Patents
Literature
150 results about "Meninges" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
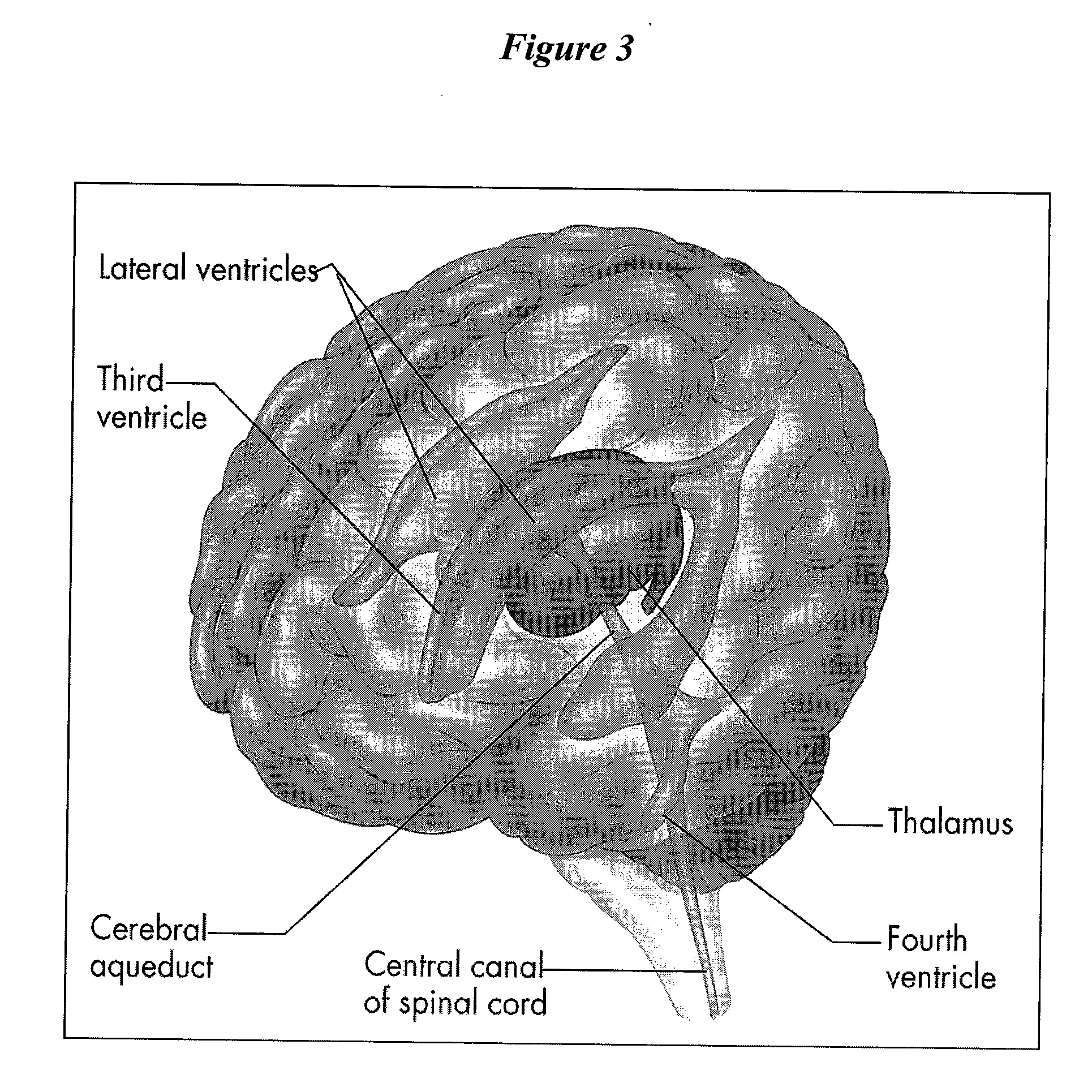
The meninges (/məˈnɪndʒiːz/, singular: meninx (/ˈmiːnɪŋks/ or /ˈmɛnɪŋks/), from Ancient Greek: μῆνιγξ, romanized: mēninx, lit. 'membrane', adjectival: meningeal /məˈnɪndʒəl/) are the three membranes that envelop the brain and spinal cord. In mammals, the meninges are the dura mater, the arachnoid mater, and the pia mater. Cerebrospinal fluid is located in the subarachnoid space between the arachnoid mater and the pia mater. The primary function of the meninges is to protect the central nervous system.
Intrathecal administration of rituximab for treatment of central nervous system lymphomas
InactiveUS20020009444A1Prevent intermolecular disulfide formationPromote recoveryBiocidePeptide/protein ingredientsMeningesImmunocompromised patient
This invention describes methods of using anti-B cell antibodies, preferably anti-CD20 antibodies, and most preferably Rituximab, to treat B cell lymphomas of the brain, especially primary central nervous system lymphomas (PCNSLs), and to prevent meningeal relapse. The antibodies can be administered intrathecally alone, or in combination with other chemotherapeutics, such as methotrexate, or other anti-B cell antibodies to treat PCNSL in both immunocompromised and non-immunocompromised patients. These antibodies can also be used to diagnose patients with CNS lymphoma, especially in immunocompromised patients.
