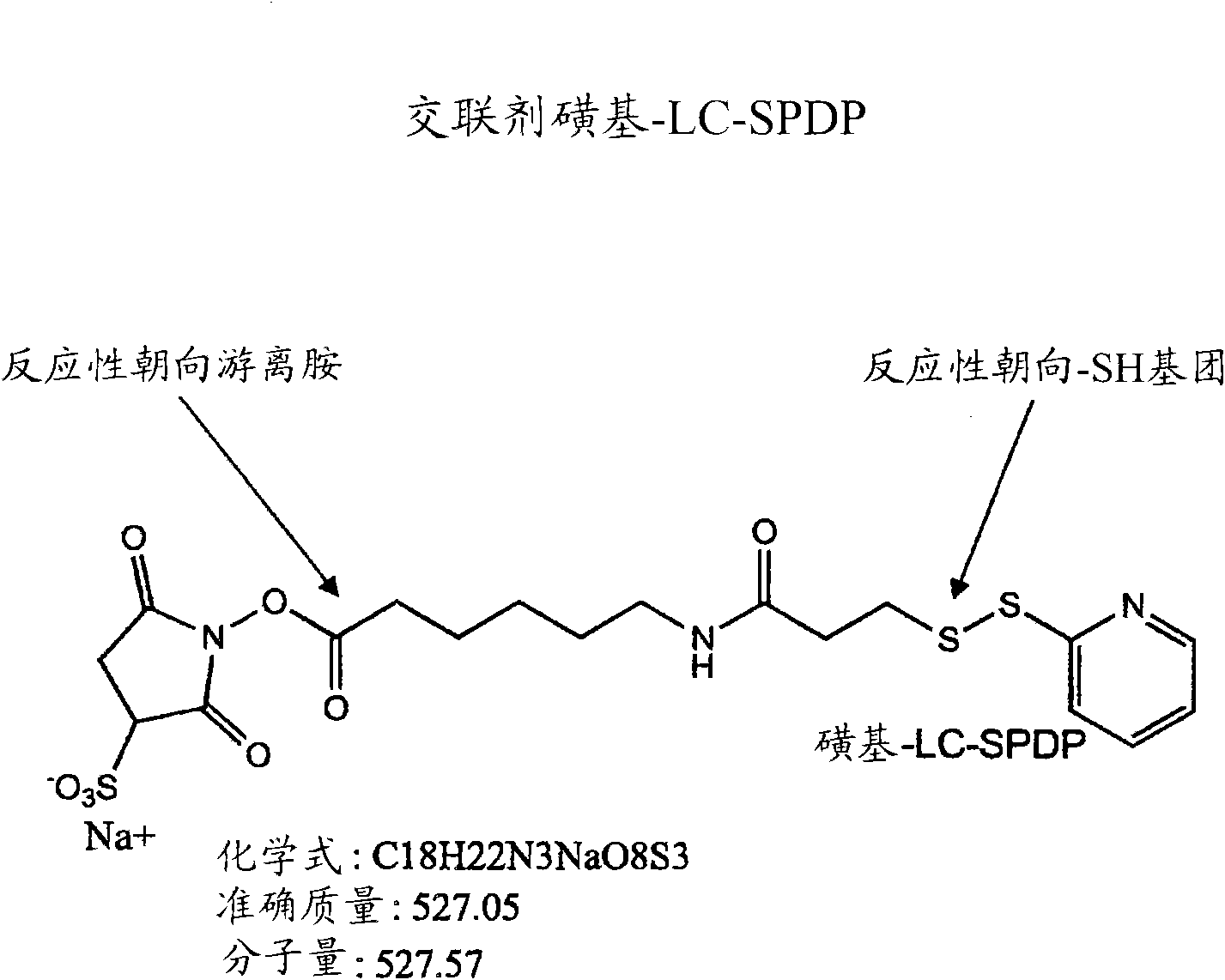
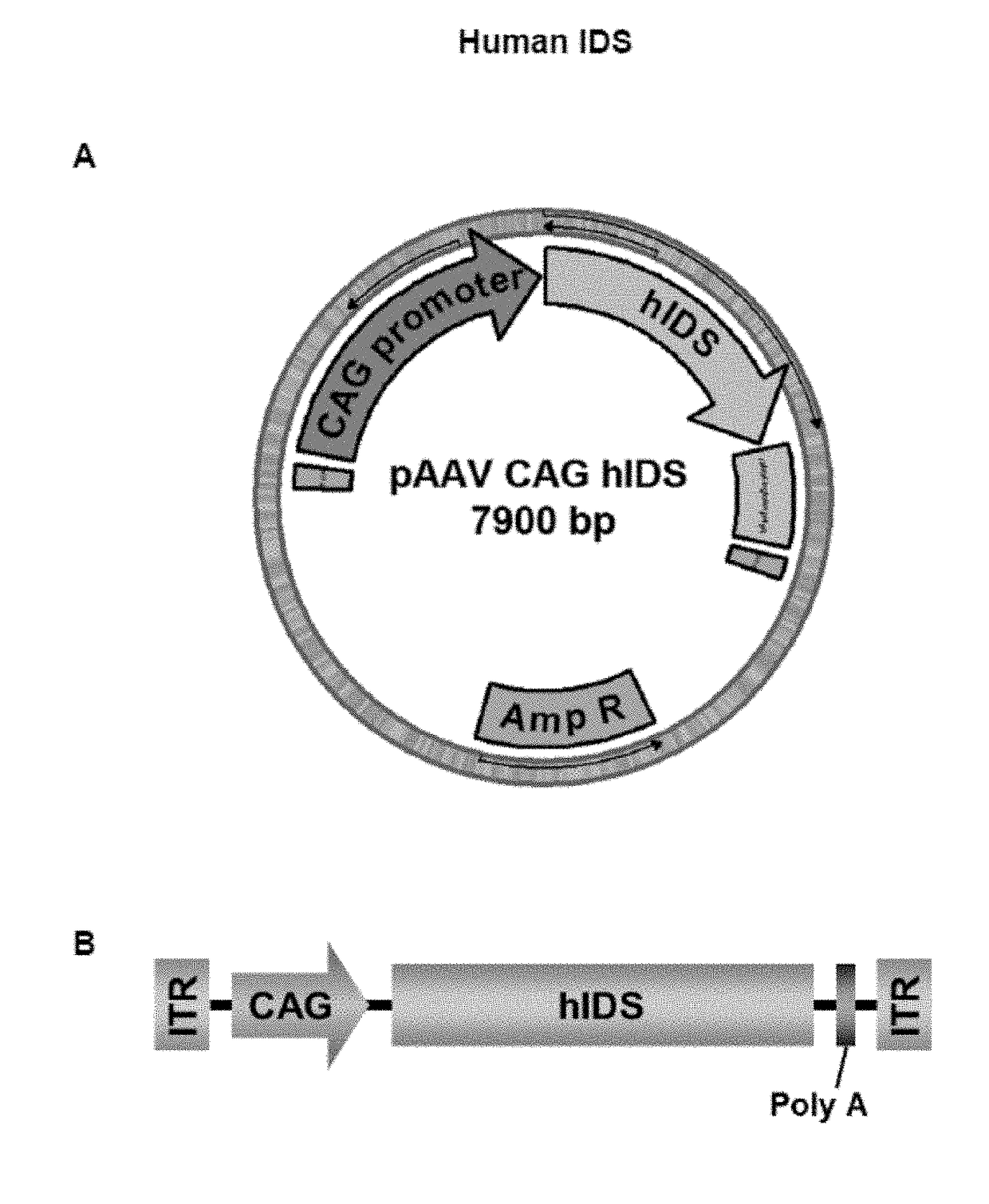
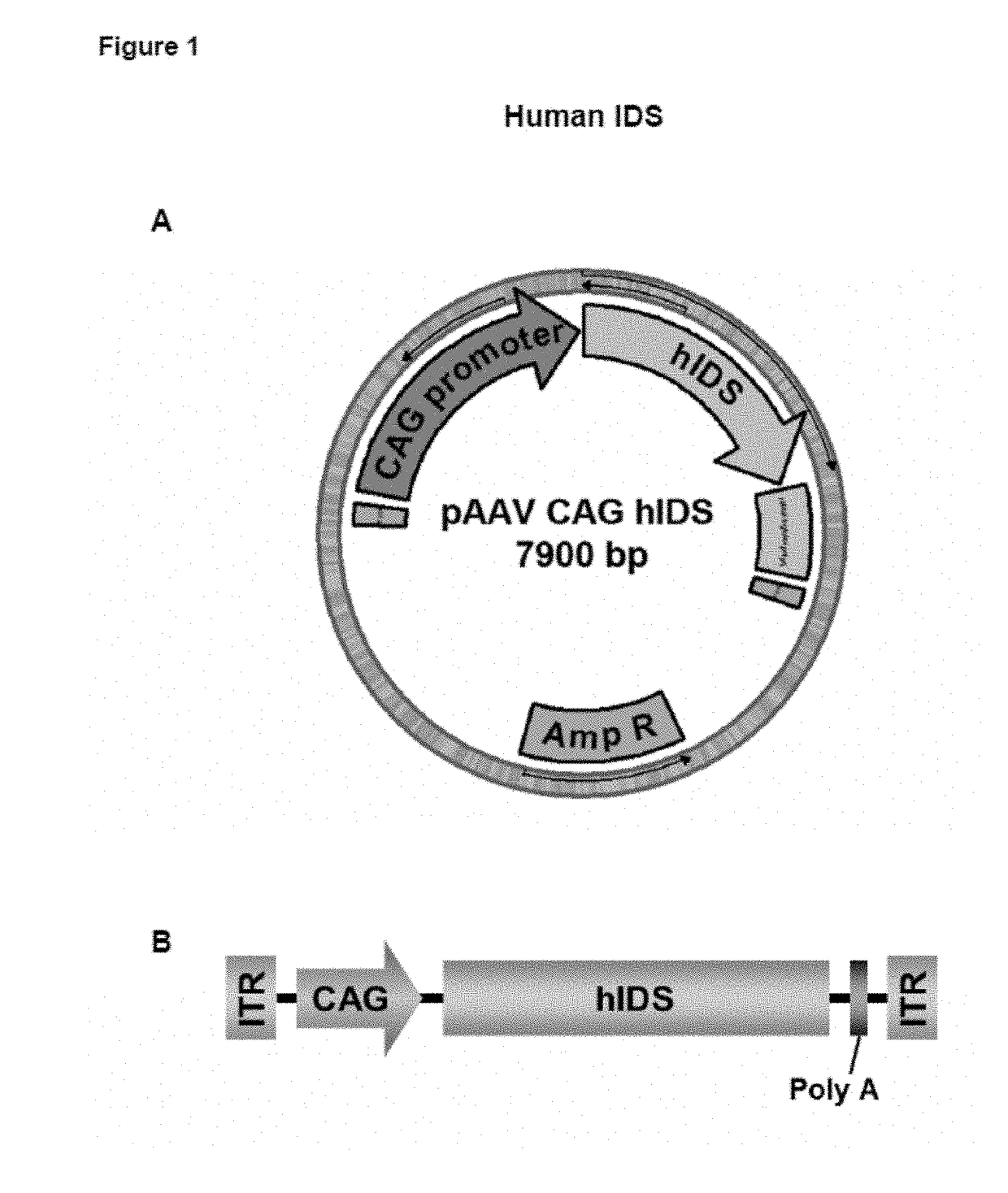
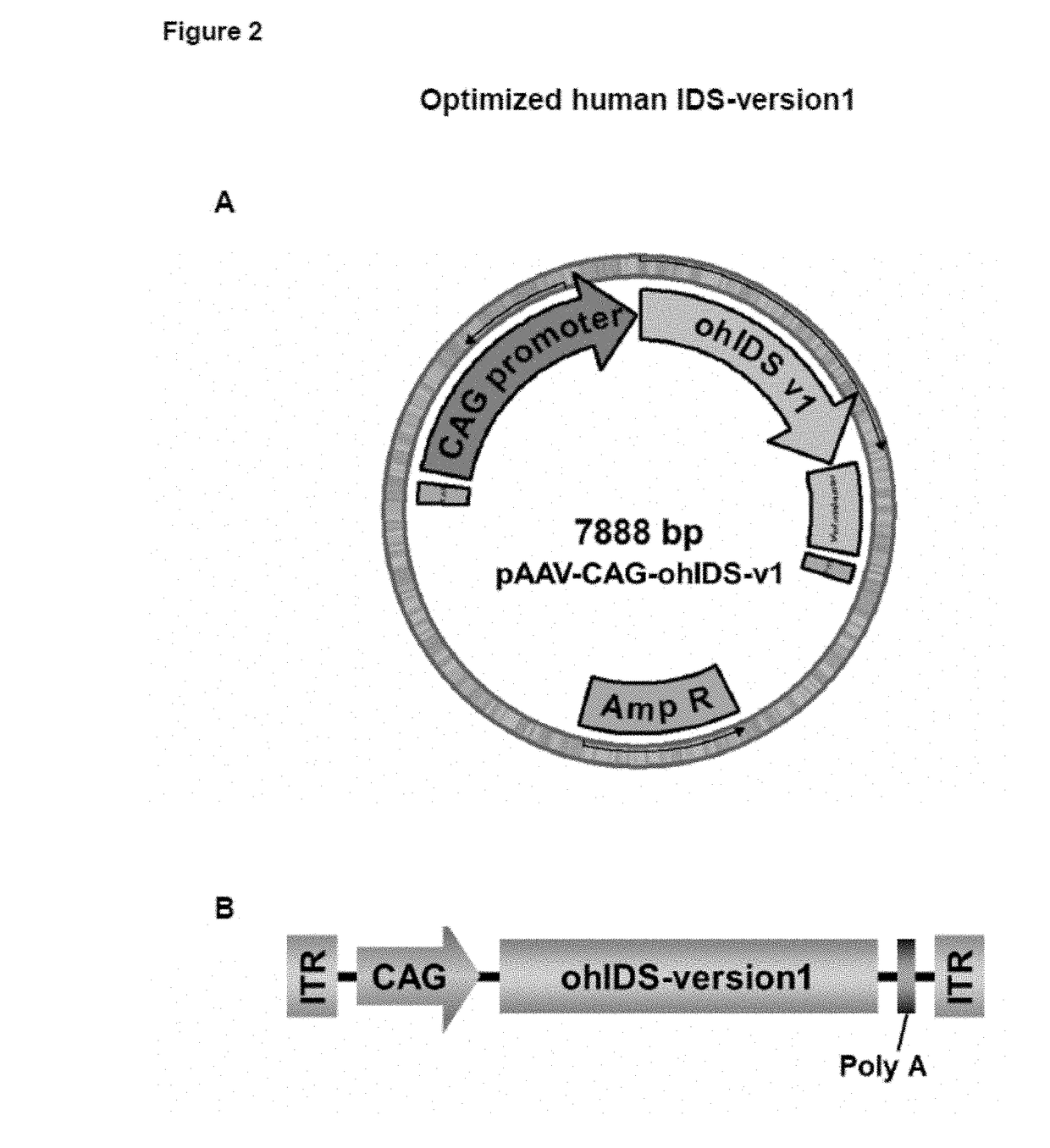
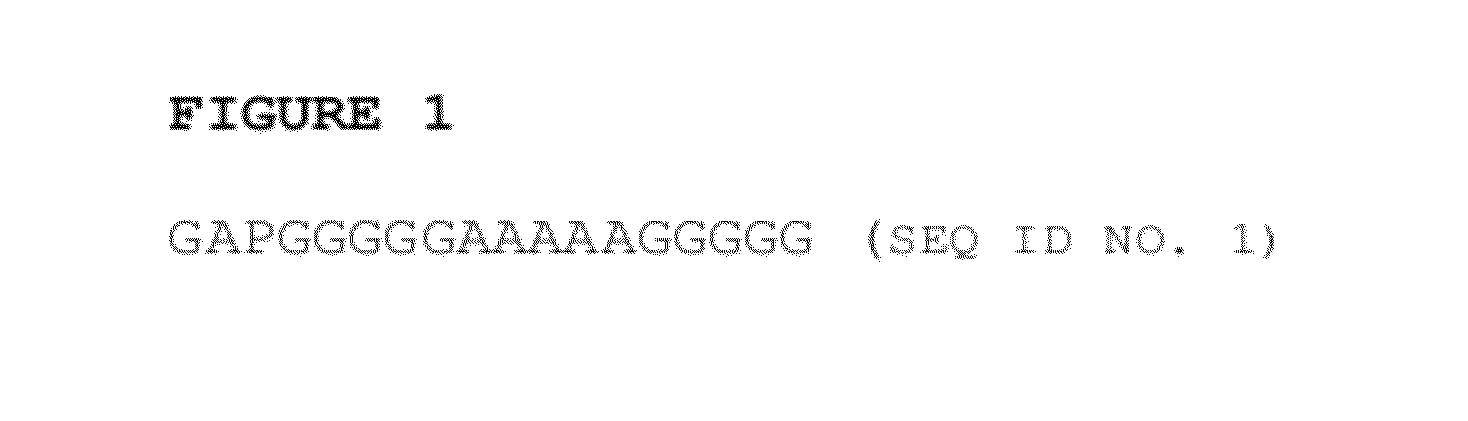Patents
Literature
83 results about "Lysosomal storage disorders" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
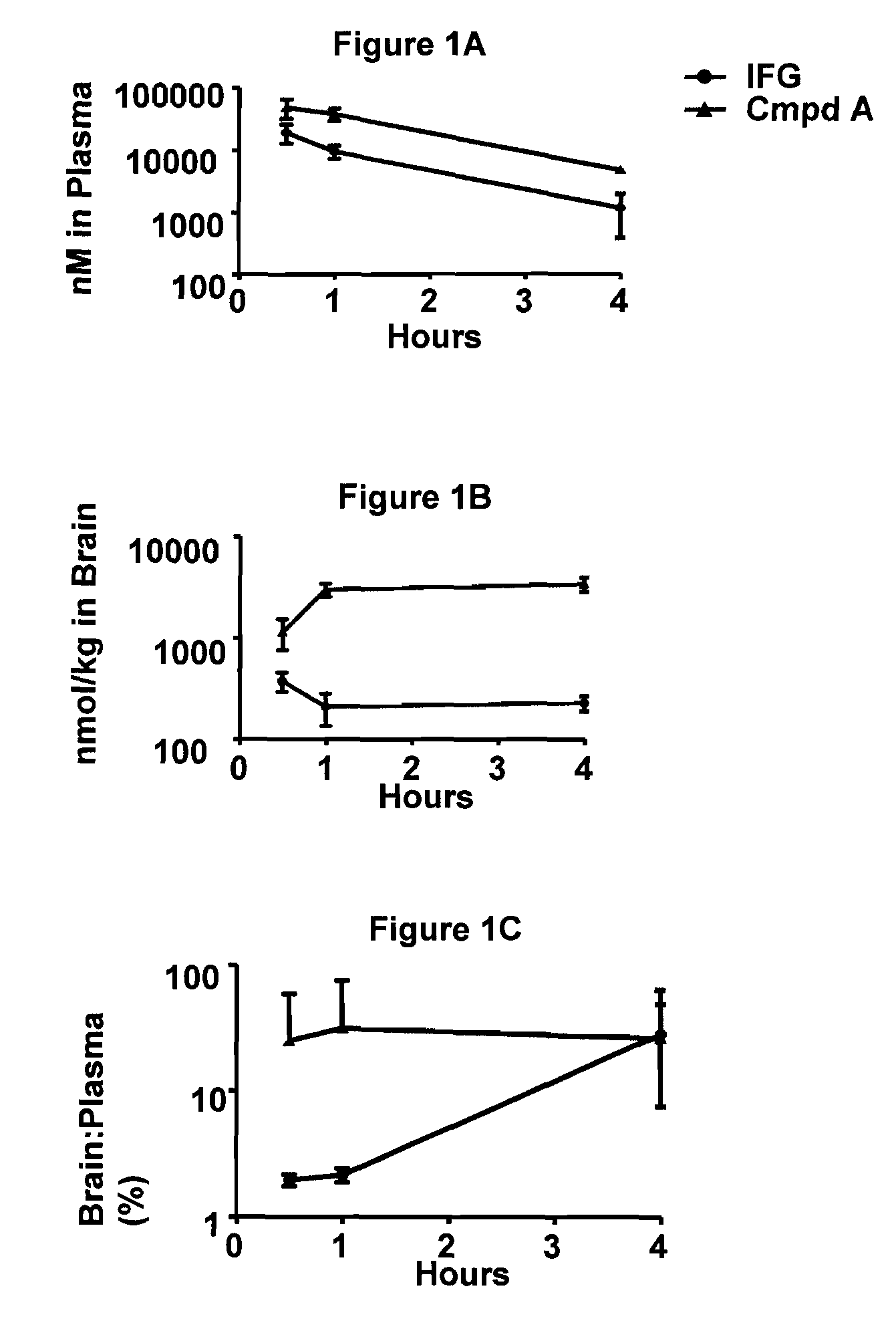
Gaucher disease is one of the most common lysosomal storage disorders (LSDs). LSDs are inherited disorders resulting from a lack of specific enzymes that break down certain lipids (fats) or carbohydrates (sugars) in the body cells.
