Patents
Literature
41 results about "Follicular lymphoma grade II" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The WHO 2008 update classifies grades 1 and 2 now as low grade follicular lymphoma, grade 3A as high grade follicular lymphoma, and grade 3B as Diffuse Large B Cell Lymphoma (DLBCL).
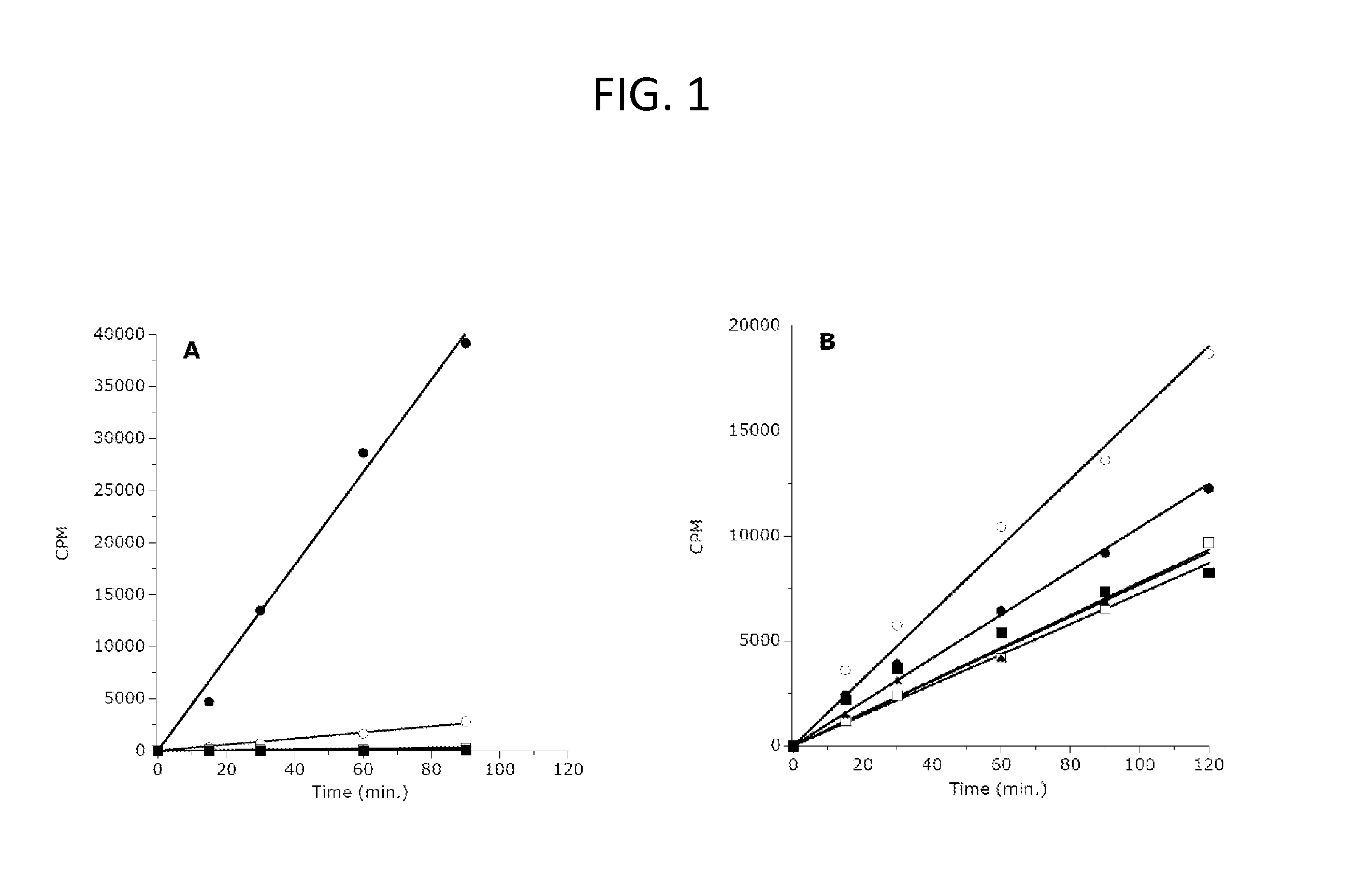
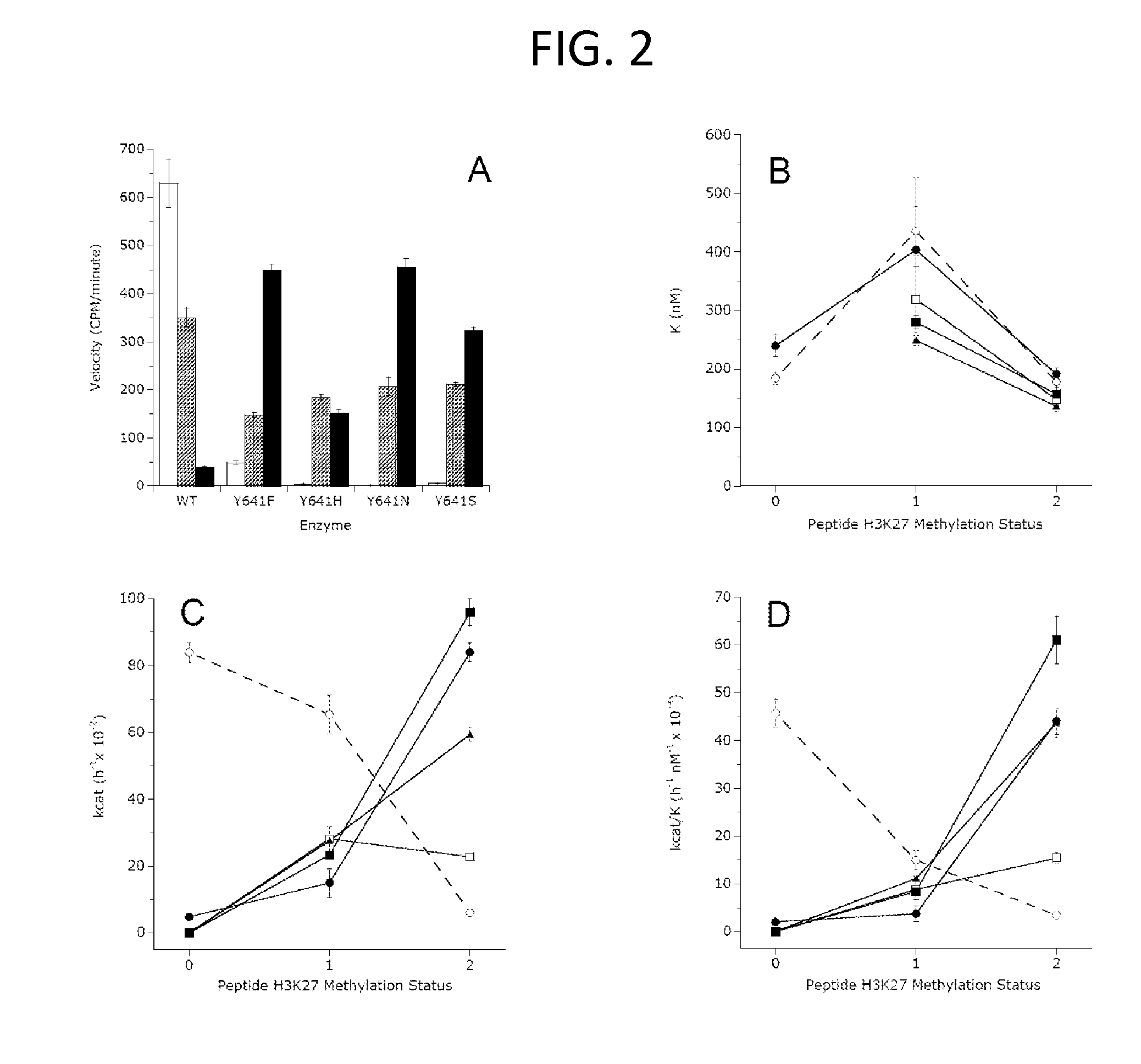
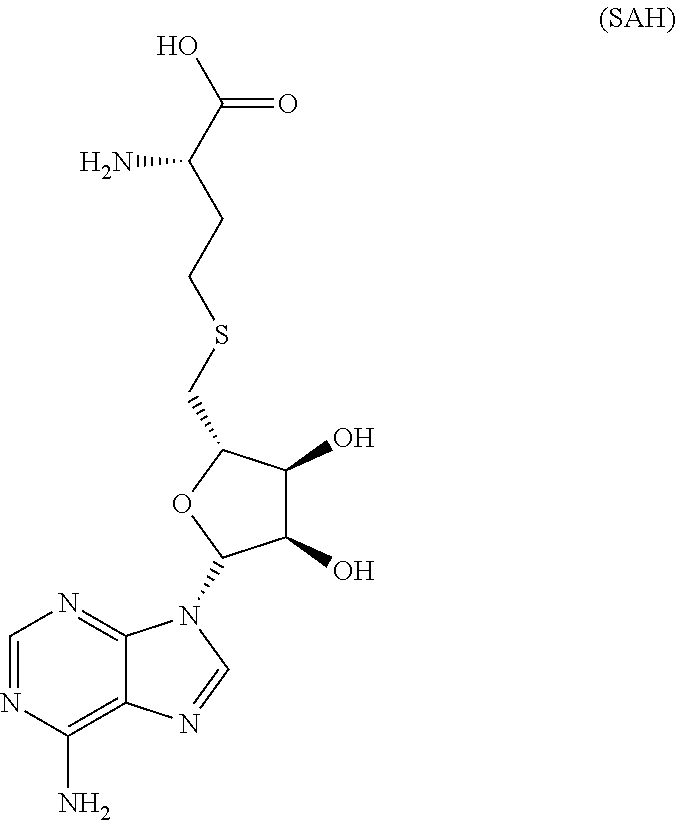
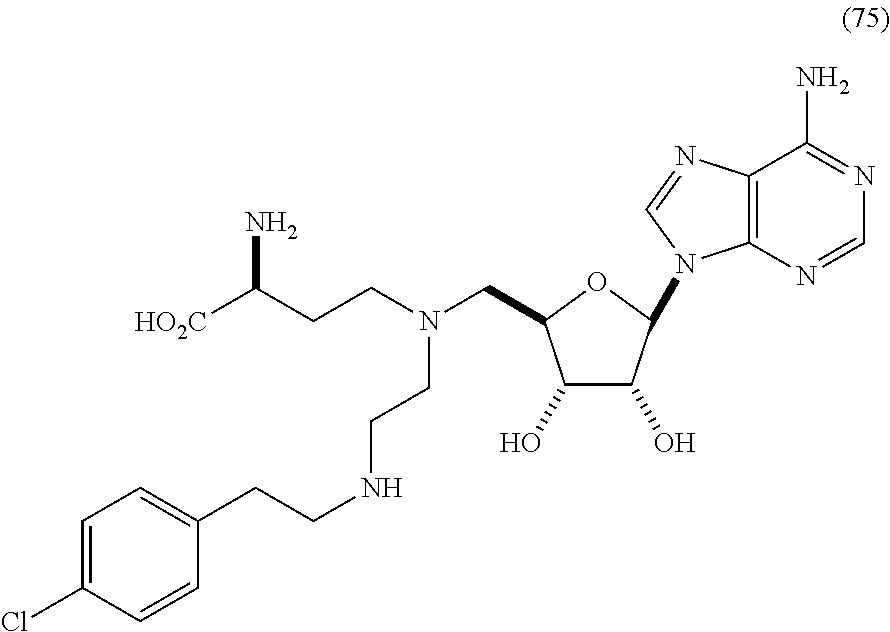
Inhibitors of Human EZH2 and Methods of Use Thereof
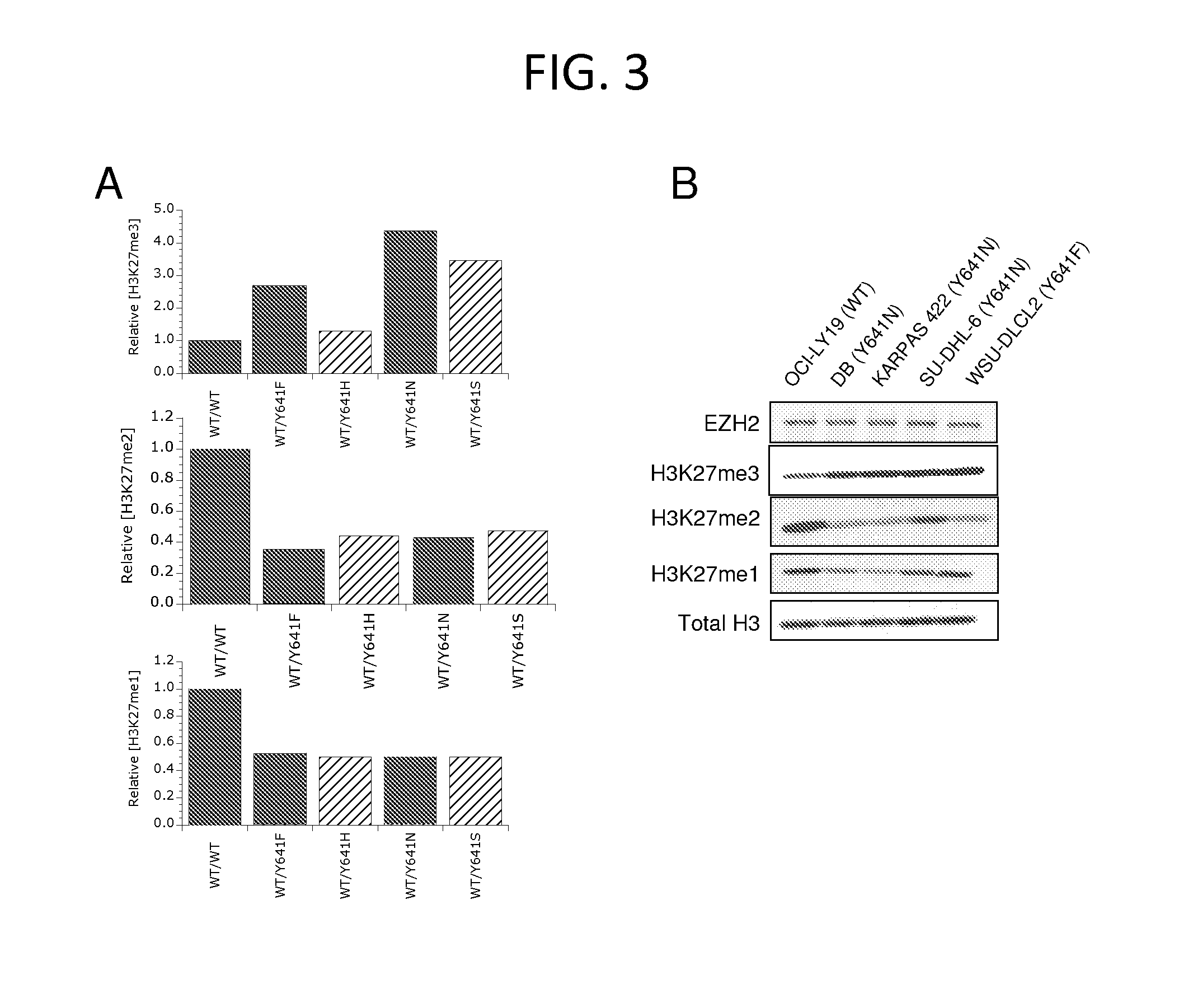
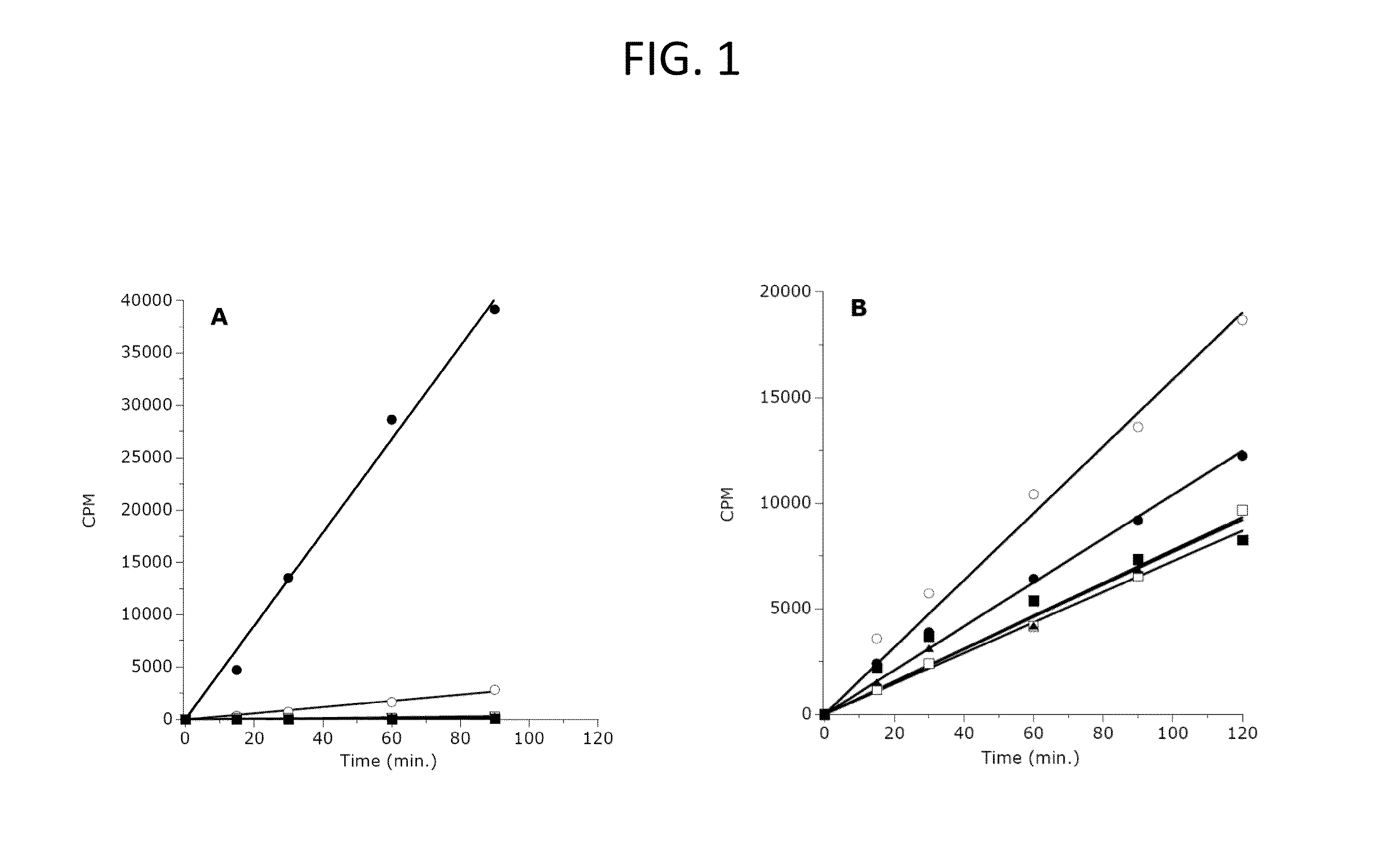
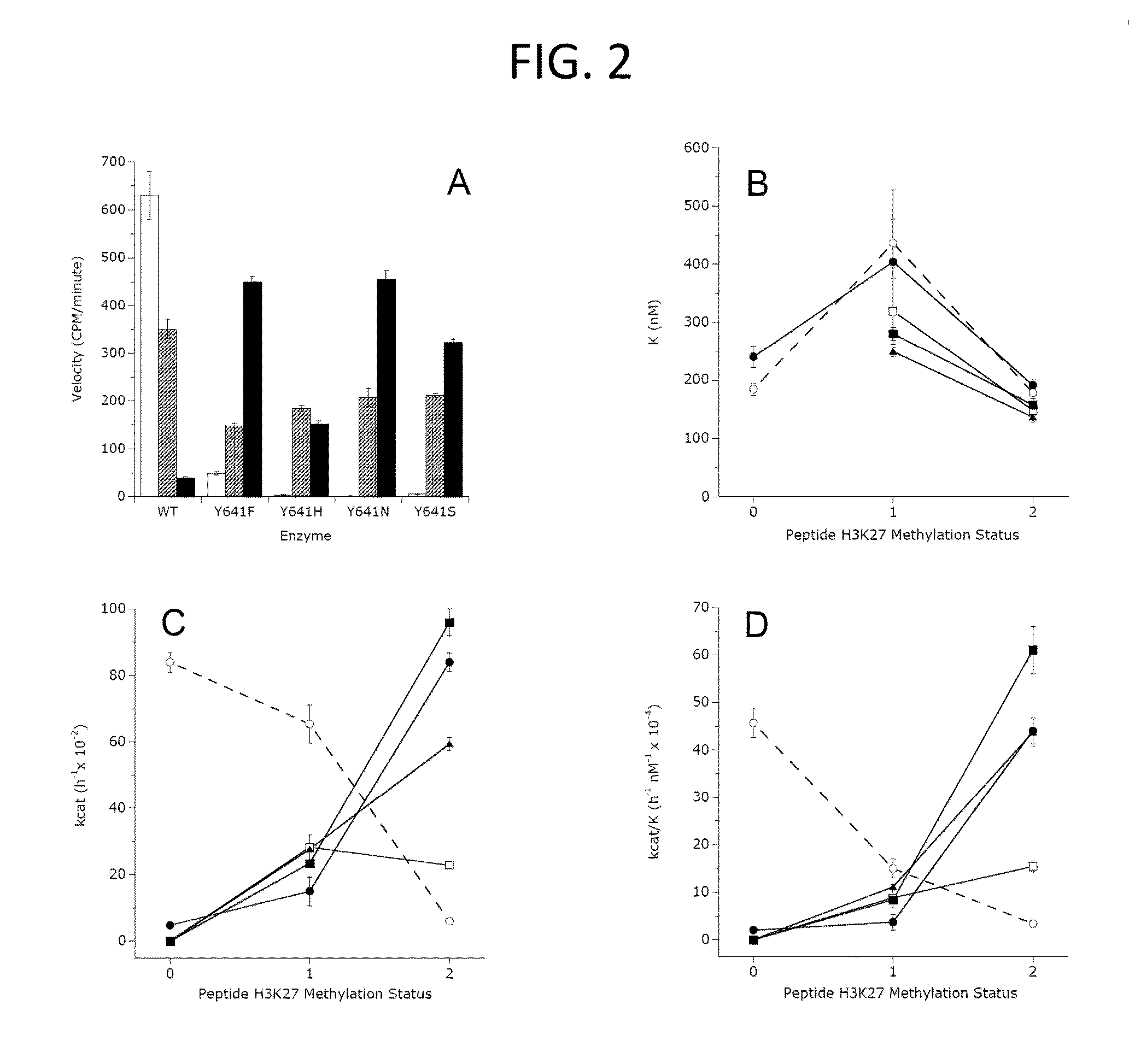
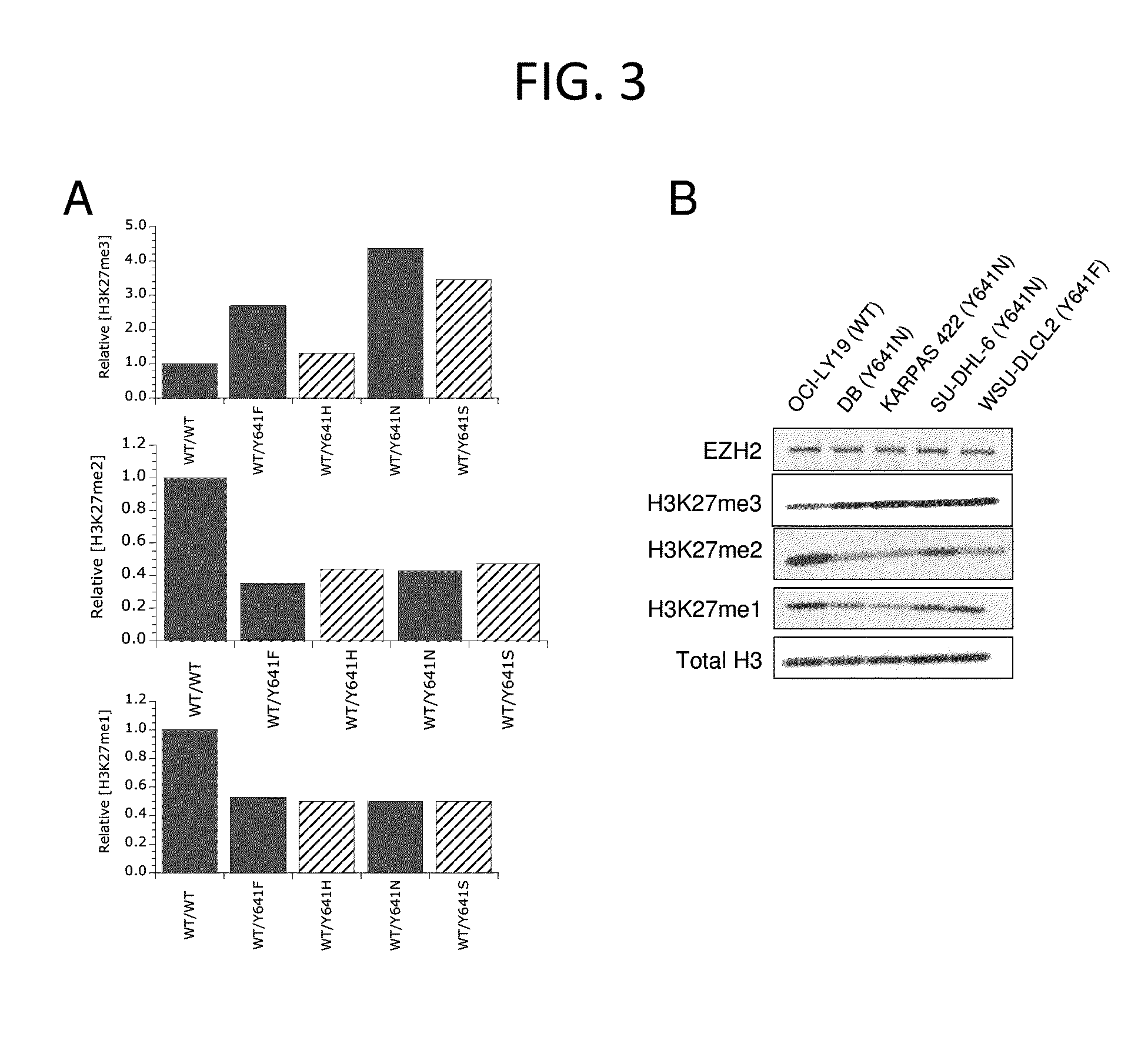
The invention relates to inhibition of wild-type and certain mutant forms of human histone methyltransferase EZH2, the catalytic subunit of the PRC2 complex which catalyzes the mono- through tri-methylation of lysine 27 on histone H3 (H3-K27). In one embodiment the inhibition is selective for the mutant form of the EZH2, such that trimethylation of H3-K27, which is associated with certain cancers, is inhibited. The methods can be used to treat cancers including follicular lymphoma and diffuse large B-cell lymphoma (DLBCL). Also provided are methods for identifying small molecule selective inhibitors of the mutant forms of EZH2 and also methods for determining responsiveness to an EZH2 inhibitor in a subject.