Owner:BIOGEN INC
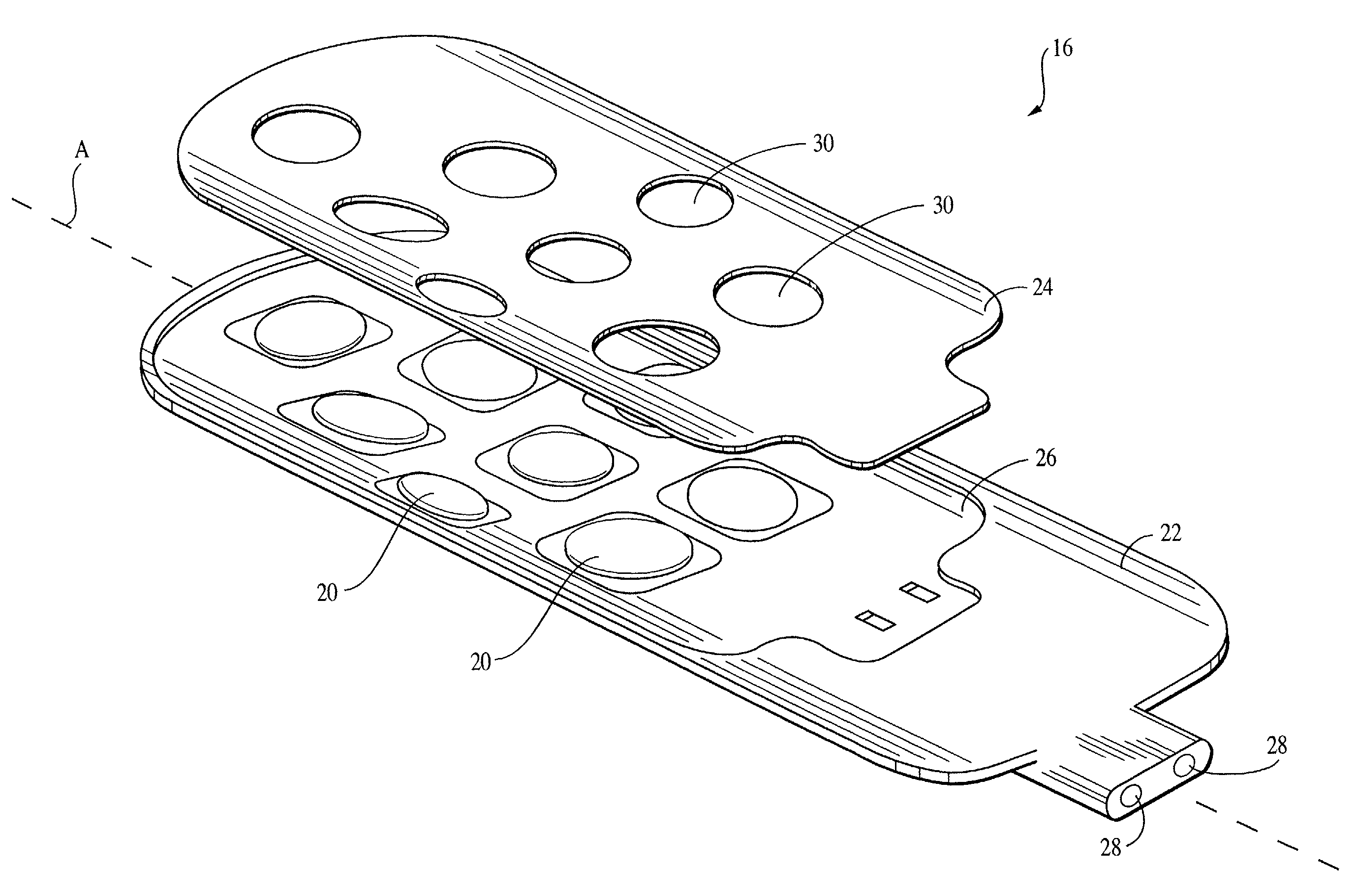
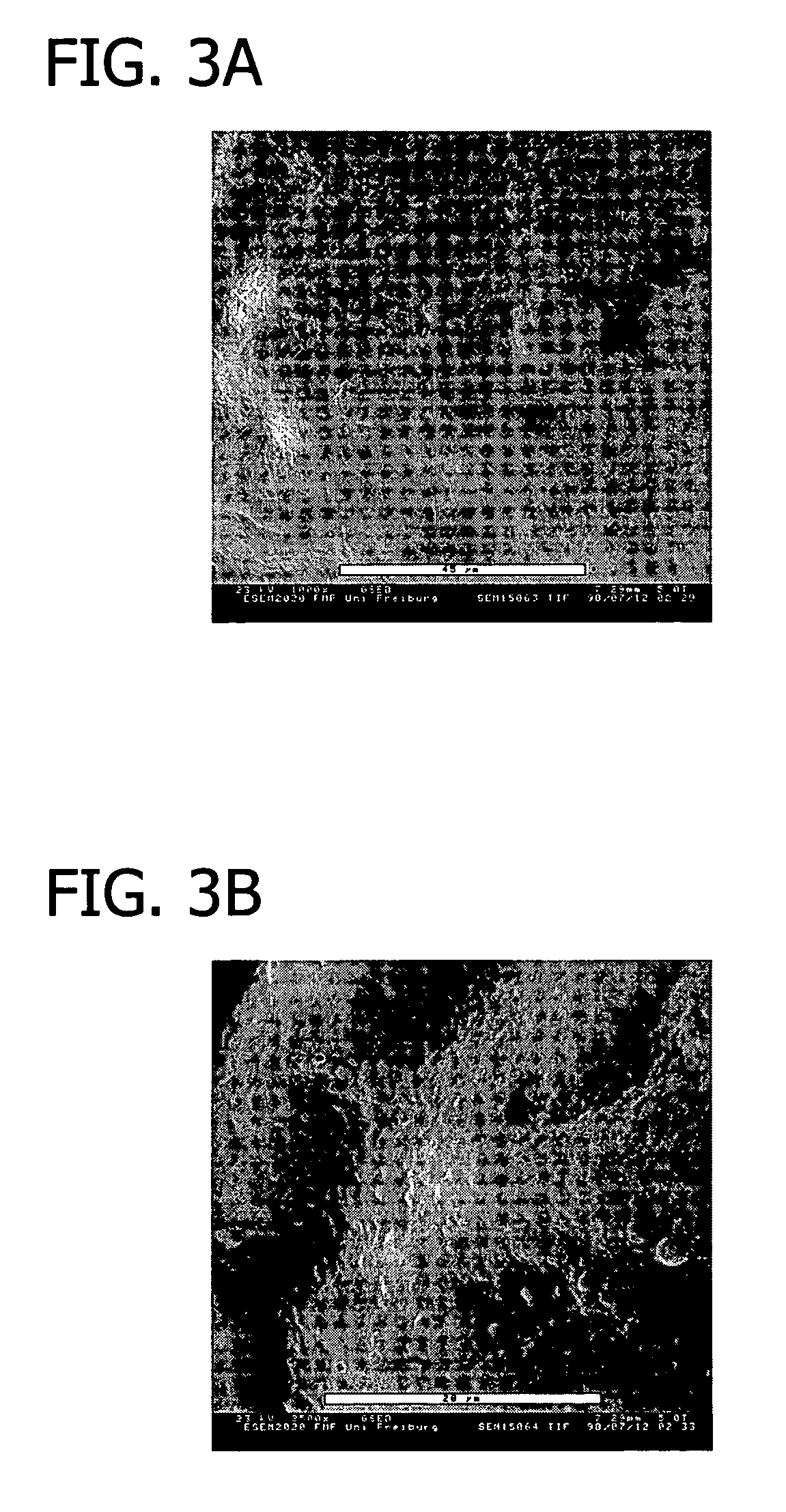
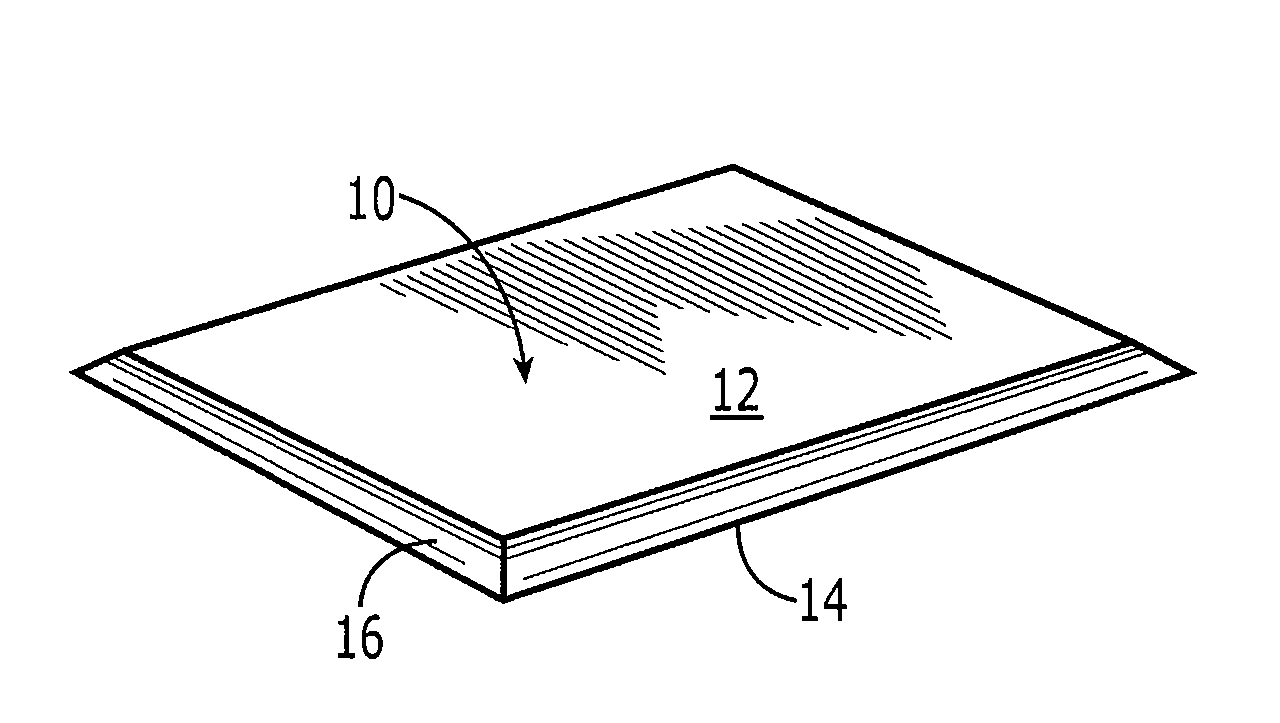
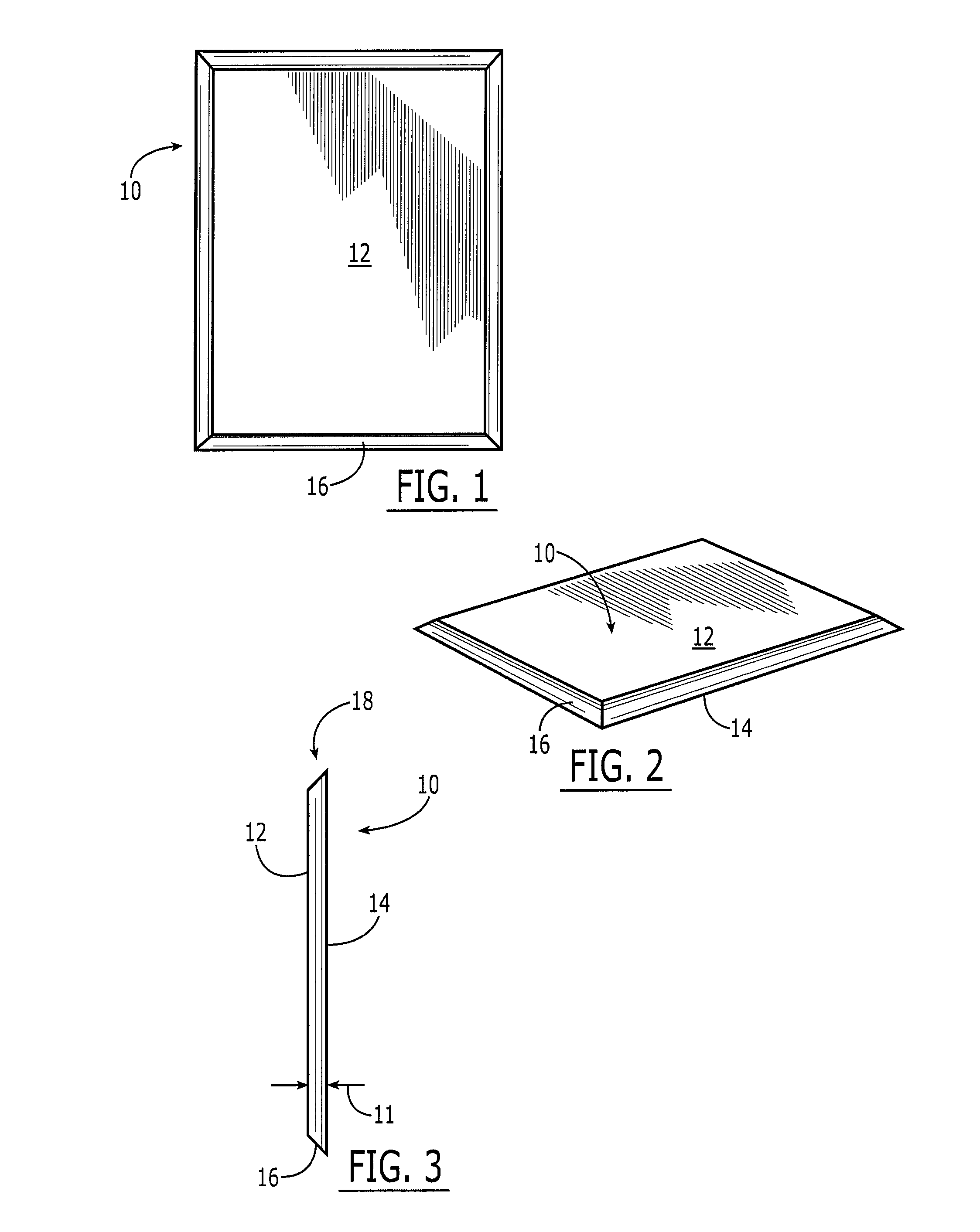
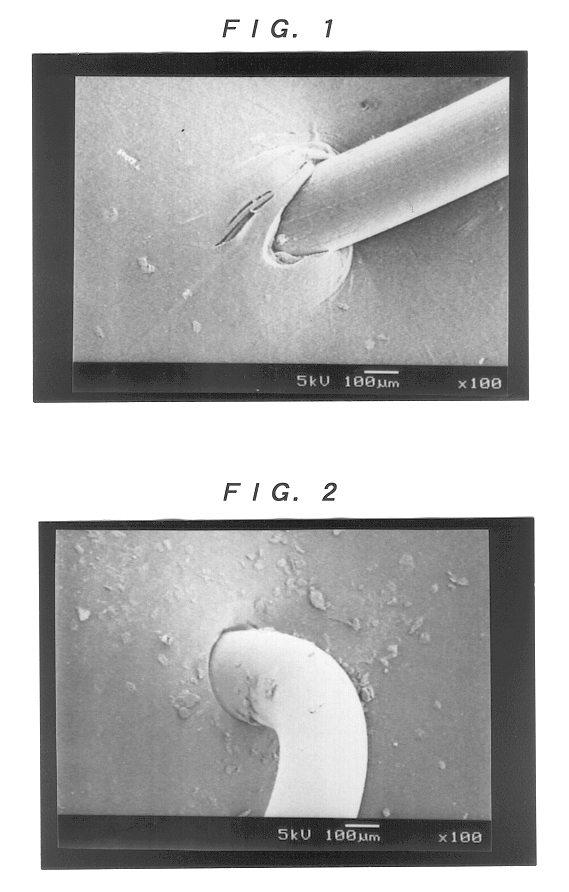
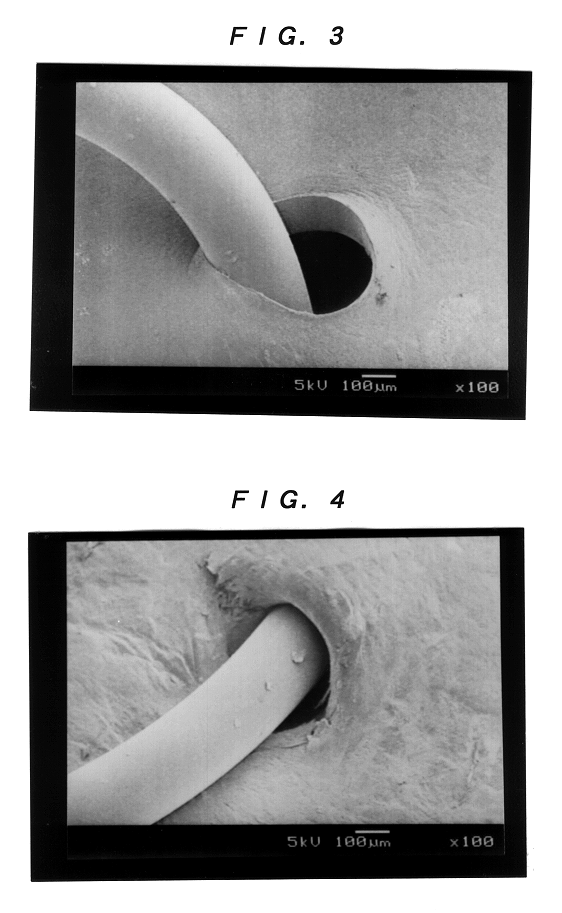
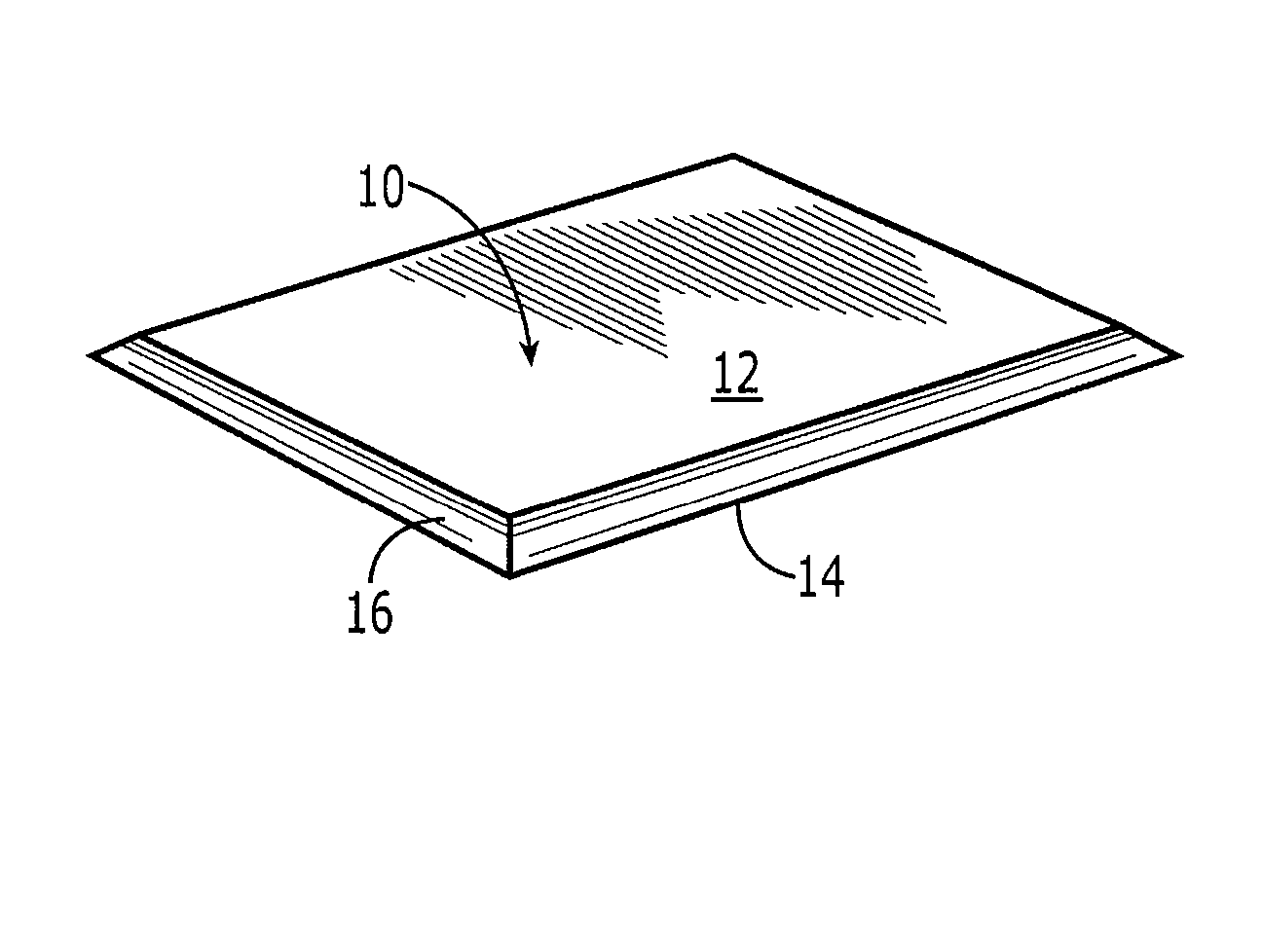
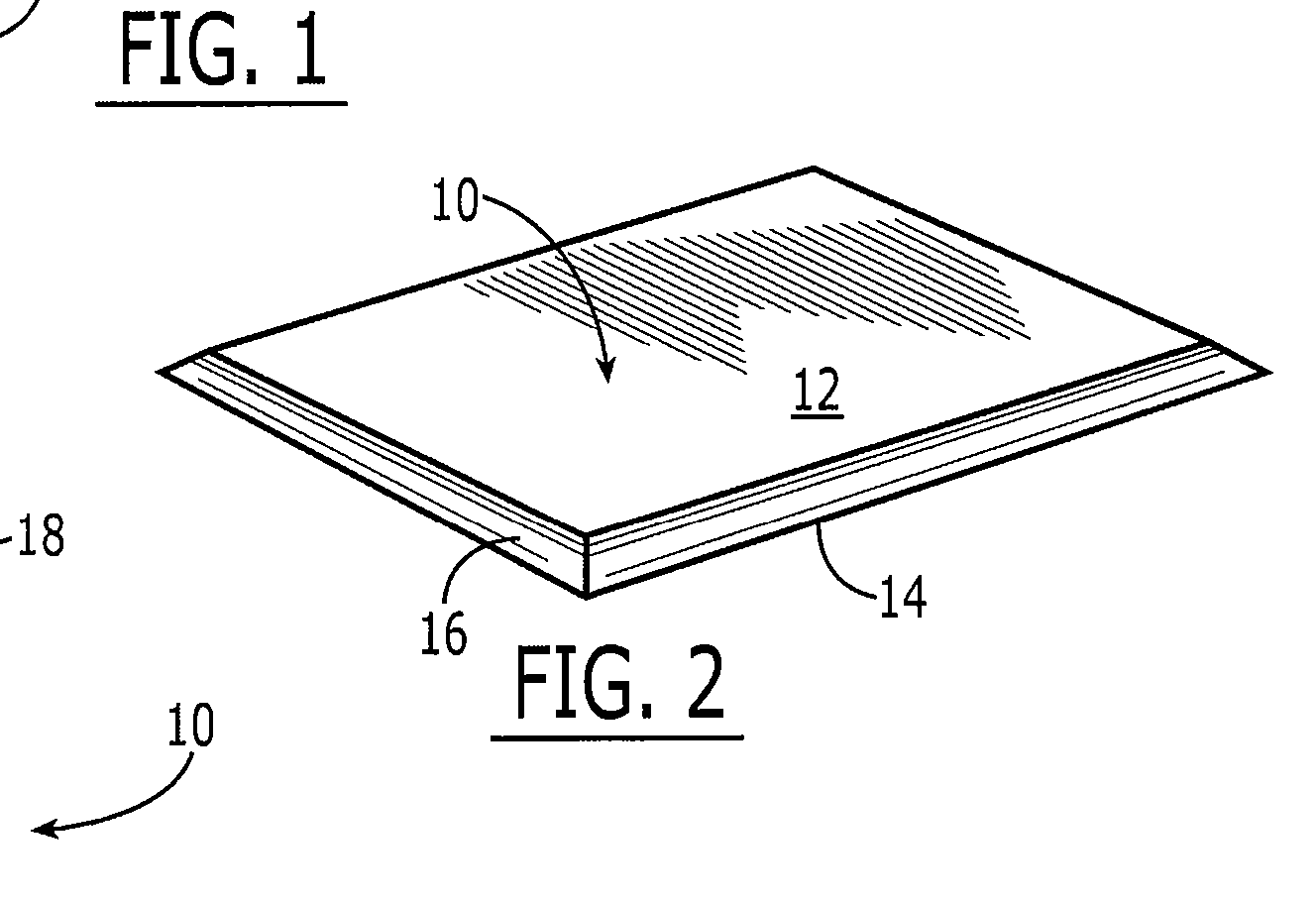
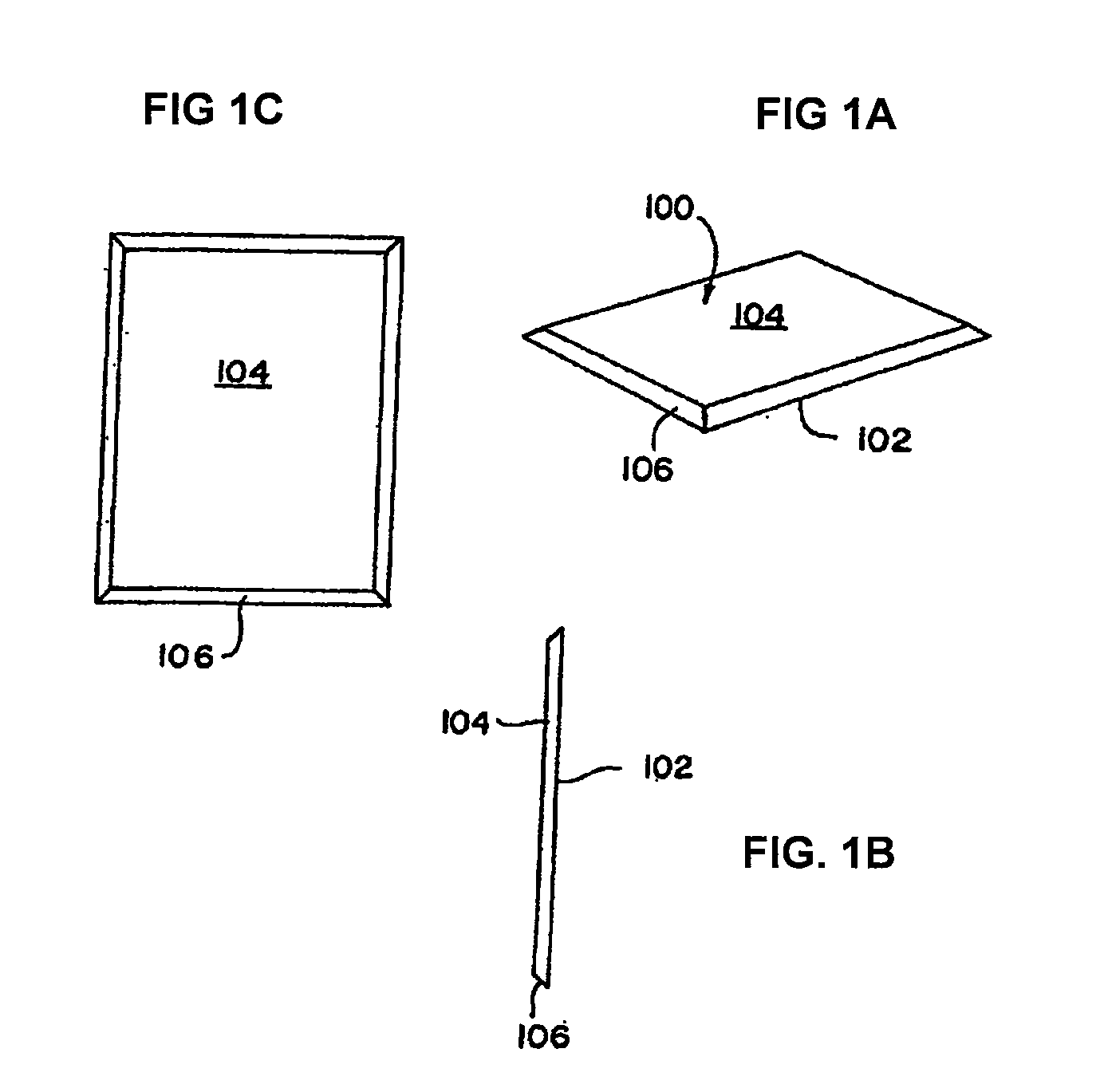
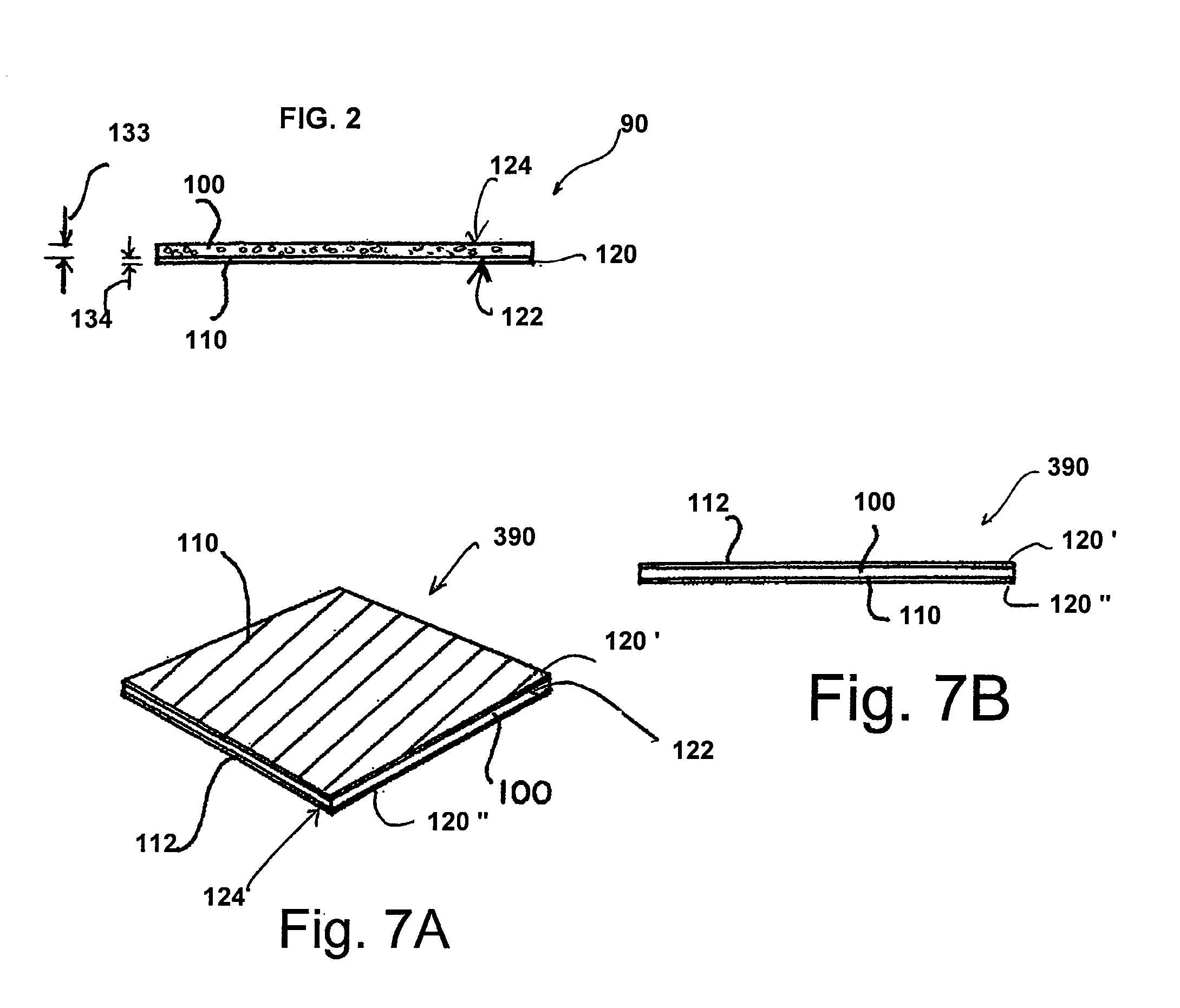
Collagen device and method of preparing the same
InactiveUS20050283256A1Minimize presenceEasy to operateSurgeryTissue regenerationMeningesUltimate tensile strength
A laminated, bioimplantable dural graft product is configured for use as both an onlay graft and a suturable graft. The dural graft product is sufficiently pliable so as to sufficiently conform to a curvature of a tissue surface to which it is applied, such as the curved surface of a meningeal membrane. The use of the graft product can have improved properties, including suture retention strength and fluid impermeability. To use the dural graft product as an implant to replace, reinforce or strengthen bodily tissue, or to act as an adhesion barrier, the dural graft is placed in contact with bodily tissue and conforms to the curvature of the bodily tissue. Sutures can be used to maintain the contact between the dural graft and the bodily tissue.
