and diagnosis of B-cell lymphomas and leukemias.
Owner:IMMUNOMEDICS INC
Heterocyclic compounds and their uses
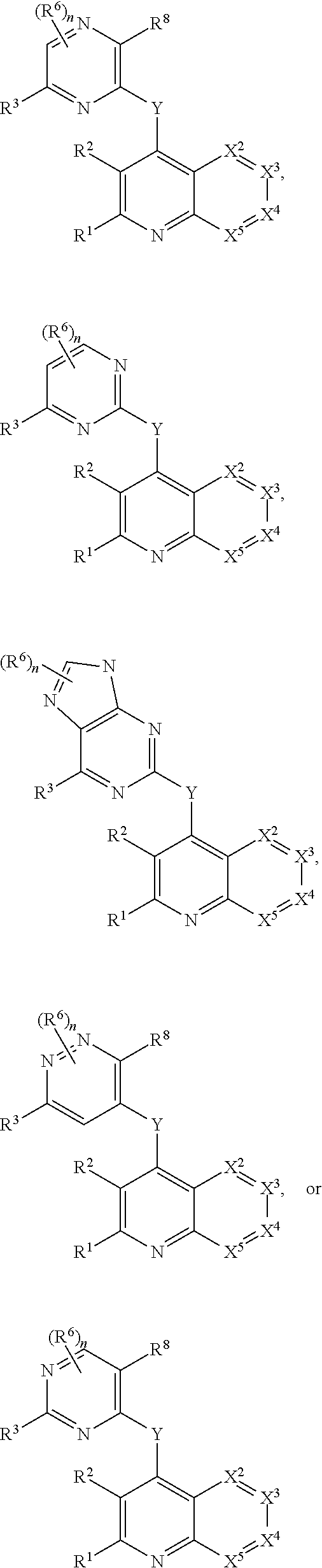
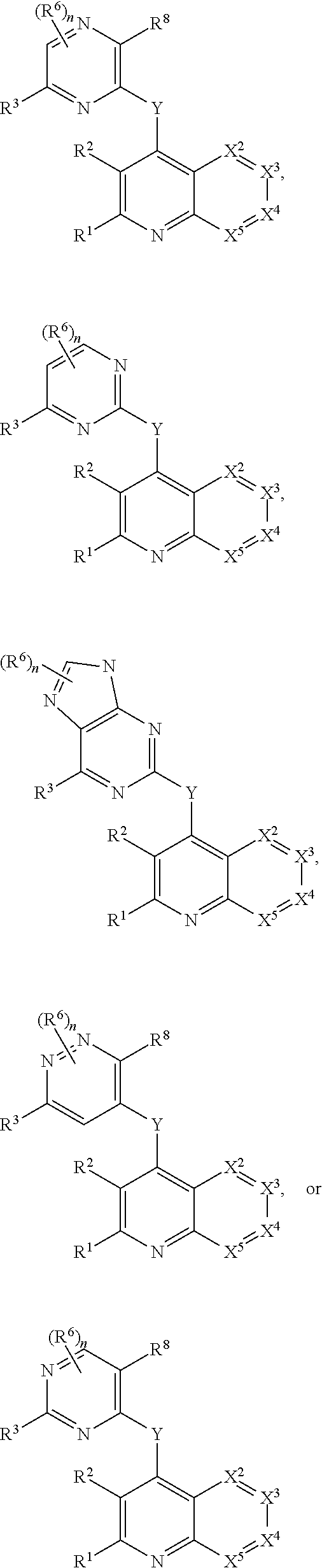
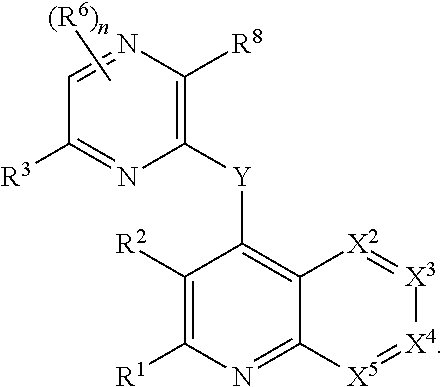
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjogren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110δ activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Il-21 antagonists
InactiveUS20070122413A1Increasing in vivo serum half-lifeModulate antibody responseNervous disorderAntibody mimetics/scaffoldsAutoimmune conditionAutoimmune disease
Monoclonal antibodies are identified that bind the IL-21 protein. These antibodies are used to identify regions of the IL-21 protein to where binding neutralizes IL-21 activity. Hybridomas and methods of producing anti-IL-21 monoclonal antibodies are described. The monoclonal antibodies are useful in treating IL-21-mediated diseases, which may include autoimmune and inflammatory diseases such as pancreatitis, type I diabetes (IDDM), Graves Disease, inflammatory bowel disease (IBD), Crohn's Disease, ulcerative colitis, irritable bowel syndrome, multiple sclerosis, rheumatoid arthritis, diverticulosis, systemic lupus erythematosus, psoriasis, ankylosing spondylitis, scleroderma, systemic sclerosis, psoriatic arthritis, osteoarthritis, atopic dermatitis, vitiligo, graft vs. host disease (GVHD), cutaneous T cell lymphoma (CTCL), Sjogren's syndrome, glomerulonephritis, IgA nephropathy, graft versous host disease, transplant rejection, atopic dermatitis, anti-phospholipid syndrome, and asthma, and other autoimmune diseases.
Owner:ZYMOGENETICS INC
Methods of treating cancer with hdac inhibitors
InactiveUS20070060614A1Better pharmacokinetic profileImprove bioavailabilityBiocideAnimal repellantsDosing regimenOncology
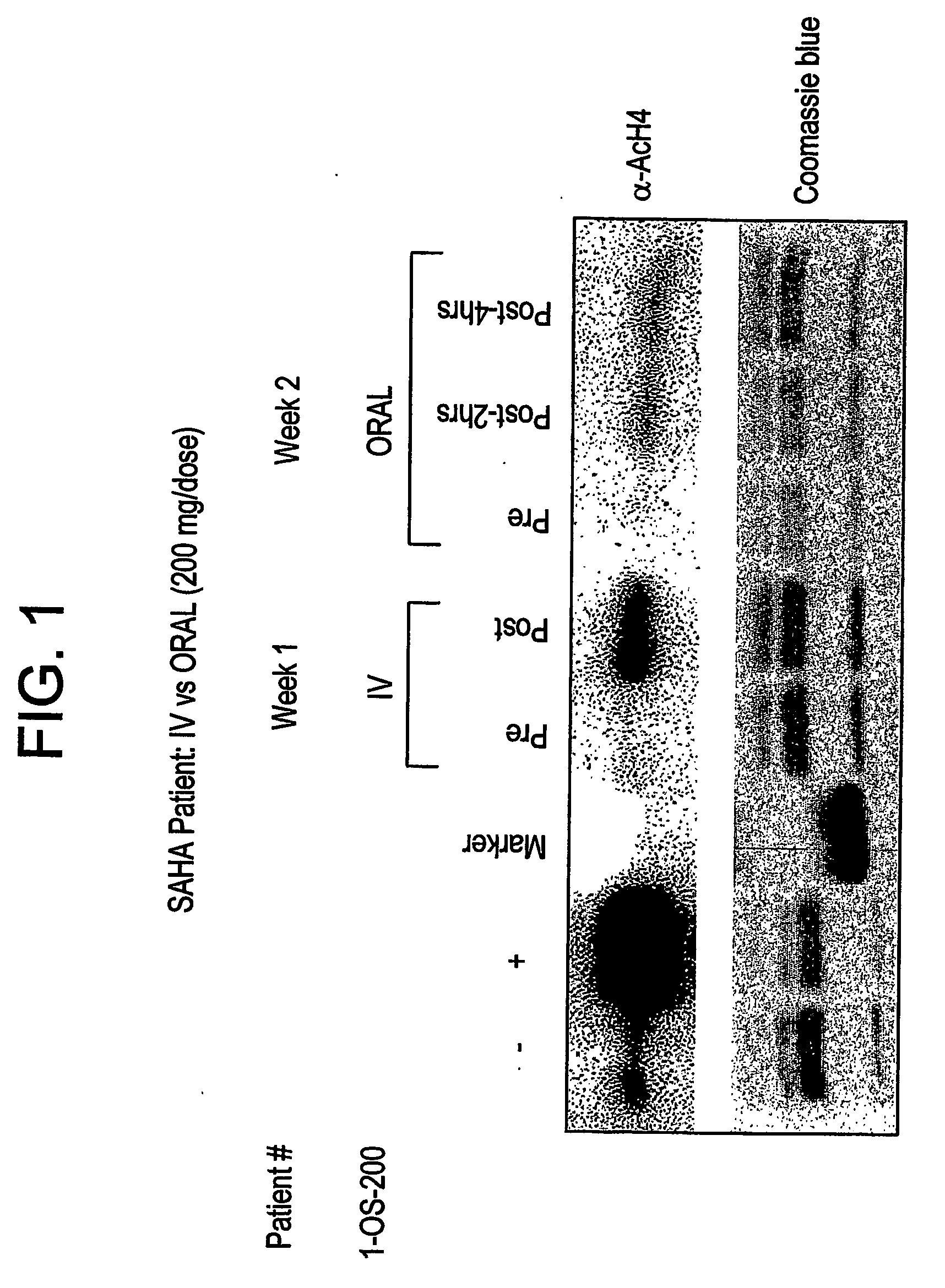
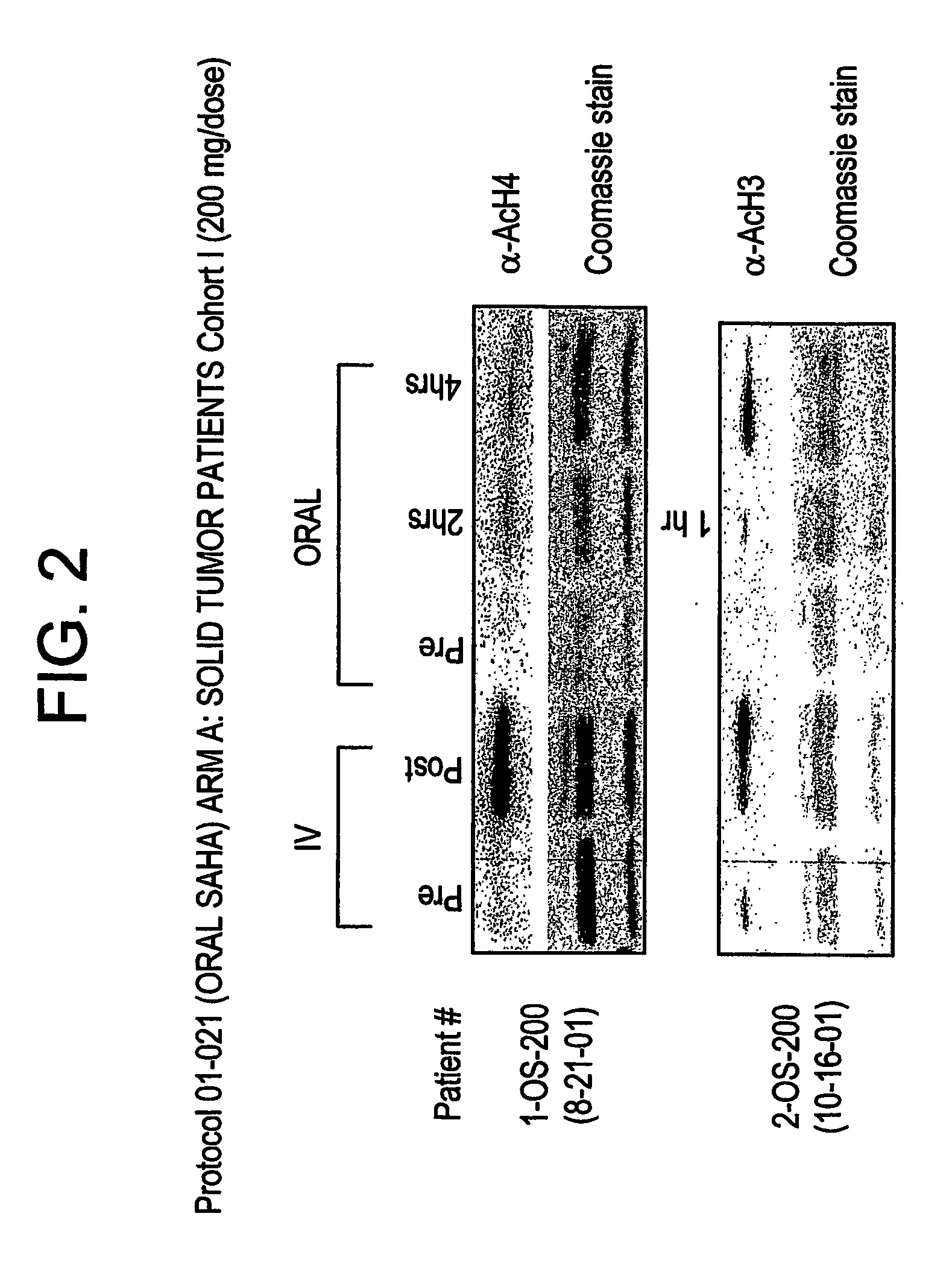
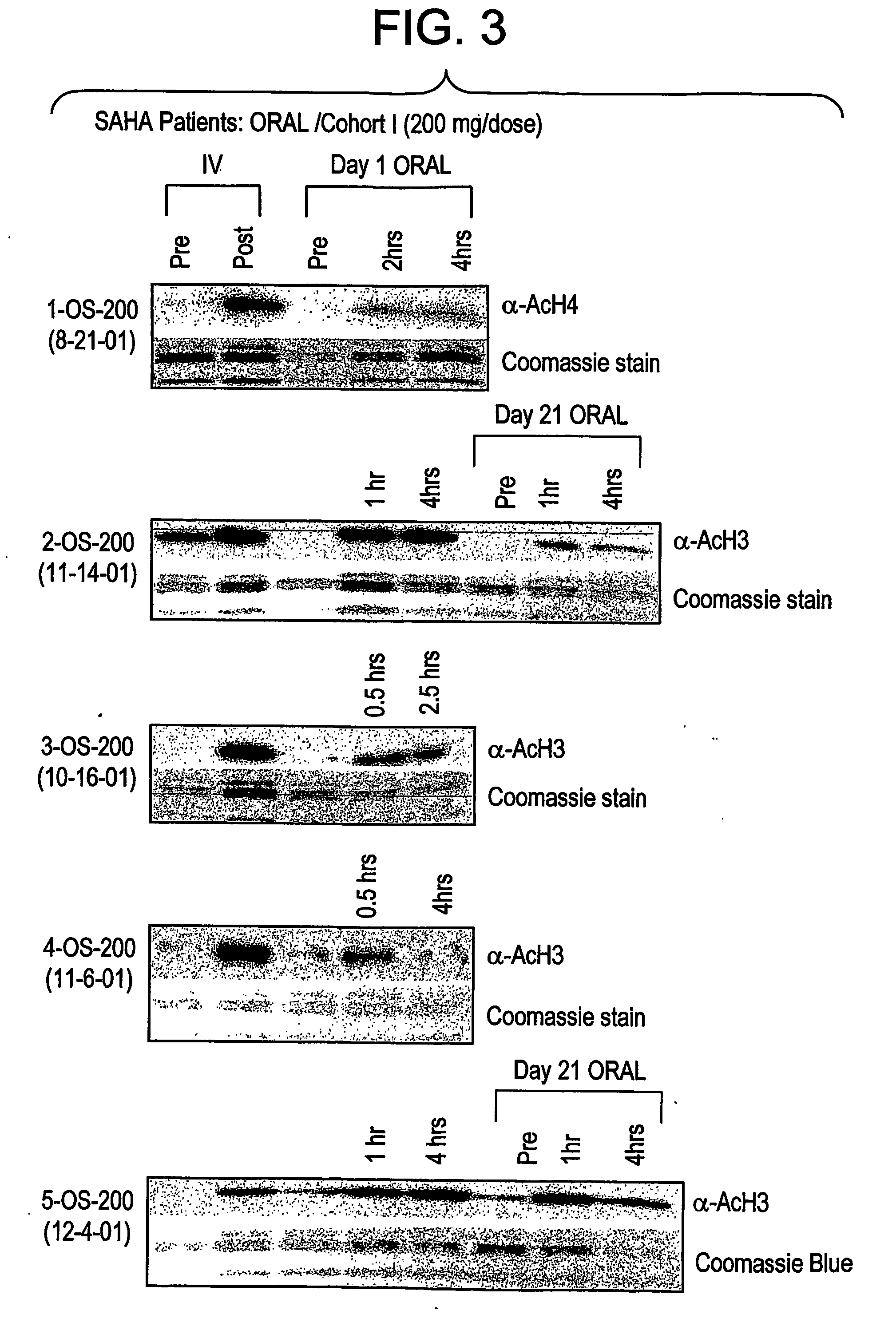
The present invention relates to methods of treating cancers, e.g., mesothelioma or lymphoma. More specifically, the present invention relates to methods of treating mesothelioma or diffuse large B-cell lymphoma (DLBCL), by administration of pharmaceutical compositions comprising HDAC inhibitors, e.g., suberoylanilide hydroxamic acid (SAHA). The oral formulations of the pharmaceutical compositions have favorable pharmacokinetic profiles such as high bioavailability and surprisingly give rise to high blood levels of the active compounds over an extended period of time. The present invention further provides a safe, daily dosing regimen of these pharmaceutical compositions, which is easy to follow, and which results in a therapeutically effective amount of the HDAC inhibitors in vivo.