Owner:EPIZYME
Inhibitors of Human EZH2, and Methods of Use Thereof
The invention relates to inhibition of wild-type and certain mutant forms of human histone methyltransferase EZH2, the catalytic subunit of the PRC2 complex which catalyzes the mono- through tri-methylation of lysine 27 on histone H3 (H3-K27). In one embodiment the inhibition is selective for the mutant form of the EZH2, such that trimethylation of H3-K27, which is associated with certain cancers, is inhibited. The methods can be used to treat cancers including follicular lymphoma and diffuse large B-cell lymphoma (DLBCL). Also provided are methods for identifying small molecule selective inhibitors of the mutant forms of EZH2 and also methods for determining responsiveness to an EZH2 inhibitor in a subject.
Owner:EPIZYME
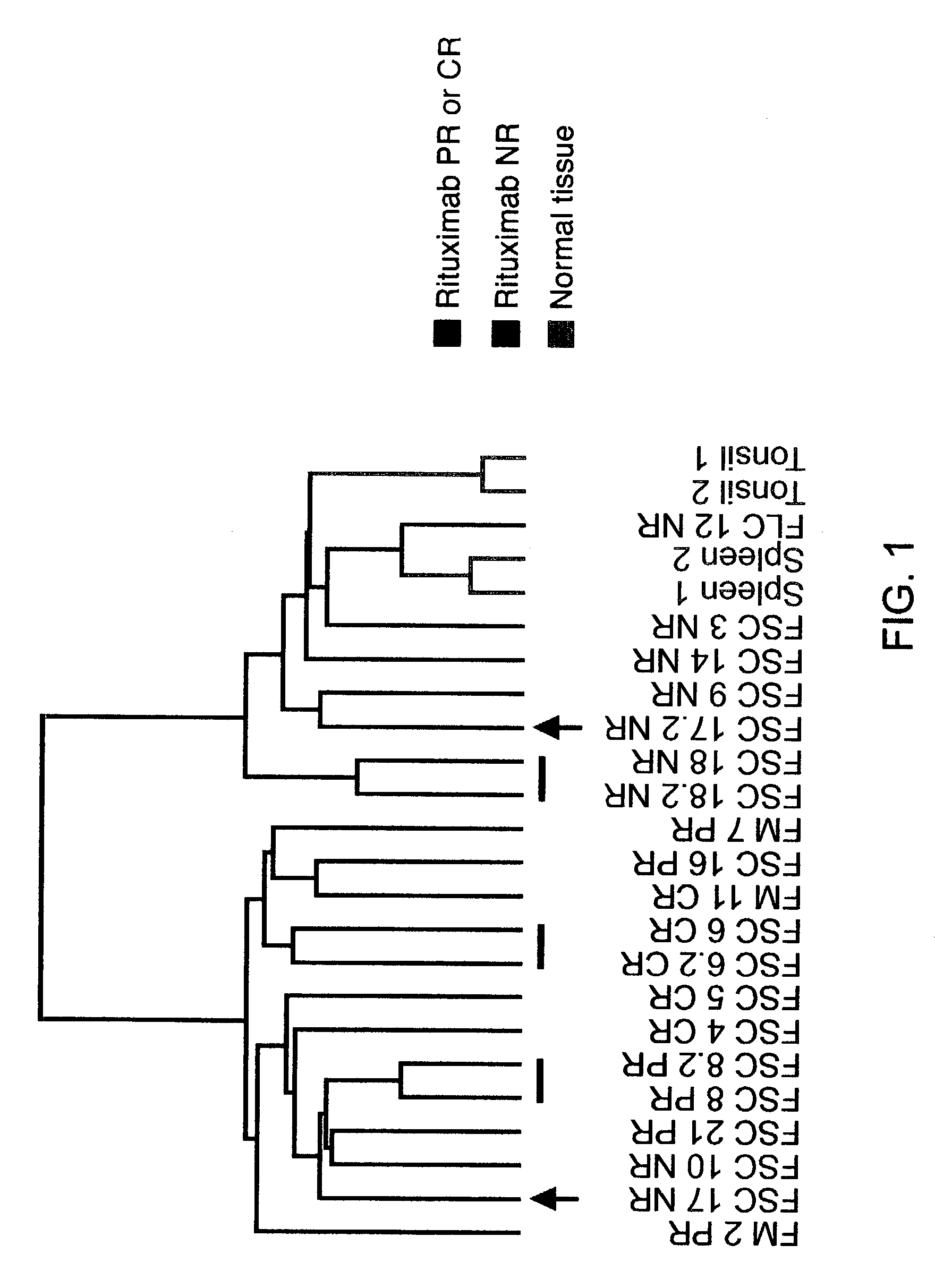
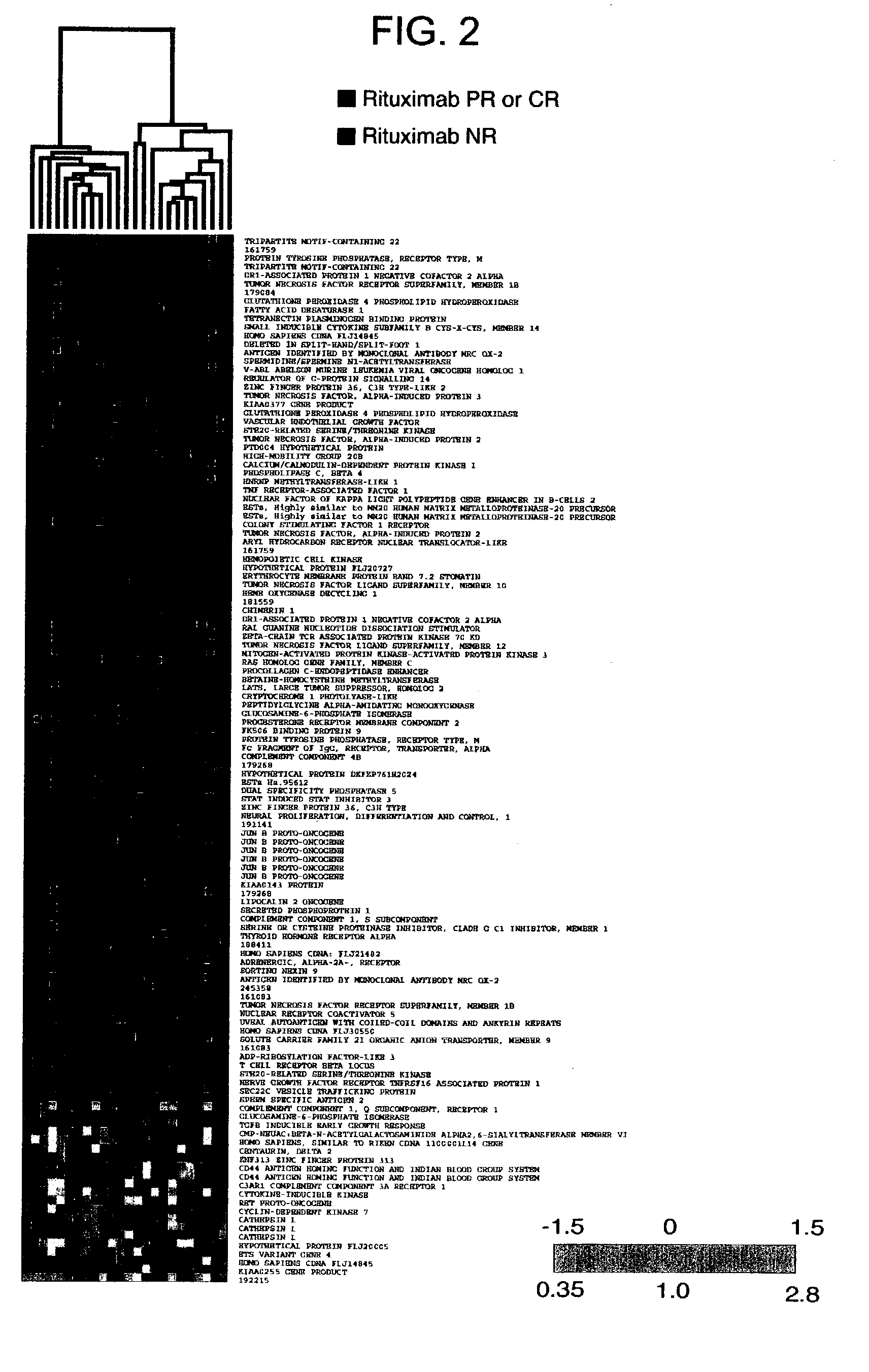
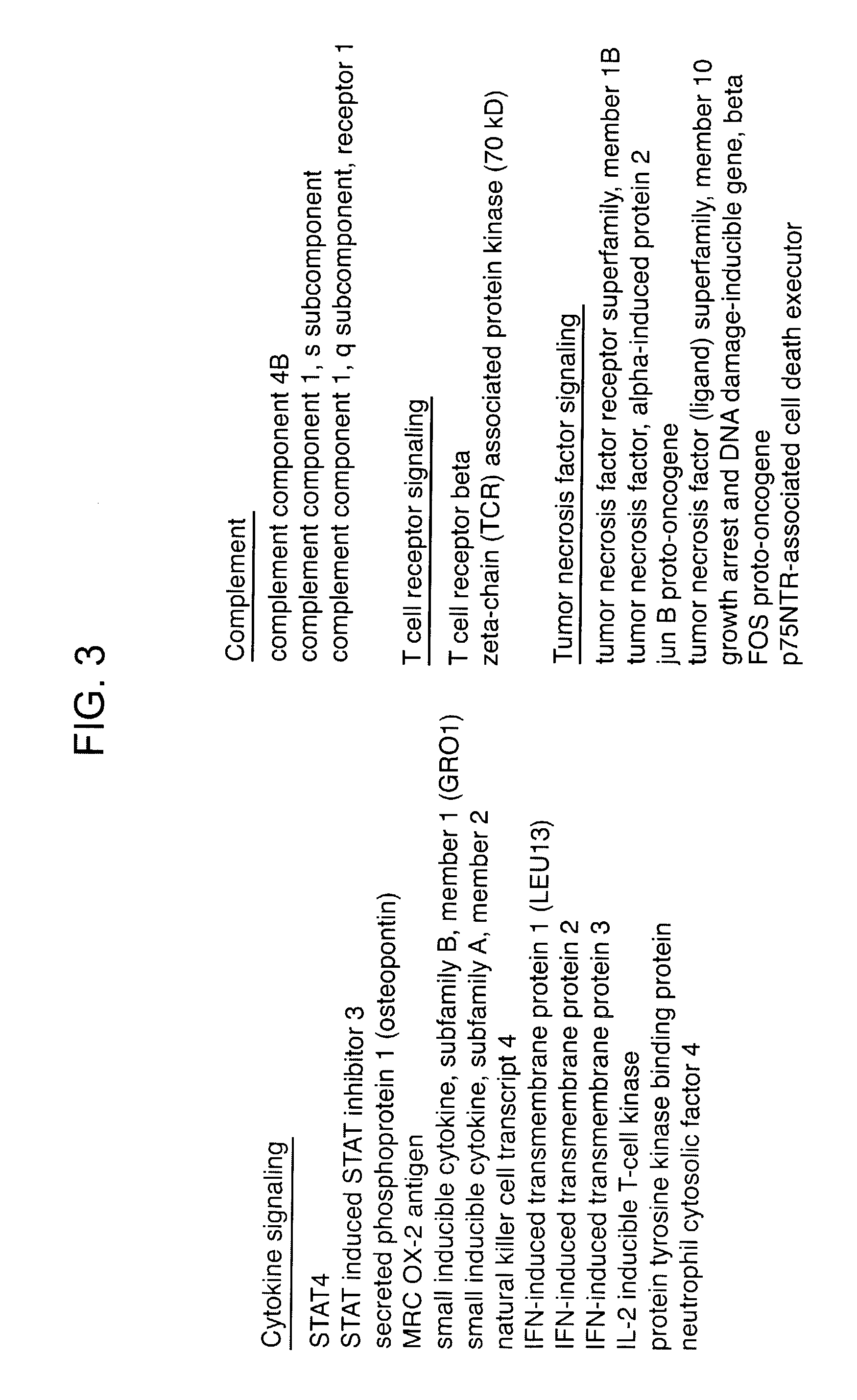
Methods and compositions for determining neoplastic disease responsiveness to antibody therapy
InactiveUS20030219818A1Microbiological testing/measurementDisease diagnosisFollicular lymphoma grade IIWilms' tumor
Methods are provided for determining whether a subject suffering from a neoplastic condition, e.g., non-Hodgkin's lymphoma (NHL), such as follicular lymphoma, is responsive to antineoplastic therapy, such as antibody therapy, e.g., Rituximab. In practicing the subject methods, an expression profile is obtained from the subject suffering from NHL and employed to determine whether the subject is responsive to antineoplastic therapy. In addition, reagents and kits thereof that find use in practicing the subject methods are provided.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
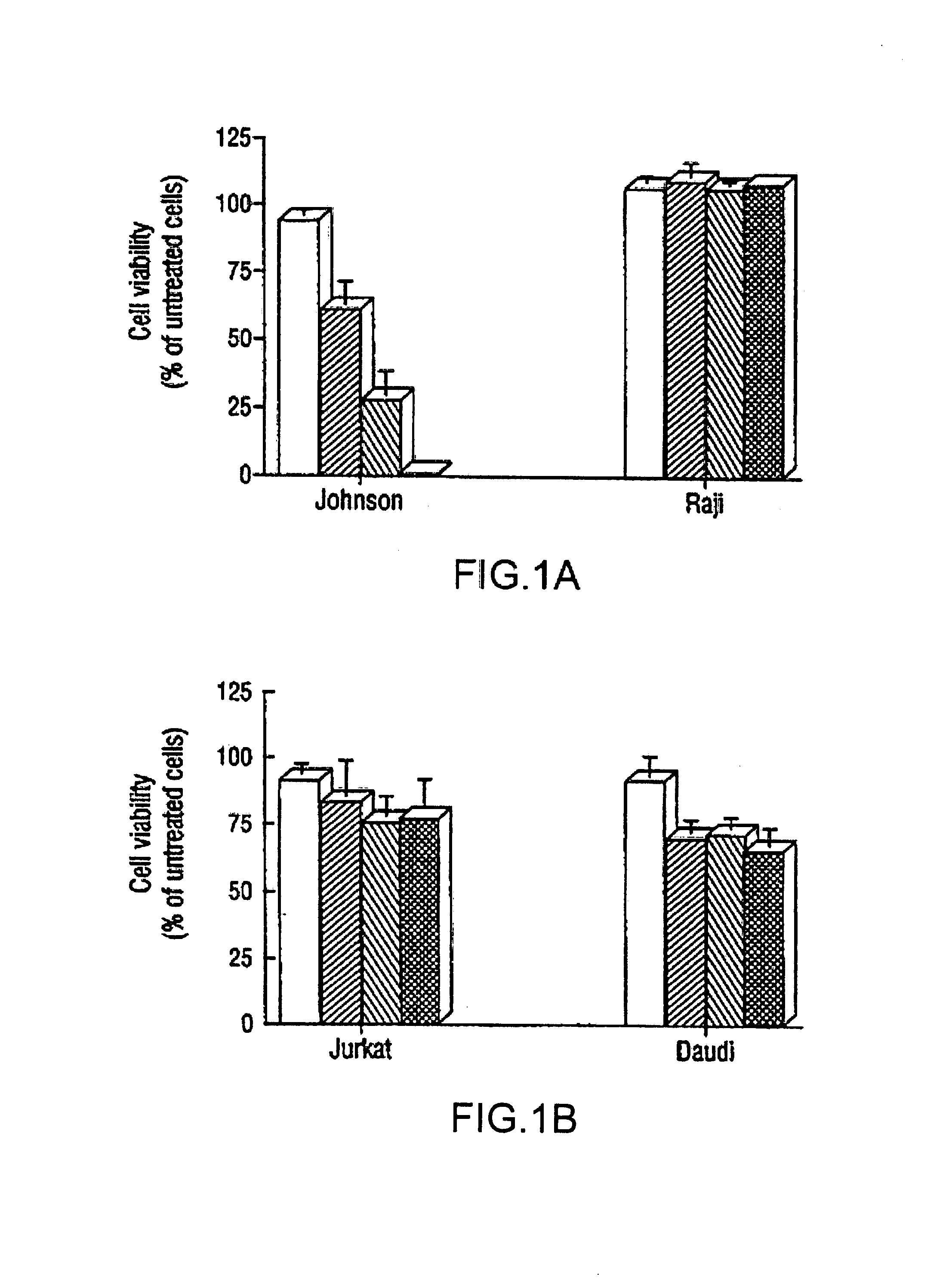
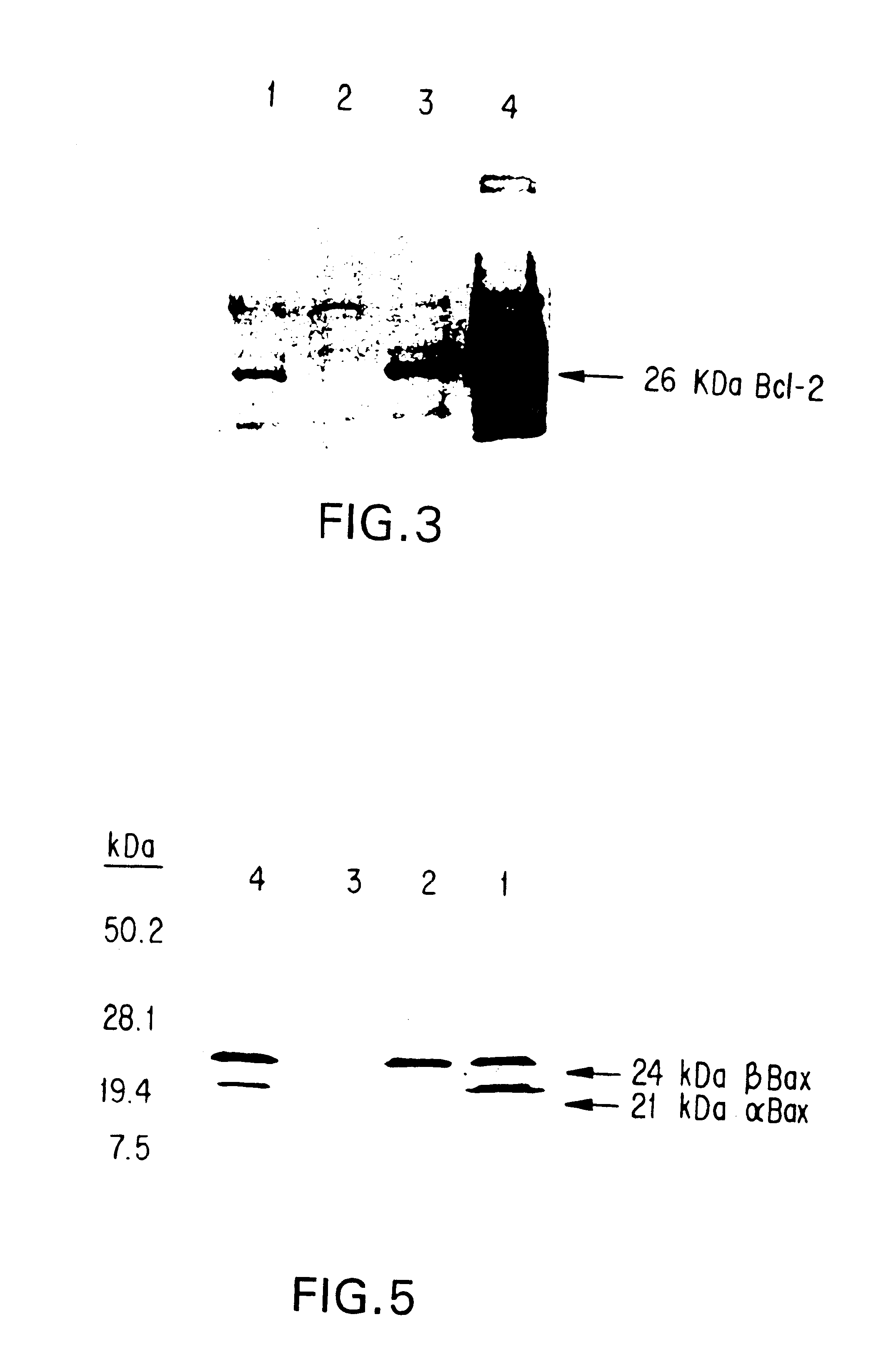
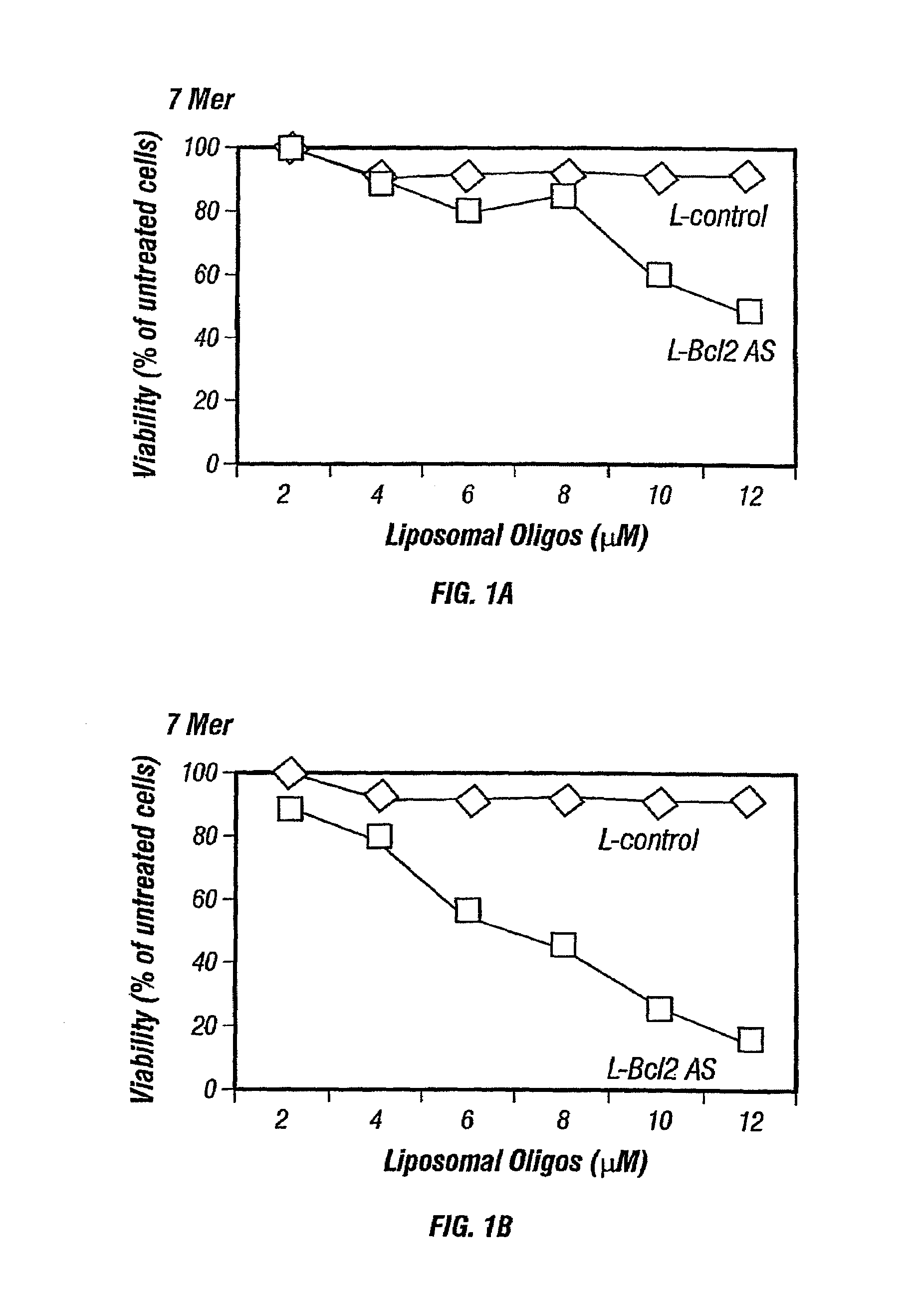
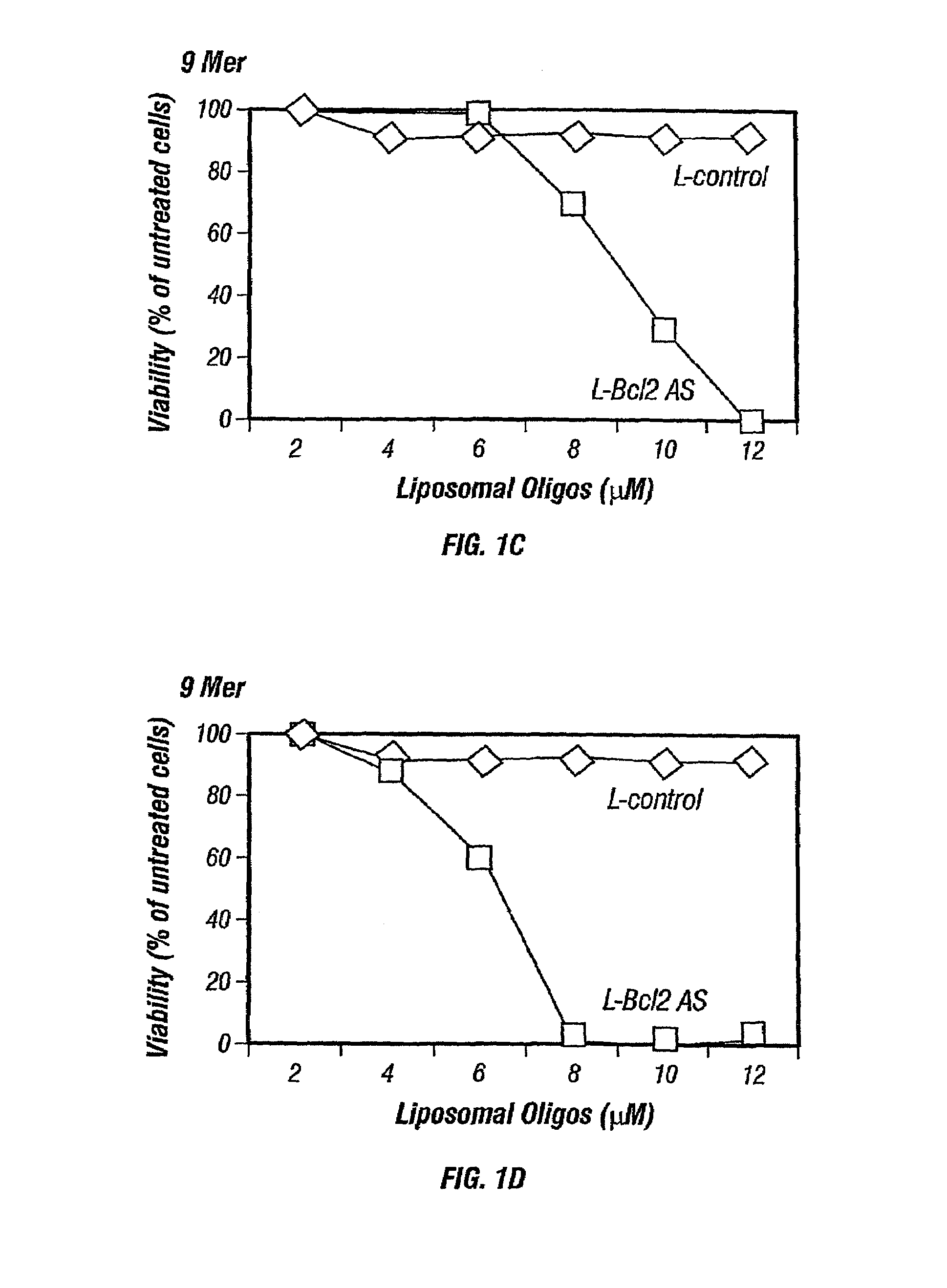
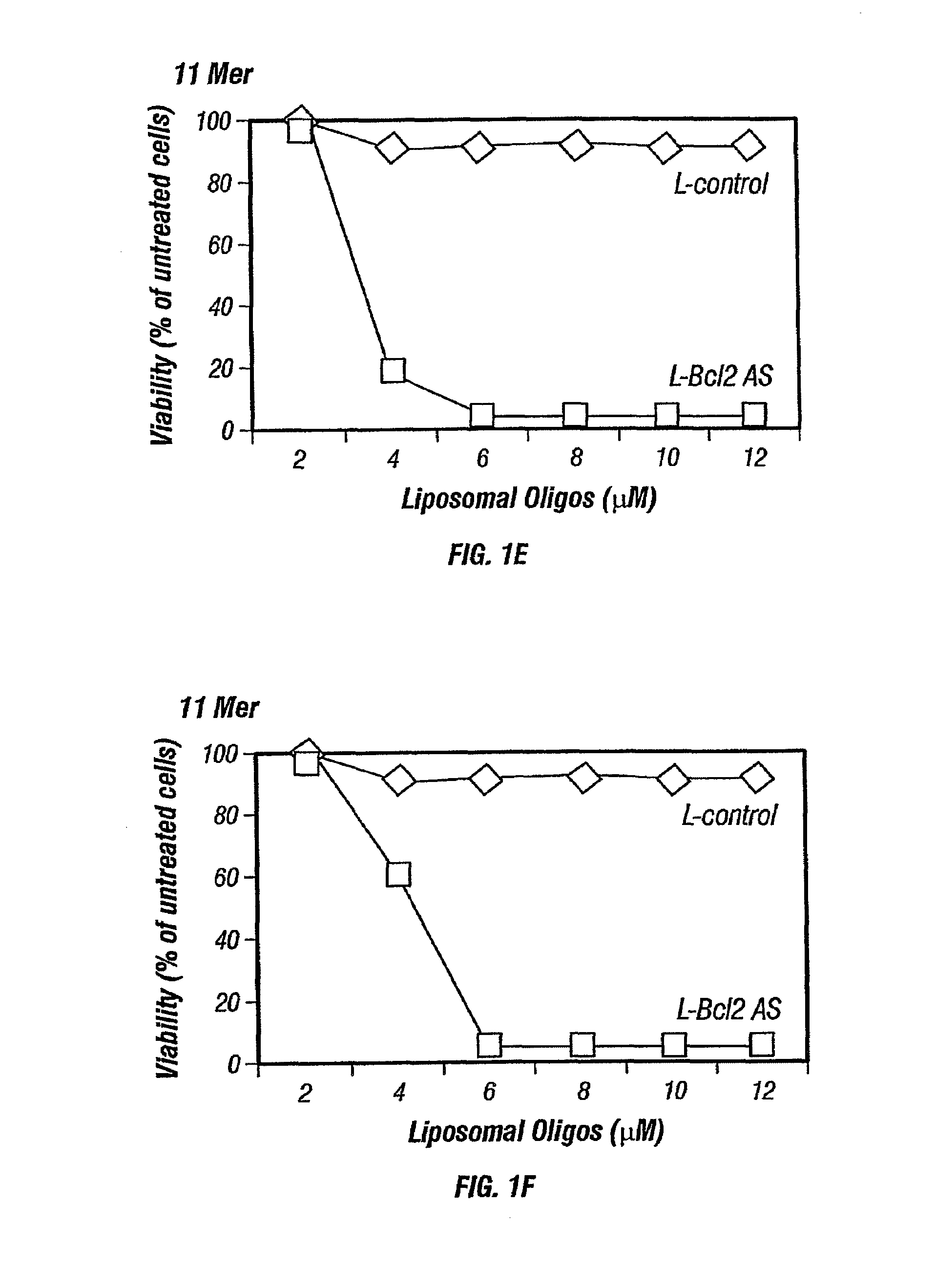
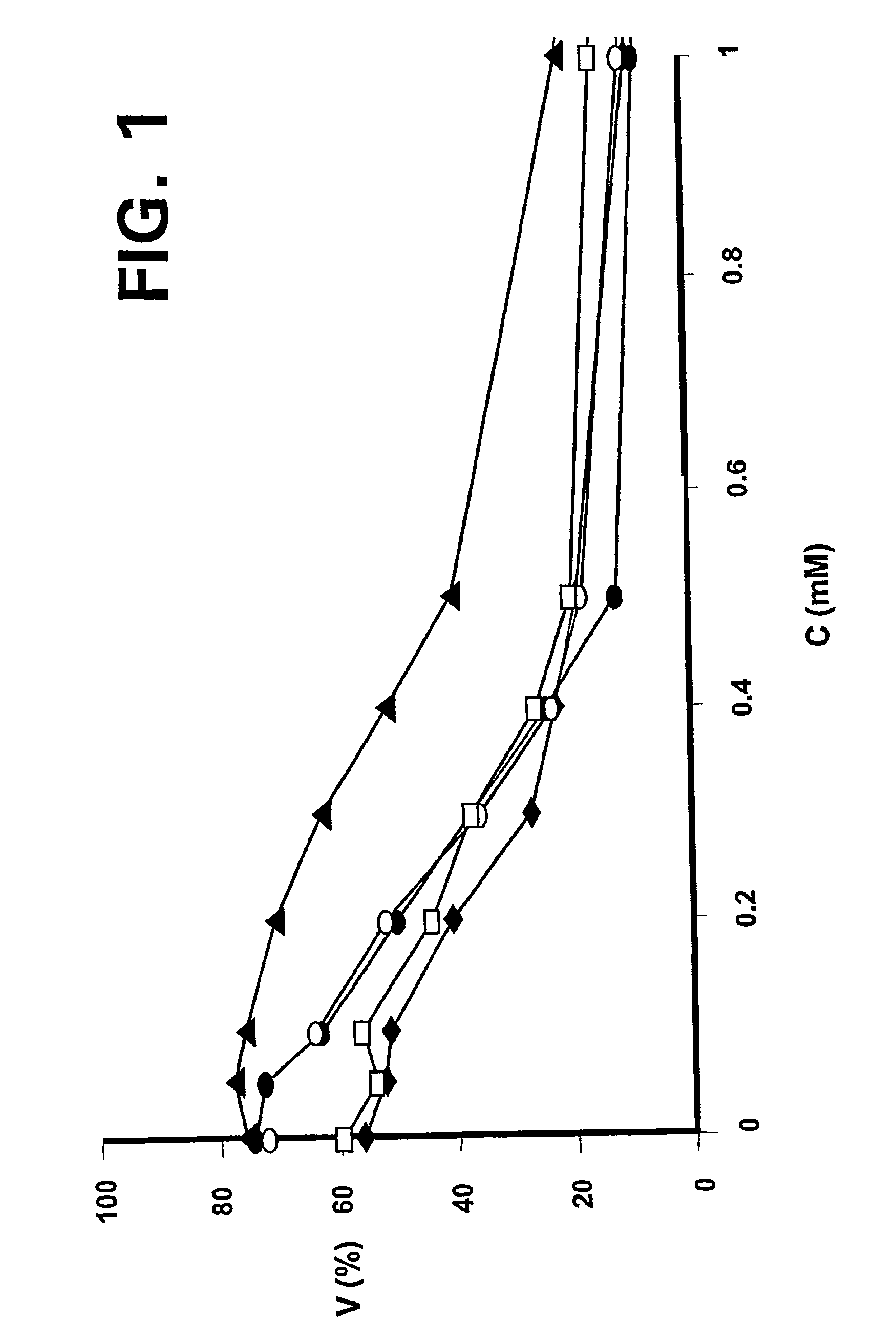
Inhibition of Bcl-2 protein expression by liposomal antisense oligodeoxynucleotides
InactiveUS7285288B1Lower the volumeIncrease the effective concentrationSugar derivativesMicrobiological testing/measurementDiseaseCancer cell
The present invention provides novel compositions and methods for use in the treatment of Bcl-2-associated diseases like cancer, specifically, in the treatment of follicular lymphoma (FL). The compositions contain antisense oligonucleotides that hybridize to Bcl-2 nucleic acids, the gene products of which are known to interact with the tumorigenic protein Bcl-2. Used alone, or in conjunction with other antisense oligonucleotides, these compositions inhibit the proliferation of FL cancer cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Monoclonal antibodies that suppress B cell growth and/or differentiation