Owner:CODMAN & SHURTLEFF INC
Delivery of therapeutic compounds to the brain and other tissues
ActiveUS20050048047A1Reduces and eliminates glycogen storage granuleReduce eliminateNervous disorderPeptide/protein ingredientsMeningesIntrathecal use
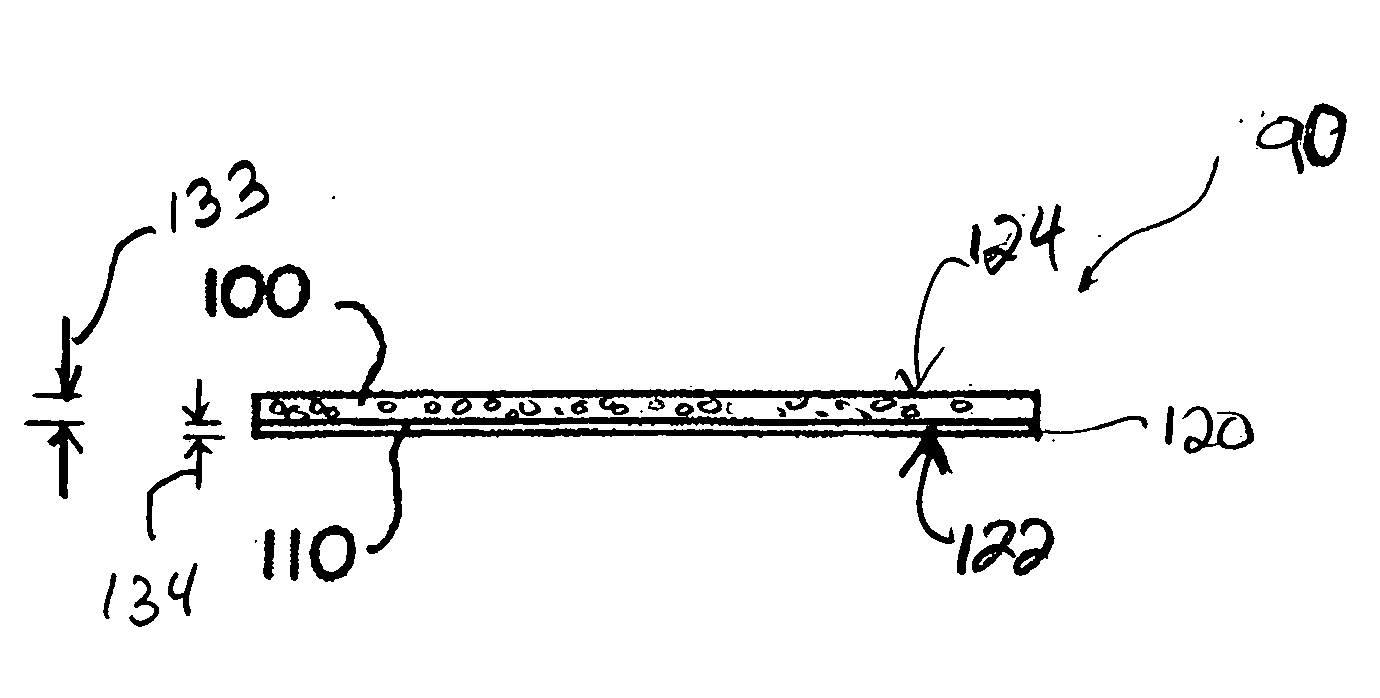
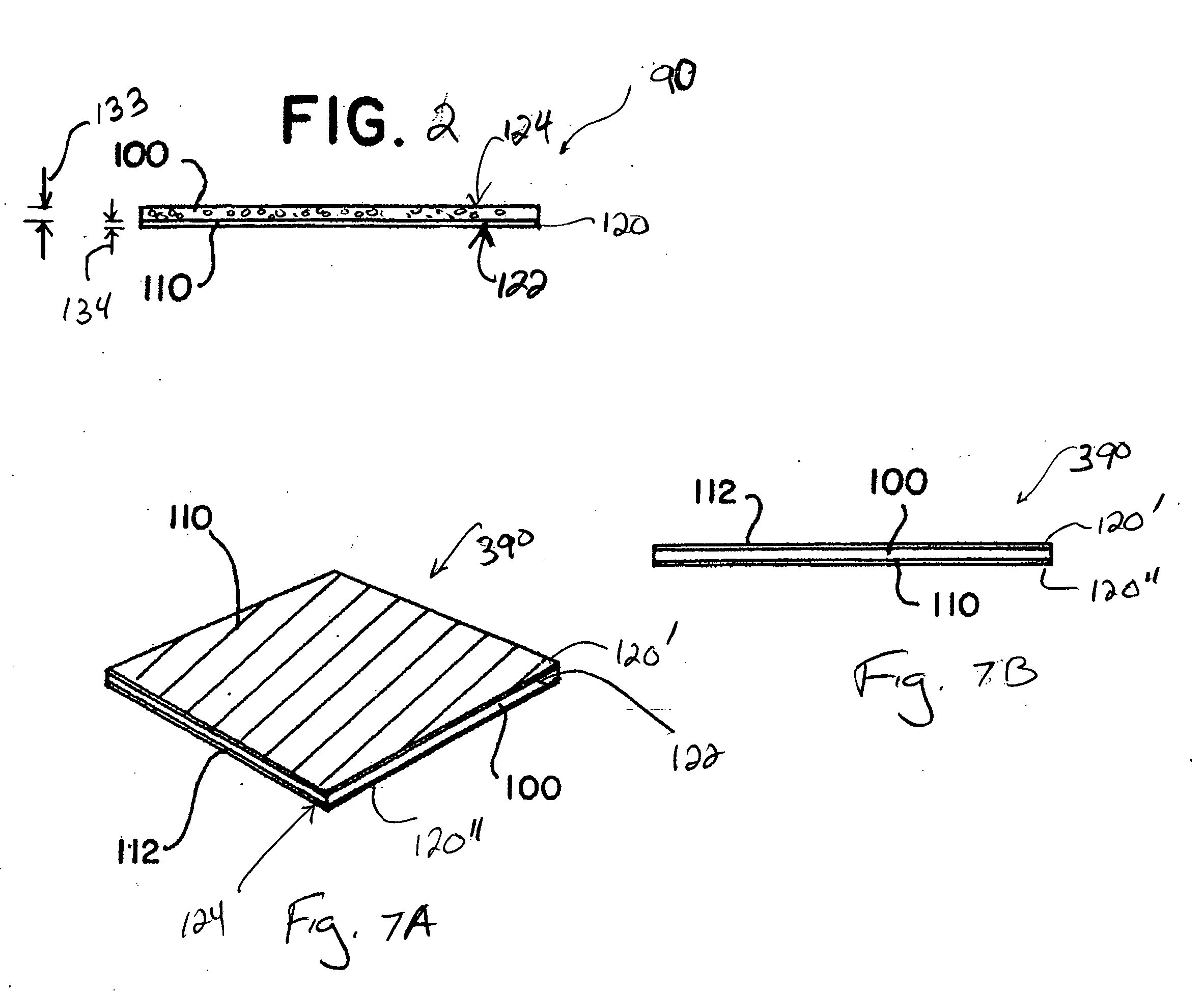
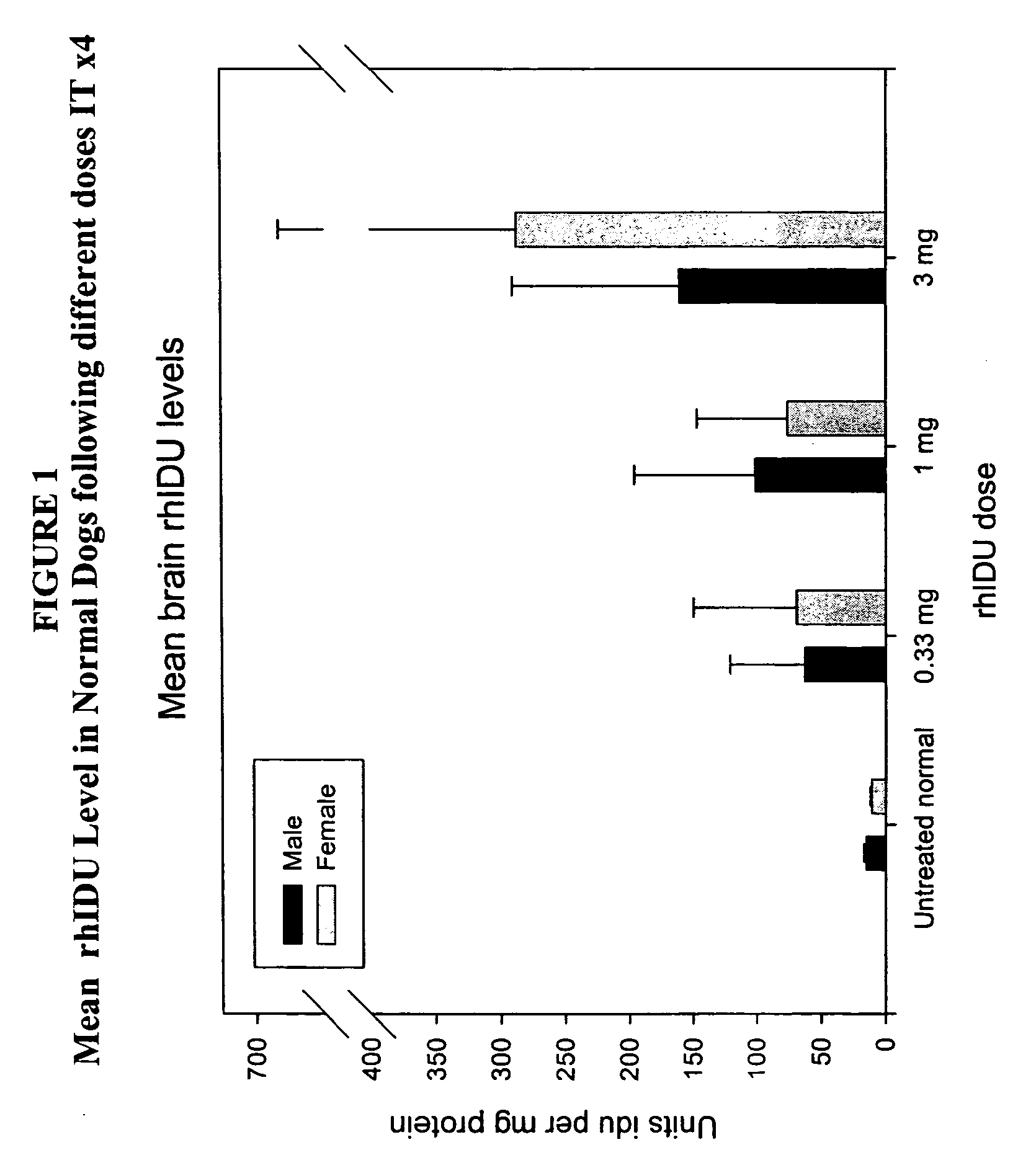
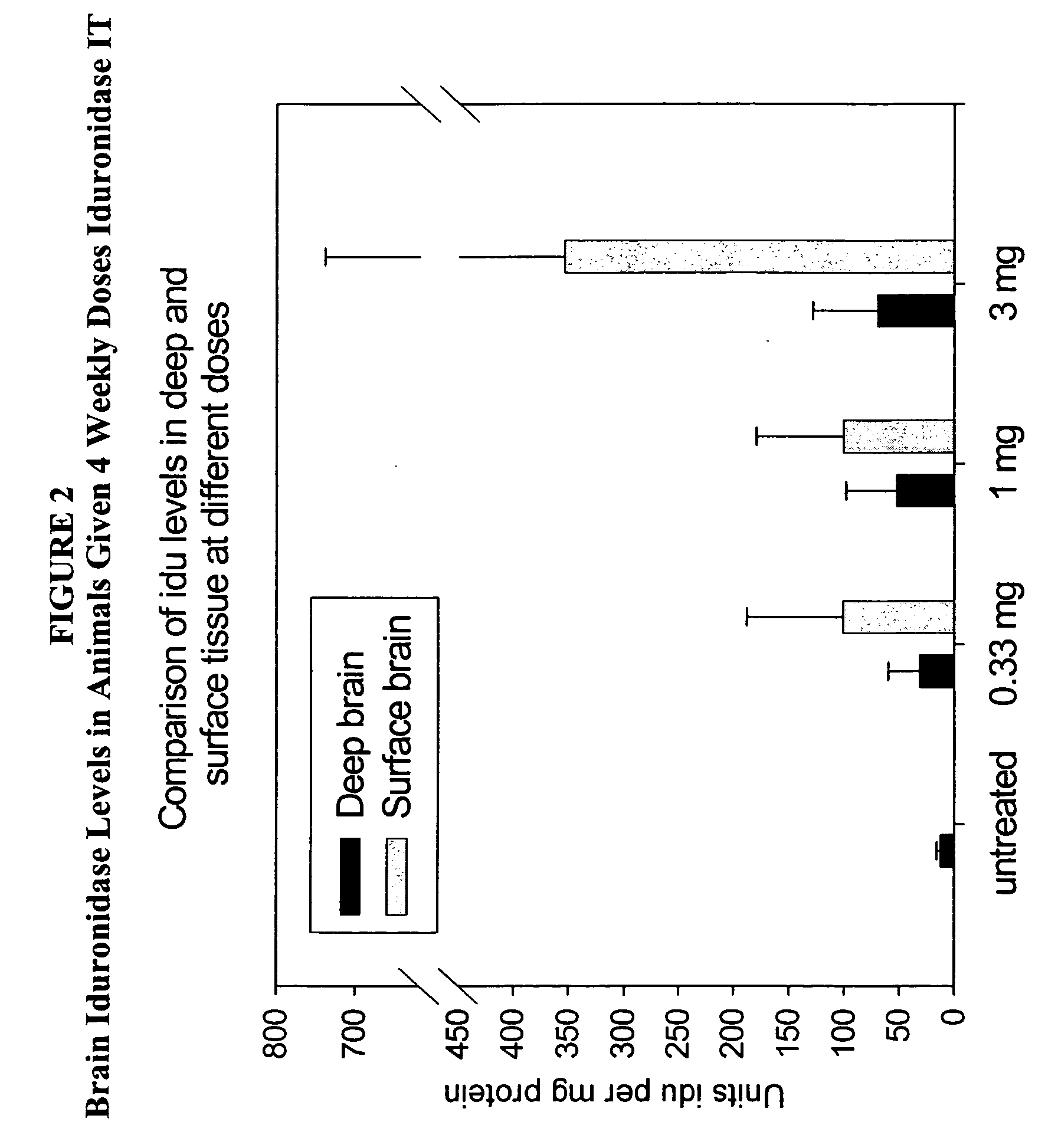
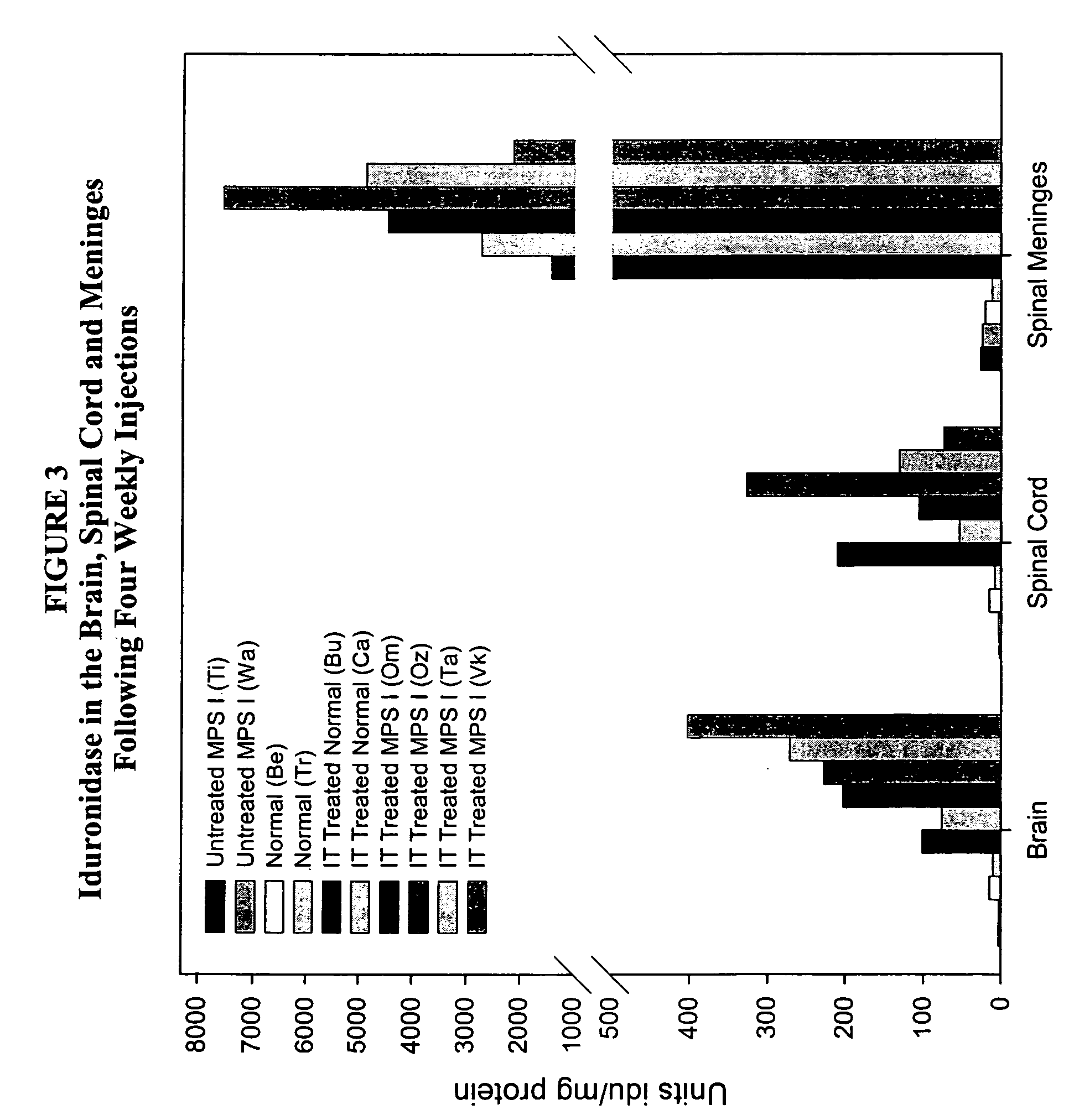
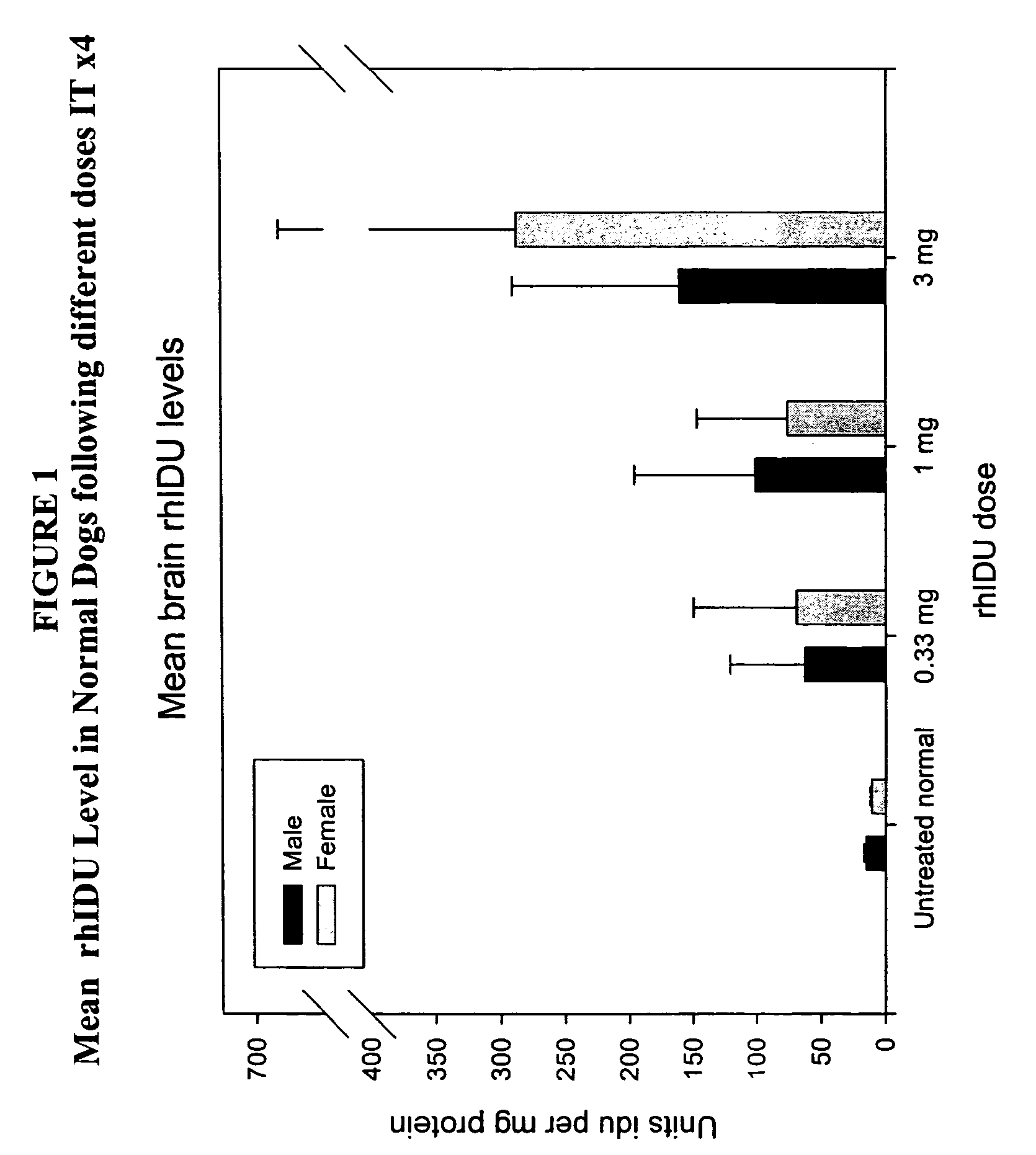
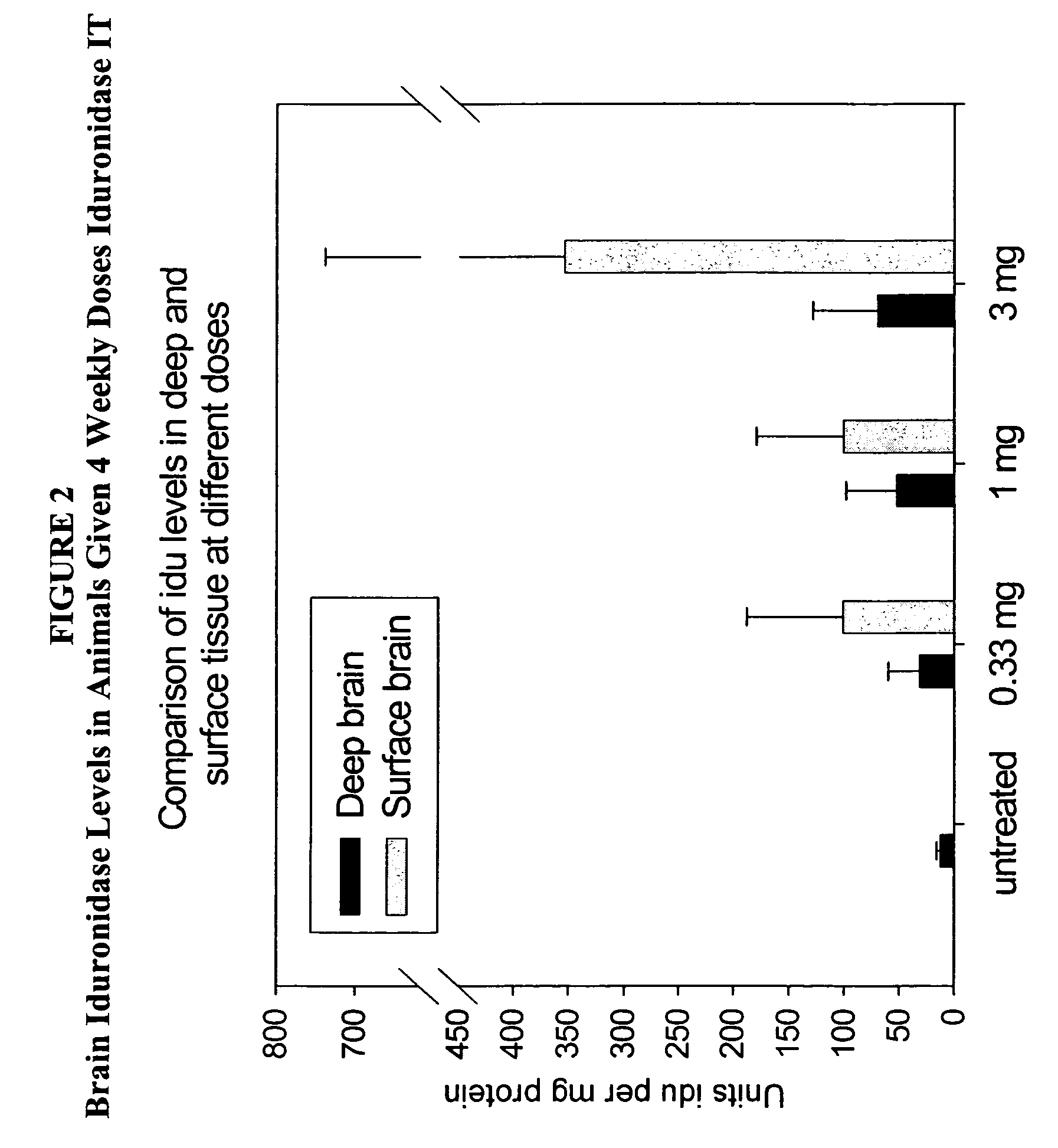
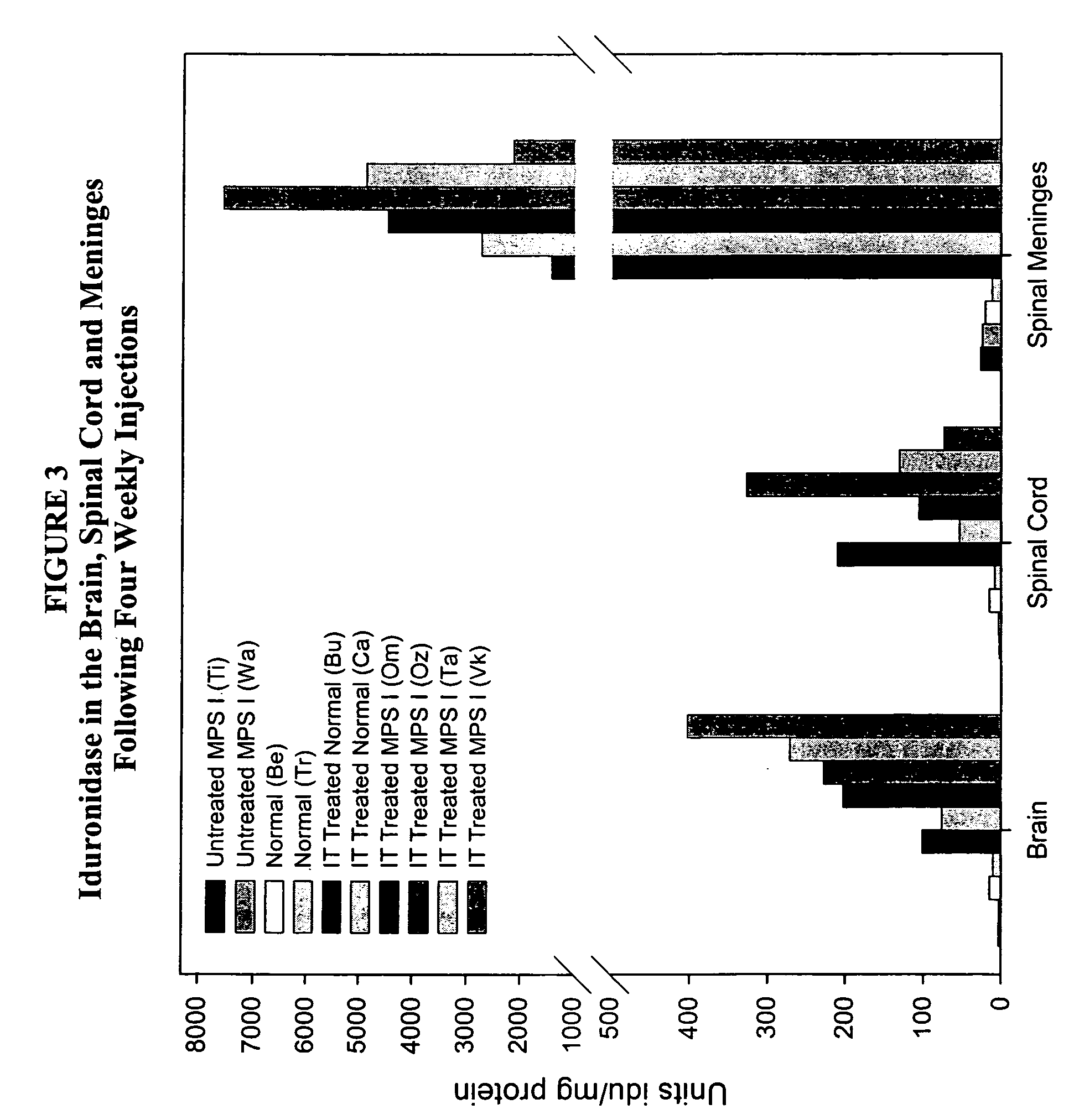
The present invention relates to the intrathecal (IT) administration of recombinant enzyme to treat lysosomal storage disorders. In an exemplary embodiment, intrathecal administration of human α-L-iduronidase (rhIDU) injections in MPS I affected animals resulted in significant enzyme uptake, significant rh-iduronidase activity in brain and meninges and a decrease of glycosaminoglycan (GAG) storage in cells of MPS I subjects to that of normal subjects. Intrathecal administration proved more effective than intravenous treatment at alleviating MPS I symptoms, indicating it is a useful method of treating lysosomal storage disorders.