Owner:MERCK HDAC RESEARCH LLC +1
Treatment for CD5+ B cell lymphoma
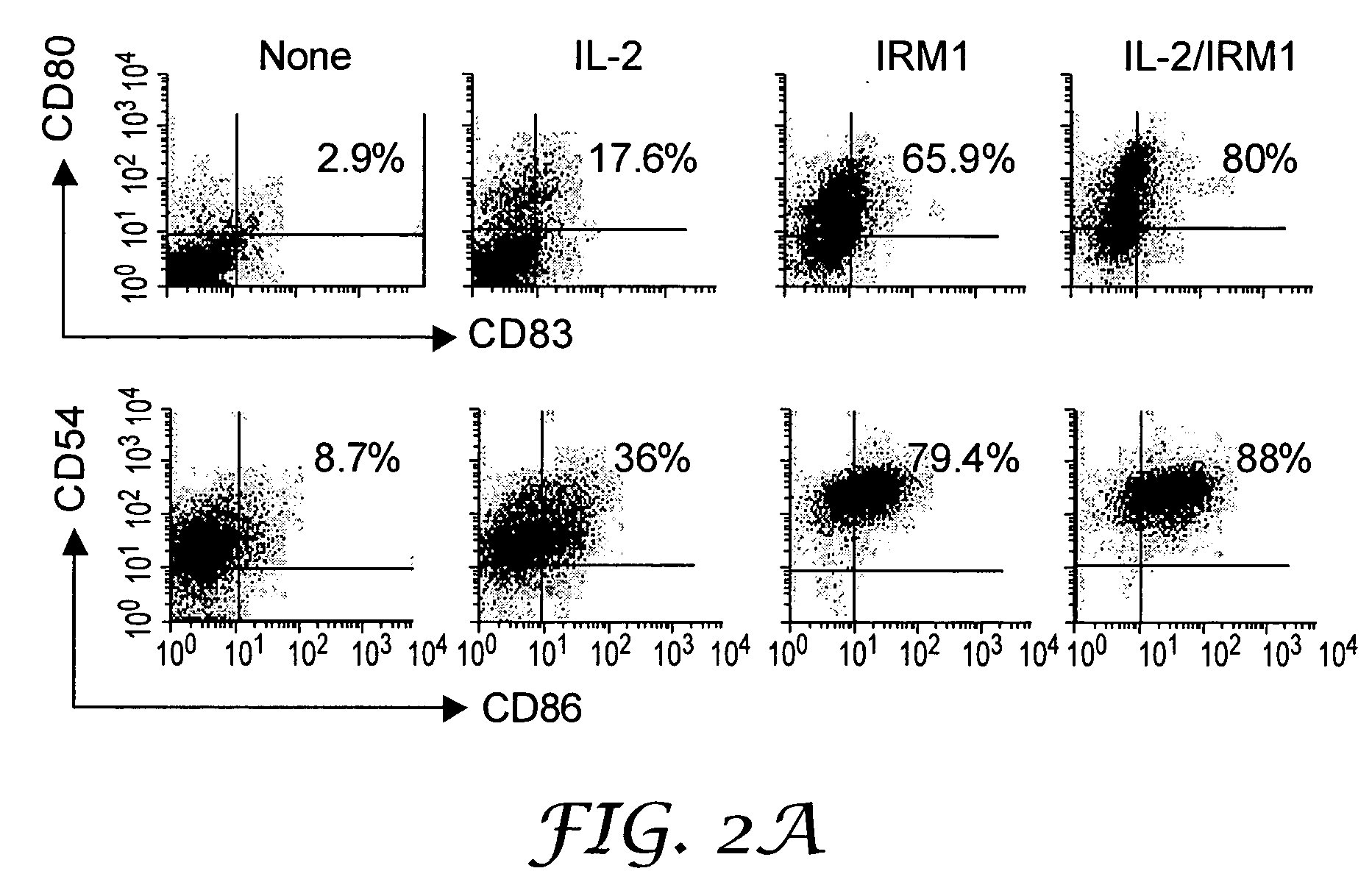
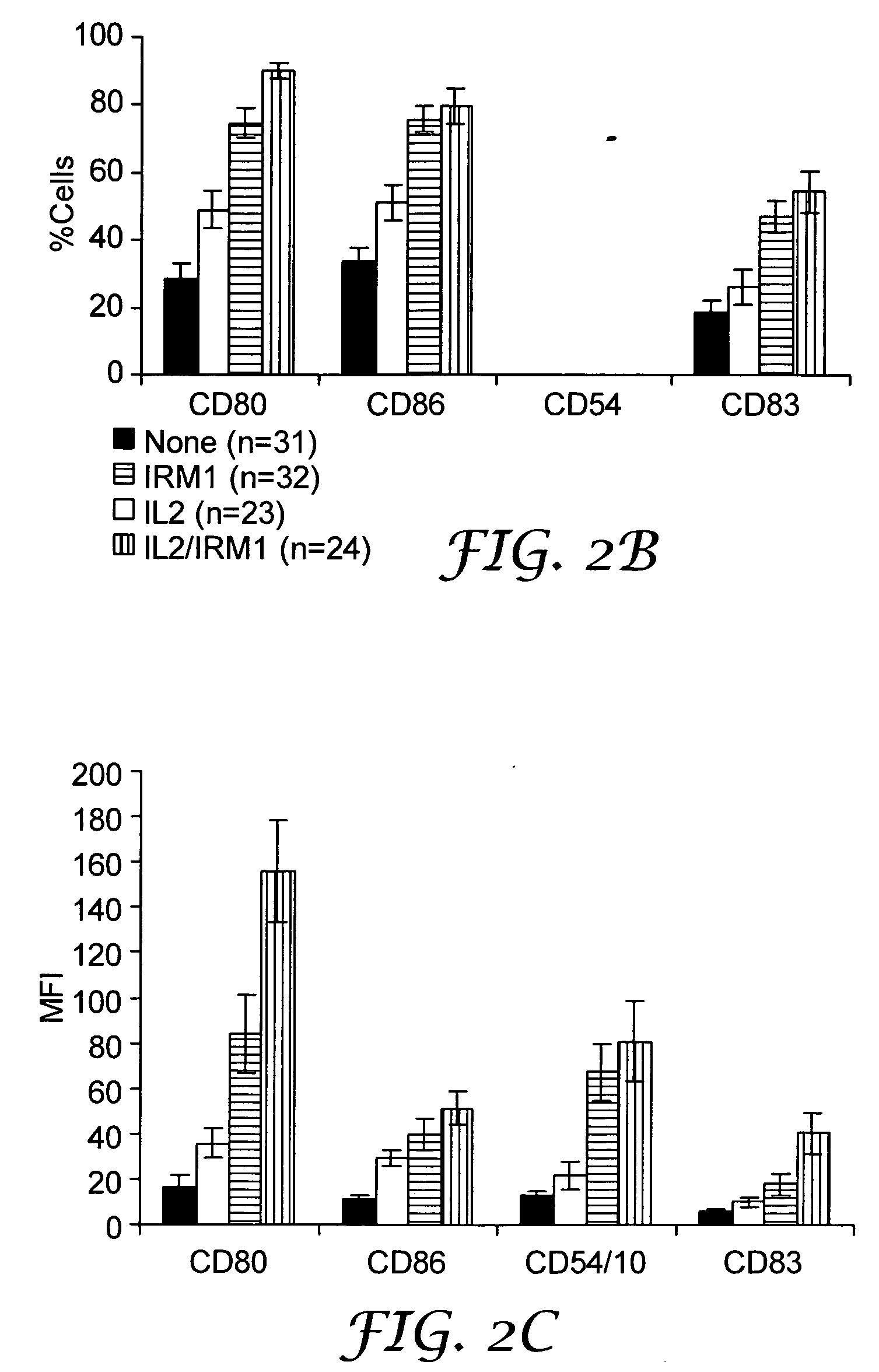
InactiveUS20050054665A1Ameliorate at least one symptomDecrease in peripheral blood lymphocytes, lymphadenopathy, or splenomegalyBiocidePeptide/protein ingredientsTLR8Agonist
The present invention provides methods for increasing expression of cell surface molecules of CD5+ B cell lymphoma cells by contacting cells with immune response modifiers. The invention also provides methods for the treatment of CD5+ B cell lymphomas, including chronic lymphocytic leukemia and small lymphocytic lymphoma, by administering immune response modifier compounds to a subject in need of such treatment. Suitable immune response modifier compounds include agonists of TLR7 and / or TLR8.
Owner:COLEY PHARMA GRP INC
Monitoring transformation of follicular lymphoma to diffuse large b-cell lymphoma by immune repertoire analysis
InactiveUS20140349883A1Raise the possibilityMicrobiological testing/measurementLibrary screeningProgenitorSomatic cell
The invention is directed to a method of prognosing in an individual a transformation from follicular lymphoma to diffuse large B-cell lymphoma (DLBCL) by measuring changes and / or lack of changes in certain groups of related clonotypes, referred to herein as “clans,” in successive clonotype profiles of the individual. A clan may arise from a single lymphocyte progenitor that gives rise to many related lymphocyte progeny, each possessing and / or expressing a slightly different immunoglobulin receptor due to somatic mutation(s), such as base substitutions, inversions, related rearrangements resulting in common V(D)J gene segment usage, or the like. A higher likelihood of transformation from follicular lymphoma to DLBCL is correlated with the persistence of clans in successive clonotype profiles whose clonotype membership fails to undergo diversification over time.
Owner:ADAPTIVE BIOTECH
Topical Use of Valproic Acid for the Prevention or Treatment of Skin Disorders
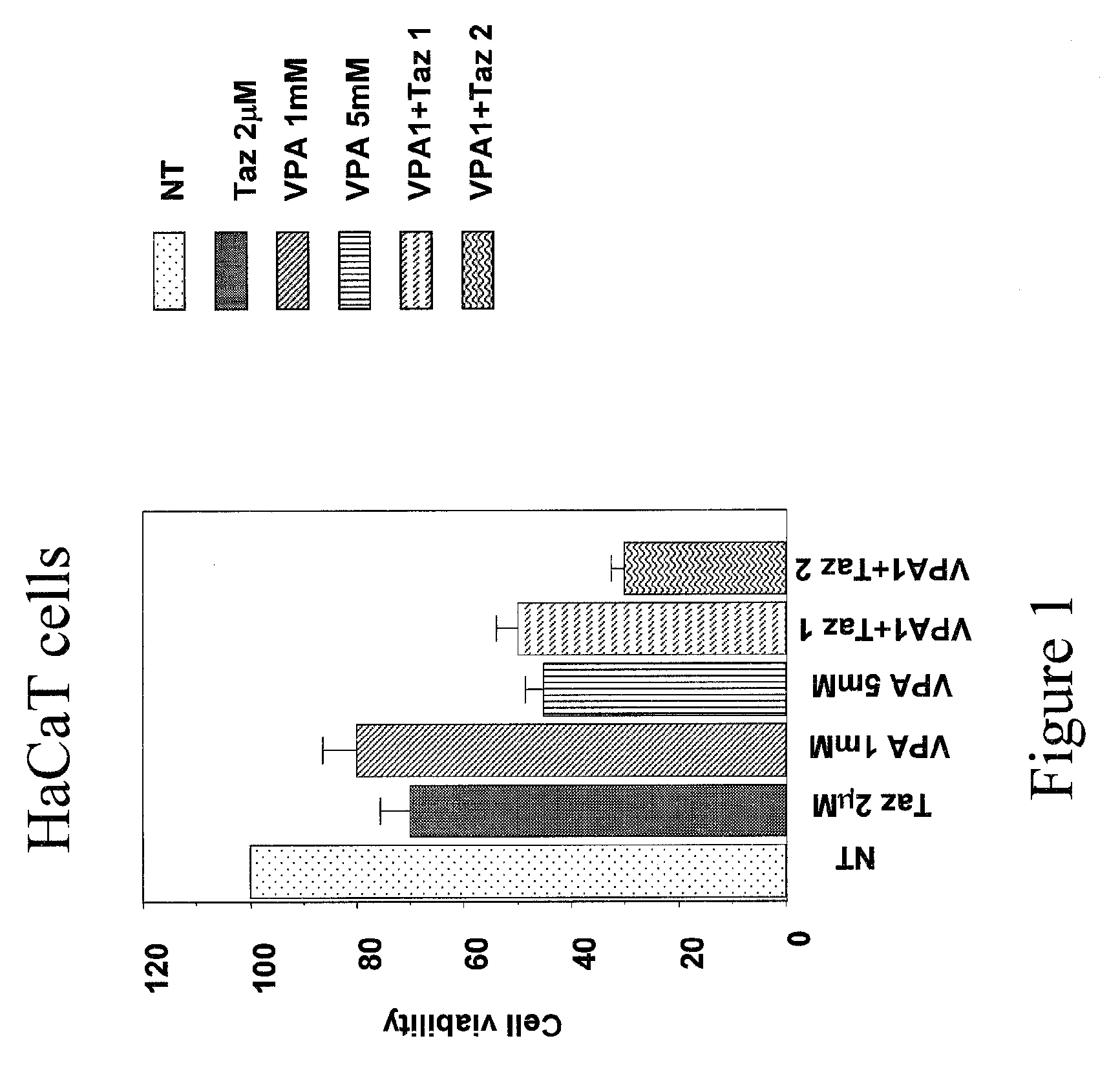
The present invention relates to a topically applicable formulation containing Valproic Acid or a derivative thereof which can be used alone or in combination with topically applicable formulations of retinoids or of nuclear receptor ligands, or of chemotherapeutic agents (e.g. 5-Fluorouracil). The formulation is useful for the topical treatment of cancerous skin disorders, such as Basal Cell Carcinoma, Squamous Cell Carcinoma, Keratoakantoma, Bowen Disease, cutaneous T-Cell Lymphoma and also for the topical treatment of pre-malignant lesions, and of inflammations of the skin and / or mucosa. The invention also relates to the use of this topically applicable formulation for the protection from UV light and for the treatment of sun burn. The invention includes the use of VPA for the manufacture of a clinically used medicament for the topical treatment of the human diseases listed above.