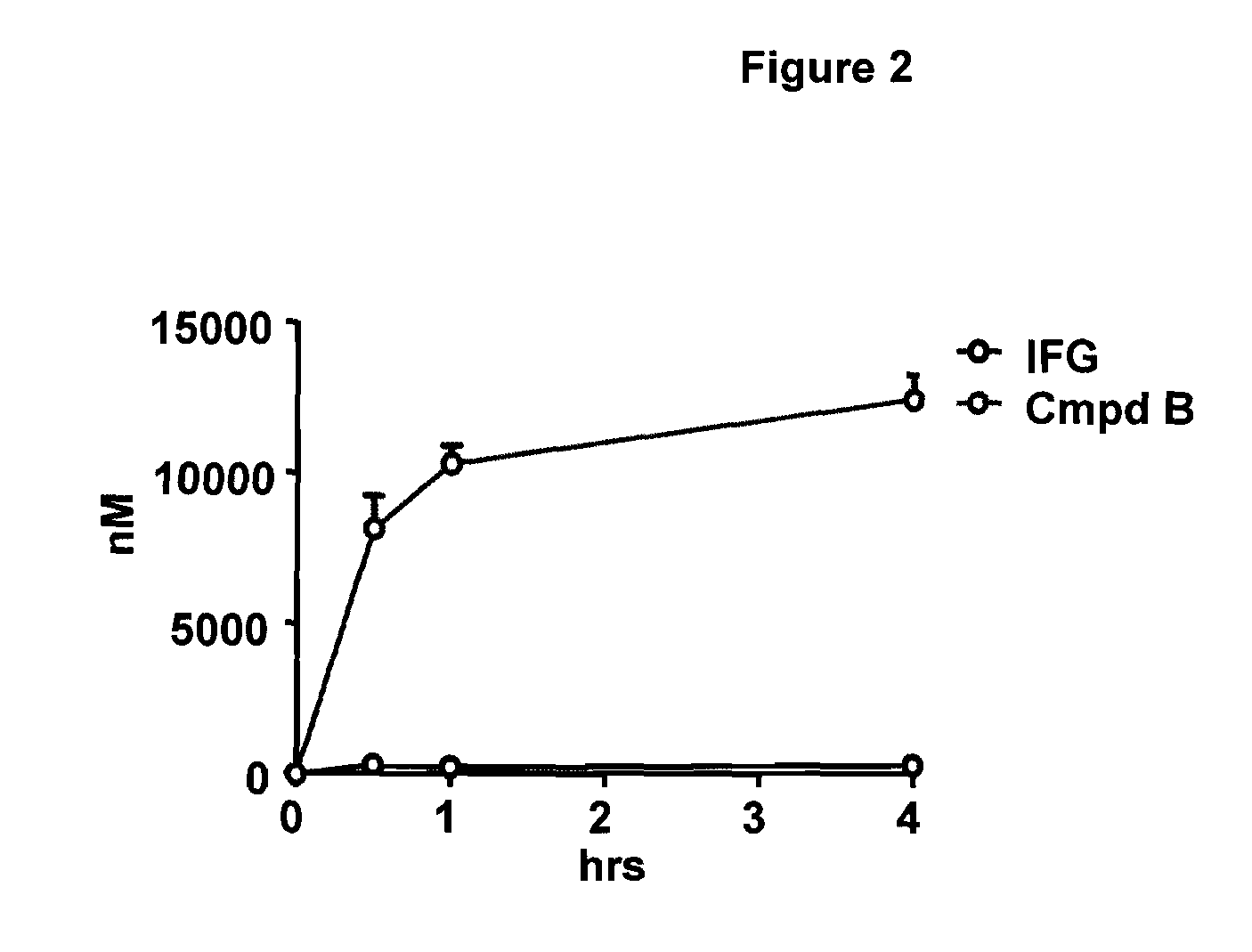
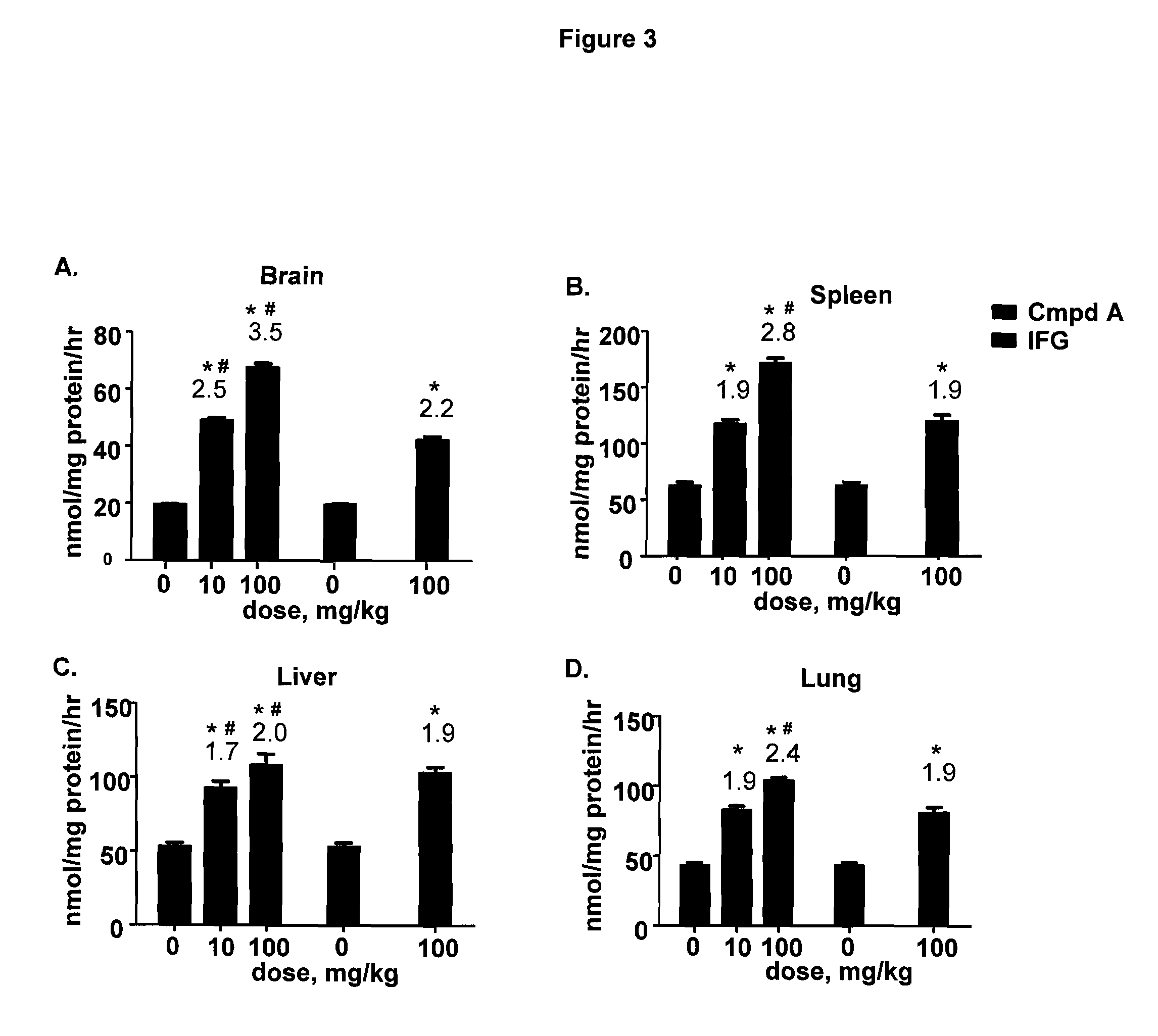
Delivery of therapeutic compounds to the brain and other tissues
ActiveUS20050048047A1Reduces and eliminates glycogen storage granuleReduce eliminateNervous disorderPeptide/protein ingredientsMeningesIntrathecal use
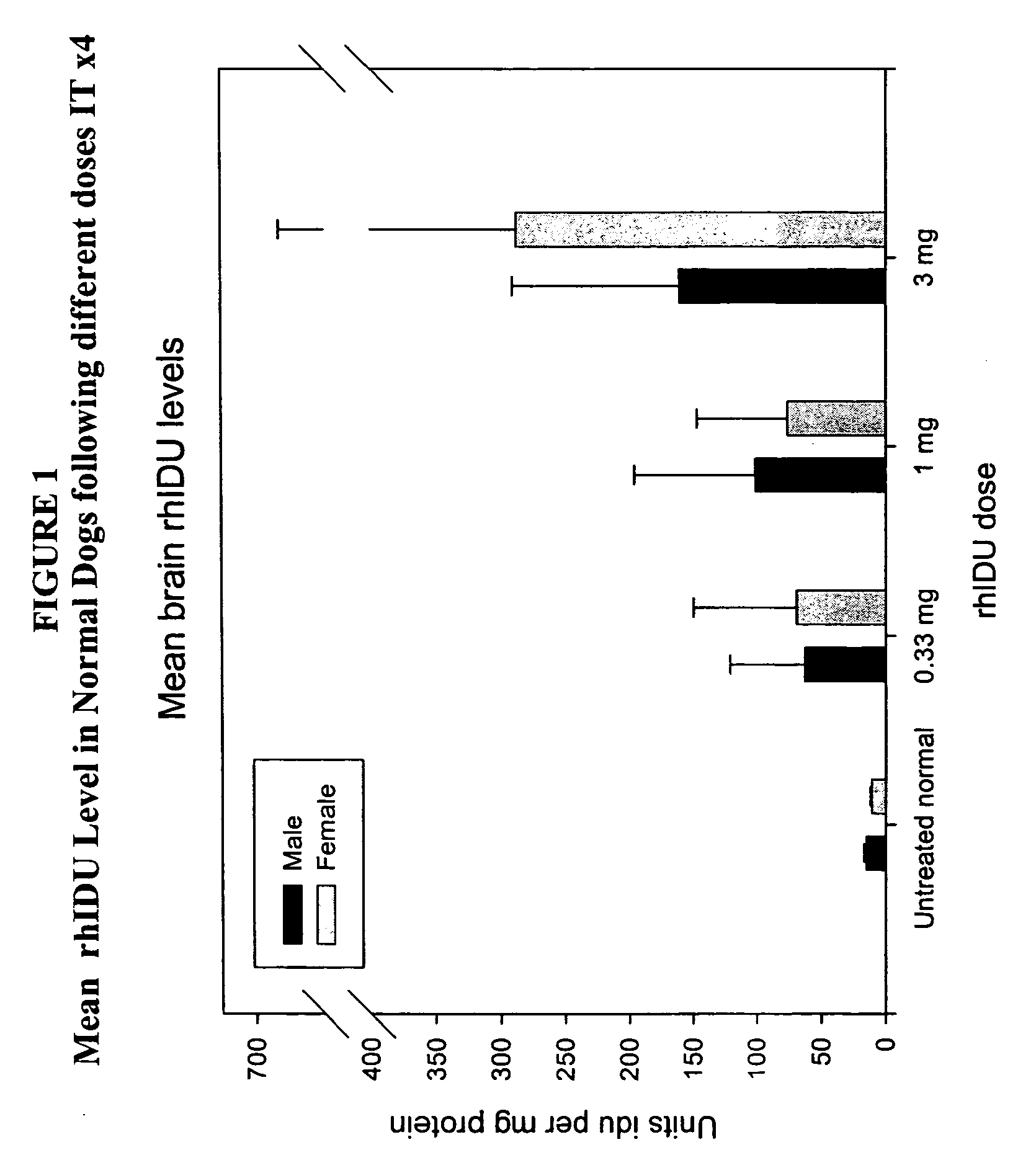
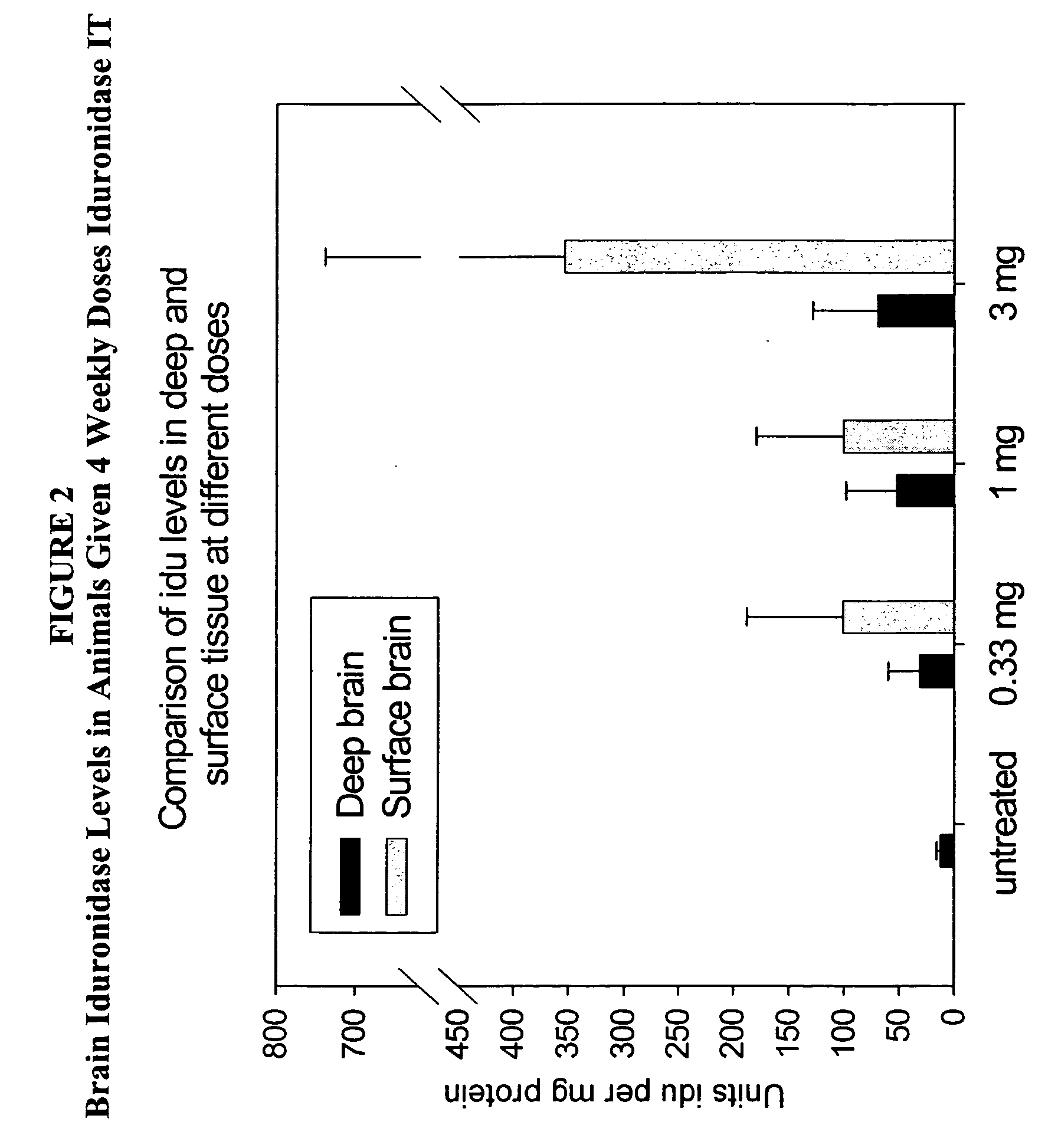
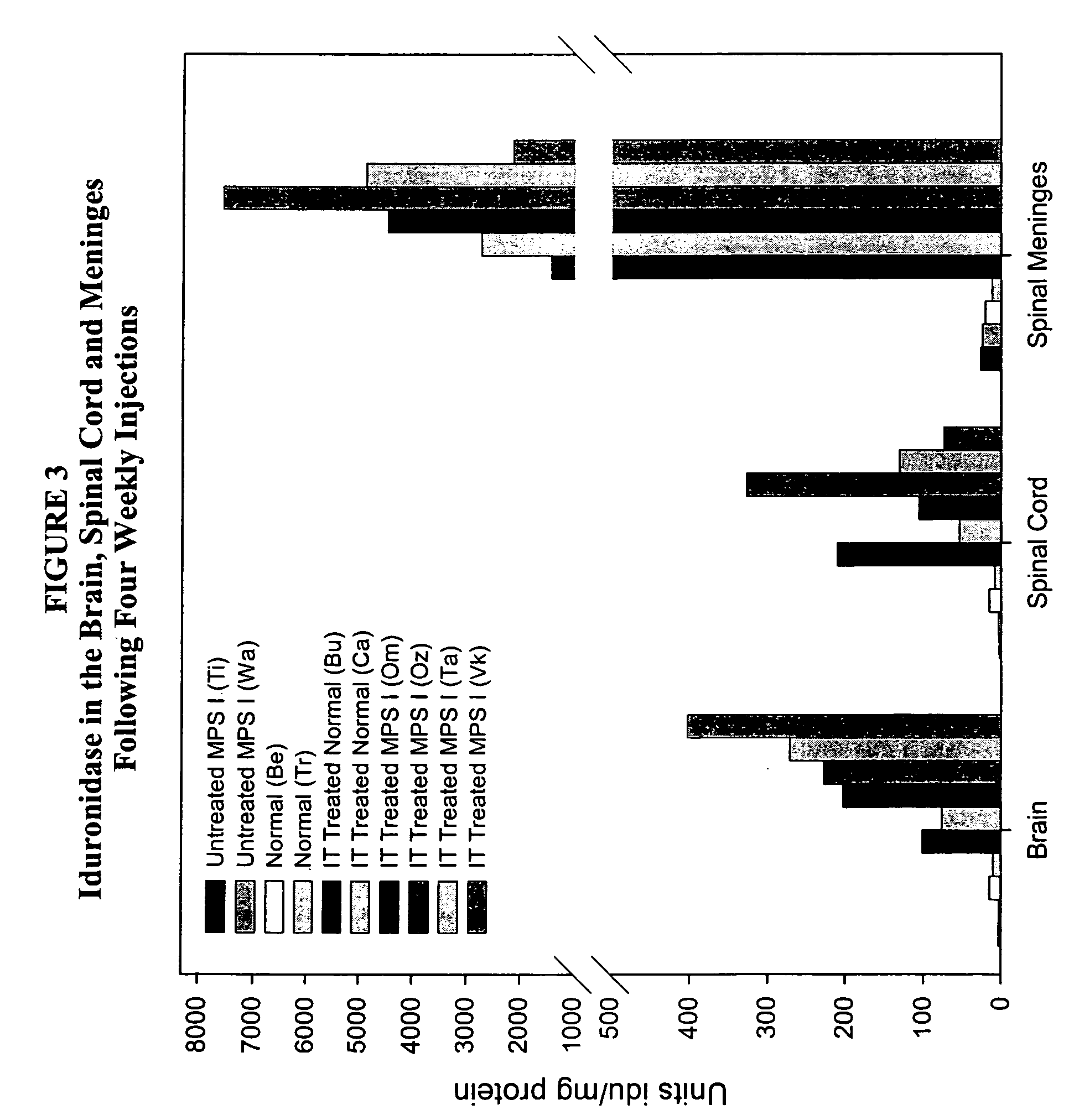
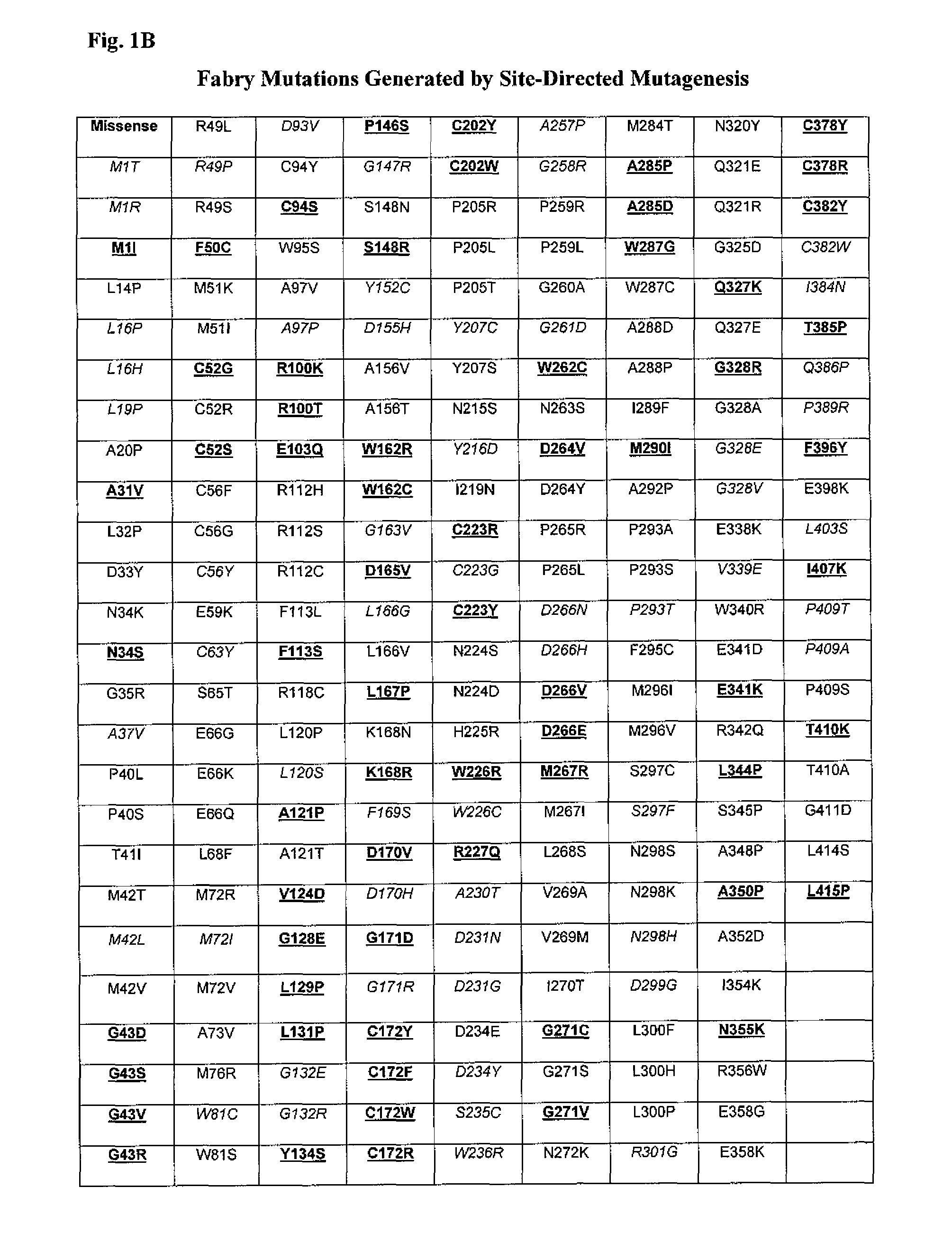
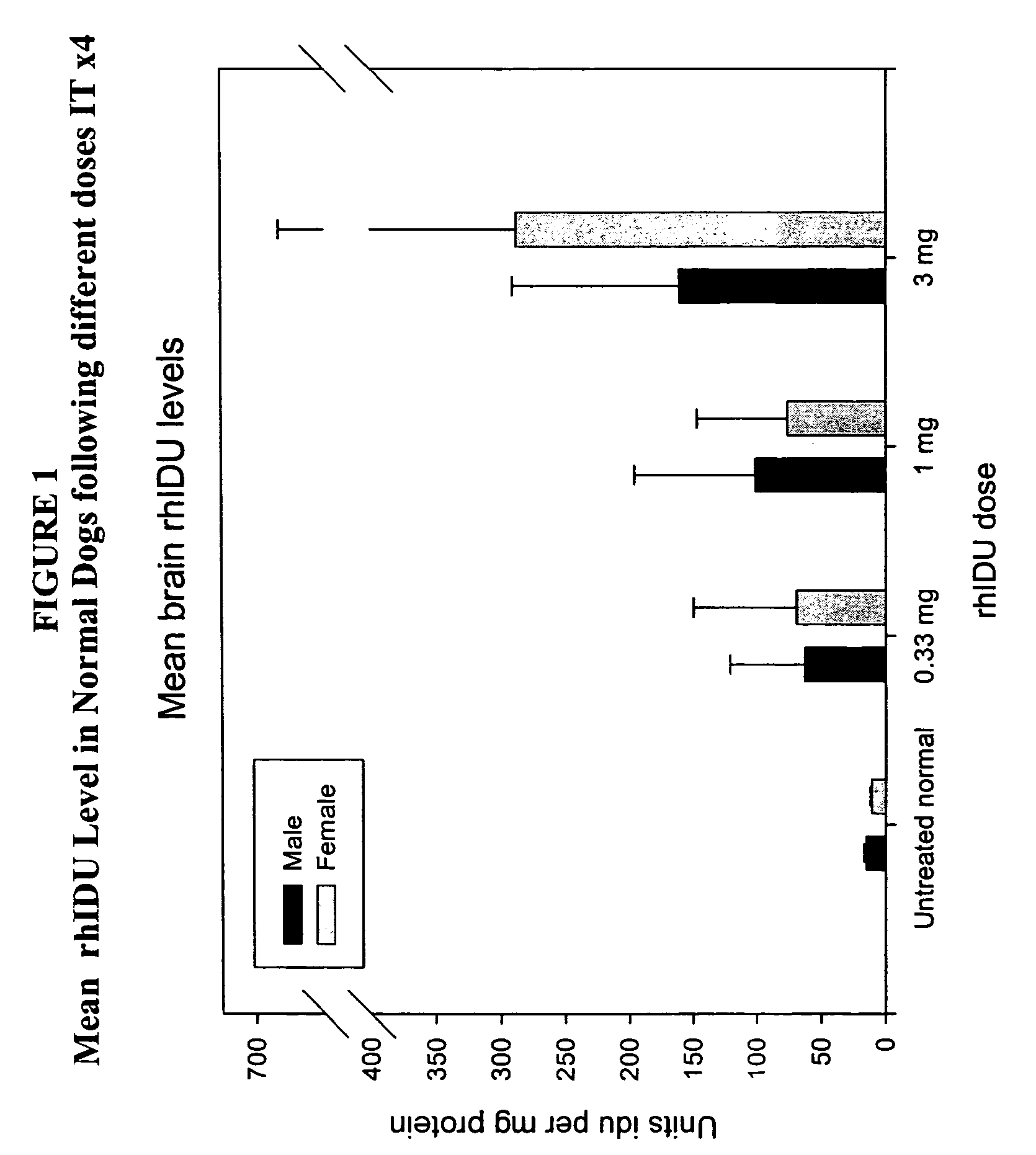
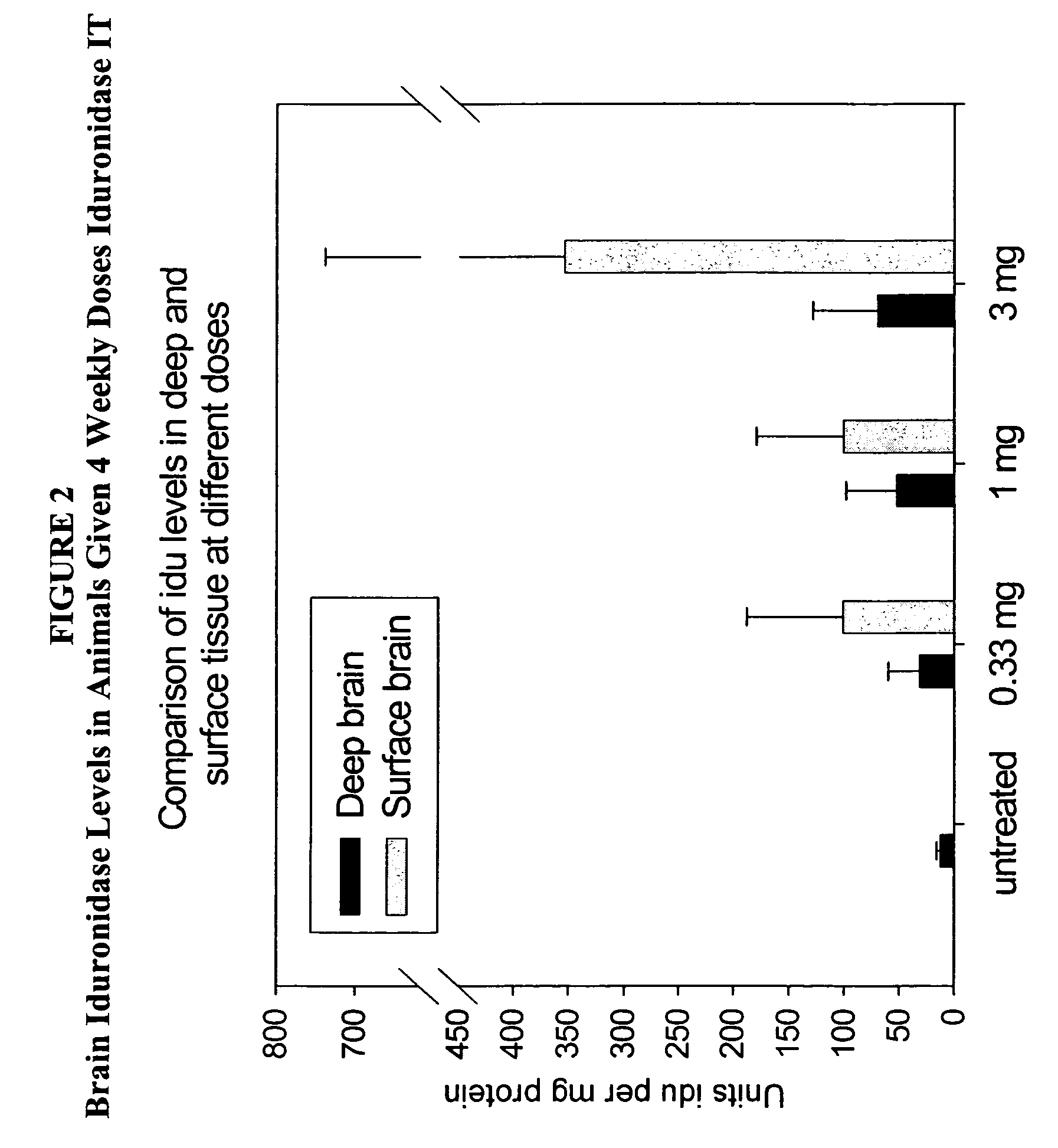
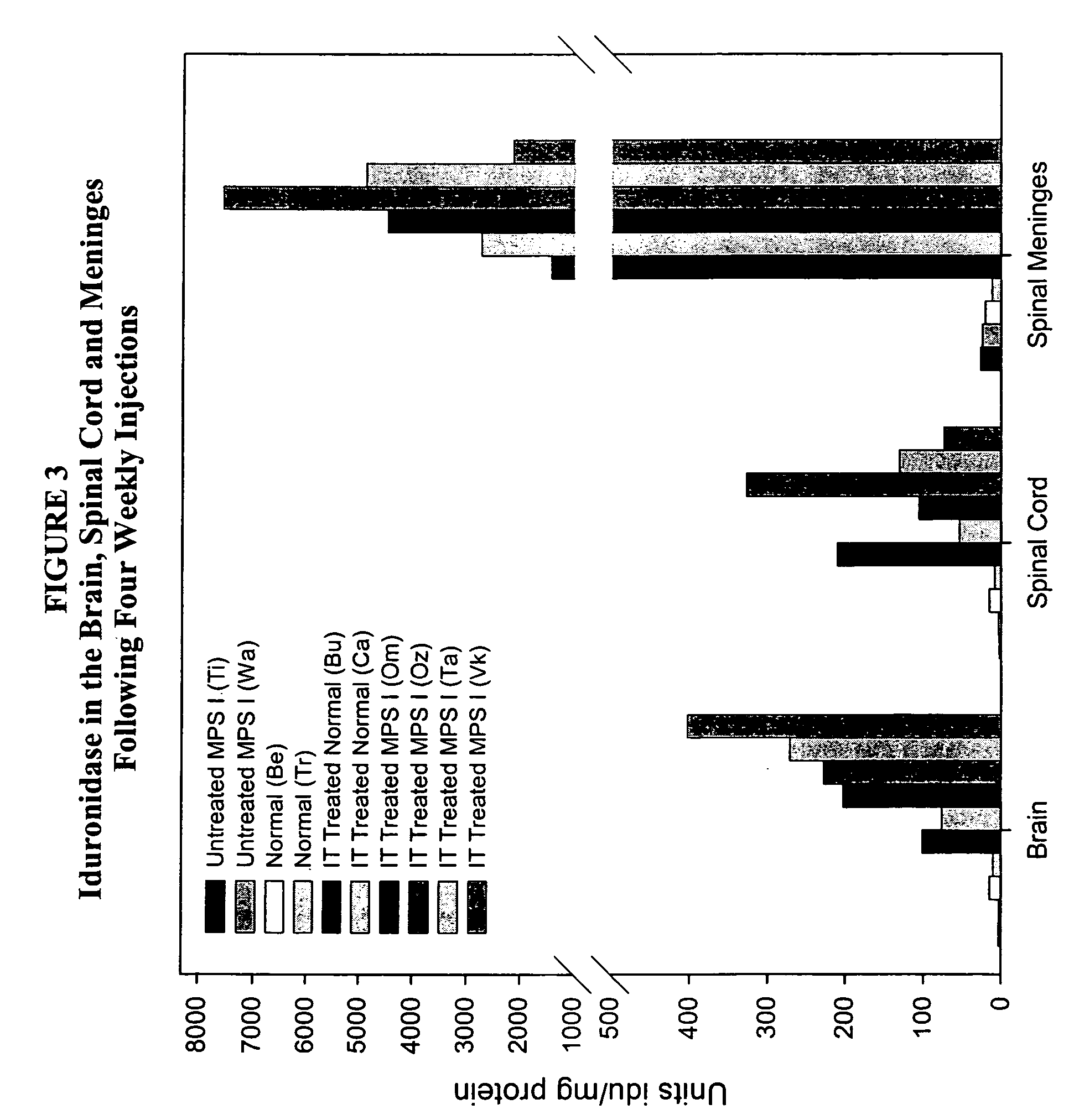
The present invention relates to the intrathecal (IT) administration of recombinant enzyme to treat lysosomal storage disorders. In an exemplary embodiment, intrathecal administration of human α-L-iduronidase (rhIDU) injections in MPS I affected animals resulted in significant enzyme uptake, significant rh-iduronidase activity in brain and meninges and a decrease of glycosaminoglycan (GAG) storage in cells of MPS I subjects to that of normal subjects. Intrathecal administration proved more effective than intravenous treatment at alleviating MPS I symptoms, indicating it is a useful method of treating lysosomal storage disorders.