Owner:BIOMARIN PHARMA INC
Surgical lead paddle
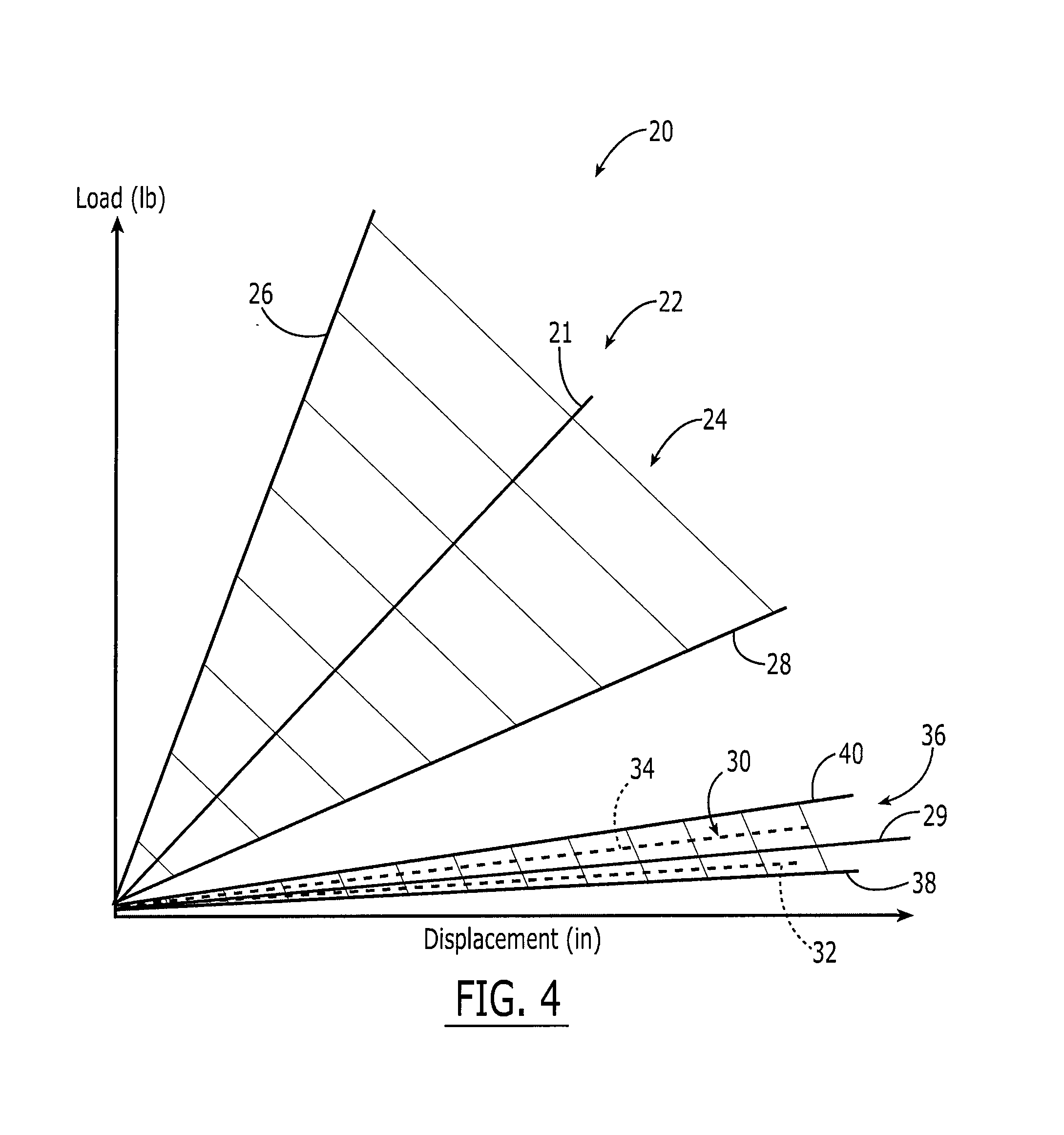
The present invention provides for an improved apparatus and method for electrical stimulation. A paddle having a thickness up to 0.030 inches is implanted adjacent the spinal cord dura mater to reduce the likelihood of paralysis due to stress on the spinal cord attributed to bulkier leads. The paddle is then positioned so that at least one of a plurality of electrodes is positioned over the area of the spinal cord requiring pain treatment; and then electric stimulation is applied to the electrodes to effect pain treatment. In another embodiment the paddle is curved about a vertical axis to substantially match the shape of a human spinal cord dura mater to help reduce lead migration.
Owner:MEDTRONIC INC
Delivery of therapeutic compounds to the brain and other tissues
ActiveUS7442372B2Reduces and eliminates glycogen storage granuleReduce eliminateNervous disorderPeptide/protein ingredientsMeningesIntrathecal use
The present invention relates to the intrathecal (IT) administration of recombinant enzyme to treat lysosomal storage disorders. In an exemplary embodiment, intrathecal administration of human α-L-iduronidase (rhIDU) injections in MPS I affected animals resulted in significant enzyme uptake, significant rh-iduronidase activity in brain and meninges and a decrease of glycosaminoglycan (GAG) storage in cells of MPS I subjects to that of normal subjects. Intrathecal administration proved more effective than intravenous treatment at alleviating MPS I symptoms, indicating it is a useful method of treating lysosomal storage disorders.
Owner:BIOMARIN PHARMA INC
Methods for repairing and regenerating human dura mater
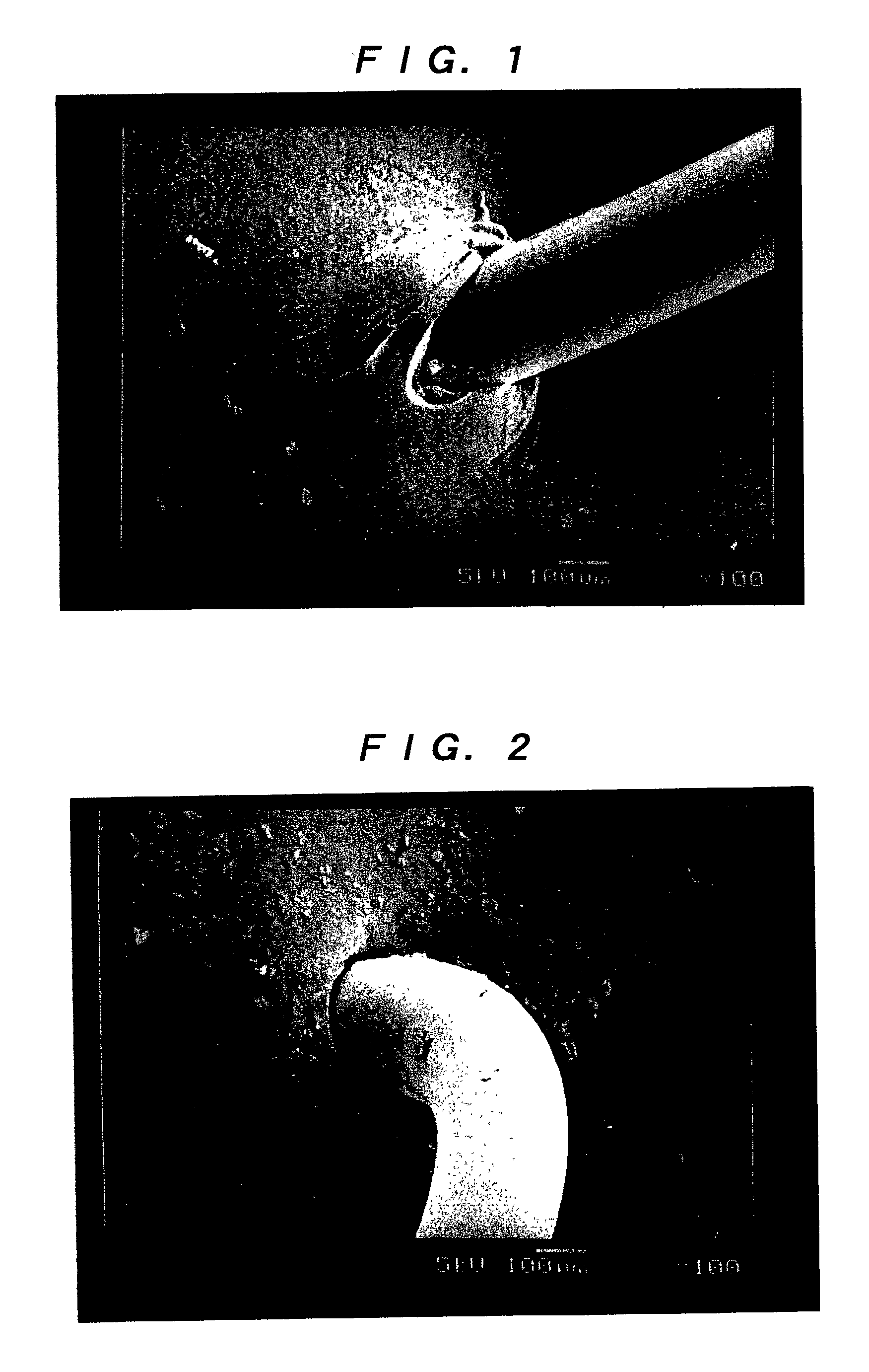
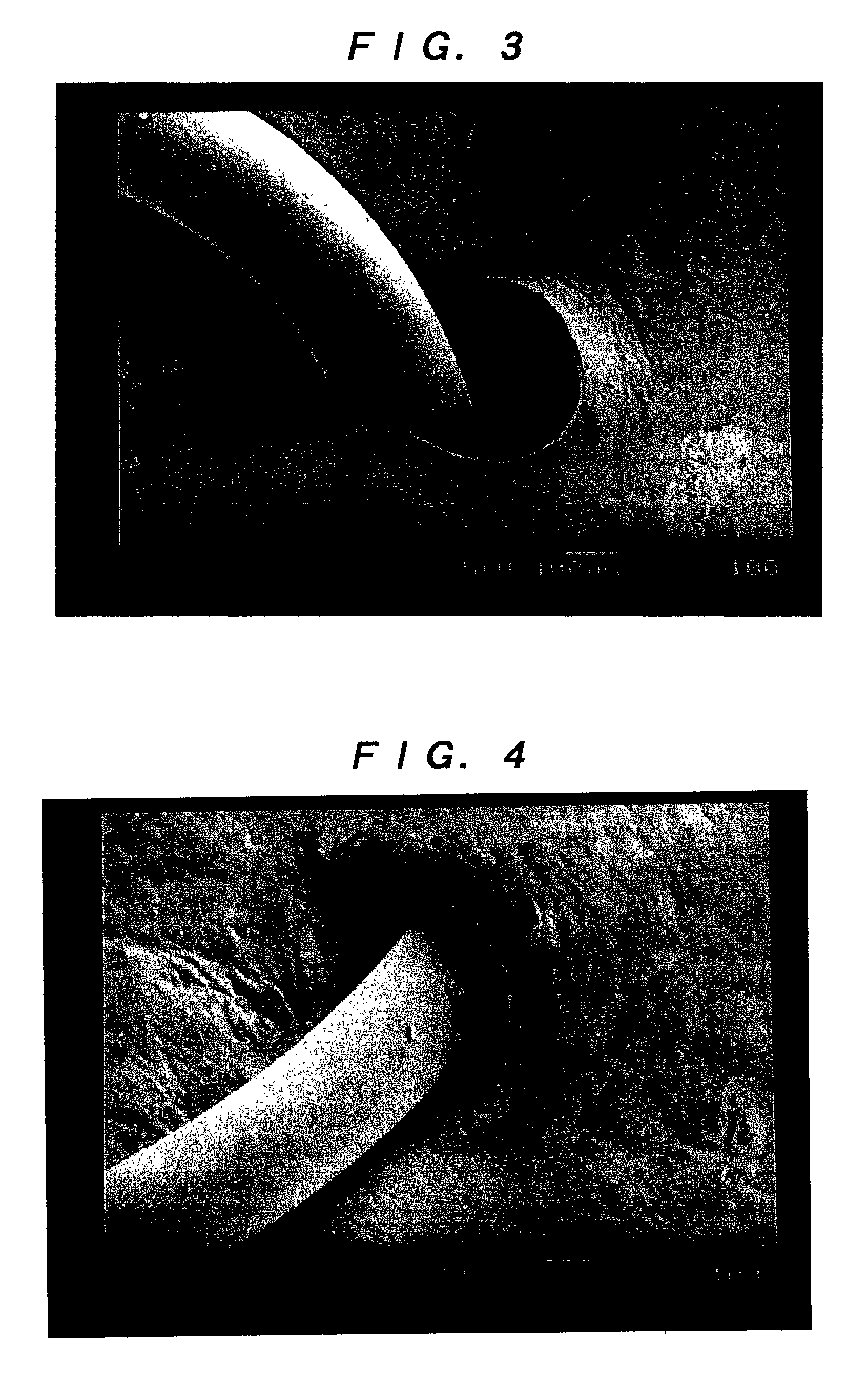
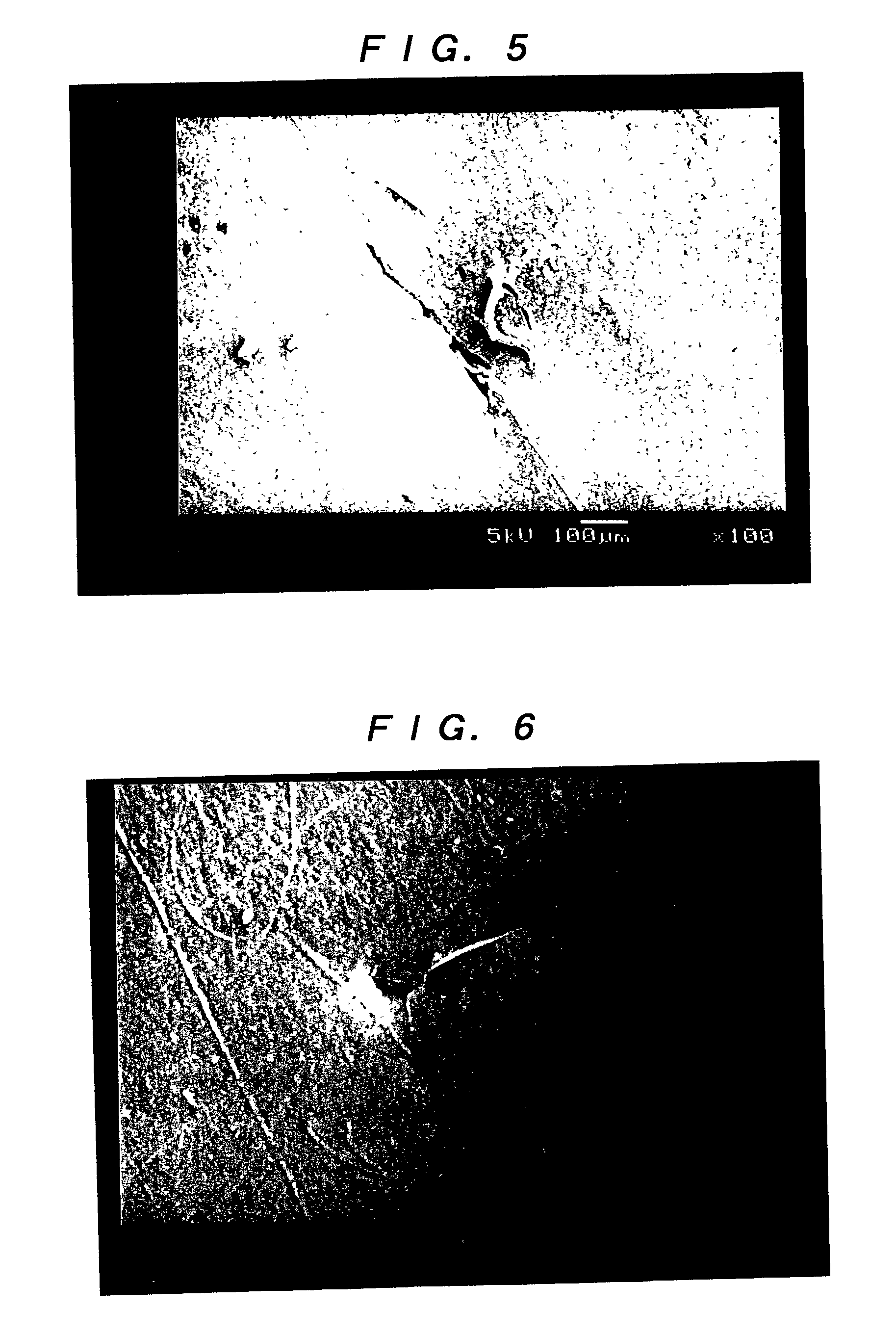
A method of using a substantially non-porous equine collagen foil to repair and regenerate dura mater tissue of mammals when the dura mater tissue is damaged as a result of injury, tumors, surgery, and the like. The non-porous equine collagen foil comprises collagen fibrils which provides a replacement dura mater composition that is elastic, liquid-tight, and which has a high tensile strength. The non-porous equine collagen foil is furthermore resorbable and provides a biomatrix, wherein a neodura is rapidly formed which becomes indistinguishable from the autologous dura mater in a matter of weeks. The process for making the equine collagen foil reduces the likelihood of disease transmission.
