Methods and compositions useful for treating diseases involving bcl-2 family proteins with isoquinoline and quinoline derivatives
a family protein and bcl-2 technology, applied in the field of methods and compositions for treating cancer and autoimmune diseases, can solve the problems of blocking the sensitivity of tumor cells to cytostatic or apoptosis inducing drugs, poor survival prognosis of patients with cll, and high cost of treatmen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
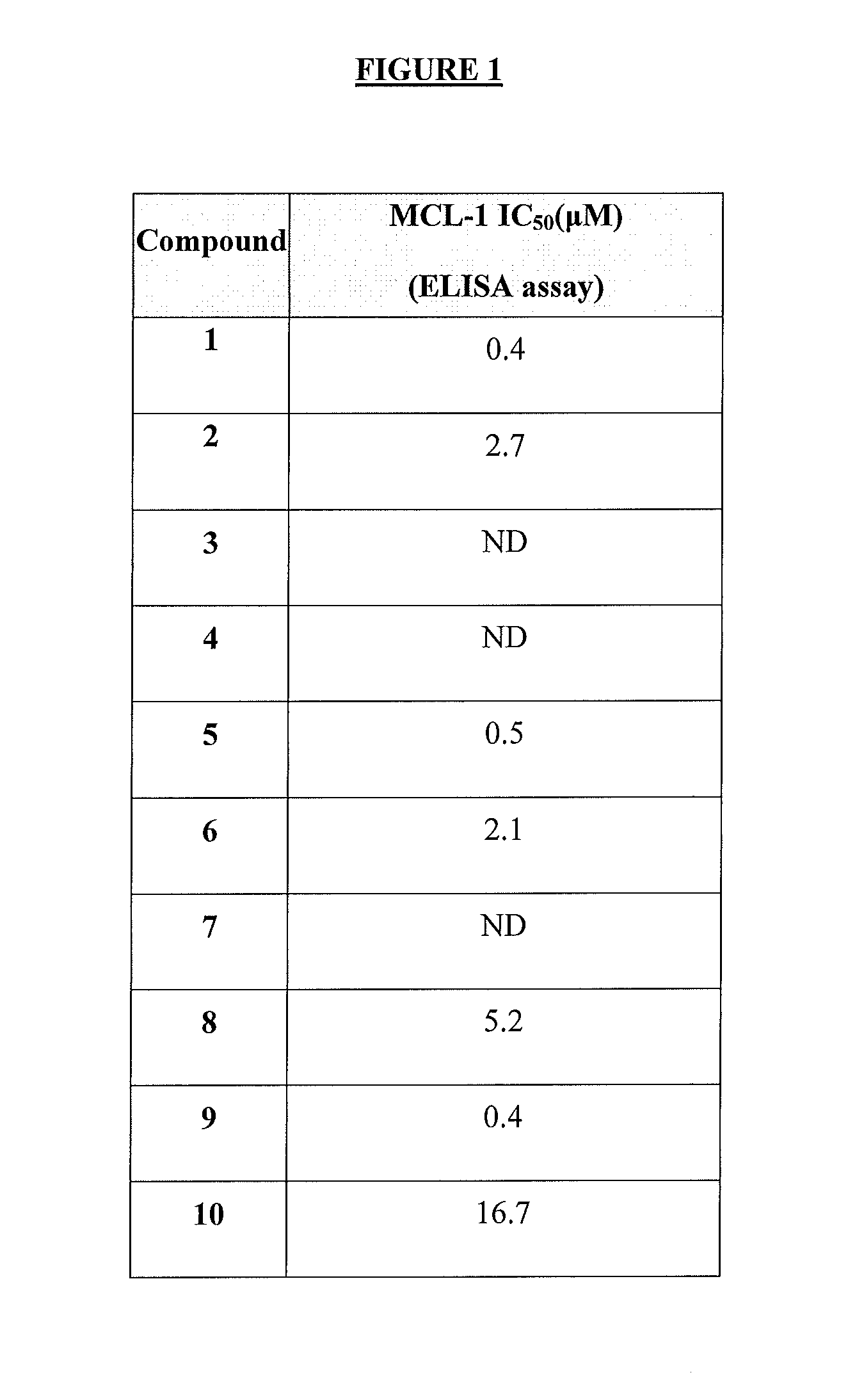
Inhibition of Mcl-1 by Compounds of Formula I or Formula II; Measurement by ELISA Assay
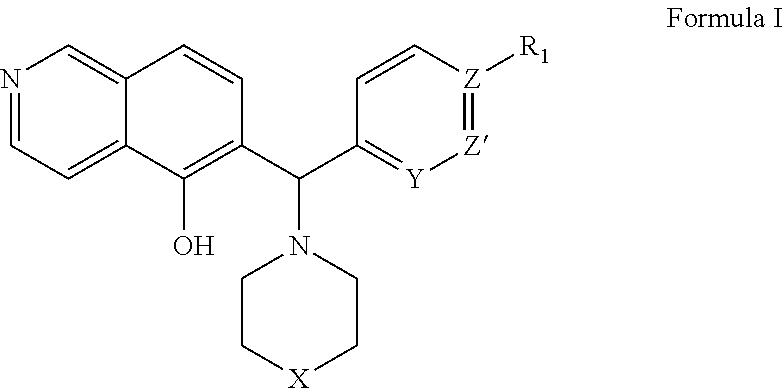
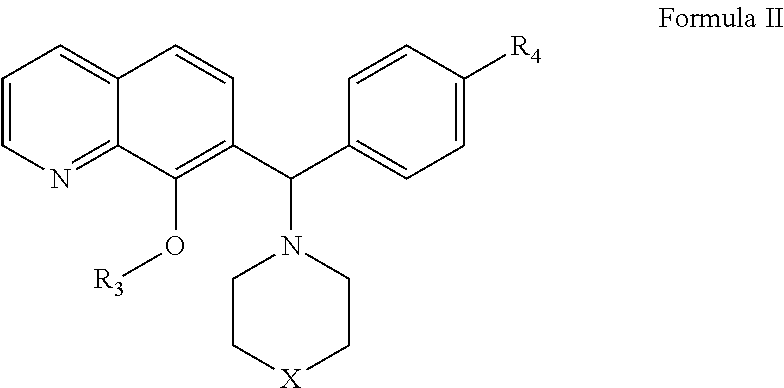
[0253]The expression level of Mcl-1 correlates directly to chemo-sensitivity and survival of certain non-Hodgkin's lymphomas (Petlickovsk, et al. (2005) Blood 105(12): 4820-7) as well as prostate cancer (Royuela, et al. (2001) Eur. Cytokine Netw. 12(4): 654-63), liver cancer (Fleischer, et al. (2006) Int. J. Oncol. 28(1): 25-32) and other cancers. Mcl-1 is therefore an ideal target for treating these cancers. This example shows that the BH3 mimic compounds of Formula I or Formula II inhibit the binding of the BH3 domain of the Bcl-2 family protein Bim to Mcl-1. Accordingly, this example indicates that compounds of Formula I or Formula II are effective in treating certain hematological malignancies that are affected principally by the Bcl-2 family protein Mcl-1.
Materials and Methods
[0254]An ELISA-like streptavidin plate assay was used to demonstrate the activity of the BH3 mimic compounds of Formul...
example 2
Inhibition of Mcl-1 and Bcl-xL by Compounds of Formula I or II; Measurement by Fluorescence Polarization Assay
[0259]In this example, a Fluorescence Polarization (FP) assay is used to demonstrate the activity of the Mcl-1 inhibitors described in Formulas I or II in inhibiting Mcl-1 as well as Bcl-xL binding to Bim BH3 as described in Degterev et al. (2001) Nature Cell Biology 3: 173-182.
[0260]A nineteen amino acid peptide, corresponding to the BH3 domain of Bim, with the sequence FITC-GGGIAQELRRIGDEFNAY (SEQ ID NO: 1) is labeled with the fluorophore FITC according to the manufacturer's instructions (Molecular Probes, Eugene, Oreg.). This sequence is identified as being able to bind to purified Bcl-xL protein (Sattler et al. (1997) Science 275(5302): 983-86) and to have biological activity in cells (Holinger et al. J. Biol. Chem. 274: 13298-1330).
[0261]In addition, recombinant GST-Mcl-1 and GST-Bcl-xL fusion proteins are generated in E. coli and purified using glutathione-sepharose be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com