The present invention provides monoclonal antibodies which interfere with the interactions between FDCs and B cells, thereby suppressing the proliferation and / or differentiation of B cells in lymphoid follicles. The monoclonal antibodies of the present invention are useful for treating follicular lymphomas, multiple myeloma as well as autoimmune diseases.
Owner:OCHSNER CLINIC FOUND
Monitoring transformation of follicular lymphoma to diffuse large b-cell lymphoma by immune repertoire analysis
InactiveUS20140349883A1Raise the possibilityMicrobiological testing/measurementLibrary screeningProgenitorSomatic cell
The invention is directed to a method of prognosing in an individual a transformation from follicular lymphoma to diffuse large B-cell lymphoma (DLBCL) by measuring changes and / or lack of changes in certain groups of related clonotypes, referred to herein as “clans,” in successive clonotype profiles of the individual. A clan may arise from a single lymphocyte progenitor that gives rise to many related lymphocyte progeny, each possessing and / or expressing a slightly different immunoglobulin receptor due to somatic mutation(s), such as base substitutions, inversions, related rearrangements resulting in common V(D)J gene segment usage, or the like. A higher likelihood of transformation from follicular lymphoma to DLBCL is correlated with the persistence of clans in successive clonotype profiles whose clonotype membership fails to undergo diversification over time.
Owner:ADAPTIVE BIOTECH
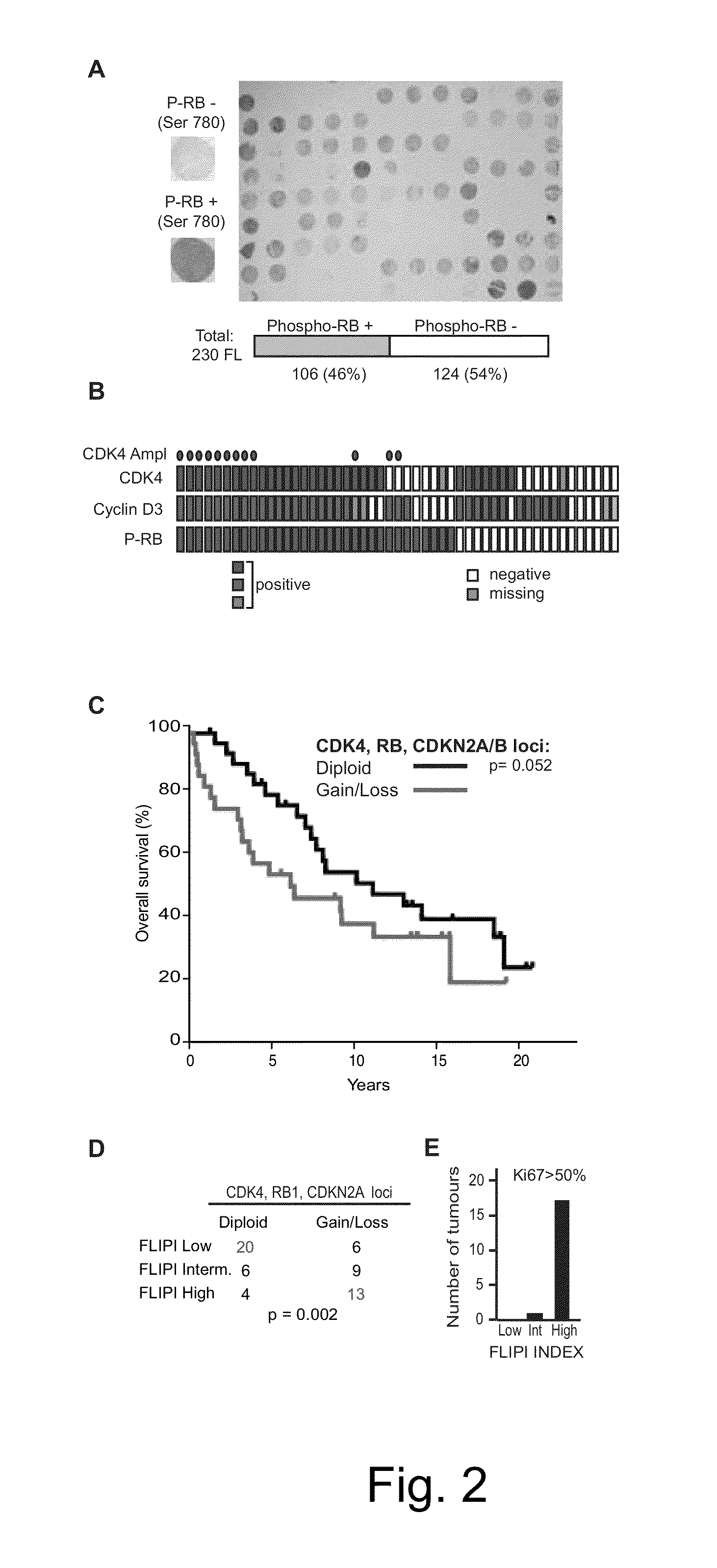
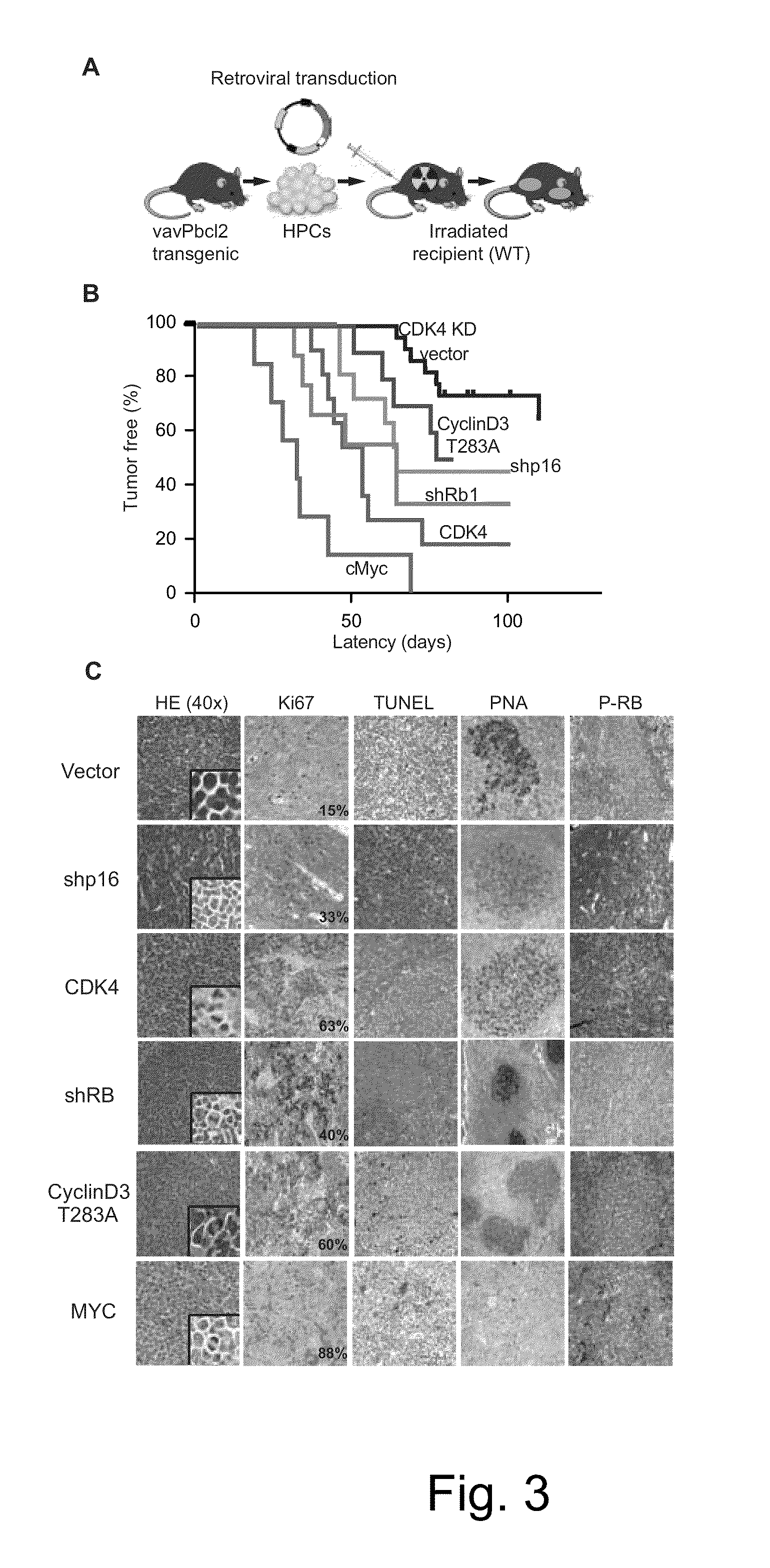
Methods for Treatment of Lymphomas with Mutations in Cell Cycle Genes
InactiveUS20140080838A1Low toxicityEnormous potential to improveOrganic active ingredientsMicrobiological testing/measurementPatient riskCell Cycle Gene
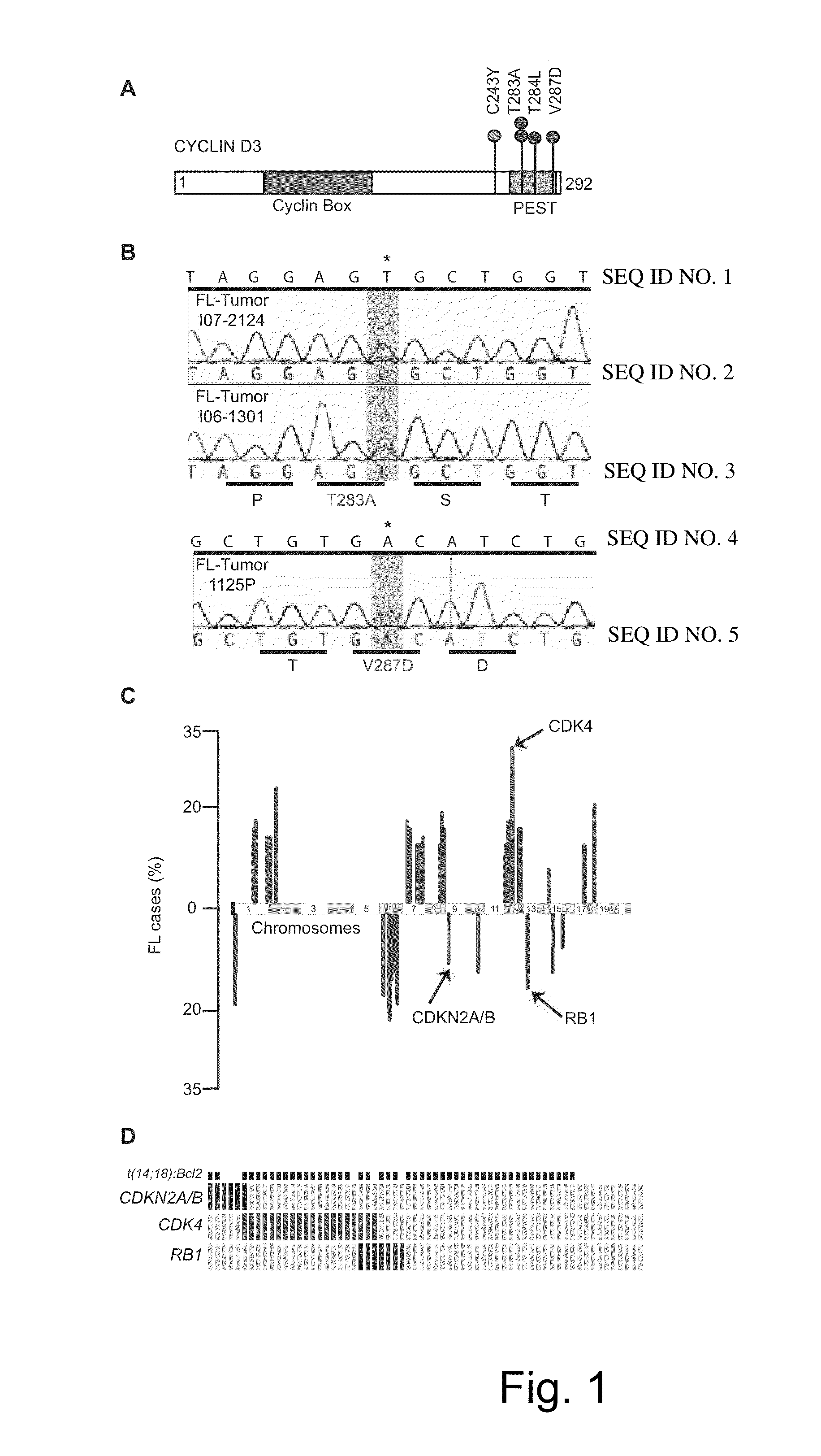
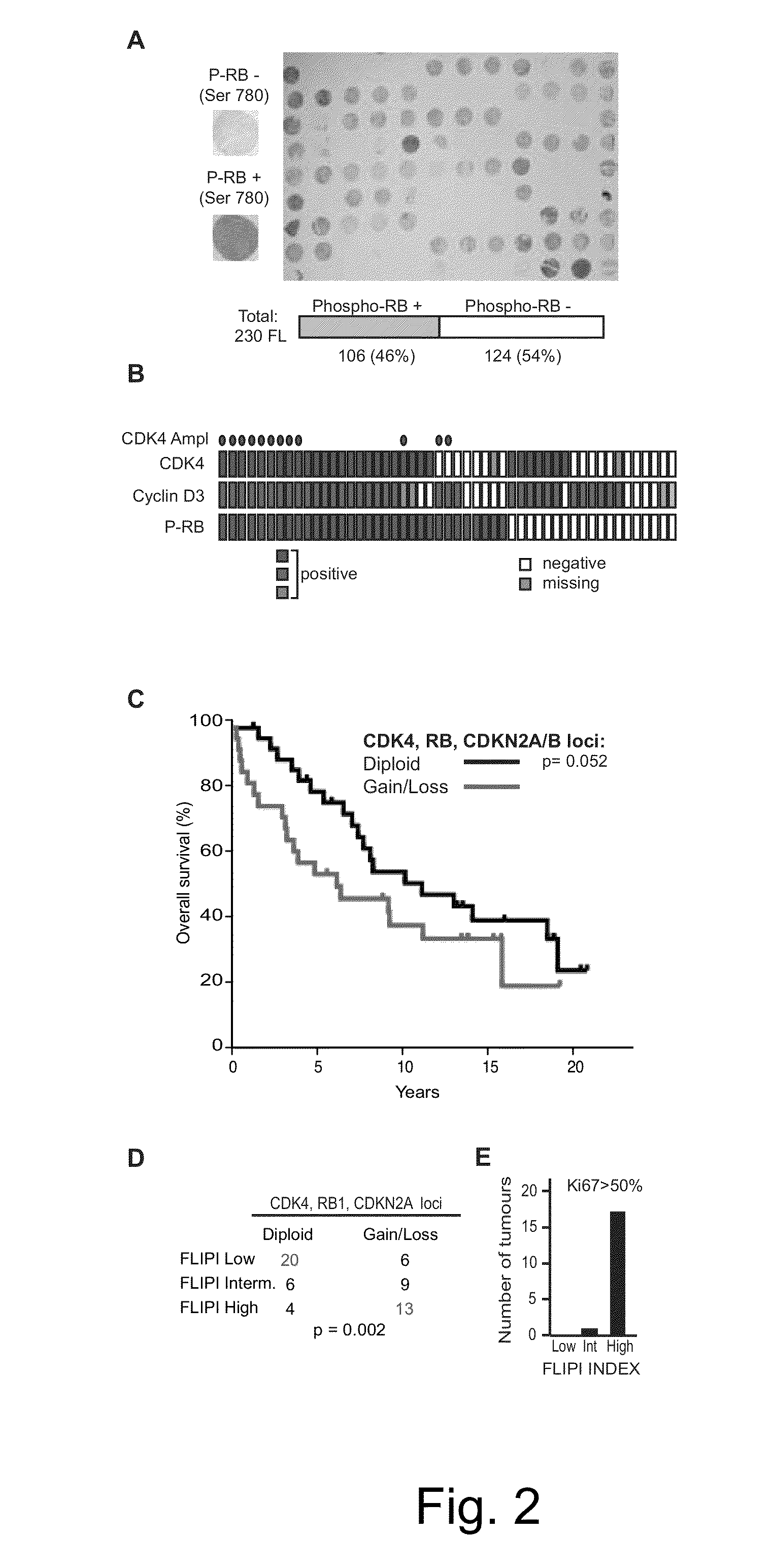
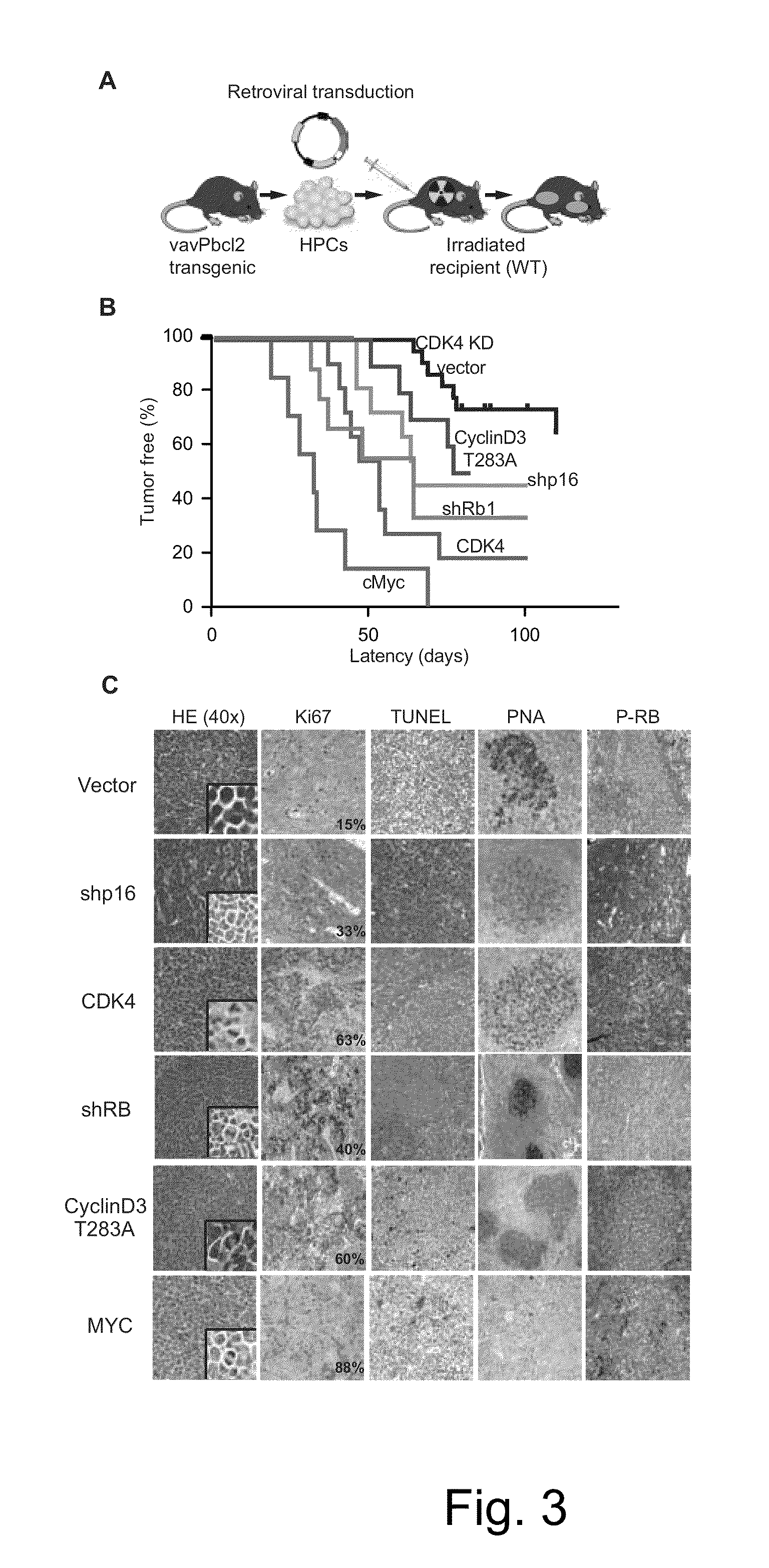
The present disclosure identifies a novel subtype of follicular lymphoma (FL) characterized by dysregulation of the cyclin / CDK / RB proliferative pathway. This subtype of FL is associated with increased malignancy and mortality, relative to FL which is not associated with cell cycle dysregulation. Accordingly, this disclosure presents novel methods to subtype FL and stratify patient risk by detection of biomarkers associated with RB inactivation. This disclosure further presents novel therapies for the treatment of FL subtyped by inactivation of RB.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Treatment of follicular lymphomas using inhibitors of the LT pathway
InactiveUS7060667B1Cosmetic preparationsPeptide/protein ingredientsFollicular lymphoma grade IIToxin
Therapeutic uses of inhibitors of the Lymphotoxin Pathway to treat tumors, specifically to treat follicular lymphomas.
