Cerdulatinib For The Treatment Of B-Cell Malignancies
A sedutinib, refractory technology, applied in the field of sedutinib for the treatment of B-cell malignant tumors, can solve the problem of not being curable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Example 1: Preclinical Models, Clinical PK / PD and Tumor Response
[0117] Materials and methods
[0118] Purified Kinase Assay: Performed at Millipore at a fixed concentration of 30OnM ATP. IC (Inhibitory Concentration) was determined using a 10-point concentration curve.
[0119] Human whole blood assay: Inhibition of SYK and JAK dependent and independent signaling was determined by stimulating human whole blood with various agonists against BCR and cytokine receptors. Use an 8-point concentration curve to determine the IC 50 , 100 μL aliquots of heparinized healthy human whole blood were stimulated as previously described (Coffey et al., J. of Pharm. and Experimental Therapeutics, 351:538-548, 2014). Signaling and activation responses were determined by fluorescence activated cell sorting ("FACS"). For clinical trials, whole blood samples were collected before and after dosing. Percent inhibition was calculated by normalizing to pre-dose stimulus response.
[01...
Embodiment 2
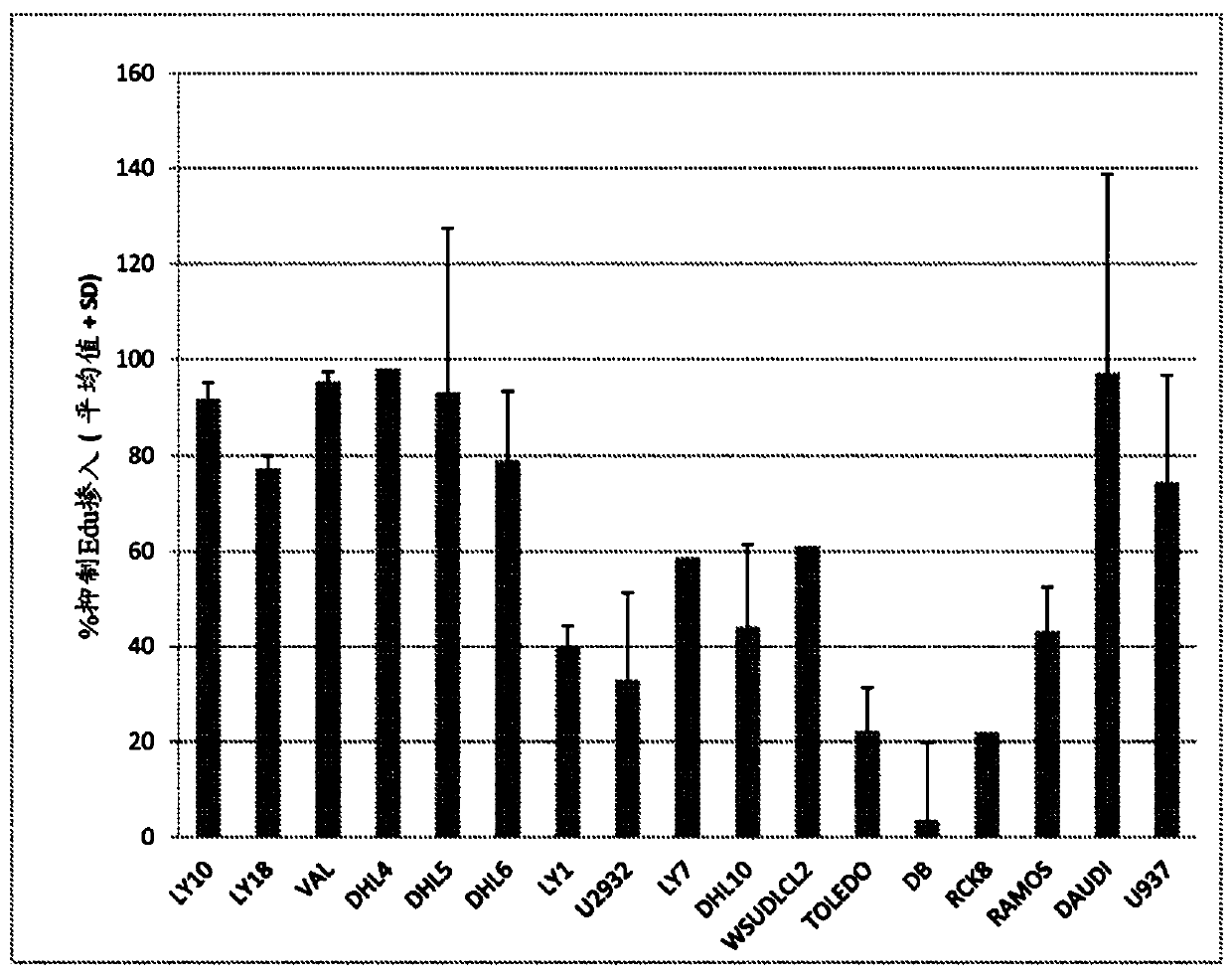
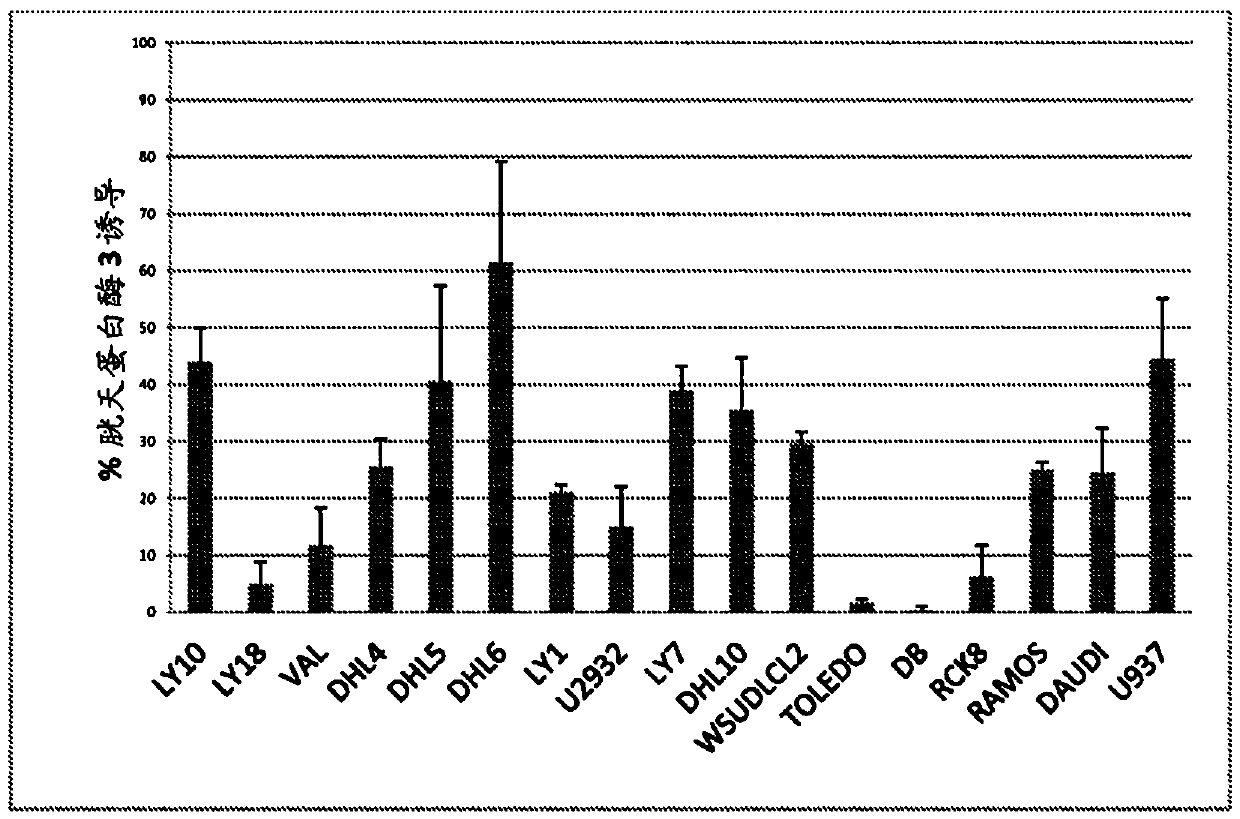
[0149] Example 2: Effect of Sadutinib on Primary Human CLL Cells
[0150] Sadutinib in 24 primary CLL samples
[0151]CLL cell isolation and culture: CLL cells (available from ATCC) were purified using the Human B Cell Enrichment Mixture Kit (Stemcell Technologies, Vancouver, BC, Canada), and stained with anti-CD5 / CD19 for verification of purity, which was present in all More than 95% of the cases. 1x10 in the presence or absence of 2.5 mg / mL CpG, 100 ng / mL CD40L, 10 ng / mL IL-4 7 Isolated CLL cells were cultured in RPMI-1640 with 15% fetal bovine serum (Gibco, Grand Island, NY, USA), penicillin (100 IU), and streptomycin ( 100 μg / mL). Anti-IgM stimulation was performed with plate-bound anti-IgM (10 μg / mL). CLL cells were stimulated with 10 ng / mL IL-6 (R&D Systems, Minneapolis, MN) to detect phosphorylation of JAK1 / JAK2 (Cell Signaling Technology, Danvers, MA) and STAT3 (Cell Signaling Technology, Danvers, MA, USA).
