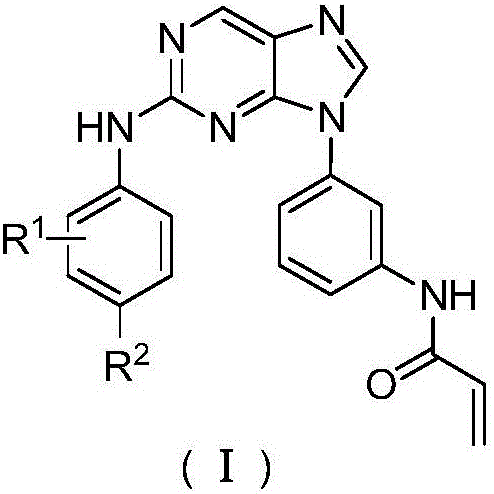
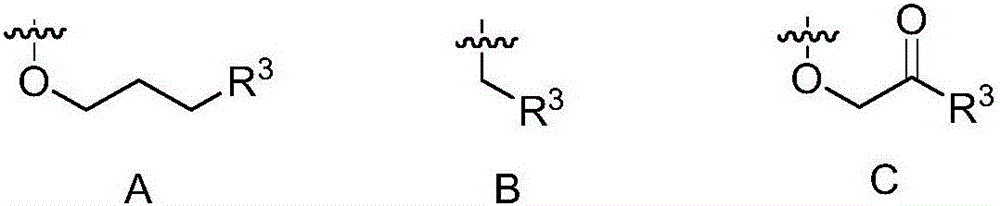
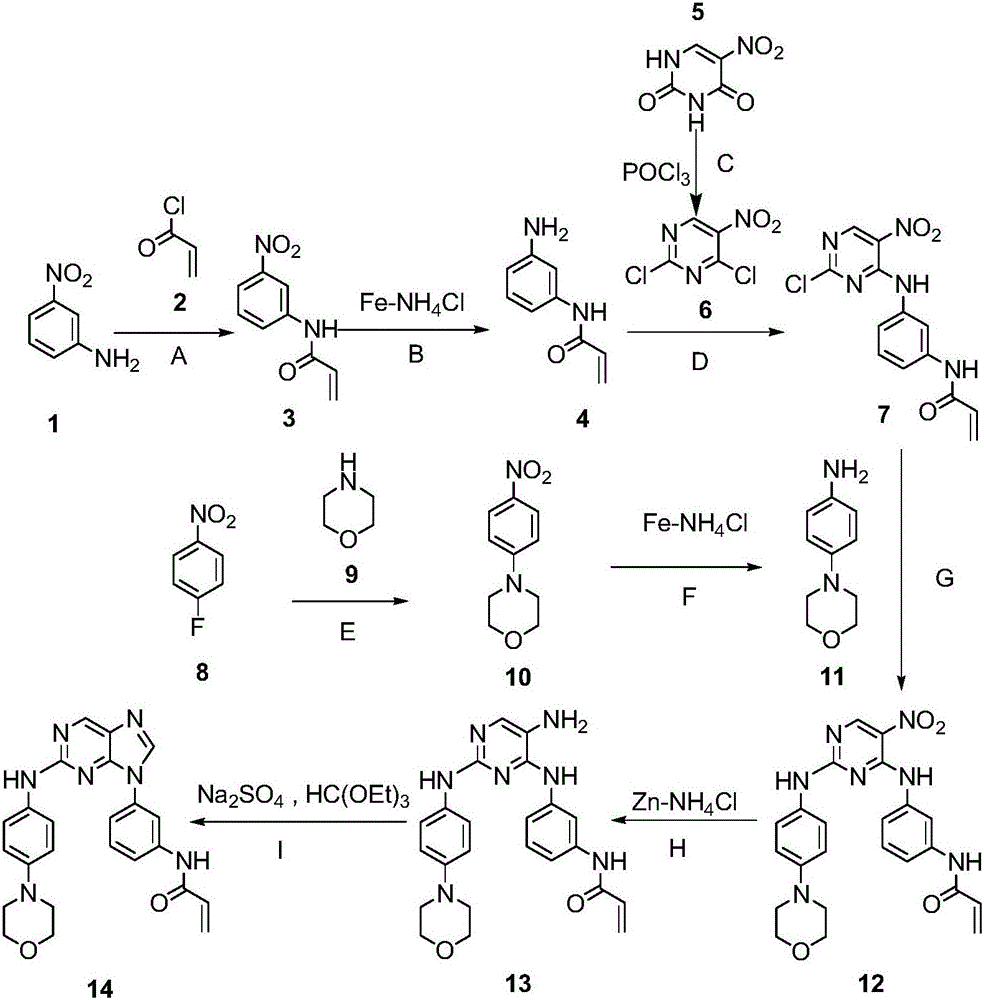
Purine compounds, composition and application
A technology of compounds and purines, applied in the field of medicine, can solve problems such as poor drug resistance and achieve good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The synthetic method of such 2-phenylaminopurine compounds is illustrated by N-(9-(2-phenylamino)purine-2-(4-morpholinephenylamino))acrylamide.