Owner:NEW YORK UNIV +1
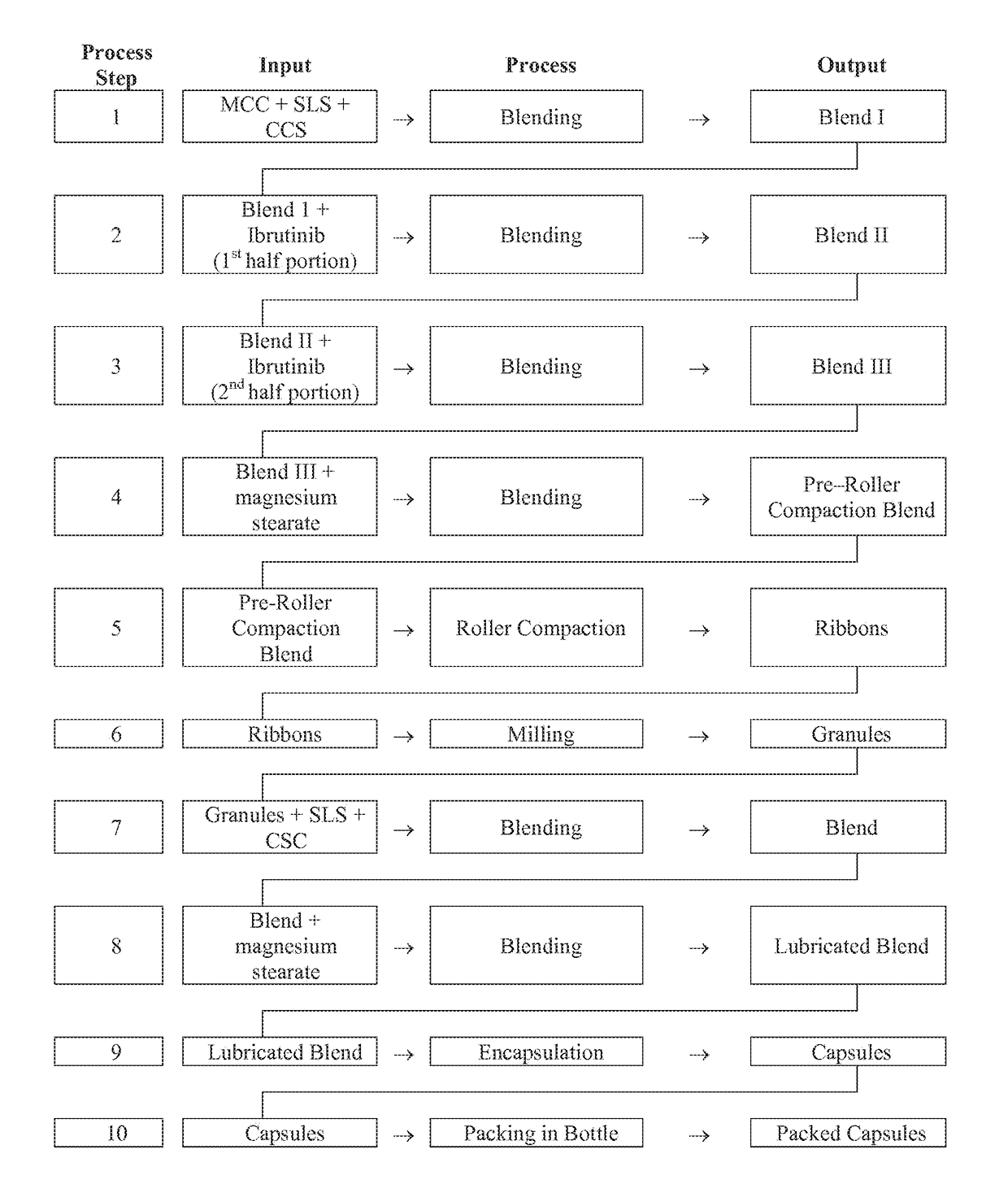
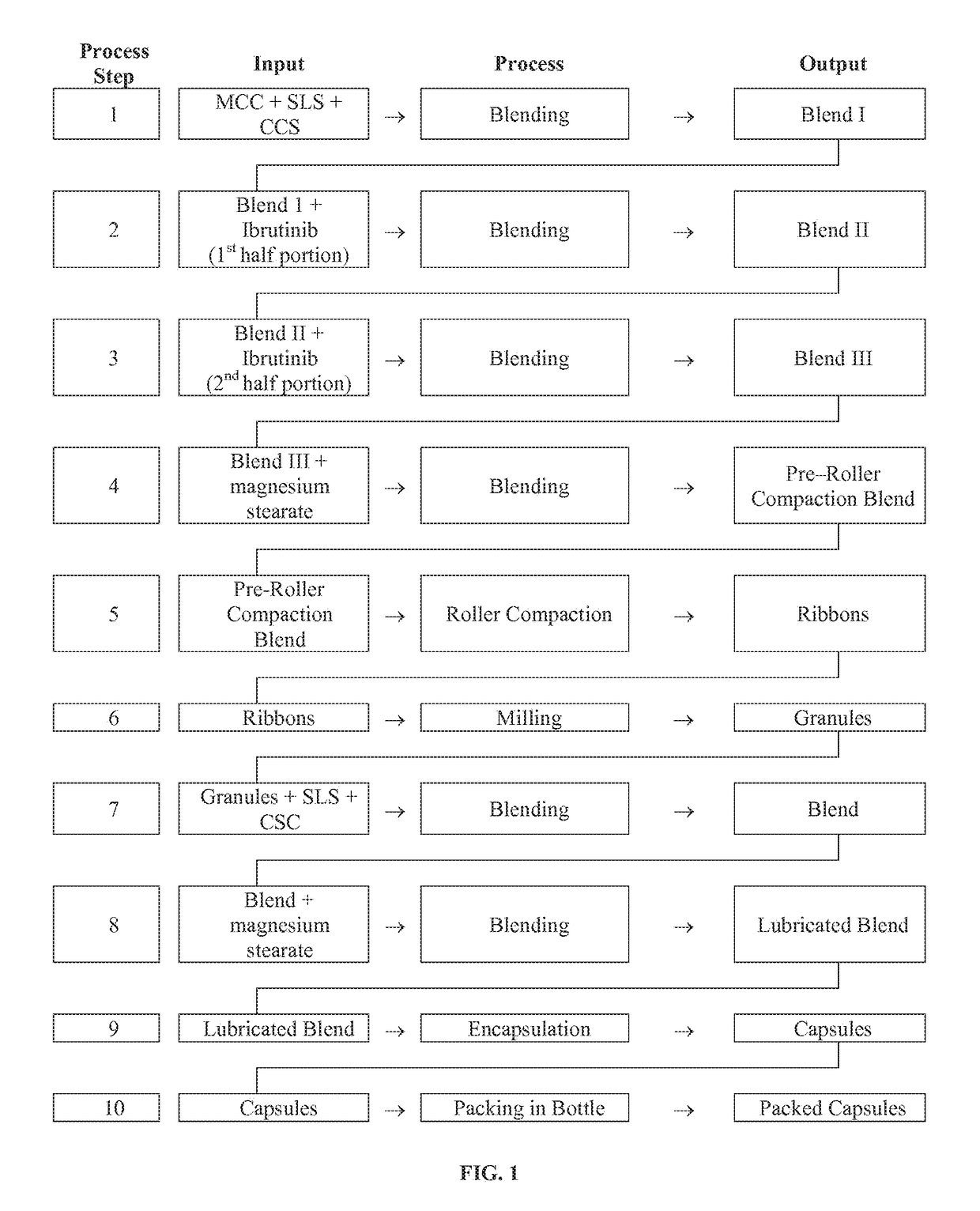
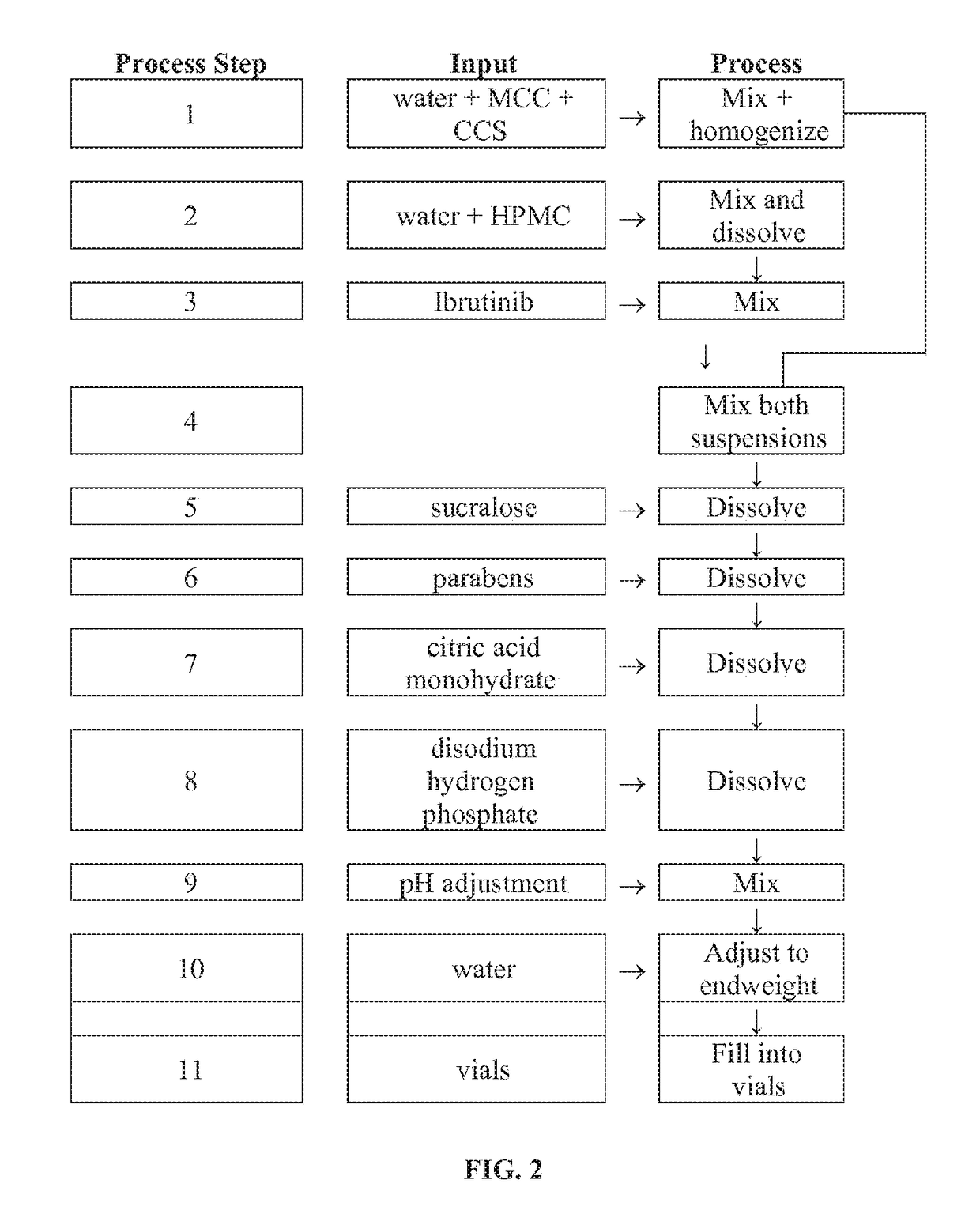
Compositions Containing Ibrutinib
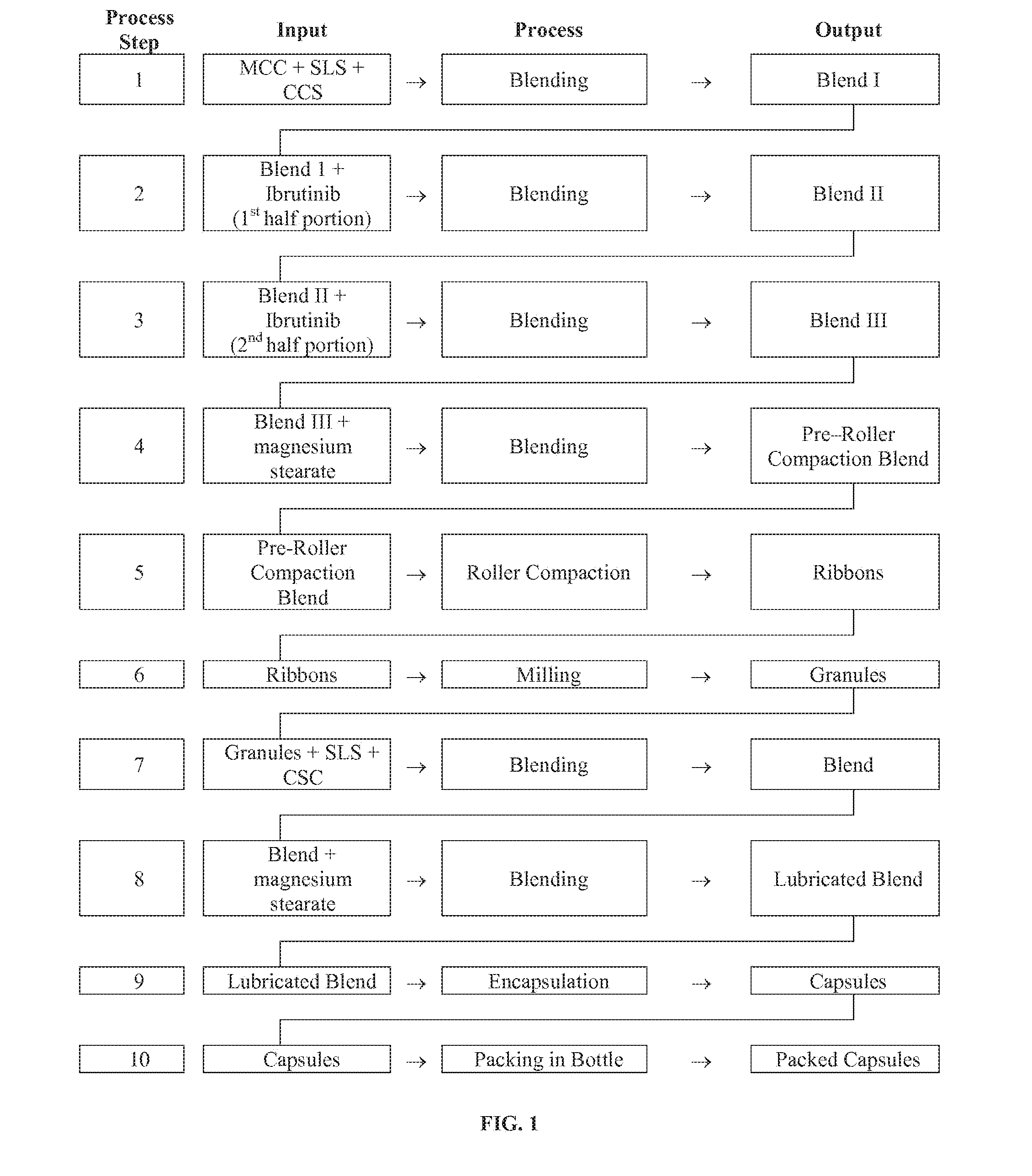
InactiveUS20160287594A1Easy to sprinkleOrganic active ingredientsInorganic non-active ingredientsWaldenstrom macroglobulinemiaMetabolite
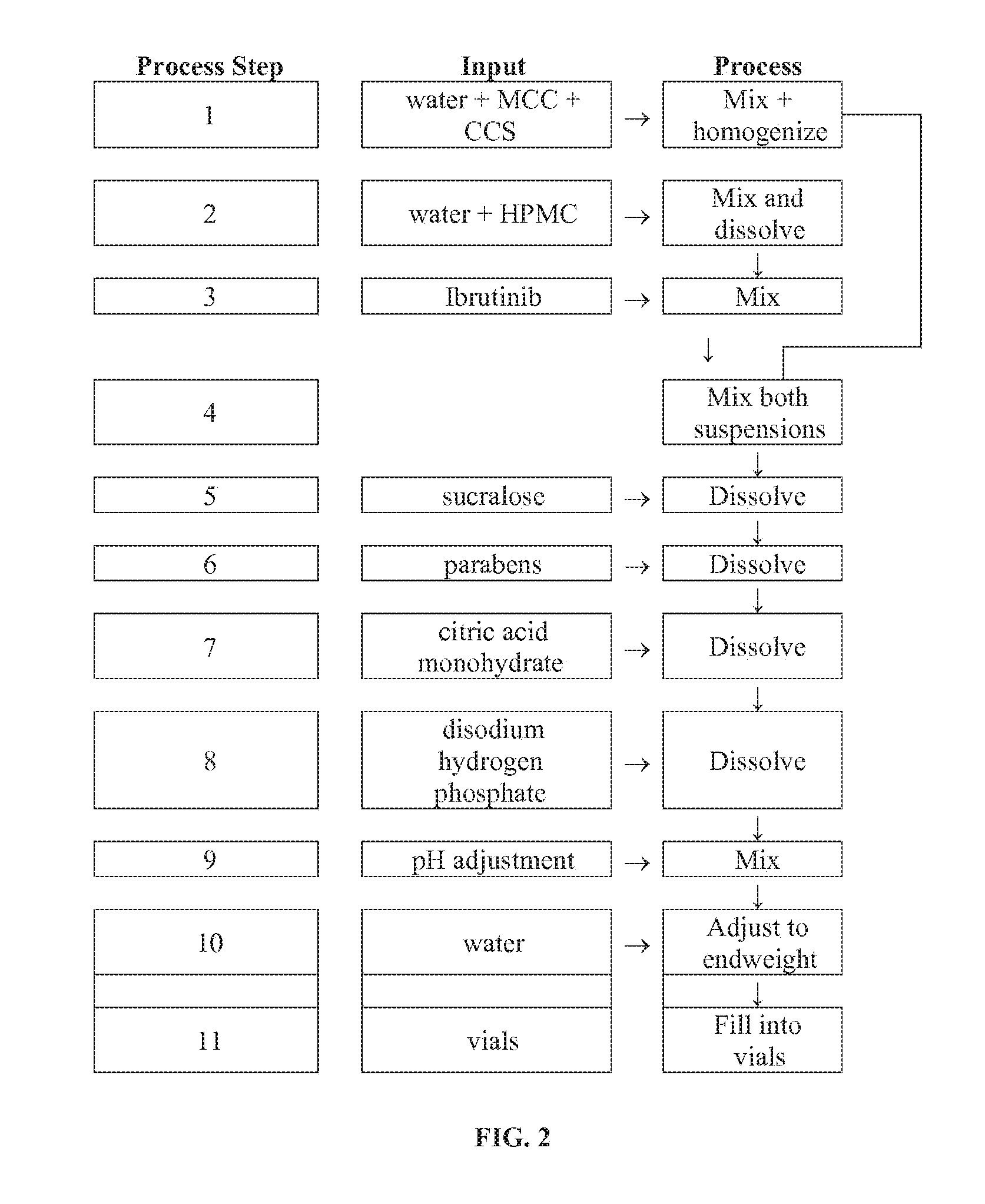
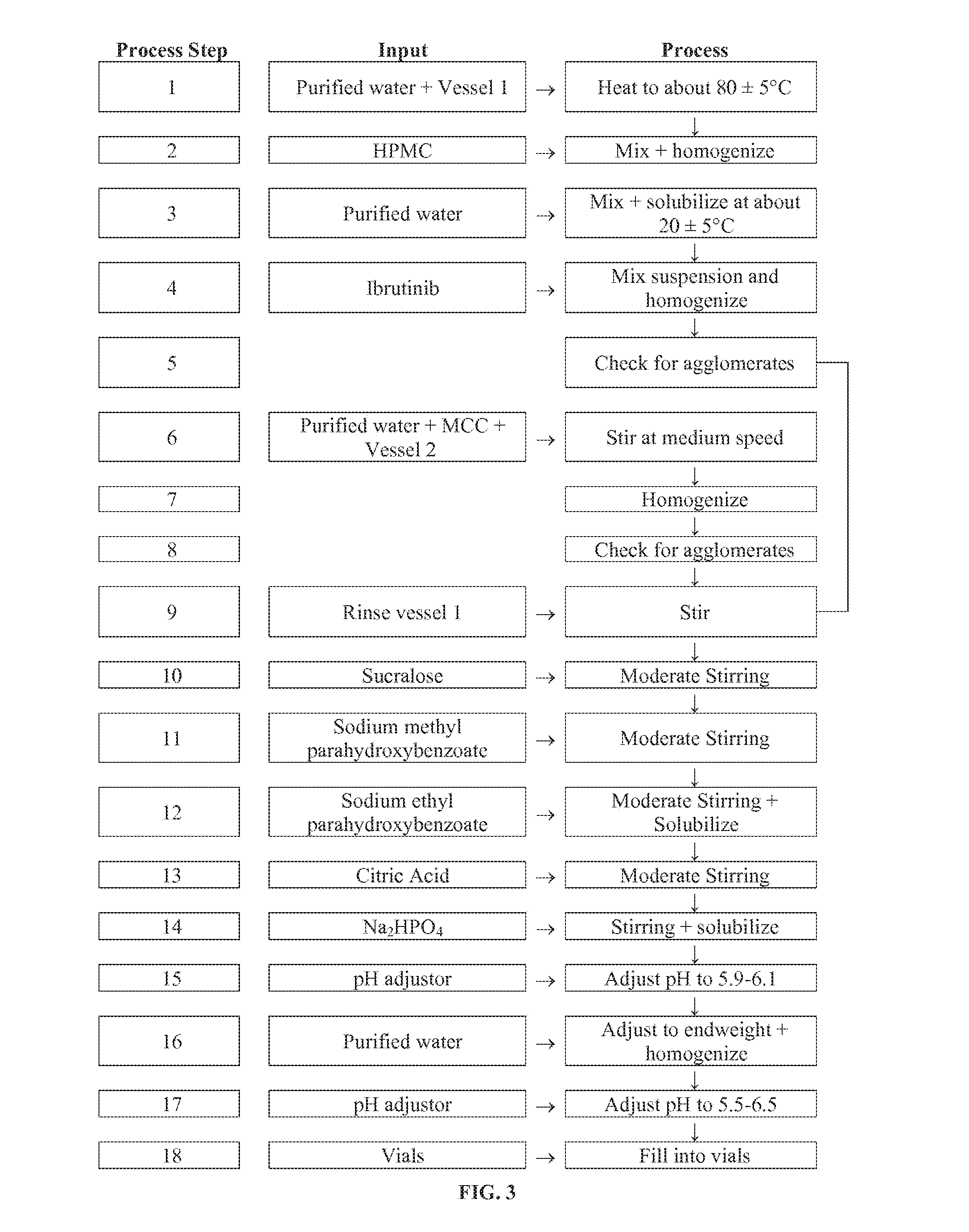
Discussed herein are pharmaceutical compositions containing Ibrutinib and processes for preparing them. The compositions may be utilized in the treatment of a variety of conditions including, without limitation, B-cell proliferative disorders such as non-Hodgkin lymphoma (diffuse large B cell lymphoma, follicular lymphoma, mantle cell lymphoma or burkitt lymphoma), Waldenstrom macroglobulinemia, plasma cell myeloma, chronic lymphocytic leukemia, lymphoma, or leukemia. These compositions are designed for oral ingestion. The compositions are contained within a capsule such as a standard or sprinkle or in a liquid formulation such as a suspension. In one embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, and magnesium stearate. In another embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, carboxymethylcellulose sodium, hydroxypropylmethylcellulose, citric acid monohydrate, disodium hydrogen phosphate, sucralose, sodium methyl parahydroxybenzoate, sodium ethyl parahydroxybenzoate, concentrated hydrochloric acid, sodium hydroxide, and water.
