Chimeric antigen receptor targeting CD22 and application of chimeric antigen receptor
A chimeric antigen receptor, targeting technology, applied in the field of biomedicine, can solve the problems of recurrence or ineffectiveness, poor killing effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Example 1: Cloning of CD22 scFv in antigen recognition region in chimeric antigen receptor
[0075] 1. Extract the total RNA of the mouse anti-human CD22 monoclonal antibody hybridoma cell line: at 5×10 6 Add 1ml of RNAiso Plus (Takara) to the cells, and mix well by pipetting. Add 200 μl of chloroform, invert up and down, and vortex to mix. Centrifuge at 12000 rpm for 5 minutes at 4°C. Pipette the supernatant into a 1.5ml EP tube, add the same volume of isopropanol, and mix by gently inverting up and down. Centrifuge at 12000 rpm for 15 minutes at 4°C. Pre-cool 75% ethanol to precipitate RNA at 4°C, and dissolve total RNA in 50 μl DEPC water.
[0076] 2. Synthesize the first strand of cDNA by reverse transcription: Prepare the PCR reaction system (20 μl) as follows: Oligo d(T)15Primers: 2 μl; M-MLV (200u / μl): 1 μl; dNTP (each 2.5mM): 1 μl; DTT ( 0.1M): 2μl; First strand buffer (5×): 4μl; CD20-RNA: 2μg; DEPC water: make up to 20μl. Reaction conditions: 37°C, 60 min...
Embodiment 2
[0091] Example 2: Construction of Chimeric Antigen Receptor Vector
[0092] 1. Digest the plasmid containing the CD8α-4-1BB-CD3ζ fragment with BamH I and EcoR I endonucleases to obtain the CD8α-4-1BB-CD3ζ fragment, the amino acid sequence of which is shown in SEQ ID NO.5. The plasmid containing the CD8α-4-1BB-CD3ζ fragment can be prepared by any suitable method in the prior art.