Owner:BIOMARIN PHARMA INC
Method to predict response to pharmacological chaperone treatment of diseases
ActiveUS8592362B2Increase activity levelHigh activityOrganic active ingredientsBiocidePharmacological chaperoneΑ galactosidase a
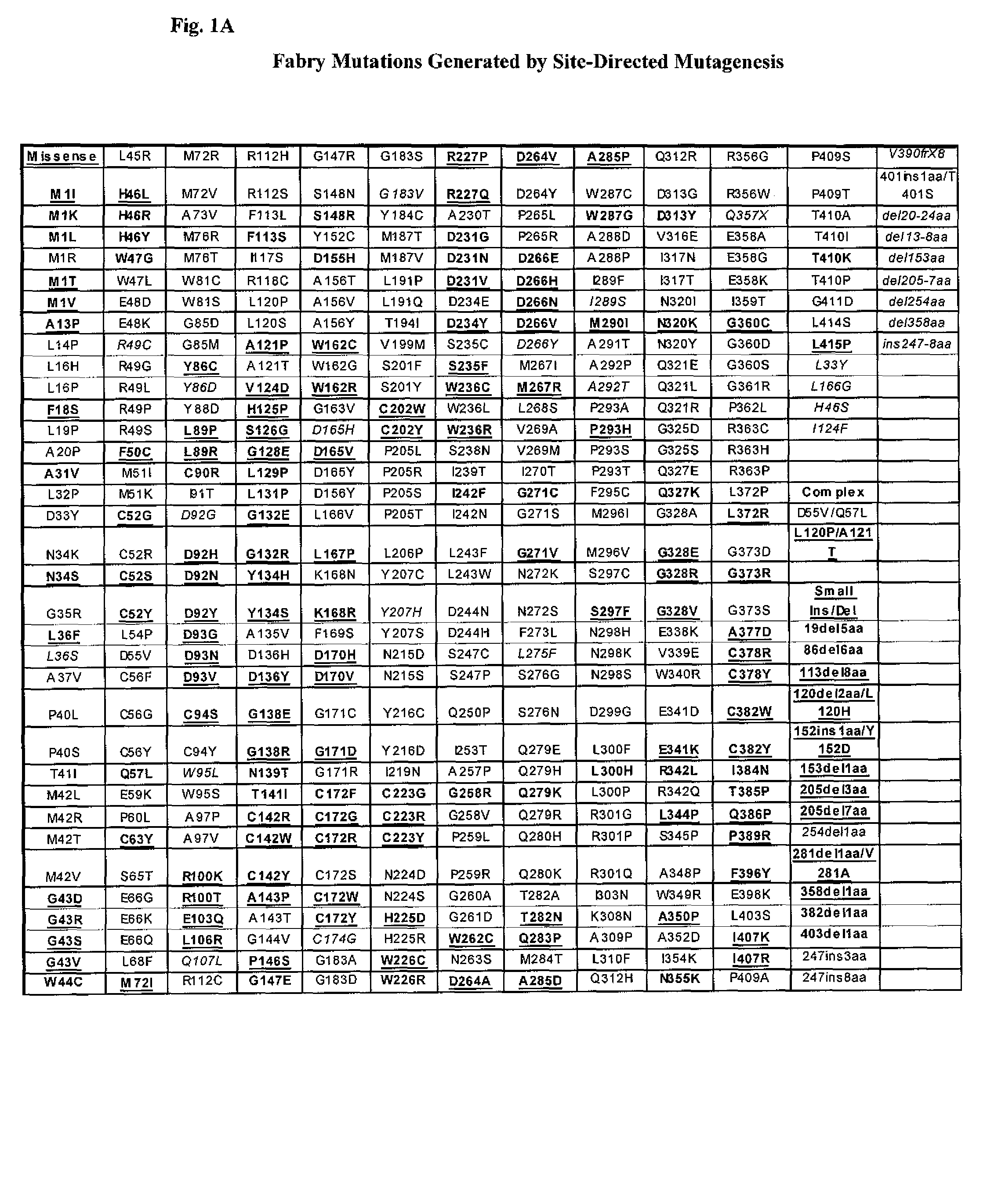
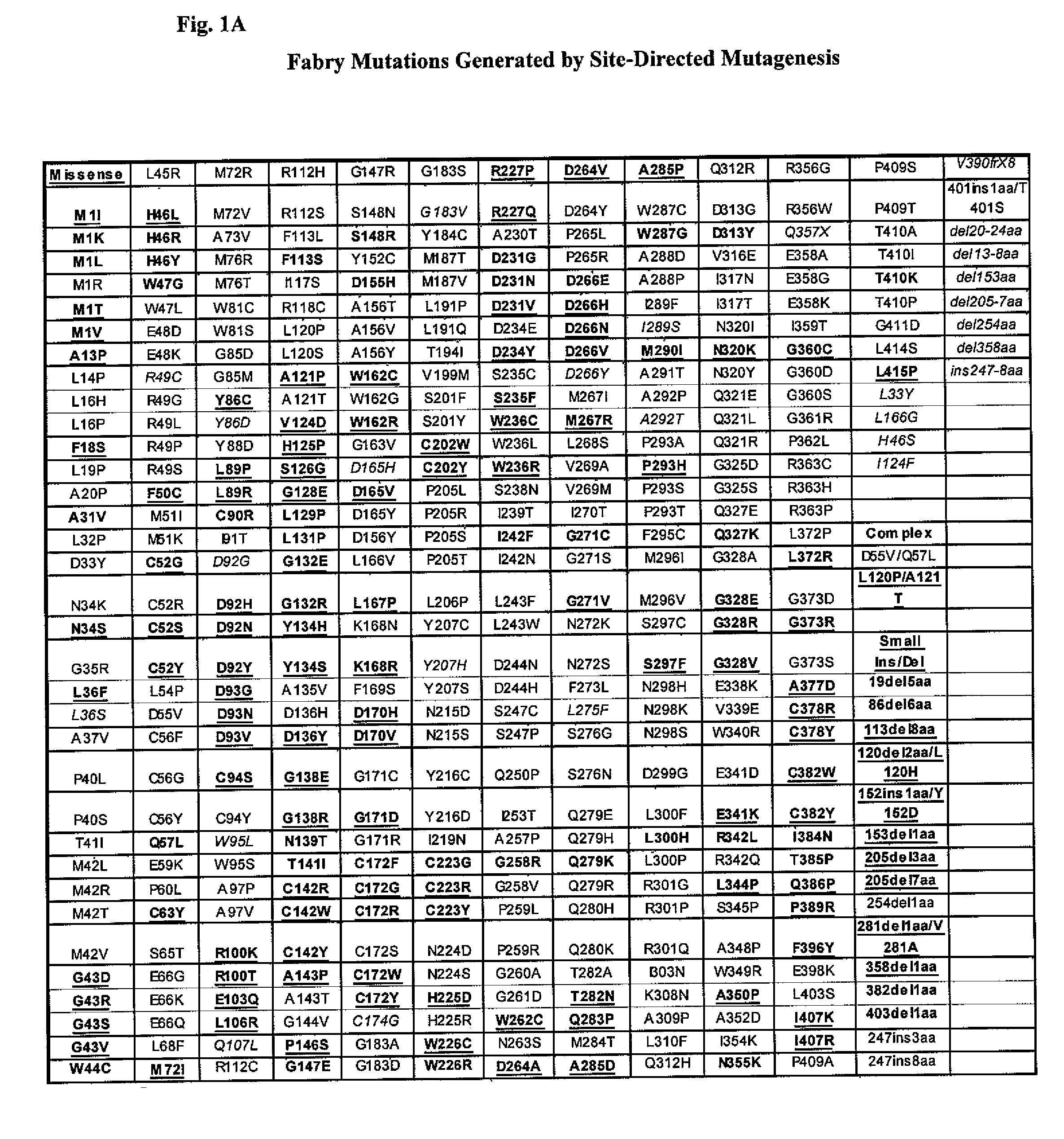
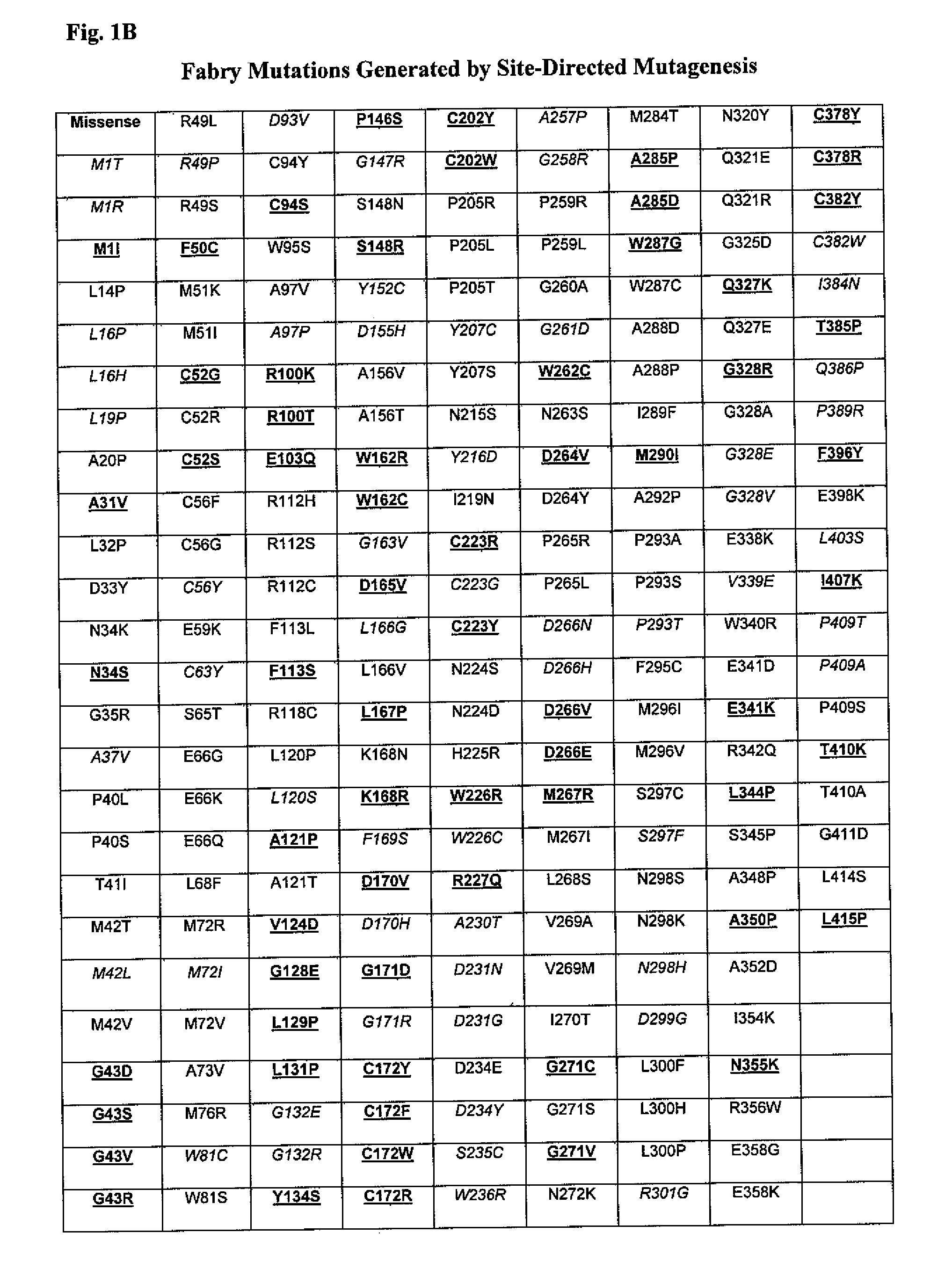
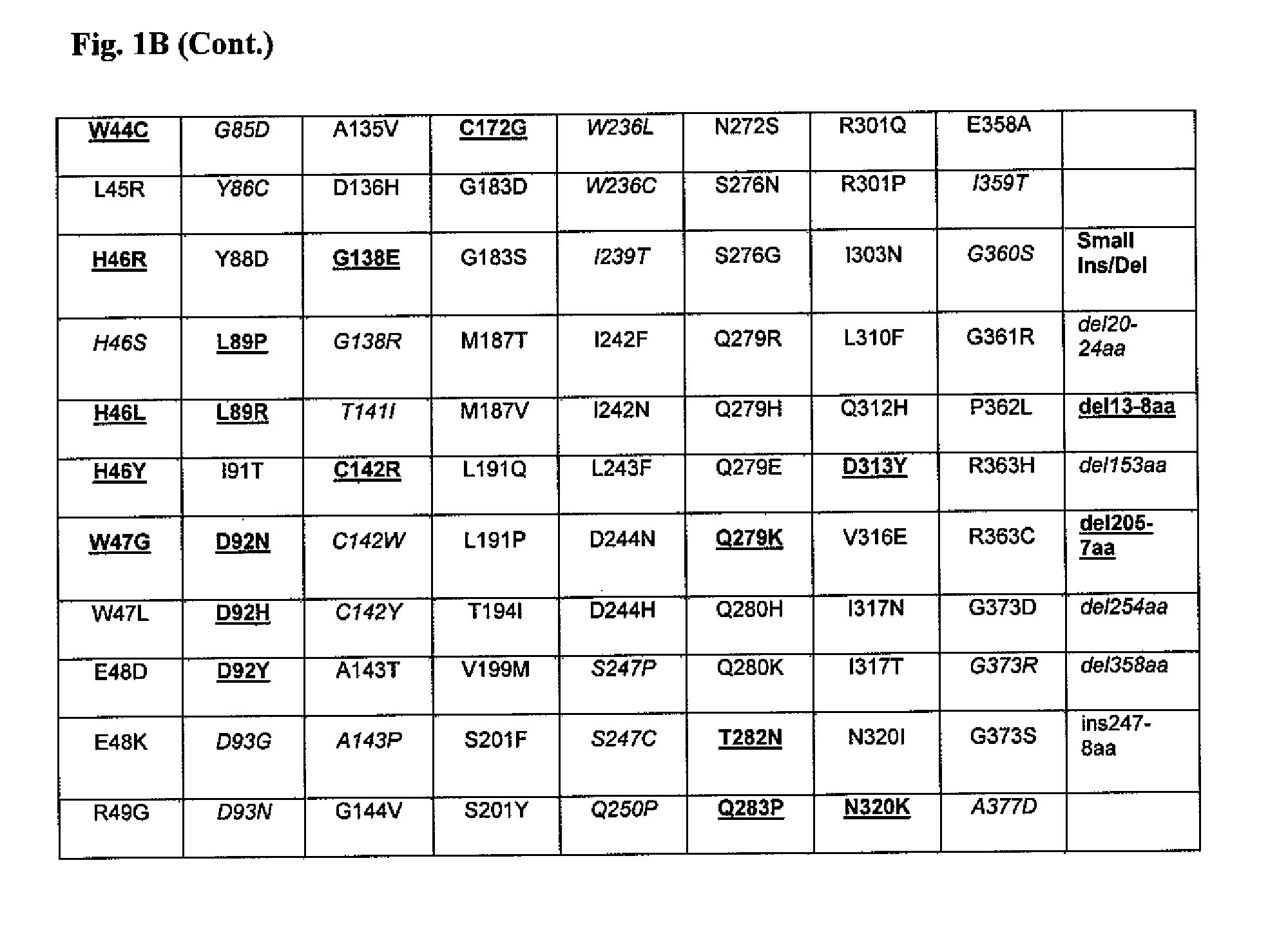
The present invention provides methods to determine whether a patient with a lysosomal storage disorder will benefit from treatment with a specific pharmacological chaperone. The present invention exemplifies an in vitro method for determining α-galactosidase A responsiveness to a pharmacological chaperone such as 1-deoxygalactonojirimycin in a cell line expressing a mutant from of α-galactosidase A. The invention also provides a method for diagnosing Fabry disease in patients suspected of having Fabry disease.
Owner:AMICUS THERAPEUTICS INC
Delivery of therapeutic compounds to the brain and other tissues
ActiveUS7442372B2Reduces and eliminates glycogen storage granuleReduce eliminateNervous disorderPeptide/protein ingredientsMeningesIntrathecal use
The present invention relates to the intrathecal (IT) administration of recombinant enzyme to treat lysosomal storage disorders. In an exemplary embodiment, intrathecal administration of human α-L-iduronidase (rhIDU) injections in MPS I affected animals resulted in significant enzyme uptake, significant rh-iduronidase activity in brain and meninges and a decrease of glycosaminoglycan (GAG) storage in cells of MPS I subjects to that of normal subjects. Intrathecal administration proved more effective than intravenous treatment at alleviating MPS I symptoms, indicating it is a useful method of treating lysosomal storage disorders.
Owner:BIOMARIN PHARMA INC
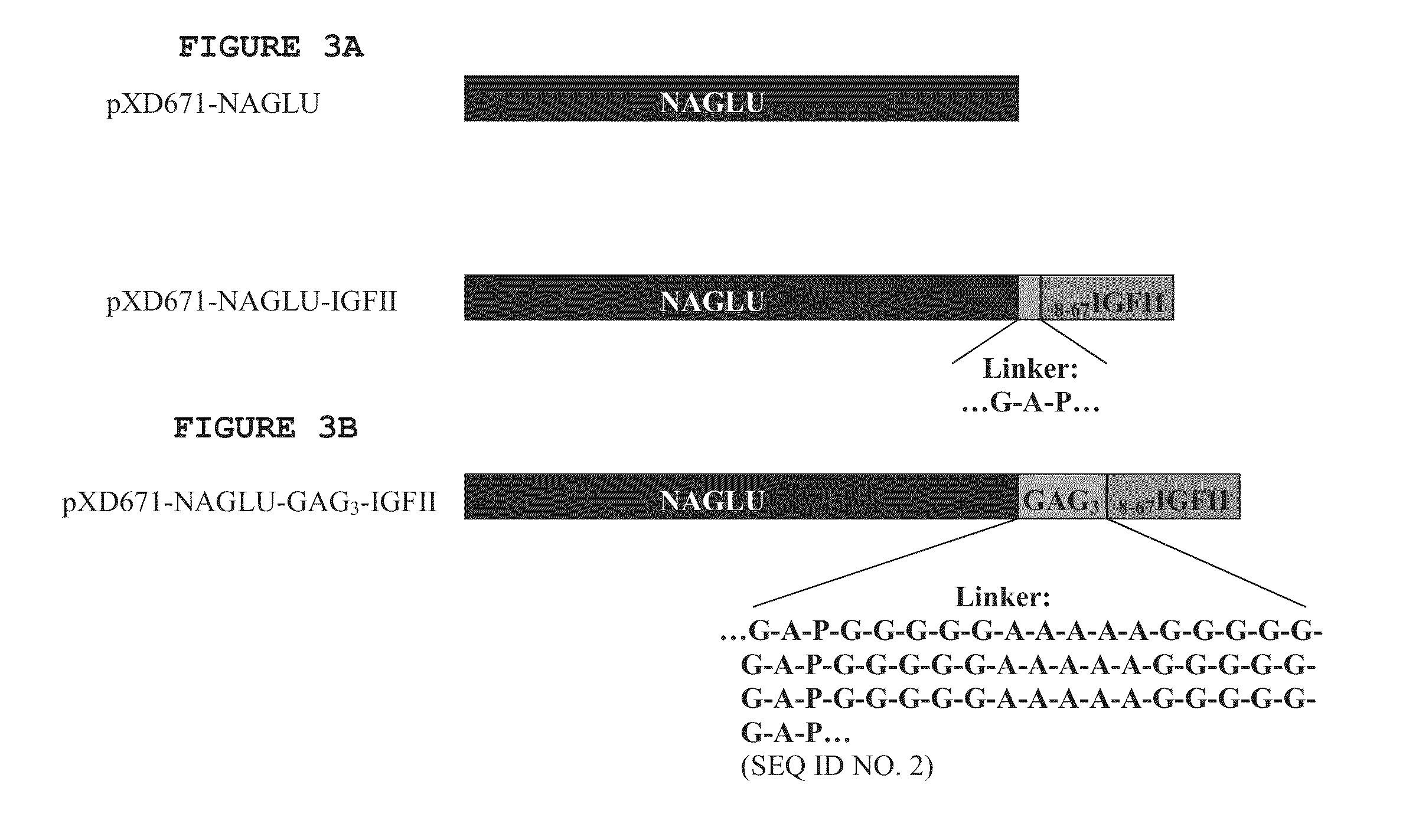
Peptide linkers for polypeptide compositions and methods for using same
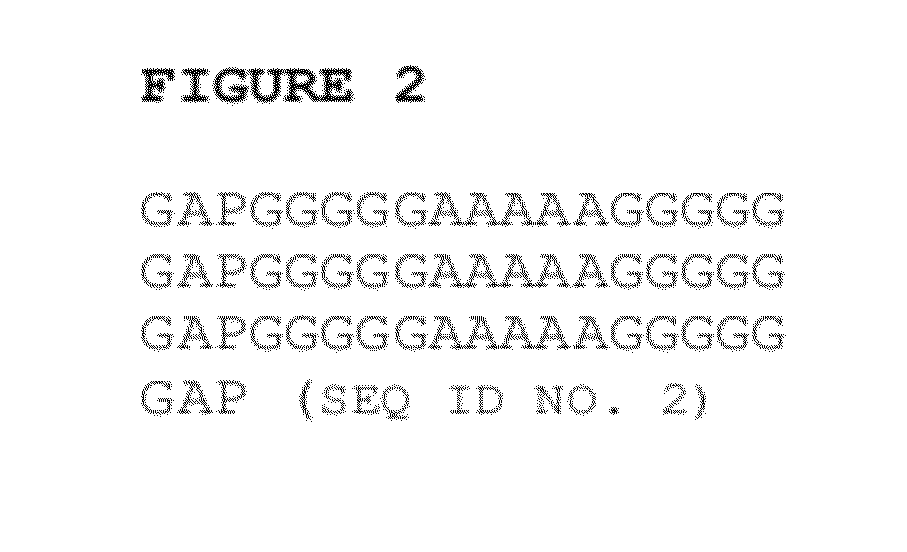
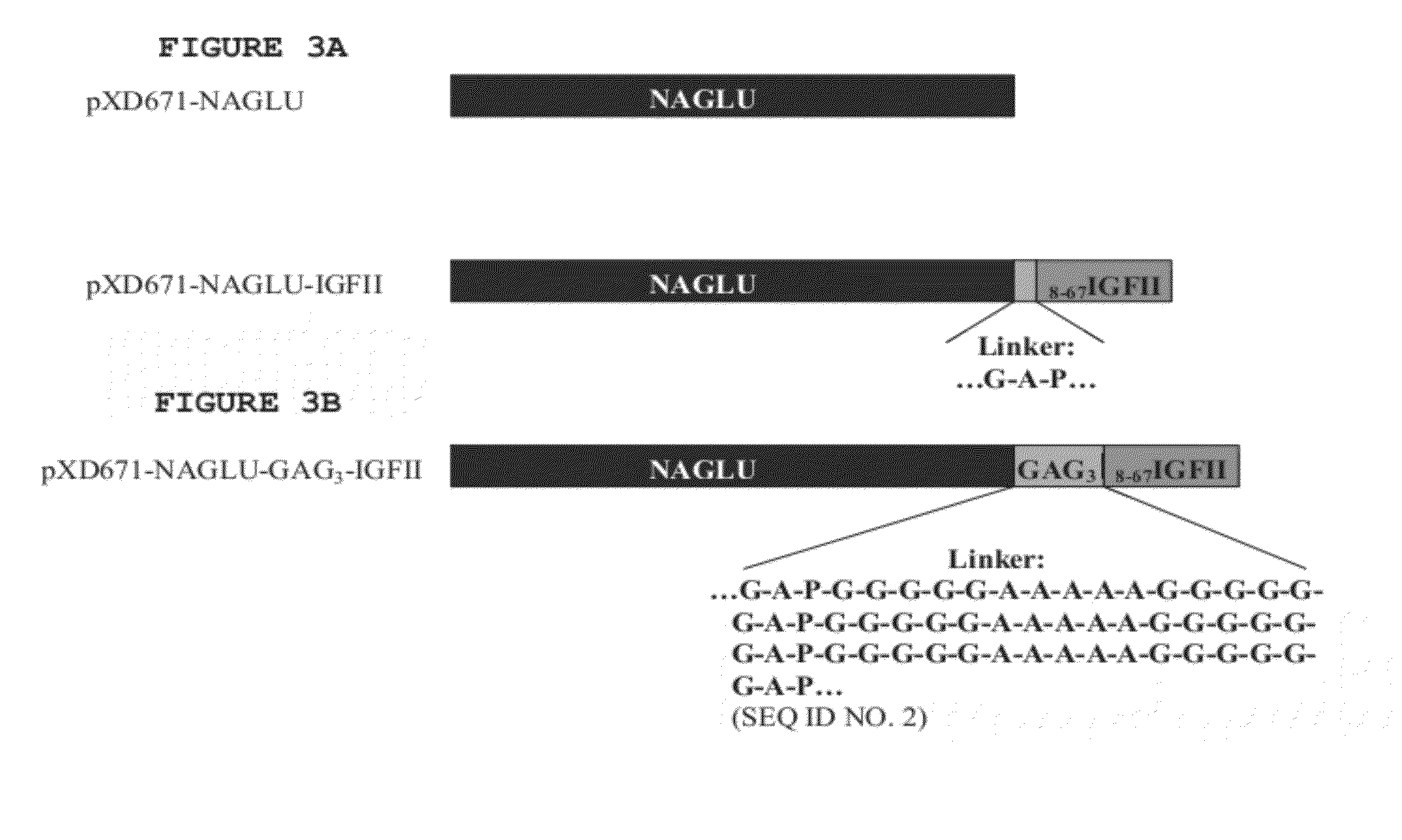
ActiveUS20120232021A1Facilitate targeted deliveryPeptide/protein ingredientsAntibody mimetics/scaffoldsLysosomal storage disordersPeptide
Disclosed herein are novel peptide linkers and polypeptide compositions comprising the linkers (e.g., chimeric polypeptides) and methods of using the polypeptide compositions. The compositions and methods are particularly useful for targeting / delivering a polypeptide or protein of interest (e.g., a therapeutic polypeptide) to a cell, tissue or organ of interest in order to treat various diseases or disorders (e.g., lysosomal storage disorders).