Owner:JANSSEN PHARMA NV
Treatment of follicular lymphomas using inhibitors of the LT pathway
InactiveUS20060280722A1Antibacterial agentsCosmetic preparationsFollicular lymphoma grade IIFollicular lymphoma
Owner:NEW YORK UNIV +1
Inhibition of Bcl-2 protein expression by liposomal antisense oligodeoxynucleotides
The present invention provides novel compositions and methods for use in the treatment of Bcl-2-associated diseases like cancer, specifically, in the treatment of follicular lymphoma (FL). The compositions contain antisense oligonucleotides that hybridize to Bcl-2 nucleic acids, the gene products of which are known to interact with the tumorigenic protein Bcl-2. Used alone, or in conjunction with other antisense oligonucleotides, these compositions inhibit the proliferation of FL cancer cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Inhibitors of human ezh2, and methods of use thereof
The invention relates to inhibition of wild-type and certain mutant forms of human histone methyltransferase EZH2, the catalytic subunit of the PRC2 complex which catalyzes the mono- through tri-methylation of lysine 27 on histone H3 (H3-K27). In one embodiment the inhibition is selective for the mutant form of the EZH2, such that trimethylation of H3-K27, which is associated with certain cancers, is inhibited. The methods can be used to treat cancers including follicular lymphoma and diffuse large B-cell lymphoma (DLBCL). Also provided are methods for identifying small molecule selective inhibitors of the mutant forms of EZH2 and also methods for determining responsiveness to an EZH2 inhibitor in a subject.
Owner:EPIZYME
Inhibitors of Human EZH2 and Methods of Use Thereof
The invention relates to determining the presence of an EZH2 gene mutation in a sample from a subject and inhibition of wild-type and certain mutant forms of human histone methyltransferase EZH2, the catalytic subunit of the PRC2 complex which catalyzes the mono-through tri-methylation of lysine 27 on histone H3 (H3-K27). In one embodiment the inhibition is selective for the mutant form of the EZH2, such that trimethylation of H3-K27, which is associated with certain cancers, is inhibited. The methods can be used to treat cancers including follicular lymphoma and diffuse large B-cell lymphoma (DLBCL). Also provided are methods for identifying small molecule selective inhibitors of the mutant forms of EZH2 and also methods for determining responsiveness to an EZH2 inhibitor in a subject.
Owner:EPIZYME
Methods for treatment of lymphomas with mutations in cell cycle genes
InactiveUS9241941B2Enormous potential to improveLow toxicityOrganic active ingredientsMicrobiological testing/measurementPatient riskCell Cycle Gene
The present disclosure identifies a novel subtype of follicular lymphoma (FL) characterized by dysregulation of the cyclin / CDK / RB proliferative pathway. This subtype of FL is associated with increased malignancy and mortality, relative to FL which is not associated with cell cycle dysregulation. Accordingly, this disclosure presents novel methods to subtype FL and stratify patient risk by detection of biomarkers associated with RB inactivation. This disclosure further presents novel therapies for the treatment of FL subtyped by inactivation of RB.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
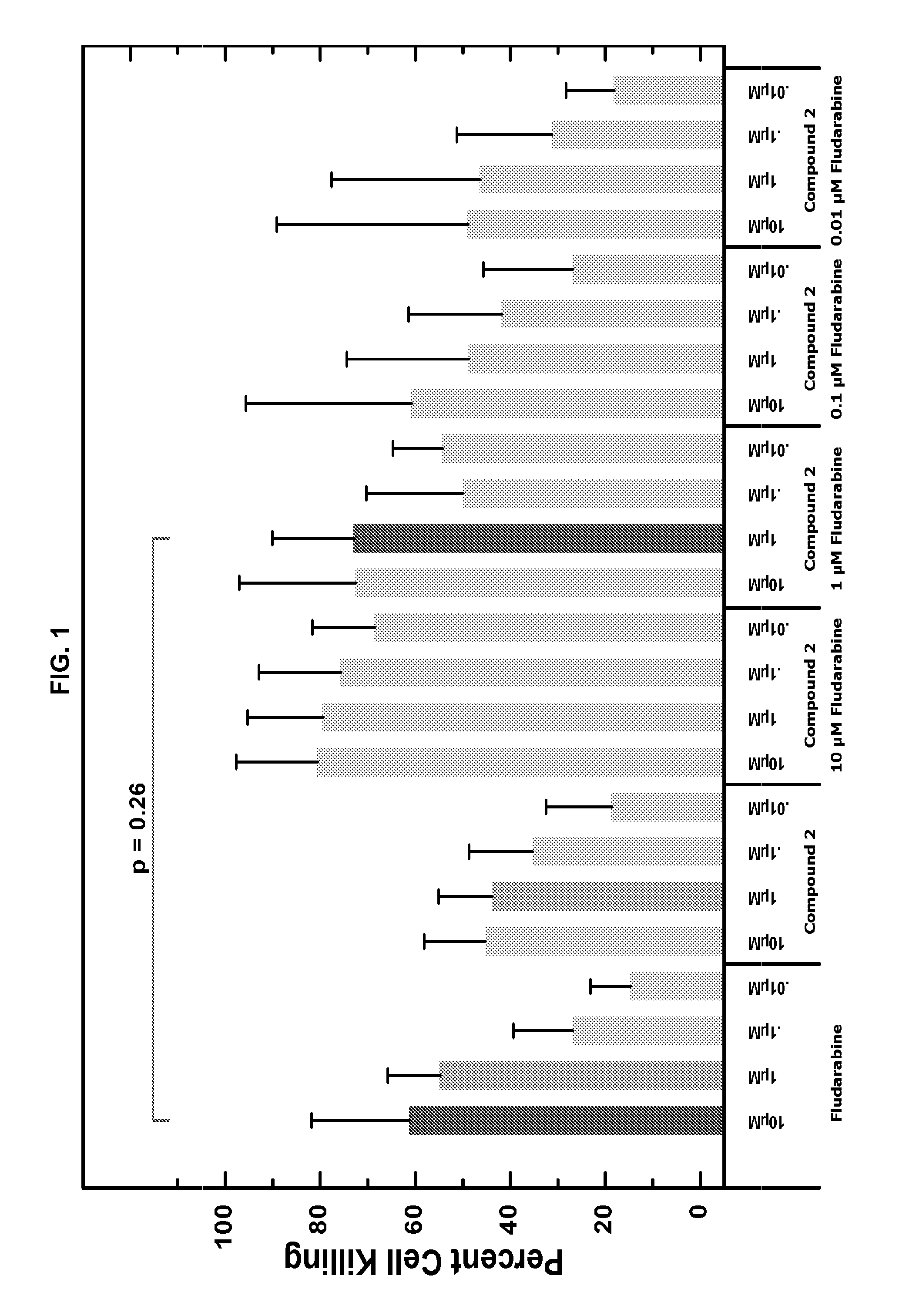
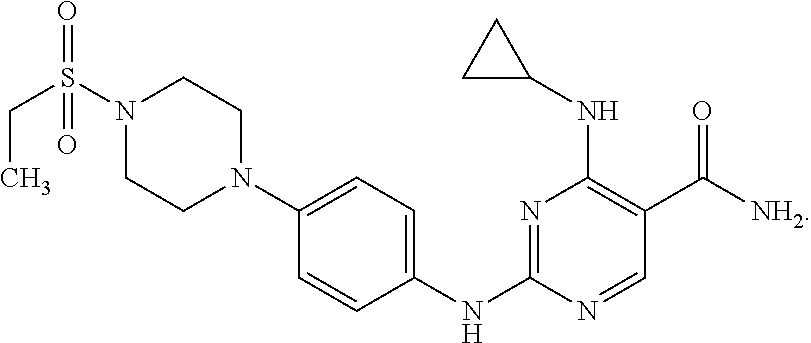
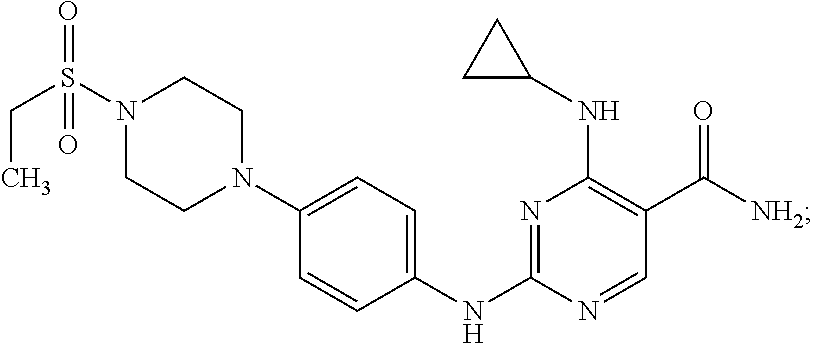
Combination therapy of 4-(cyclopropylamino)-2-(4-(4-(ethylsulfonyl)piperazin-1-yl)phenylamino)pyrimidine-5-carboxamide and fludarabine
InactiveUS20130237493A1Good effectGood treatment effectBiocideCarbohydrate active ingredientsMantle lymphomaCombination therapy
The present invention is directed to pharmaceutical compositions and methods of using combination therapies containing 4-(cyclopropylamino)-2-(4-(4-(ethylsulfonyl)piperazin-1-yl)phenylamino)pyrimidine-5-carboxamide, or a pharmaceutically acceptable salt thereof, and fludarabine for the treatment of cell proliferative disorders, such as undesired acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), including diffuse large B cell lymphoma (DLBCL); mantle cell lymphoma, acute lymphocytic leukemia (ALL), follicular lymphoma, Burkitt's lymphoma, small Lymphocytic Lymphoma (SLL) and multiple myeloma.
Owner:ALEXION PHARMA INC
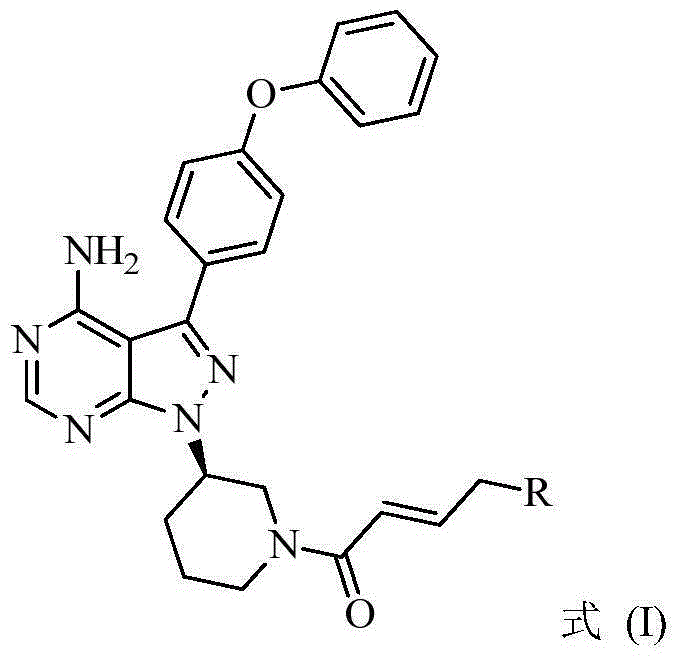
BTK inhibitor and uses thereof
ActiveCN105399756AEnhanced inhibitory effectOrganic active ingredientsOrganic chemistryChronic lymphocytic leukemiaFollicular lymphoma grade II
The present invention provides a BTK inhibitor compound (having s structure represented by a formula (I)) and uses of the BTK inhibitor compound in medicines. According to the present invention, the compound and the pharmaceutical composition can be used for treatment of diffuse large B-cell lymphoma, follicular lymphoma or chronic lymphocytic leukemia. The formula (I) is defined in the specification.
Owner:SUNSHINE LAKE PHARM CO LTD
Small oligonucleotides with anti-tumor activity
InactiveUS7704962B1Growth inhibitionEasy to controlBiocidePeptide/protein ingredientsDiseaseCancer cell
The present invention provides short antisense oligonucleotide compositions and methods for their use in the treatment of Bcl-2-associated diseases like cancer, such as follicular lymphoma (FL). The antisense oligonucleotides contain sequences that hybridize to Bcl-2 nucleic acids, the gene products of which are known to interact with the tumorigenic protein Bcl-2. The use of novel short antisense oligonucleotides, from 7 bases to 9 bases in length, is described in this invention. The invention also describes certain specific sequences which are longer than 9 bases and are 11 or 15 bases long. Used alone, or in conjunction with other antisense oligonucleotides, these antisense oligonucleotide compositions inhibit the proliferation of cancer cells.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Therapeutic use of riboside of 5-aminoimidazole-4-carboxamide (acadesine)
The present disclosure relates to a method of treatment of a human patient suffering from a B-cell lymphoproliferative disorders such as B-cell chronic lymphocytic leukemia (B-CLL), splenic marginal zone lymphoma (SMZL), mantle cell lymphoma (MCL), follicular lymphoma (FL), lymphoplasmacytic lymphoma (LPL), and Waldenström syndrome (WS), by the administration of a therapeutically effective amount of 5-aminoimidazole-4-carboxamide riboside (acadesine) or its precursors (eg. its mono-, di- and tri-5′-phosphates). This makes acadesine and its bioprecursors (eg. its mono-, di- and tri-5′-phosphates) useful as therapeutic agents for B-cell lymphoproliferative disorders in humans. The surprising feature that T cells are virtually not affected means that the side effect (immunosuppression) is minor, what represents a therapeutical advantage of acadesine over cladribine, fludarabine and other nucleosides known in the art.
Owner:ADVANCELL ADVANCED IN VITRO CELL TECH
Purine compounds, composition and application
InactiveCN106432239AGood curative effectOrganic chemistryAntineoplastic agentsDiseaseChronic lymphocytic leukemia
The invention belongs to the technical field of medicine and relates to purine compounds, composition and an application of the composition. The compounds represented as a general formula (I) as well as all possible isomers, pharmaceutical salts or hydrates or the composition of the compounds are used for treating diseases caused by BTK (Bruton tyrosine kinase) and particularly used for treating diffuse large B cell lymphoma, follicular lymphoma or chronic lymphocytic leukemia.
Owner:DALIAN MEDICAL UNIVERSITY
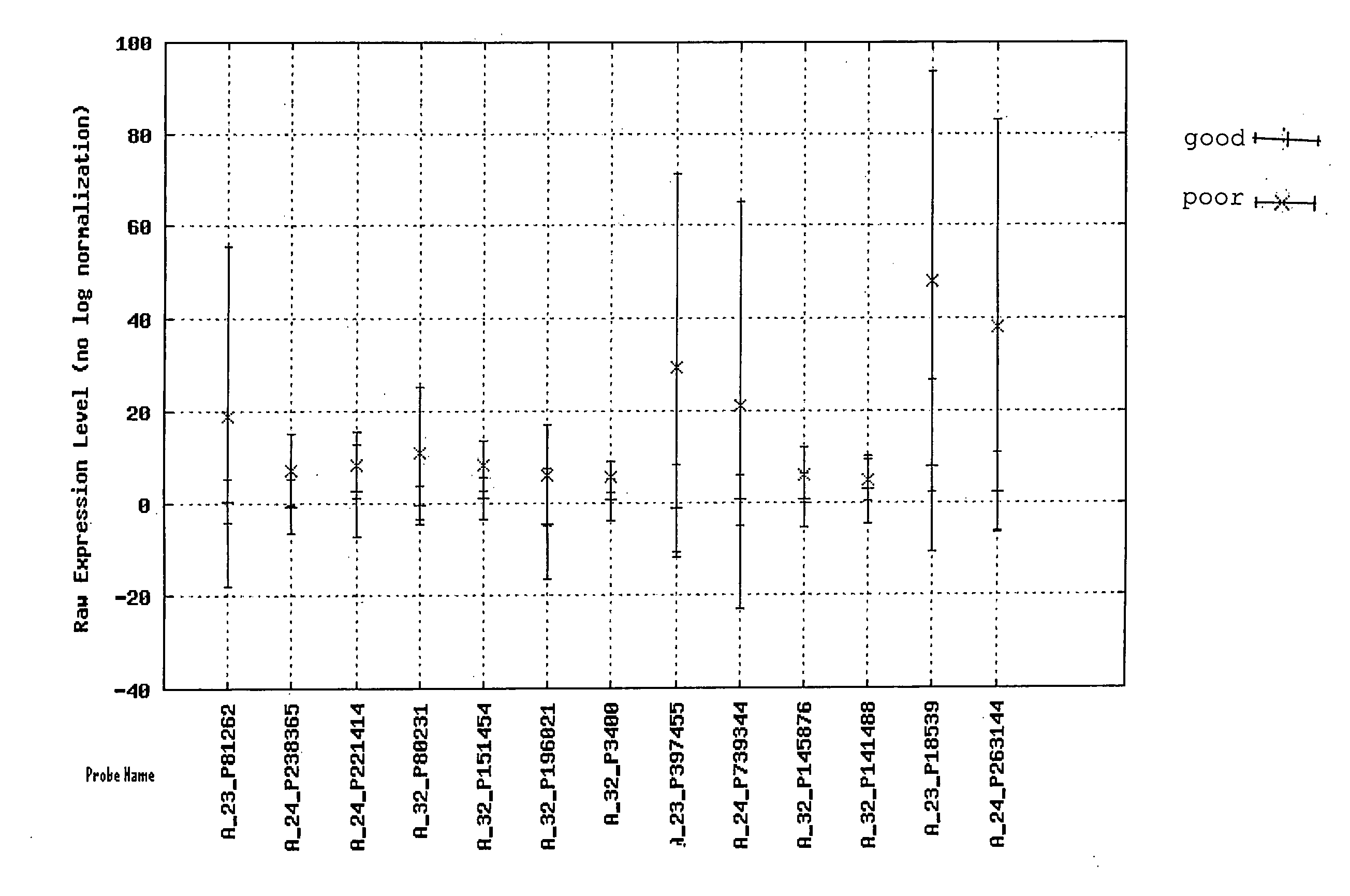
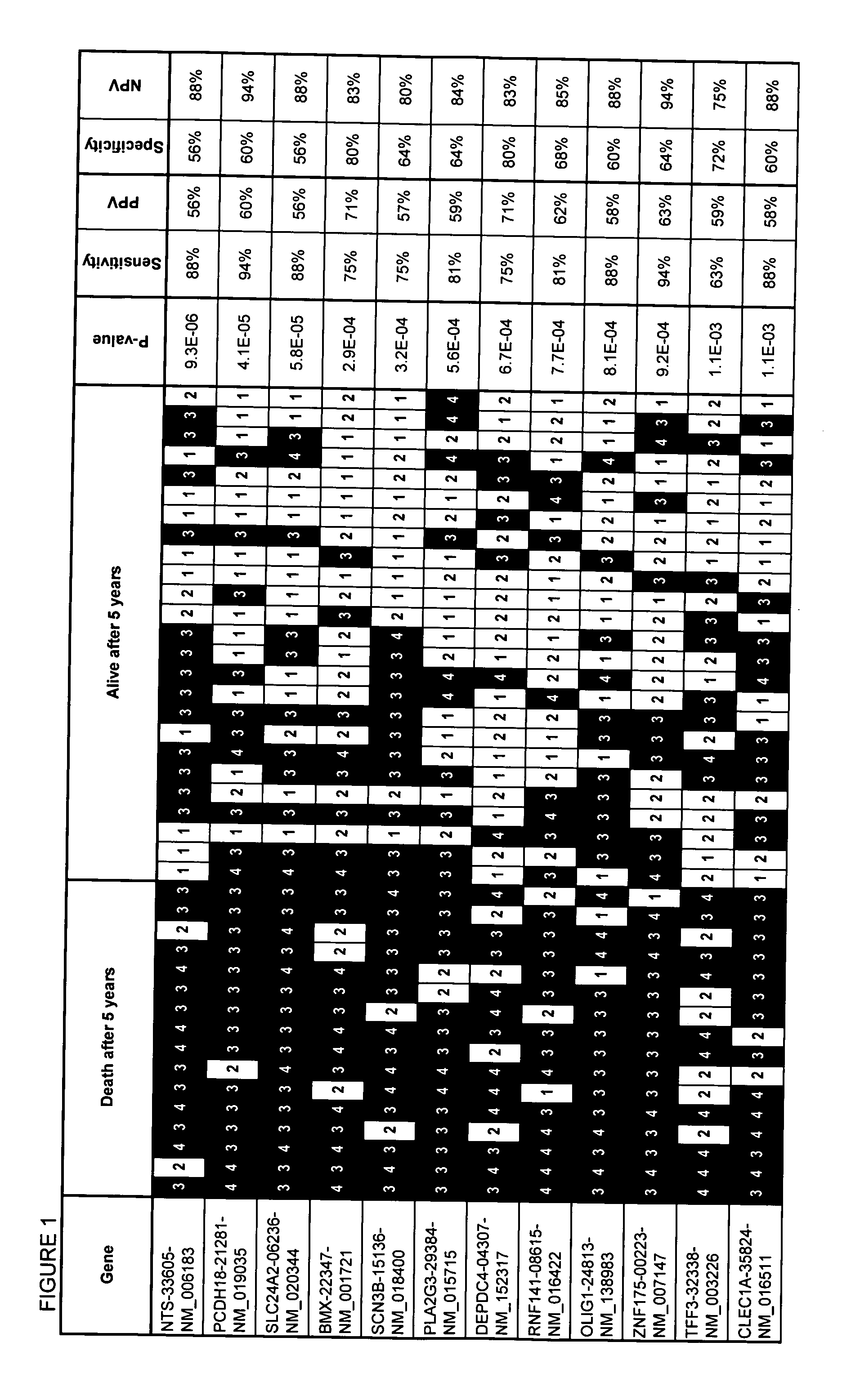
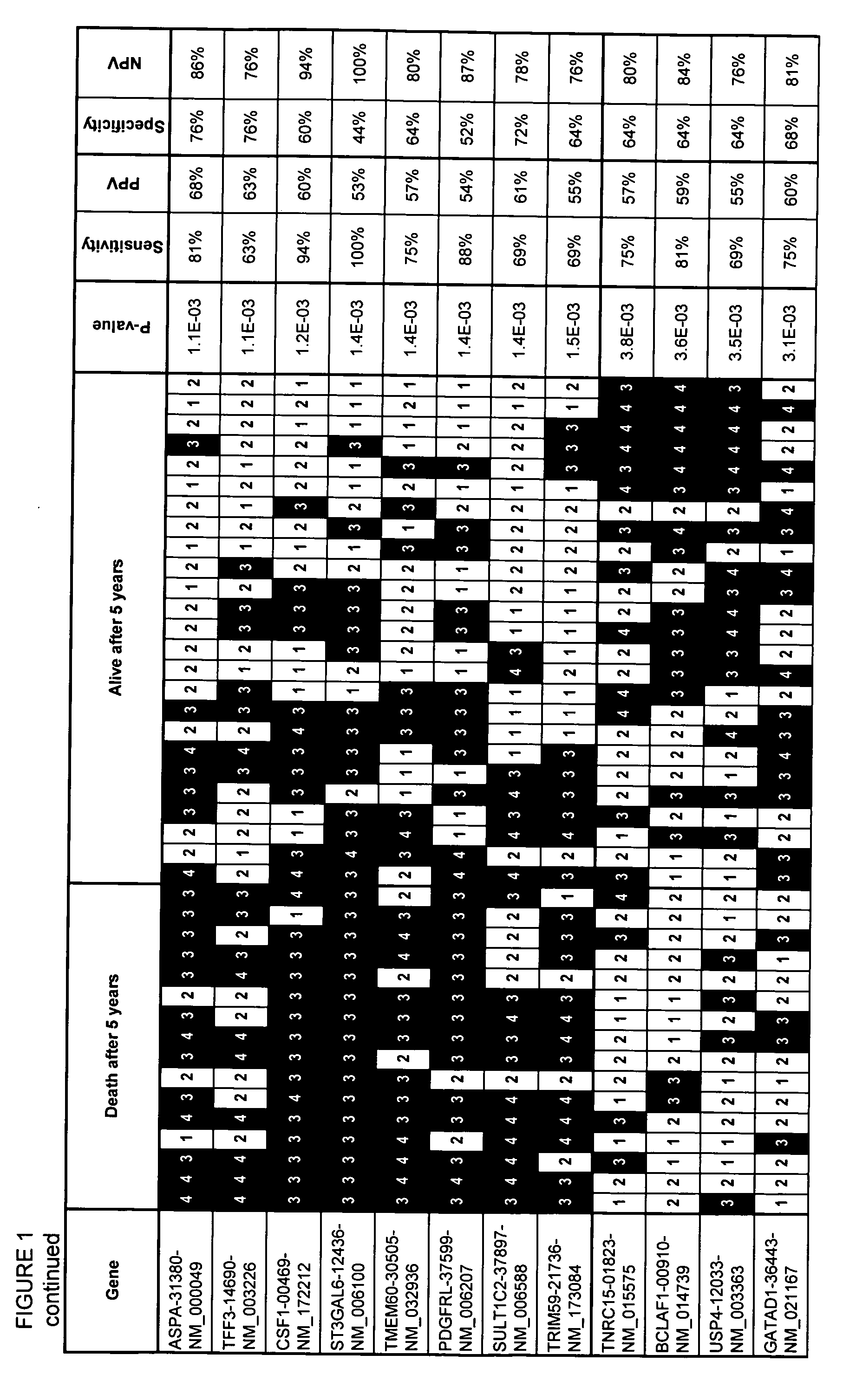
Assays, methods and systems for predicting follicular lymphoma outcome
InactiveUS20090092991A1Predictable outcomeMicrobiological testing/measurementBiological testingFollicular lymphoma grade IICancer research
Assays, kits, methods and systems for predicting outcome in patients with follicular lymphoma based upon measurement of one or more phenomenologically competitive or synergistic gene pairs or a set of classifier genes are provided.
