Radiolabeling kit and binding assay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Radioincorporation--Kits and Assays
[0341] I. Summary One objective of the present invention was to devise radiolabeling kit protocols for preparation of .sup.111In and .sup.90Y-labeled 2B8-MX-DTPA (In2B8 and Y2B8, respectively) and to establish release specifications for clinical products. The radiolabeling kit protocols are reproducible with respect to radioincorporation and binding to antigen-positive SB cells and indicate the suitability of the radiolabeling kit for use in the clinical trials. It is recommended that In2B8 and Y2B8 release specifications for radioincorporation and binding be established at .gtoreq.95% and .gtoreq.70%, respectively.
[0342] II. Introduction
[0343] A .sup.90Y-labeled murine monoclonal anti-CD20 antibody (Y2B8) is currently being evaluated in clinical trials for the treatment of relapsed B-cell lymphoma. The yttrium isotope lacks a gamma component making it unsuitable for imaging systems. Therefore, .sup.111In-labeled 2B8-MX-DTPA (In2B8) will be used to...
example 2
Radioincorporation and Binding--Kits and Assays
[0510] I. Summary
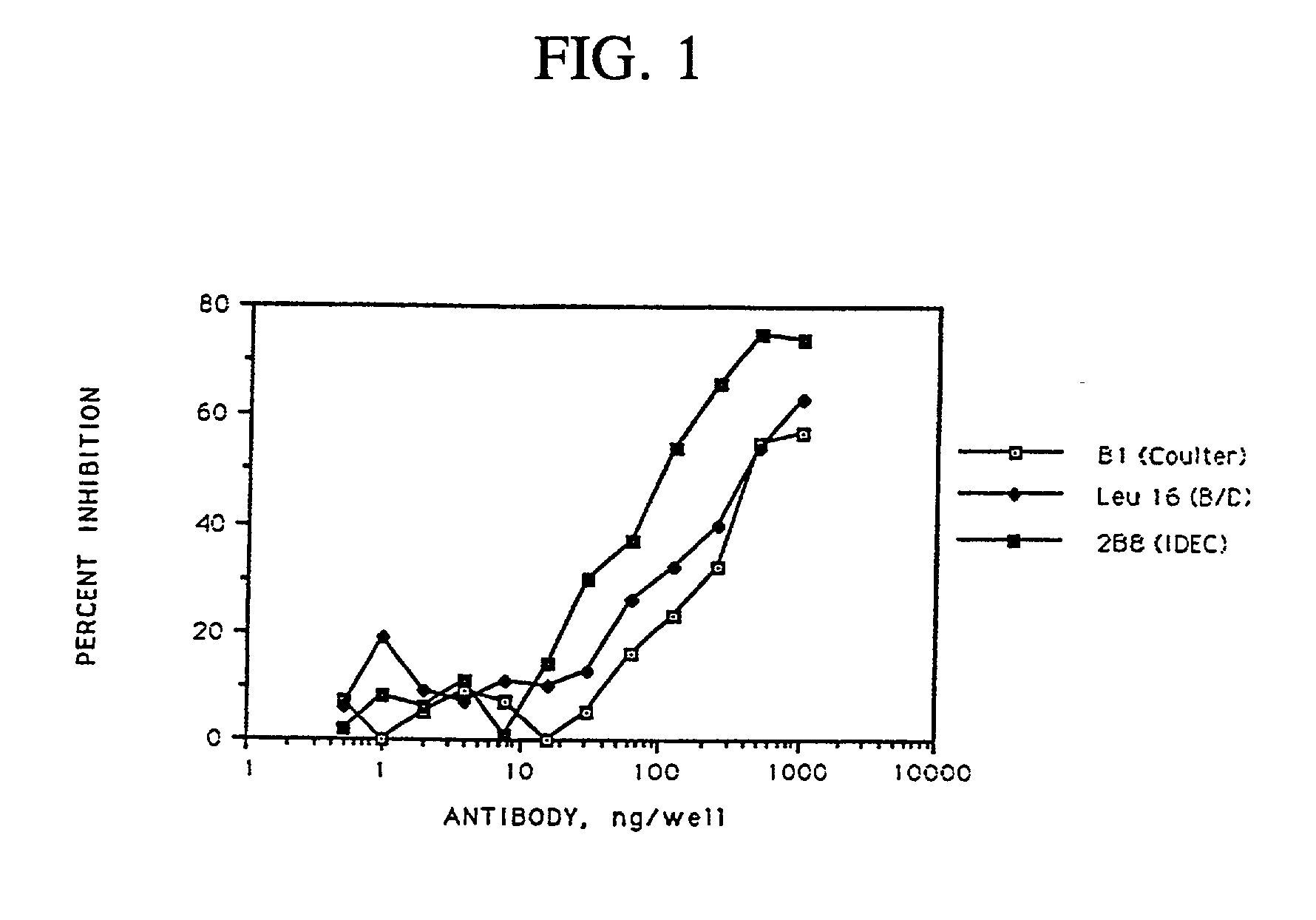
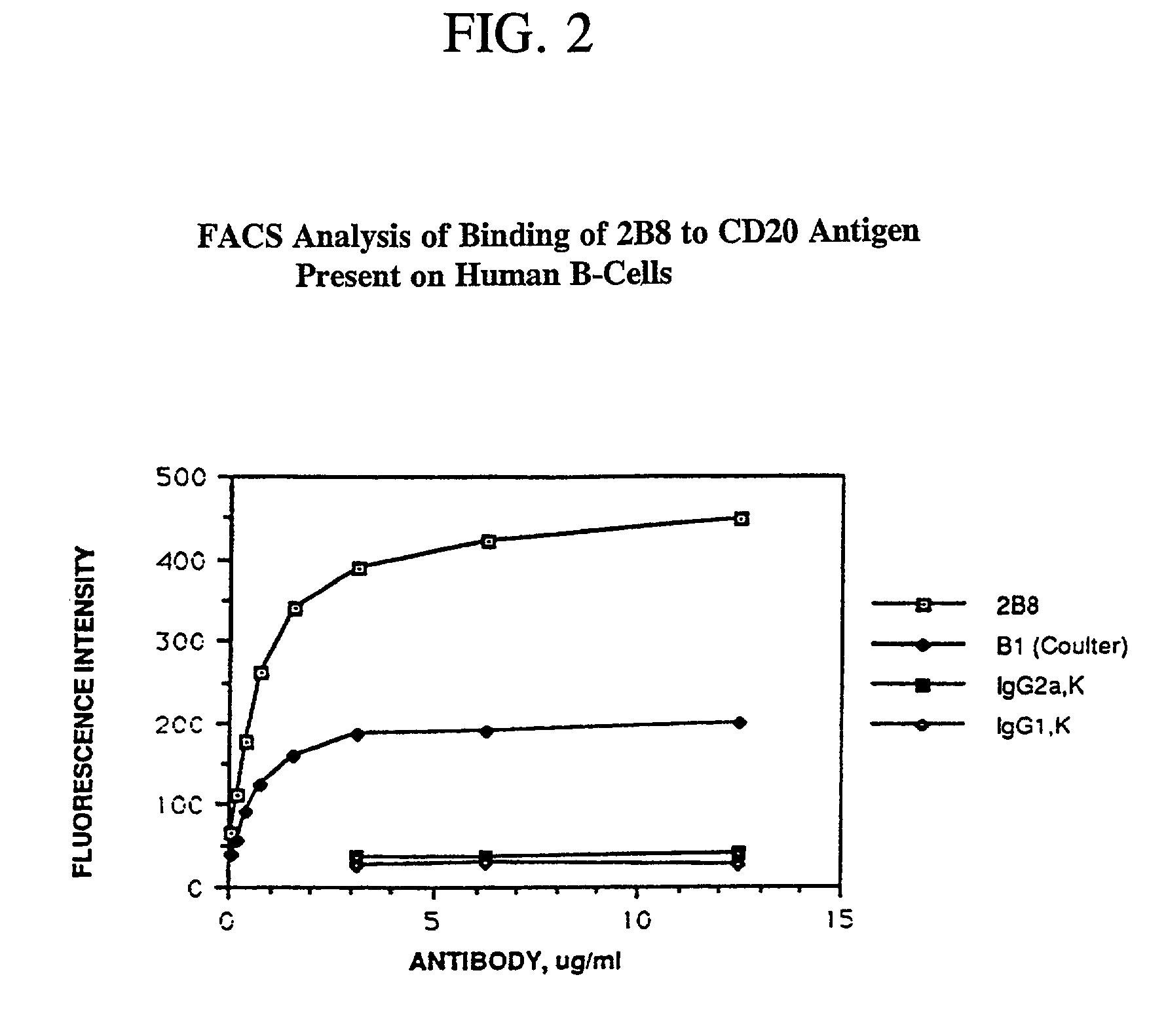
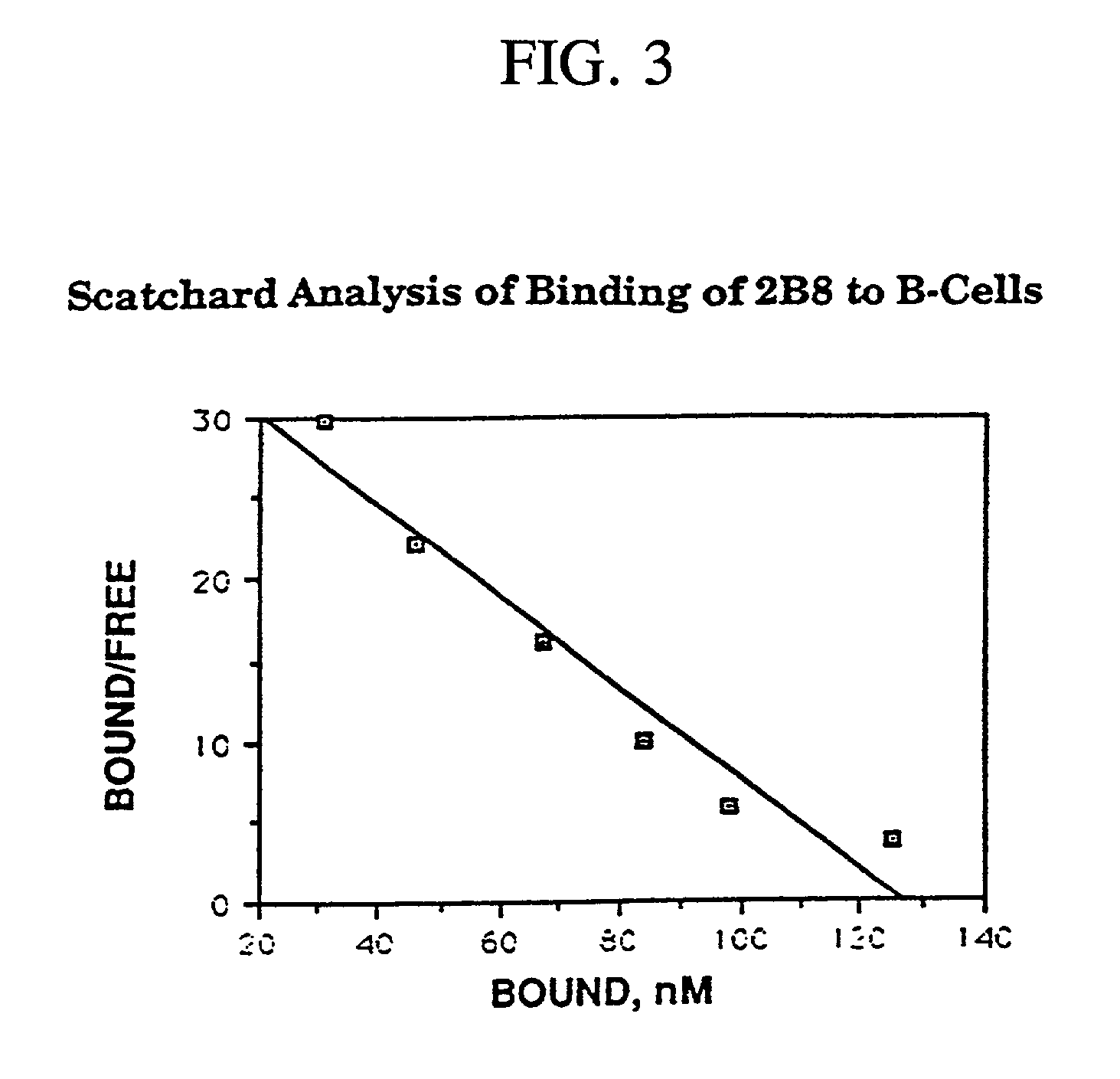
[0511] The murine anti-CD20 monoclonal antibody designated 2B8 has been cloned in CHO cells to yield a high expression cell line. Specificity of the CHO-derived antibody for CD20-positive human cells was demonstrated by FACS analysis and competitive binding. Negligible binding was observed to human T-cells. The affinity of the antibody for CD20-positive cells was determined to be 1.3.times.10.sup.-10 M using a competitive binding assay. The antibody was reacted with the chelating agent MX-DTPA to form a conjugate, 2B8-MX-DTPA, with negligible loss of immunoreactivity (affinity value was 4.4.times.10.sup.-10 M. Optimal chelator conjugation, as determined by measuring radioincorporation of .sup.111In, was achieved after eight hours reaction. Radiolabeling protocols for 2B8-MX-DTPA were optimized for .sup.90Y or .sup.111In with respect to pH and incubation time to insure maximal radioincorporation (>95%) and retention of i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com