Owner:TOPOTARGET GERMANY AG
Heterocyclic compounds and their uses
Substituted bicyclic heteroaryls and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity. The present invention also enables methods for treating cancers that are mediated, dependent on or associated with pi 105 activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia (B-ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
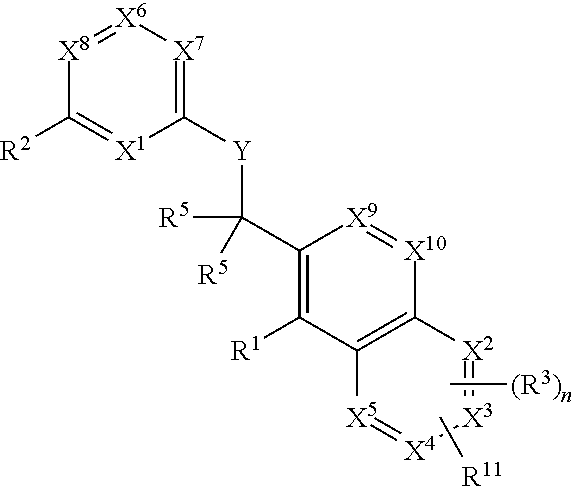
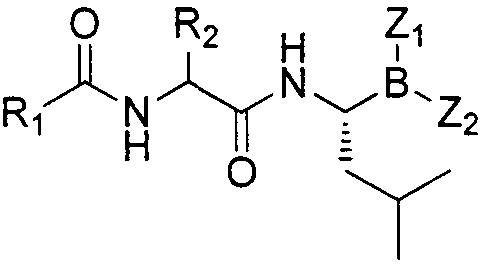
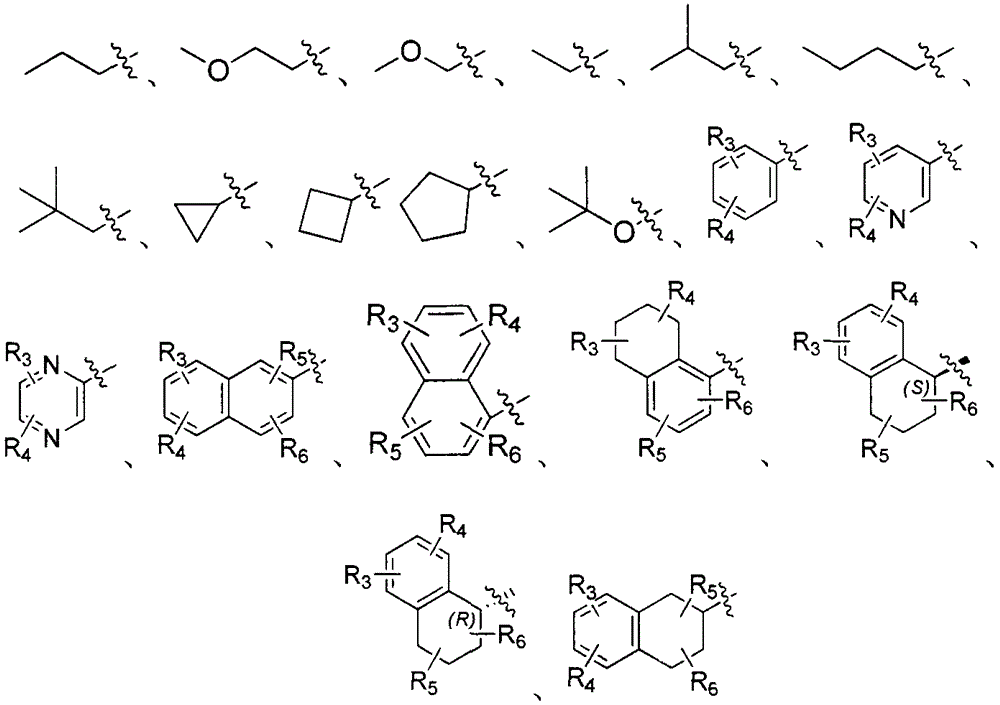
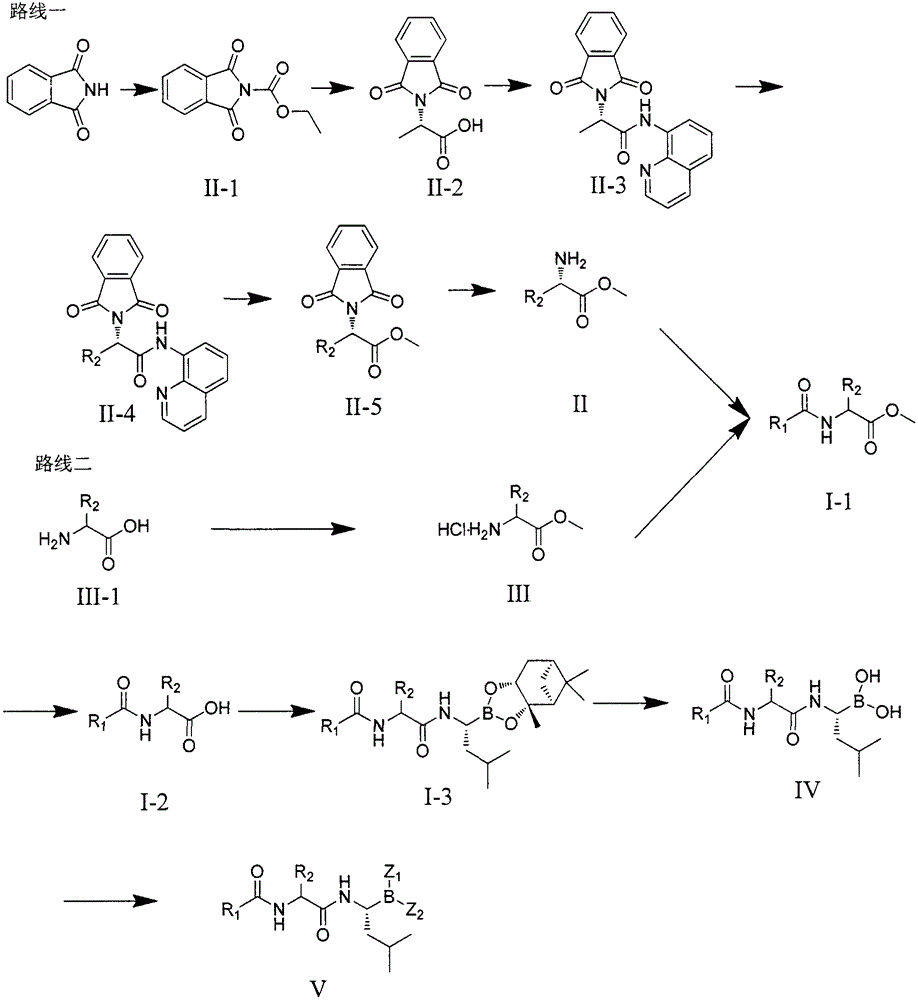
Dipeptide boric acid composed of carboxylic acid and alpha-amino acid as well as ester compound thereof, and preparation method and application of dipeptide boric acid and ester compound thereof
ActiveCN105732683AHigh yieldHigh activityBoron compound active ingredientsGroup 3/13 element organic compoundsProstate cancerProteasome inhibitor
The invention belongs to the field of drug synthesis and in particular relates to a series of novel peptide boric acids as well as an ester compound or pharmaceutical salt thereof, and a preparation method and application of the peptide boric acids as well as the ester compound or pharmaceutical salt thereof in pharmacodynamics. A structure of the peptide boric acid and the ester compound or pharmaceutical salt thereof is shown in a formula I (described in the specification). The compound provided by the invention can be used for preparing a proteasome inhibitor and can further be used for treating solid tumours and blood tumours, wherein the solid tumours are selected from non-small cell lung cancer, small cell lung cancer, lung adenocarcinoma, lung squamous carcinoma, pancreatic cancer, breast cancer, prostate cancer, liver cancer, skin cancer, epithelial cell cancer, gastrointestinal stromal tumor, nasopharynx cancer and leukemia; and the blood tumours are selected from multiple myeloma, mantle cell lymphoma and histiocytic lymphoma.
Owner:JIANGSU CHIA TAI FENGHAI PHARMA
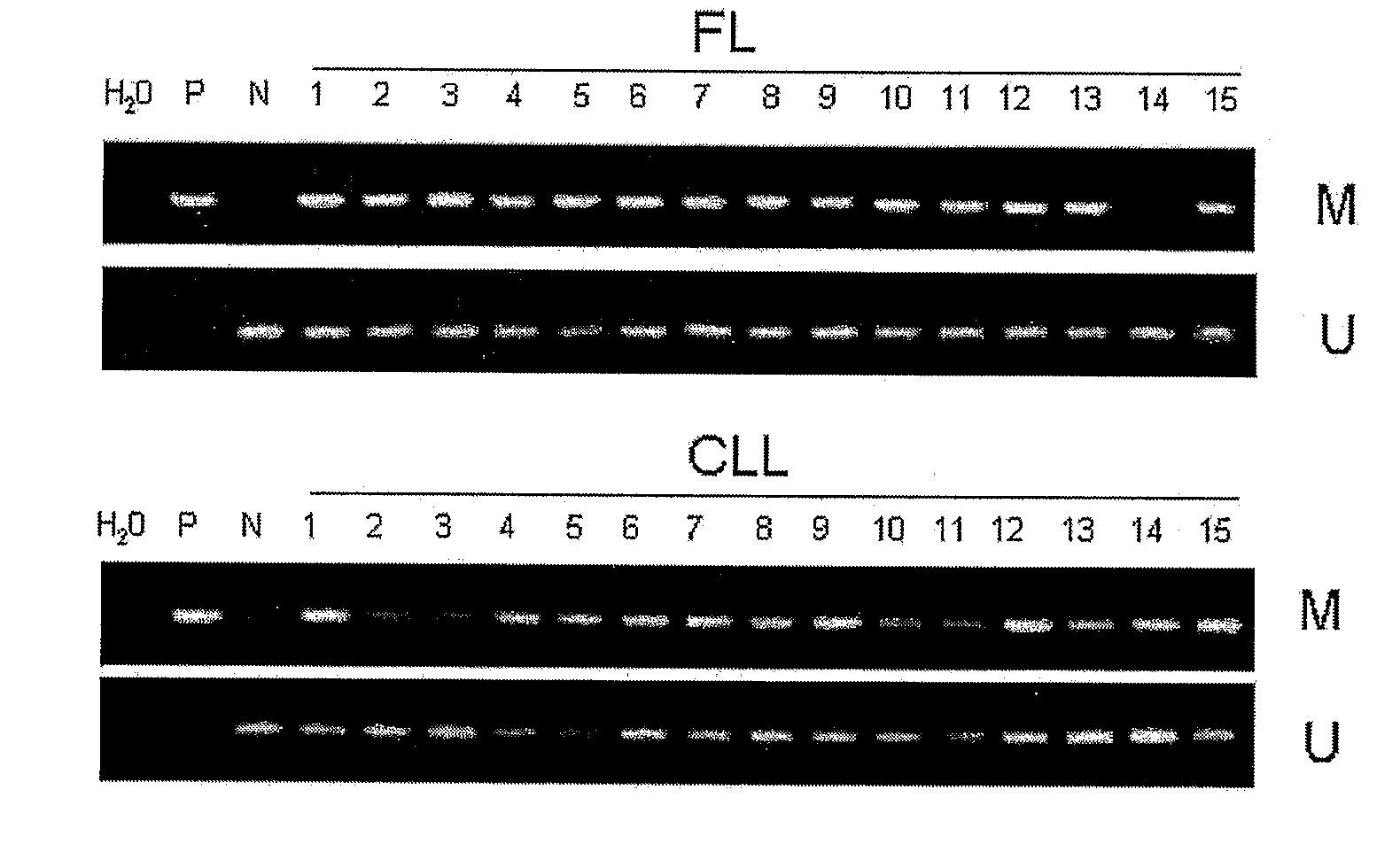
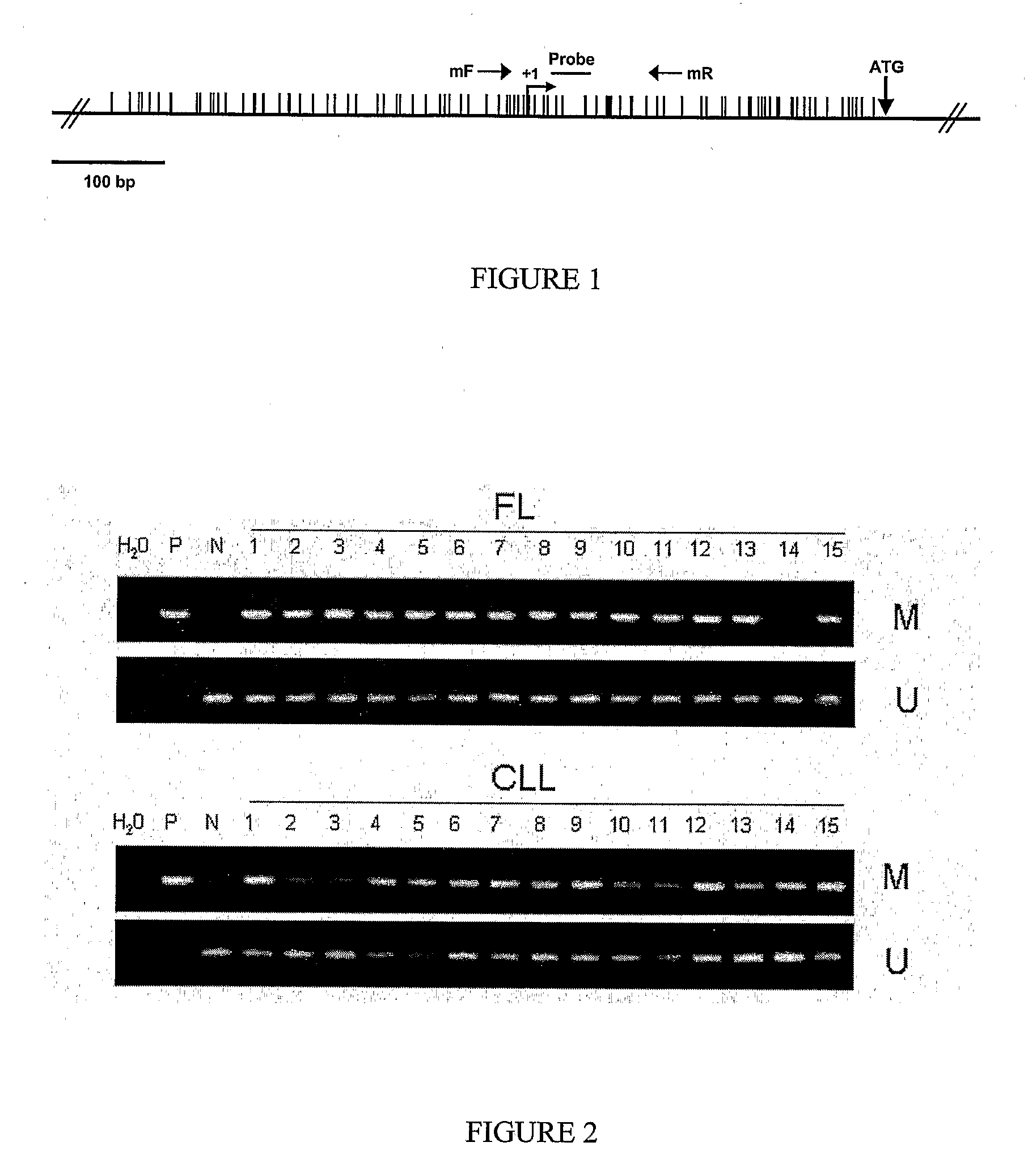
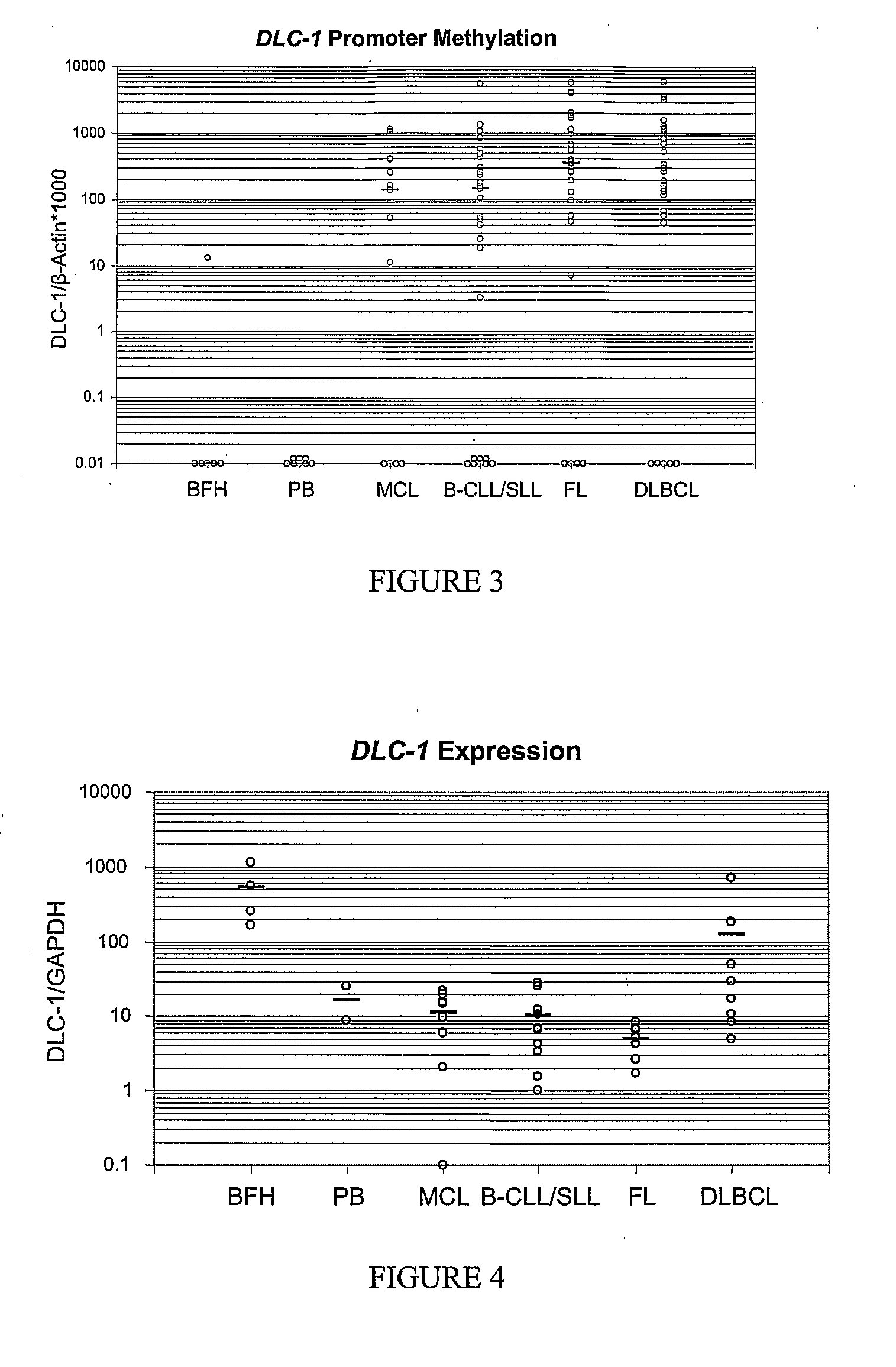
DNA methylation biomarkers in lymphoid and hematopoietic malignancies
InactiveUS20090264306A1Guaranteed maximum utilizationImprove the detection rateMicrobiological testing/measurementLibrary screeningDNA methylationLymphocytic cell