Owner:BIOSYSTIX +2
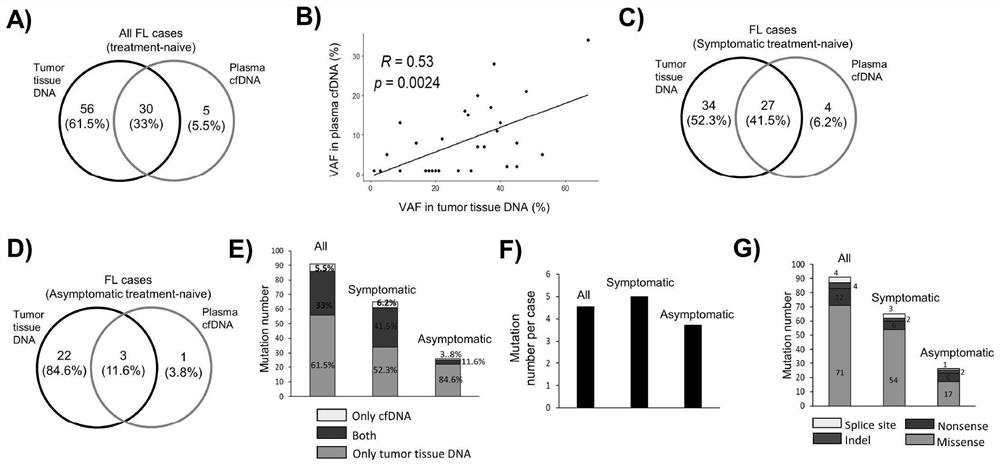
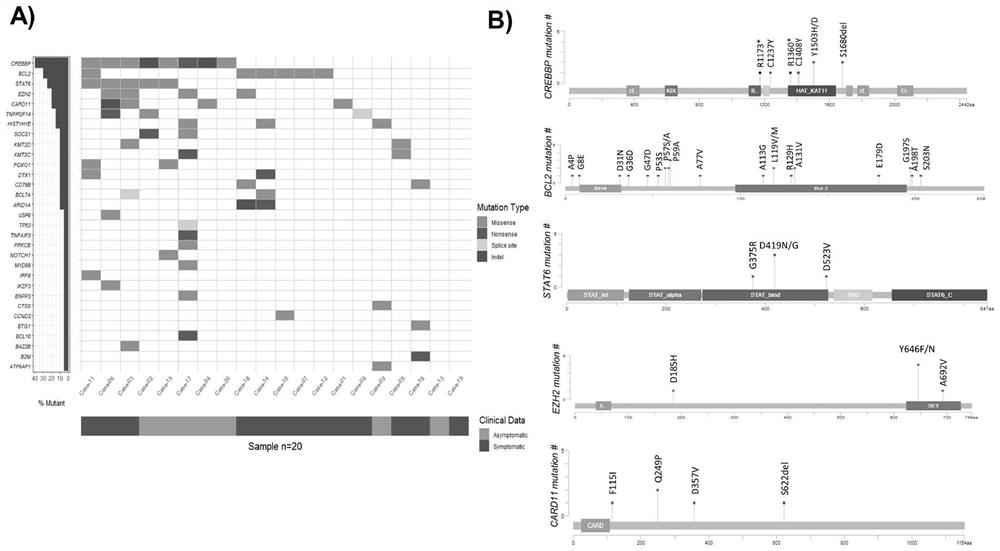
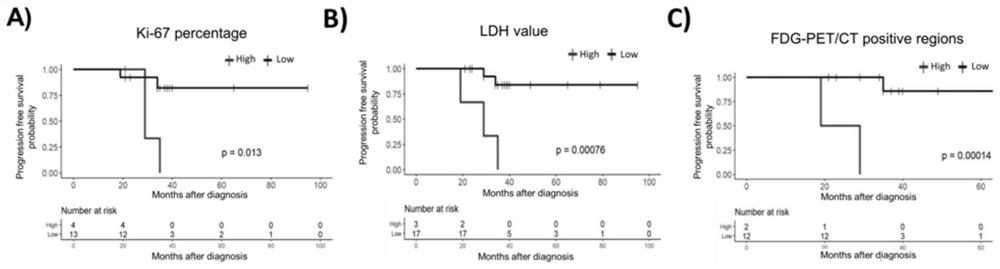
Detection panel for follicular lymphoma prognosis evaluation based on circulating free DNA mutation, kit and application
PendingCN114752672AImproved prognosisHas clinical application valueMicrobiological testing/measurementDNA/RNA fragmentationFollicular lymphoma grade IIOncology
The invention discloses a detection panel for prognosis evaluation of follicular lymphoma based on circulating free DNA mutation, a kit and application. The detection panel comprises detection of a mutation gene related to follicular lymphoma prognosis. The invention provides a non-invasive detection method for prognosis evaluation of follicular lymphoma, and the non-invasive detection method has clinical application value so as to better guide treatment and improve prognosis of FL patients. A gene combination closely related to follicular lymphoma is creatively selected, based on a second-generation sequencing technology, the detection and analysis content comprises the following steps: performing second-generation detection on plasma cfDNA (plasma cfDNA), paraffin lymphoma tissue DNA (tumor tissue gDNA) and granulocyte gDNA of an FL clinical patient, and the gene combination disclosed by the invention has guiding significance on prognosis evaluation and treatment and has a good application prospect. And more prognosis evaluation dimensions are provided for doctors.
Owner:CANCER CENT OF GUANGZHOU MEDICAL UNIV
Monoclonal antibodies that suppress B cell growth and/or differentiation
Owner:OCHSNER CLINIC FOUND
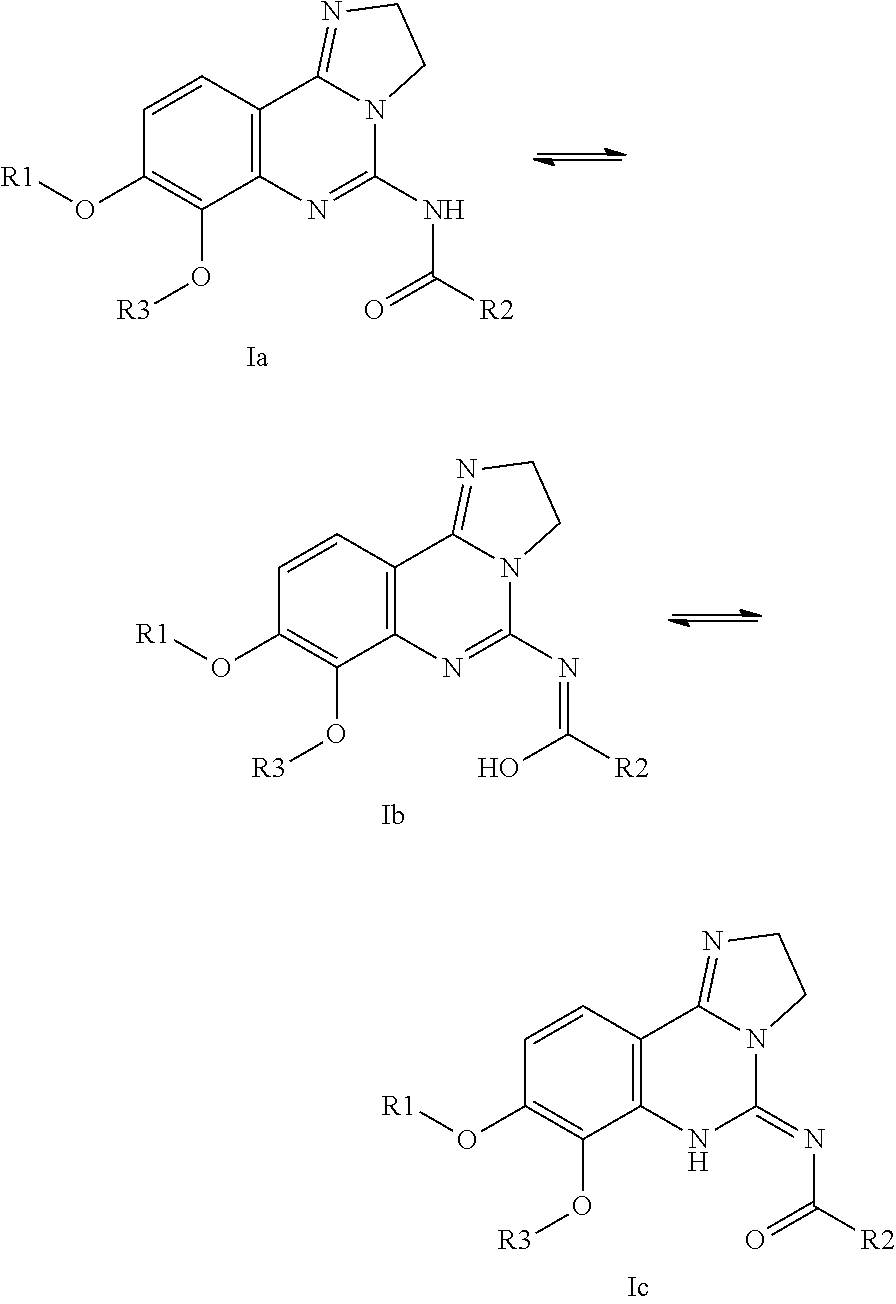
Use of substituted 2,3-dihydroimidazo[1,2-c]quinazolines for treating lymphomas
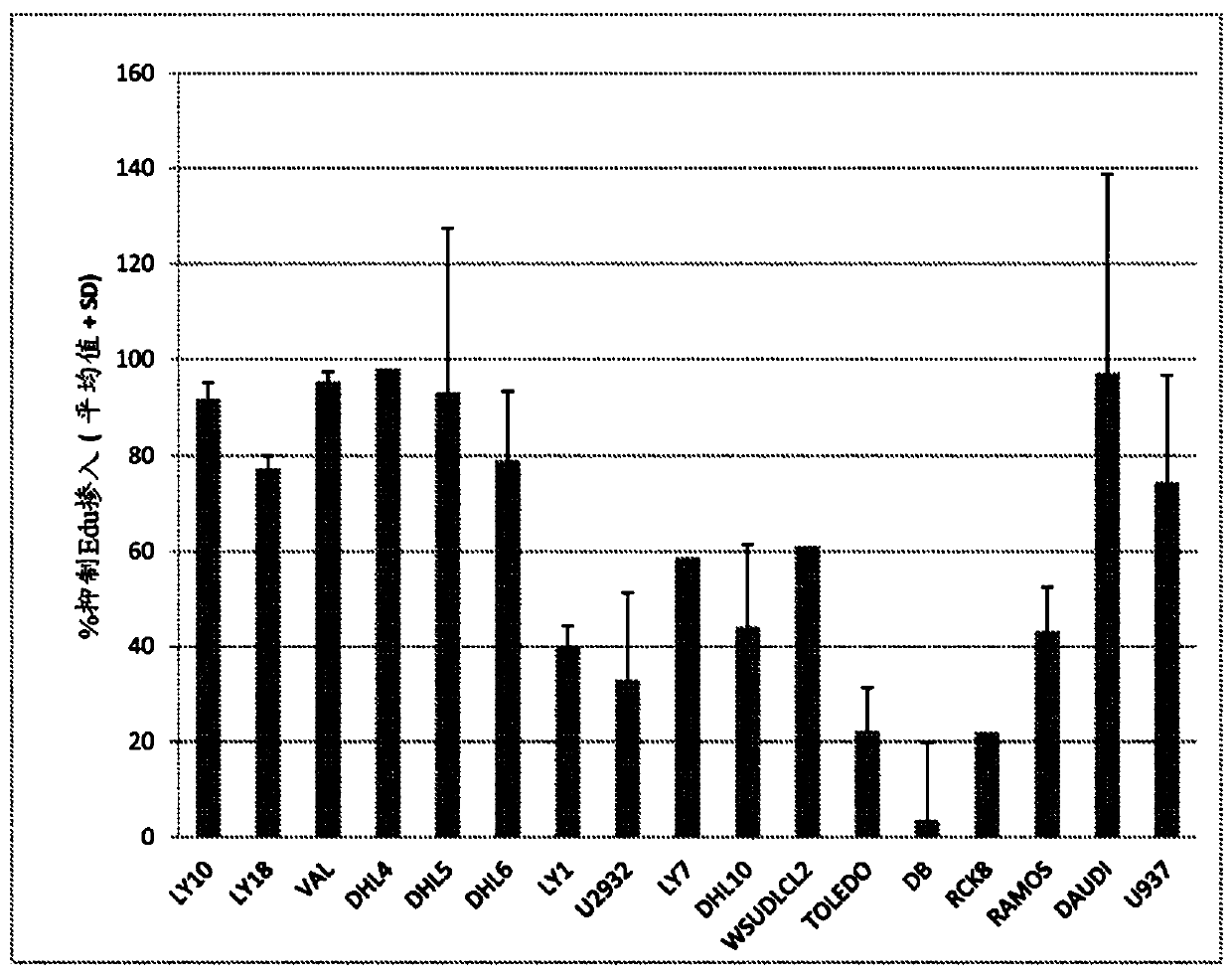
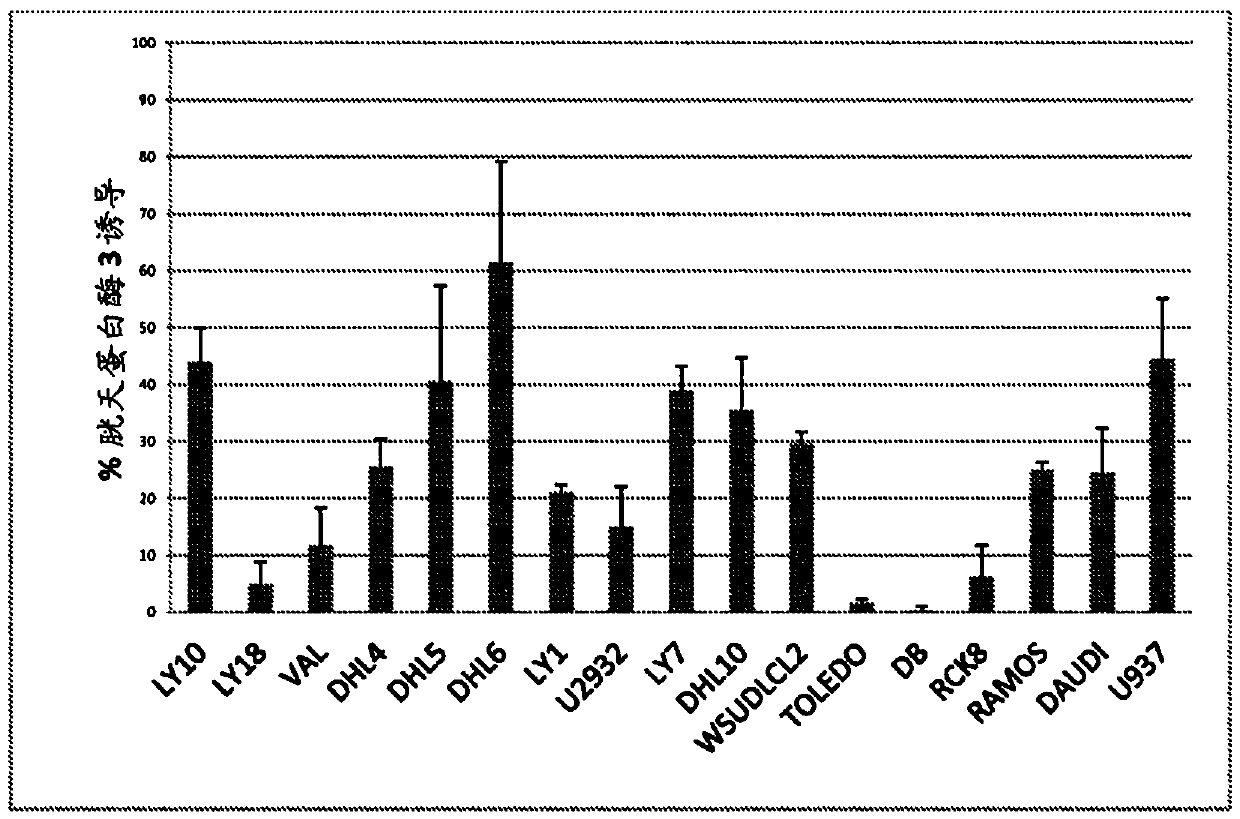
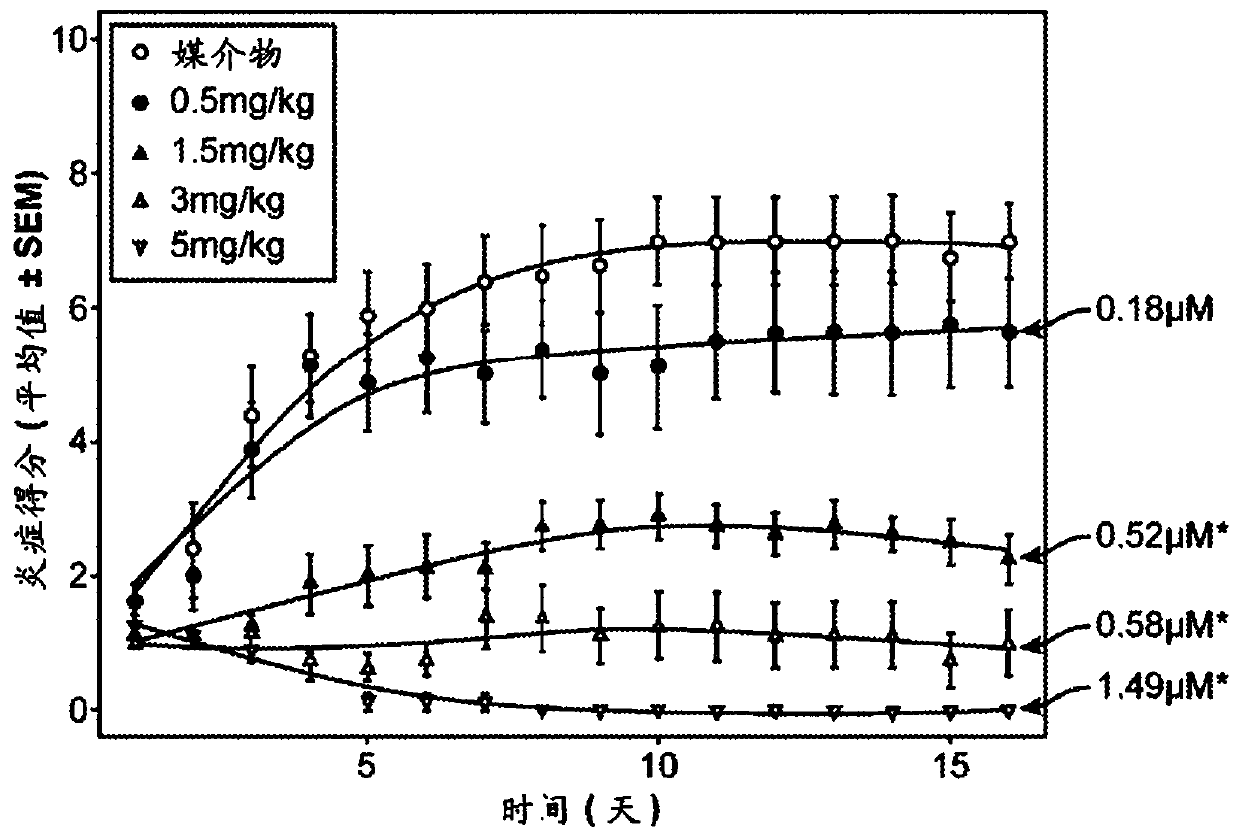
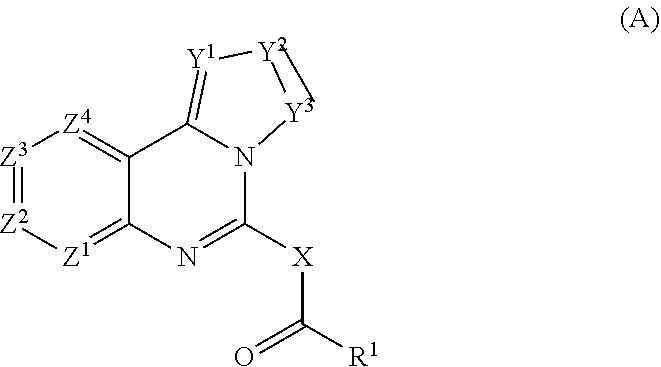
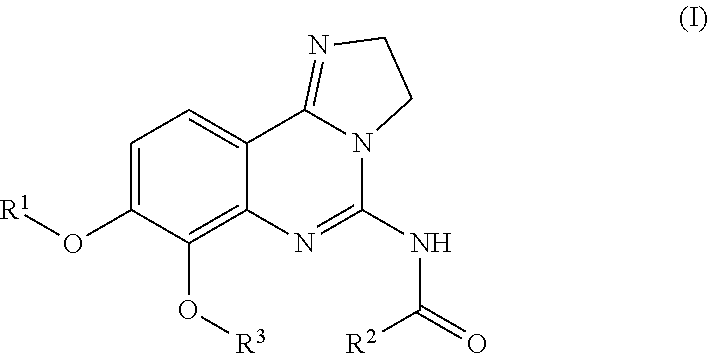
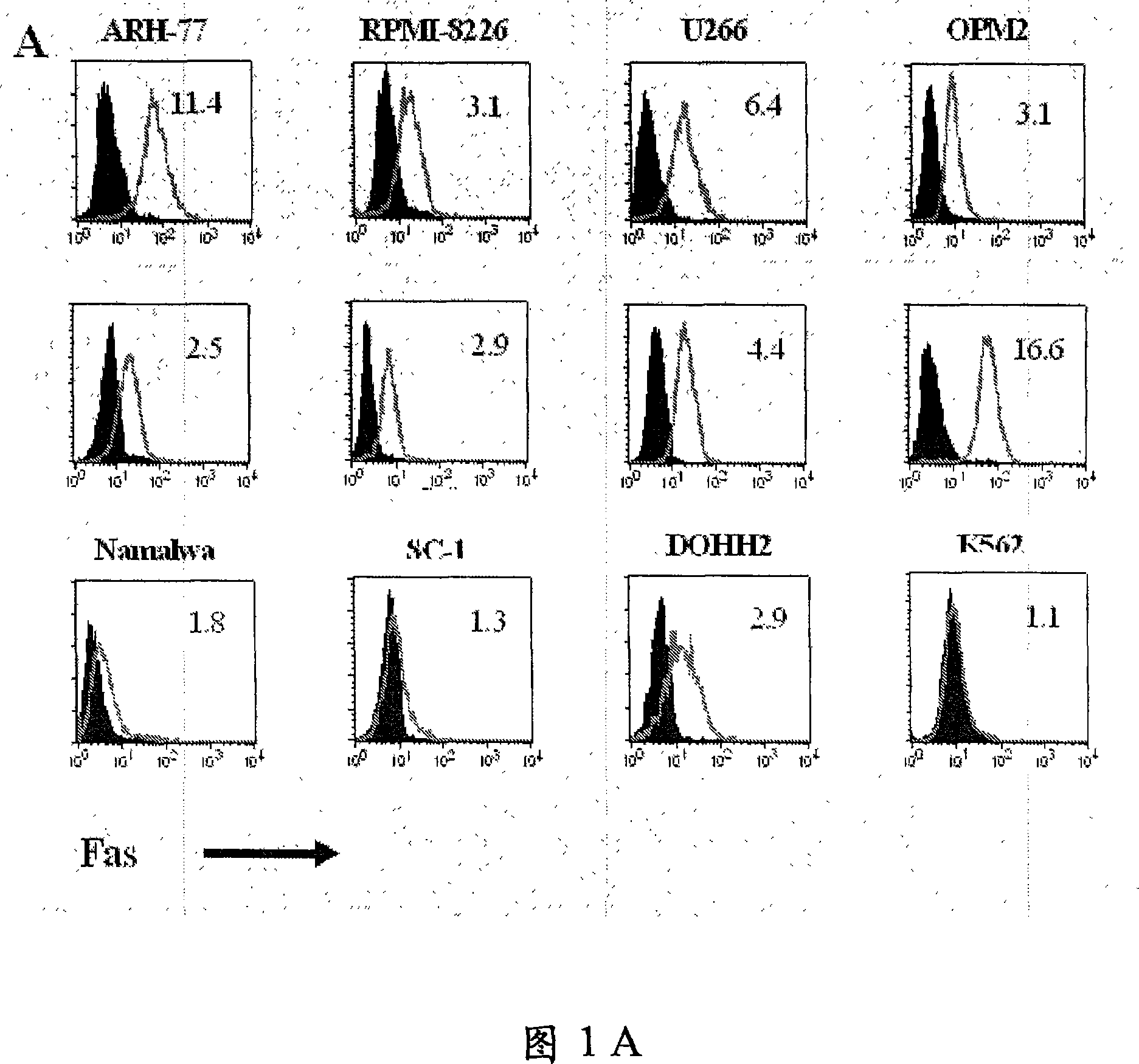
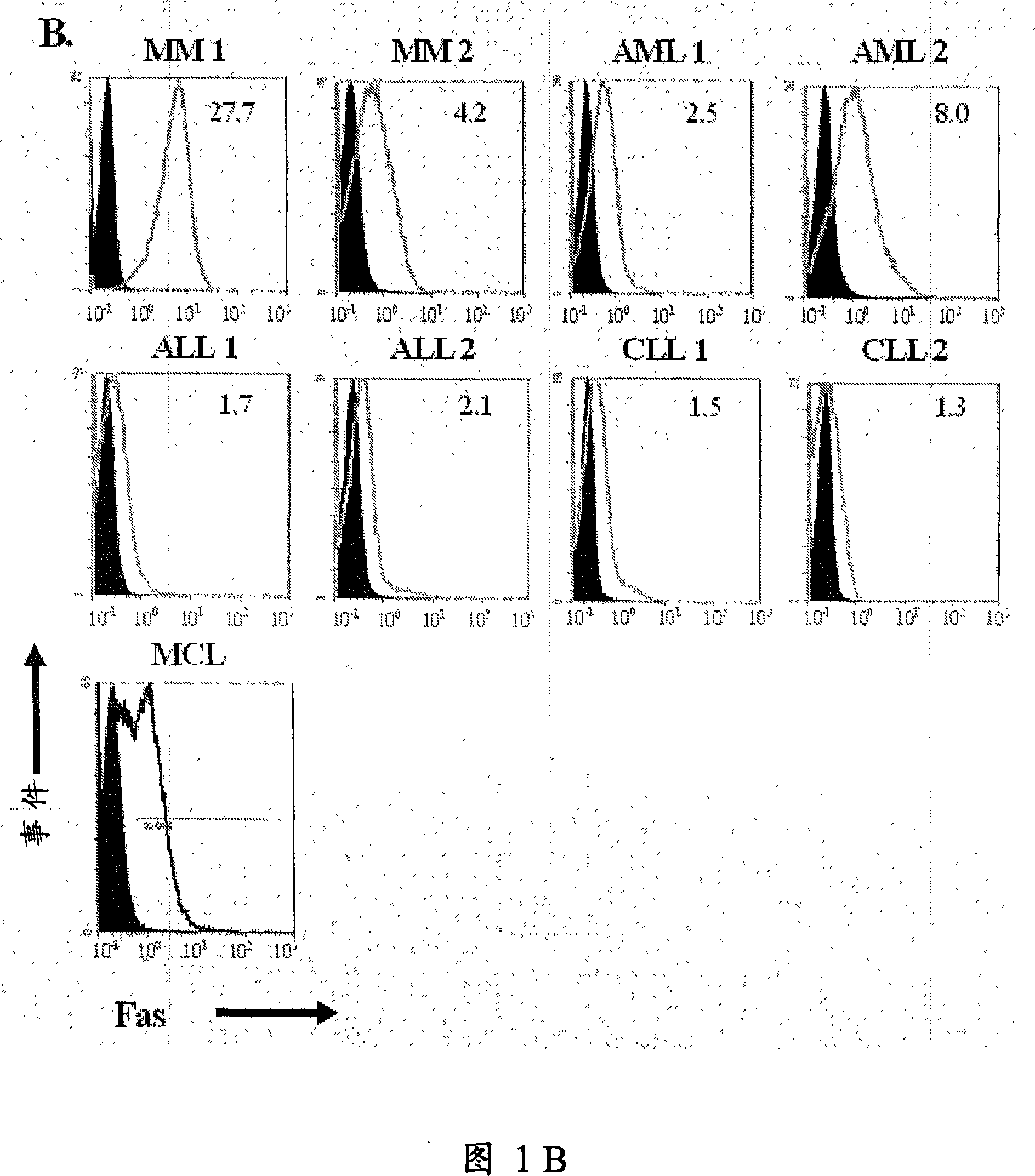
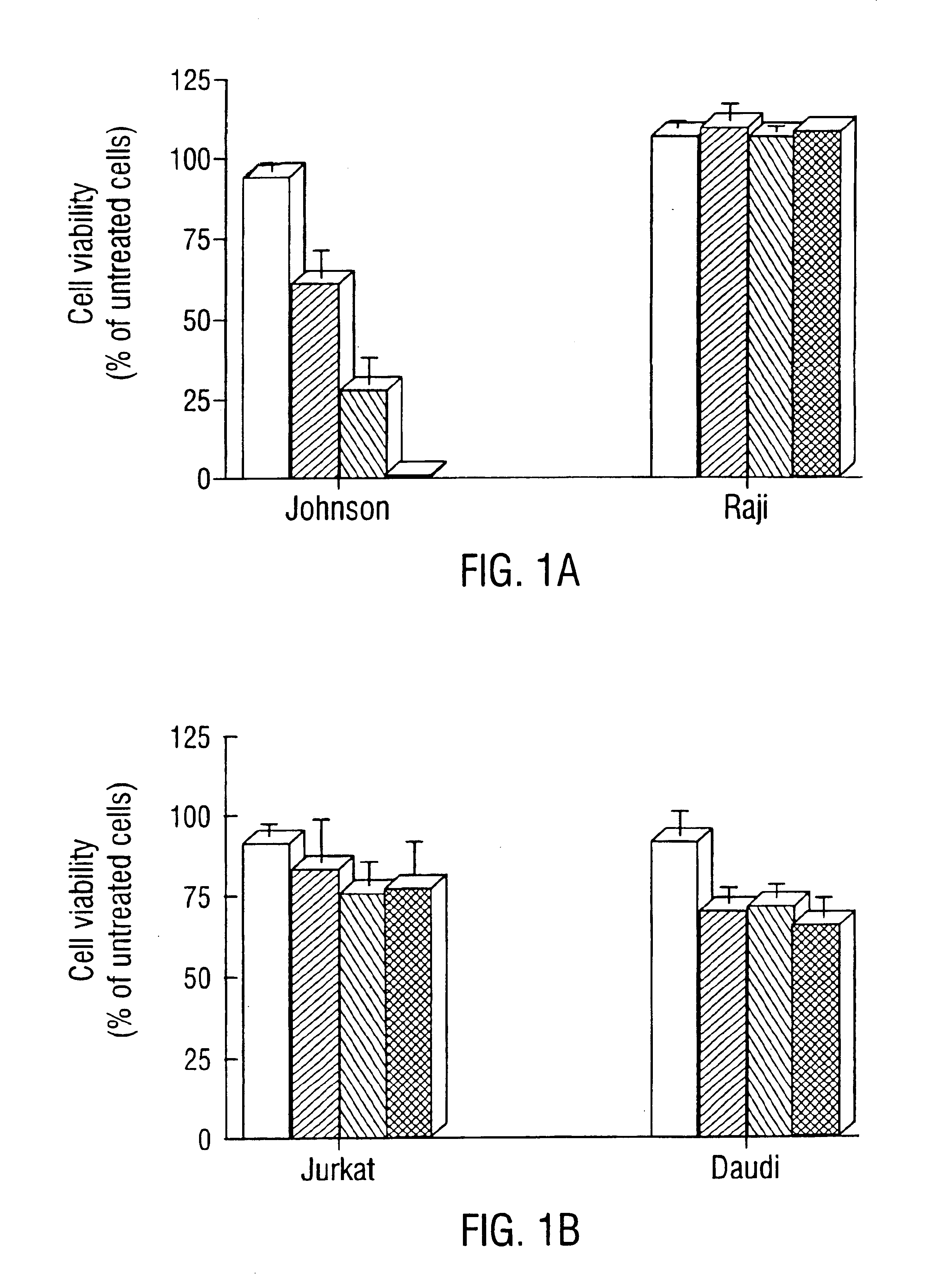
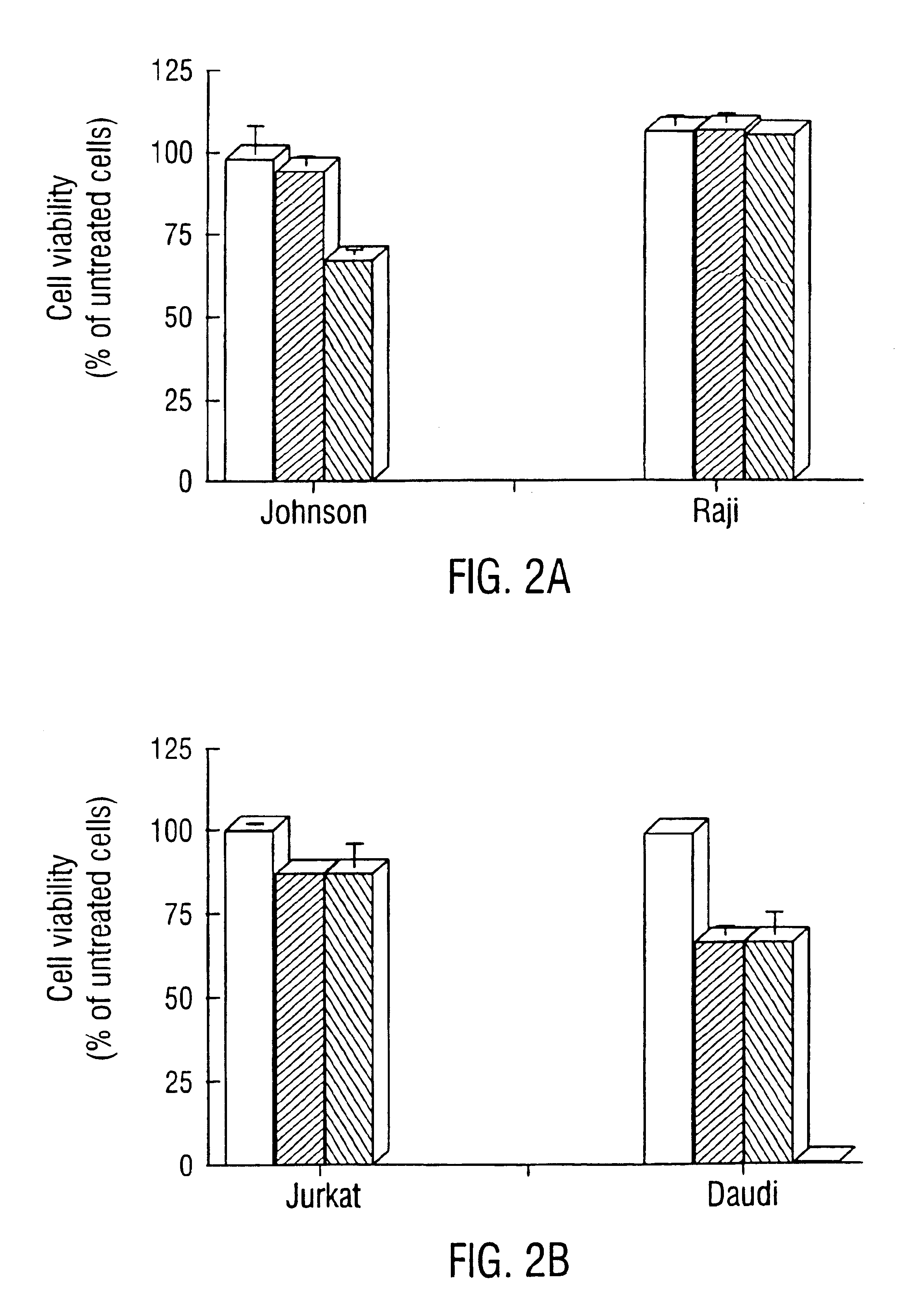
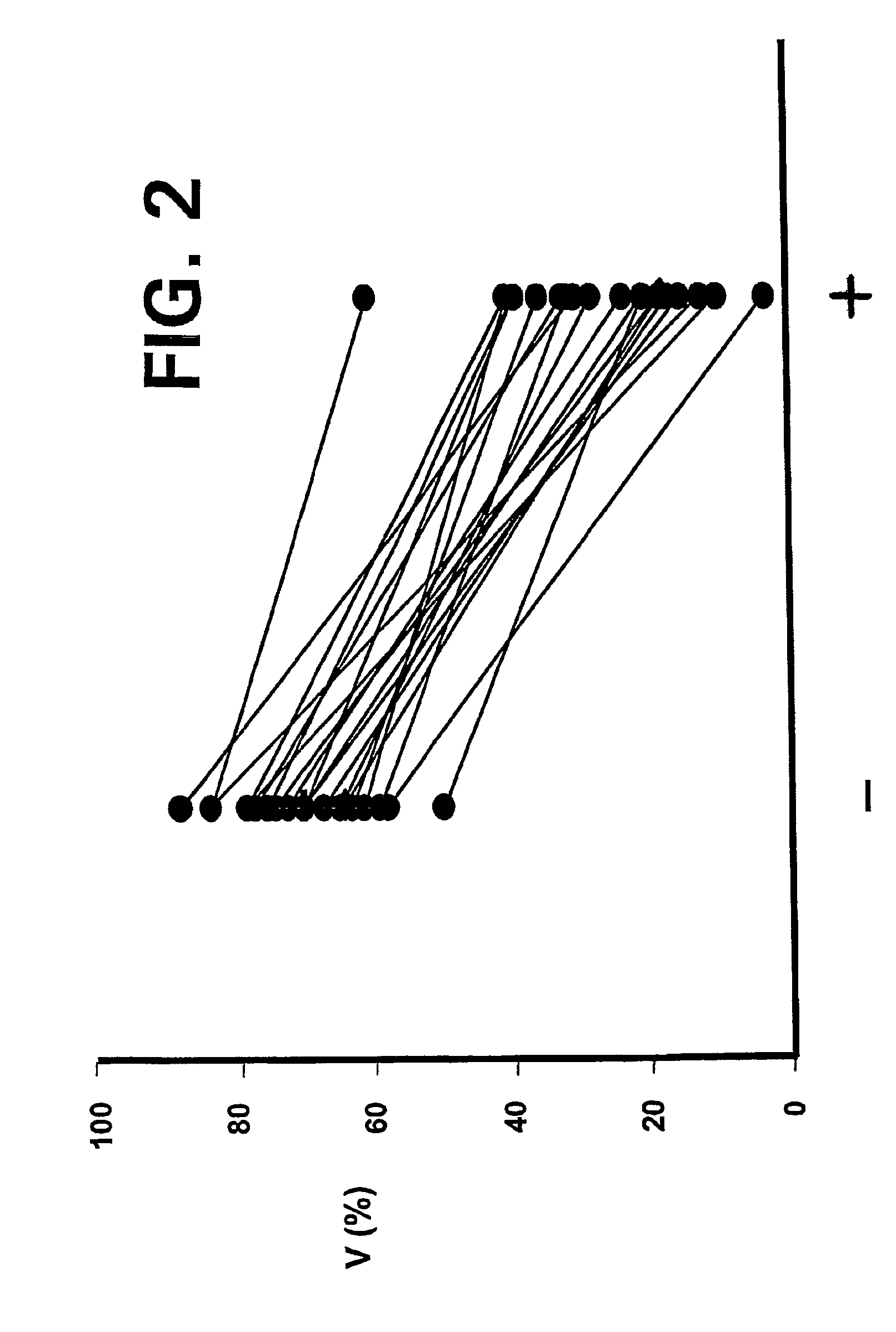
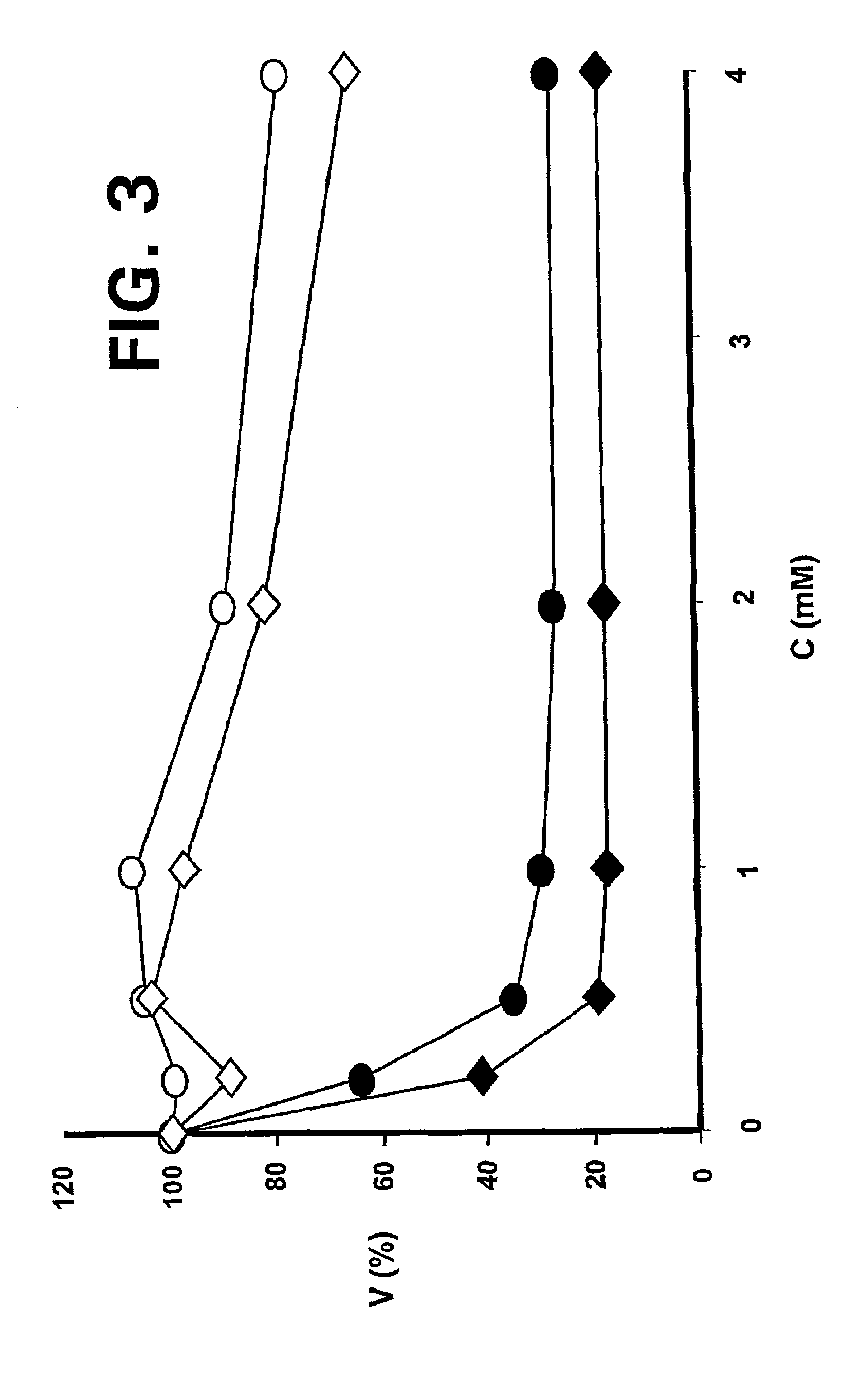