[0065] The reaction path is as follows:
[0066]
[0067] Step A:
[0068] Take 1 (13.81g, 0.1mol) and sodium bicarbonate (12.6g, 0.15mol) in 30ml of acetonitrile, slowly add acryloyl chloride (9.5g, 0.105mol) dropwise under stirring, and react at room temperature for 5 minutes after the dropwise addition is completed. After the reaction was completed, 400ml of water was added, and a white solid was precipitated, which was suction filtered to obtain 18g of the product. (Yield: 93.8%)
[0069] Step B:
[0070] Take 3 (6g, 31.23mmol) and ammonium chloride (3.3g, 62.46mmol) in a reaction flask, add 20ml of MeOH and 20ml of water, add iron powder (7g, 125mmol) under stirring, raise the temperature to 60°C for 2 hours, and draw while hot Filter, wash the filter residue with ethyl acetate, extract the filtrate with ethyl acet...
Embodiment 2
[0086] The synthetic method of this kind of compound is illustrated with N-[9-(2-phenylamino)purine-2-[[4-(1-morpholine)propoxy]phenylamino]]acrylamide.
[0087] The reaction path is as follows:
[0088]
[0089] The title compound was prepared according to the schemes, procedures and intermediates described below.
[0090]
[0091] Steps A-D are the same as the intermediate preparation steps in the Example 1 scheme.
[0092] Step E:
[0093] Dissolve 8 (10g, 72mmol) in 30ml of acetonitrile, add potassium carbonate (14.9g, 108mmol) and potassium iodide (1.2g, 7.2mmol), slowly add 1-bromo-3-chloropropane (13.6g, 86mmol) dropwise, Raise the temperature to 70°C and react for 2 hours. After the reaction, the solvent was drained and 400ml of water was added to precipitate a yellow-white solid, which was filtered by suction to obtain 14g of solid. (yield: 90%)
[0094] Step F:
[0095] Dissolve 10 (4g, 18.56mmol) in 20ml of DMF, add potassium carbonate (3.8g, 27.84mmol) a...
Embodiment 3
[0098]The synthesis of this class is illustrated with N-[9-(2-phenylamino)purine-2-[[4-[(1-morpholine)acetyl]phenoxy]amino]]acrylamide.
[0099] The reaction path is as follows:
[0100]
[0101] The title compound was prepared according to the schemes, procedures and intermediates described below.
[0102]
[0103] Steps A-D are the same as the intermediate preparation steps in the Example 1 scheme.
[0104] Step E:
[0105] Dissolve 8 (20g, 143.88mmol) in 150ml of acetonitrile, add potassium carbonate (29g, 215.83mmol), potassium iodide (2.4g, 14.39mmol), slowly add ethyl bromoacetate (24g, 143.88mmol) dropwise, and heat up to 70°C The reaction was carried out for 2 hours. After the reaction was completed, the solvent was sucked dry, washed with water and filtered to obtain 27 g of a yellow-white solid. (yield: 85%)
[0106] Step F:
[0107] Take 10 (6g, 26.67mmol) in 50ml of water, slowly add potassium hydroxide (3g, 53.3mmol), heat up to 50°C for 1 hour, cool to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com