Owner:SHIRE HUMAN GENETIC THERAPIES INC
Targeting proteins to cells expressing mannose receptors via expression in insect cells
The present invention is based on the discovery that proteins produced in insect cell cultures are glycosylated in a unique manner that causes them to be selectively imported by cells that express mannose receptors on their membranes, particularly macrophages. Proteins that are selectively imported into cells containing mannose receptors are provided, as well as pharmaceutical compositions containing such proteins and methods for producing such proteins. Application of the present invention to produce proteins useful for treating lysosomal storage disorders is also disclosed. Engineering of cells to express mannose receptors so that they will selectively import proteins produced in insect cells is also taught, as well as a protein targeting system using such cells and proteins. Finally, an improved elution buffer for the purification of proteins produced in insect cells from a Concanavalin A column is provided.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
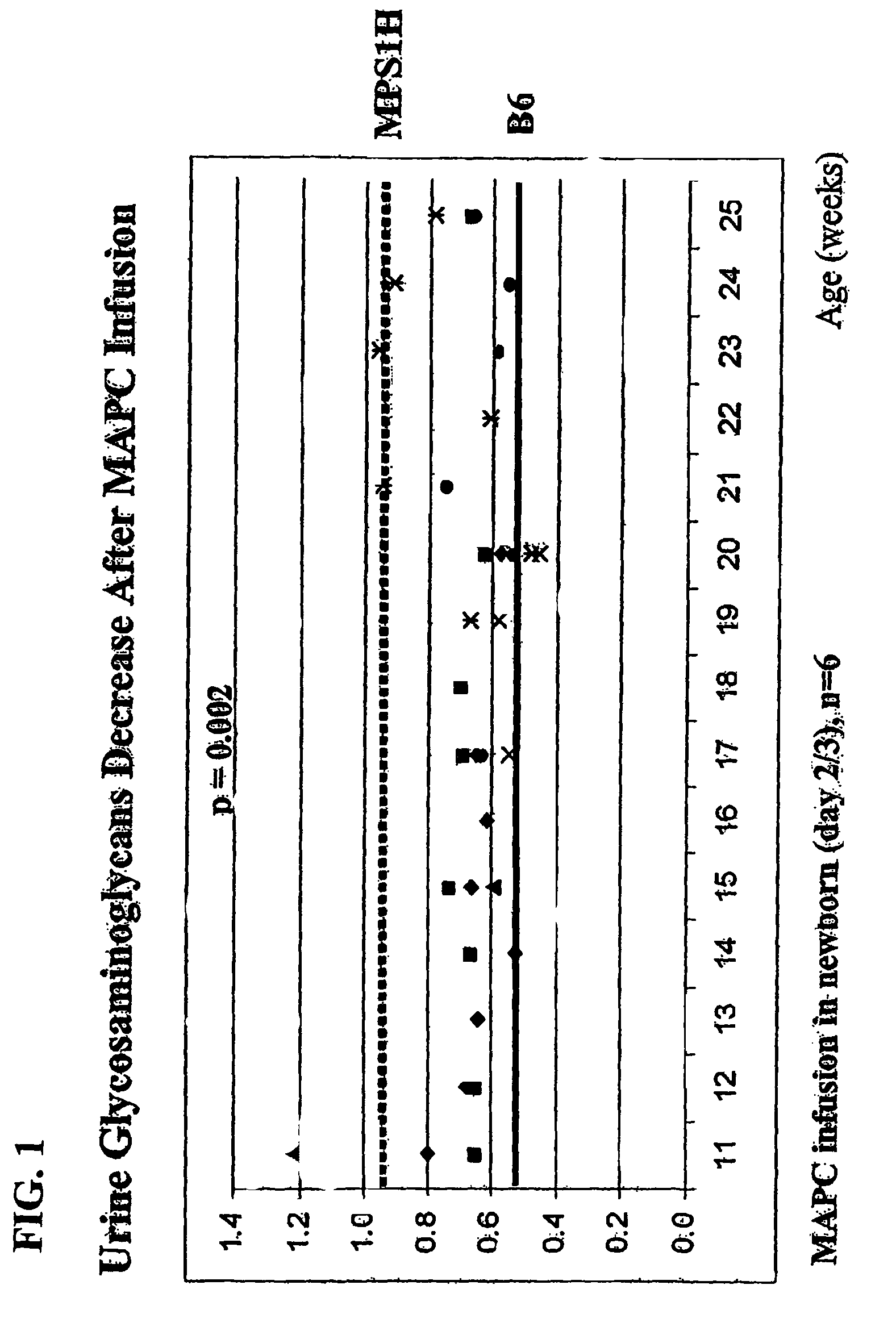
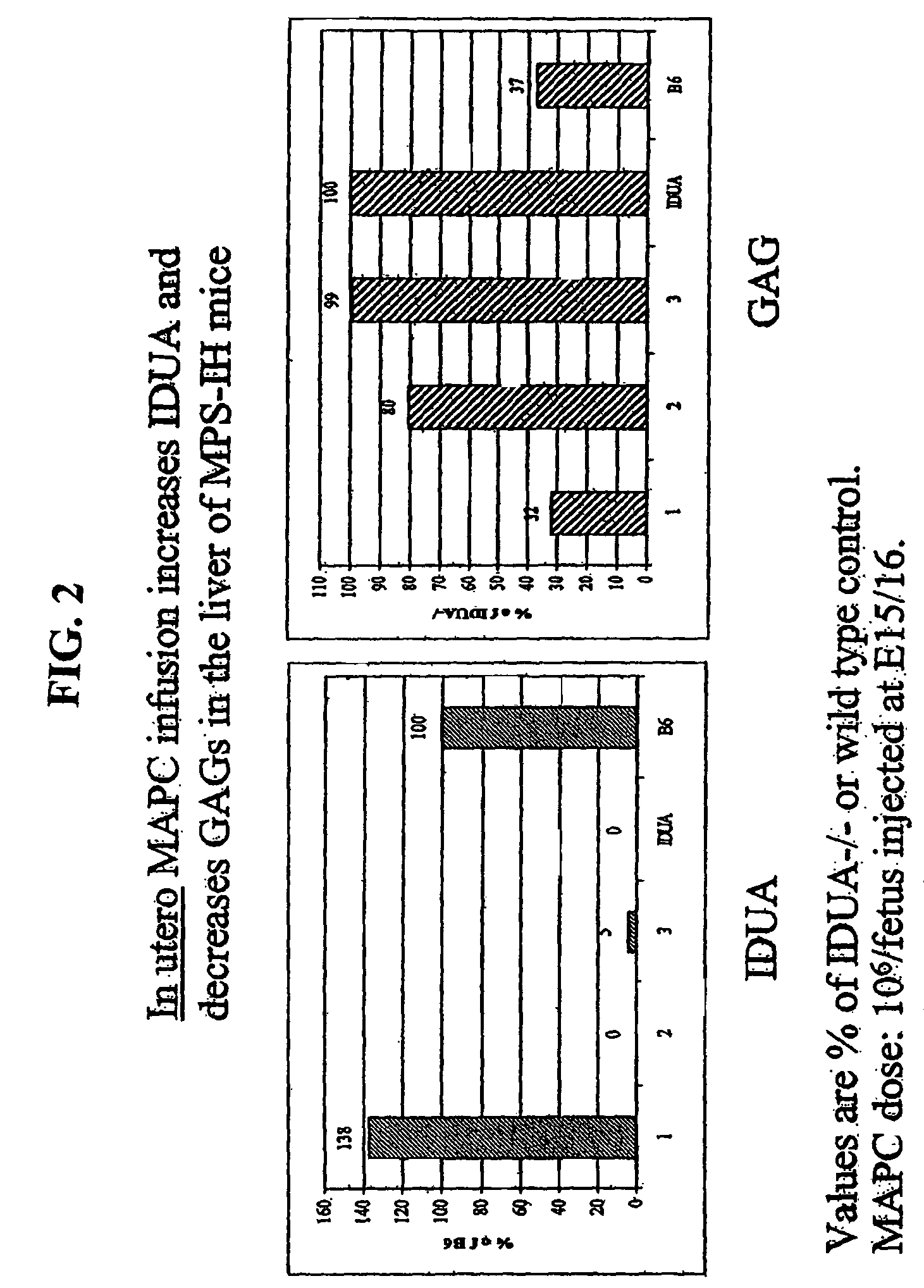
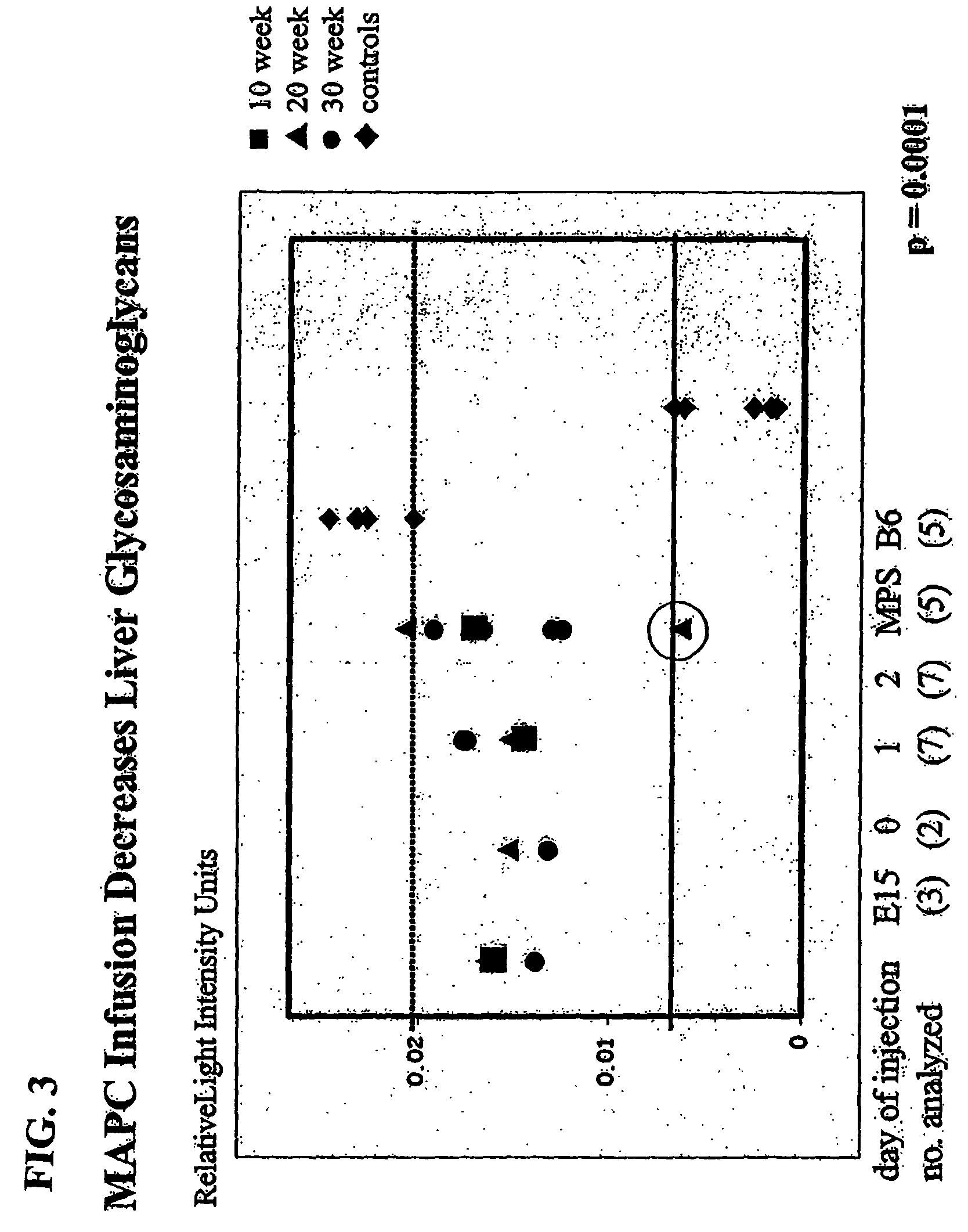
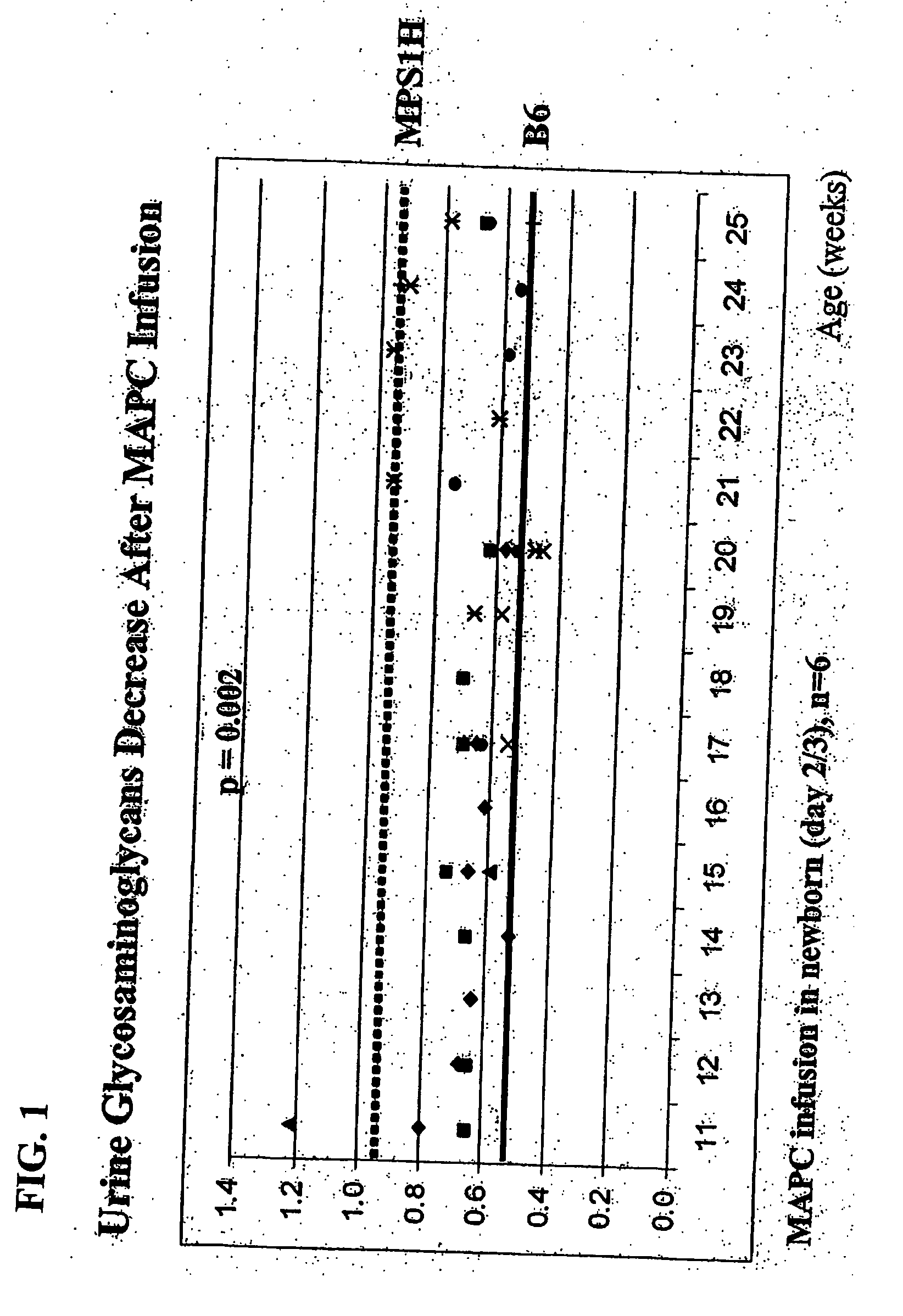
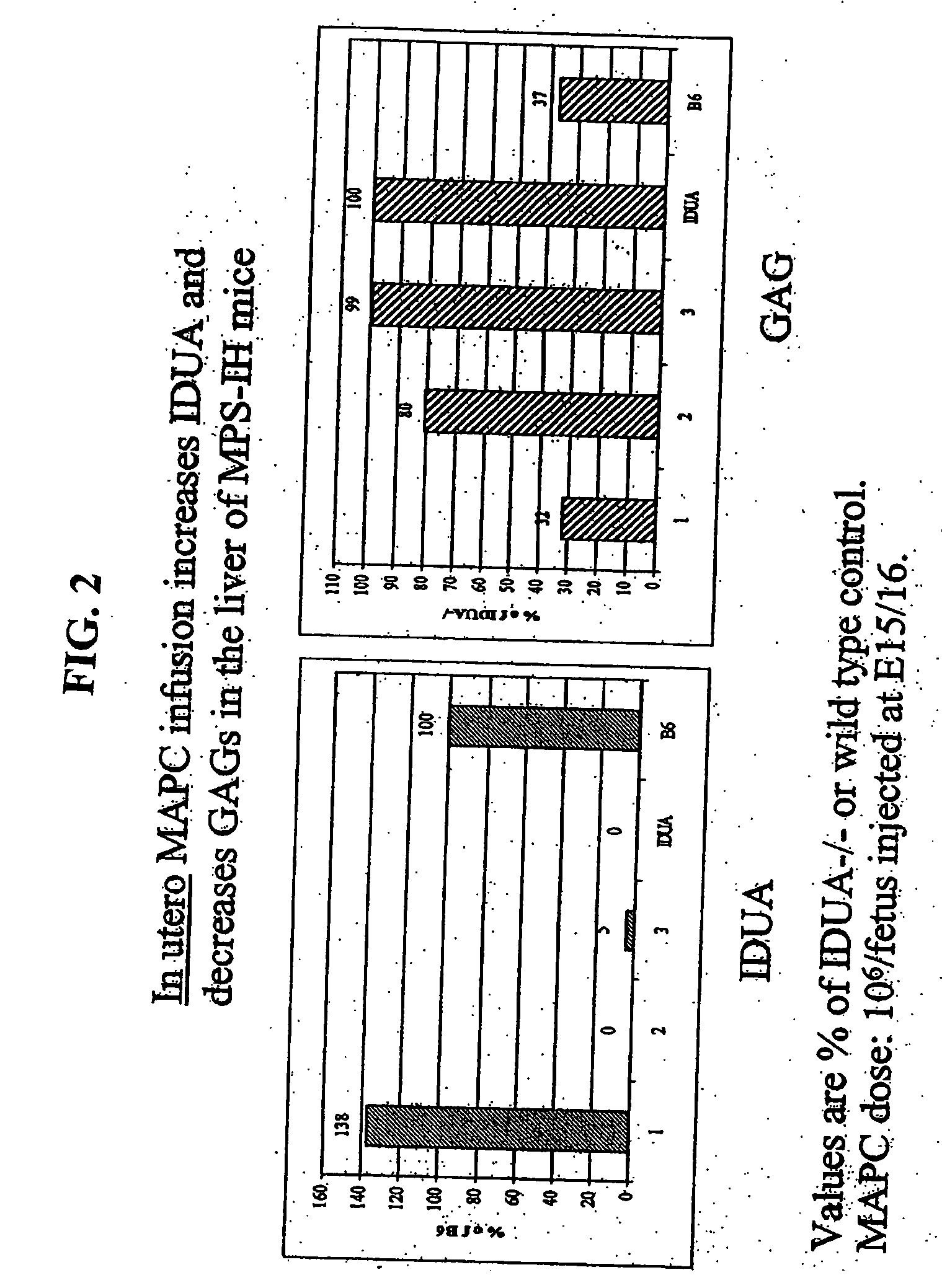
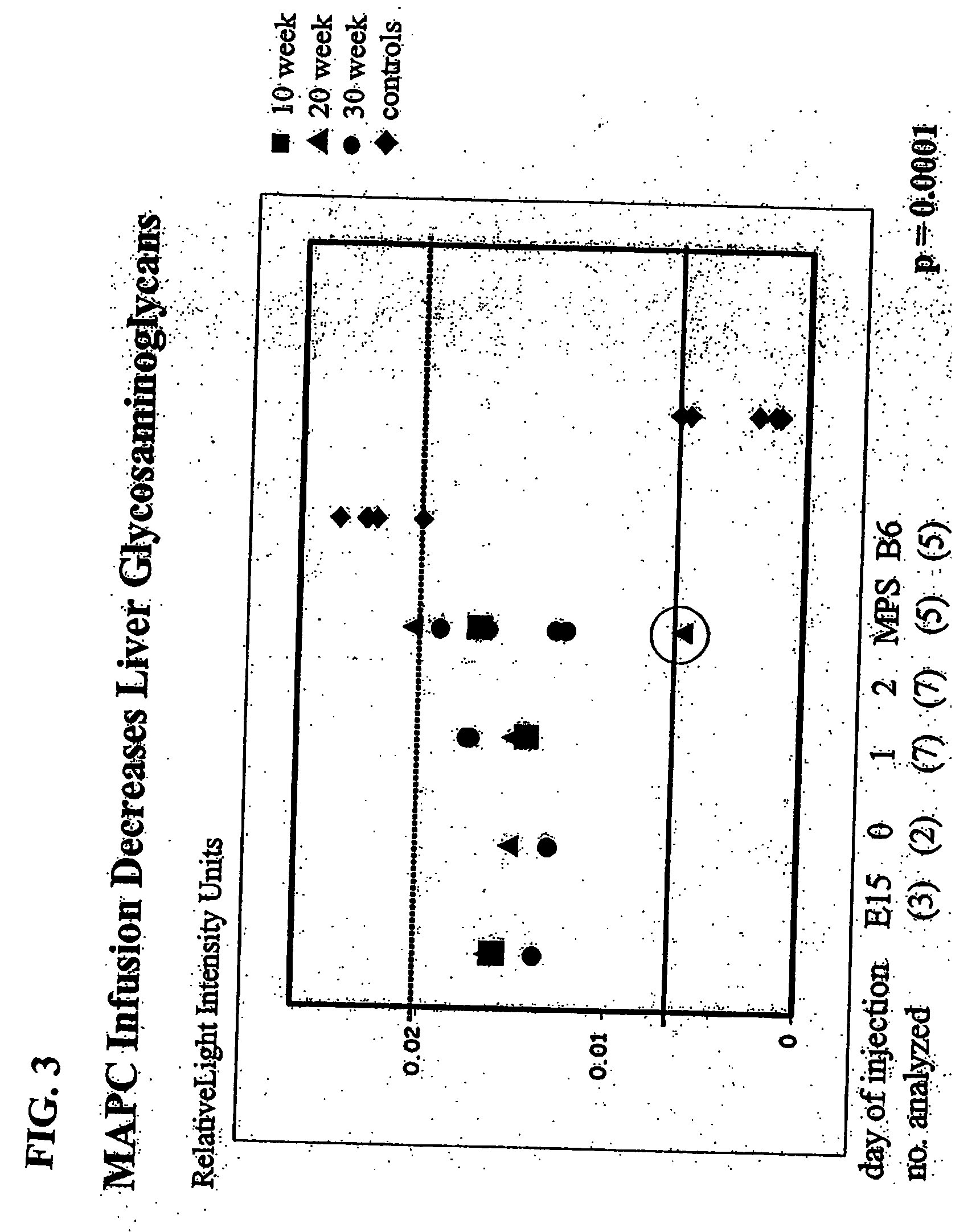
MAPC administration for the treatment of lysosomal storage disorders
ActiveUS7927587B2Preventing, decreasing or eliminating said accumulationBiocidePeptide/protein ingredientsProgenitorLysosome
The present invention relates to methods for providing lysosomal enzymes to a subject by administering stem cells, preferably Multipotent Adult Progenitor Cells (MAPCs). The invention further relates to methods for treating lysosomal storage disorders by administering stem cells.
Owner:RGT UNIV OF MINNESOTA
Compositions and methods for the treatment of lysosomal storage disorders
ActiveUS20070009500A1ModulatingAvoid accumulationBiocidePeptide/protein ingredientsProgenitorLysosome
The present invention relates to methods for providing lysosomal enzymes to a subject by administering stem cells, preferably Multipotent Adult Progenitor Cells (MAPCs). The invention further relates to methods for treating lysosomal storage disorders by administering stem cells.
Owner:RGT UNIV OF MINNESOTA
Compositions and methods for the transport of therapeutic agents
InactiveUS20120277158A1Reducing and eliminating risk of developingReduce the numberPowder deliveryObesity gene productsTherapeutic effectCell type
The present invention is directed to conjugates that include a polypeptide capable of crossing the blood-brain barrier or entering one or more cell types attached to a transport vector, i.e., a composition capable of transporting an agent (e.g., a therapeutic agent). In certain cases, the polypeptides are directly conjugated to a lipid or polymeric vector to allow targeted application of a therapeutic agent to treat, for example, a cancer, a neurodegenerative disease, or a lysosomal storage disorder.
Owner:ANGLACHEM INC
Methods for preventing and/or treating lysosomal storage disorders
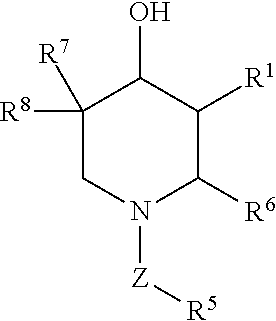
The present invention provides methods for preventing and / or treating lysosomal storage disorders using 5-(fluoromethyl)piperidine-3,4-diol, 5-(chloromethyl)piperidine-3,4-diol, or a pharmaceutically acceptable salt, solvate, or prodrug thereof, or any combination of two or more thereof. In particular, the present invention provides methods for preventing and / or treating Gaucher's disease.
Owner:AMICUS THERAPEUTICS INC
Methods for preventing and/or treating lysosomal storage disorders
The present invention provides methods for preventing and / or treating lysosomal storage disorders using 5-(fluoromethyl)piperidine-3,4-diol, 5-(chloromethyl)piperidine-3,4-diol, or a pharmaceutically acceptable salt, solvate, or prodrug thereof, or any combination of two or more thereof. In particular, the present invention provides methods for preventing and / or treating Gaucher's disease.
Owner:AMICUS THERAPEUTICS INC
Treatment of protein folding disorders
InactiveUS20100317690A1Enhance normal foldingBiocideOrganic chemistryImino SugarsLysosomal enzyme defect
Described are various compounds and methods for the treatment of disorders arising from aberrant protein folding, including in particular lysosomal storage diseases. In particular, polyhydroxylated alkaloids and imino sugars which are pharmacoperones of an enzyme and which do not bind to a catalytic site of said enzyme are described.