Differential Methylation Hybridization (DMH) was used to identify novel methylation markers and methylation profiles for hematopoieetic malignancies, leukemia, lymphomas, etc. (e.g., non-Hodgkin's lymphomas (NHL), small B-cell lymphomas (SBCL), diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), B-cell chronic lymphocytic leukemia / small lymphocytic lymphoma (B-CLL / SLL), chronic lymphocytic leukemia (CLL), multiple myeloma (MM), acute myelogenous leukemia (AML), acute lymphoblastic leukemia (ALL), etc.). Particular aspects provide novel biomarkers for NHL and subtypes thereof (e.g., MCL, B-CLL / SLL, FL, DLBCL, etc.), AML, ALL and MM, and further provide non-invasive tests (e.g. blood tests) for lymphomas and leukemias. Additional aspects provide markers for diagnosis, prognosis, monitoring responses to therapies, relapse, etc., and further provide targets and methods for therapeutic demethylating treatments. Further aspects provide cancer staging markers, and expression assays and approaches comprising idealized methylation and / or patterns” (IMP and / or IEP) and fusion of gene rankings.
Owner:UNIVERSITY OF MISSOURI
Anti CD20 tetravalent antibody, preparation method and uses thereof
InactiveCN101205255AReduce severityPrevent other symptomsHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsCD20Medicine
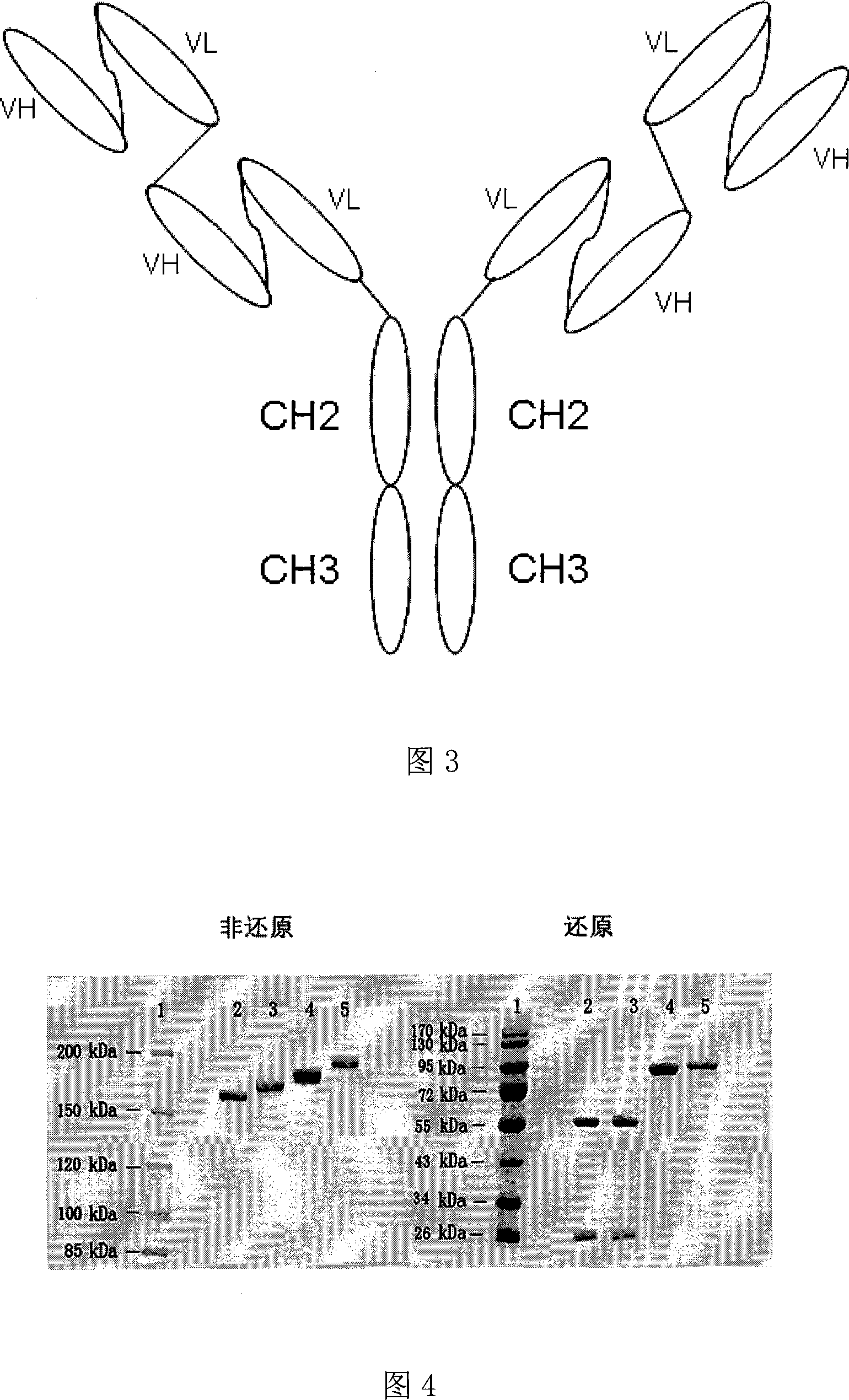
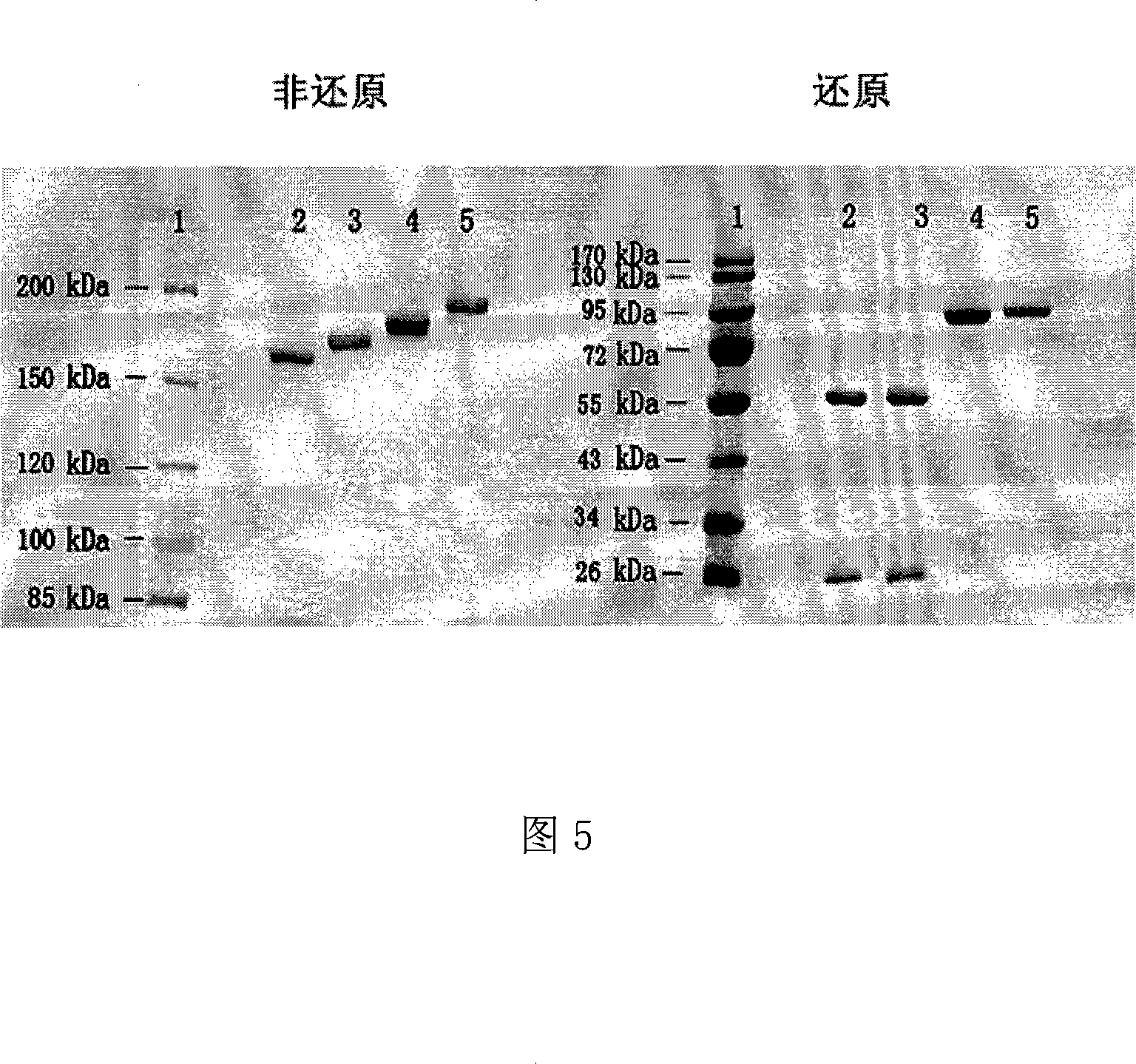
The invention discloses an anti- CD20 tetravalent antibody and a preparation method as well as application thereof, in particular the invention disclsoes an anti- CD20 tetravalent antibody C2B8(ScFvHL)4-Fc and 2F2(ScFvHL)4-Fc, and the preparation method as well as the use thereof in the preparation of drugs for inhibiting B-cell lymphoma.
Owner:SHANGHAI NAT ENG RES CENT OF ANTIBODY MEDICINE
Treatment of B-cell lymphoma
InactiveUS20060029543A1Increase ratingsConducive to survivalBiocideIn-vivo radioactive preparationsRegimenChemotherapy regimen
A method of treating B-cell lymphoma comprises administering to a patient a chemotherapeutic regimen, followed by treatment with a radiolabeled anti-CD20 antibody, wherein at the time of said treatment with said radiolabeled antibody said patient is not refractory to said chemotherapeutic regimen and has not relapsed.
