Nano artificial dura mater capable of being used as medicine sustained-release system and preparation method thereof
An artificial dura mater and drug technology, applied in the field of biomedicine, can solve the problems of no artificial meninges
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
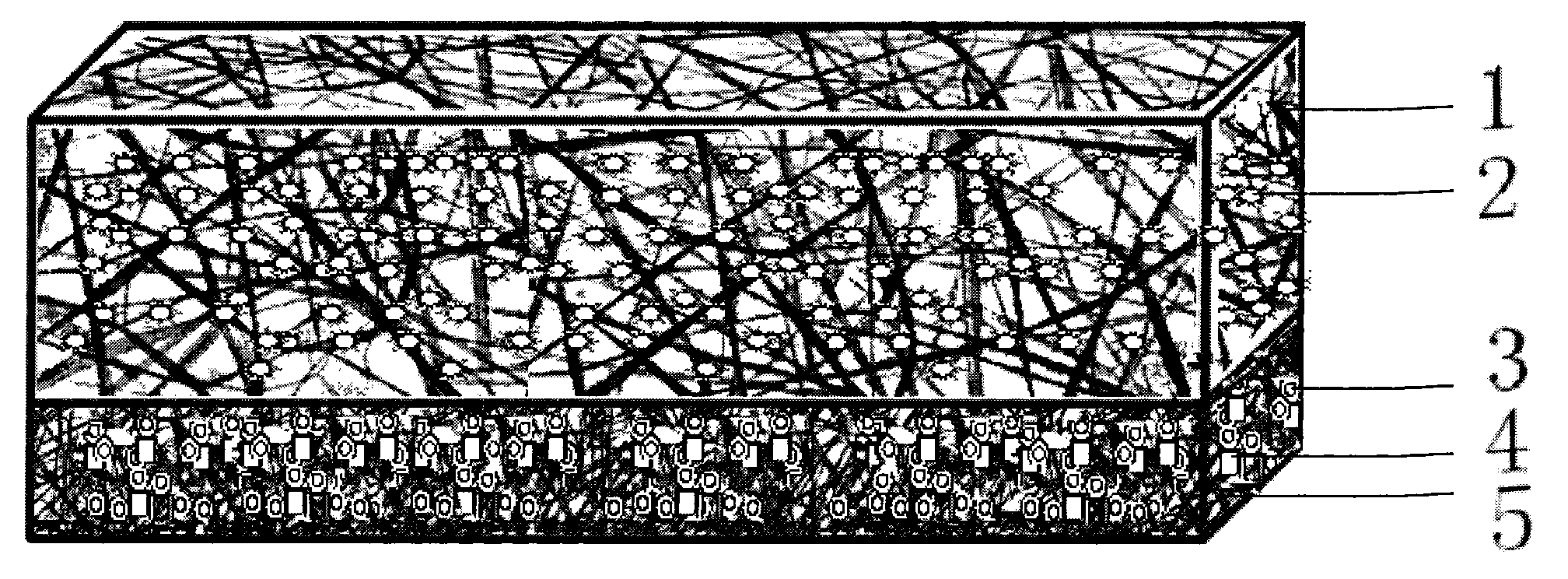
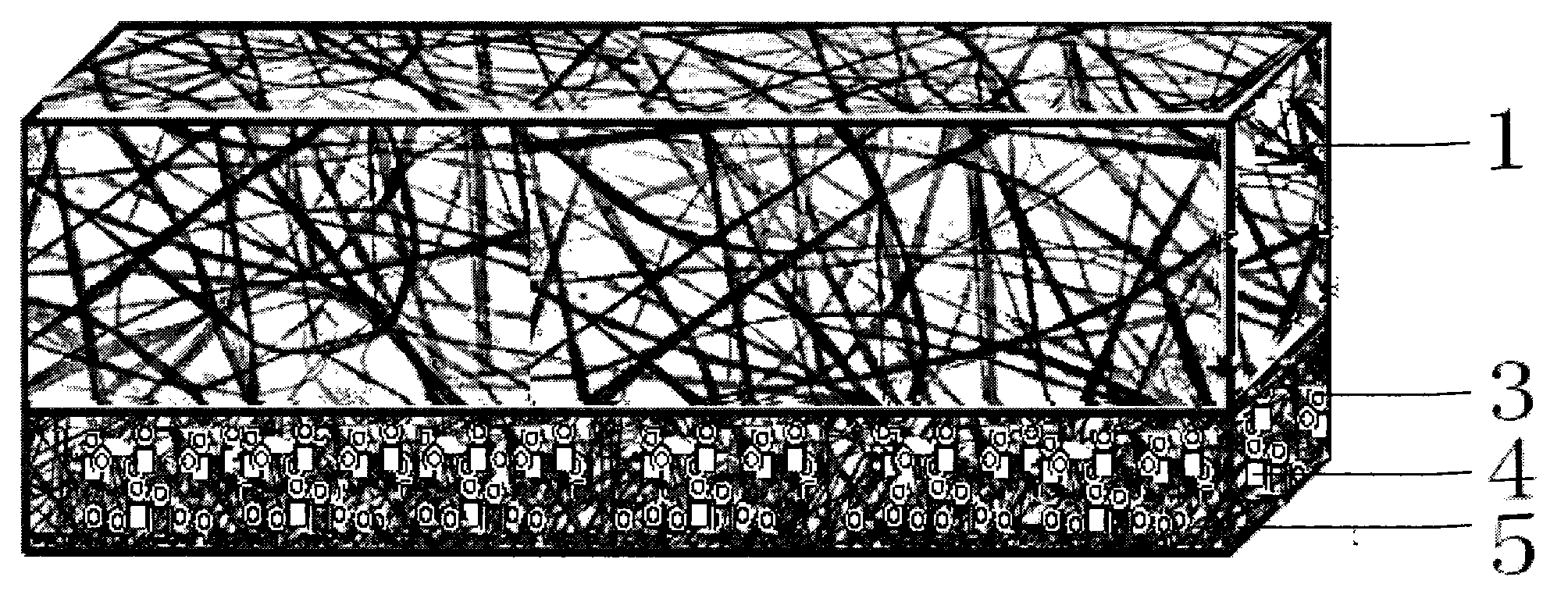
[0057] (1) Preparation of anti-adhesion layer: choose hydrophobic polycaprolactone, chloroform / methanol mixed solvent ratio of 1:1, mix ceftriaxone sodium at a concentration of 1%. Mix the hemostatic drug promethazine to a final concentration of 10 mg / ml. A homogeneous solution was obtained.
[0058]Add the above solution into the syringe of the electrospinning device, adjust the rate of the micro-injection pump to 0.8 ml / hour, adjust the voltage of the high-voltage generator to 12KV, adjust the receiving distance of the receiving device to 15 cm, and receive the fiber into a film-like structure . A fiber diameter of 600 nm was obtained.
[0059] Turn off the electrostatic device.
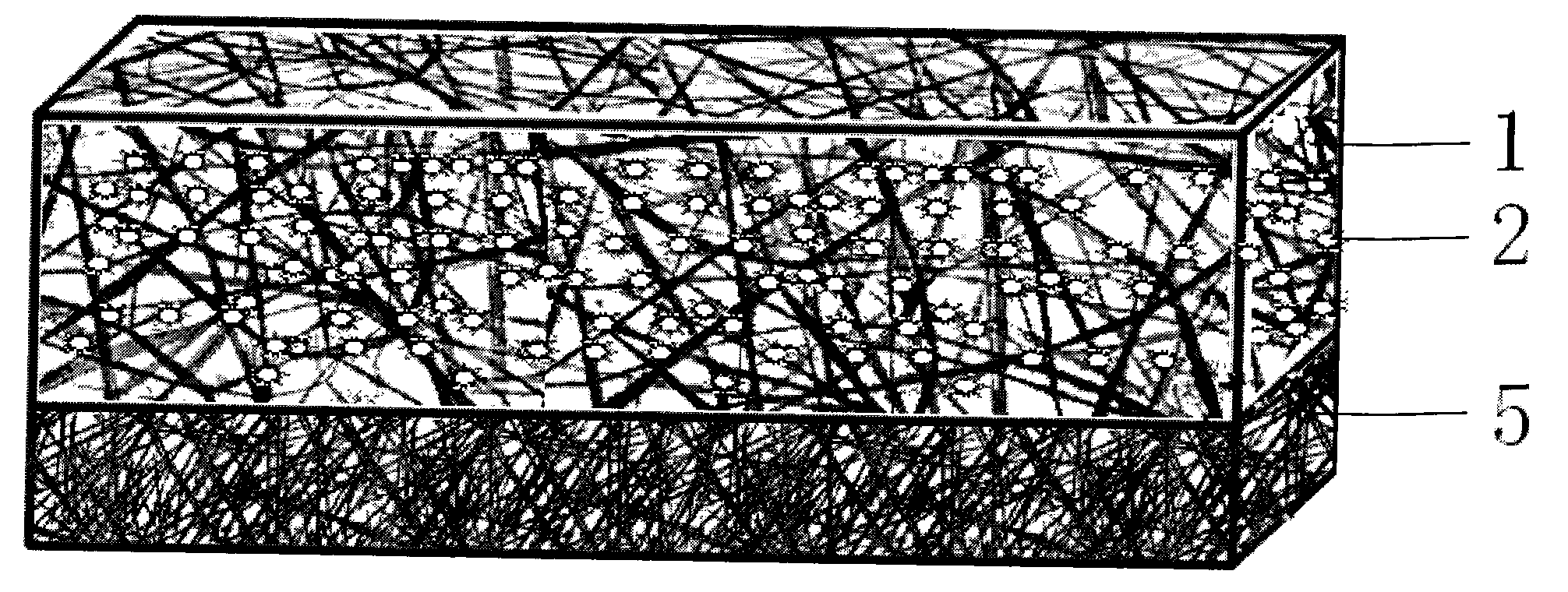