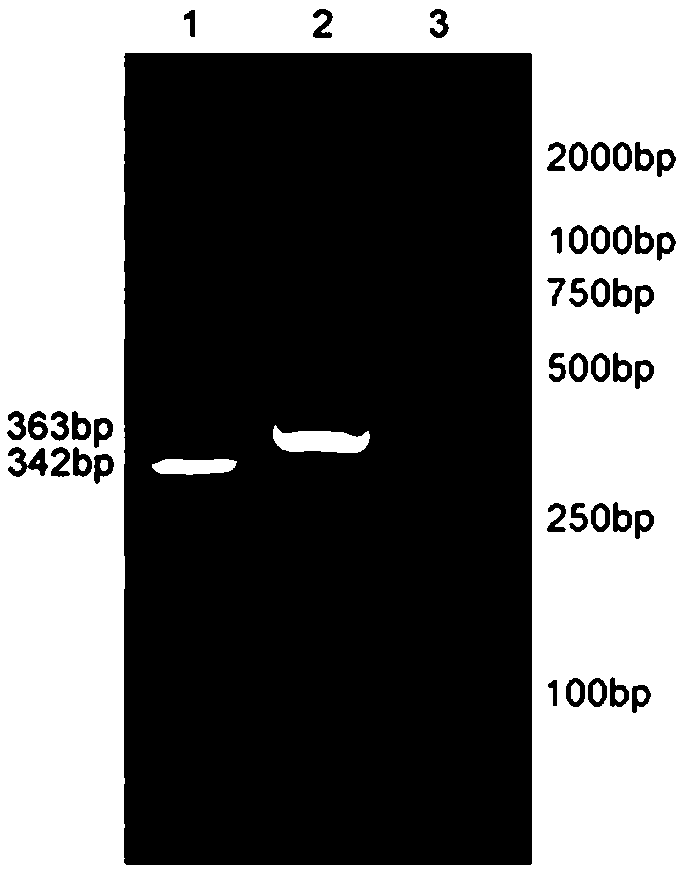
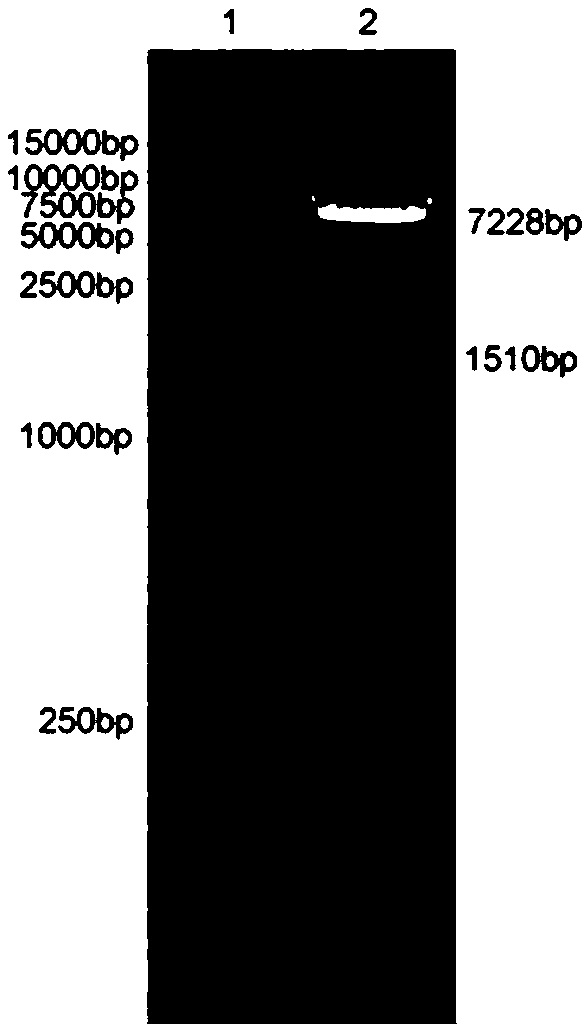
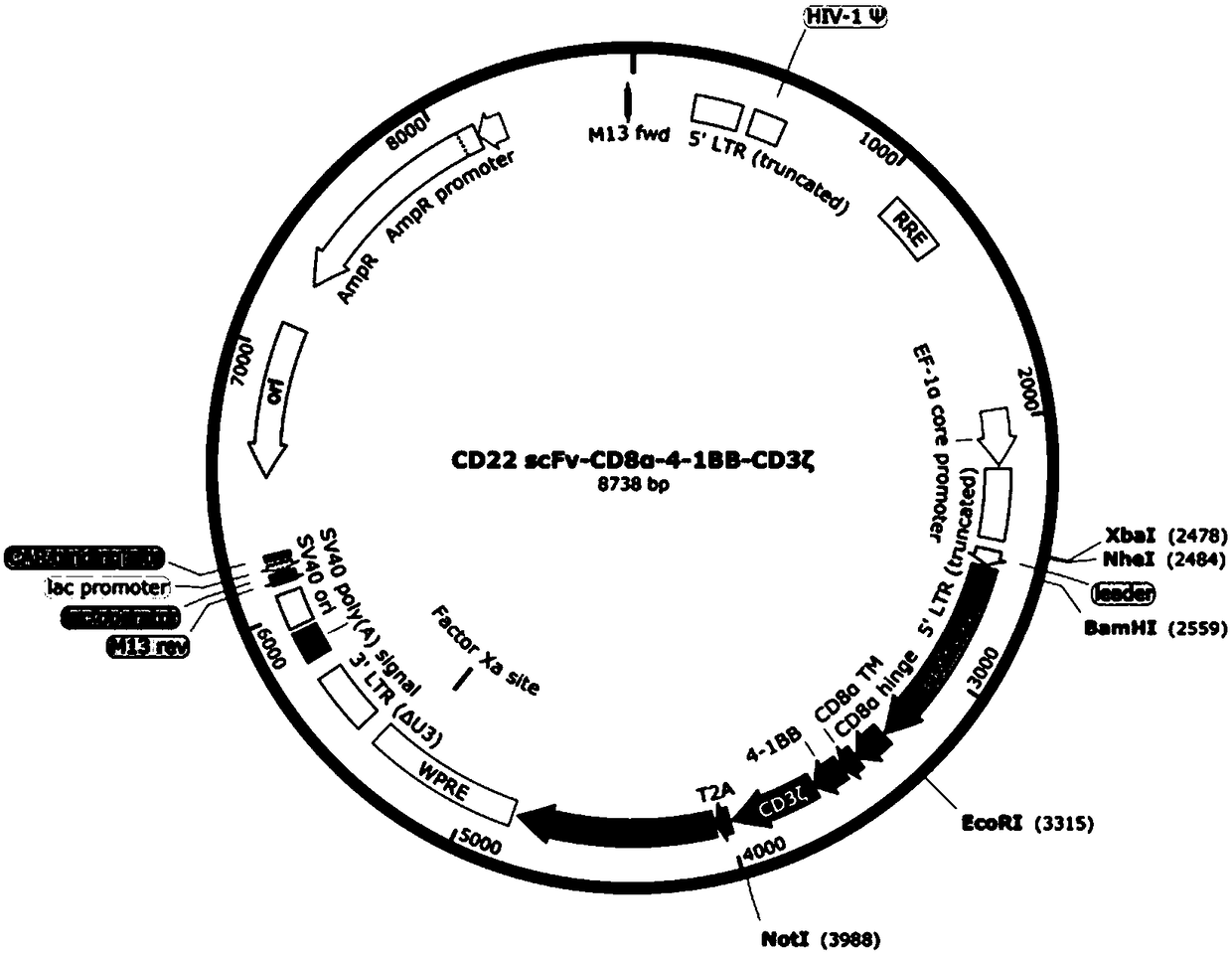
[0093] 2. Ligate the CD22 scFv fragment obtained in Example 1 with the destination vector, and identify the constructed CD22 scFv-CD8α-4-1BB-CD3ζ CAR destination vector with XbaI and NotI. The result is as figure 2 As shown, the enzyme digestion results showed that the positive clone contained the target band and was correctly identified by sequencing. The schematic diagram of the carrier is as image 3 shown.
Embodiment 3
[0094] Example 3: Preparation of chimeric antigen receptor CD22 scFv-CD8α-4-1BB-CD3ζ lentivirus modified T cells
[0095] 1. The CD22scFv-CD8α-4-1BB-CD3ζ expression plasmid and packaging plasmid psPAX2 and pMD.2G were extracted using the EndoFree Plasmid Maxi Plasmid Extraction Kit (QIAGEN Company). The three plasmids were transfected with PEI transfection reagent (polyscience company) at a ratio of 4:3:1 (see the instructions of PEI transfection reagent for specific methods). Replace the fresh culture medium 12 hours after transfection, collect the virus supernatant 24 hours and 48 hours later, centrifuge at 4°C, 3000rpm for 15 minutes, filter through a 0.45μm filter, and use 50000g, 4°C, 1.5 hours after ultracentrifugation Concentrate 10 times, then transfer to -80°C for storage.
[0096]2. Preparation of T cells: Take 10 ml of fresh healthy human peripheral blood, and use RosetteSep T cell enrichment Cocktail (Stemcell) and Ficoll-Paque PLUS (GE Healthcare) to extract T ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com