Immunoconjugates and humanized antibodies specific for b-cell lymphoma and leukemia cells
a technology of humanized antibodies and immunoconjugates, which is applied in the direction of peptides, drug compositions, and injected cells, can solve the problems of increased radiolabel concentration to increase the dosage to the tumor, and limited clinical use of mll2, just as with most other promising murine antibodies, and achieves the effect of lowering the hama reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Choice of Human Frameworks and Sequence Design for the Humanization of LL2 Monoclonal Antibody
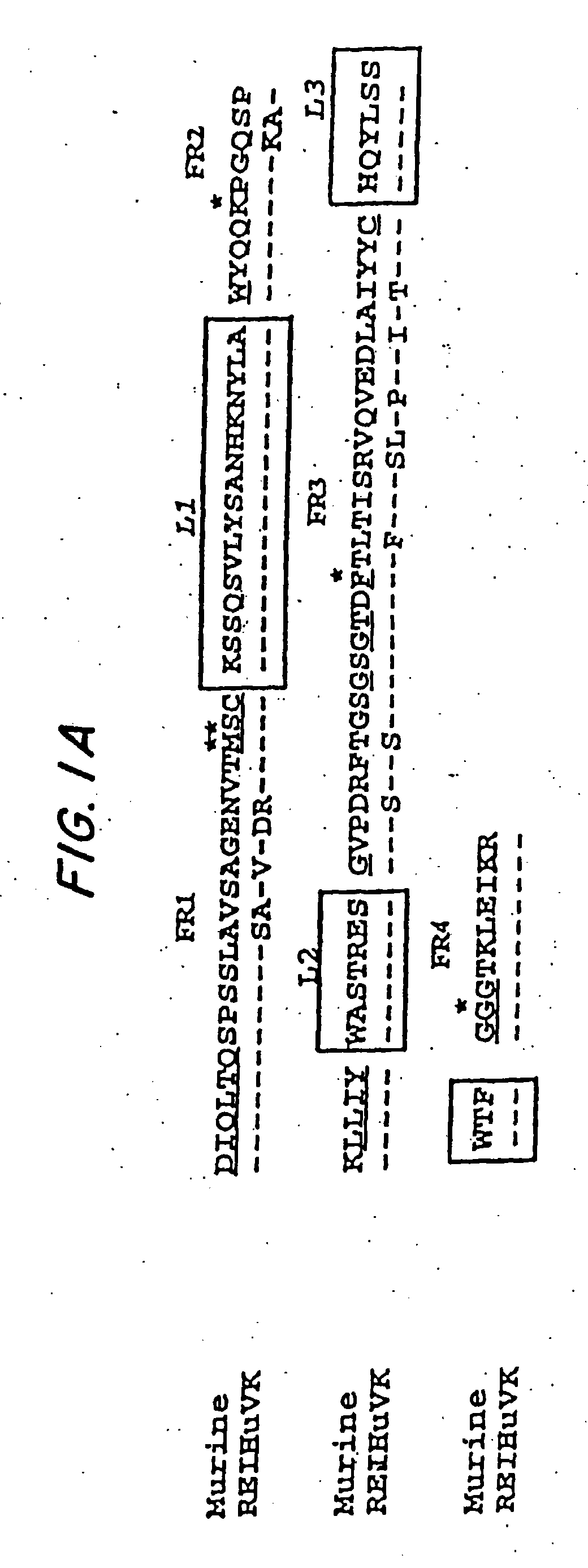
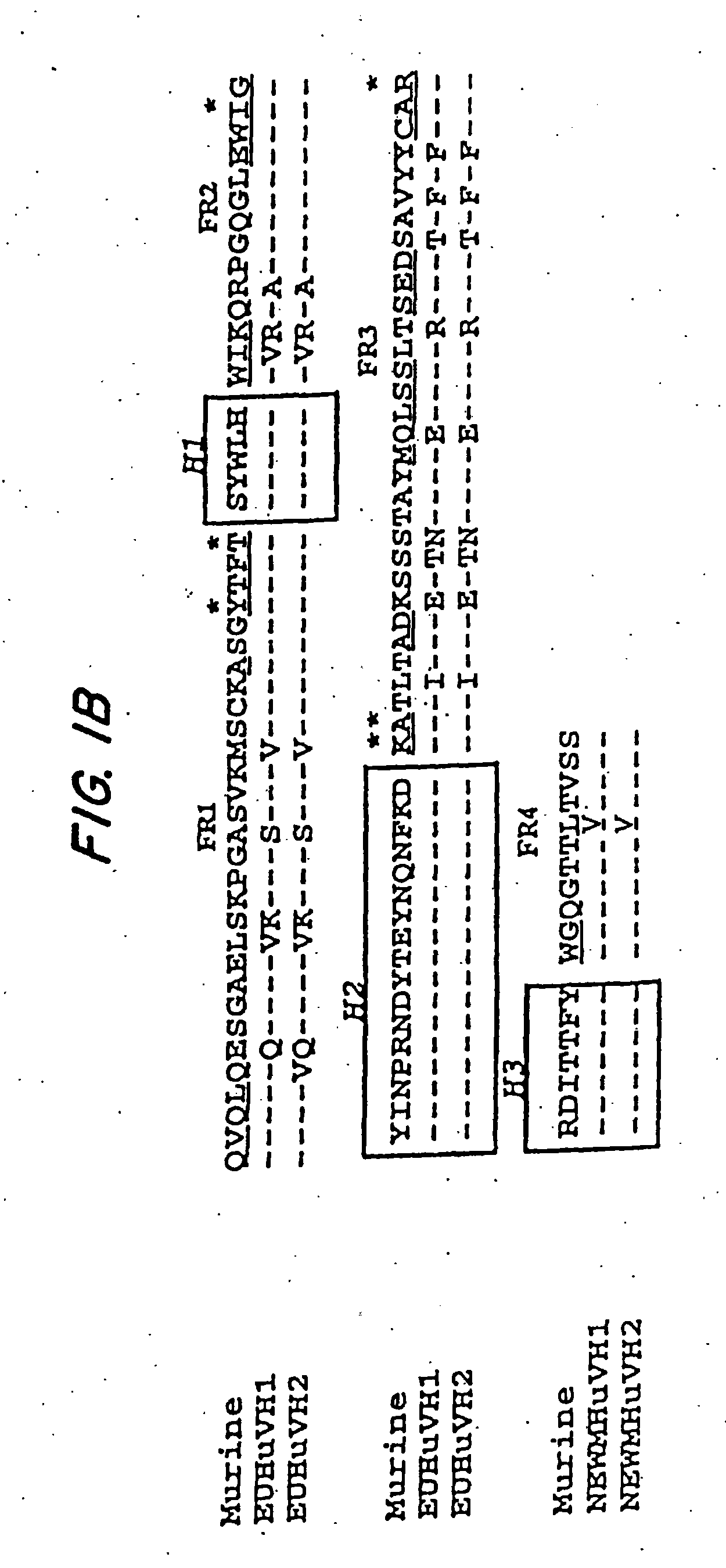
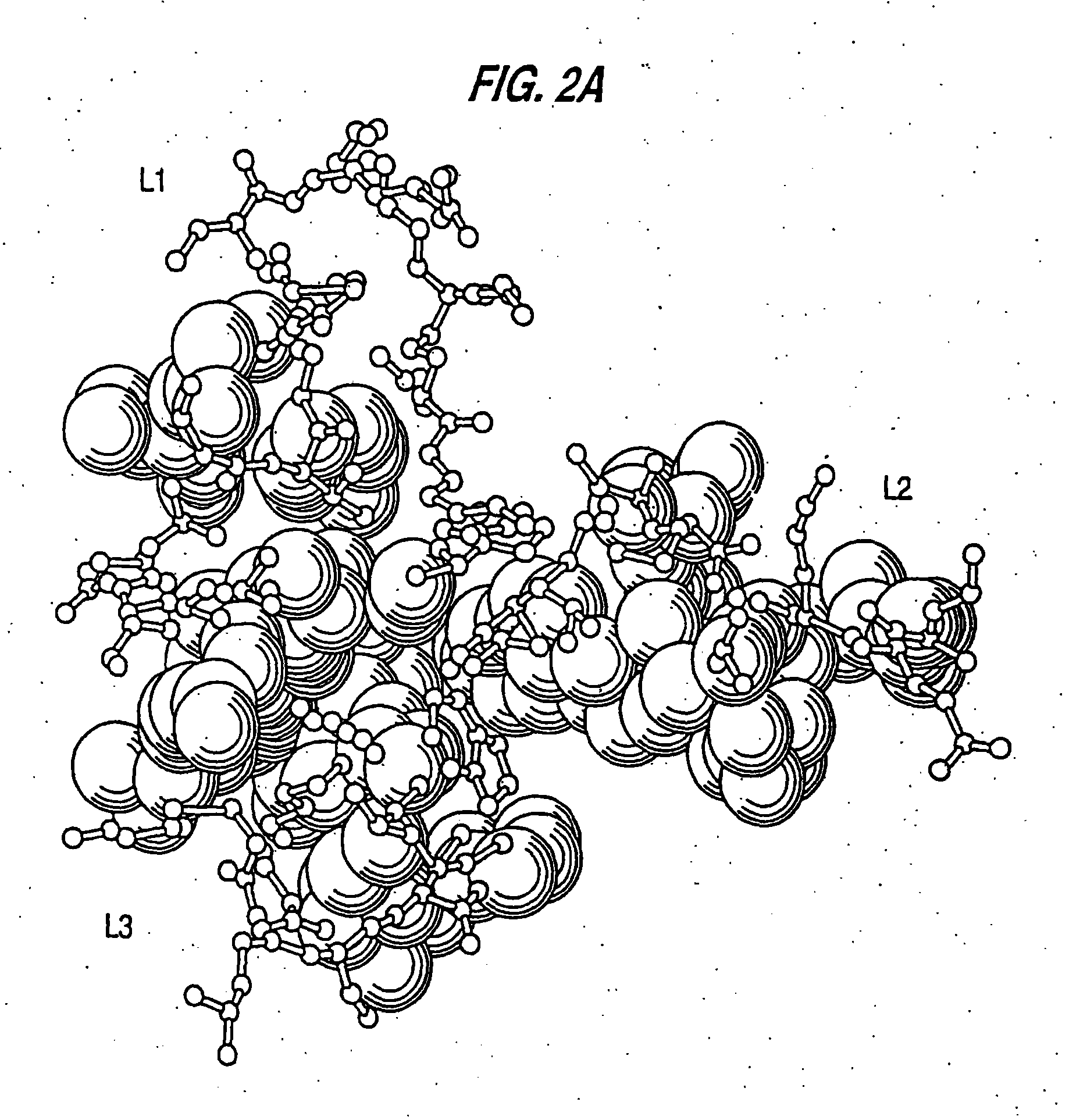
[0067] By comparing the murine variable (V) region framework (FR) sequences of LL2 to that of human antibodies in the Kabat data base (Kabat et al., Sequences of Proteins of Immunological Interest, 5th ed., U.S. Department of Health and Human Services, U.S. Government Printing Office, Washington, D.C.), which is incorporated by reference, the human REI (FIG. 1A, Sequence ID No. 1) and EU (FIG. 1B, Sequence ID No. 2) sequences were found to exhibit the highest degree of sequence homology to the FRs