Owner:SUMMIT
Peptide linkers for polypeptide compositions and methods for using same
ActiveUS8580922B2Facilitate targeted deliveryNervous disorderPeptide/protein ingredientsLysosomal storage disordersPeptide
Owner:SHIRE HUMAN GENETIC THERAPIES INC
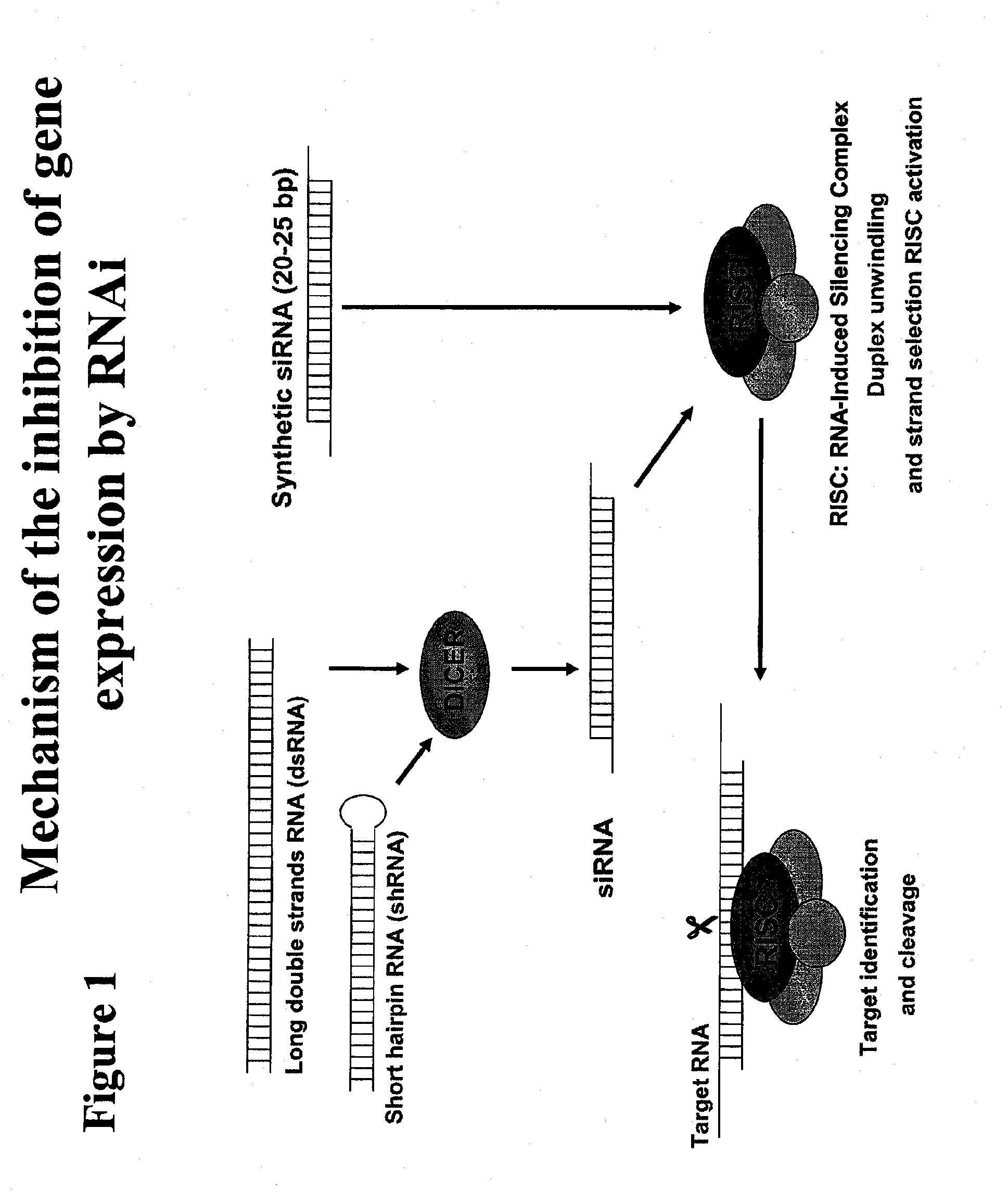
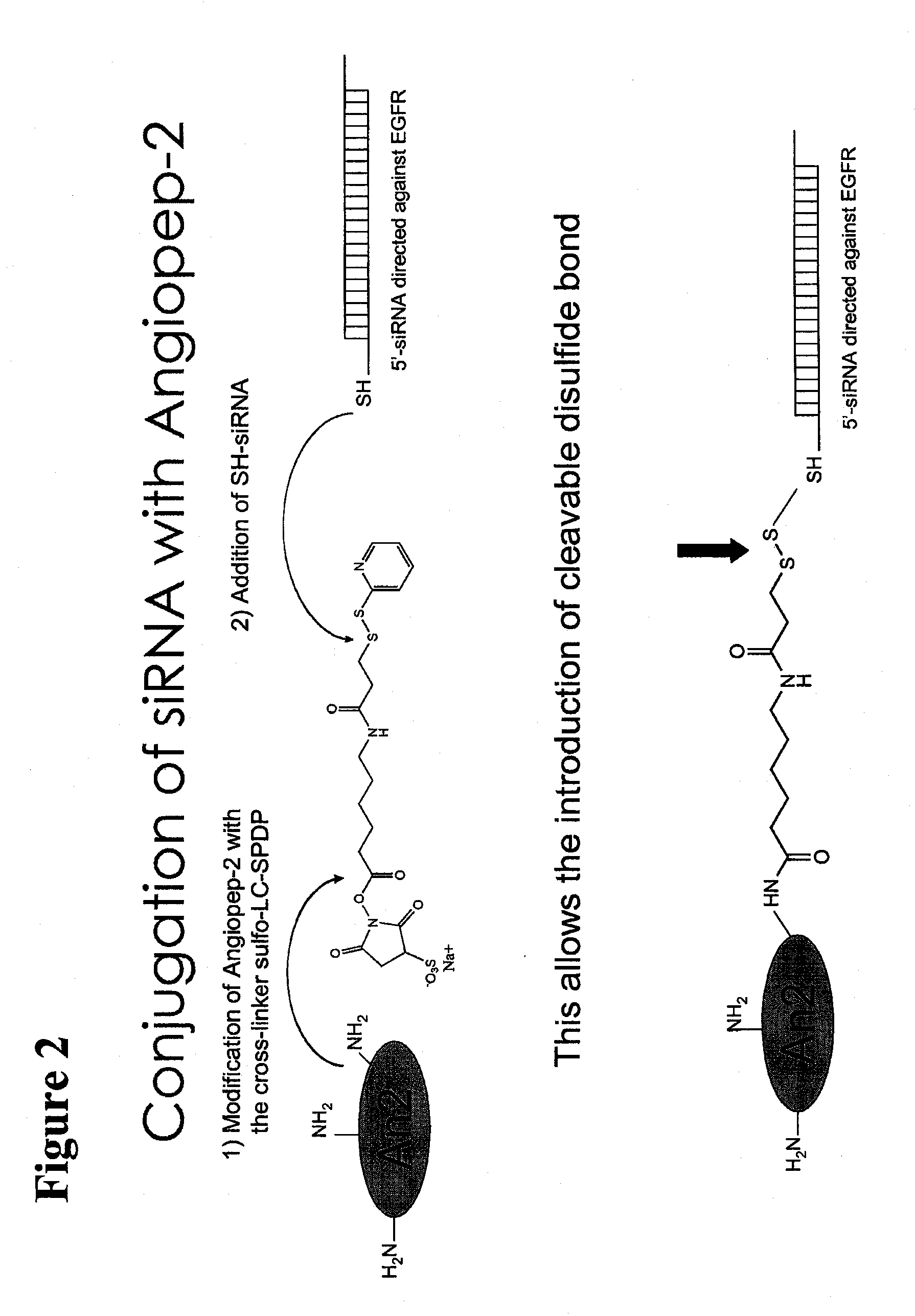
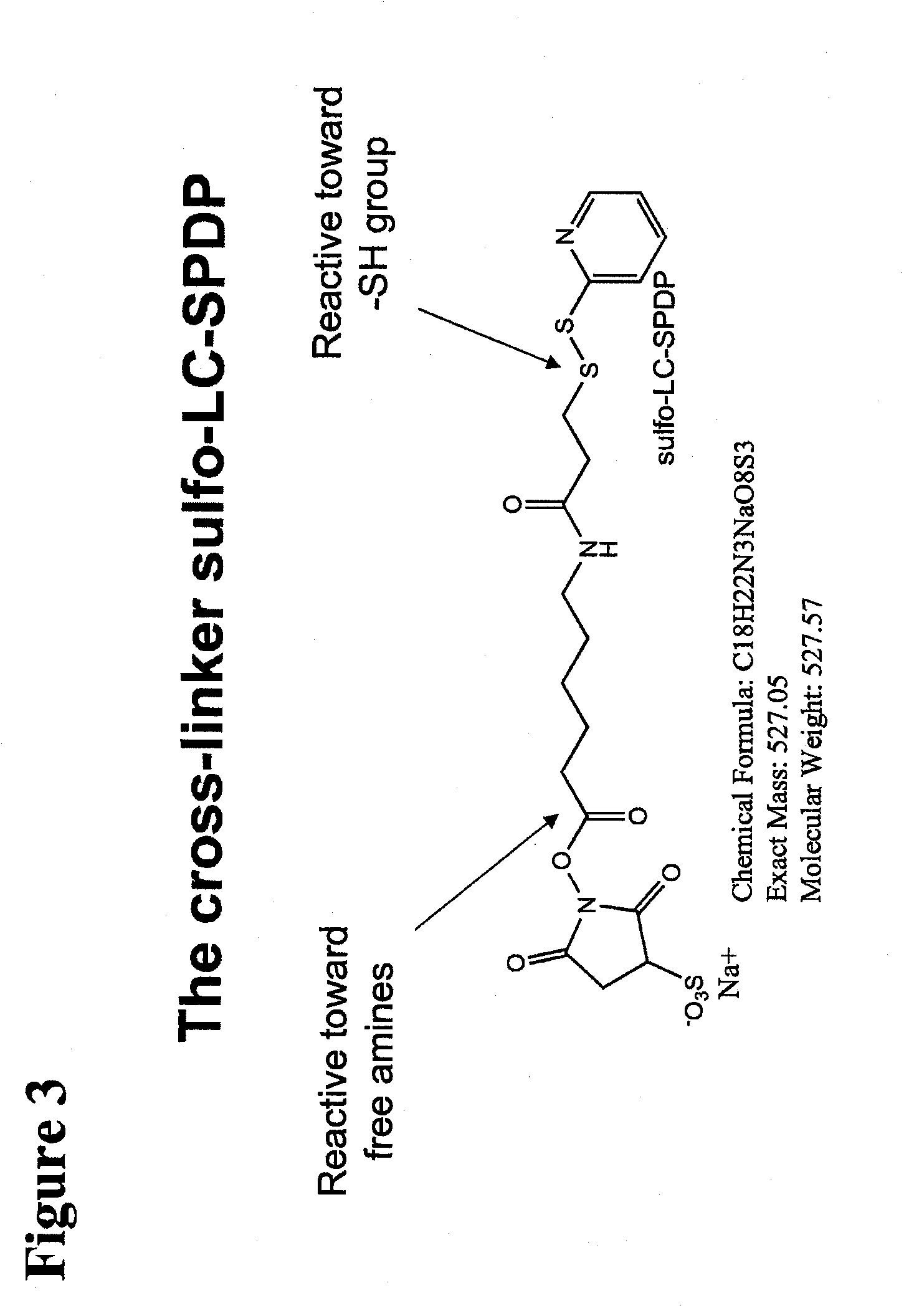
Polypeptide-nucleic acid conjugates and uses thereof
InactiveUS20110039785A1Reduce frequencyPrevent reduce appearsSenses disorderNervous disorderLysosomal storage disordersStereochemistry
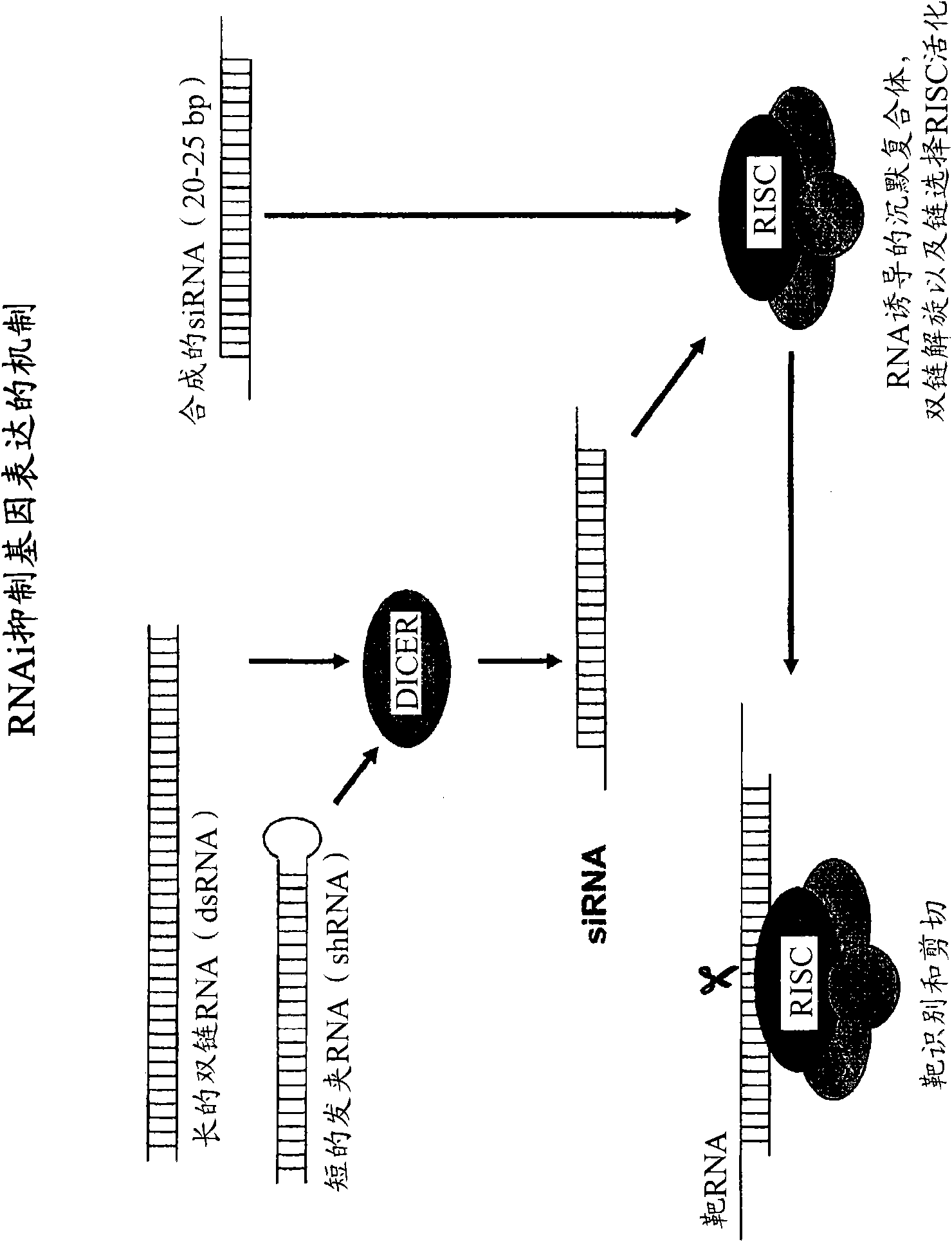
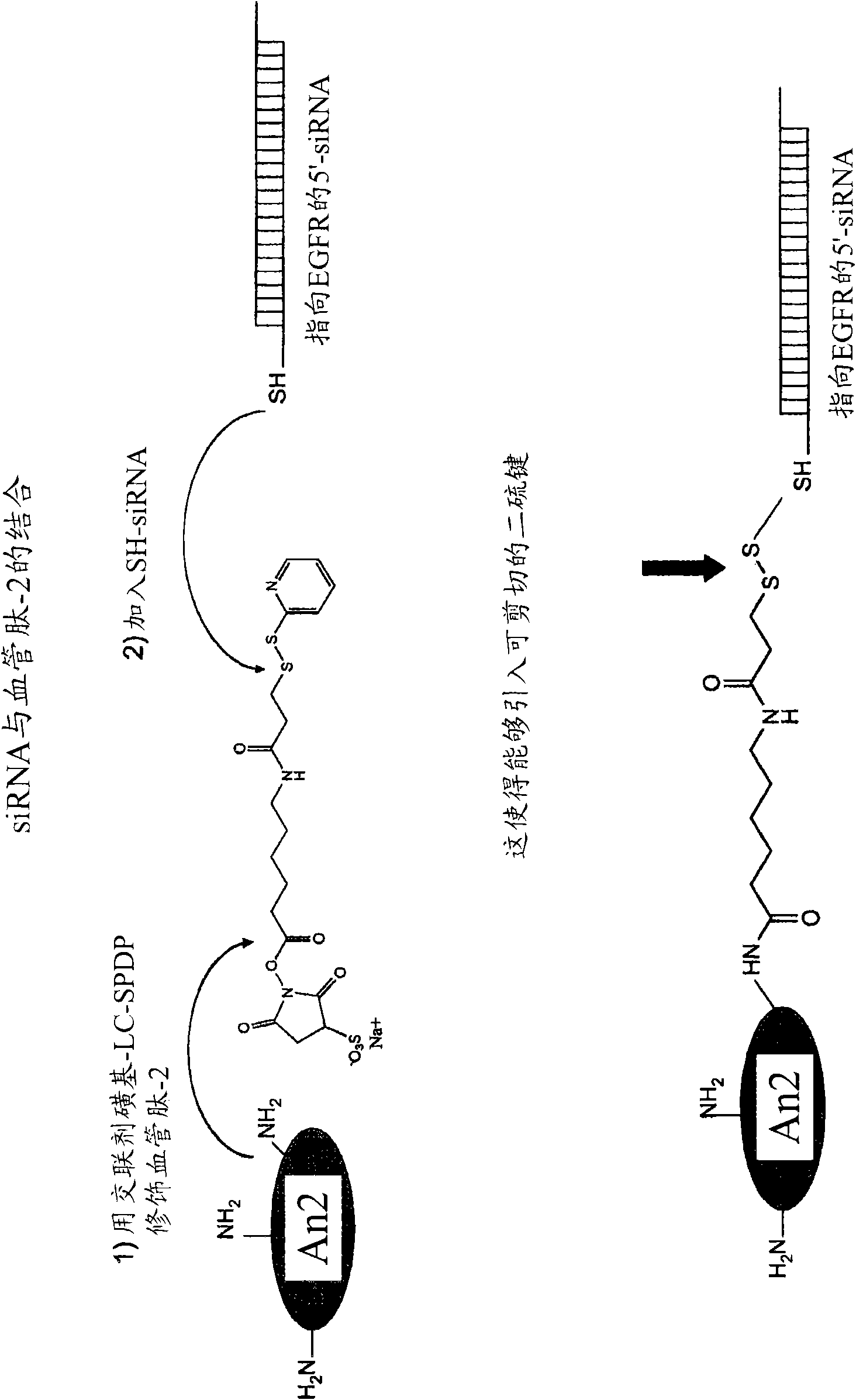
The present invention is directed to polypeptide-nucleic acid conjugates. These conjugates can allow for targeted application of a therapeutic RNAi agent across the blood-brain barrier to treat, for example, a cancer, neurodegenerative disease, or lysosomal storage disorder.
Owner:ANGLACHEM INC
Method to predict response to pharmacological chaperone treatment of diseases
InactiveUS20110152319A1Increase activity levelHigh activityBiocideMetabolism disorderPharmacological chaperoneΑ galactosidase a
The present invention provides methods to determine whether a patient with a lysosomal storage disorder will benefit from treatment with a specific pharmacological chaperone. The present invention exemplifies an in vitro method for determining α-galactosidase A responsiveness to a pharmacological chaperone such as 1-deoxygalactonojirimycin in a cell line expressing a mutant from of α-galactosidase A. The invention also provides a method for diagnosing Fabry disease in patients suspected of having Fabry disease.
Owner:AMICUS THERAPEUTICS INC
Method for producing proteins suitable for treating lysosomal storage disorders
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Compositions for preventing and/or treating lysosomal storage disorders
The present invention provides novel compositions as well as methods for preventing and / or treating lysosomal storage disorders. In particular, the present invention provides methods for preventing and / or treating Gaucher's disease.
Owner:AMICUS THERAPEUTICS INC
Compositions and methods for the transport of therapeutic agents
InactiveUS20150174267A1Improve compoundExtended half-lifePowder deliveryObesity gene productsTherapeutic effectCell type
The present invention is directed to conjugates that include a polypeptide capable of crossing the blood-brain barrier or entering one or more cell types attached to a transport vector, i.e., a composition capable of transporting an agent (e.g., a therapeutic agent). In certain cases, the polypeptides are directly conjugated to a lipid or polymeric vector to allow targeted application of a therapeutic agent to treat, for example, a cancer, a neurodegenerative disease, or a lysosomal storage disorder.
Owner:ANGLACHEM INC
Compositions and methods for treating diseases and disorders of the central nervous system
PendingUS20200038439A1Improve efficiencyEnhanced drug releaseNervous disorderGenetic material ingredientsHematopoietic cellNervous system