Owner:BAYER PHARMA AG
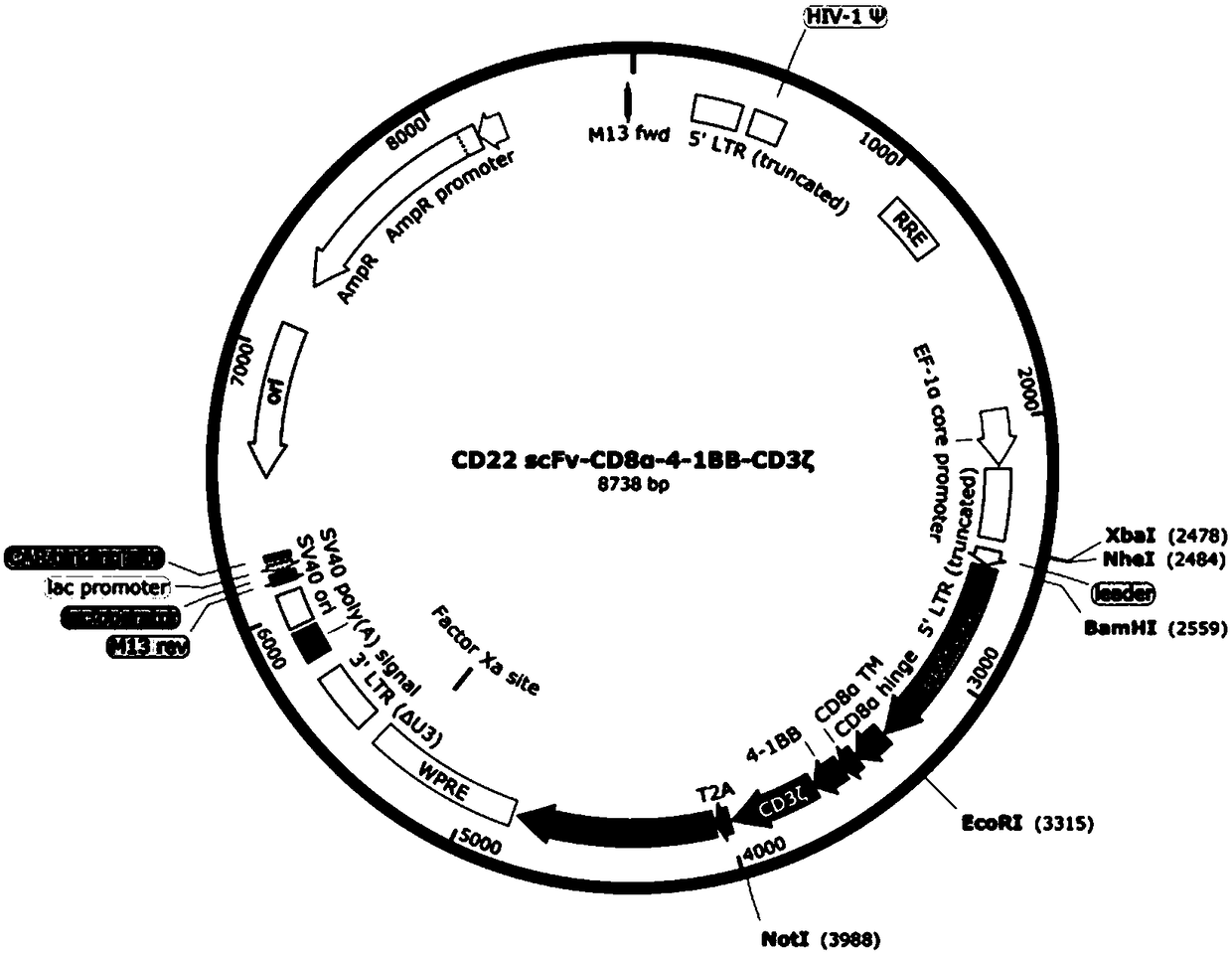
Chimeric antigen receptor targeting CD22 and application of chimeric antigen receptor
ActiveCN108715859AGood killing effectNo lethal effectPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigenOn cells
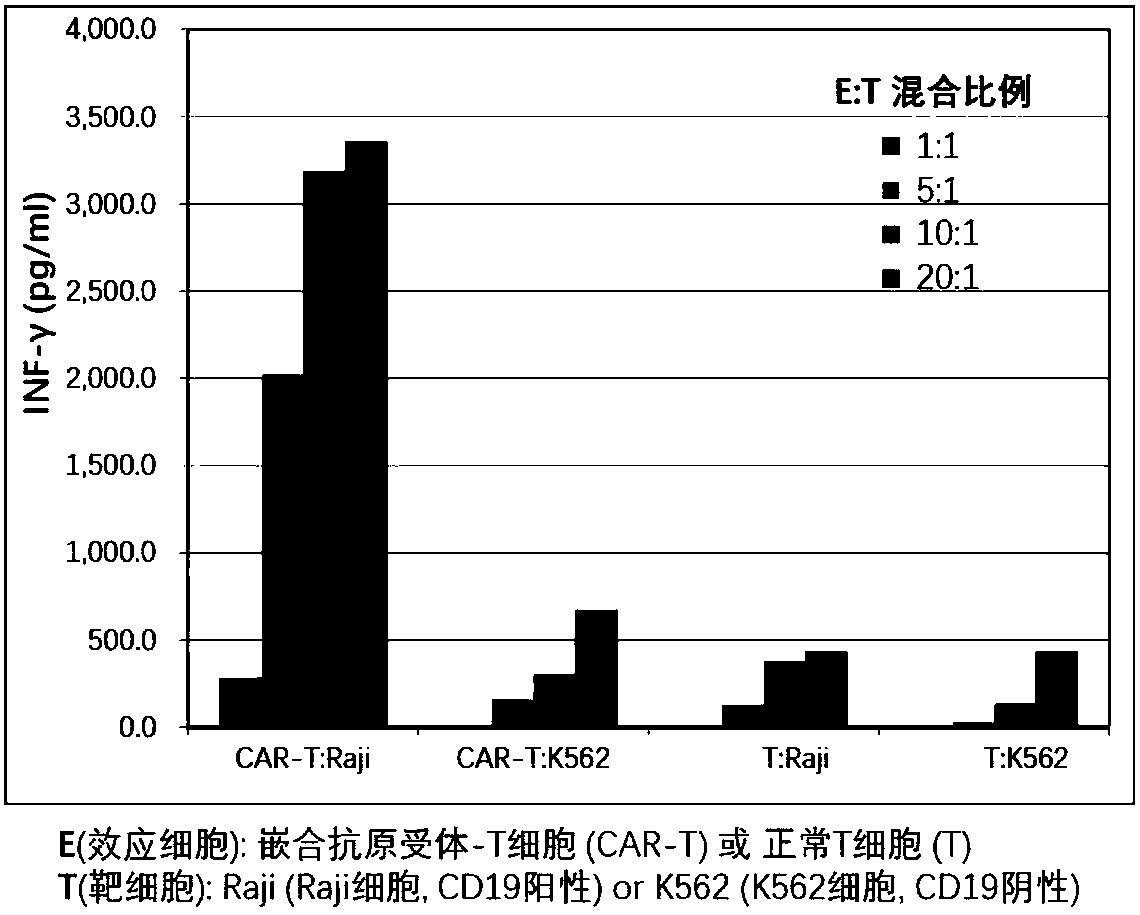
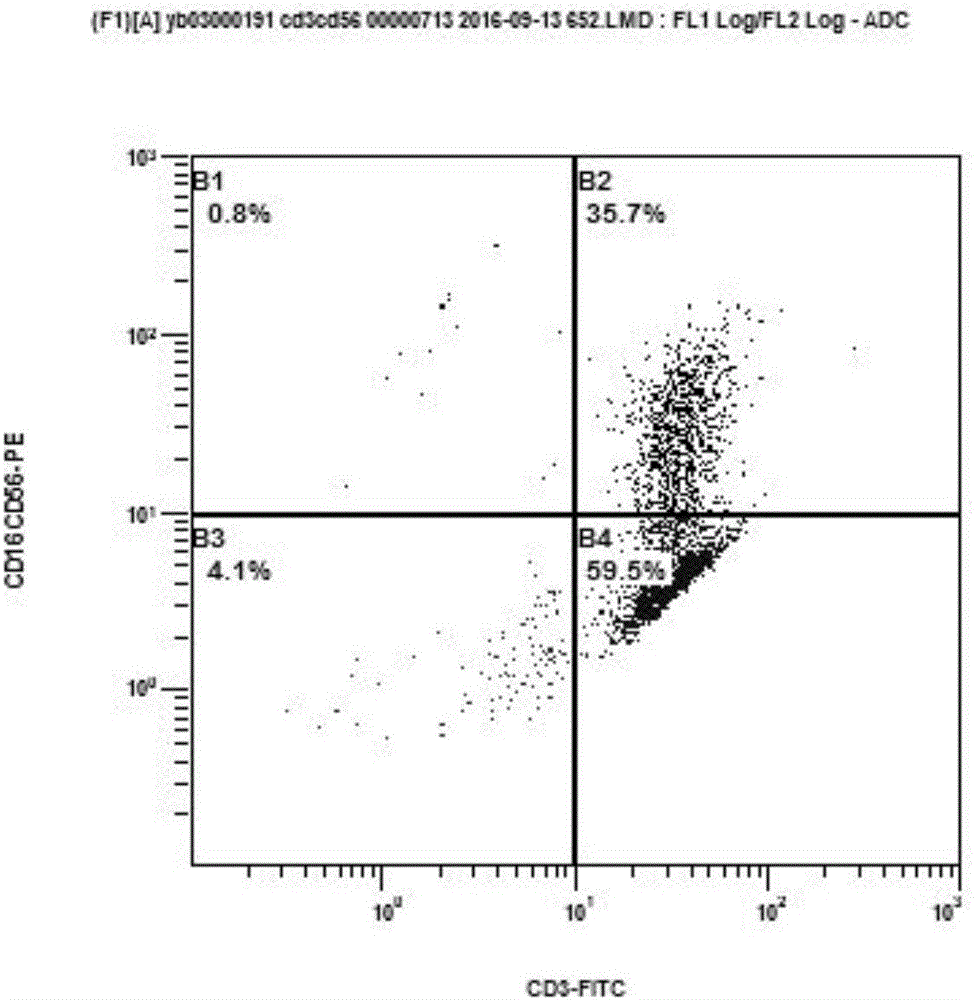
The invention provides a nucleic acid molecule for encoding a chimeric antigen receptor targeting CD22. The chimeric antigen receptor comprises an extracellular region, a transmembrane region and a intracellular signal transduction region, the extracellular region encoded by the nucleic acid molecule comprises a CD22 binding domain, and the CD22 binding domain is amino acid sequence as shown in SEQ ID No. 3. By using flow cytometry, a degranulation analysis experiment and ELISA to detect cell factors secreted by a T cell, a fact that the CAR-T cell has a high lethal effect on B cell lymphoma cells and acute B cell lymphoma cell leukemia cells expressing CD22 and hardly has a lethal effect on cells which do not express CD22, and an off-target effect is prevented effectively is proved. The chimeric antigen receptor CD22scFv-CD8alpha-4-1BB-CD3zeta can be used for treating CD22<+>B cell hematologic neoplasms and is applicable to combined treatment with a CD19 CAR-T cell.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE
Immunoconjugates and humanized antibodies specific for b-cell lymphoma and leukemia cells
InactiveUS20070172920A1Peptide/protein ingredientsAntibody mimetics/scaffoldsHuman antimouse AntibodyComplementarity determining region
A chimeric LL2 monoclonal antibody is described in which the complementarity determining regions (CDRs) of the light and heavy chains of the murine LL2 anti-B-lymphoma, anti-leukemia cell monoclonal antibody has been recombinantly joined to the human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-cell lymphoma and leukemia cell internalization capacity of the parental murine LL2 monoclonal antibody, and which has the potential of exhibiting reduced human anti-mouse antibody production activity. A humanized LL2 monoclonal antibody is described in which the CDRs of the light and heavy chains have been recombinantly joined to a framework sequence of human light and heavy chains variable regions, respectively, and subsequently linked to human kappa and IgG1 constant region domains, respectively, which retains the immunospecificity and B-lymphoma and leukemia cell internalization capacities of the parental murine and chimeric LL2 monoclonal antibodies, and which has the potential for exhibiting reduced human anti-mouse antibody production activity. Vectors for producing recombinant chimeric and humanized chimeric monoclonal antibodies are provided. Isolated DNAs encoding the amino acid sequences of the LL2 variable light and heavy chain and CDR framework regions are described. Conjugates of chimeric and humanized chimeric LL2 antibodies with cytotoxic agents or labels find use in therapy and diagnosis of B-cell lymphomas and leukemias.
Owner:IMMUNOMEDICS INC
Il-12 for radiation protection and radiation-induced toxicity mitigation
ActiveUS20140369958A1Increase opportunitiesReduce impactNervous disorderPeptide/protein ingredientsBiochemistryRadiation induced toxicity
Aspects and embodiments of the instant disclosure provide therapeutic methods and compositions comprising inter-leukin 12 (IL-12) useful for treating radiation-induced damage in a subject. In particular, the instant disclosure provides methods and compositions for radiation protection and / or radiation toxicity mitigation for the treatment of acute radiation syndrome and radiation induced toxicity associated with the treatment of cutaneous T-cell lymphoma.