Owner:BAXTER INT INC +1
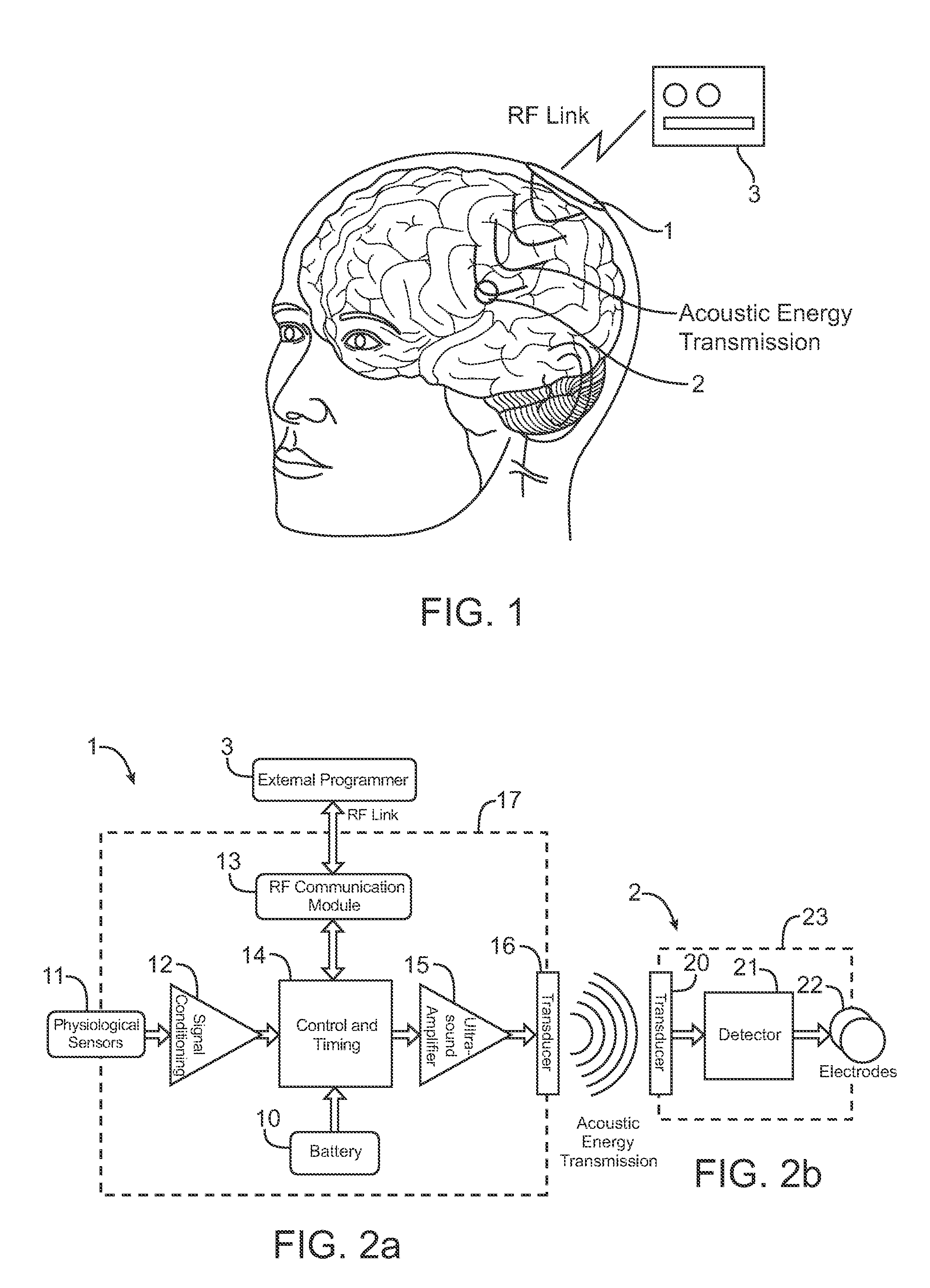
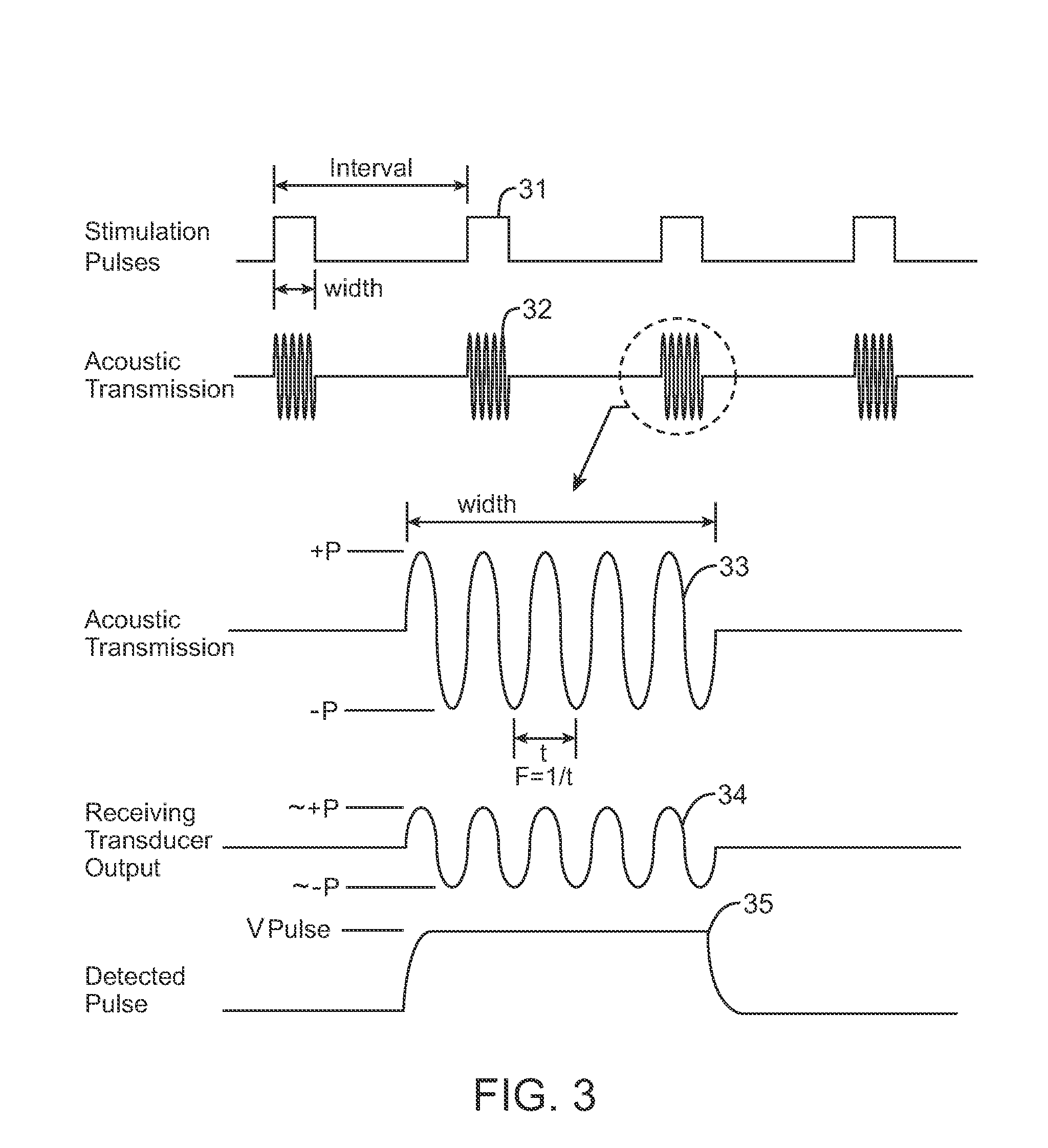
Systems and methods for implantable leadless brain stimulation
ActiveUS20070293908A1Relieve painRelieve symptomsHead electrodesTherapeutic treatmentElectrical stimulations
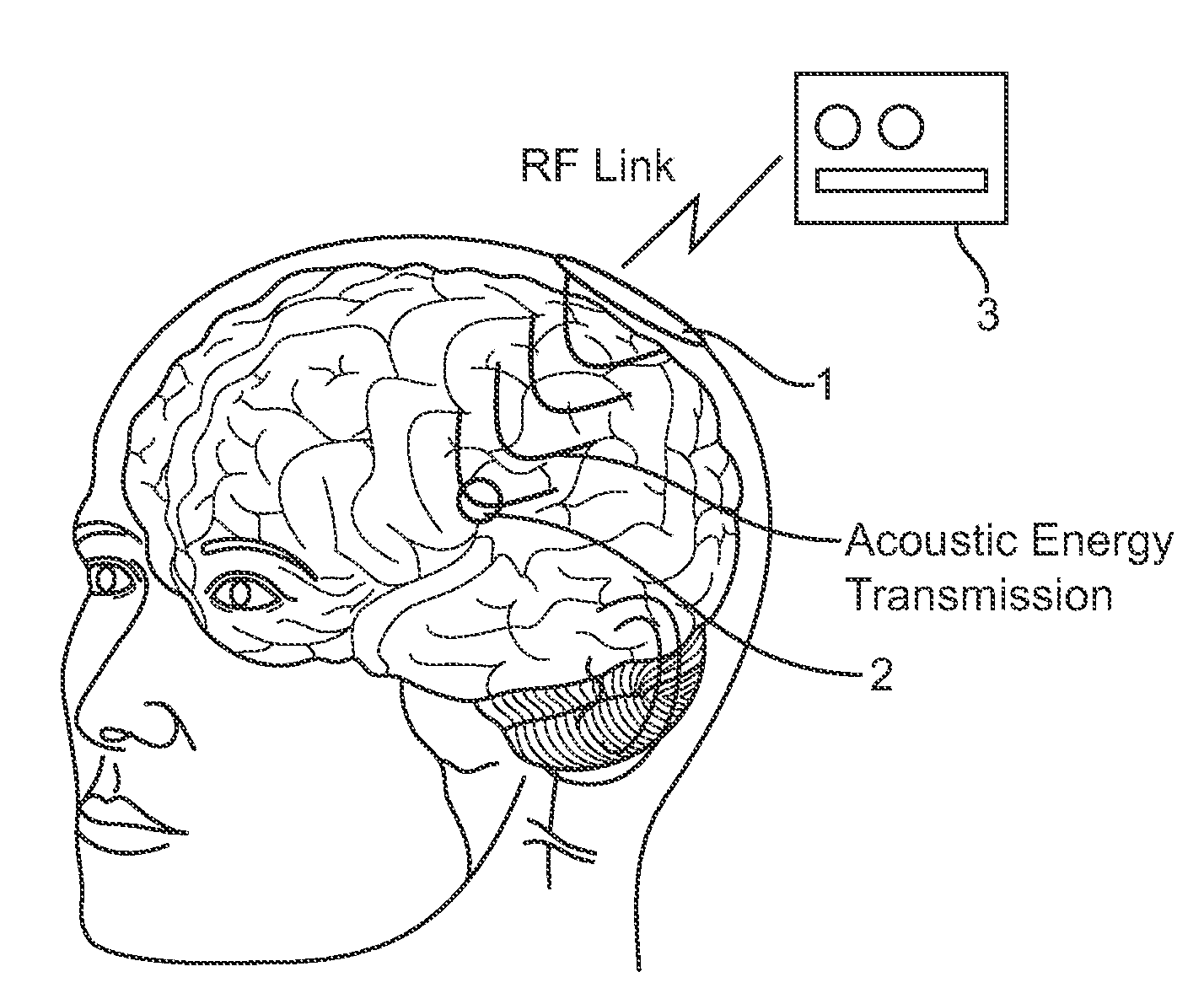
Systems and methods are disclosed to stimulate brain tissue to treat medical conditions such as movement disorders, pain and epilepsy. The disclosed invention uses electrical stimulation of the brain tissue, where vibrational energy from a source is received by an implanted device and converted to electrical energy and the converted electrical energy is used by implanted electrodes to stimulate the pre-determined brain site. The vibrational energy is generated by a controller-transmitter, which could be either implanted or located externally. The vibrational energy is received by a receiver-stimulator, which could be located under the skull, within the brain, on the dura, or in the cranial space close to the brain. As a therapeutic treatment, the implantable receiver-stimulator stimulates the brain sites that are effective in altering brain activity.
Owner:EBR SYST
Cerebrospinal Fluid Purification System
The present invention provides methods and systems for conditioning cerebrospinal fluid (CSF). The methods provide for efficiently removing target compounds from CSF. The systems provide for a multilumen flow path and exchange of a majority volume portion of CSF in the CSF space. The removal and / or delivery of specific compounds can be tailored to the pathology of the specific disease. The removal is targeted and specific, for example, through the use of specific size-exclusion thresholds, antibodies against specific toxins, and other chromatographic techniques, as well as delivery and / or removal of targeted therapeutic agents. The invention finds use as a diagnostic, therapeutic and drug delivery platform for a variety of diseases affecting the CNS by accessing the CSF space. Exemplified disease conditions treatable by the present CSF processing systems and methods include, but are not limited to: Cerebral Vasospasm, Guillain Bane Syndrome, illustrating multi-lumen lumbar approach Alzheimer's, Parkinson's, Huntington's, Multiple Sclerosis, Amyotrophic Lateral Sclerosis, Spinal Cord Injury, Traumatic Brain Injury, Stroke, Cancer affecting the brain or spinal cord, Prion disease, Encephalitis from various causes, Meningitis from various causes, diseases secondary to enzymatic or metabolic imbalances, Biological Warfare, etc. For the first time, the present invention offers patients a disease-modifying, disruptive technology treatment platform that addresses the known disease pathogenesis of a number of neurologic conditions to which there are presently limited and ineffective treatment options.
Owner:NEUROFLUIDICS
Suturable dural and meningeal repair product comprising collagen matrix
A matrix for tissue growth includes: (a) a first layer including a first assembly of collagen fibers; (b) a plurality of projections on a top surface of the first layer; and (c) a second layer bonded to a bottom portion of the first layer and including a second assembly of collagen fibers, wherein the second layer has a lower density than the first layer, and the matrix includes pores effective to support cell growth into the matrix. A method for providing the matrix is also described.
Owner:INTEGRA LIFESCI
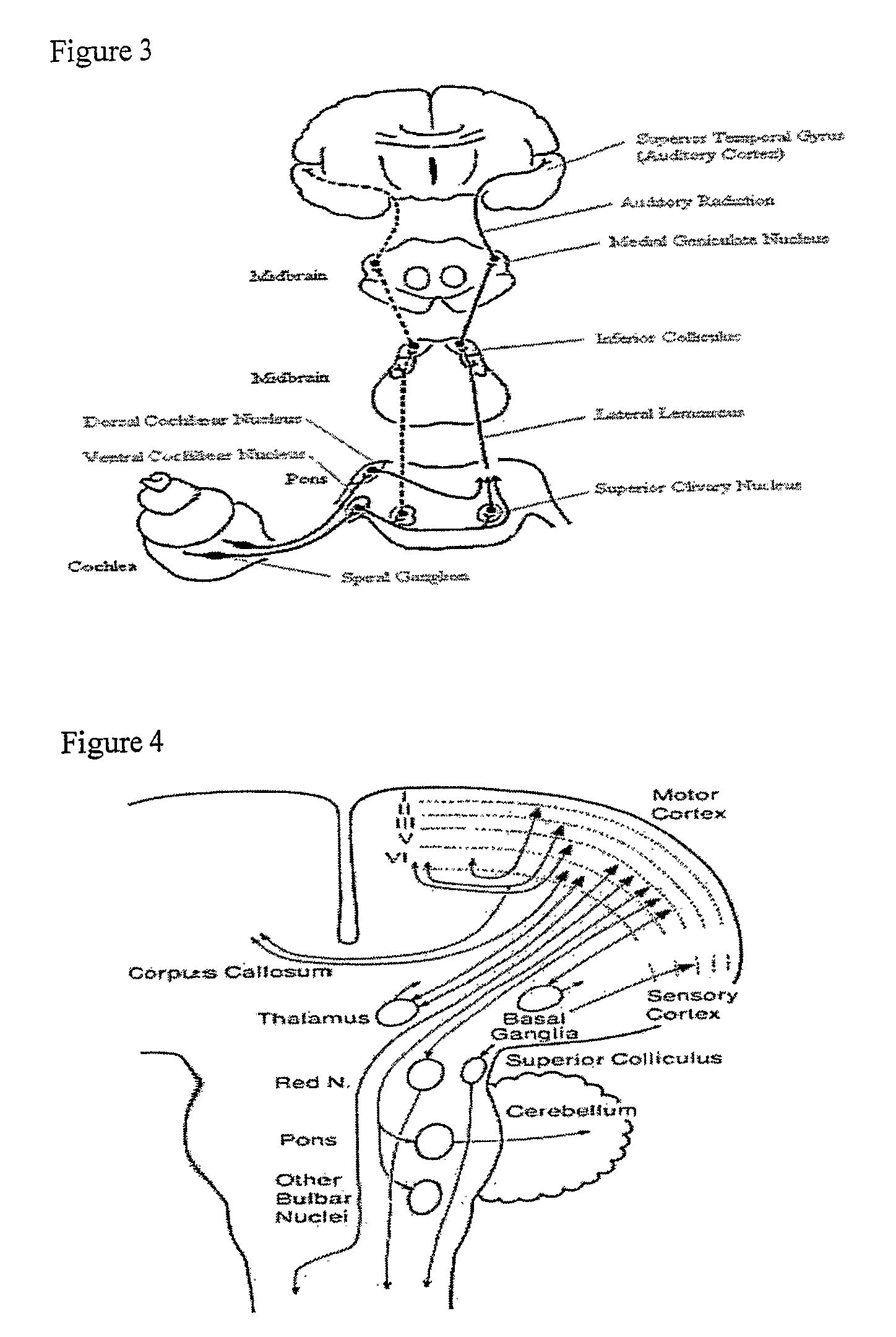
Method and Apparatus for Treatment of Tinnitus and Other Neurological Disorders by Brain Stimulation in the Inferior Colliculi and/or In Adjacent Areas
InactiveUS20070265683A1Reducing and eliminating effectSuppress and eliminate neural activityHead electrodesImplantable neurostimulatorsPeriaqueductal grayElectrode placement
Electrical stimulation is applied to the inferior colliculus or colliculi (IC), in order to diminish tinnitus by revising auditory pathway neuronal activity. This intervention diminishes tinnitus and treats other neurological and otological disorders. The locations and methods of electrode placement and anchoring and the structure of the electrodes are an advance over prior treatments. Other treatment locations in the nearby region of the IC, including the superior colliculi (SC) and Peri-aqueductal gray (PAG), provide treatments for other disorders and symptoms such as partial hearing loss and pain. The IC is a unique choice for the treatment of tinnitus and other disorders because an electrode placed in that region enables minimal invasiveness. The anchoring location also uniquely minimizes invasiveness by providing the option of residing in the meninges instead of the brain tissue. The shape of the electrode and its anchoring process uniquely match the brain's anatomy in order to provide greater specificity in diagnosis and treatment. Stimulation of areas near the IC, particularly the superior colliculus and peri-aqueductal gray, can be used to treat various neurological disorders. Customized feedback from the implantable system enables the creation of customized treatment programs.
Owner:EHRLICH DOV
Suturable dural and meningeal repair product comprising collagen matrix
A matrix for tissue growth includes: (a) a first layer including a first assembly of collagen fibers; (b) a plurality of projections on a top surface of the first layer; and (c) a second layer bonded to a bottom portion of the first layer and including a second assembly of collagen fibers, wherein the second layer has a lower density than the first layer, and the matrix includes pores effective to support cell growth into the matrix. A method for providing the matrix is also described.
Owner:INTEGRA LIFESCI
Methods for repairing and regenerating human dura mater
A method of using a substantially non-porous equine collagen foil to repair and regenerate dura mater tissue of mammals when the dura mater tissue is damaged as a result of injury, tumors, surgery, and the like. The non-porous equine collagen foil comprises collagen fibrils which provides a replacement dura mater composition that is elastic, liquid-tight, and which has a high tensile strength. The non-porous equine collagen foil is furthermore resorbable and provides a biomatrix, wherein a neodura is rapidly formed which becomes indistinguishable from the autologous dura mater in a matter of weeks. The process for making the equine collagen foil reduces the likelihood of disease transmission.
Owner:BAXTER INT INC +1
Implantable micro-system for treatment of hydrocephalus
An implantable system for the treatment of hydrocephalus includes a plurality of microneedles in a fixed array relative to each other adapted to extend from the subarachnoid space containing CSF surrounding the brain, through dura mater forming the wall of the superior sagital sinus. A microvalve is associated with a proximal end of each of the microneedles and is adapted to permit the flow of cerebrospinal fluid (CSF) from the subarachnoid space through the wall of the superior sagital sinus and deposited in the venous return of the brain. The method of treating hydrocephalus with the system of this invention also constitutes a part of the invention.
Owner:DREXEL UNIV
Neural scaffolds
Disclosed herein are compositions and methods useful for preparing neural scaffolds. The scaffolds comprise tissue taken from the spinal cord and / or dura mater of vertebrate and can be processed to form gels or sheets. Methods of treating patient with CNS injury are also presented.
Owner:UNIVERSITY OF PITTSBURGH
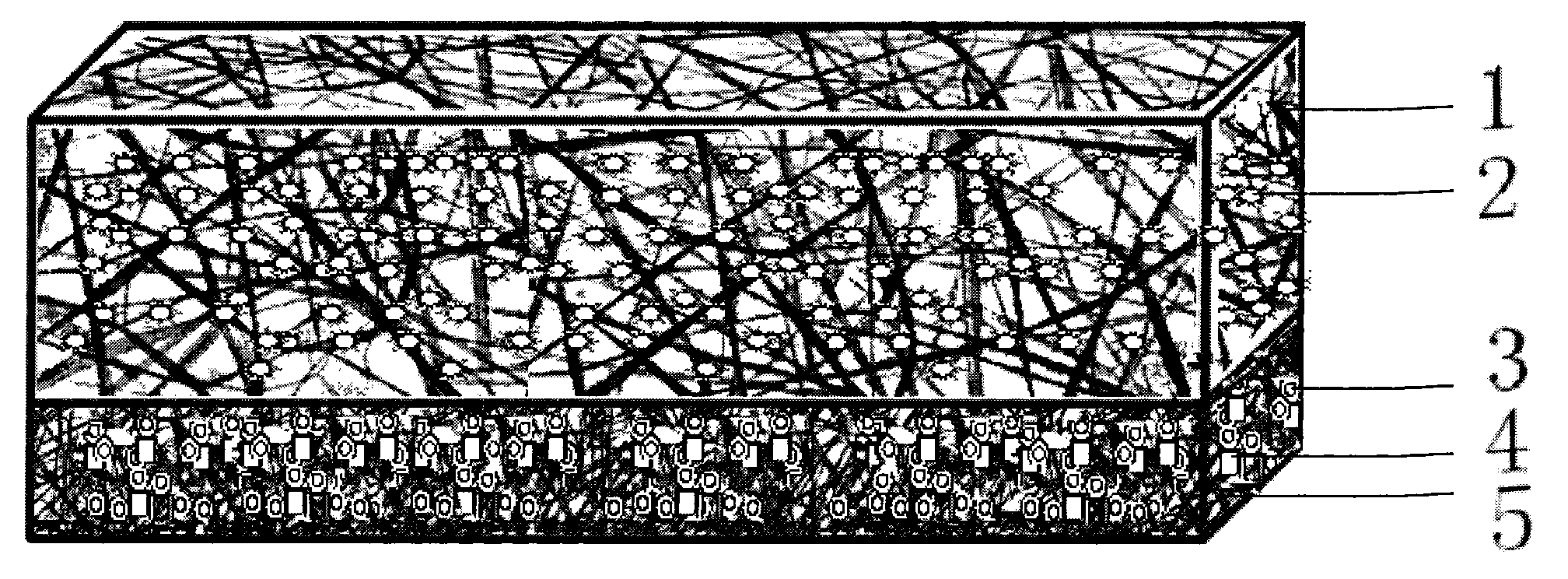
Nano artificial dura mater capable of being used as medicine sustained-release system and preparation method thereof
The invention provides a nano artificial dura mater capable of being used as a medicine sustained-release system, having the structure which comprises at least two layers, i.e. a hydrophobic anti-blocking electro-spun layer which faces the cerebrum, and a hydrophilic nano cytoskeleton layer which backs on to the cerebrum; and cell factors and / or medicines are arranged in any layer of the artificial dura mater by way of blended spinning. The invention also provides a method for preparing the nano biomimic artificial dura mater. Compared with an artificial mater prepared by the single utilization of the electro-spinning technology, the artificial mater to which the medicines and the cell factors are added by blending technology can effectively prevent infection and faster promote the regeneration process of the artificial mater. The invention also provides a novel medicine loading and releasing mode for treating cerebral diseases, the loaded medicines can be directly and efficiently transferred into the cranial cavity along with the implantation of the dura mate and can be released according to requirements, and therefore the invention realizes favorable treatment effect and has broad application prospect.