[0060] (2) Preparation of cell scaffold layer: use hydrophilic silk fibroin and natural gelatin in a ratio of 20-80:80-20, and the mass fraction of spinning solution is 9%.
[0061] Prepare basic fibroblast cytokine solution, mix it with the above electrospinning solution evenly, so that the fi...
Embodiment 2
[0064] (1) Preparation of anti-adhesion layer: choose hydrophobic polycaprolactone, chloroform / methanol mixed solvent ratio of 1:1, mix ceftriaxone sodium at a concentration of 1%. Mix the hemostatic drug promethazine to a final concentration of 10 mg / ml.
[0065] Add the above solution into the syringe of the electrospinning device, adjust the rate of the micro-injection pump to 0.8 ml / hour, adjust the voltage of the high-voltage generator to 12KV, adjust the receiving distance of the receiving device to 15 cm, and receive the fiber into a film-like structure . A fiber diameter of 600 nm was obtained.
[0066] Turn off the electrostatic device.
[0067] (2) Preparation of the transition layer: the following scheme is selected in this embodiment: the mass ratio of polyurethane to hyaluronic acid is 70:30, and the mass fraction of spinning solution is 10%. Mix ampicillin at a concentration of 3%. A homogeneous solution was obtained.
[0068] Turn on the electrospinning, an...
Embodiment 3
[0075] (1) Preparation of anti-adhesion layer: choose hydrophobic polycaprolactone, chloroform / methanol mixed solvent ratio of 1:1, mix ceftriaxone sodium and 1% concentration. Mix the hemostatic drug promethazine to a final concentration of 10 mg / ml. A homogeneous solution was obtained.
[0076] Add the above solution into the syringe of the electrospinning device, adjust the rate of the micro-injection pump to 0.8 ml / hour, adjust the voltage of the high-voltage generator to 12KV, adjust the receiving distance of the receiving device to 15 cm, and receive the fiber into a film-like structure . A fiber diameter of 600 nm was obtained.
[0077] Turn off the electrostatic device.
[0078] (2) Preparation of cell scaffold layer: use hydrophilic silk fibroin and natural gelatin in a ratio of 20-80:80-20, and the mass fraction of spinning solution is 9%.
[0079] Put the above solution into the syringe of the electrospinning device, the receiving distance is 10cm, the voltage i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com