Owner:KARYOPHARM THERAPEUTICS INC
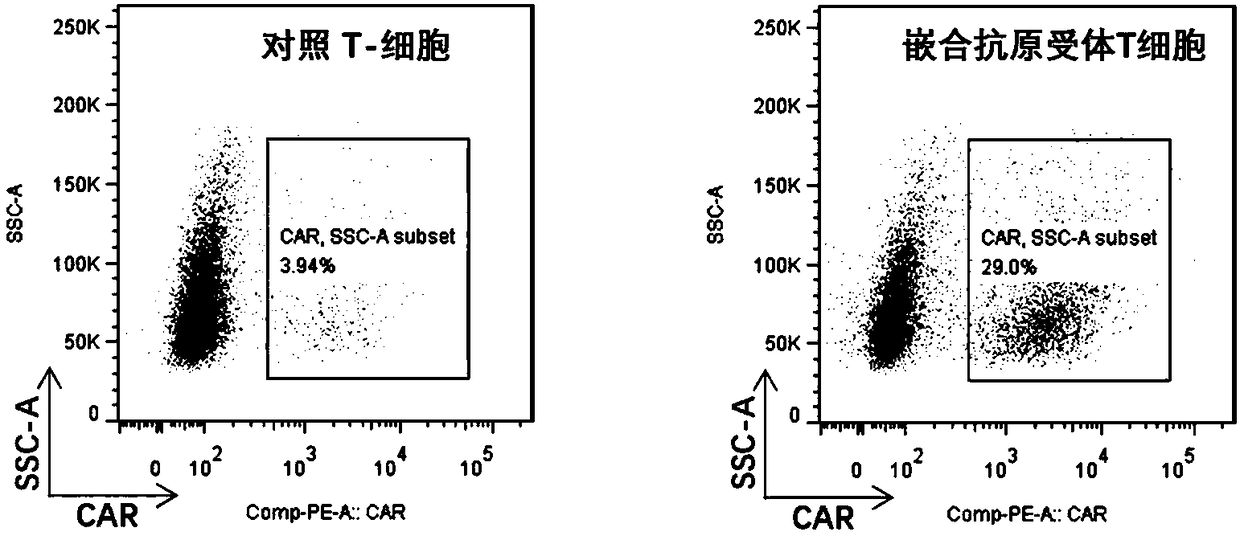
Chimeric antigen receptor
InactiveUS20170066827A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorBinding domain
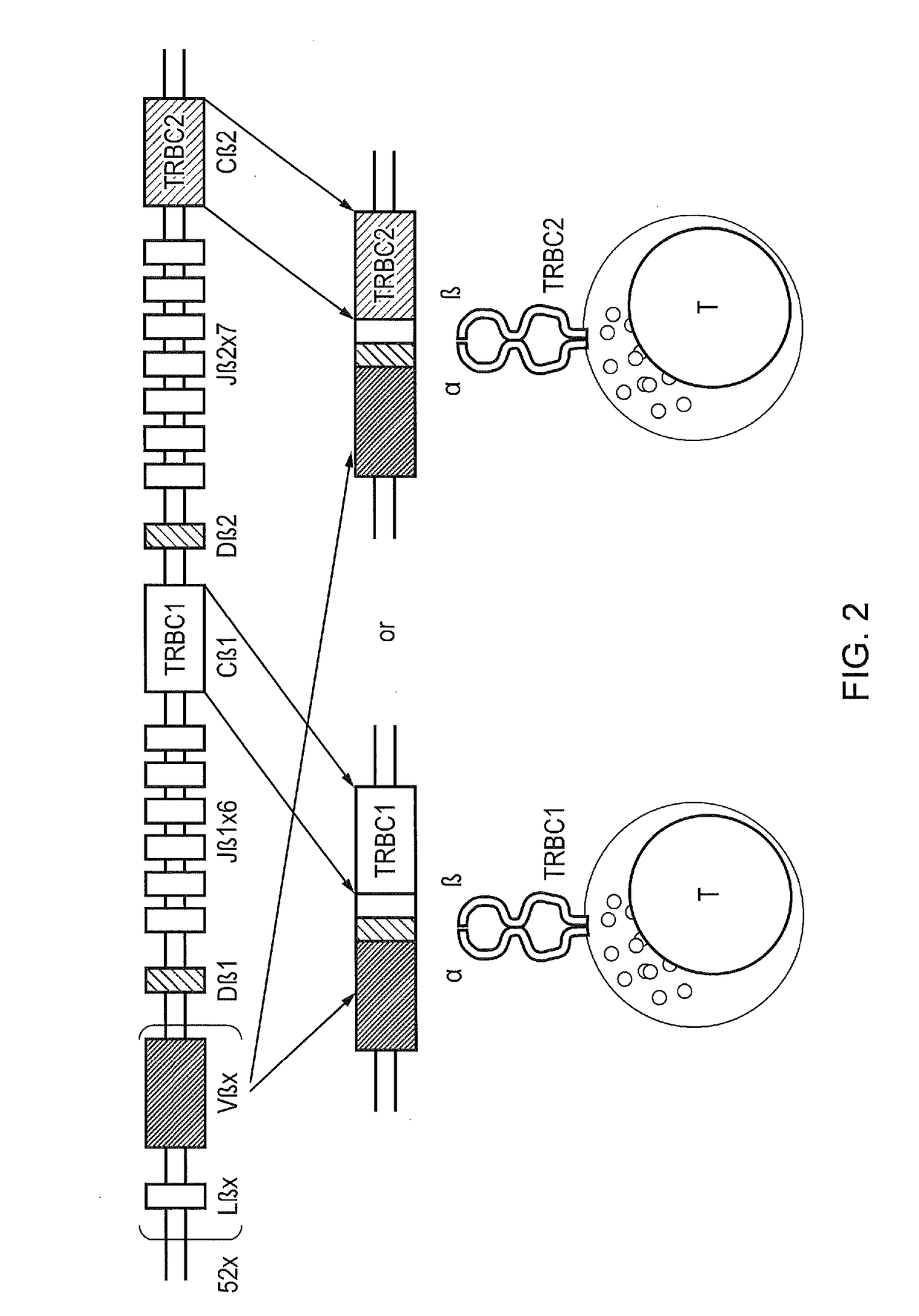
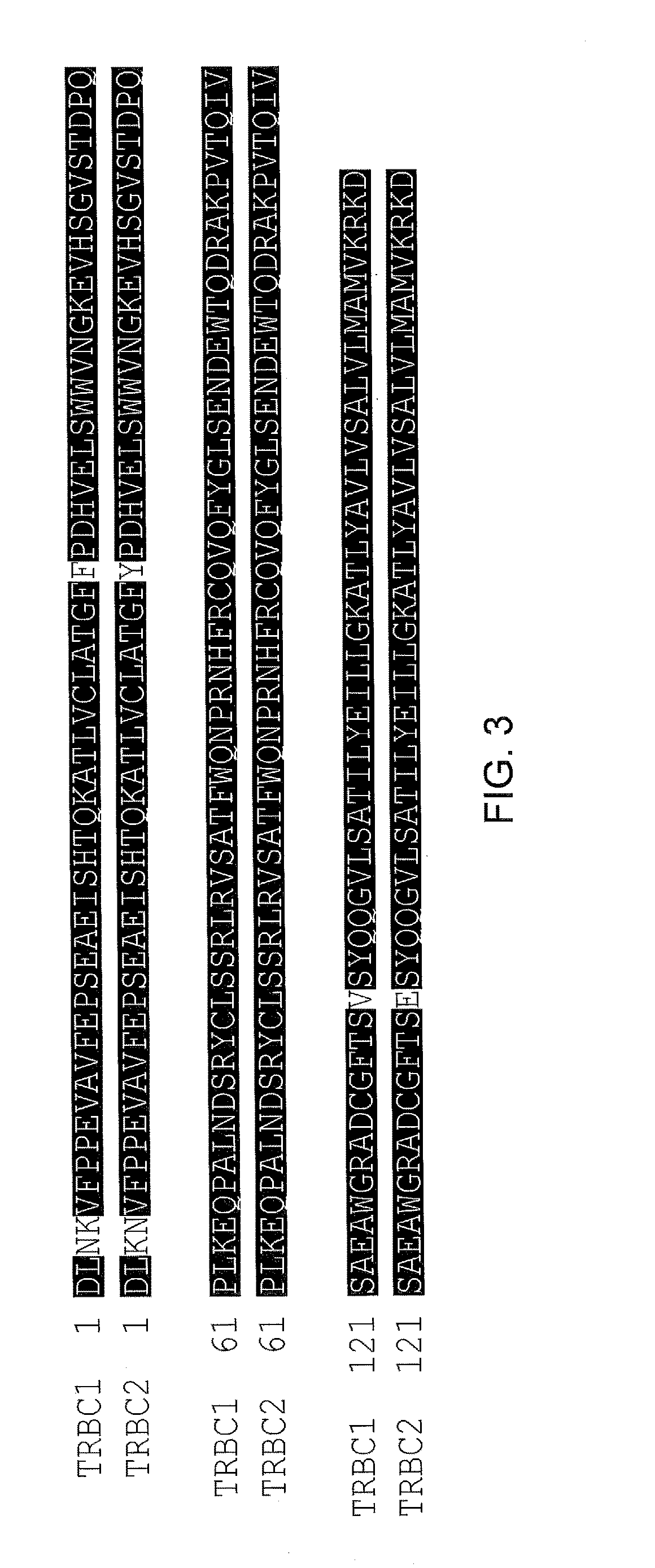
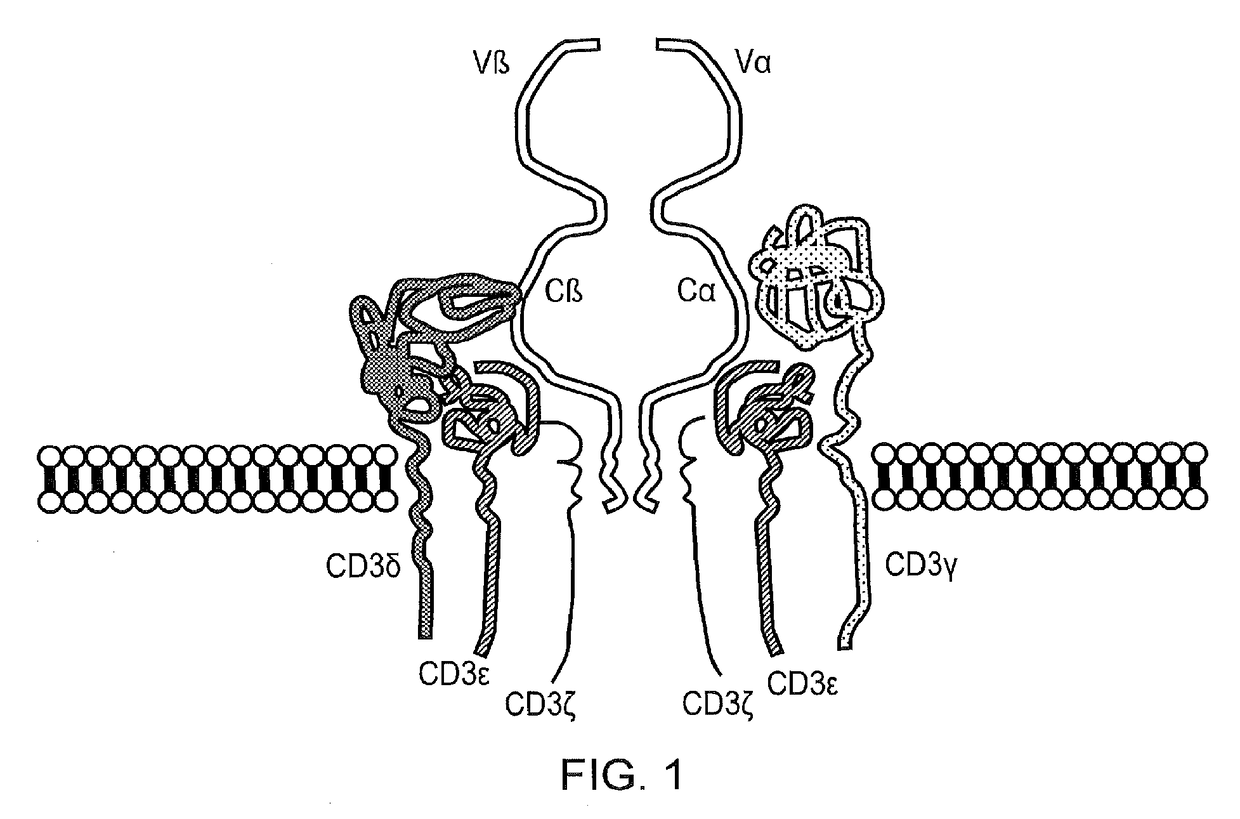
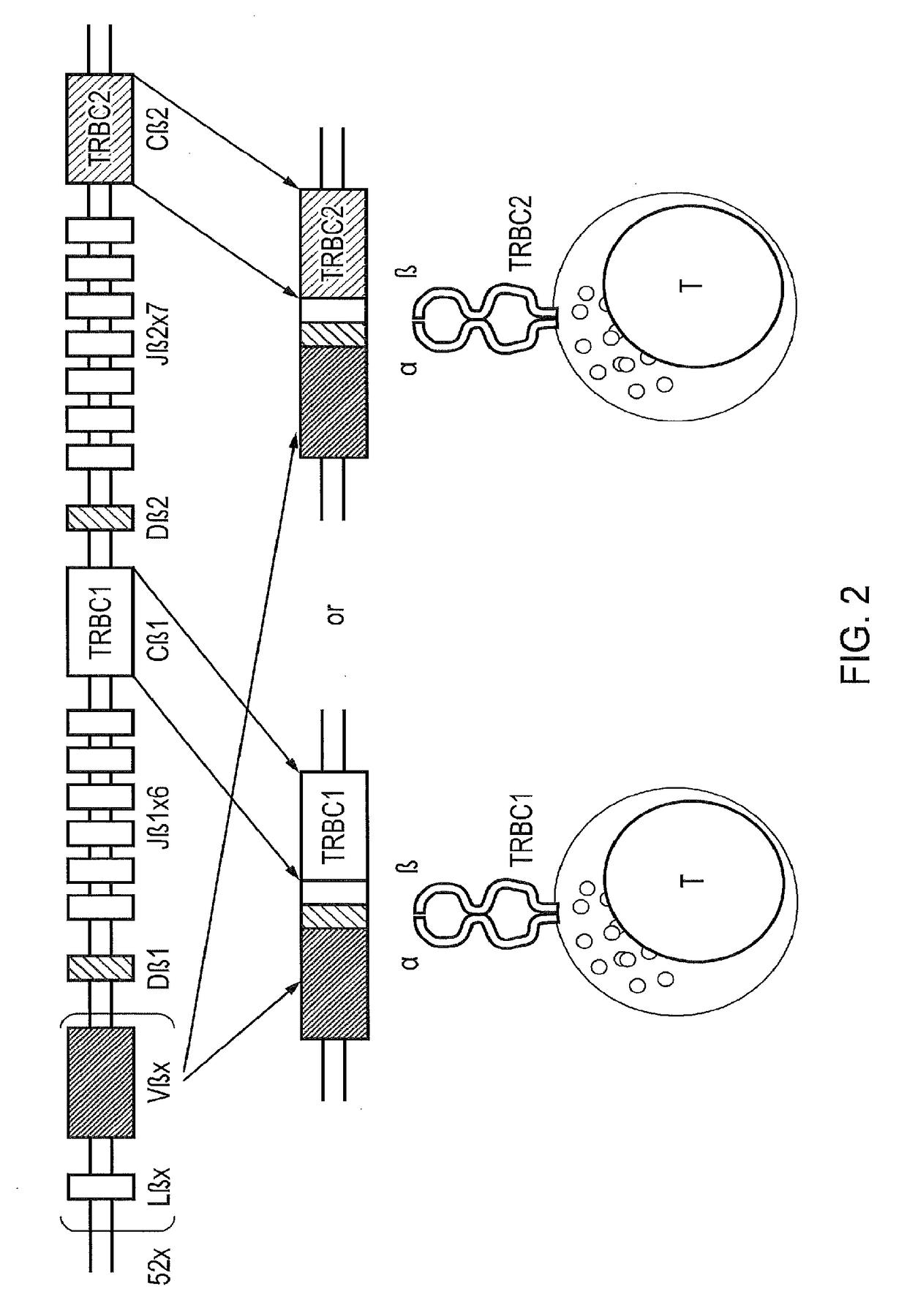
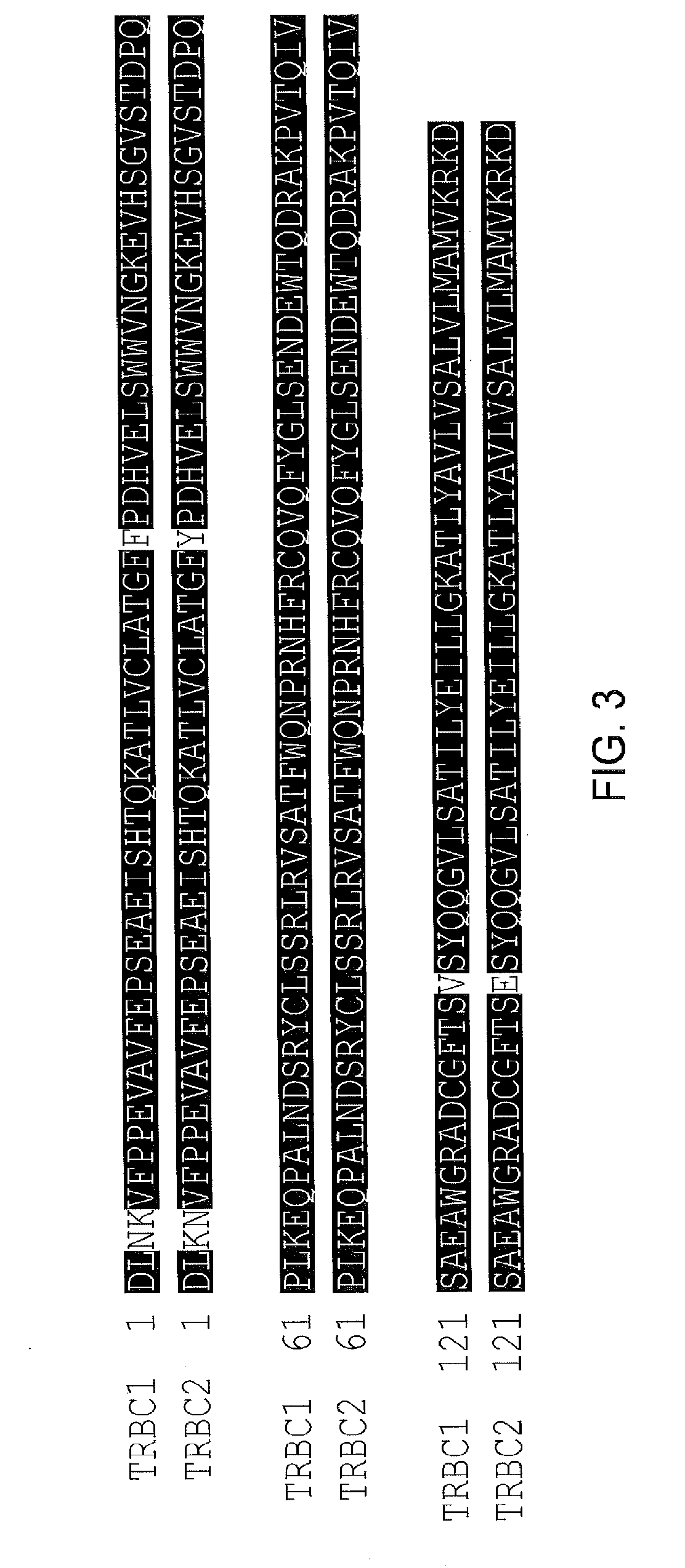
The present disclosure relates to a chimeric antigen receptor (CAR) which comprises an antigen-binding domain which selectively binds TCR beta constant region 1 (TRBC1) or TRBC2; cells; such a T cells comprising such a CAR; and the use of such cells for the treatment of a T-cell lymphoma or leukaemia in a subject.