Owner:MEDPRIN REGENERATIVE MEDICAL TECH
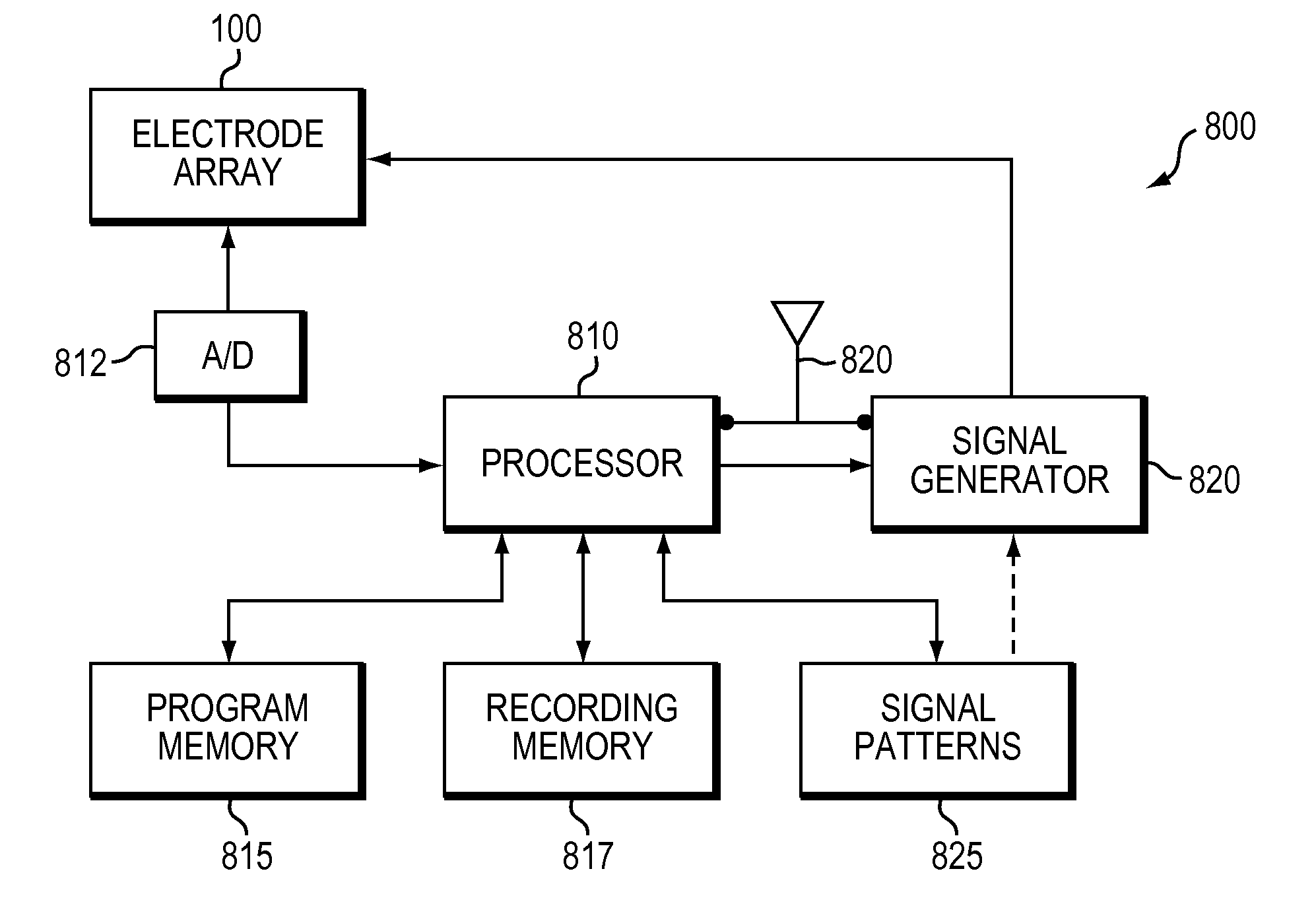
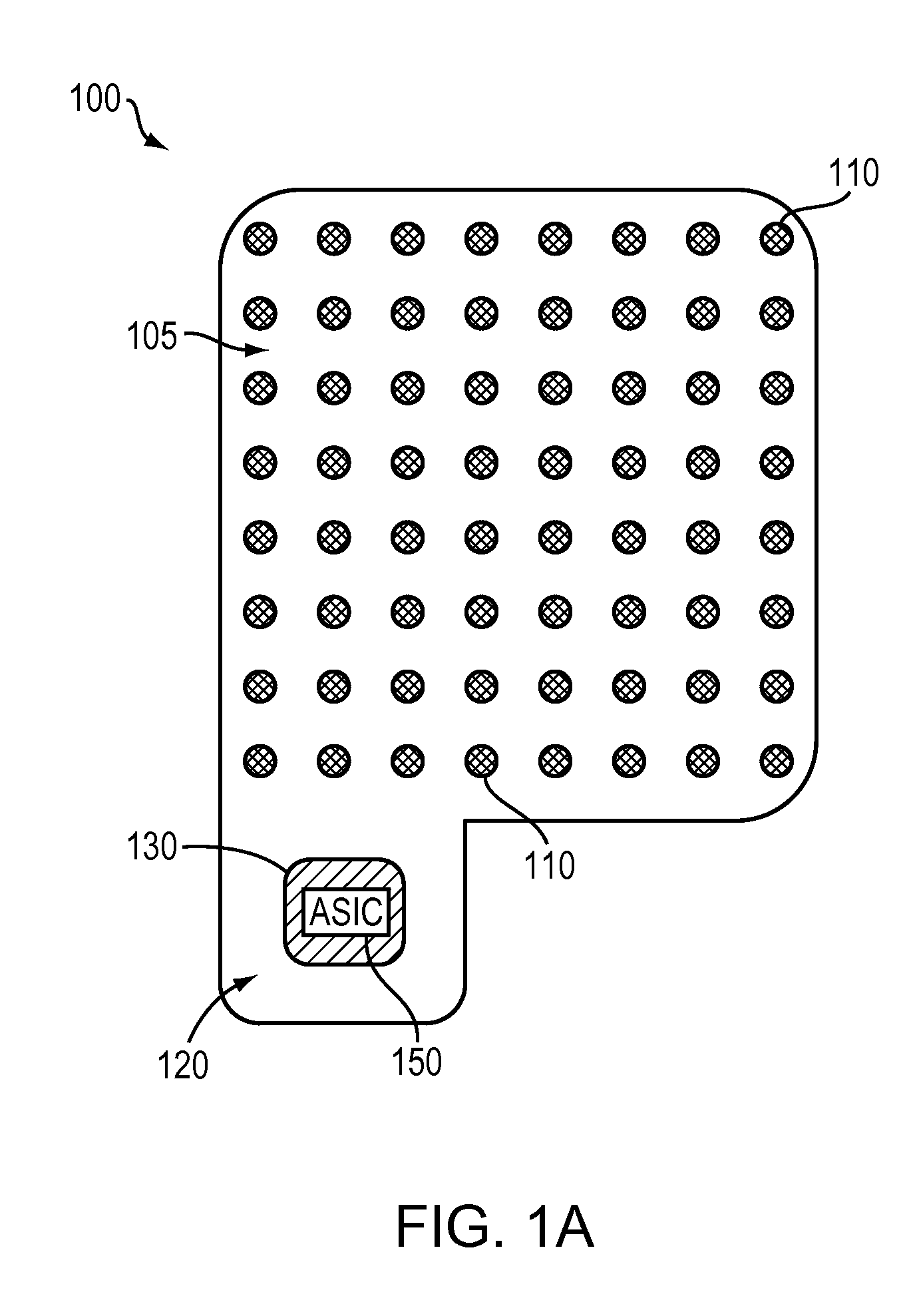
Wireless Recording and Stimulation of Brain Activity
InactiveUS20100198297A1Avoid lengthAvoiding costly hospitalizationElectroencephalographyHead electrodesMedicineElectroencephalography
Subdural arrays transmit electrocorticogram recordings wirelessly, across the patient's skull, allowing the craniotomy used for surgical placement of the arrays to be completely closed. In various embodiments, the arrays also respond to commands, applying signal patterns to the patient's brain for diagnostic and treatment purposes.
Owner:SIGENICS +2
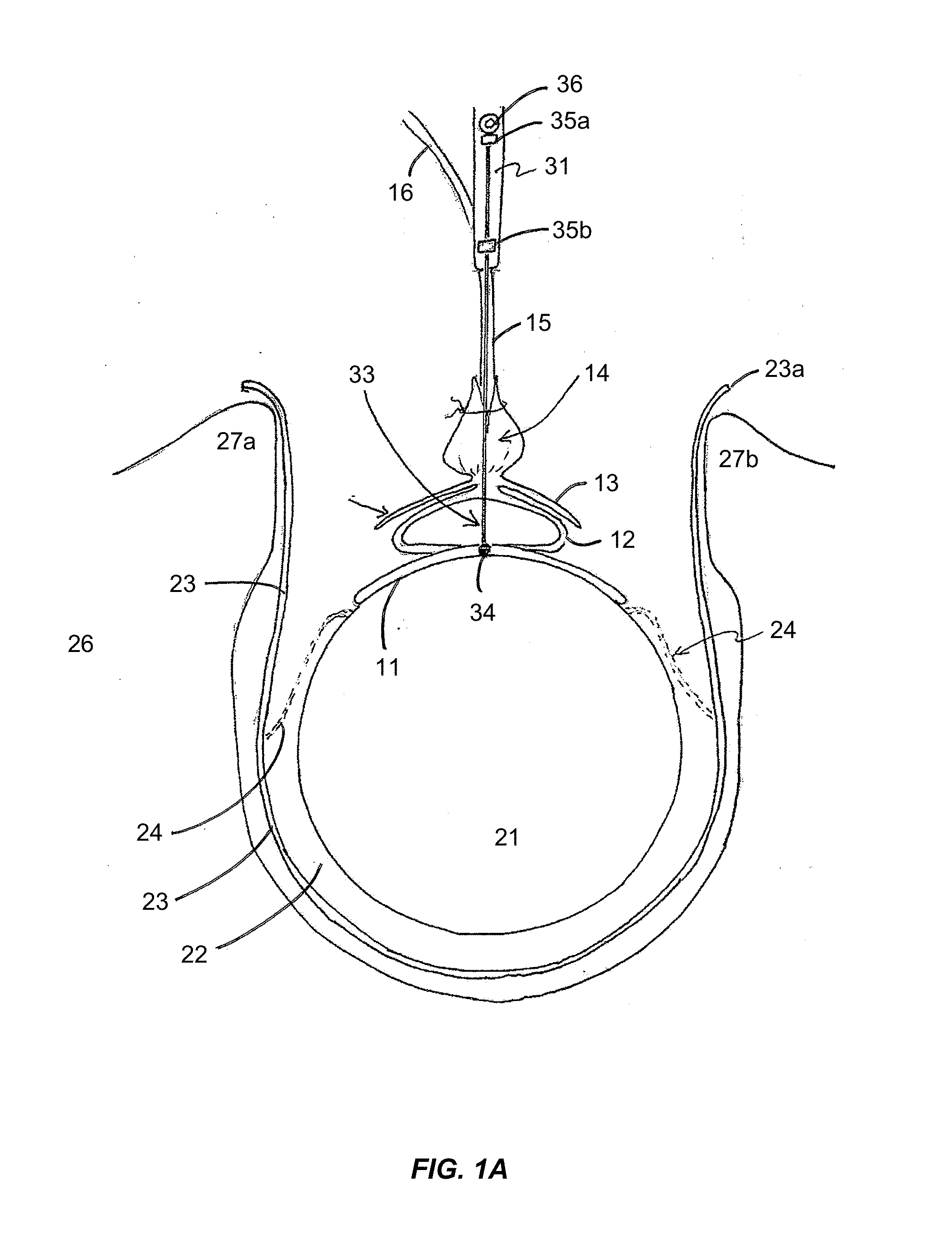
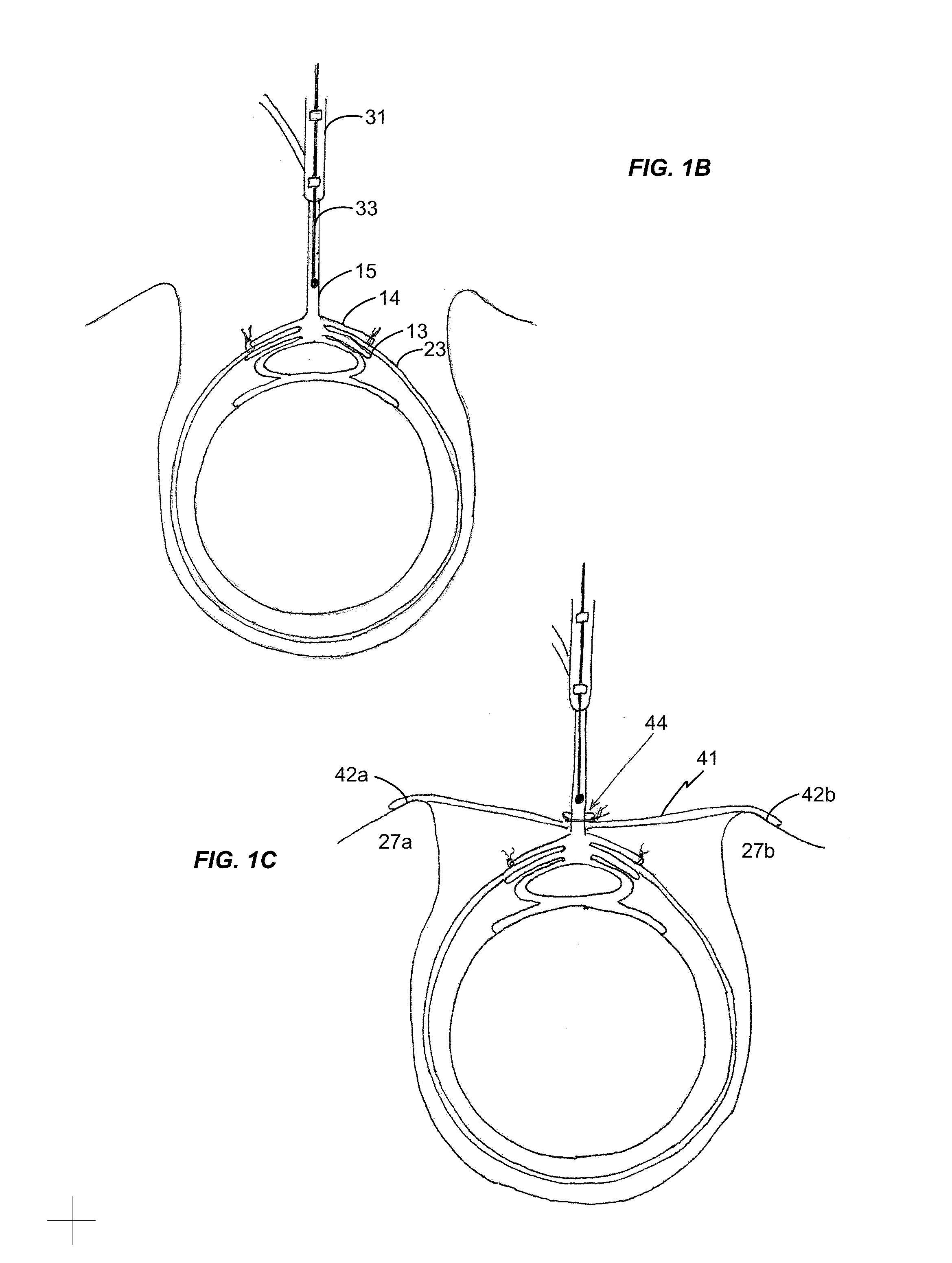
Methods and Apparatus for Occluding Reproductive Tracts to Effect Contraception
Systems and methods of visibly marking a reproductive tract, e.g., at least one ostium of the Fallopian tubes accessed transvaginally and transcervically, to indicate delivery of an occlusion device into the corresponding at least one Fallopian tube to effect contraception are disclosed. Marking is effected by delivery of a dye that stains the ostium and / or extension of the marking member from the delivered occlusion device into view in the uterine cavity or ostium. A catheter (or catheters) preferably introduced through a hysteroscope that illuminates and provides visualization of the uterine cavity and the ostia of the Fallopian tubes is employed to insert each contracted, stent-like, occluding device into each Fallopian tube and to mechanically expand or release to self-expand the occluding device.
Owner:BAYER HEALTHCARE LLC
Hydrogel tissue adhesive having increased degradation time
InactiveUS20100160960A1Long degradation timeProlong degradation timeCosmetic preparationsSurgical adhesivesSurgical operationFistula
A hydrogel tissue adhesive having increased degradation time is described. The hydrogel tissue adhesive is formed by reacting an oxidized polysaccharide with a water-dispersible, multi-arm amine in the presence of a polyol additive, which retards the degradation of the hydrogel. The hydrogel may be useful as a tissue adhesive or sealant for medical applications, including but not limited to, ophthalmic applications such as sealing wounds resulting from trauma such as corneal lacerations, or from surgical procedures such as vitrectomy procedures, cataract surgery, LASIK surgery, glaucoma surgery, and corneal transplants; neurosurgery applications, such as sealing the dura; as a plug to seal a fistula or the punctum; adhesion prevention to prevent undesired tissue to tissue adhesions resulting from trauma or surgery; and as a hemostat sealant.
Owner:ACTAMAX SURGICAL MATERIALS
Implantable micro-system for treatment of hydrocephalus
An implantable system for the treatment of hydrocephalus has a plurality of hollow microneedles in a fixed array. The microneedles have passages there through and are of a size to extend through dura mater with a proximal end on one side of the dura mater and a distal end communicating with the venous system in an area on a second side of the dura mater. An array of microvalves associated with a proximal end of the microneedles include segments movable by a pressure build up of cerebrospinal fluid (CSF) within the interior of the brain to provide open communication between the interior of the brain on one side of the dura mater and the venous system on the second side of the dura mater. A plurality of fluid transmitting members has distal ends communicating with the microvalves and a proximal end communicating with the ventricular space of the brain. The fluid transmitting members transmit CSF from the ventricular space through the microvalves and micro needles in fluid communication with the fluid transmitting members to prevent an excessive pressure build up of CSF within the interior of the brain
Owner:DREXEL UNIV
Dural graft and method of preparing the same
Owner:DEPUY SYNTHES PROD INC
Artificial dura mater and process for producing dura mater
The present invention provides an artificial dura mater characterized by comprising at least a sheet of a biodegradable and bioabsorbable synthetic polymer and having a storage modulus at ordinary temperature of from 1x107 to 5x108 (Pa); and a process for producing an artificial dura mater characterized by dissolving a lactide / epsi-caprolactone copolymer (in a molar ratio ranging from 40 / 60 to 60 / 40) in a solvent, filtering the resultant solution and casting the same followed by air drying.
Owner:GUNZE LTD
Novel composite biological dura mater
The invention discloses a novel composite biological dura mater. According to the dura mater, a dura mater substitute is prepared by compounding SIS (small intestinal submucosa) and collagen sponge, is relatively close to a natural dura mater, has the characteristics of being degradable, plastic and modifiable and does not need stitching. The product is relatively substantially improved in performances, mechanical strength and safety. An innovative crosslinking manner enables the product degradation time to be consistent with newborn tissue growth time, and the product is better than clinical a same kind of products. The state of the artificial dura mater patch is innovatively designed according to the thickness difference of different parts of human dura mater, and defects of conventional clinical dura mater products are effectively solved.
Owner:王伟
Dural graft and method of preparing the same
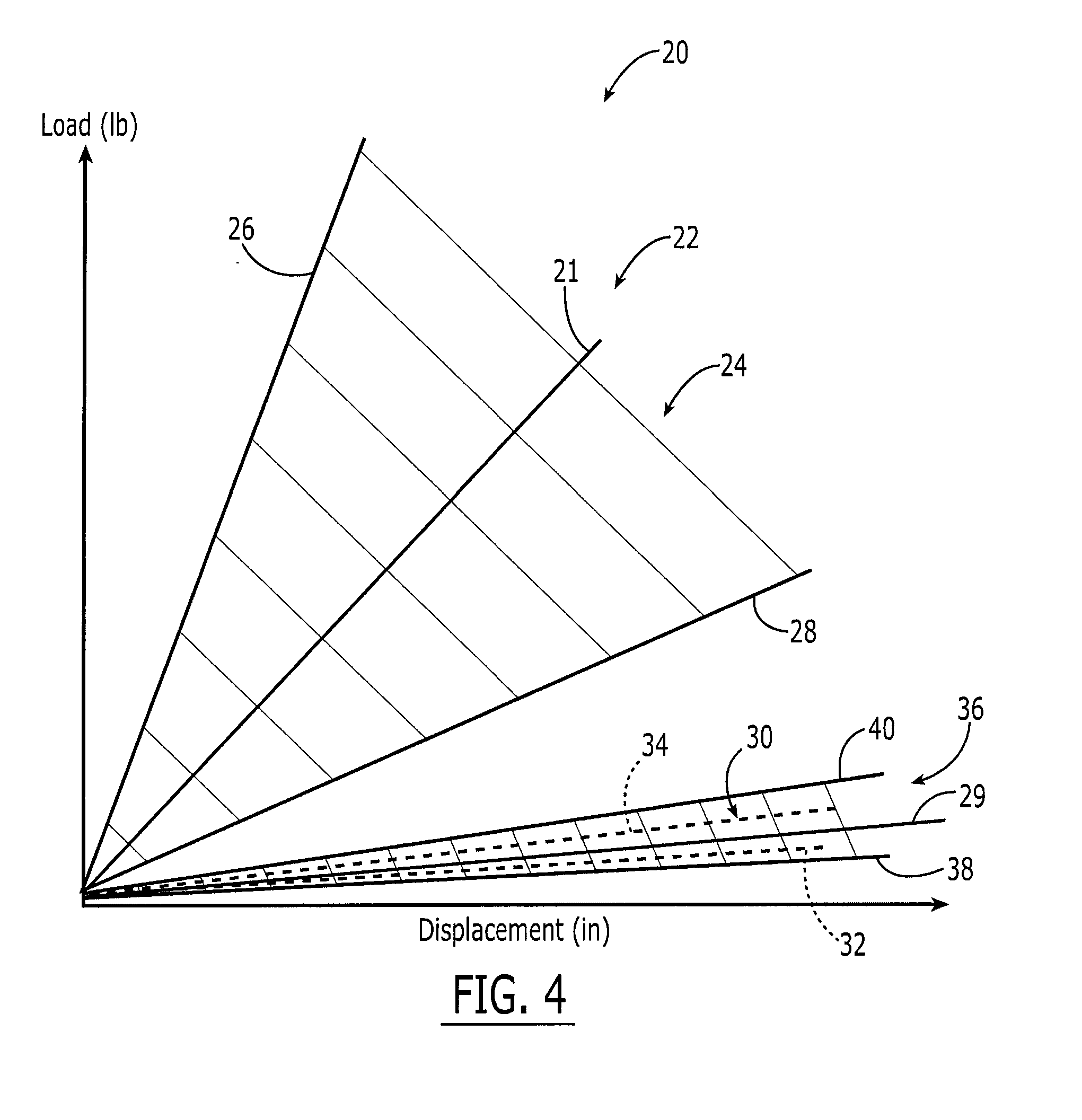
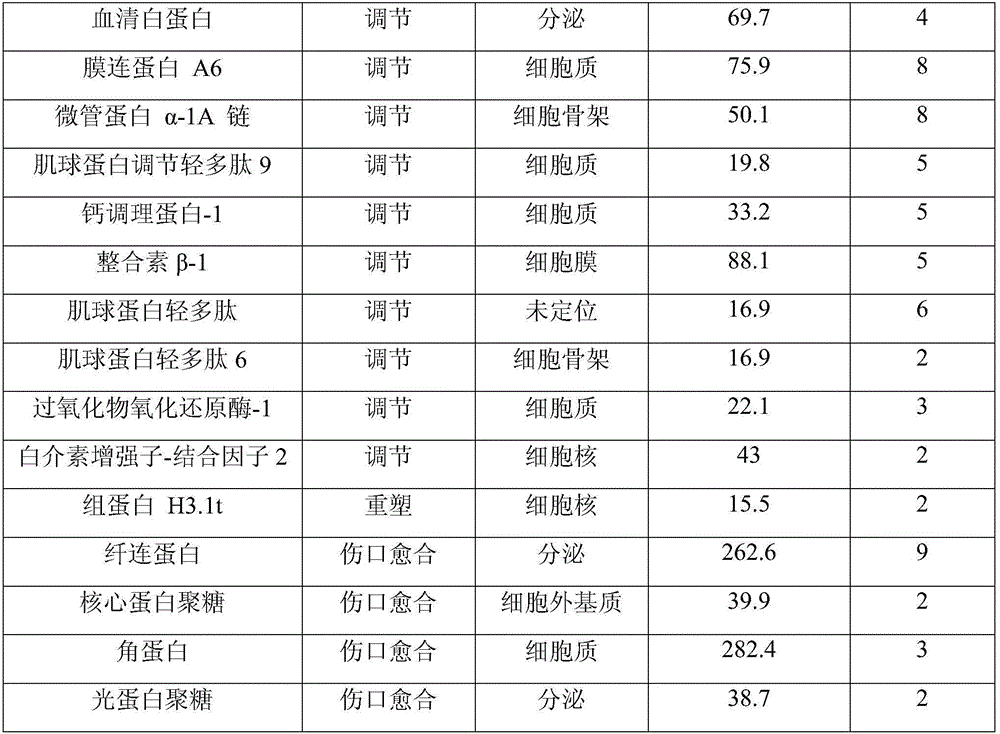
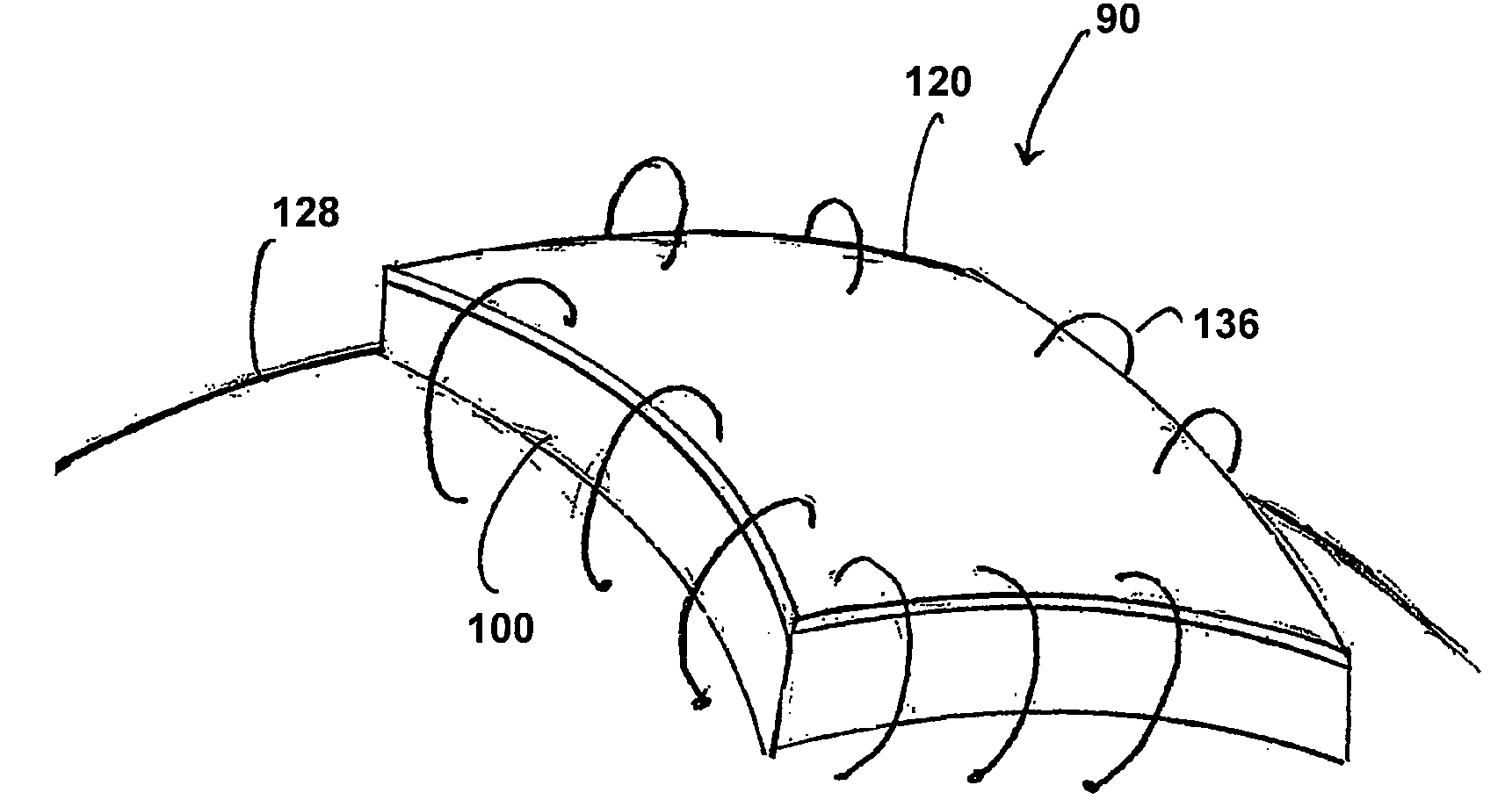
ActiveUS20070073415A1Reduce stiffnessReduced stiffness of materialTubular organ implantsTissue regenerationMeningesPliability
A dural graft is provided having improved stiffness characteristics relative to conventional dural substitutes. The dural graft can be formed from a collagen material having a stiffness between about 0.1 pounds per inch (lb. / in.) and 0.25 lb. / in. Relative to the collagen material forming conventional dural graft substitutes, the decreased stiffness of the collagen material of the present dural graft can provide the graft with a relatively improved or increased pliability. As a result of the increased pliability, the dural graft can sufficiently conform to a curvature of a tissue surface to which it is applied, such as the curved surface of a meningeal membrane. The reduced stiffness of the collagen material can also provide for a relatively improved or increased flexibility or elasticity of the dural graft. The increased flexibility of the dural graft minimizes tearing of the graft when handled or manipulated during an implantation procedure.
