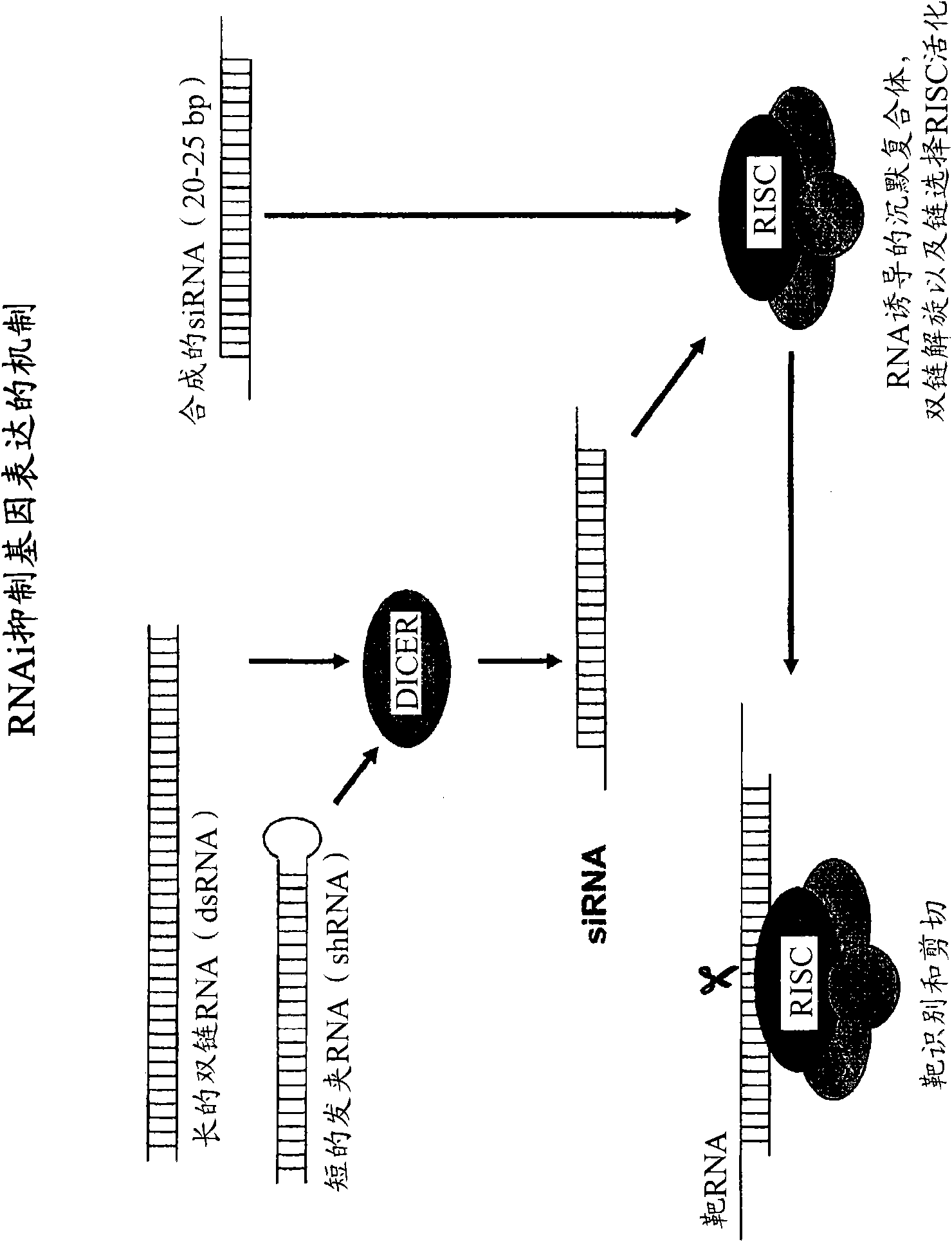
Polypeptide-nucleic acid conjugates and uses thereof
A nucleic acid and compound technology, applied in the fields of applications, peptides, and decapeptides, can solve problems such as gene silencing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0230] Preparation of Peptide Derivatives and Mimetic Peptides
[0231]In addition to polypeptides consisting only of naturally occurring amino acids, the invention also includes peptidomimetics or polypeptide analogs. Peptide analogs are generally used as non-peptide drugs in the pharmaceutical industry, and their properties are similar to those of template polypeptides. Non-peptidic compounds are known as "peptidomimetics" or "peptidomimetics" (Fauchere et al., Infect. Immun. 54:283-287, 1986; Evans et al, J.Med.Chem. 30:1229-1239, 1987) . Peptidomimetics that are structurally related to a therapeutically useful peptide or polypeptide can be used to produce equivalent or enhanced therapeutic or prophylactic effects. Typically, a peptidomimetic is structurally similar to a paradigm polypeptide (i.e., a polypeptide having biological or pharmaceutical activity), such as a polypeptide that binds a naturally occurring receptor, but has one or more peptide linkages , the peptid...