—use of a 2,3-dihydroimidazo[1,2-c]quinazoline compound, or of a pharmaceutical composition containing same, as a sole active agent, or of a combination of a) said compound or a pharmaceutical composition containing said compound and b) one or more further active agents, for the preparation of a medicament for the treatment or prophylaxis of non-Hodgkin's lymphoma (NHL), particularly 1st line, 2nd line, relapsed, refractory, indolent or aggressive non-Hodgkin's lymphoma (NHL), in particular follicular lymphoma (FL), chronic lymphocytic leukaemia (CLL), marginal zone lymphoma (MZL), diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), transformed lymphoma (TL), or peripheral T-cell lymphoma (PTCL); —combinations of a) said compound and b) one or more further active agents; —a pharmaceutical composition comprising said compound as a sole active agent for the treatment of non-Hodgkin's lymphoma (NHL), particularly 1st line, 2nd line, relapsed, refractory, indolent or aggressive non-Hodgkin's lymphoma (NHL), in particular follicular lymphoma (FL), chronic lymphocytic leukaemia (CLL), marginal zone lymphoma (MZL), diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), transformed lymphoma (TL), or peripheral T-cell lymphoma (PTCL); —a pharmaceutical composition comprising a combination of a) said compound and b) one or more further active agents; —use of biomarkers involved in the modification of the expression of PI3K isoforms, BTK and IKK, BCR activation, BCR downstream activation of NFKB pathway, c-Myc, EZH2, for predicting the sensitivity and / or resistance of a cancer patient to said compound and providing a rationale-based synergistic combination as defined herein to increase sensitivity and / or to overcome resistance; and —a method of determining the level of a component of one or more of the expression of PI3K isoforms, BTK and IKK, BCR activation, BCR downstream activation of NFKB pathway, c-Myc, EZH2.
Owner:BAYER PHARMA AG
Cerdulatinib For The Treatment Of B-Cell Malignancies
PendingCN107683139AOrganic active ingredientsAntineoplastic agentsChronic lymphocytic leukemiaFollicular lymphoma grade II
Provided herein are compositions and methods for treating a relapsed or refractory hematologic cancer in a human patient in need thereof. The methods entail administering to the patient a daily dose of about 10 mg to about 75 mg of cerdulatinib or a pharmaceutically acceptable salt thereof, wherein the patients suffer one or more of a B-cell malignancy, chronic lymphocytic leukemia (CLL), follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL) or other transformed FL and / or have relapsed or not responded to a prior chemotherapy.