Owner:INTEGRA LIFESCI
Artificial dura mater and process for producing dura mater
InactiveUS20030132546A1Easy to degradeExcessive degradationSurgical adhesivesCeramic shaping apparatusPolymer scienceMeninges
The present invention provides an artificial dura mater characterized by comprising at least a sheet of a biodegradable and bioabsorbable synthetic polymer and having a storage modulus at ordinary temperature of from 1x107 to 5x108 (Pa); and a process for producing an artificial dura mater characterized by dissolving a lactide / epsi-caprolactone copolymer (in a molar ratio ranging from 40 / 60 to 60 / 40) in a solvent, filtering the resultant solution and casting the same followed by air drying.
Owner:GUNZE LTD
3D scanning transcranial magnetic stimulation system and transcranial magnetic stimulation method
InactiveCN109173063ASimple methodEasy to operateMagnetotherapy using coils/electromagnetsMeningesImage matching
The invention discloses a 3D scanning transcranial magnetic stimulation system and a transcranial magnetic stimulation method. The system comprises: a 3D scanning modeling system. The 3D scanning modeling system uses a 3D scanner to scan a head image of a tester and establish a head mold image in real time; 2, a matching positioning system, wherein the matching positioning system matches the headmold image established by the 3D scanning modeling system with meninges to precisely locate stimulation points; 3, a t system, wherein that test system manipulate a robot to perform transcranial magnetic stimulation according to a positioning stimulation point; 4, a fNIRS signal acquisition system, wherein that fNIRS signal acquisition system is use for acquiring near-infrared signal of a tester'sbrain region, comparing changes of near-infrared signal in real time, accurately finding out insufficient stimulation or excessive stimulation, and feeding back the result to the test system. In order to accurately perform transcranial magnetic stimulation (TMS), 3D head-mold images are matched with meninges and a real-time fNIRS signal acquisition system is used to establish a simulation experiment.
Owner:WUHAN ZNION TECH CO LTD
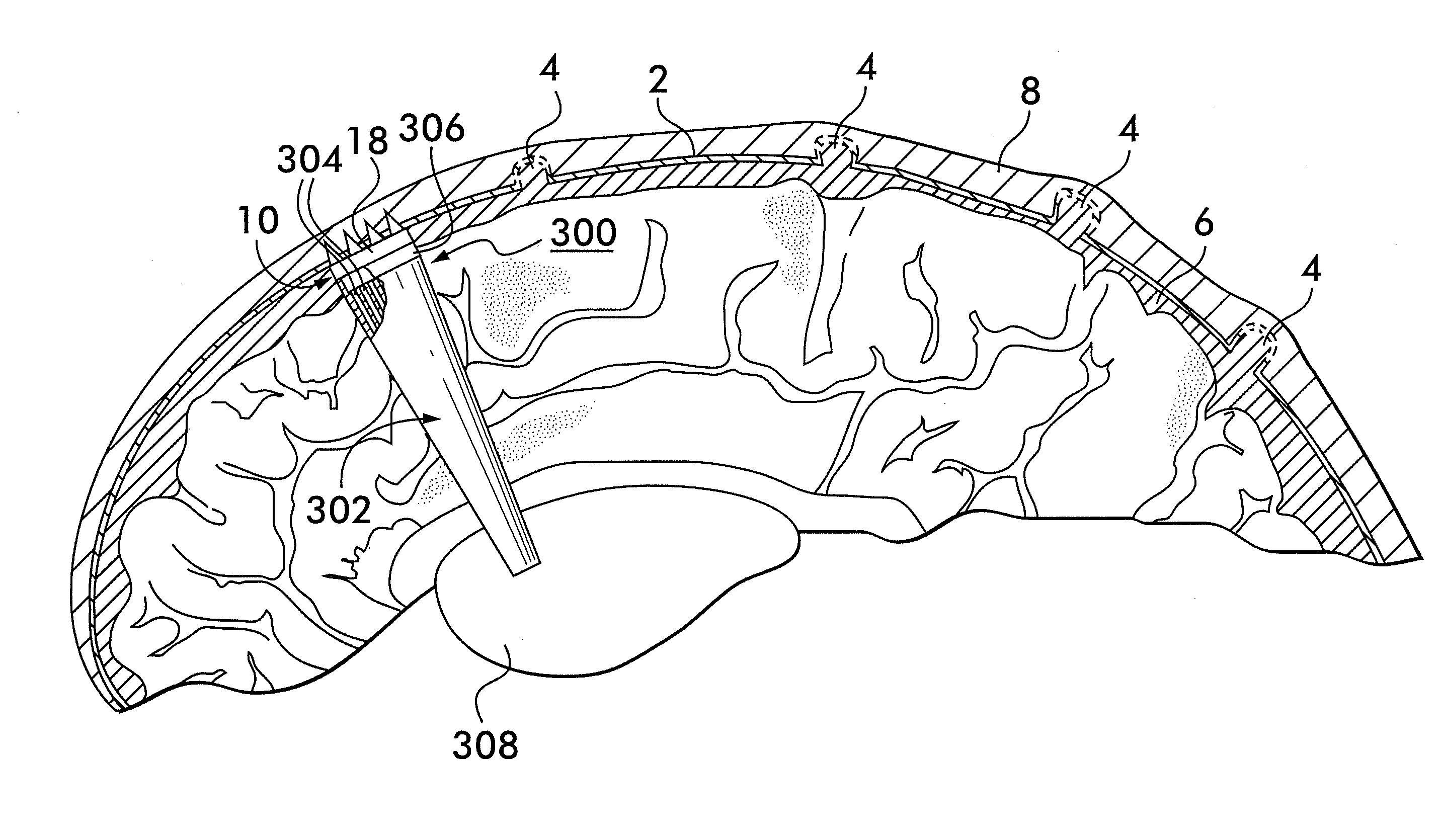
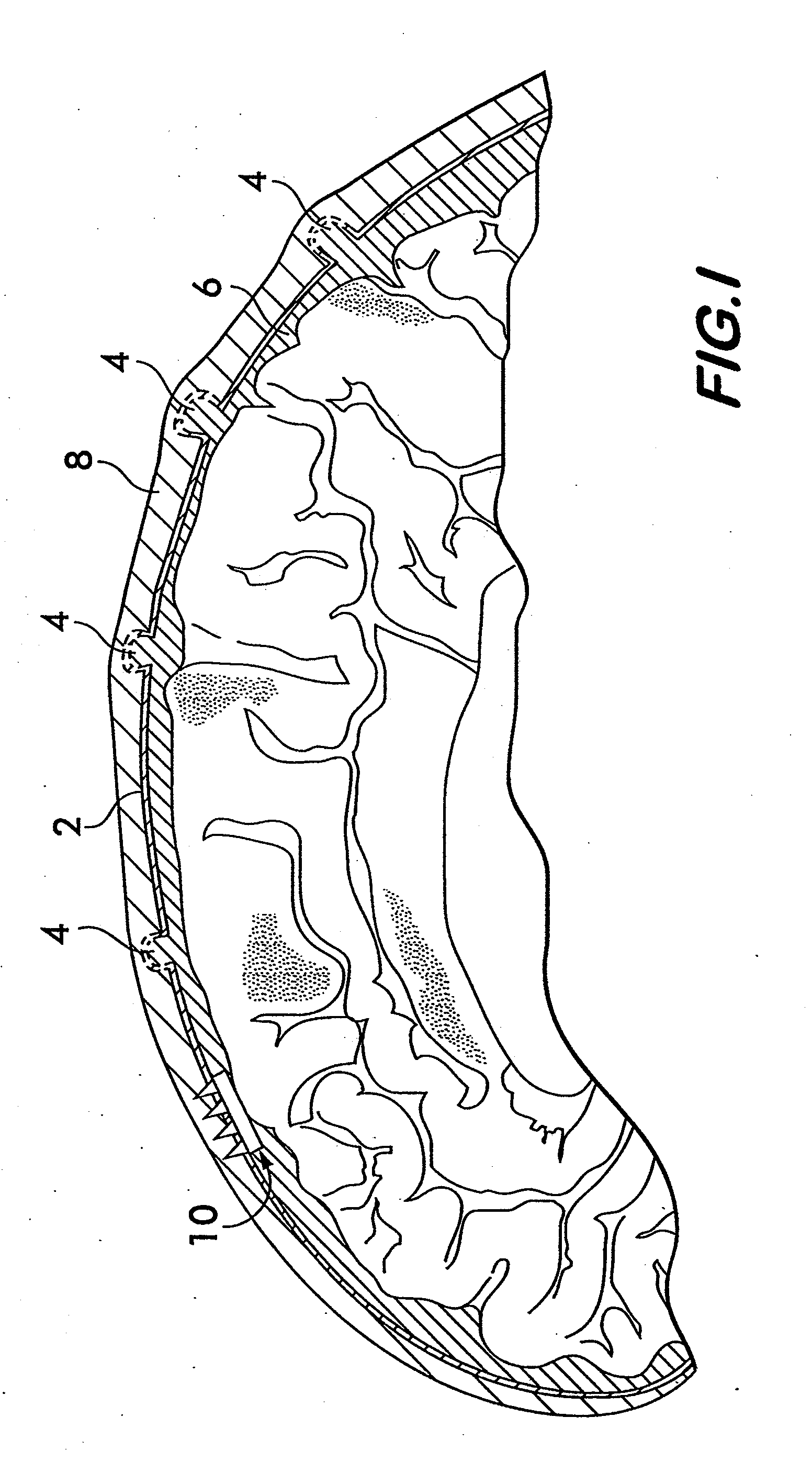
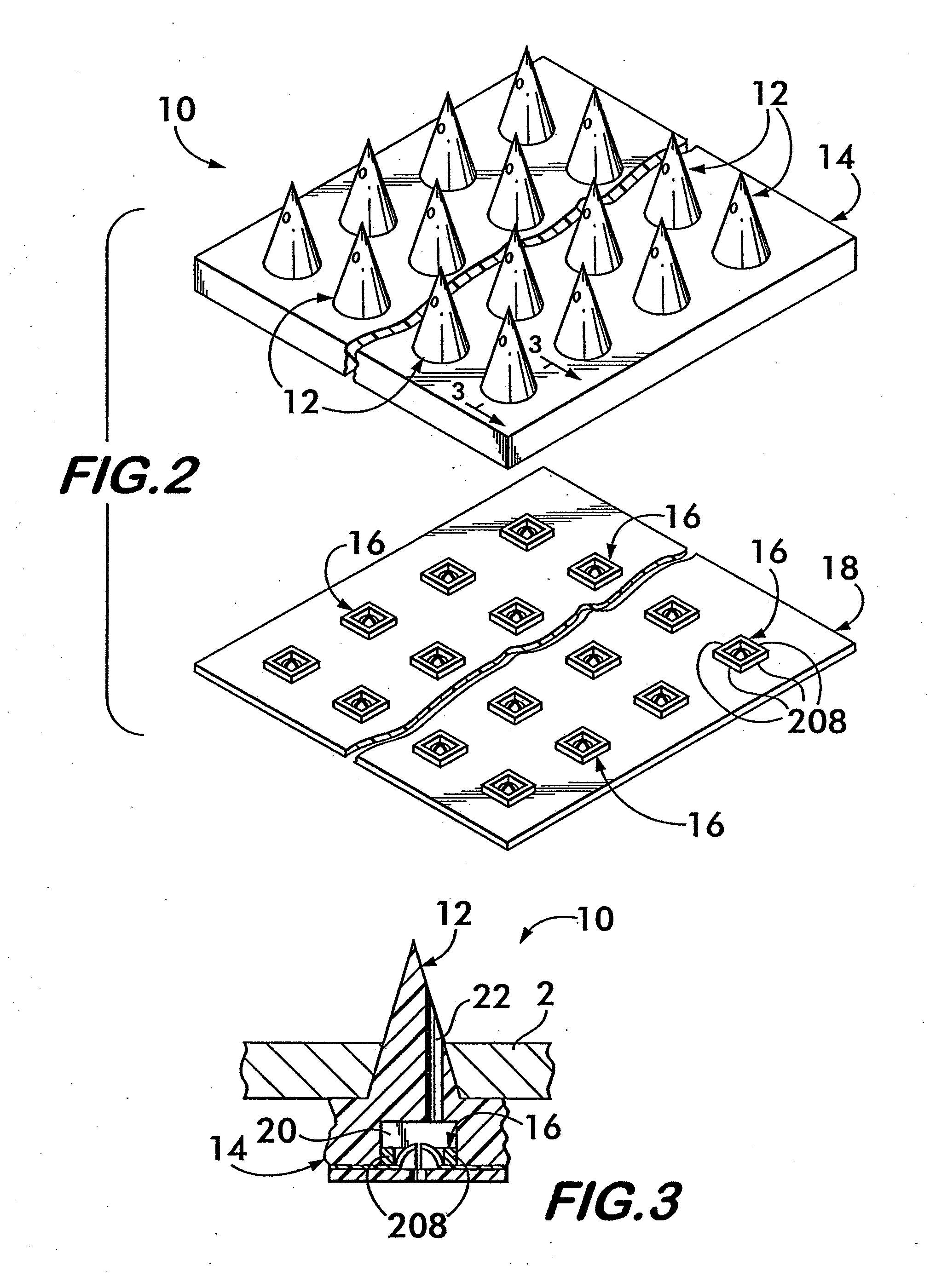
Method and apparatus for securing a neuromodulation lead to nervous tissue or tissue surrounding the nervous system
An apparatus for securing a neuromodulation lead to nervous tissue or surrounding tissue (i.e. meninges) is provided. The neuromodulation lead includes an elongated member having a proximal end, a distal end, and a passage extending there between. The lead further includes at least one electrical contact secured around an outer surface of the distal end of the elongated member for stimulating nervous tissue. The apparatus comprises first and second tubular members are disposed within the passage of the elongated member. Each of the tubular members terminates at the distal end of the elongated member. First and second glue components are provided for injecting into the first and second tubular members, respectively. The first and second glue components, when injected, exit the respective tubular members at the distal end of the elongated member and mix to create a solidified glue that anchors the distal end of the elongated member within the nervous tissue.
Owner:THE CLEVELAND CLINIC FOUND
Composite extracellular matrix ingredient biological material
ActiveCN105920669ALight adhesionImprove adhesionTissue regenerationProsthesisCell-Extracellular MatrixAdditive ingredient
Owner:SHANGHAI EXCELLENCE MEDICAL TECH CO LTD
Collagen device and method of preparing the same
ActiveUS20090030526A1Easy to operateGood strength performanceTissue regenerationProsthesisMeningesUltimate tensile strength
A laminated, bioimplantable dural graft product is configured for use as both an onlay graft and a suturable graft. The dural graft product is sufficiently pliable so as to sufficiently conform to a curvature of a tissue surface to which it is applied, such as the curved surface of a meningeal membrane. The use of the graft product can have improved properties, including suture retention strength and fluid impermeability. To use the dural graft product as an implant to replace, reinforce or strengthen bodily tissue, or to act as an adhesion barrier, the dural graft is placed in contact with bodily tissue and conforms to the curvature of the bodily tissue. Sutures can be used to maintain the contact between the dural graft and the bodily tissue.