Embodiment 3
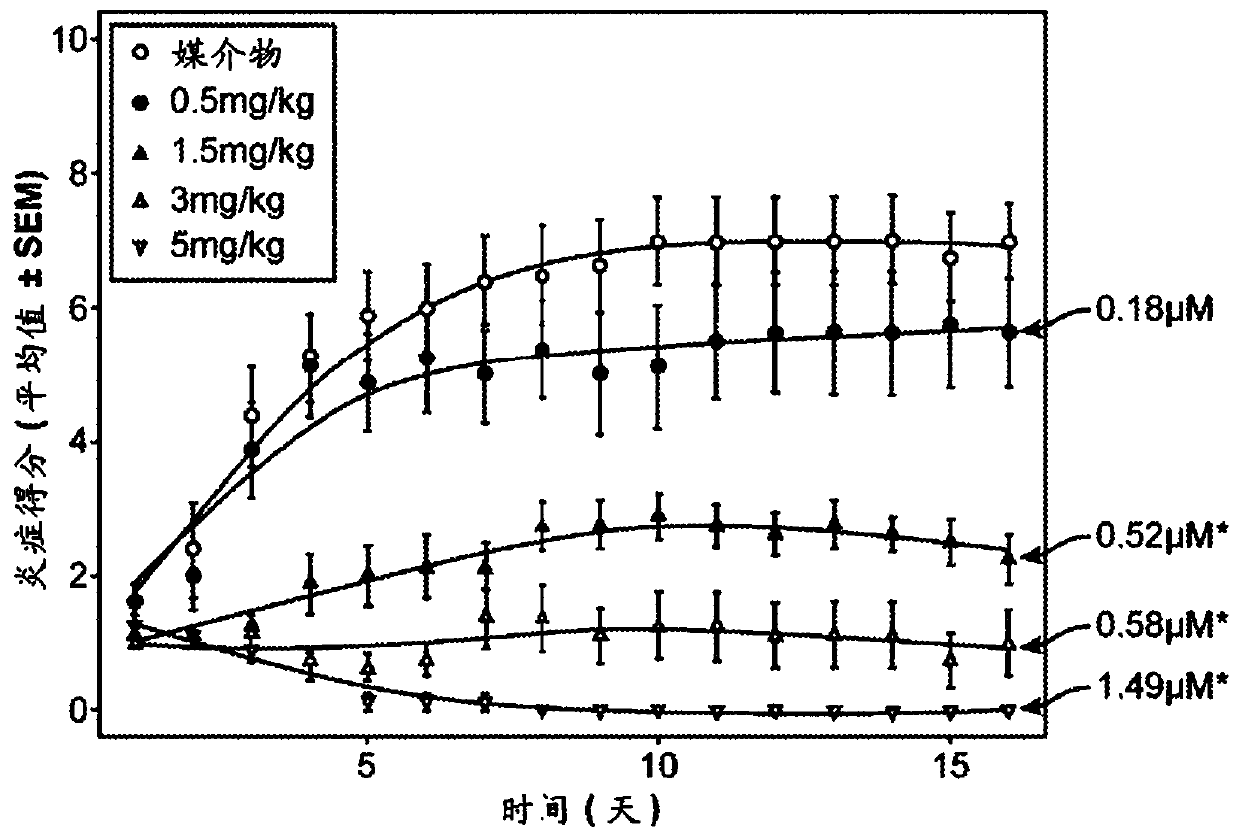
[0166] Example 3: Clinical and Relevant Results of a Phase I Study of Sarutinib
[0167] A first-in-human study of cerdutinib in patients with relapsed / refractory CLL / SLL or B-cell non-Hodgkin's lymphoma (NHL). A 3+3 dose-escalation study with a 28-day cycle was conducted; the doses studied ranged from 15 mg to 65 mg once daily and up to 45 mg twice daily. Patients received a single dose on Day 1 for a 72-hour PK assessment. Continuous dosing was initiated on day 4. Dosed in 43 patients with CLL / SLL or B-cell NHL. The median age was 67 years (range 23-85), and the median prior therapy (tx) was 3 (range 1-8).
[0168] Pharmacokinetics ("PK"), pharmacodynamics ("PD"), and safety were monitored. Responses were assessed by standard norms. Inhibition levels of SYK and JAK were determined using various whole blood assays measuring signaling through receptors for B cell antigens, IL2, IL4, IL6, and GM-CSF. Serum markers of tumor burden including CCL3, CCL4, and other inflammato...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com