The present invention provides compositions and methods for the treatment or prevention of a neurological disease or disorder of the central nervous system (e.g., a storage disorder, lysosomal storage disorder, neurodegenerative disease, etc.) by reconstitution of brain myeloid cell and microglia upon transplantation of hematopoietic cells enriched in microglia reconstitution potential. The invention also provides compositions and methods for ablating and reconstituting microglia.
Owner:CHILDRENS MEDICAL CENT CORP +3
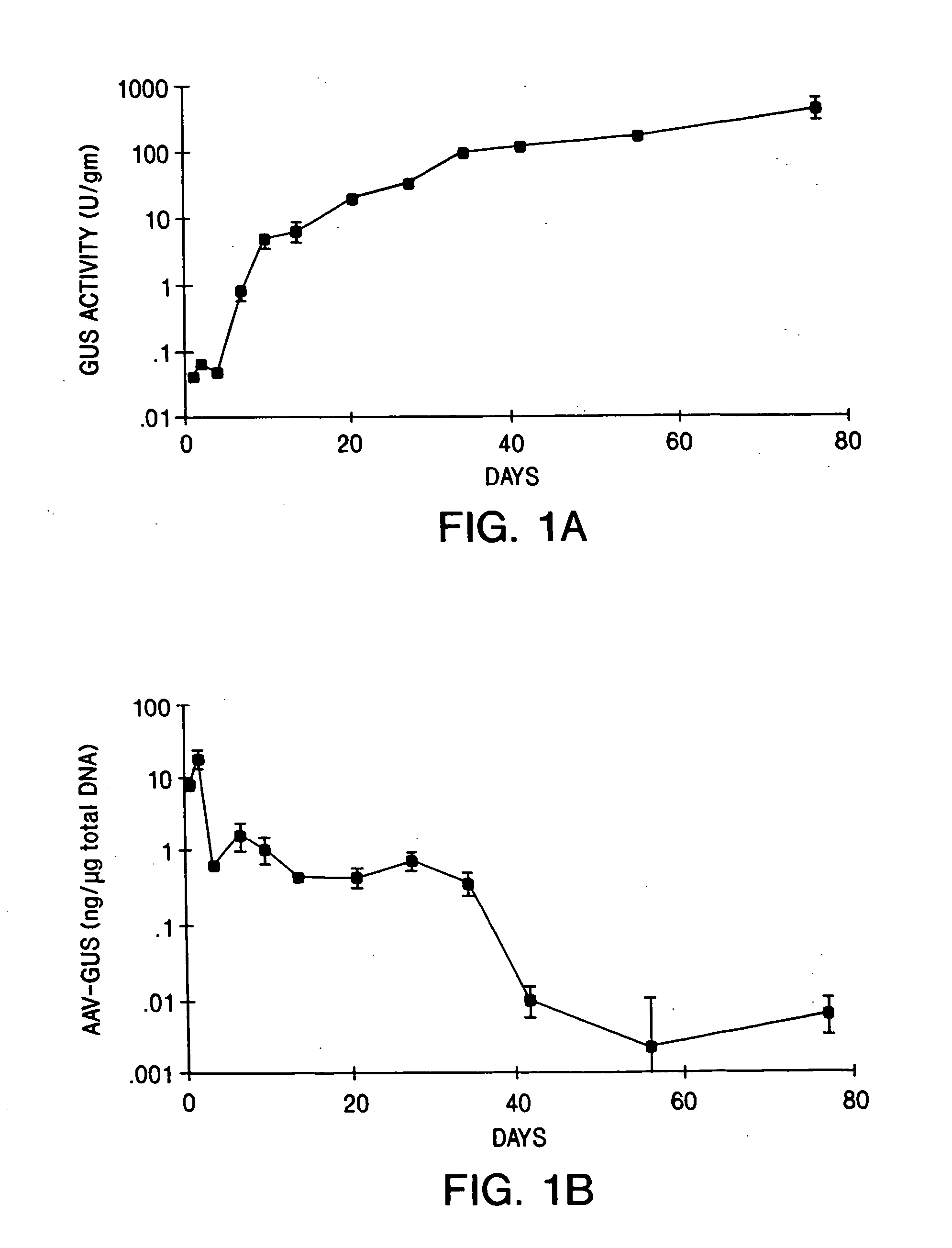
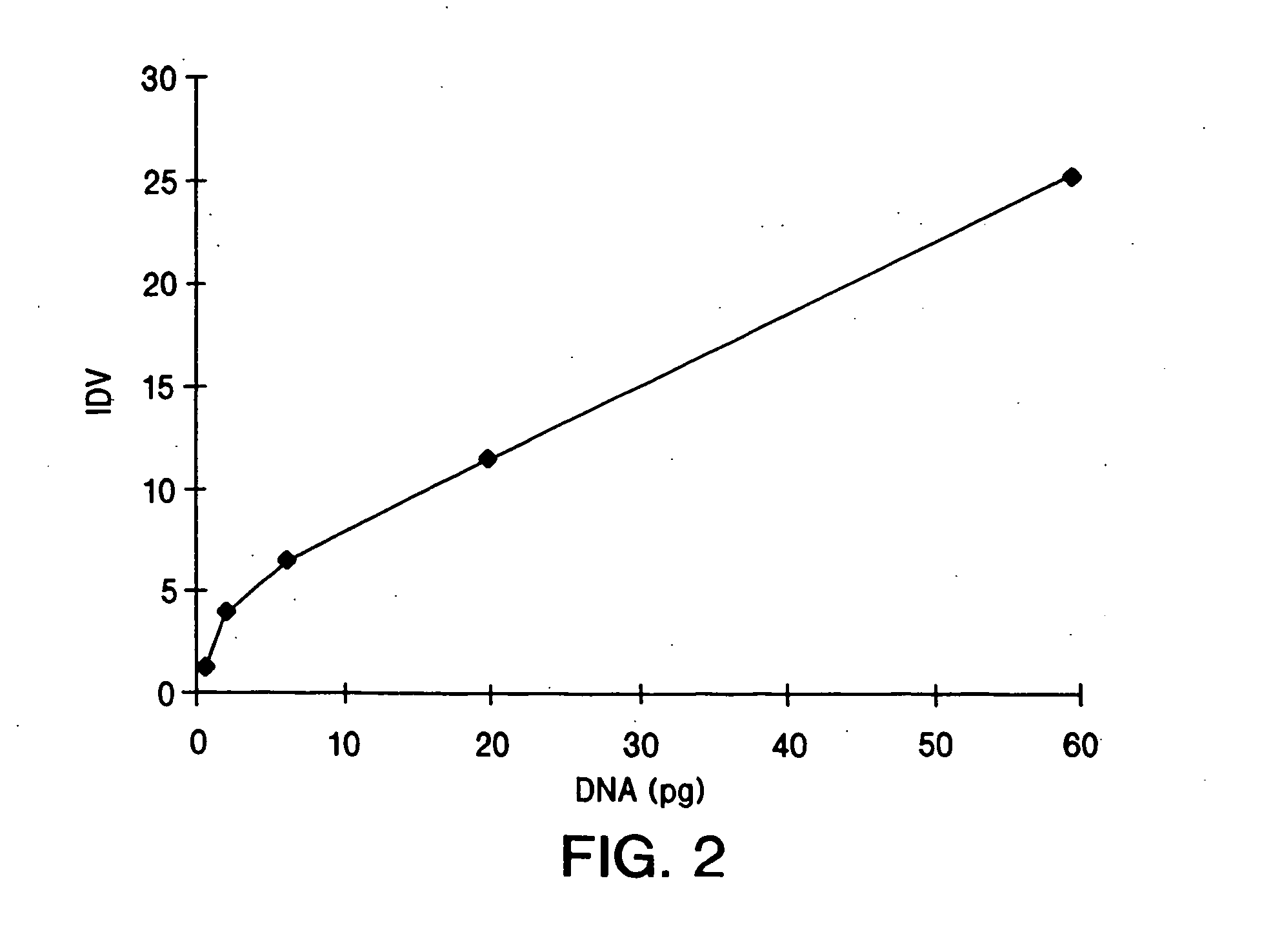
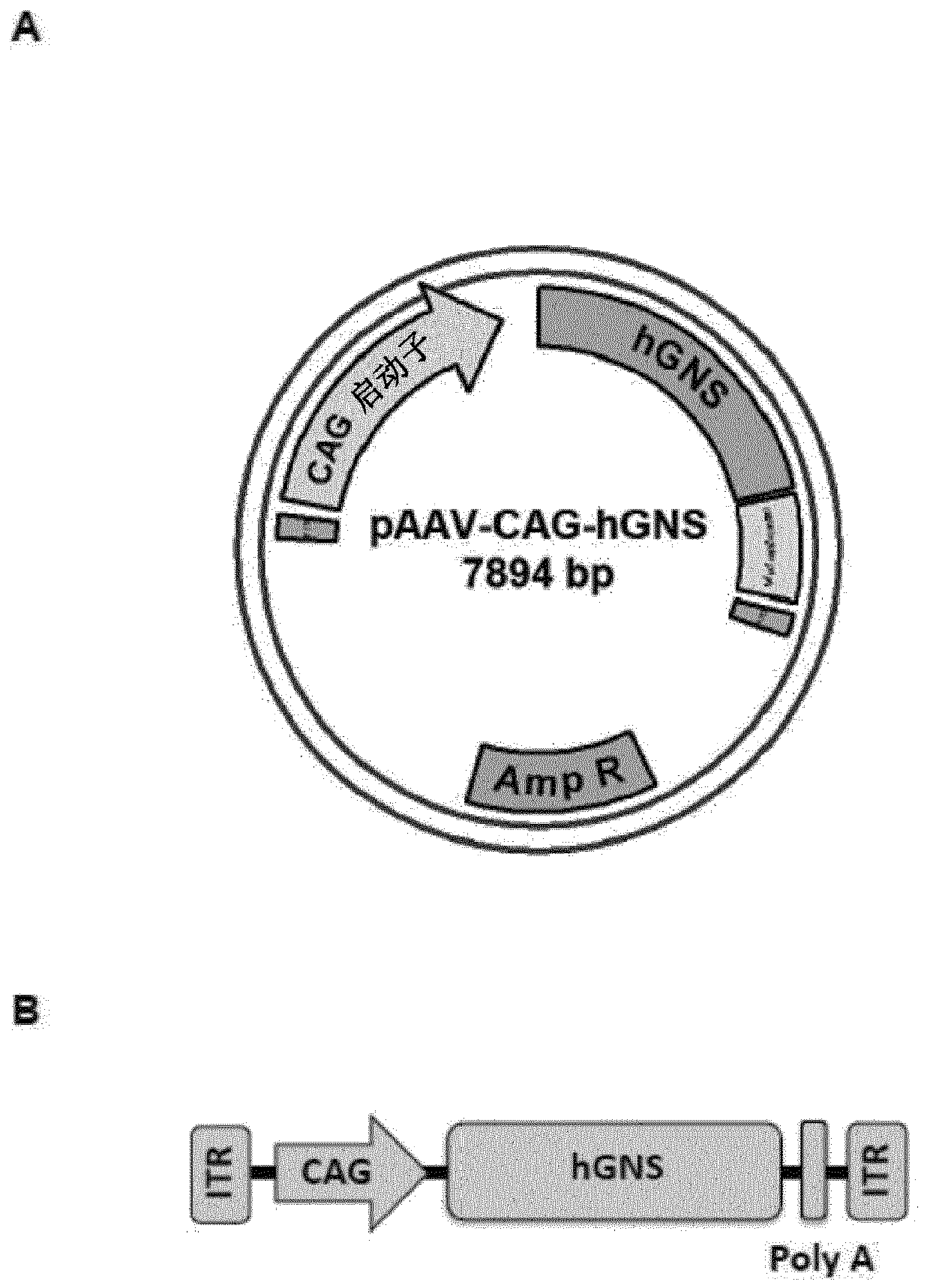
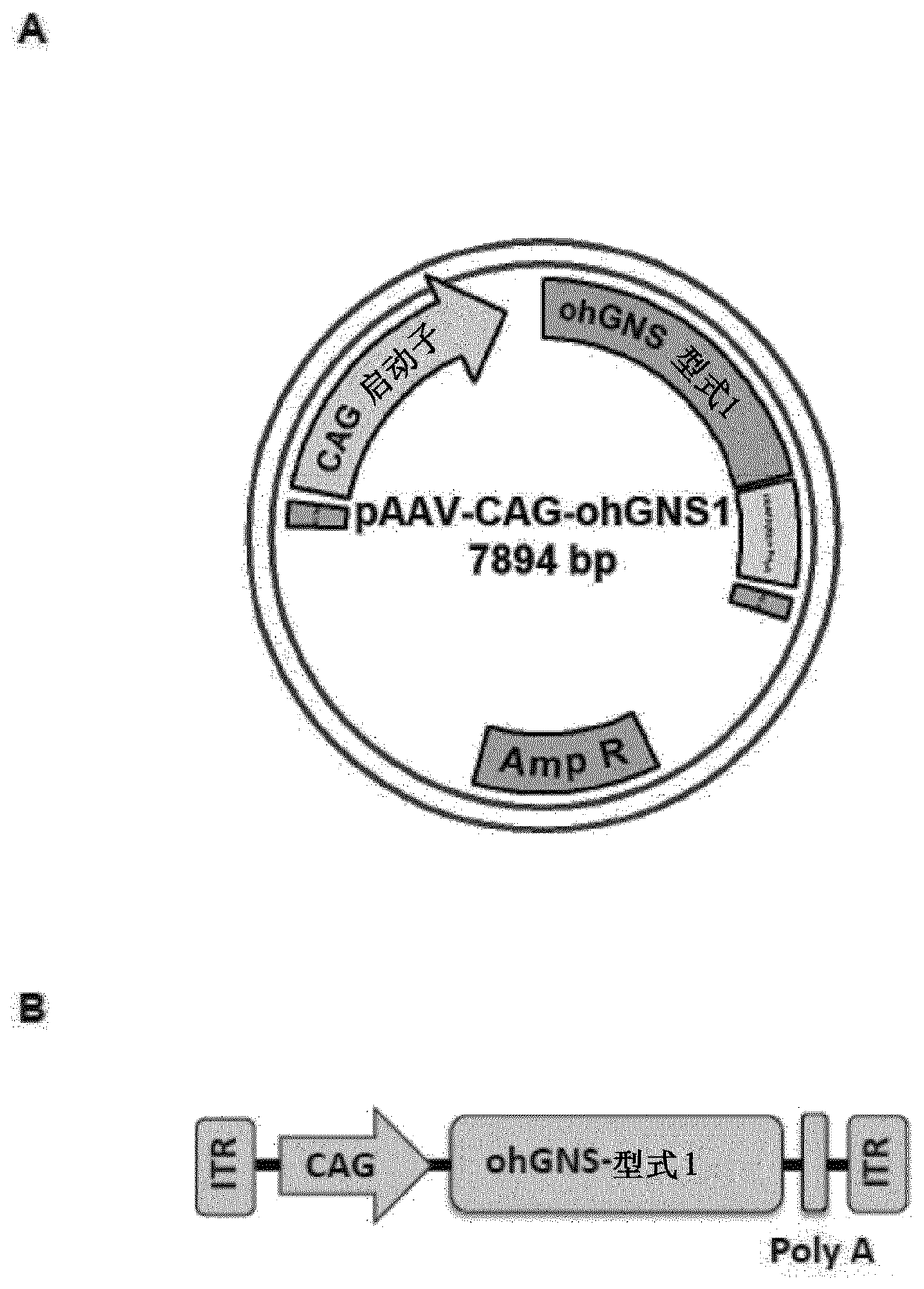
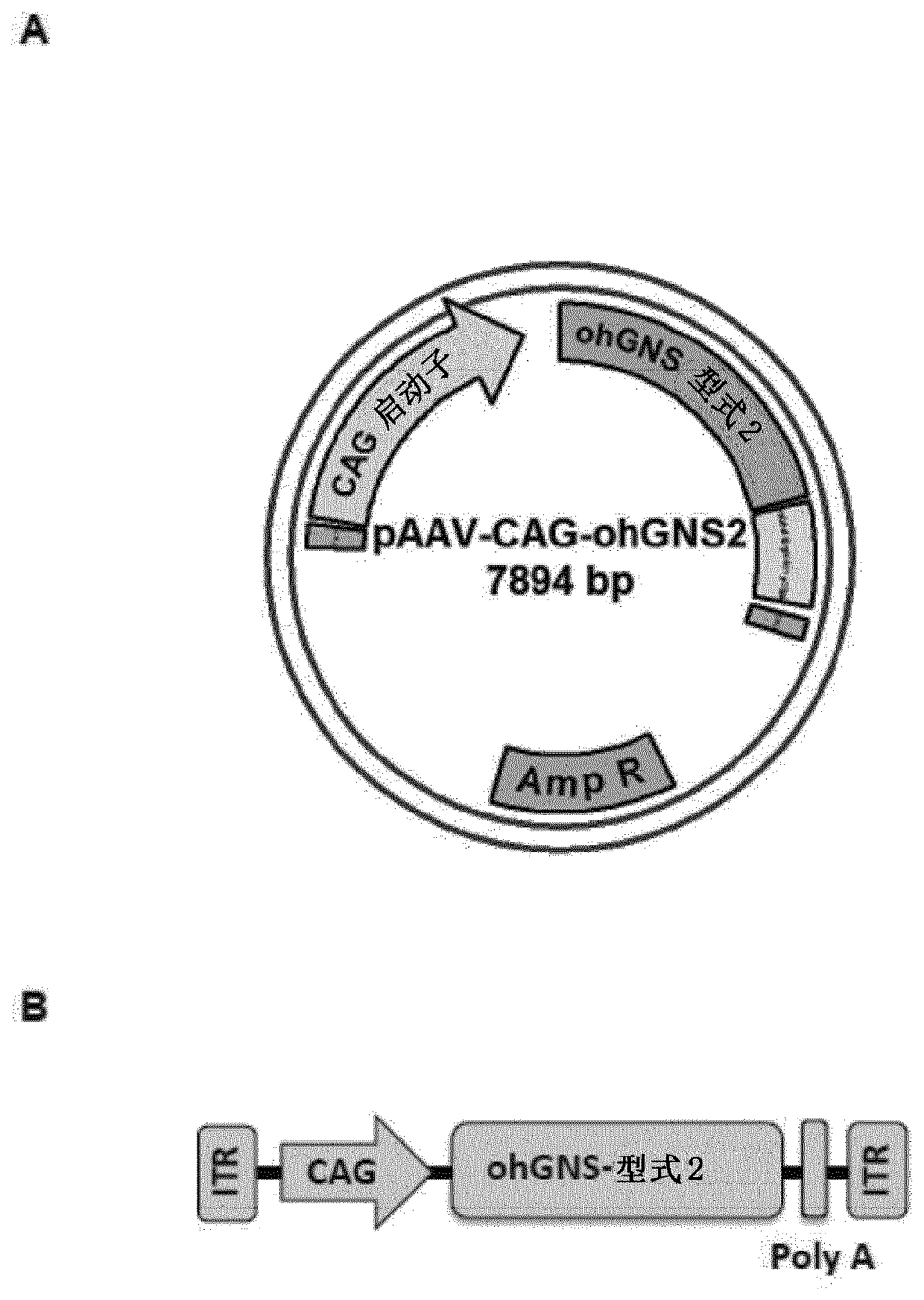
Recombinant adeno-associated virus virions for the treatment of lysosomal disorders
InactiveUS20060104954A1Reduces and eliminates GAG storageBeneficial effect on subjectBiocideVectorsLysosomeLysosomal storage disorders
AAV expression vectors and recombinant virions produced using these vectors, which include genes coding for enzymes defective or missing in lysosomal storage disorders, are described. These recombinant AAV virions are useful in the treatment of a variety of lysosomal storage disorders and the methods described herein provide for long-term, sustained expression of the defective or missing enzyme.
Owner:GENZYME CORP
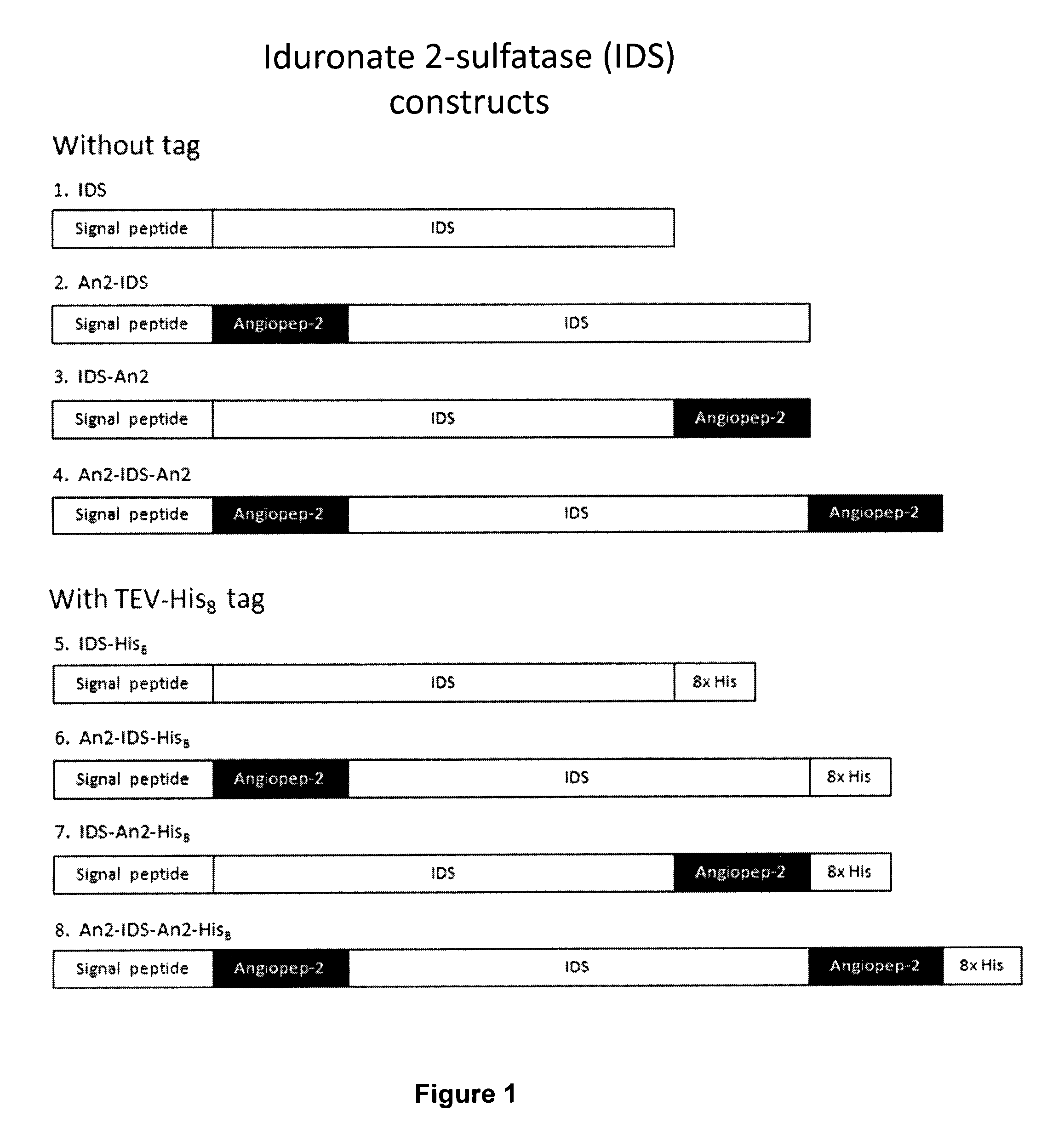
Targeted iduronate-2-sulfatase compounds
InactiveUS20140335163A1Treat symptomsEfficient transportPolypeptide with localisation/targeting motifNervous disorderLysosomeIduronate Sulfatase
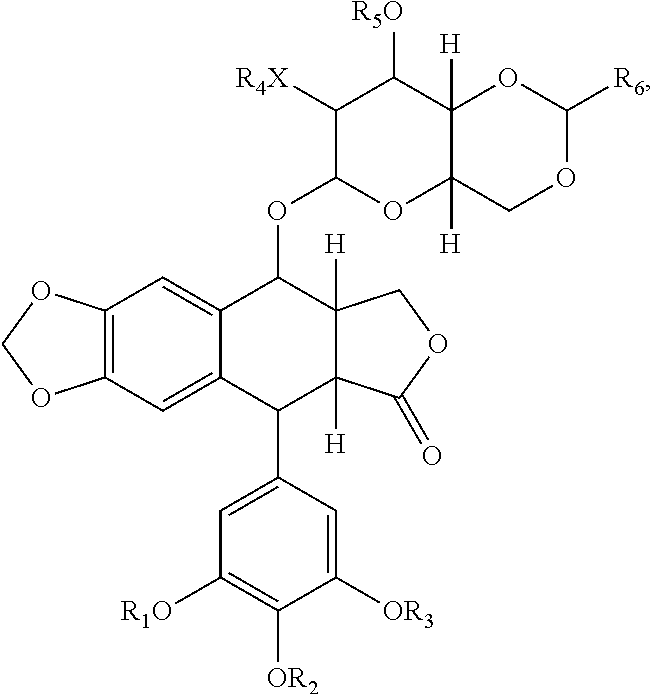
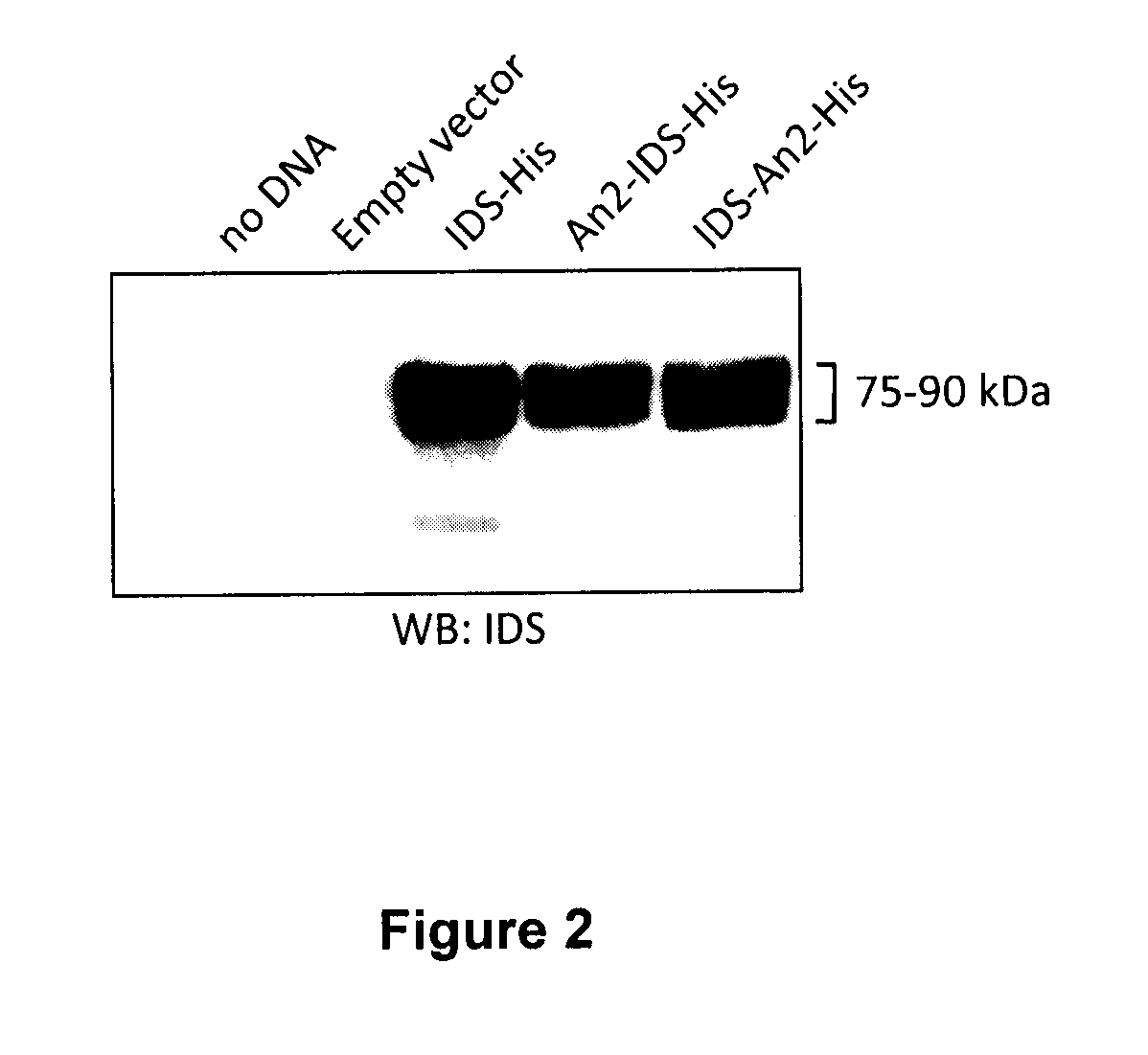
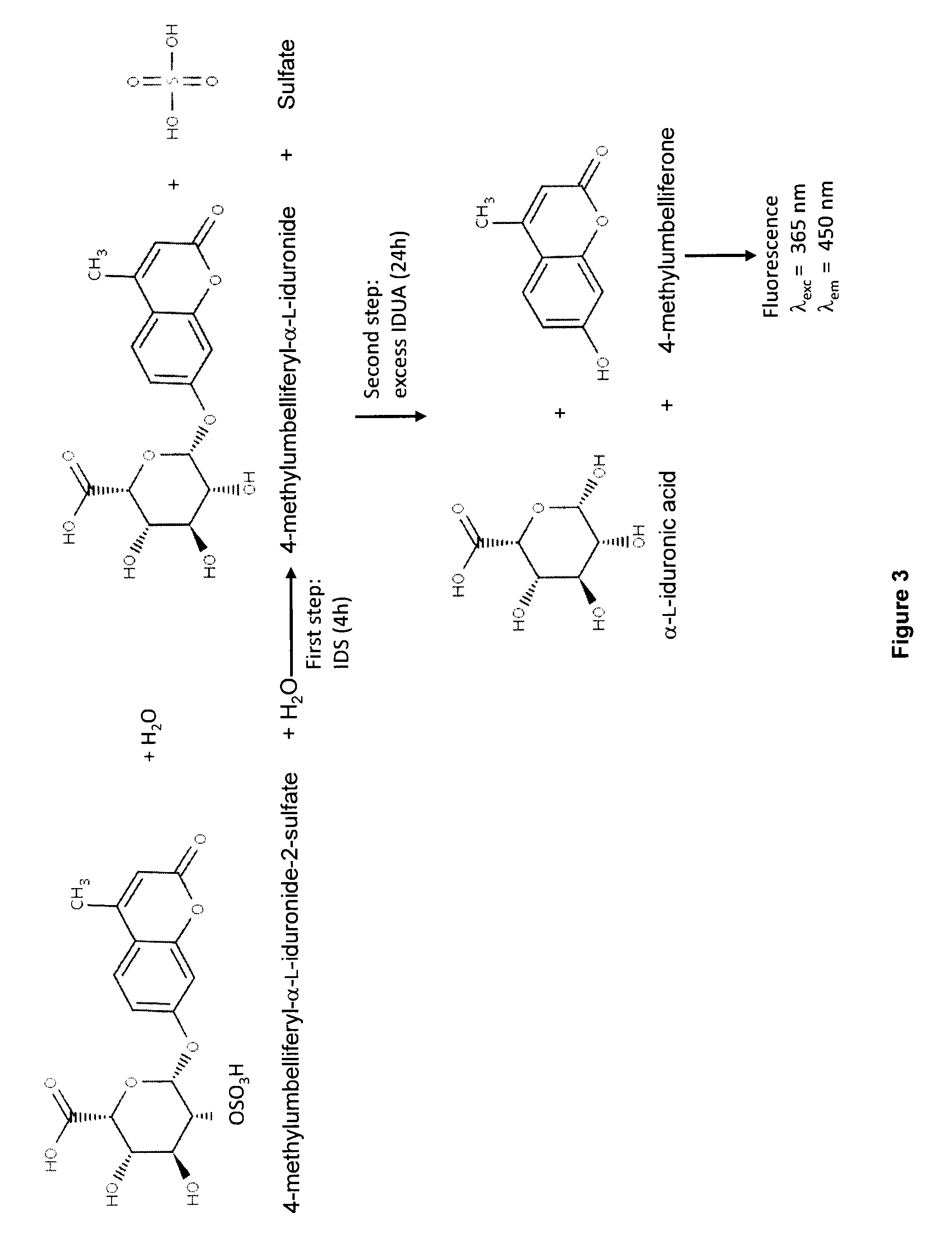
The present invention is related to a compound that includes a lysosomal enzyme and a targeting moiety, for example, where compound is a fusion protein including iduronate-2-sulfatase and Angiopep-2. In certain embodiments, these compounds, owning to the presence of the targeting moiety can crossing the blood-brain barrier or accumulate in the lysosome more effectively than the enzyme alone. The invention also features methods for treating lysosomal storage disorders (e.g., mucopolysaccharidosis Type II) using such compounds.
Owner:ANGLACHEM INC
Polypeptide-nucleic acid conjugates and uses thereof
InactiveCN101946001AOrganic active ingredientsSenses disorderLysosomal storage disordersStereochemistry
The present invention is directed to polypeptide-nucleic acid conjugates. These conjugates can allow for targeted application of a therapeutic RNAi agent across the blood-brain barrier to treat, for example, a cancer, neurodegenerative disease, or lysosomal storage disorder.
Owner:ANGLACHEM INC
Adenoassociated virus vectors for the treatment of mucopolysaccharidoses
ActiveUS20180169272A1HydrolasesPharmaceutical delivery mechanismAdenoassociated virusAdeno associate virus
The present invention provides new Adeno-associated virus-derived vectors and pharmaceutical compositions containing the same for the treatment of lysosomal storage disorders and specially, for the treatment of mucopolysaccharidoses Type II.
Owner:ESTEVE PHARMA SA +1
Adenoassociated virus vectors for the treatment of mucopolysaccharidoses
The present invention provides new adeno-associated virus-derived vectors and pharmaceutical compositions containing the same for the treatment of lysosomal storage disorders and specially, for the treatment of mucopolysaccharidoses Type IIID.