Owner:INTEGRA LIFESCI
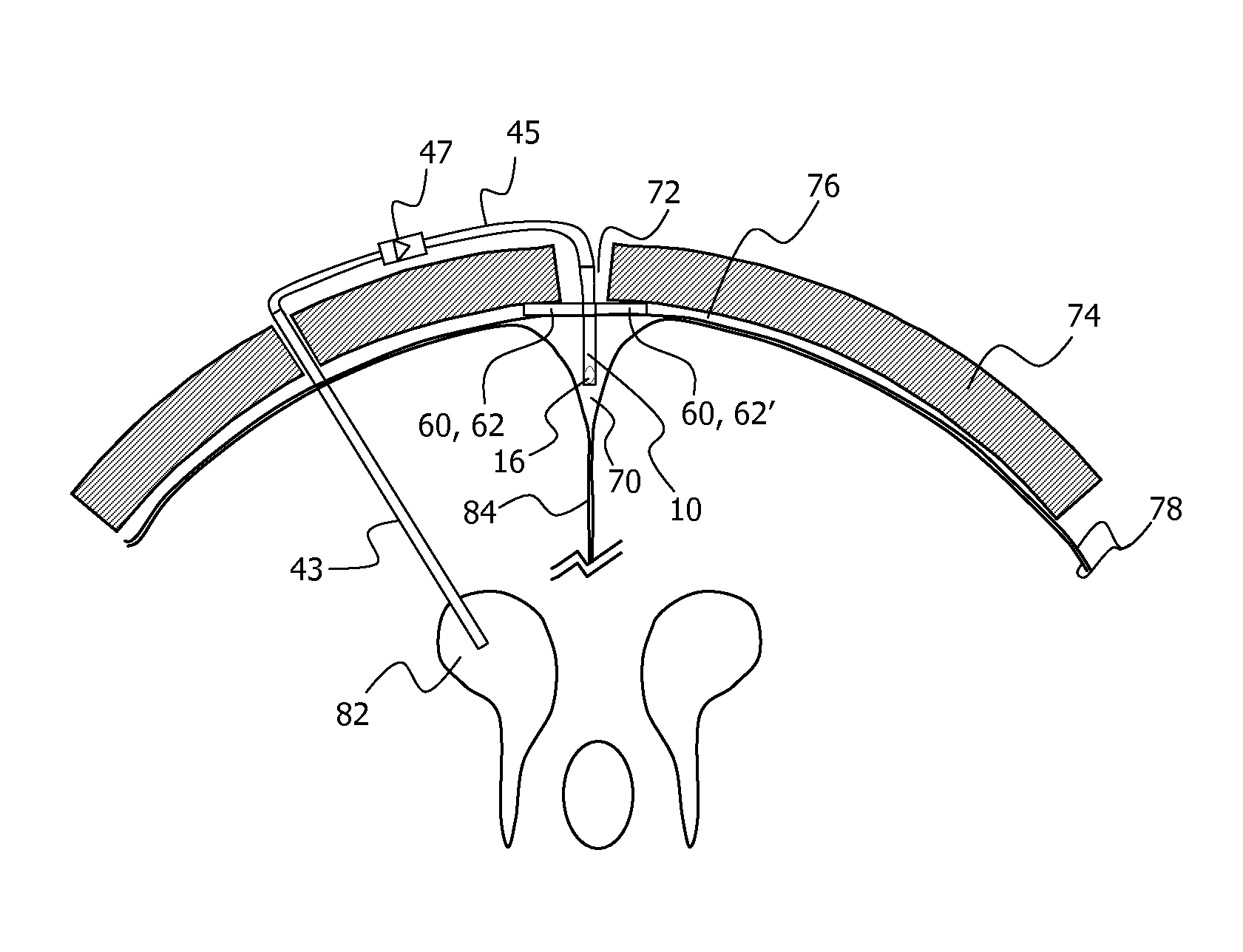
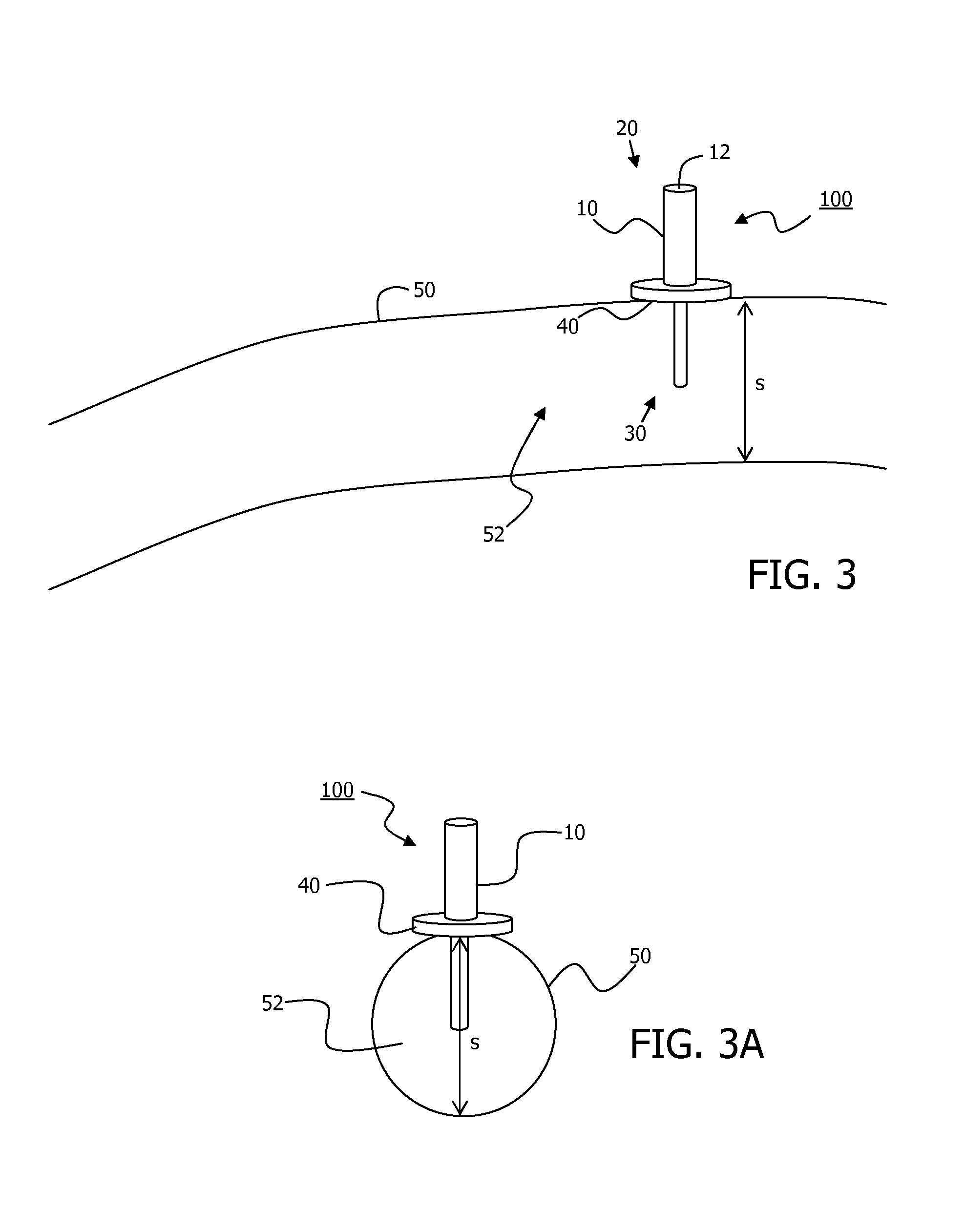
Minimally-advancing luminal catheter
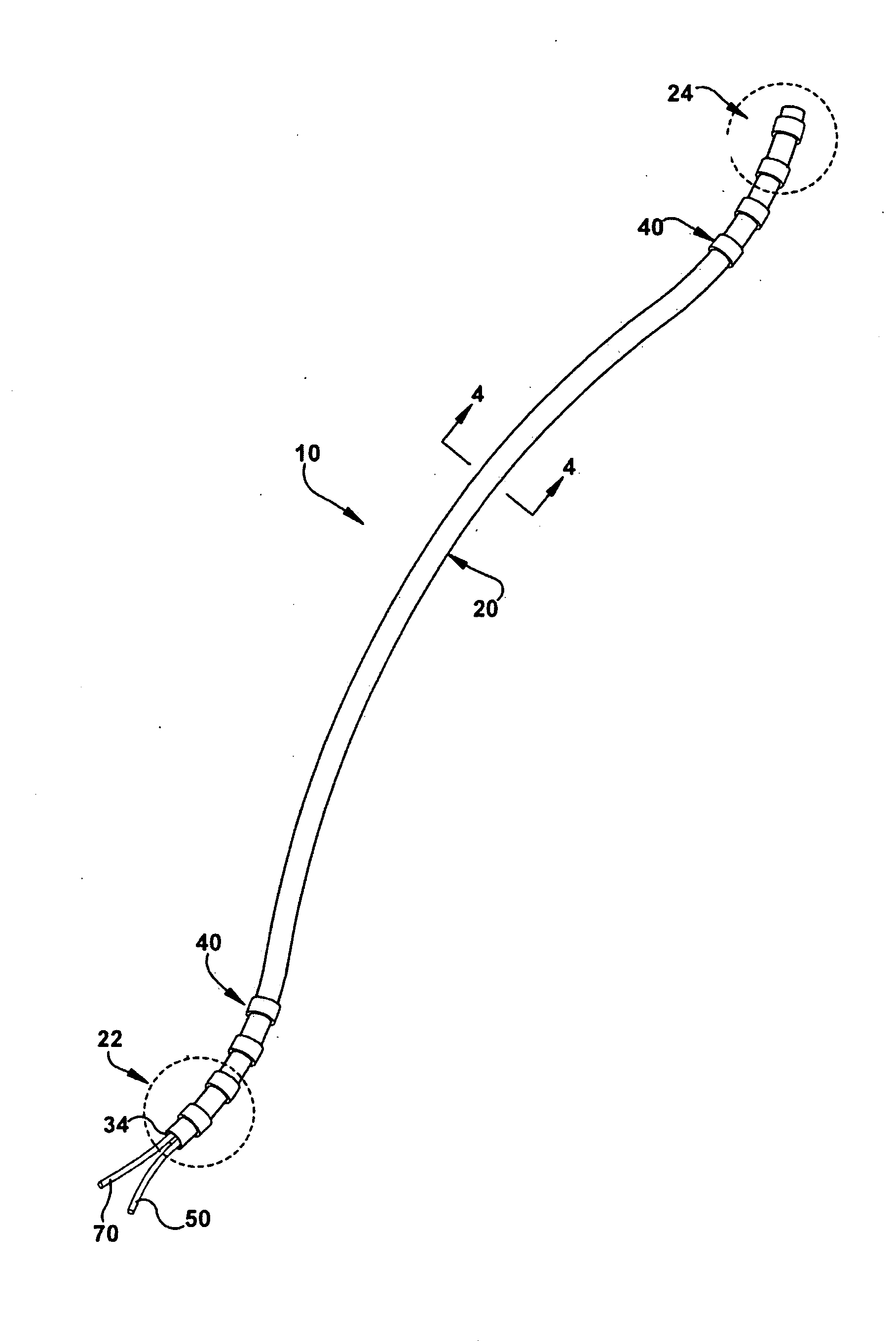
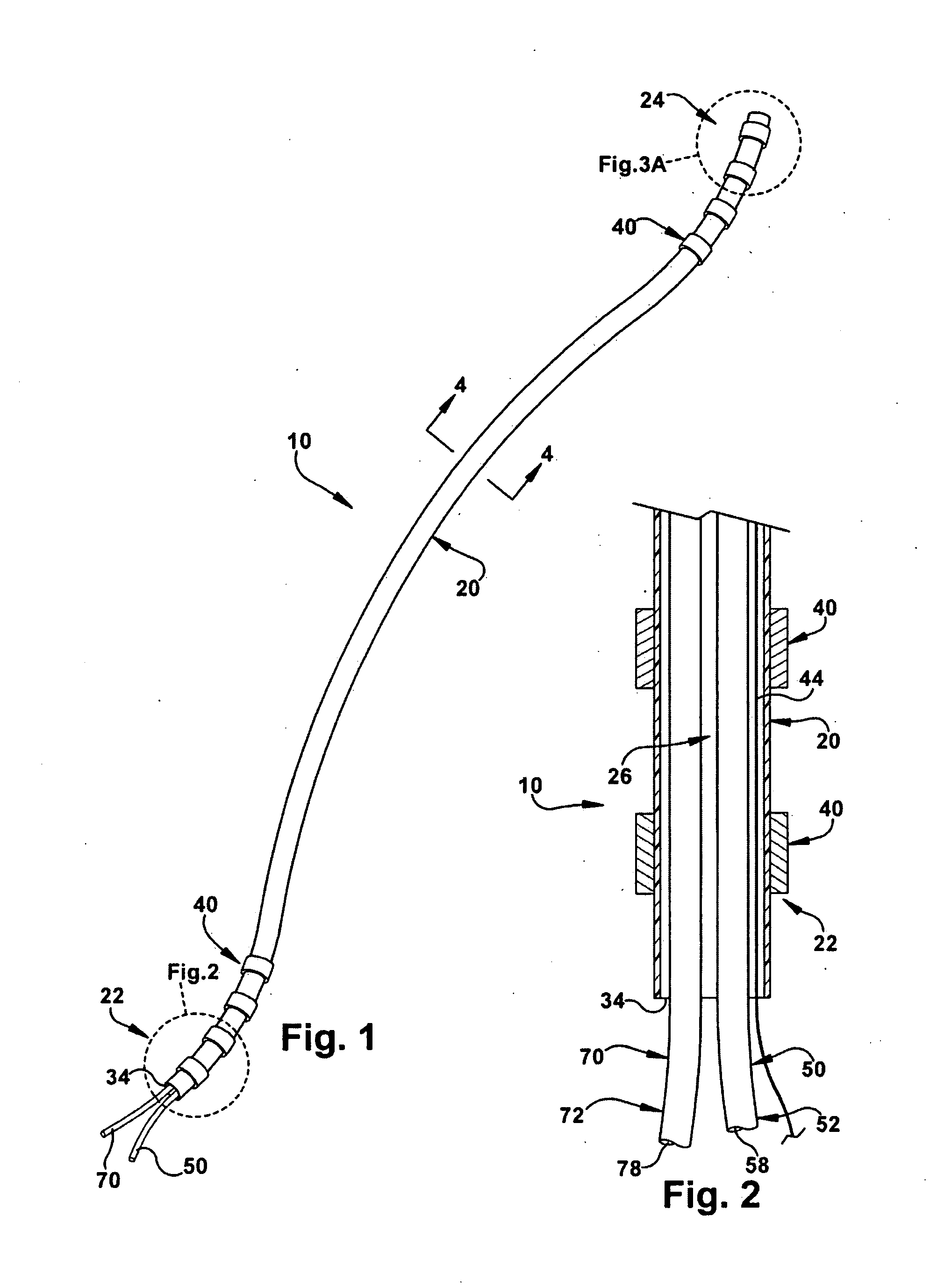
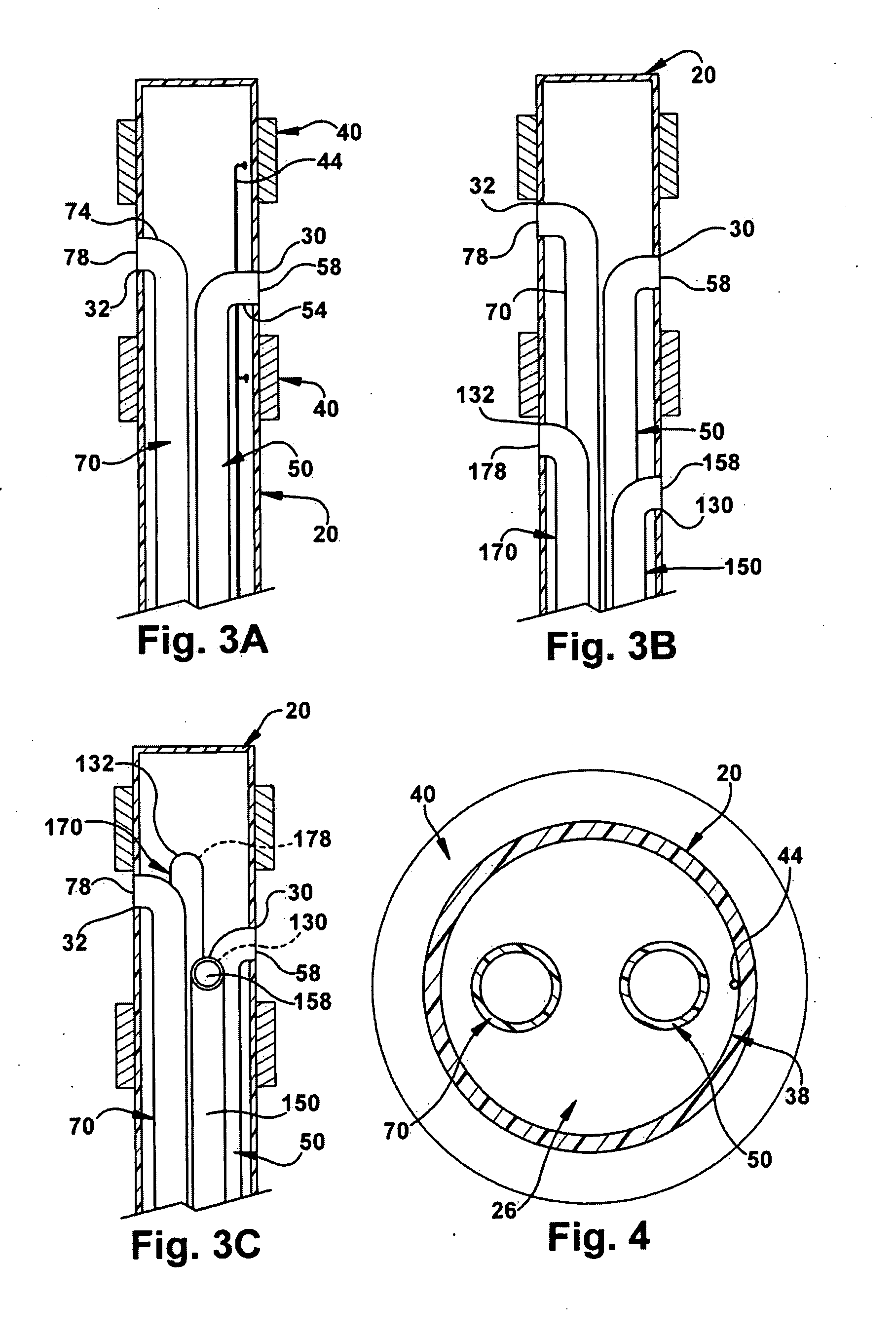
The present invention relates to an implantable catheter (100) provided for insertion through a wall (50) of a dural venous sinus (70) in a subject, having a proximal (20) and distal end (30), comprising: —a tubular shaft (10) for insertion through the wall (50) of the venous sinus (70) into the sinus (52), provided with a shaft lumen (12) in fluid connection with a proximal port (14) at the proximal end and a distal port (16) at the distal end of the shaft (10), and —a stop element (40) disposed on an outer surface of the tubular shaft (10), configured to limit the depth of insertion of the tubular shaft (10) into the sinus.
Owner:STEERABLE INSTR NV +1
System that secures an electrode array to the spinal cord for treating back pain
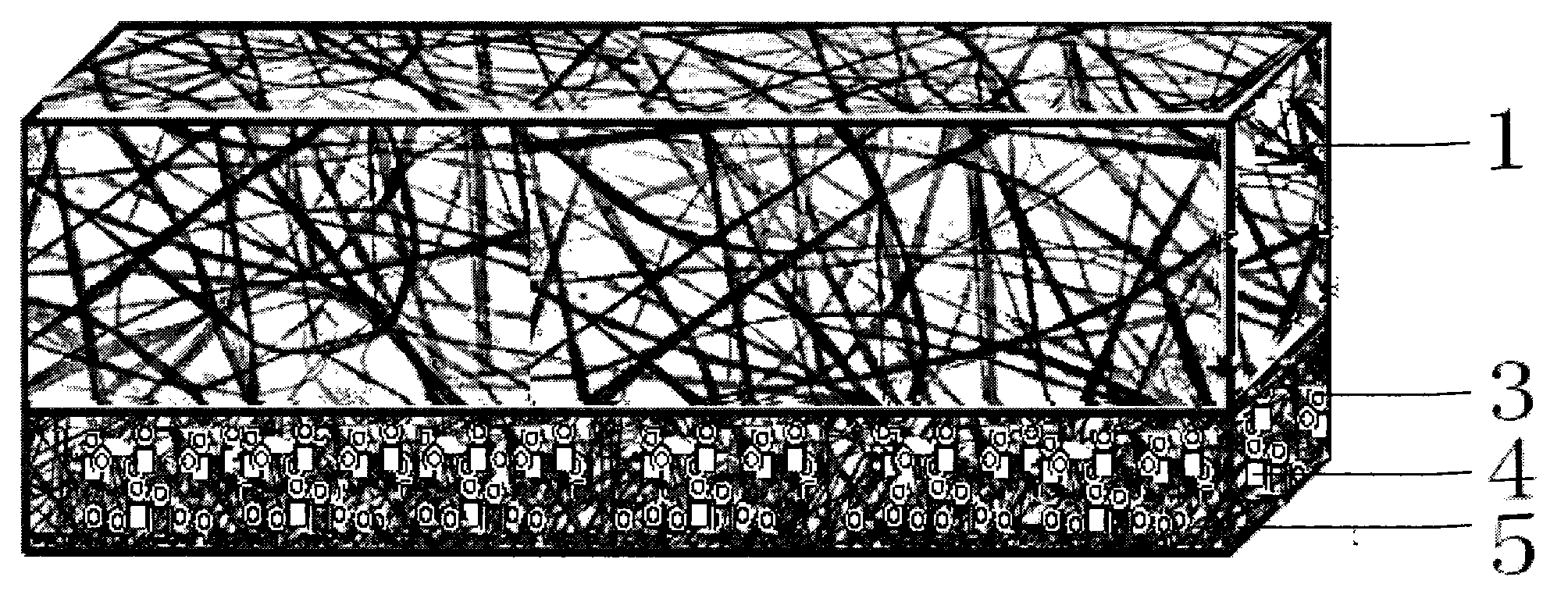
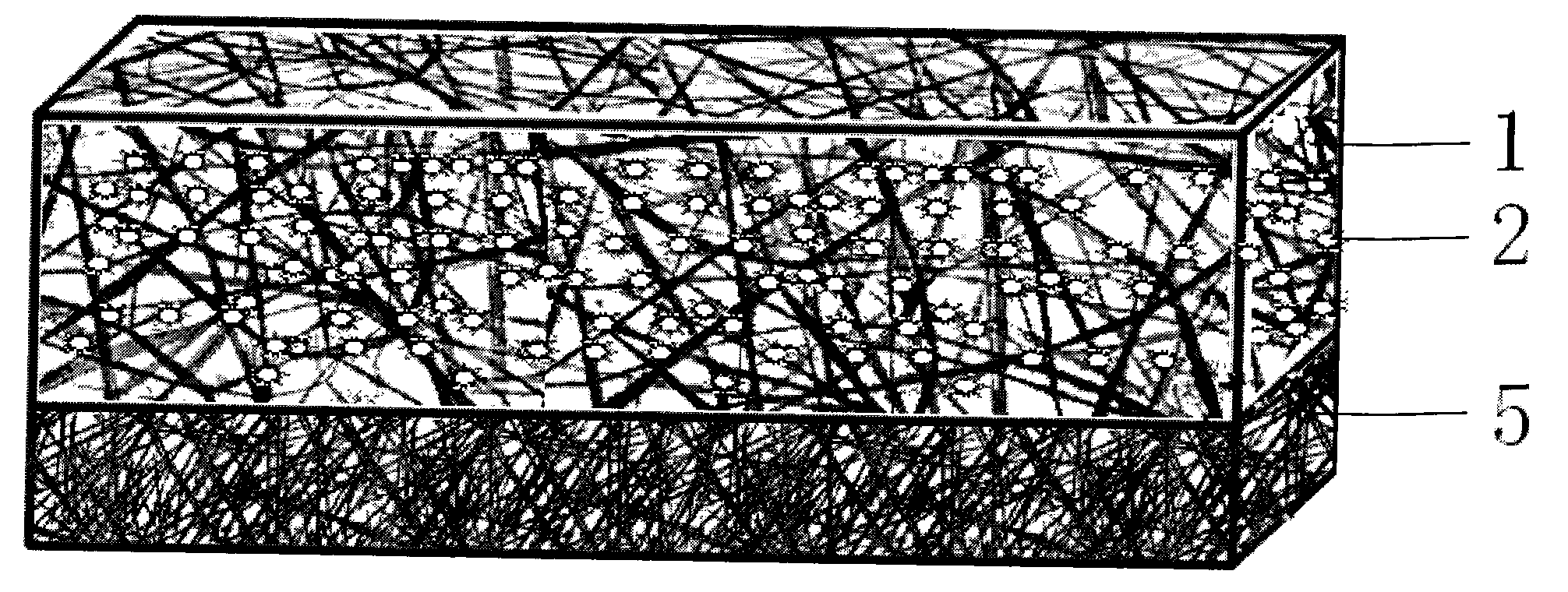
ActiveUS20140371830A1Increase surface areaAvoid liftingSpinal electrodesMachines/enginesAnatomical structuresMedicine
This invention provides a device for implantation directly into the spinal cord for the purpose of treating back pain. Electrodes on a backing that conforms directly to the spinal cord are installed as a source of electrical stimulation and pain relief. The electrode array is maintained on the spinal cord at a chosen location by way of a spring or support structure that is anchored to an anatomical structure outside the spinal cord but near the site of implantation. Suitable anchoring structures include the vertebrae and the dura. Secured in this fashion, the support structure maintains a gentle pressure of the electrode array against the spinal cord so as to stay in electrical contact but minimize injury or inflammation. The device may accommodate and buffer movement of the spinal cord both laterally and in a caudal-rostral fashion so that the electrode array remains in place.
Owner:UNIV OF IOWA RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com