Owner:AUTOLUS LIMIED
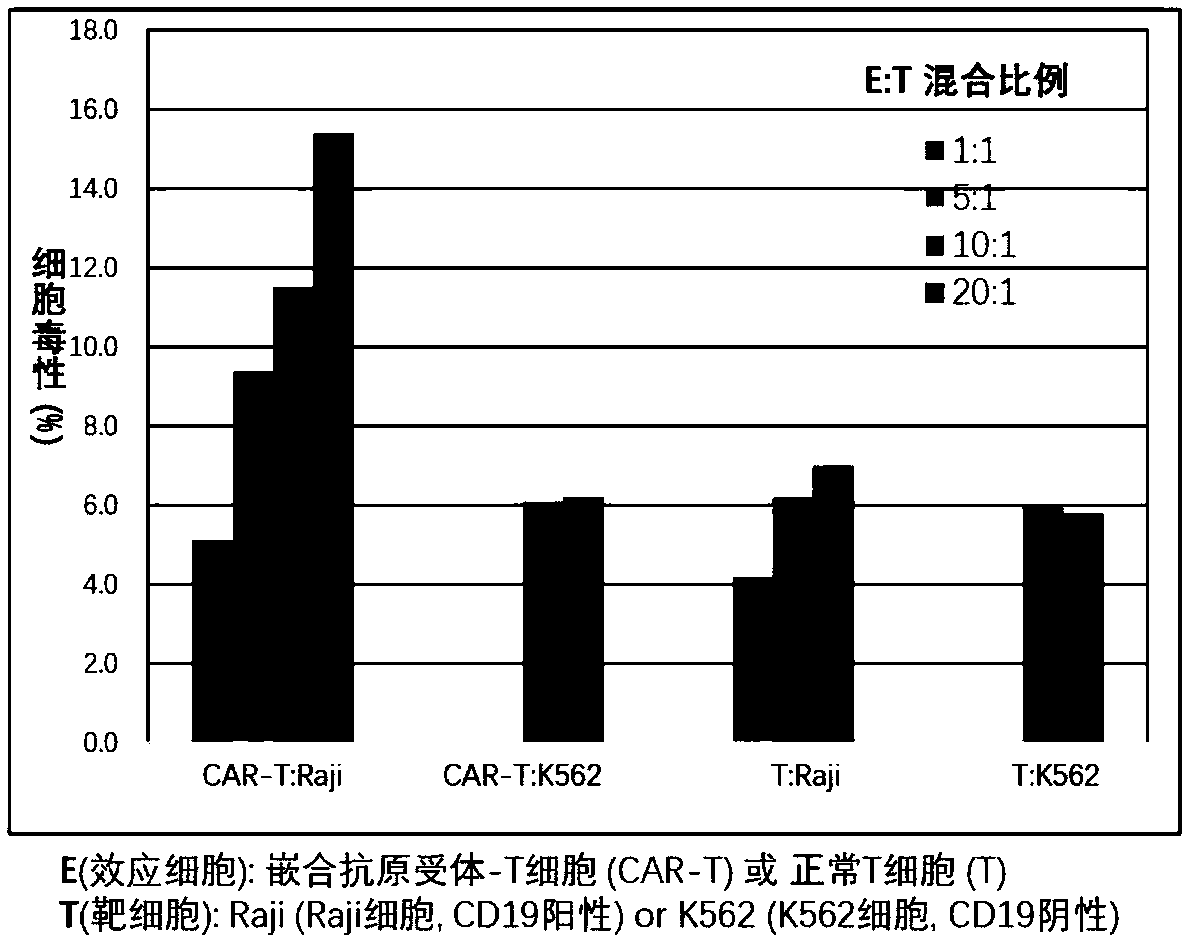
Chimeric antigen receptor T cells targeting CD19, and application of chimeric antigen receptor T cells
InactiveCN108276497AInhibit tumor cell proliferationActivate the killing mechanismMammal material medical ingredientsImmunoglobulinsNH lymphomaInterleukin 2
The invention discloses chimeric antigen receptor T cells targeting CD19, and application of the chimeric antigen receptor T cells. A chimeric antigen receptor for preparing the chimeric antigen receptor T cells comprises interleukin 2 signal peptide, an anti-CD19 single chain antibody, a CD8 protein molecular hinge region, a transmembrane region, an intracellular signal structural domain, and anintracellular signal transduction structural domain of CD3 zeta protein molecules which are sequentially connected in series. The chimeric antigen receptor T cells are used for preparing medicines orpreparations for treating hematological malignancies, wherein the hematological malignancies comprise CD19-positive acute B-lymphocytic leukemia, diffuse large B-cell lymphoma and non-Hodgkin lymphoma.
Owner:英普乐孚生物技术(上海)有限公司
Heterocyclic compounds and their uses
InactiveUS20130079342A1Organic active ingredientsSenses disorderB-cell acute lymphoblastic leukaemiaAutoimmune disease
Substituted bicyclic heteroaryls of the following formulae and compositions containing them, for the treatment of general inflammation, arthritis, rheumatic diseases, osteoarthritis, inflammatory bowel disorders, inflammatory eye disorders, inflammatory or unstable bladder disorders, psoriasis, skin complaints with inflammatory components, chronic inflammatory conditions, including but not restricted to autoimmune diseases such as systemic lupus erythematosis (SLE), myestenia gravis, rheumatoid arthritis, acute disseminated encephalomyelitis, idiopathic thrombocytopenic purpura, multiples sclerosis, Sjoegren's syndrome and autoimmune hemolytic anemia, allergic conditions including all forms of hypersensitivity, The present invention also enables methods for treating cancers that are mediated, dependent on or associated with p110 activity, including but not restricted to leukemias, such as Acute Myeloid leukaemia (AML) Myelo-dysplastic syndrome (MDS) myelo-proliferative diseases (MPD) Chronic Myeloid Leukemia (CML) T-cell Acute Lymphoblastic leukaemia (T-ALL) B-cell Acute Lymphoblastic leukaemia ALL) Non Hodgkins Lymphoma (NHL) B-cell lymphoma and solid tumors, such as breast cancer.
Owner:AMGEN INC
Bispecific antibody capable of resisting B cell lymphoma and application thereof
InactiveCN102250245AHighlighting the role of anti-B-cell lymphomaFungiHybrid immunoglobulinsAntiendomysial antibodiesT lymphocyte
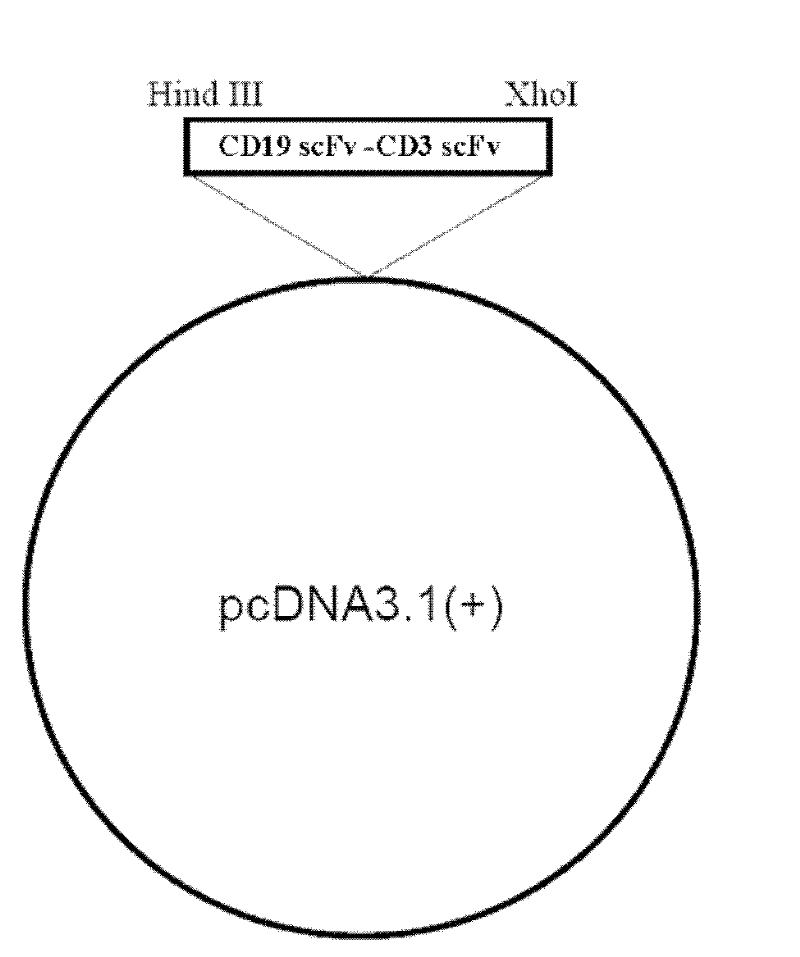
The invention relates to the technical fields of genetic engineering and protein engineering, in particular relates to DNA (deoxyribonucleic acid) for encoding recombinant fusion protein containing human CD19 antibody variable region and human CD3 antibody variable region fragments, fusion protein encoded by DNA, a production method of the fusion protein, pharmaceutical application of the fusion protein and a treatment method using the fusion protein. The invention provides bispecific antibody protein containing human CD19scFv and CD3scFv. The bispecific antibody protein can be combined with positive CD19 and CD3 positive cells, has good bioactivities in vivo and vitro, can activate human T lymphocyte, kill B lymphoma cells, and has good application prospects.
Owner:SICHUAN UNIV
Methods and compositions useful for treating diseases involving bcl-2 family proteins with isoquinoline and quinoline derivatives
InactiveUS20160038503A1BiocideBoron compound active ingredientsAbnormal tissue growthAutoimmune condition
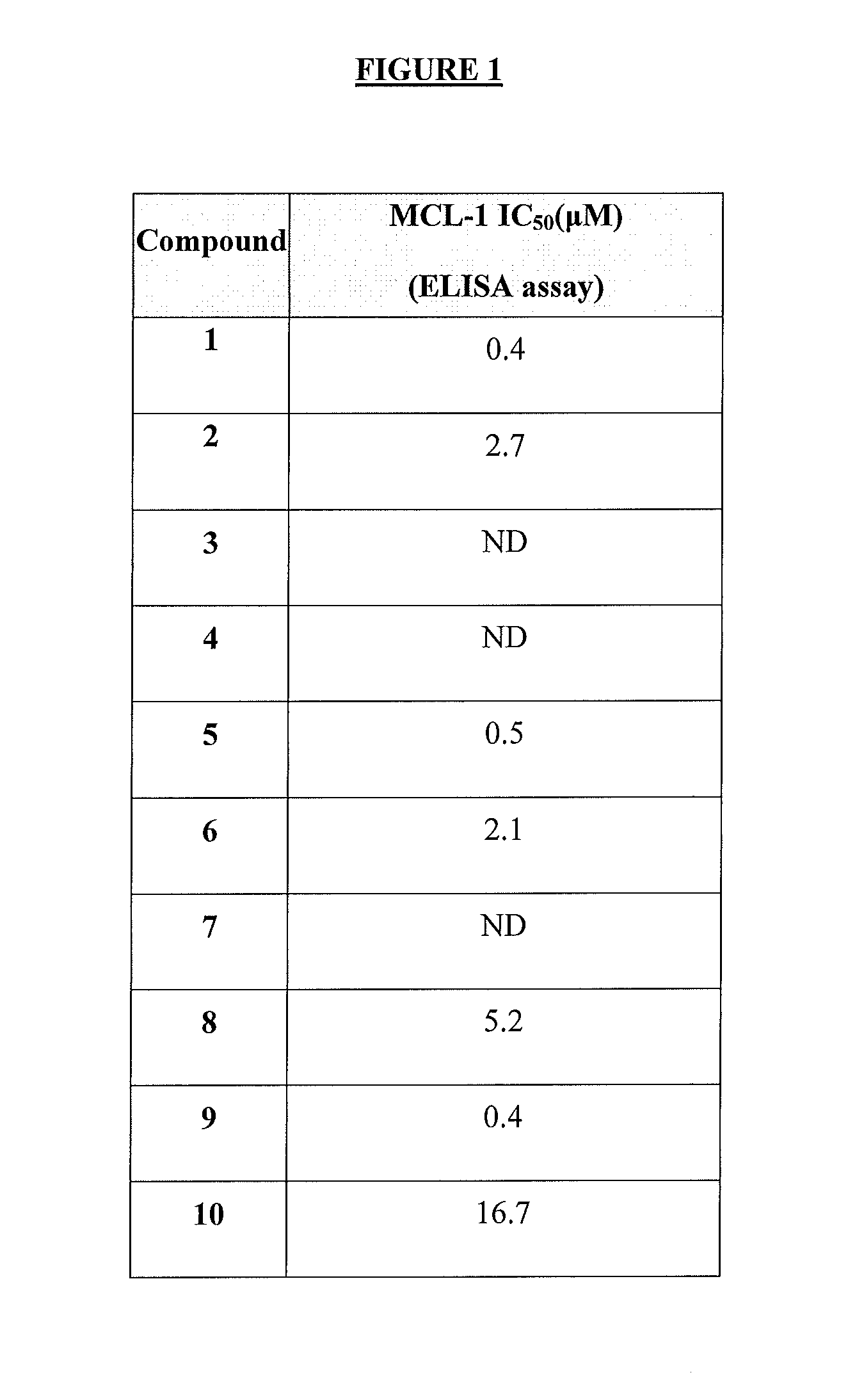
The present invention relates to a compositions for and methods for cancer treatment, for example, hematopoietic cancers (e.g. B-cell Lymphoma). In other aspects, the invention provides methods for treating particular types of hematopoietic cancers, such as B-cell lymphoma, using a combination of one or more of the disclosed compounds and, for example, 26S proteasome inhibitors, such as, for example, Bortezomib. In another aspect the present invention relates to autoimmune treatment with the disclosed compounds. In another aspect, this invention relates to methods for identifying compounds, for example, compounds of the BH3 mimic class, that have unique in vitro properties that predict in vivo efficacy against B-cell lymphoma tumors and other cancers as well as autoimmune disease.
