Recombinant adeno-associated virus virions for the treatment of lysosomal disorders
a technology of lysosomal disorders and adenovirus, which is applied in the field of lysosomal disorders treated with lysosomal disorders, can solve the problems of organ failure, premature death, and cellular structure distortion and compromise function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
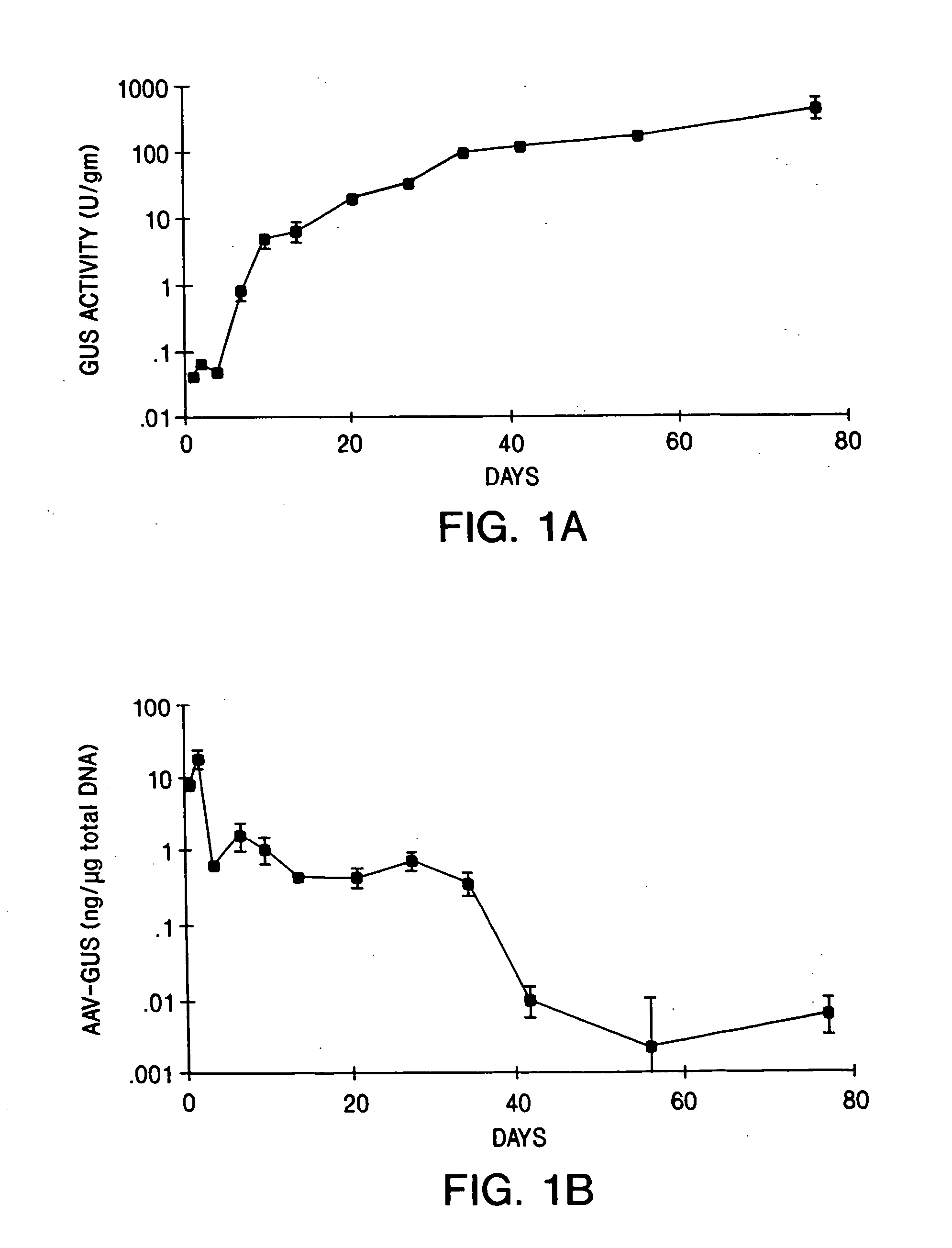
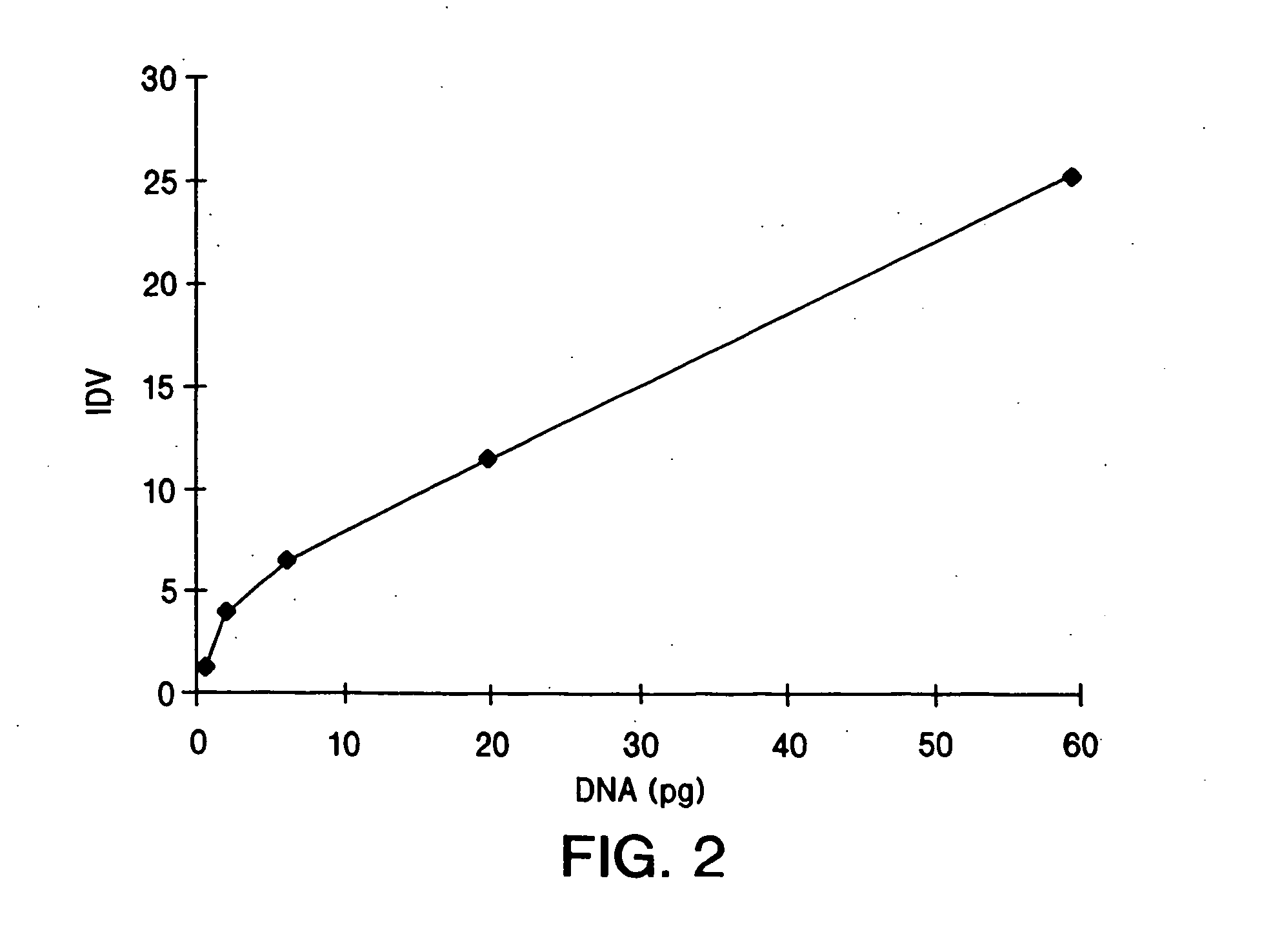
IM and IV AAV-GUS Virion Administration for the Treatment of MPS VII
[0149] The following examples detail intramuscular and intravenous delivery of AAV virions, which include the gene encoding β-glucuronidase gene (GUS), the enzyme deficient in individuals suffering from MPS VII (Sly Syndrome).
example 1a
Production of an MPS Animal Model
[0150] All procedures using mice were first reviewed and approved by an Institutional Animal Care and Use Committee and adhered to the principles of the NIH ‘Guide for the Care and Use of Laboratory Animals.’
[0151] Gusmps / + mice in the original C57BL / 6 (B6) background were obtained from E. Birkenmeier (Jackson Laboratory, Bar Harbor, Me., USA) and were crossed to the congenic strain B6.Gusa (Pfister et al. (1982) Biochem Genet 20: 519-535). From the progeny, Gusmps / a ice were selected and were used to establish a breeding colony in which both parents were always Gusmps / a and progeny carried mps / a, a / a or mps / mps allele combinations. The introduction of the a allele was done to aid genotyping by PCR. The homozygous, mps / mps mice are homozygous for the mutant allele, mps, of the β-glucuronidasegene, Gus (Birkenmeier et al. (1989) J Clin Invest 83: 1258-1266), displayed disease symptoms and were essentially identical to the original mutants. Presumably...
example 1b
Recombinant AAV-GUS Virion Construction
[0154] Vector construction and virion production and purification were analogous to those in previous reports (Kessler et al. (1996) Proc Natl Acad Sci USA 93: 14082-14087; Malik et al. (1997) J Virol (1997) 71: 1776-1783; Matsushita et al. (1998) Gene Ther 5:938-945). AAV-GUS was made by inserting a mouse GUS cDNA sequence (SEQ ID NO:3) into the XhoI site of pV4.1, a plasmid containing a CMV promoter and flanking ITRs. The mouse DNA included 82 bp of genomic DNA 5′ to the GUS transcriptional start site, a full-length cDNA coding for the Gus-sa structural allele, and a piece of polylinker DNA including an XhoI site at the 3′ end.
[0155] Plasmid pV4.1 was constructed as follows.
[0156] A synthetic DNA encoding the restriction enzyme sites NotI-MluI-Ecl136II-SstII-SfuI-SmaI-SfuI-ClaI-BglII-SnaBI-BstEII-PmlI-RsrII-NotI and having the sequence CGGCCGCACGCGTGAGCTCCGCGGTTCGAATCCCGGGATTCGAACATCGATA AAAGATCTACGTAGGTAACCACGTGCGGACCGAGCGGCCGC (SEQ ID NO...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com