Application of chlorogenic acid in preparing drugs for prevention and treatment of primary cutaneous T-cell lymphoma
A chlorogenic acid, primary technology, applied in drug combinations, antineoplastic drugs, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
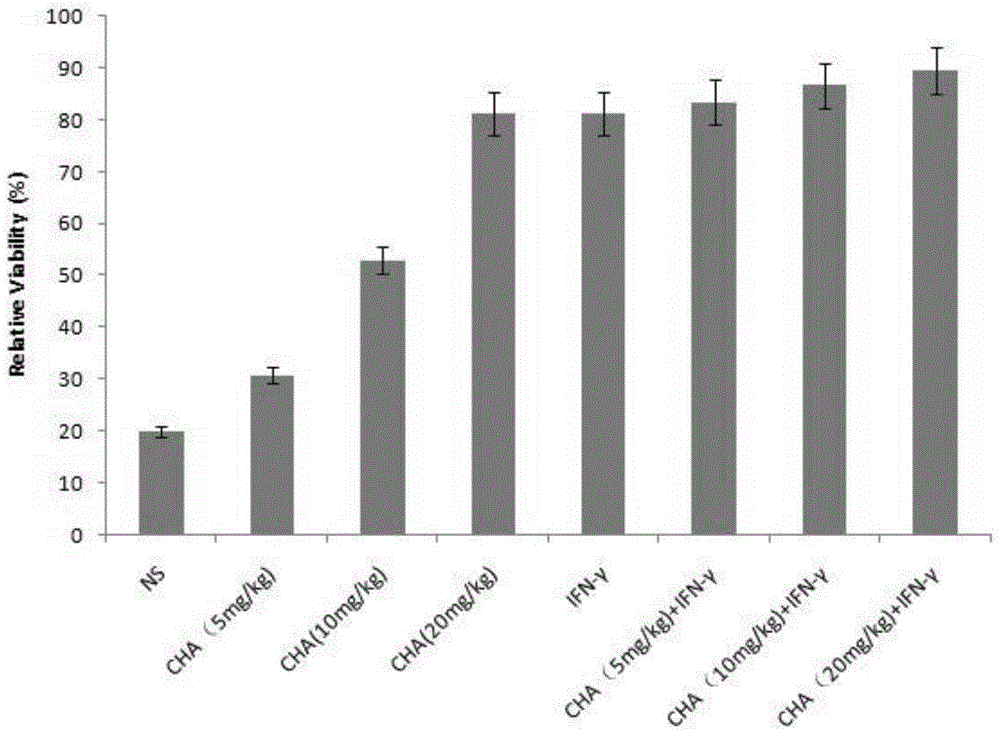
[0031] Example 1 Targeting CD4 T lymphocytes in the treatment of primary cutaneous T cell lymphoma
[0032] (1) Animal model establishment and grouping
[0033] Mouse primary cutaneous T-cell lymphoma model, select adult BABL / c mice, male, 18-22g. During the experiment, take the well-grown tumor tissue, cut it into pieces, grind it, filter it, and dilute it with sterile normal saline at a ratio of 1:3 to make a tumor cell suspension, and inoculate 0.2ml of the tumor cell suspension on the back of the armpit of each mouse . The next day after inoculation, the animals were randomly divided into 8 groups, namely: (1) negative control group (normal saline group, NS), (2) chlorogenic acid administration group (CHA, 5 mg / kg), (3) Chlorogenic acid administration group (CHA, 10mg / kg), (4) Chlorogenic acid administration group (CHA, 20mg / kg), (5) Interferon administration group (IFN-γ), (6) Chlorogen Acid and interferon administration group (CHA+IFN-γ, 5mg / kg), (7) Chlorogenic acid ...