Owner:ESTEVE PHARMA SA +1
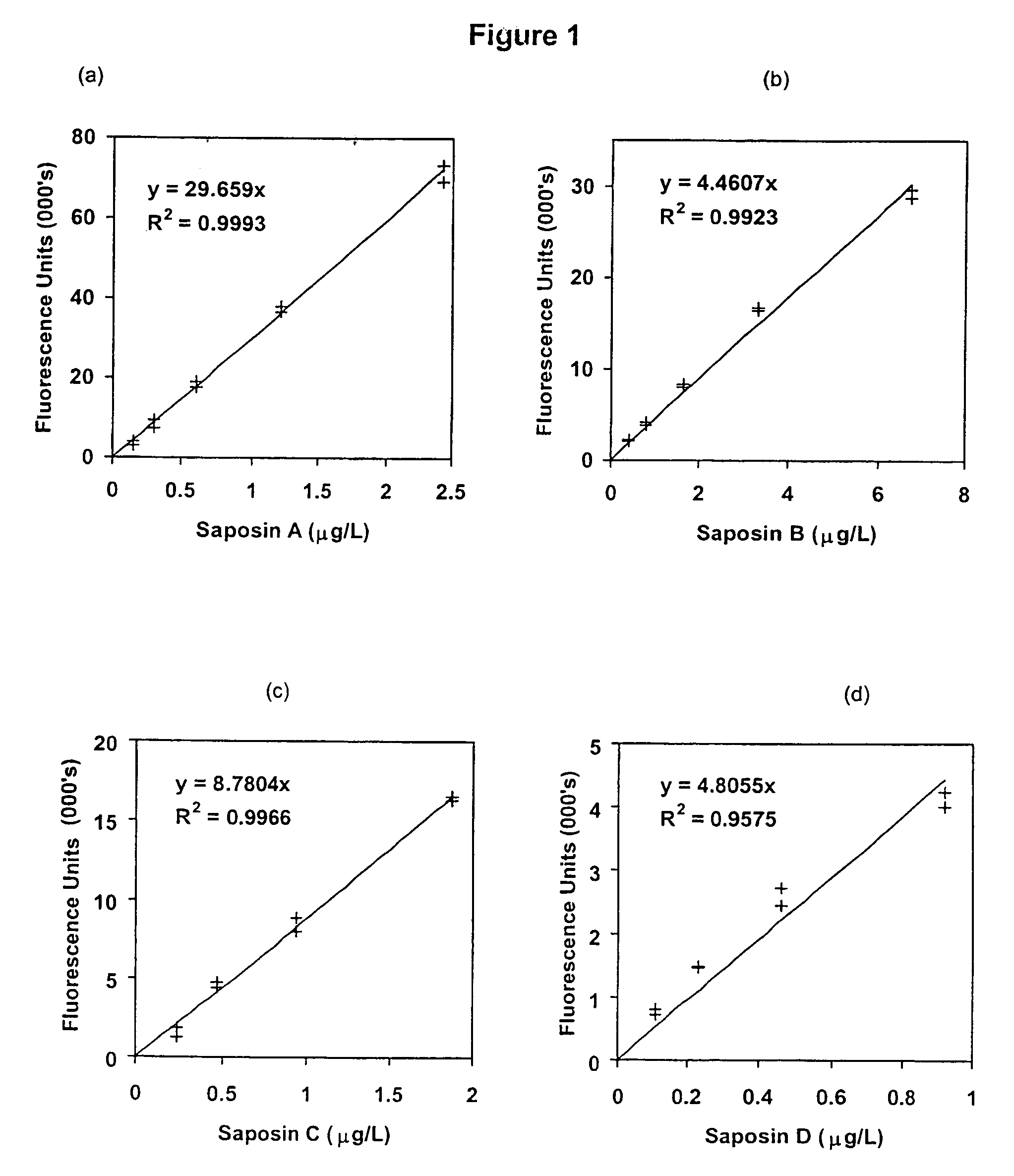
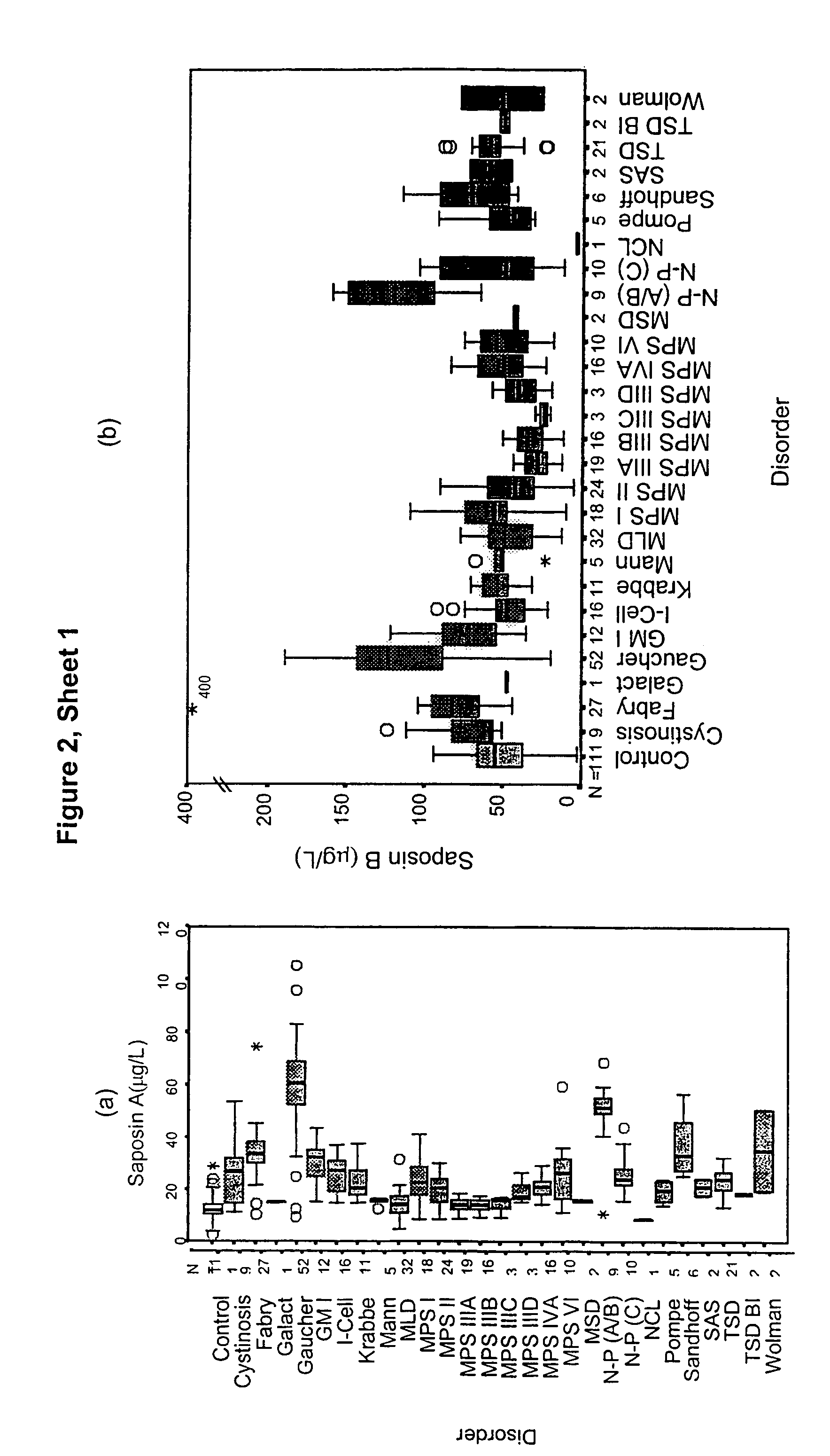
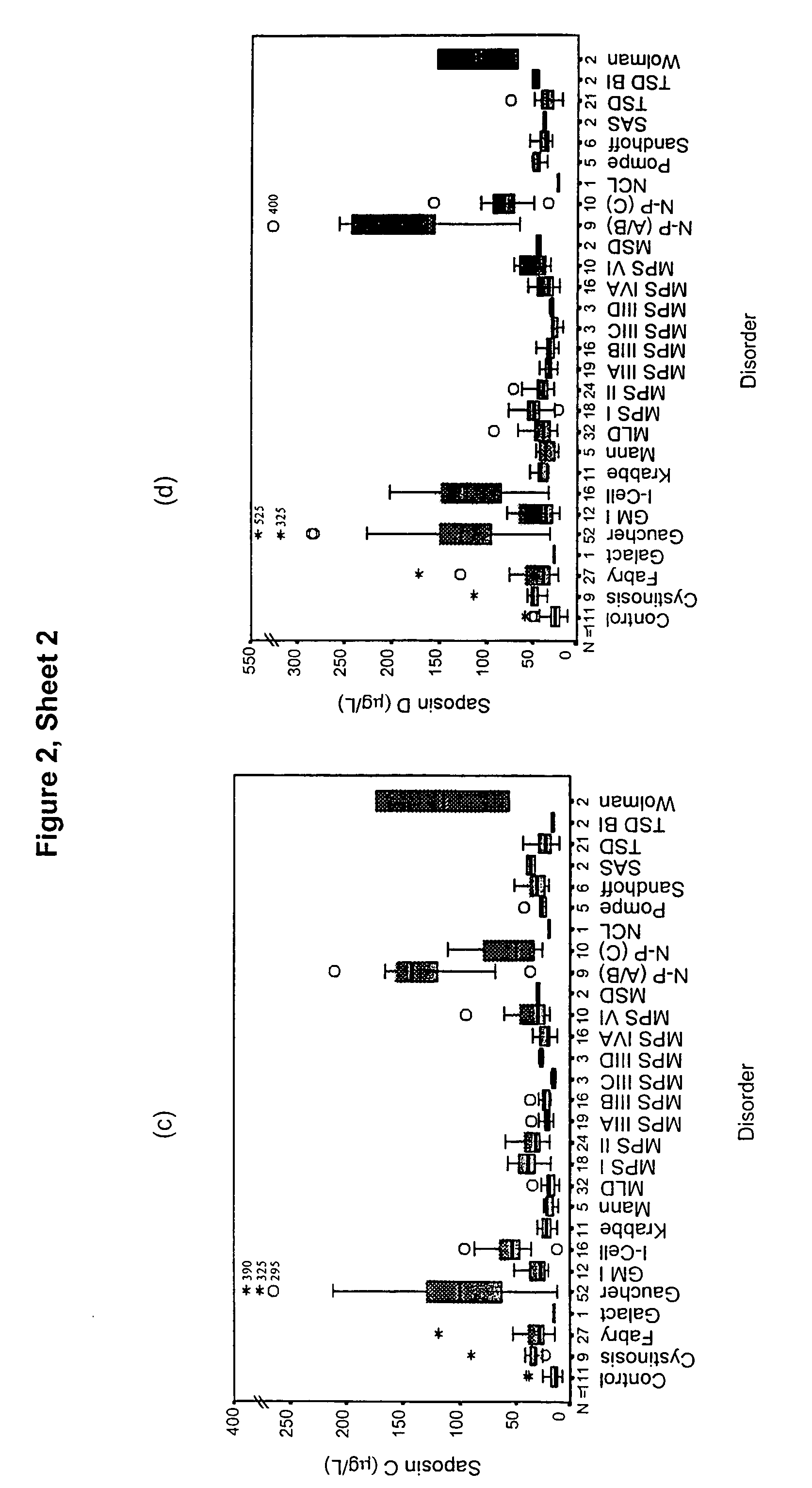
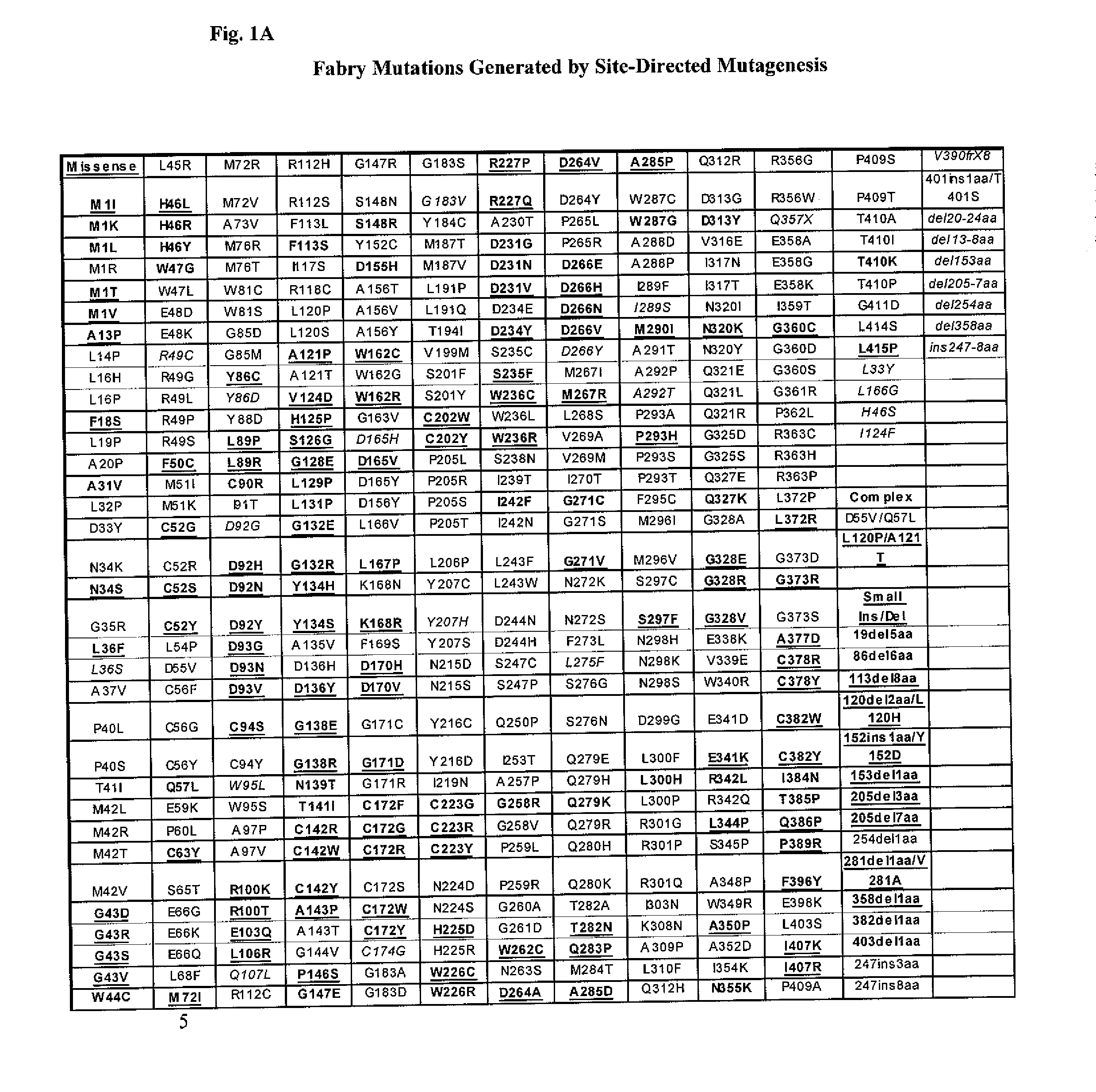
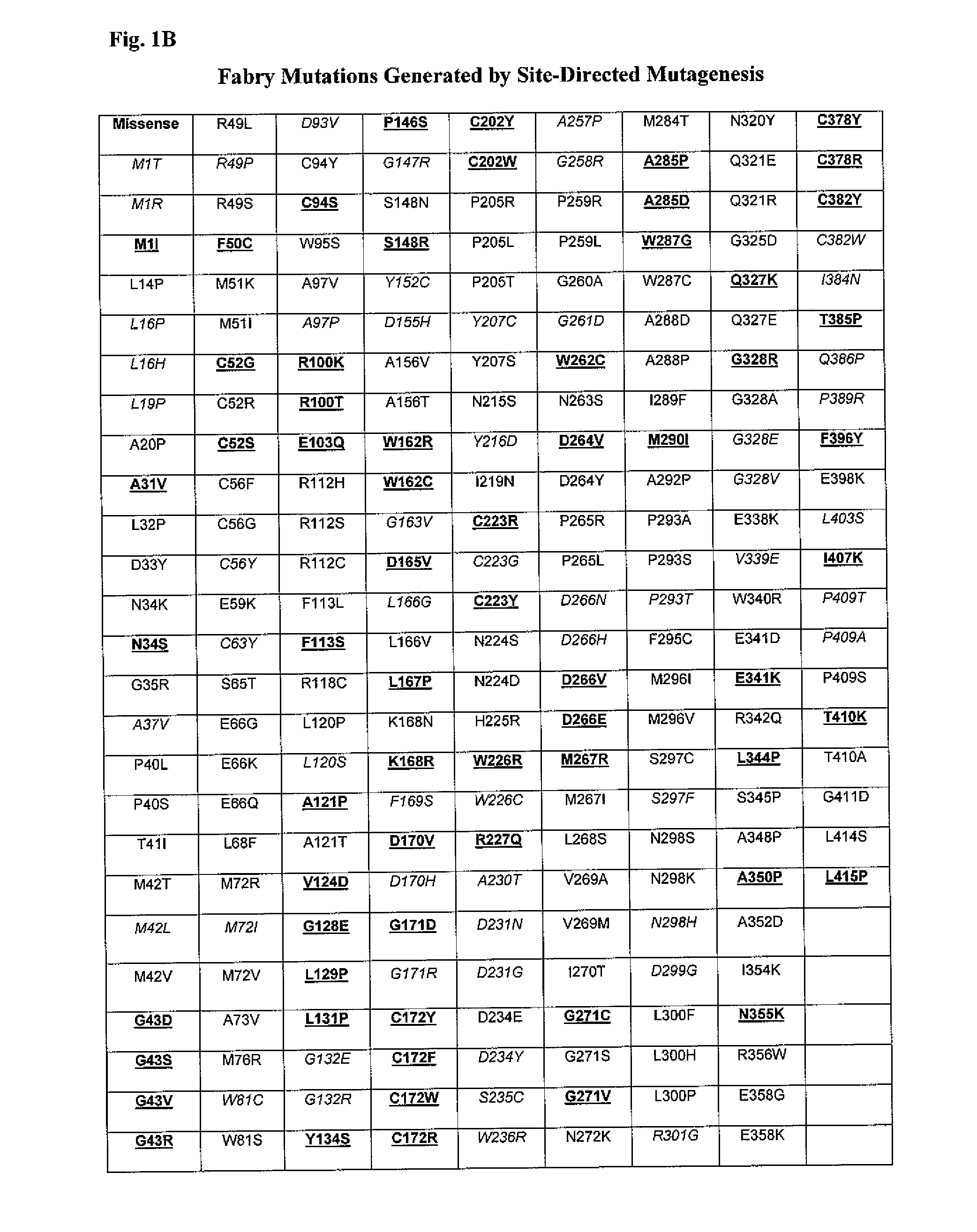
Diagnosis of lysosomal storage disorders using saposins and other markers
InactiveUS7378231B1Positive treatment outcomeMicrobiological testing/measurementDisease diagnosisPediatricsLysosomal storage disorders
The invention provides methods of diagnosing or monitoring lysosomal storage disorders based on detecting levels of saposins, LAMPs and / or α-glucosidase in patient sample. Elevated levels of saposins and / or LAMPs are indicative of a disorder. Elevated levels of α glucosidase are indicative of some types of lysosomal storage disorders and decreased levels of α glucosidase are indicative of other types of lysosomal storage disorder. In some methods, the profile of elevation of different saposins, LAMPs and α glucosidase allows distinction between different types of lysosomal storage disorder.
Owner:WOMENS & CHILDRENS HOSPITAL
Method to predict response to pharmacological chaperone treatment of diseases
ActiveUS20110212996A1Increase activity levelHigh activityBiocideMetabolism disorderPharmacological chaperoneΑ galactosidase a
The present invention provides methods to determine whether a patient with a lysosomal storage disorder will benefit from treatment with a specific pharmacological chaperone. The present invention exemplifies an in vitro method for determining α-galactosidase A responsiveness to a pharmacological chaperone such as 1-deoxygalactonojirimycin in a cell line expressing a mutant from of α-galactosidase A. The invention also provides a method for diagnosing Fabry disease in patients suspected of having Fabry disease.
Owner:AMICUS THERAPEUTICS INC
Novel Compounds For Preventing And/Or Treating Lysosomal Storage Disorders And/Or Degenerative Disorders Of The Central Nervous System
Described are novel salts of the compound (3R,4R,5S)-5-(difluoromethyl) piperidine-3,4-diol, as well as methods of using the same for preventing and / or treating lysosomal storage disorders and / or degenerative disorders of the central nervous system. In particular, the present invention provides methods for preventing and / or treating Gaucher's disease and / or Parkinson's disease.
Owner:AMICUS THERAPEUTICS INC
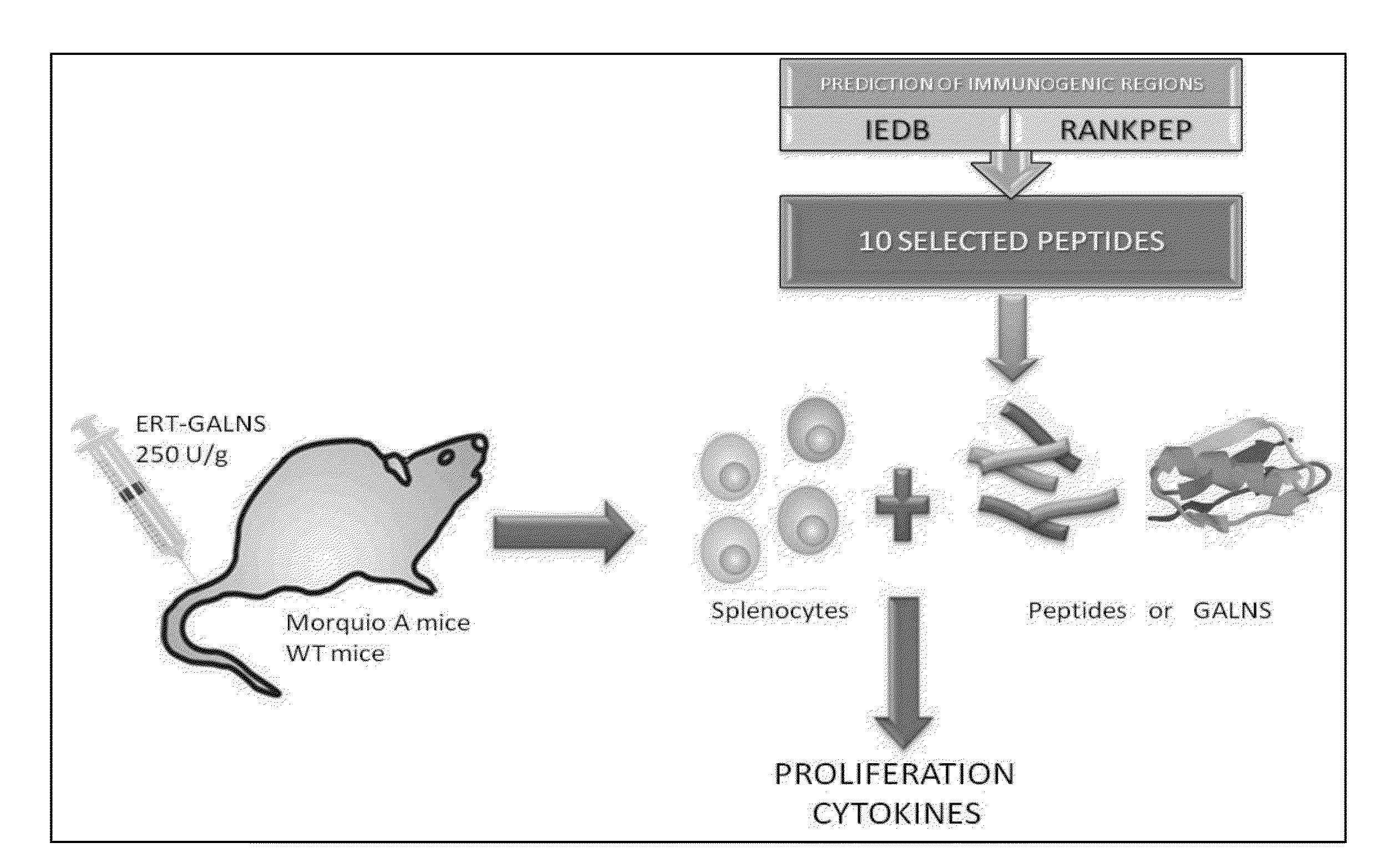
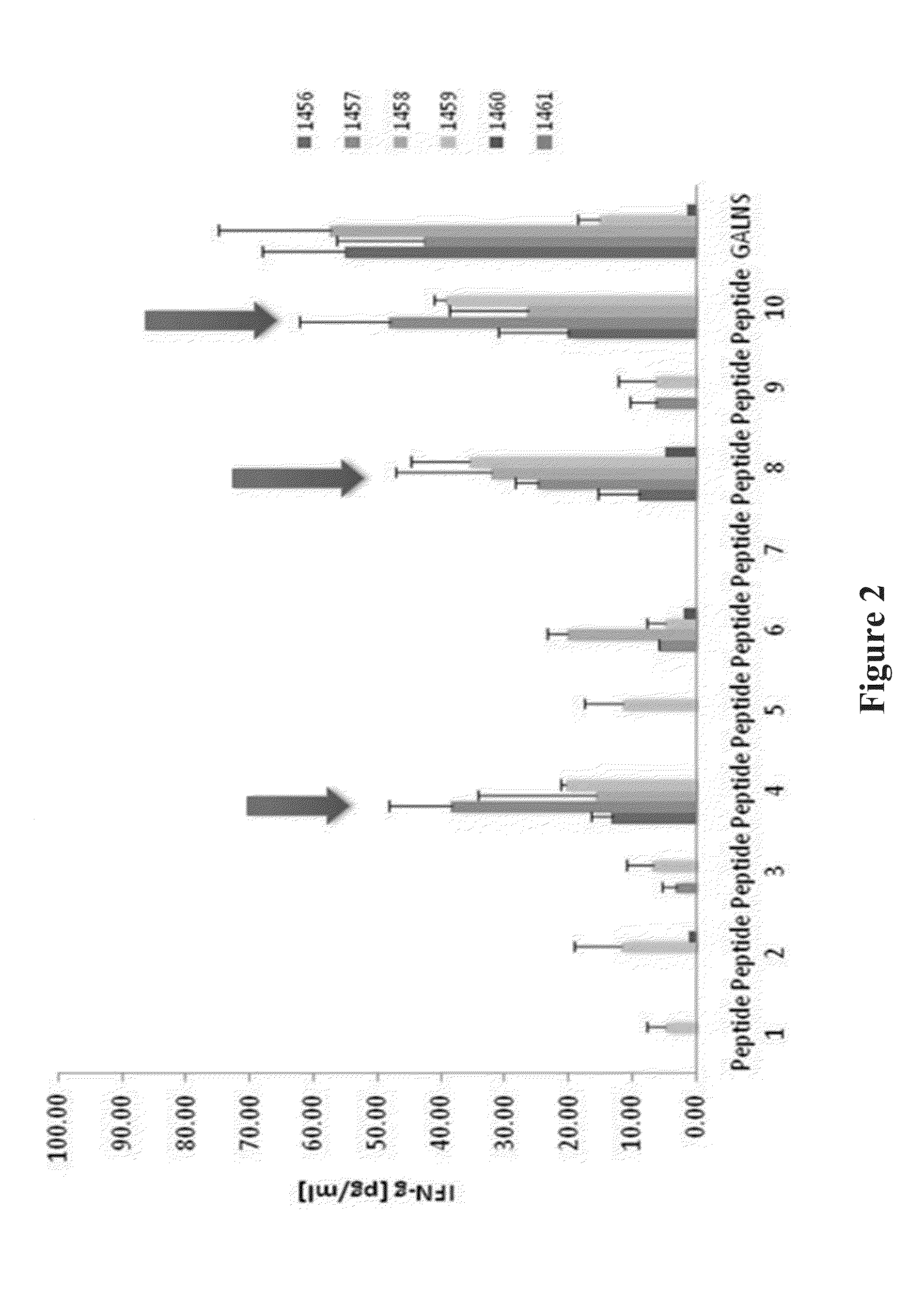
Determination of immunogenic peptides in lysosomal enzymes and induction of oral tolerance
Disclosed are methods and compositions for determining immunodominant peptides of target enzymes used in enzyme replacement therapy for lysosomal storage disorders. More specifically disclosed are immunodominant peptides for N-acetylgalactosamine-6-sulfatase (GALNS). Also disclosed are methods of inducing oral tolerance towards a target enzyme through oral administration of immunodominant peptides prior to commencing enzyme replacement therapy. More specifically disclosed is a method of inducing oral tolerance for GALNS, by orally administering specific immunodominant peptides for GALNS; in subjects suffering from mucopolysaccharidosis type IVA prior to commencing enzyme replacement therapy using GALNS.