Owner:EUTROPICS PHARMA
Novel therapeutic use of riboside of 5-aminoimidazole-4-carboxamide (acadesine)
InactiveUS20050233987A1Improve toleranceImprove therapeutic potentialBiocideSugar derivativesDiseaseApoptosis
Owner:ADVANCELL ADVANCED IN VITRO CELL TECH
BTK inhibitor and uses thereof
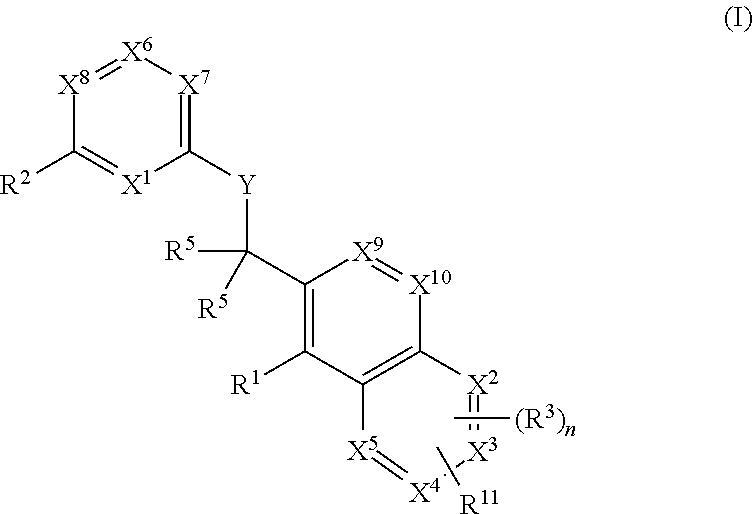
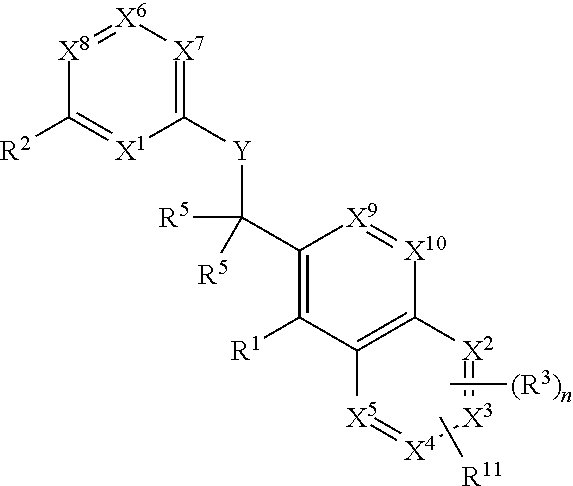
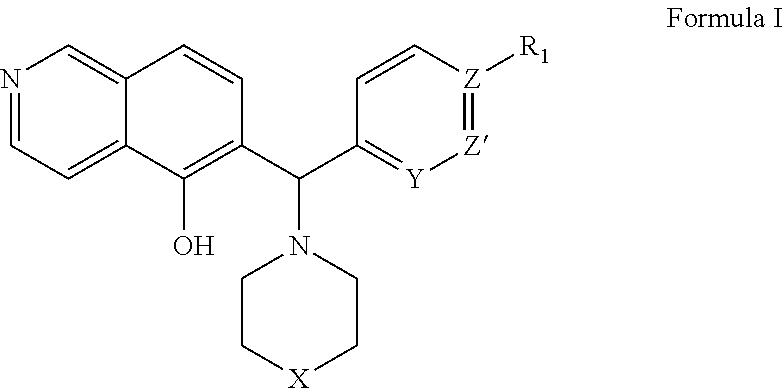
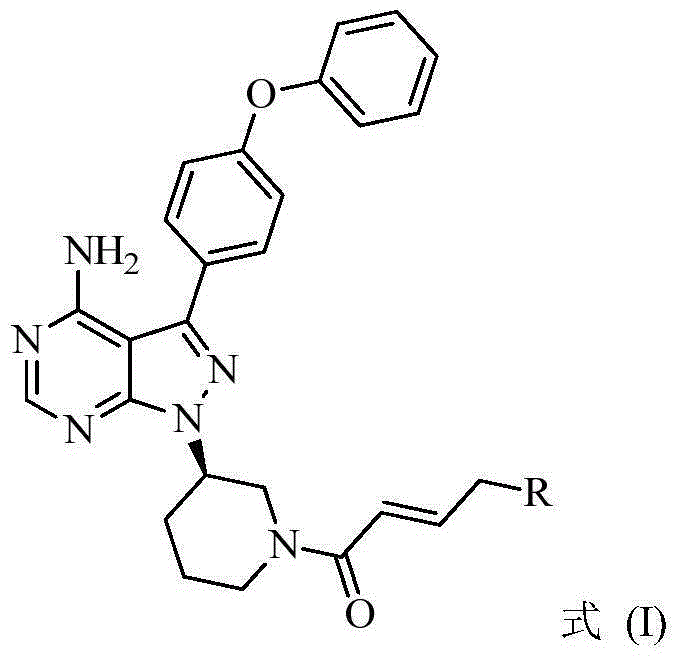
ActiveCN105399756AEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryChronic lymphocytic leukemiaFollicular lymphoma grade II
The present invention provides a BTK inhibitor compound (having s structure represented by a formula (I)) and uses of the BTK inhibitor compound in medicines. According to the present invention, the compound and the pharmaceutical composition can be used for treatment of diffuse large B-cell lymphoma, follicular lymphoma or chronic lymphocytic leukemia. The formula (I) is defined in the specification.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method and application of CIK (Cytokine-induced Killer) modified by anti-human CD19 chimeric antigen receptor
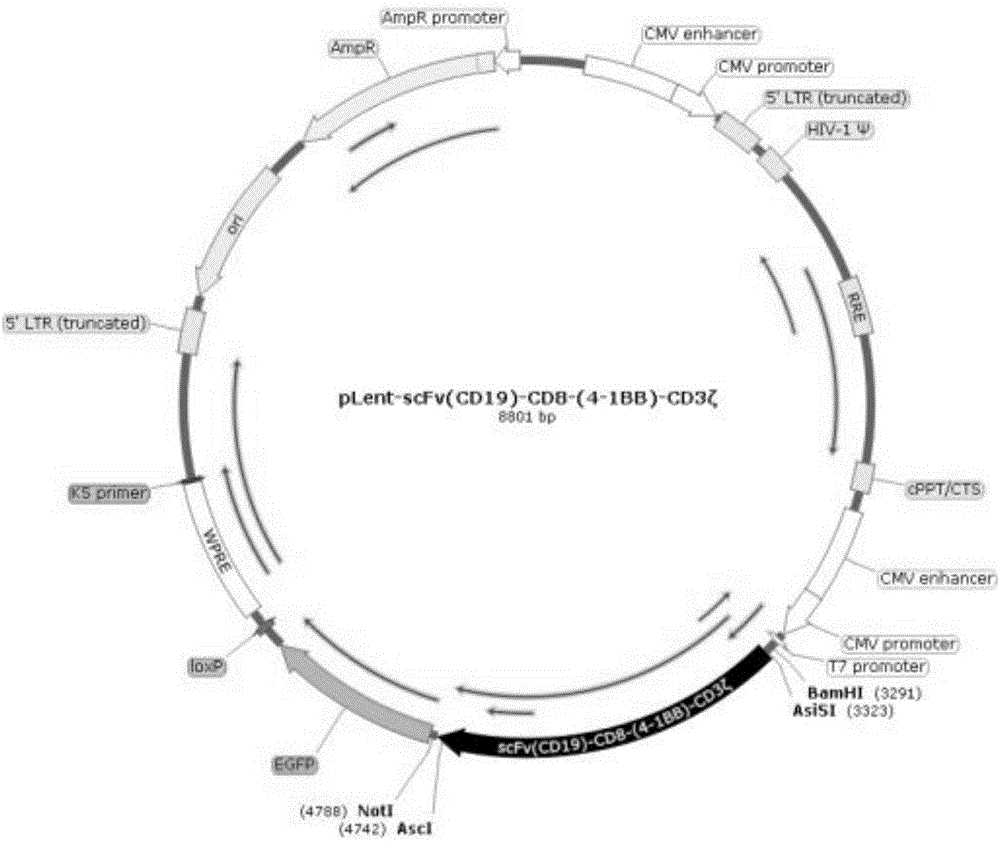
ActiveCN106544365AStrong killing effectProliferation effect is goodGenetically modified cellsMammal material medical ingredientsAntigen receptorLentivirus
The invention discloses a preparation method and application of a CIK (Cytokine-induced Killer) modified by an anti-human CD19 chimeric antigen receptor. The preparation method comprises the steps of coding a fusion gene segment of the chimeric antigen receptor scFv (CD19)-CD8-(4-1BB)-CD3 zeta; inserting the fusion gene segment into a lentiviral expression vector; packaging into a lentivirus carried with a scFv (CD19)-CD8-(4-1BB)-CD3 zeta coding gene; and infecting the CIK induced by autologous lymphocytes of a patient on the lentivirus carried with the scFv (CD19)-CD8-(4-1BB)-CD3 zeta coding gene. The CIK modified by the chimeric antigen receptor scFv (CD19)-CD8-(4-1BB)-CD3 zeta can be applied in treatment of B cell lymphoma leukemia.
Owner:SHANDONG XINRUI BIOTECH CO LTD
Chimeric antigen receptor (CAR) with antigen binding domains to the t cell receptor beta constant region
InactiveUS20170334998A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorT-Cell Receptor Beta
The present disclosure relates to a chimeric antigen receptor (CAR) which comprises an antigen-binding domain which selectively binds TCR beta constant region 1 (TRBC1) or TRBC2; cells; such a T cells comprising such a CAR; and the use of such cells for the treatment of a T-cell lymphoma or leukaemia in a subject.
Owner:AUTOLUS LIMIED
Methionine aminopeptidase-2 inhibitors and methods of use thereof
InactiveUS7348307B2Low toxicityInhibit methionine aminopeptidase 2BiocideDipeptide ingredientsMethionine aminopeptidaseBiochemistry
Owner:GLAXO SMITHKLINE LLC
Application of chlorogenic acid in preparing drugs for prevention and treatment of primary cutaneous T-cell lymphoma
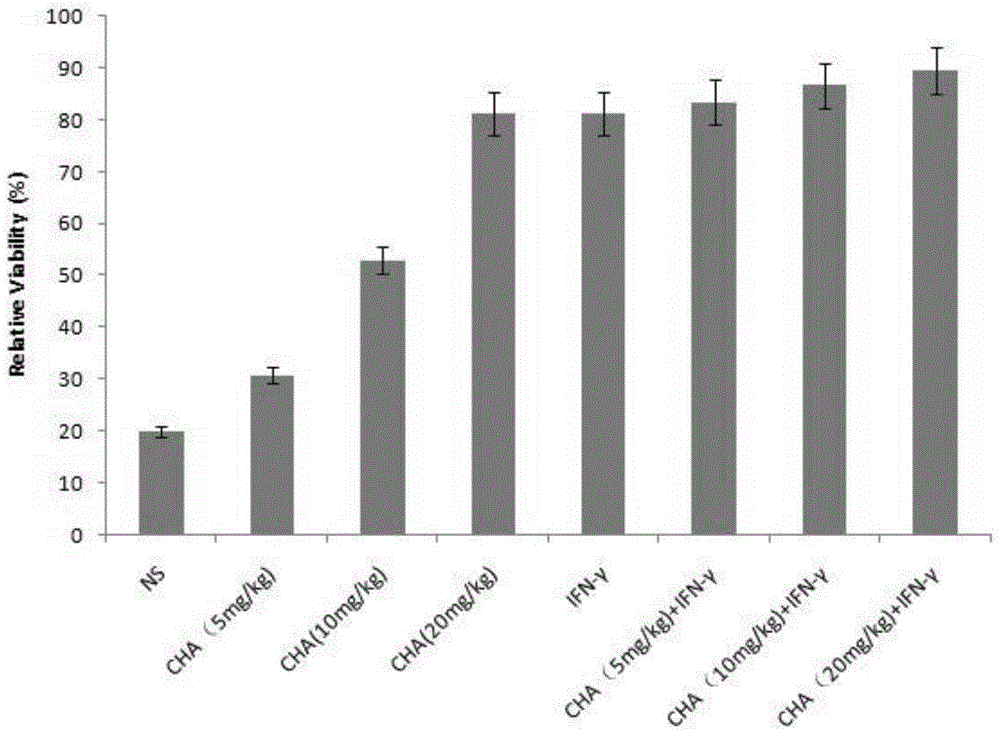
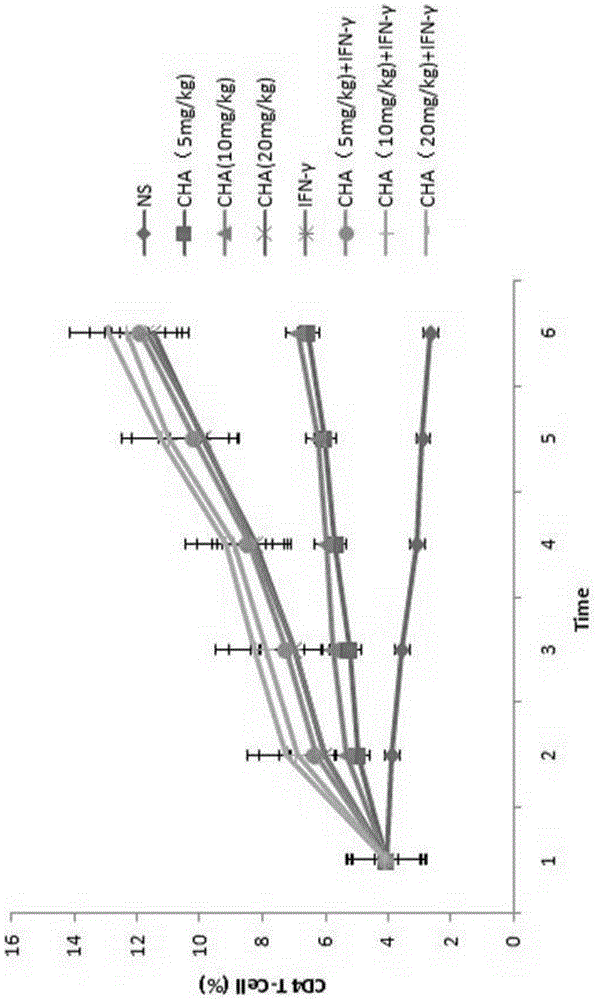
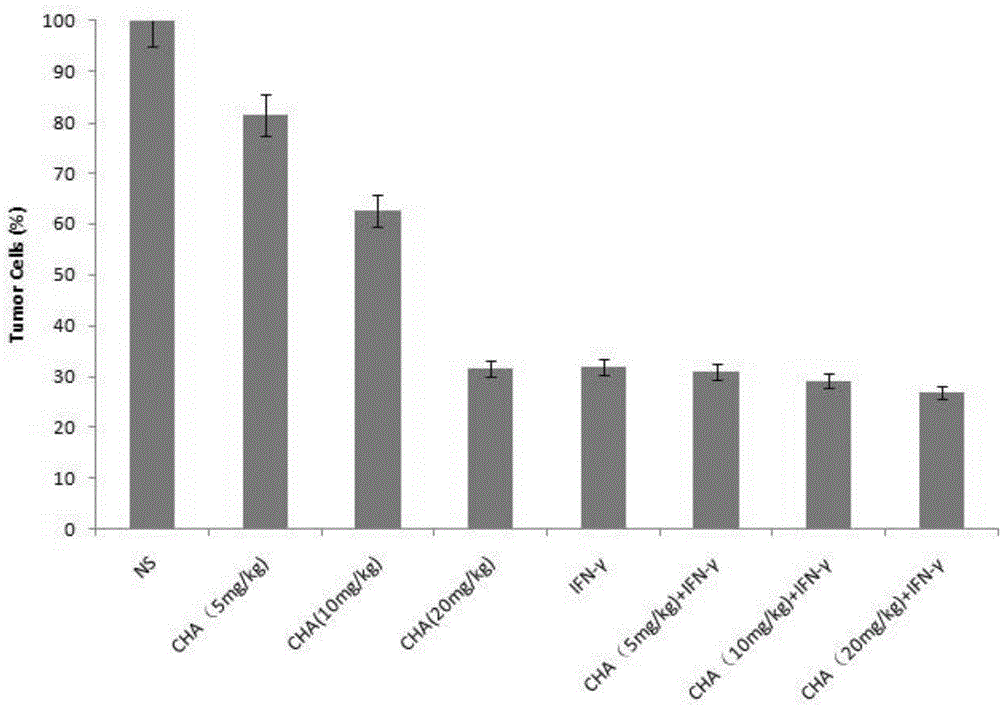
ActiveCN104622864APromote proliferationPrevent proliferationOrganic active ingredientsPeptide/protein ingredientsChlorogenic acidPrimary cutaneous T-cell lymphoma
The invention aims to provide an application of chlorogenic acid in preparing drugs for prevention and treatment of primary cutaneous T-cell lymphoma. Experiments show that the chlorogenic acid can activate CD4 T-lymphocytes and CD8 T-lymphocytes, and prevent and treat primary cutaneous T-cell lymphoma by taking the CD4 T-lymphocytes and the CD8 T-lymphocytes as targets. According to the invention, the chlorogenic acid is adopted for promoting the proliferation of T-lymphocytes of a body and activating the T-lymphocytes so as to achieve the purpose of treating primary cutaneous T-cell lymphoma. Meanwhile, the chlorogenic acid also can inhibit the proliferation of primary cutaneous T-cell lymphoma cells, and the inhibition effect of the chlorogenic acid is similar to the inhibition effect of interferon.
Owner:SICHUAN JIUZHANG BIO TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com