Owner:PORTOLA PHARMA INC
Combination of pi3k-inhibitors
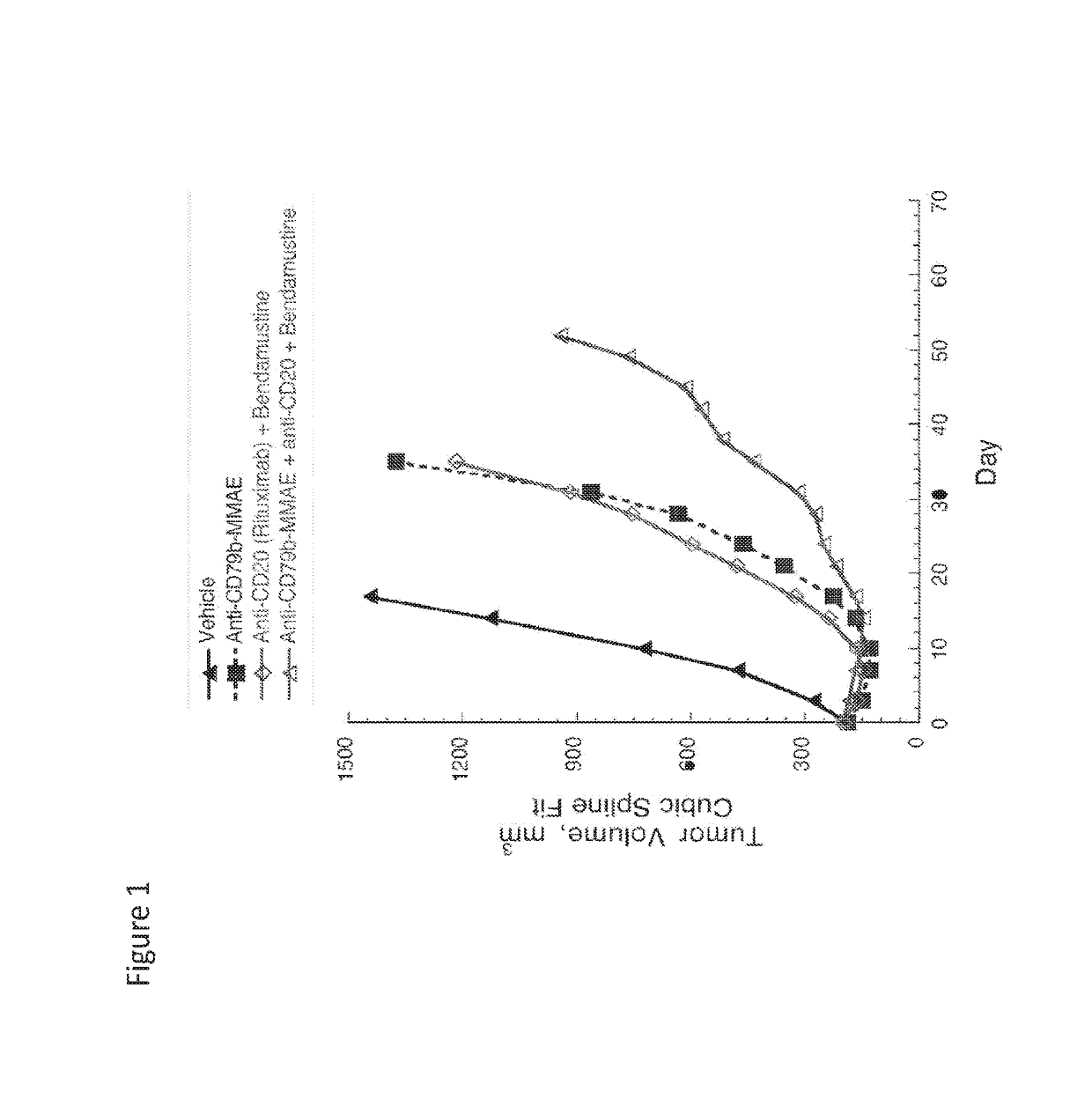
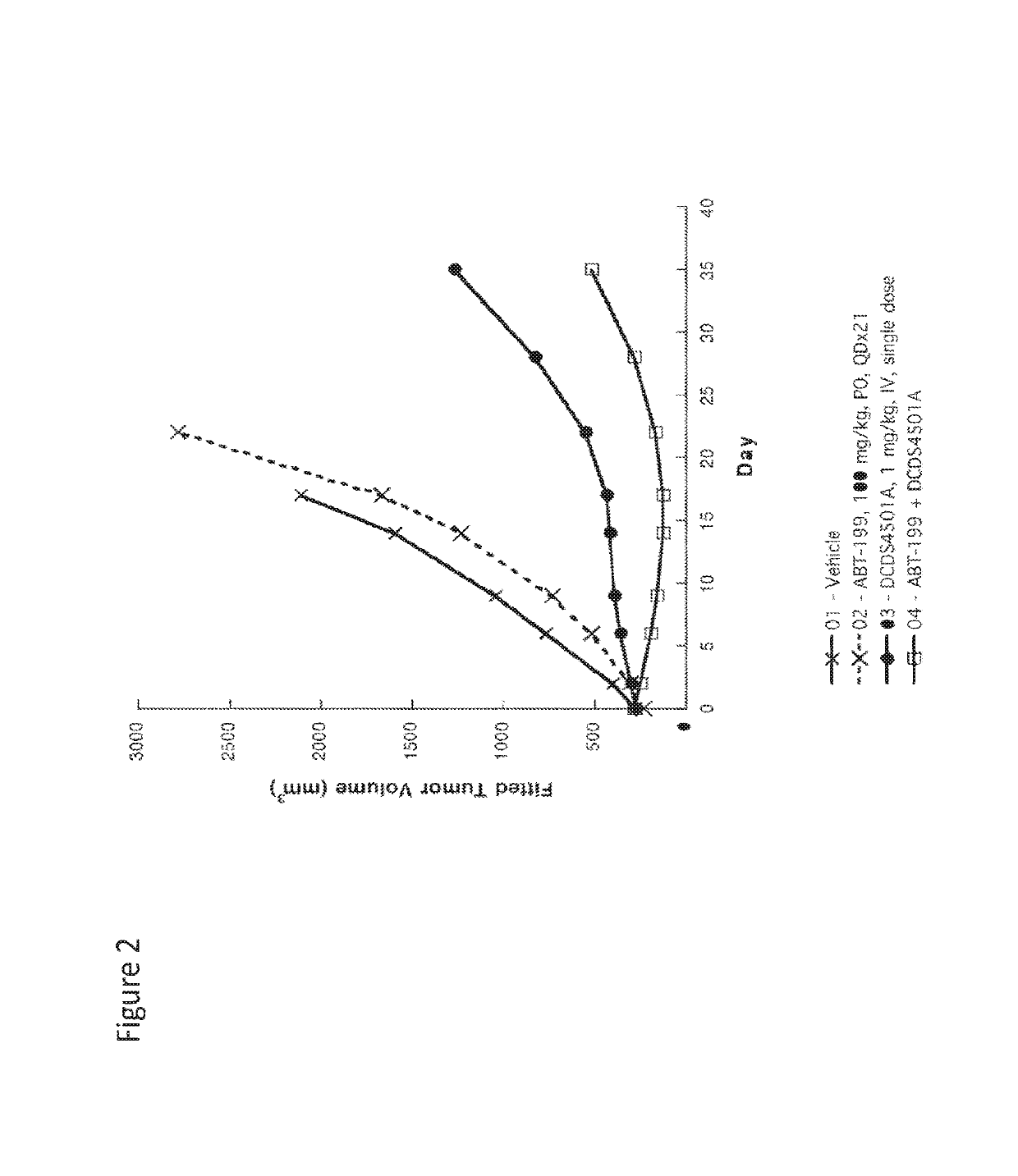
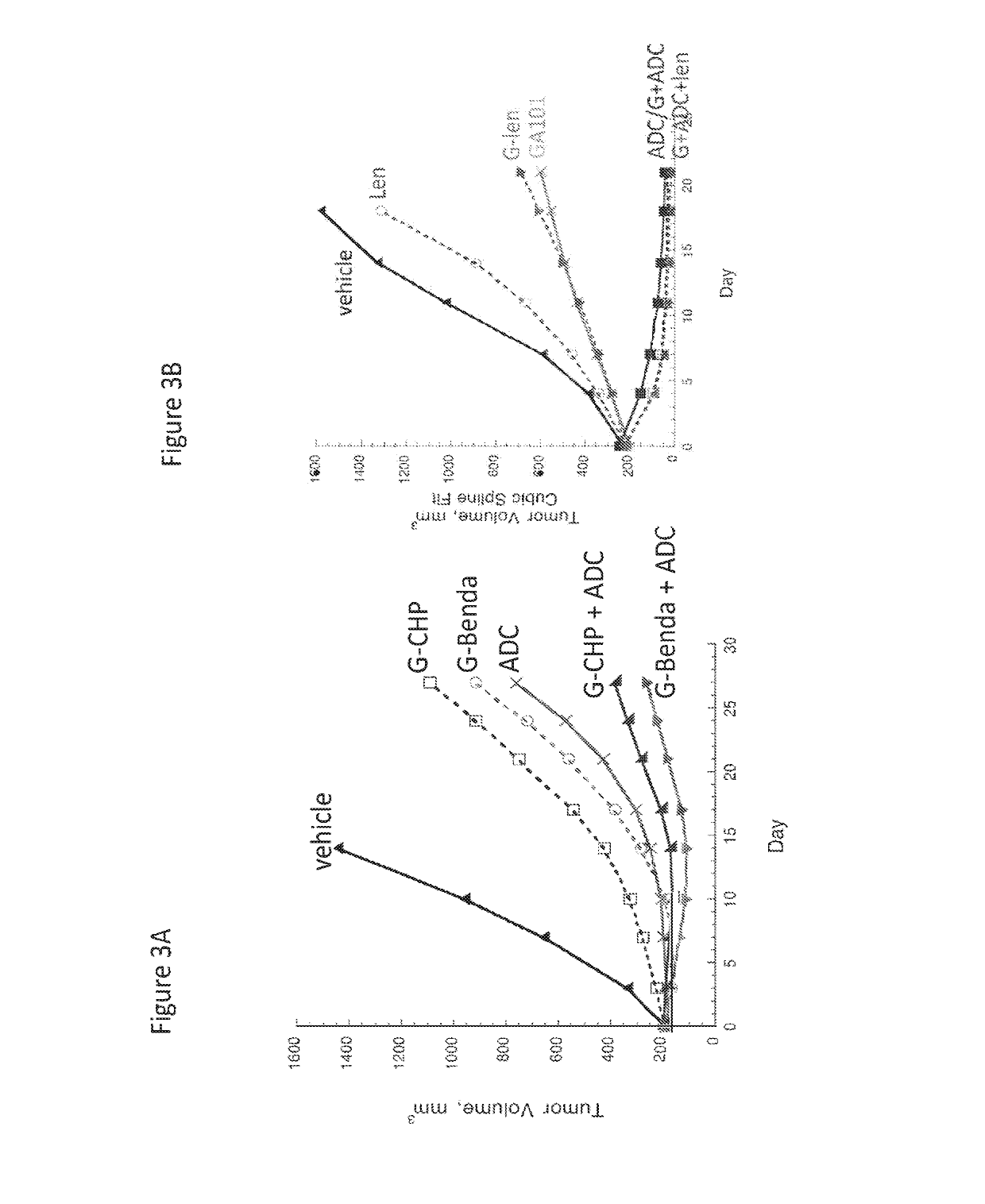
ActiveUS20190255063A1Synergistic effectOrganic active ingredientsOrganic chemistryDiseaseTransformed Lymphoma
The present invention relates to combinations of at least two components, component A and component B, component A being an inhibitor of PI3K kinase, and component B being venetoclax or palbociclib. Another aspect of the present invention relates to the use of such combinations as described herein for the preparation of a medicament for the treatment or prophylaxis of a disease, particurlarly for the treatment or prophylaxis of non-Hodgkin's lymphoma (hereinafter abbreviated to “NHL”), particularly 1st line, 2nd line, relapsed, refractory, indolent or aggressive non-Hodgkin's lymphoma (NHL), in particular follicular lymphoma (hereinafter abbreviated to “FL”), chronic lymphocytic leukaemia (hereinafter abbreviated to “CLL”), marginal zone lymphoma (hereinafter abbreviated to “MZL”), splenic marginal zone lymphoma (hereinafter abbreviated to “SMZL”), diffuse large B-cell lymphoma (hereinafter abbreviated to “DLBCL”), mantle cell lymphoma (MCL), transformed lymphoma (hereinafter abbreviated to “TL”), or peripheral T-cell lymphoma (hereinafter abbreviated to “PTCL”).
Owner:BAYER PHARMA AG
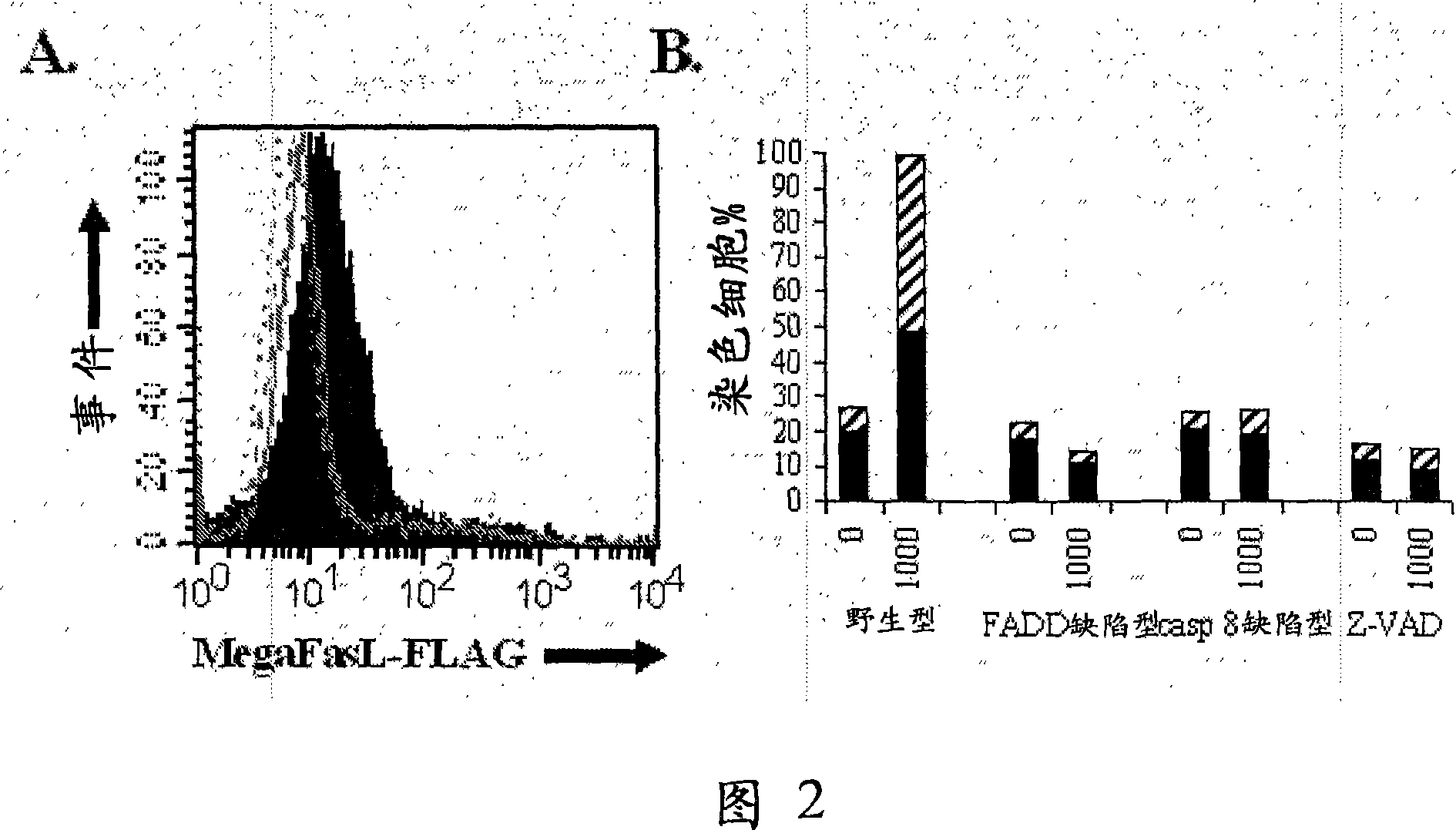
Method for ex-vivo purging in autologous transplantation
The present invention concerns a new method for ex-vivo purging of cells in autologous transplantation, wherein the sample of taken cells is treated with a sufficient amount of a multimeric form of the soluble portion of FasL to kill malignant cells without substantially affecting viability of cells to be transplanted. Autologous stem cell transplantation (ASCT) following high-dose chemotherapy with or without radiotherapy has become the standard therapy for the majority of patients with large-cell lymphomas, multiple myeloma, and refractory / recidivating Hodgkin's disease. Such therapy is nowadays also contemplated for selected patients with low-grade lymphomas (chronic lymphocytic leukemia, follicular lymphoma, mantle cell lymphoma) and for patients with acute myeloid leukemia (AML). Current treatments for cell purging include chemotherapy and antibody cocktails. These treatments are often toxic on stem cells and not efficient in eliminating cancer cells. Thus, there is an unmet medical need for cell purging in ASCT which this project will address.
Owner:APOXIS
METHODS OF USING ANTI-CD79b IMMUNOCONJUGATES
ActiveUS20190201382A1Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAntiendomysial antibodies
Provided herein are methods of treating B-cell proliferative disorders in particular Follicular Lymphoma and / or Diffuse Large B-Cell Lymphoma using immunoconjugates comprising anti-CD79b antibodies in combination with additional therapeutic agents.
Owner:GENENTECH INC
Compositions Containing Ibrutinib
InactiveUS20170252344A1Organic active ingredientsInorganic non-active ingredientsWaldenstrom macroglobulinemiaMetabolite
Discussed herein are pharmaceutical compositions containing Ibrutinib and processes for preparing them. The compositions may be utilized in the treatment of a variety of conditions including, without limitation, B-cell proliferative disorders such as non-Hodgkin lymphoma (diffuse large B cell lymphoma, follicular lymphoma, mantle cell lymphoma or burkitt lymphoma), Waldenstrom macroglobulinemia, plasma cell myeloma, chronic lymphocytic leukemia, lymphoma, or leukemia. These compositions are designed for oral ingestion. The compositions are contained within a capsule such as a standard or sprinkle or in a liquid formulation such as a suspension. In one embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, croscarmellose sodium, sodium lauryl sulfate, and magnesium stearate. In another embodiment, the pharmaceutical composition contains Ibrutinib, a salt, prodrug, or metabolite thereof, microcrystalline cellulose, carboxymethylcellulose sodium, hydroxypropylmethylcellulose, citric acid monohydrate, disodium hydrogen phosphate, sucralose, sodium methyl parahydroxybenzoate, sodium ethyl parahydroxybenzoate, concentrated hydrochloric acid, sodium hydroxide, and water.
Owner:JANSSEN PHARMA NV
Treatment of follicular lymphomas using inhibitors of the lymphotoxin (LT) pathway
The application of lymphotoxin pathway inhibitors in the treatment of tumors, especially follicular lymphoma.
Owner:BIOGEN IDEC MA INC +1
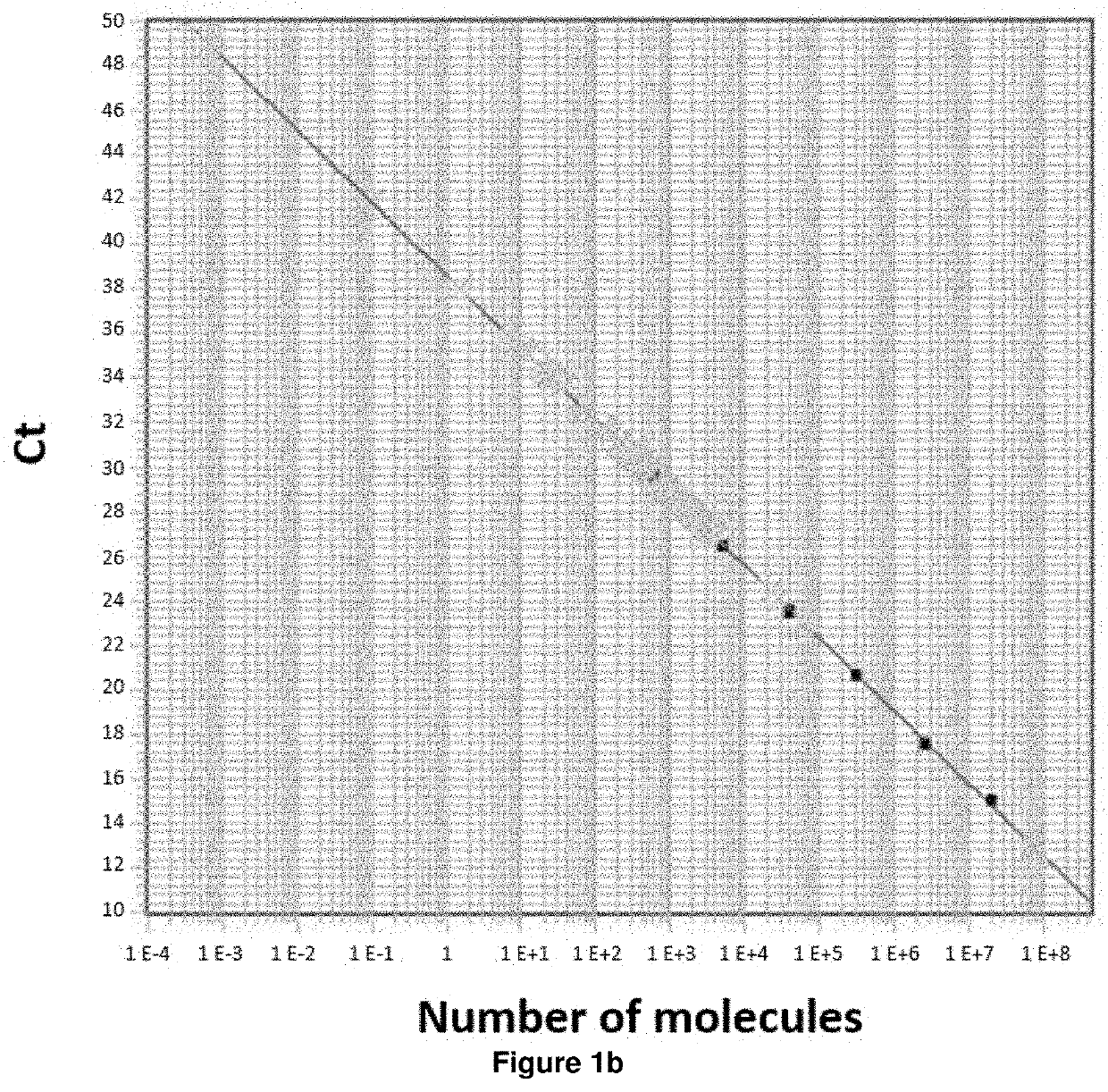
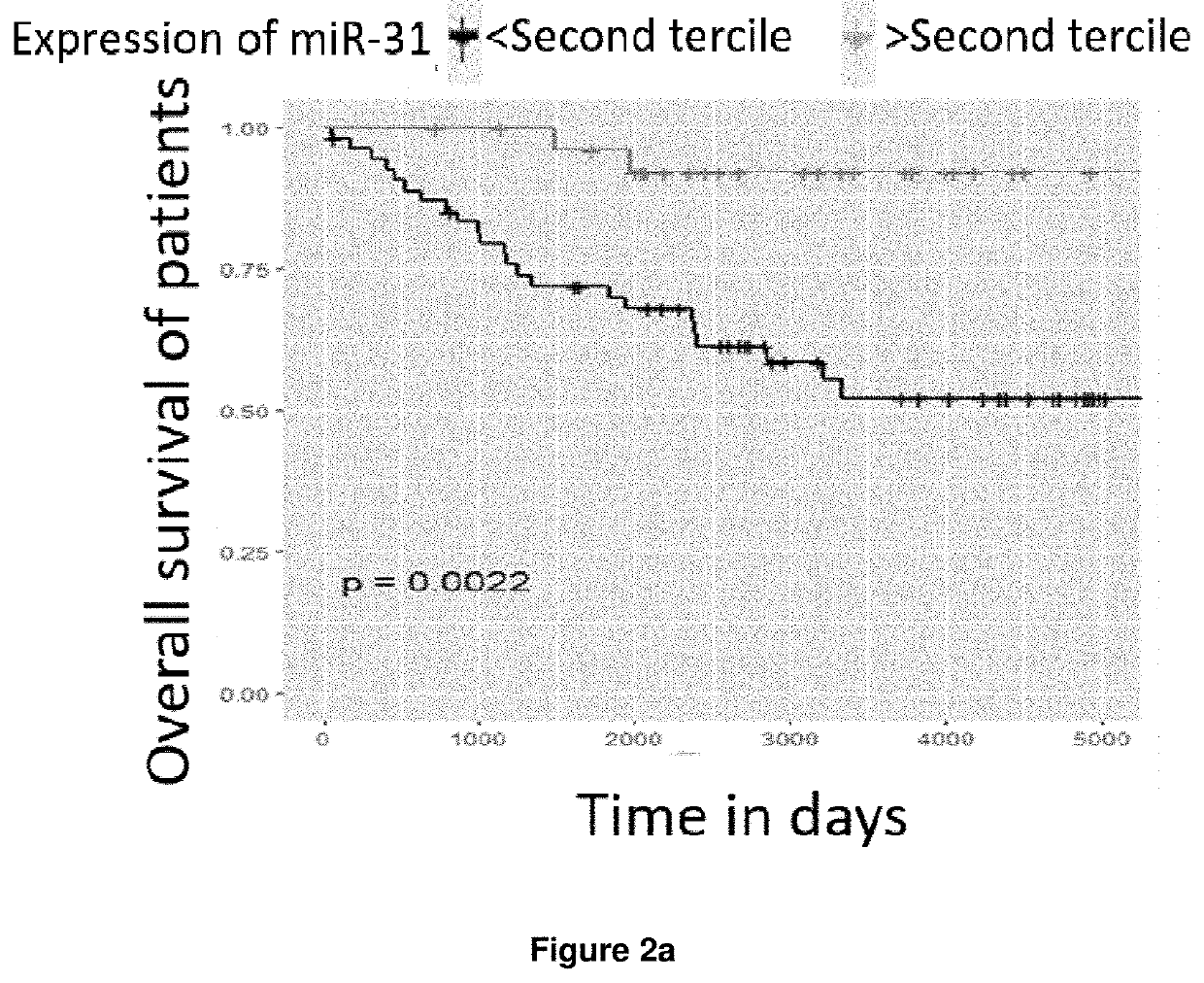
Method of determining prognosis in patients with follicular lymphoma
PendingUS20210388448A1Shorten survival timeEasy to getMicrobiological testing/measurementGenetic engineeringFollicular lymphoma grade IIOncology
A method is disclosed for determining prognosis for a patient suffering from follicular lymphoma, that determines the amount of miR-31 in a biological sample taken from the body of the patient, and assigns the patient to a prognostic group based on the determined amount of miR-31, wherein the prognostic groups and the threshold values for assignment to the prognostic groups are obtained by analyzing the amount of miR-31 in biological samples of patients with known prognosis. In the biological sample taken from the patient, the miR-31 expression may be determined along with the expression of an endogenous control, which is a small nuclear RNA or stably expressed miRNA, and, in case of absolute quantification, the expression of synthetic standards of these miRNAs and endogenous controls of known number of molecules are determined. The method allows the assignment of patients to prognostic groups by determining miR-31 expression.
Owner:MASARYK UNIVERSITY +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
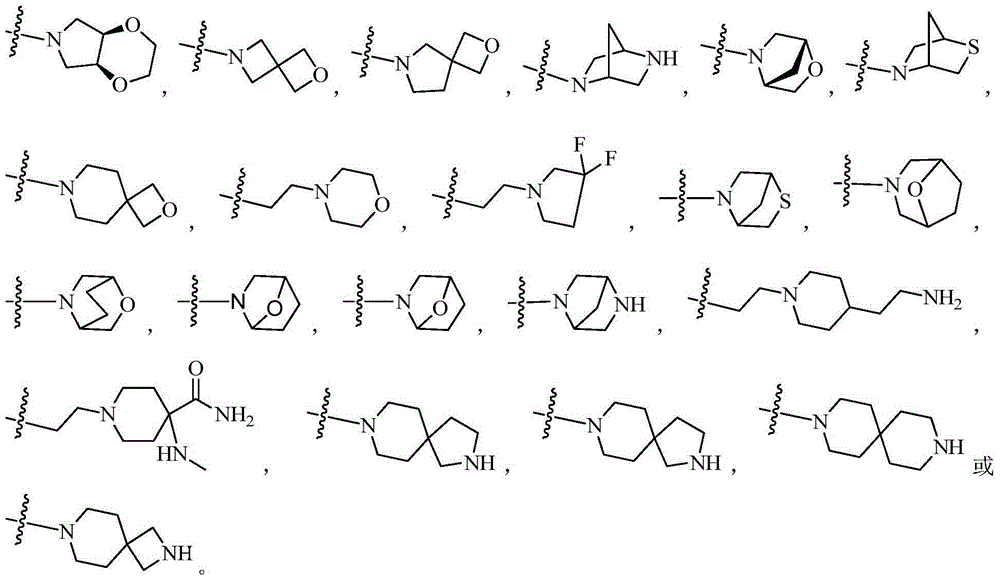
![Use of substituted 2,3-dihydroimidazo[1,2-c]quinazolines for treating lymphomas Use of substituted 2,3-dihydroimidazo[1,2-c]quinazolines for treating lymphomas](https://images-eureka.patsnap.com/patent_img/03834f4c-ee55-4bc5-8cc4-647e11bfeed1/US20160058770A1-20160303-D00001.PNG)
![Use of substituted 2,3-dihydroimidazo[1,2-c]quinazolines for treating lymphomas Use of substituted 2,3-dihydroimidazo[1,2-c]quinazolines for treating lymphomas](https://images-eureka.patsnap.com/patent_img/03834f4c-ee55-4bc5-8cc4-647e11bfeed1/US20160058770A1-20160303-D00002.PNG)
![Use of substituted 2,3-dihydroimidazo[1,2-c]quinazolines for treating lymphomas Use of substituted 2,3-dihydroimidazo[1,2-c]quinazolines for treating lymphomas](https://images-eureka.patsnap.com/patent_img/03834f4c-ee55-4bc5-8cc4-647e11bfeed1/US20160058770A1-20160303-D00003.PNG)