Owner:SAINT LOUIS UNIVERSITY
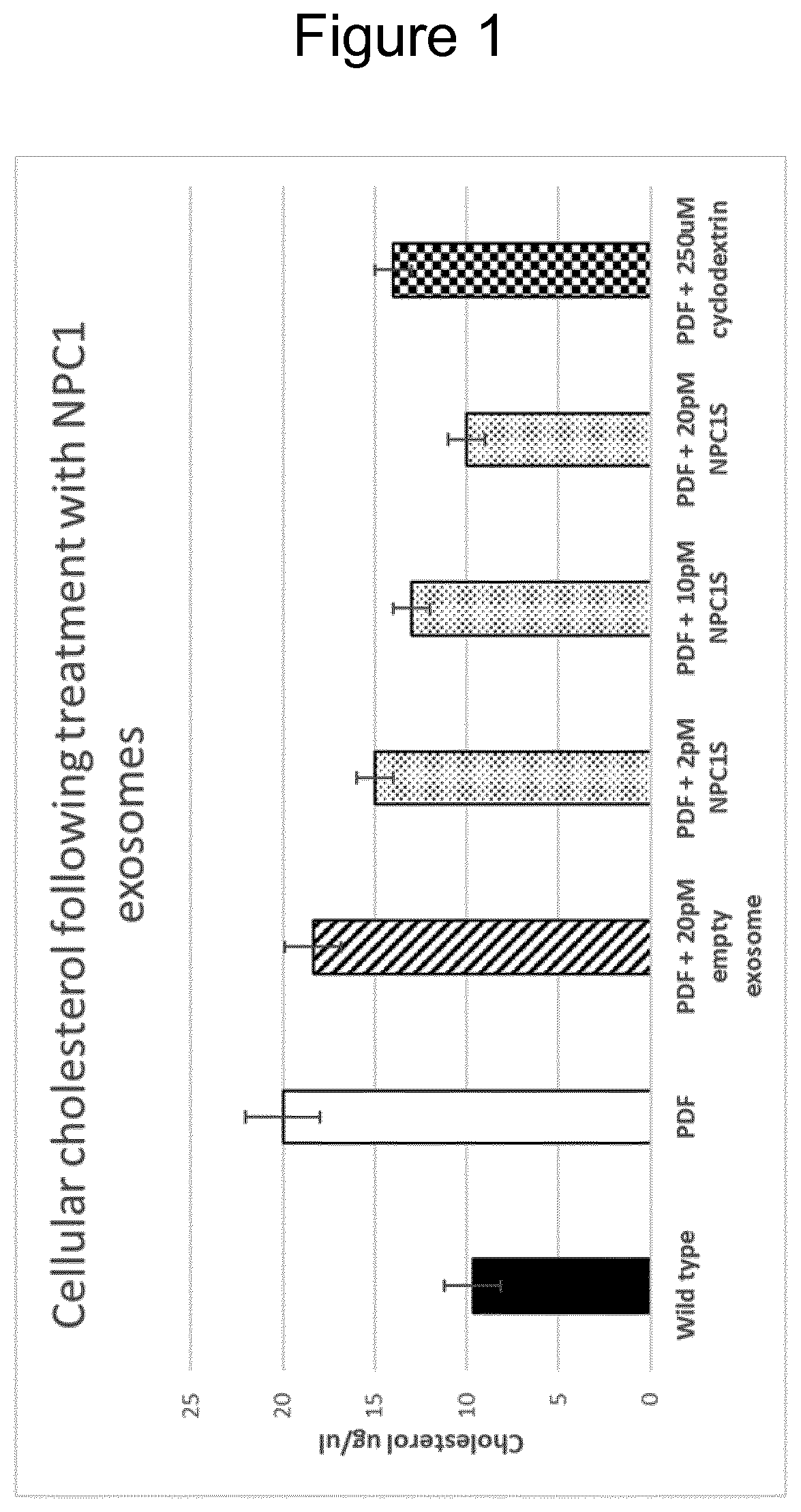
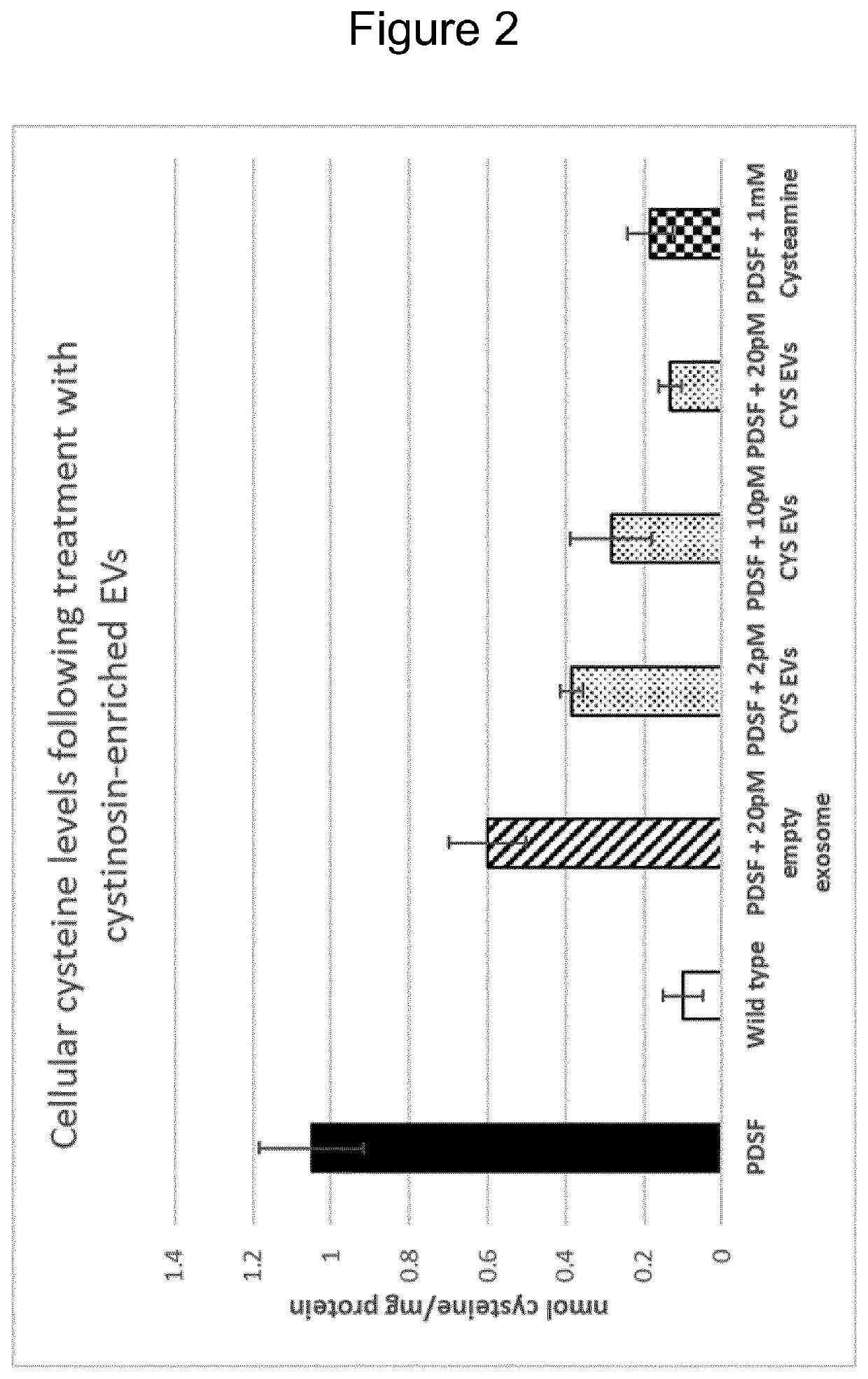
Protein engineered extracellular vesicles
PendingUS20200399591A1High trafficPolypeptide with localisation/targeting motifCell dissociation methodsExtracellular vesicleLysosome
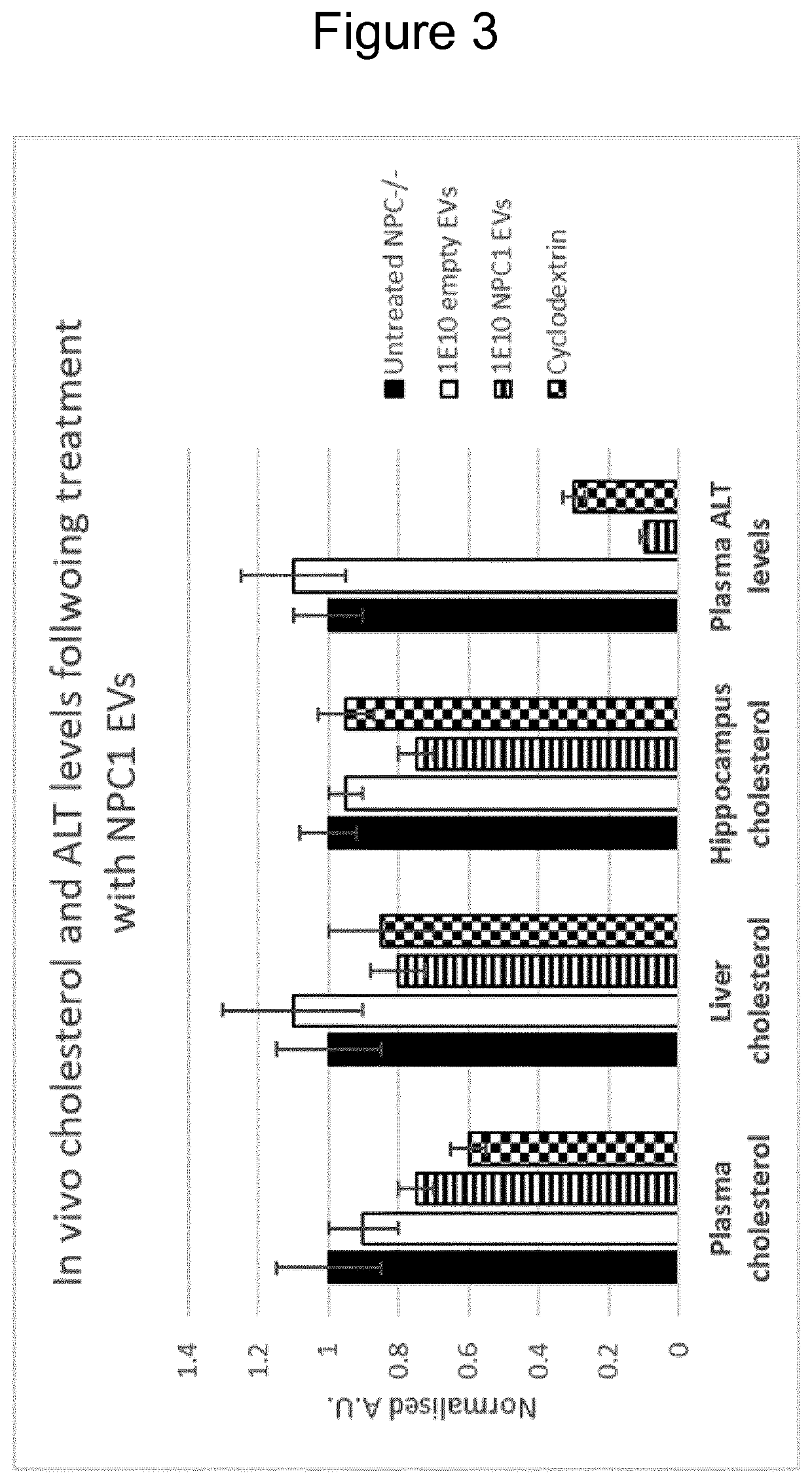
The present invention relates to extracellular vesicles (EVs) as a novel therapeutic approach to lysosomal storage disorders (LSD). More specifically, the invention relates to the use of various protein engineering strategies for improving loading of hard-to-load LSD-related proteins and targeting of the resultant EVs to tissues and organs of interest.
Owner:EVOX THERAPEUTICS LTD
Molecules able to modulate the expression of at least a gene involved in degradative pathways and uses thereof
InactiveUS20120040451A1Enhancement—of the production of the lysosomal proteinsModulate expression of target geneNervous disorderSugar derivativesTFEBDegradative Pathway
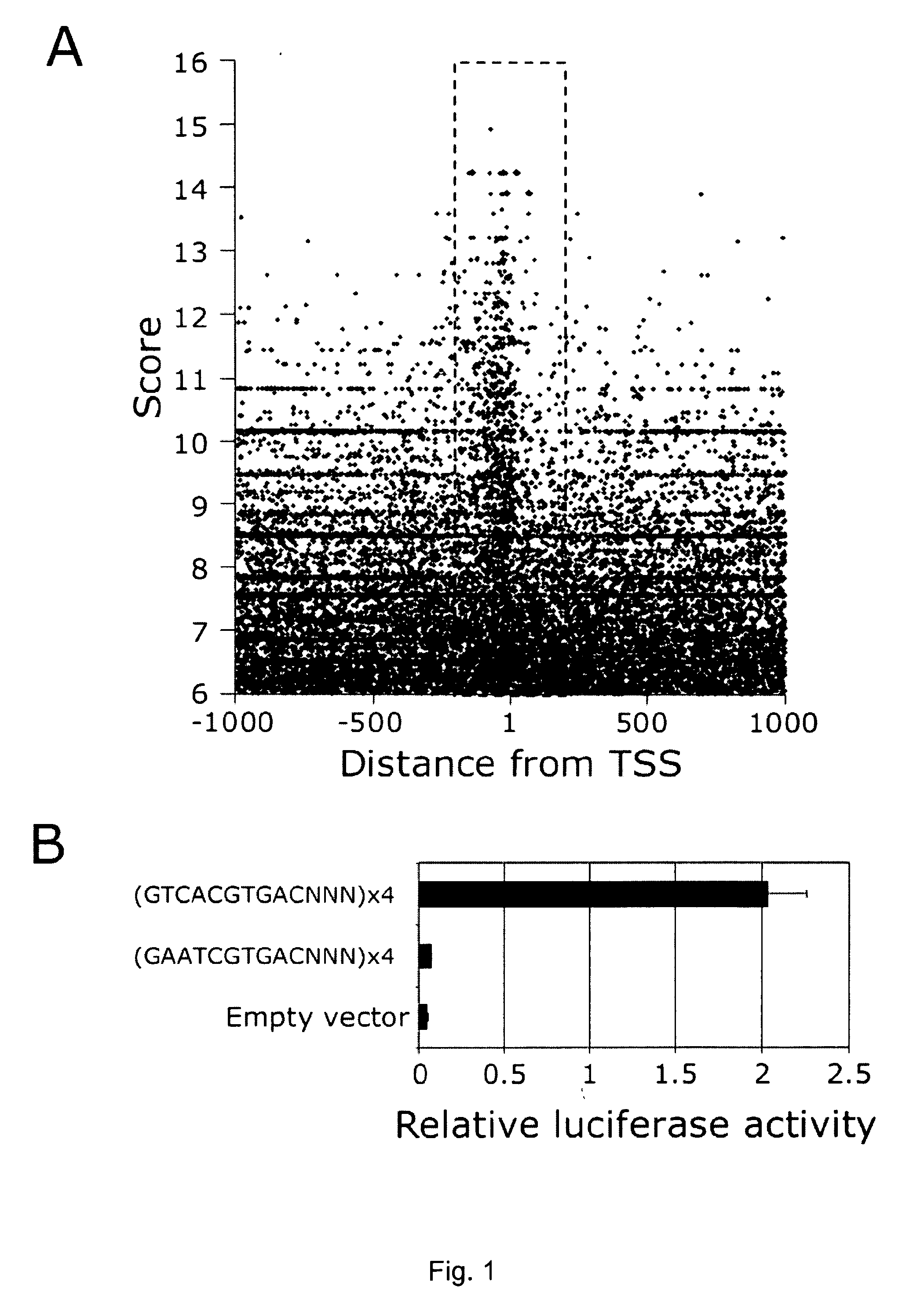
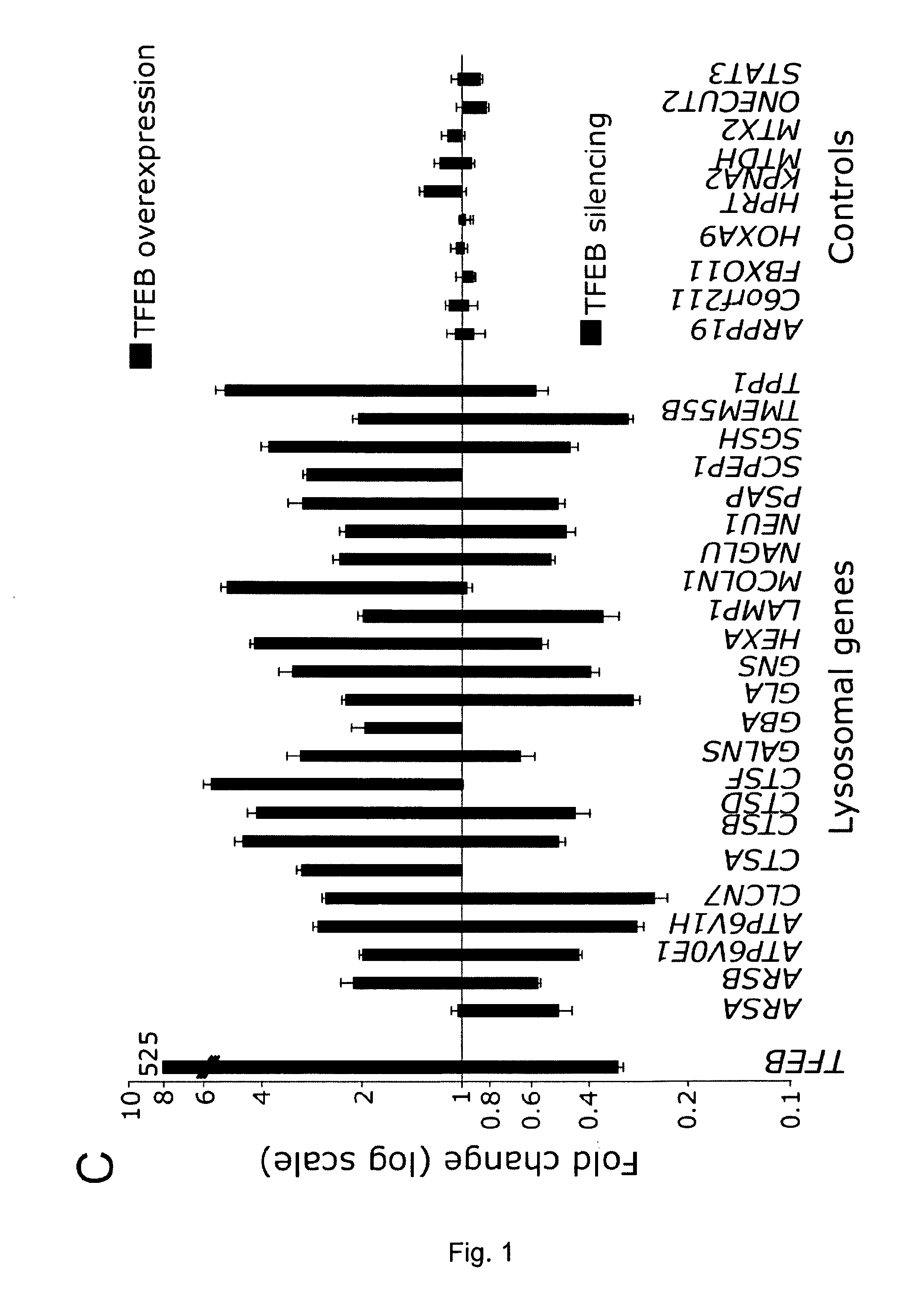
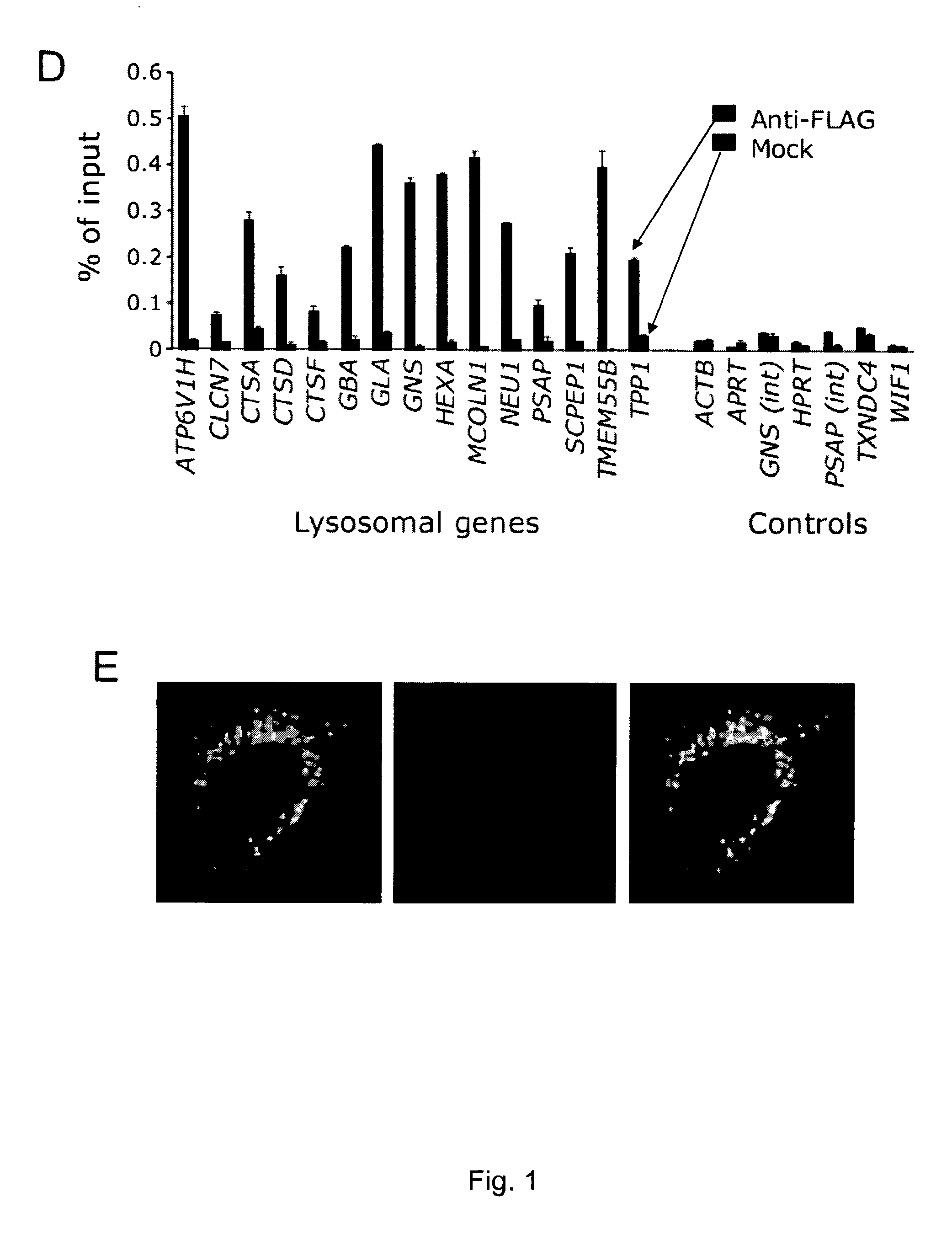
A molecule being able to modulate the expression of at least a gene involved in degradative pathways so to enhance the cellular degradative pathways and prevent or antagonize the accumulation of toxic compounds in a cell and acting on a CLEAR element. Preferred molecules are: the TFEB protein, synthetic or biotechnological functional derivative thereof; chimeric molecule comprising the TFEB protein, synthetic or biotechnological functional derivative thereof; modulator of the TFEB protein activity and / or expression level. The molecule may be used in the treatment of neurodegenerative and / or lysosomal storage disorders.
Owner:FOND AZIONE TELETHON
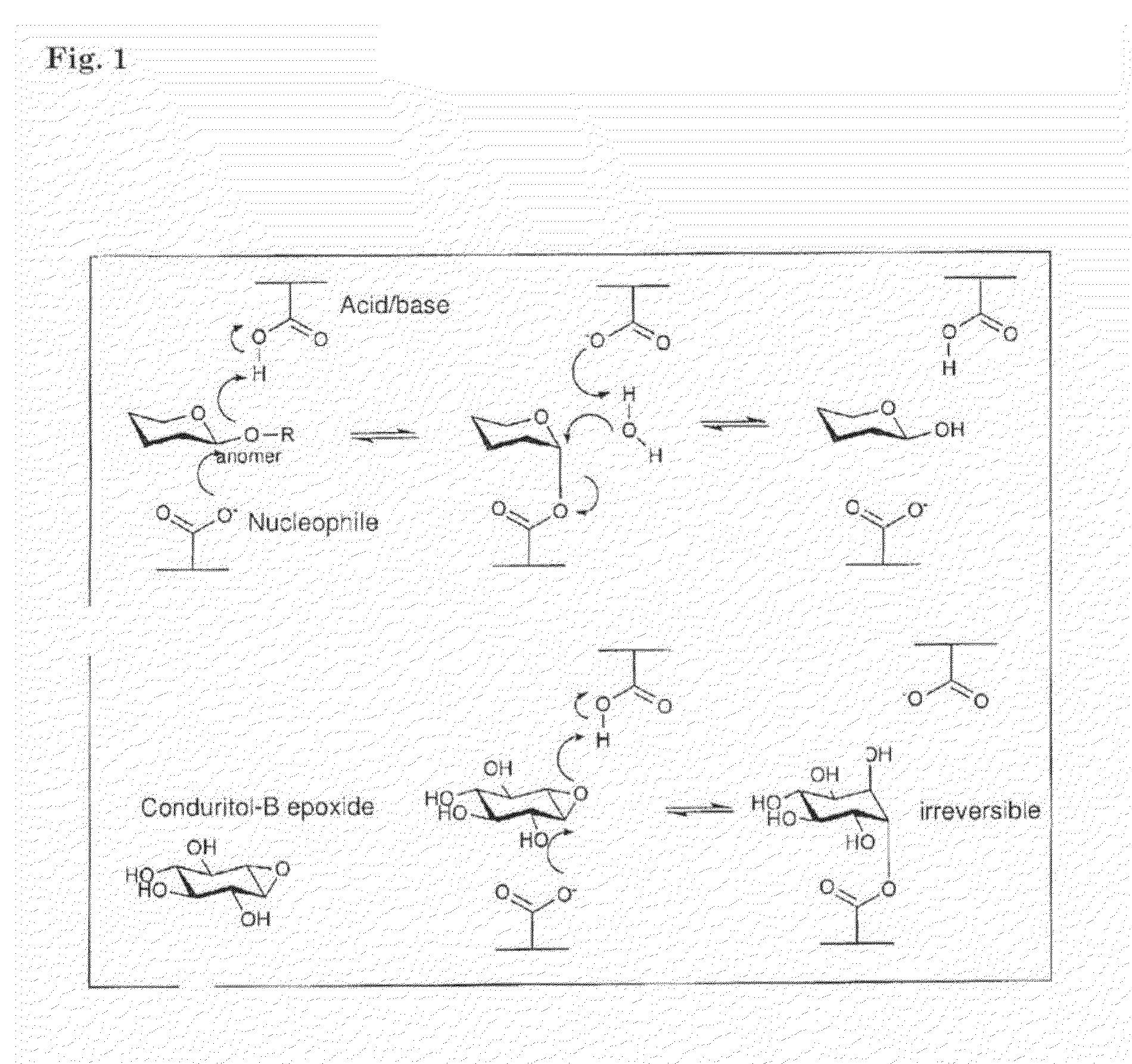
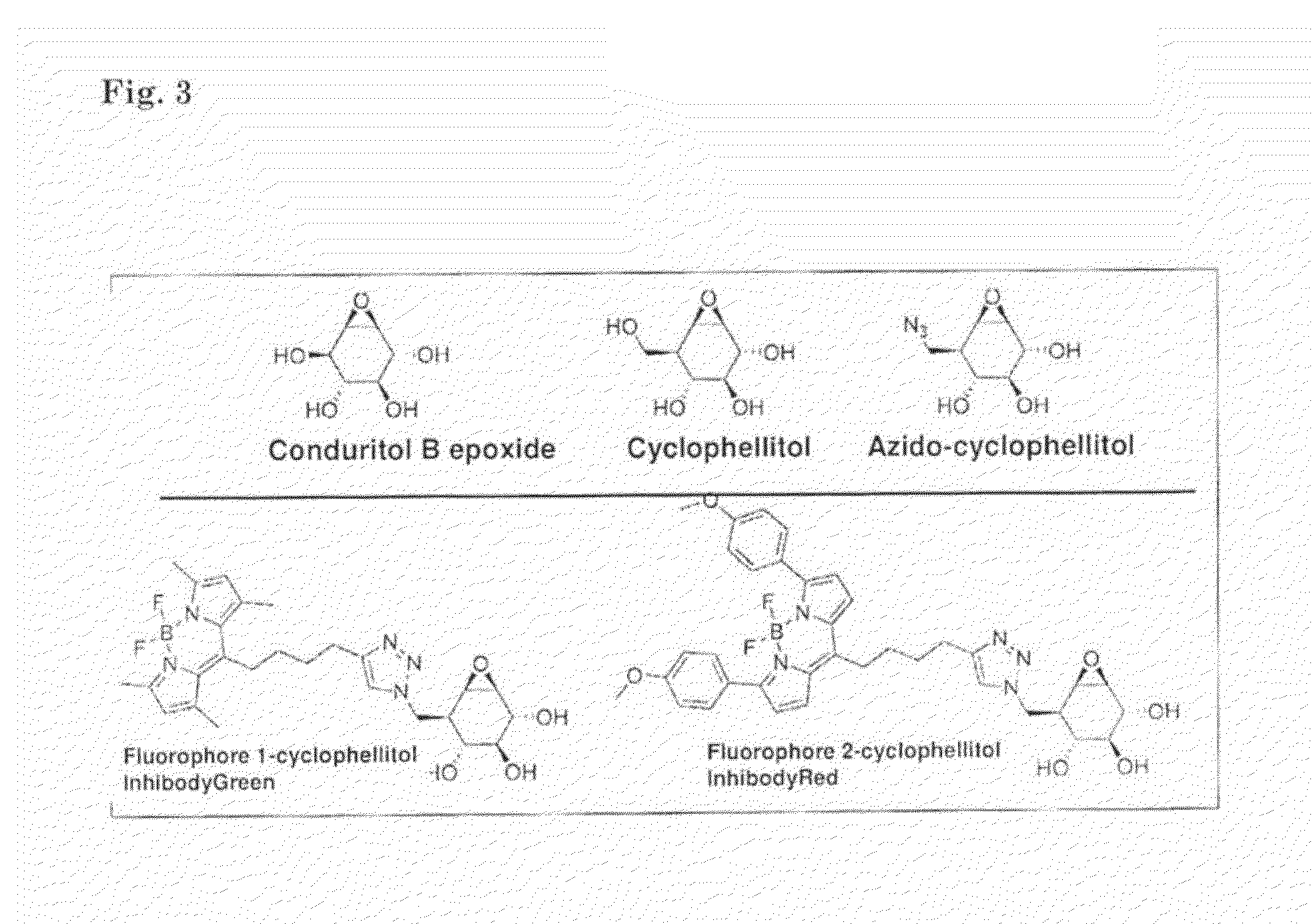
ACTIVITY BASED PROBES (ABPs) INTERACTING WITH GLYCOSIDASES
InactiveUS20130143228A1Improved affinity/sensitivityGood inhibitorSilicon organic compoundsCompound screeningGlycosidase inhibitorMolecular imaging
An activity based probe (ABP) comprising a glycosidase inhibitor, and a detection-group. The ABPs of the inventions are used for diagnosing storage disorder for screening of compounds suitable for preventing and / or treating a storage disorder, for monitoring of therapeutic enzymes for lysosomal storage disorders, and for ultra-sensitive visualization of glycosidase-fusion proteins in molecular imaging.
Owner:ACADEMISCH MEDISCH CENT BIJ DE UNIV VAN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com