Embodiment 1
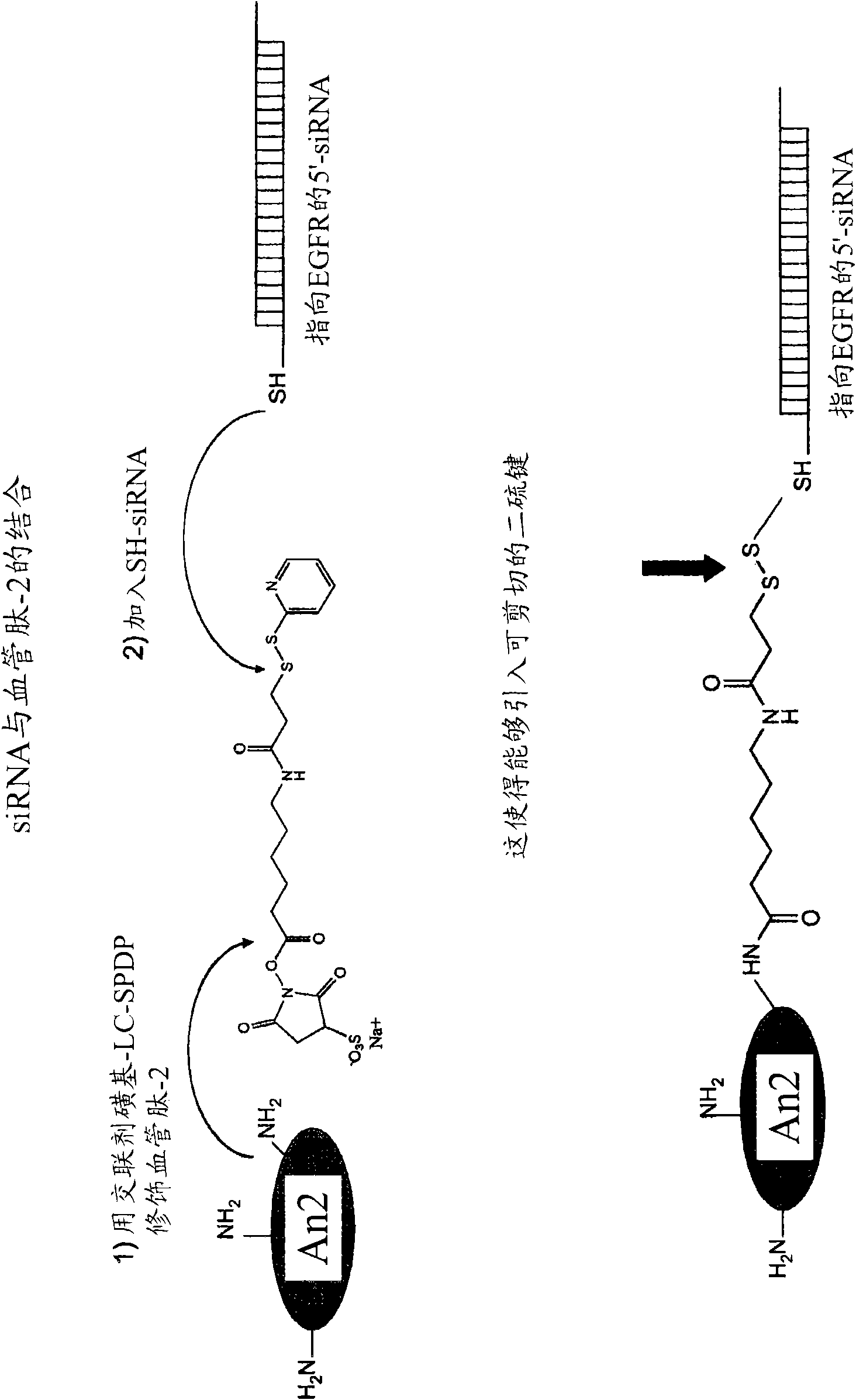
[0324] Peptide-Nucleic Acid Binding
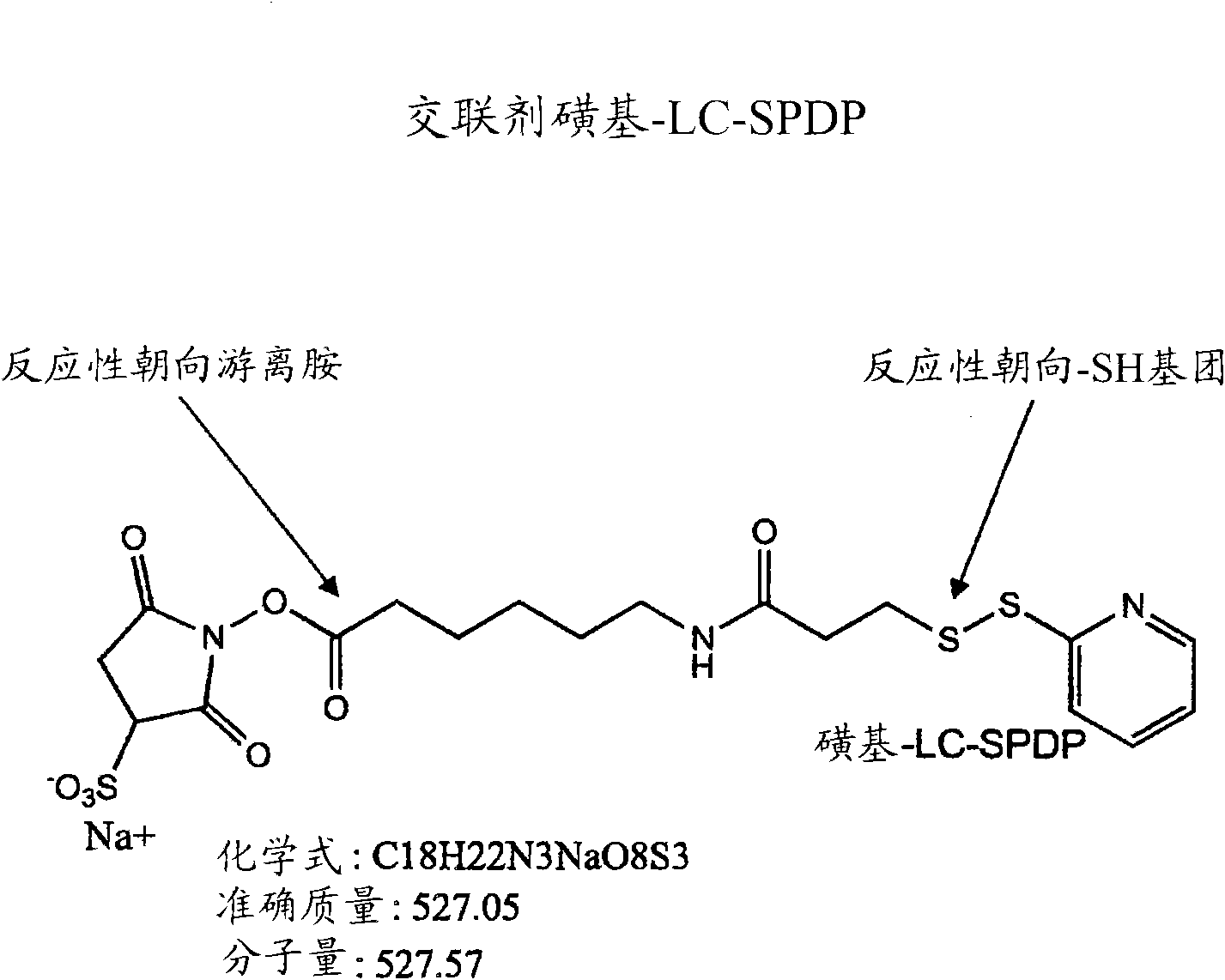
[0325] Incubate at 90°C in annealing buffer (100 mM potassium acetate, 30 mM HEPES-KOH pH 7.2, 2 mM magnesium acetate) with 35 μM single-stranded siRNA sequence encoding epidermal growth factor receptor (EGFR) containing a 5' thiol group RNA oligonucleotides were used for 1 min, followed by incubation at 37°C for 1 h. In Eppendorf tubes, desalt the annealed siRNA oligonucleotides by incubating the hybridization mix well in pre-made 1% agarose in 100 mM glucose for 7 min on ice (reserve 100 μL of Spare (tip) and allow it to adjust (set)). To the desalted siRNA molecules, 1 volume of reaction buffer (10 mM HEPES, 1 mM EDTA, pH 8.0) was added to adjust the final concentration of siRNA to 17.5 μM. Equimolar amounts of EGFR siRNA, angiopeptide-2 polypeptide and thiol oxidant diamide (sigma, USA) were mixed and incubated at 40° C. for 1 h. The polypeptide-nucleic acid conjugate / diamide solution is mixed with culture media and applied to targe...
Embodiment 2
[0327] The N-terminus and C-terminus of the siRNA are bound to the peptide carrier
[0328] Such as Figure 4 As shown, peptide vectors with N-terminal or C-terminal cysteines (eg, SEQ ID NO: 113 and 114) can be bound to SH-siRNA directly or through a linker. Depending on the linker chosen, this linkage can be cleavable or non-cleavable. Here, the peptide carrier is bound to the sense strand of the siRNA duplex.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com