of VK and VH domains of LL2, respectively. Therefore, the REI and EU FRs were selected as the human frameworks onto which the CDRs for LL2 VK and VH were grafted, respectively. The FR4 sequence of NEWM, however, rather than that of EU, was used to replace the EU FR4 sequence for the humanization of LL2 heavy chain. Based on the results of computer modeling studies (FIGS. 2A and 2B), murine FR res...
example 2
PCR Cloning and Sequence Elucidation for LL2 Heavy and Light Chain Variable Regions
[0070] The variable regions for both heavy (VH) and light (VK) chains of mLL2 (IgG2a) were obtained by PCR cloning using DNA primers as described in general above and in greater detail in Example 3, below. As PCR is prone to mutations, the variable region sequence of multiple individual clones for either the heavy or light chains was determined for six clones and confirmed to be identical prior to use for the construction of the chimeric antibody.
[0071] The PCR products for VK were subcloned into a pBR327-based staging vector, VKpBR, which contained an Ig promoter, a signal peptide sequence and convenient restriction sites to facilitate in-frame ligation of the VK PCR products (FIG. 3A). The PCR products for VH were subcloned into a similar pBluescript-based staging vector, VHpBS (FIG. 3B).
[0072] As noted above, at least six individual clones containing the respective PCR products were sequenced ac...
example 3
PCR / Gene Synthesis of the Humanized V Genes
[0074] The designed sequence for the hLL2 VH domain, the construction of the hLL2 VH domain by long oligonucleotides and PCR, and the staging vector VHpBS containing the hLL2 VH domain are summarized in the sketch shown in FIG. 6.
[0075] For the construction of the hLL2 VH domain, oligo A (149-mer) and oligo B (140-mer) were synthesized on an automated CYCLONE PLUS™ DNA synthesizer (Milligen Bioresearch).
[0076] Oligo A (Sequence ID No. 7 below) represents the minus strand of the hLL2 VH domain complementary to nt 24 to 172.
Sequence ID No. 75′-TAT AAT CAT TCC TAG GAT TAA TGT ATC CAA TCC ATTCCA GAC CCT GTC CAG GTG CCT GCC TGA CCC AGT GCAGCC AGT AGC TAG TAA AGG TGT AGC CAG AAG CCT TGCAGG AGA CCT TCA CTG ATG ACC CAG GTT TCT TGA CTTCAG CC-3′
[0077] Oligo B (Sequence ID No. 8 below) represents the minus strand of the hLL2 VH domain complementary to nt 180 to 320.
Sequence ID No. 85′-CCC CAG TAG AAC GTA ATA TCC CTT GCA CAA AAA TAAAAT GCC GTG T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com