Embodiment 2
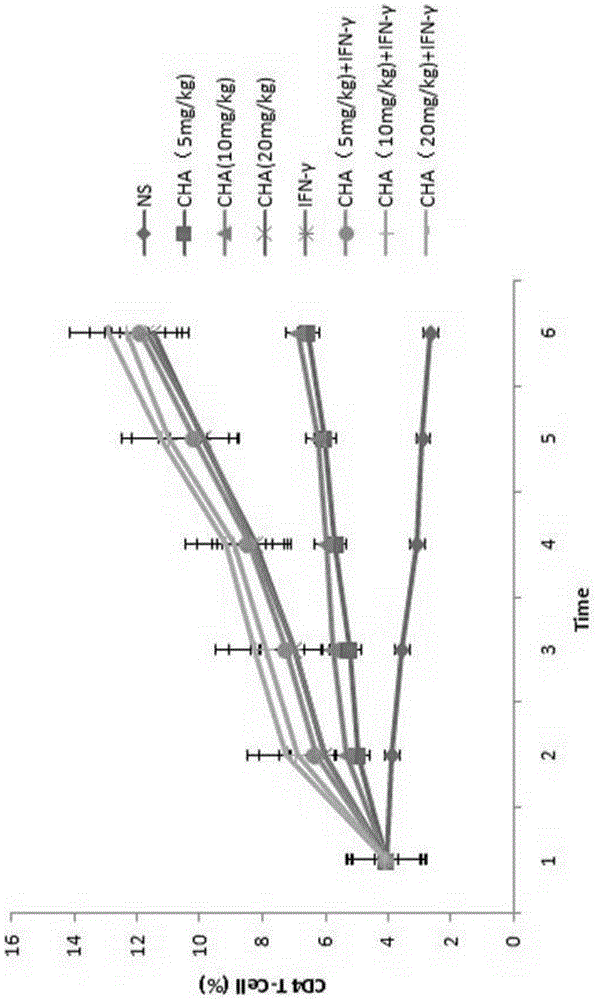
[0046] Example 2 Targeting CD8 T lymphocytes in the treatment of primary cutaneous T cell lymphoma
[0047] (1) Animal model establishment and grouping
[0048] Mouse primary cutaneous T-cell lymphoma model, select adult BABL / c mice, male, 18-22g. During the experiment, take the well-grown tumor tissue, cut it into pieces, grind it, filter it, and dilute it with sterile normal saline at a ratio of 1:3 to make a tumor cell suspension, and inoculate 0.2ml of the tumor cell suspension on the back of the armpit of each mouse . The next day after inoculation, the animals were randomly divided into 8 groups, namely: (1) negative control group (normal saline group, NS), (2) chlorogenic acid administration group (CHA, 5 mg / kg), (3) Chlorogenic acid administration group (CHA, 10mg / kg), (4) Chlorogenic acid administration group (CHA, 20mg / kg), (5) Interferon administration group (IFN-γ), (6) Chlorogen Acid and interferon administration group (CHA+IFN-γ, 5mg / kg), (7) Chlorogenic acid ...
Embodiment 3
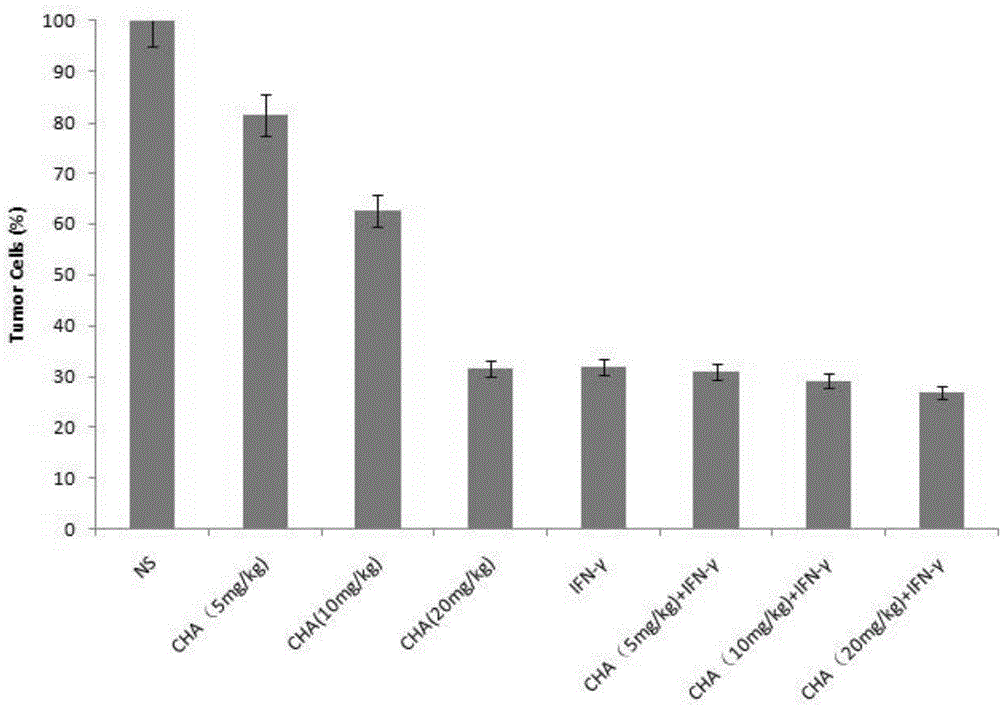
[0061] Example 3 Experiment of chlorogenic acid on tumor suppression rate of primary cutaneous T-cell lymphoma
[0062] (1) Animal model establishment and grouping
[0063] Mouse primary cutaneous T-cell lymphoma model, select adult BABL / c mice, male, 18-22g. During the experiment, take well-grown tumor tissue, cut it into pieces, grind it, filter it, and dilute it with sterile normal saline at a ratio of 1:3 to make a tumor cell suspension, and inoculate 0.2ml of the tumor cell suspension on the back of each mouse’s armpit . On the second day after inoculation, the animals were randomly divided into 8 groups, namely: (1) negative control group (normal saline group, NS), (2) chlorogenic acid administration group (CHA, 5 mg / kg), (3) Chlorogenic acid administration group (CHA, 10mg / kg), (4) Chlorogenic acid administration group (CHA, 20mg / kg), (5) Interferon administration group (IFN-γ), (6) Chlorogen Acid and interferon administration group (CHA+IFN-γ, 5mg / kg), (7) Chlorogen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com