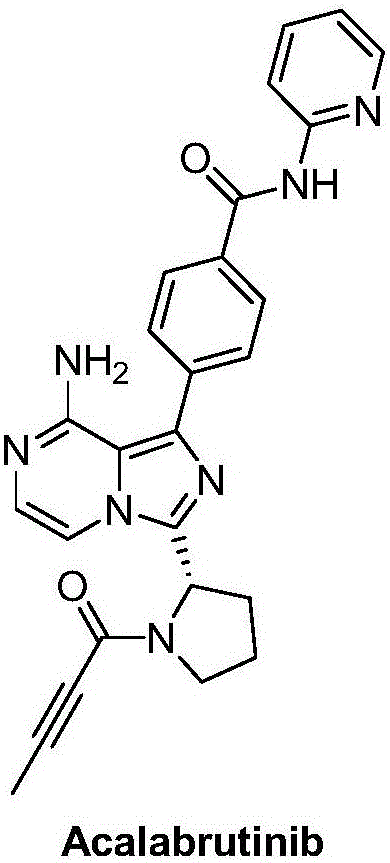
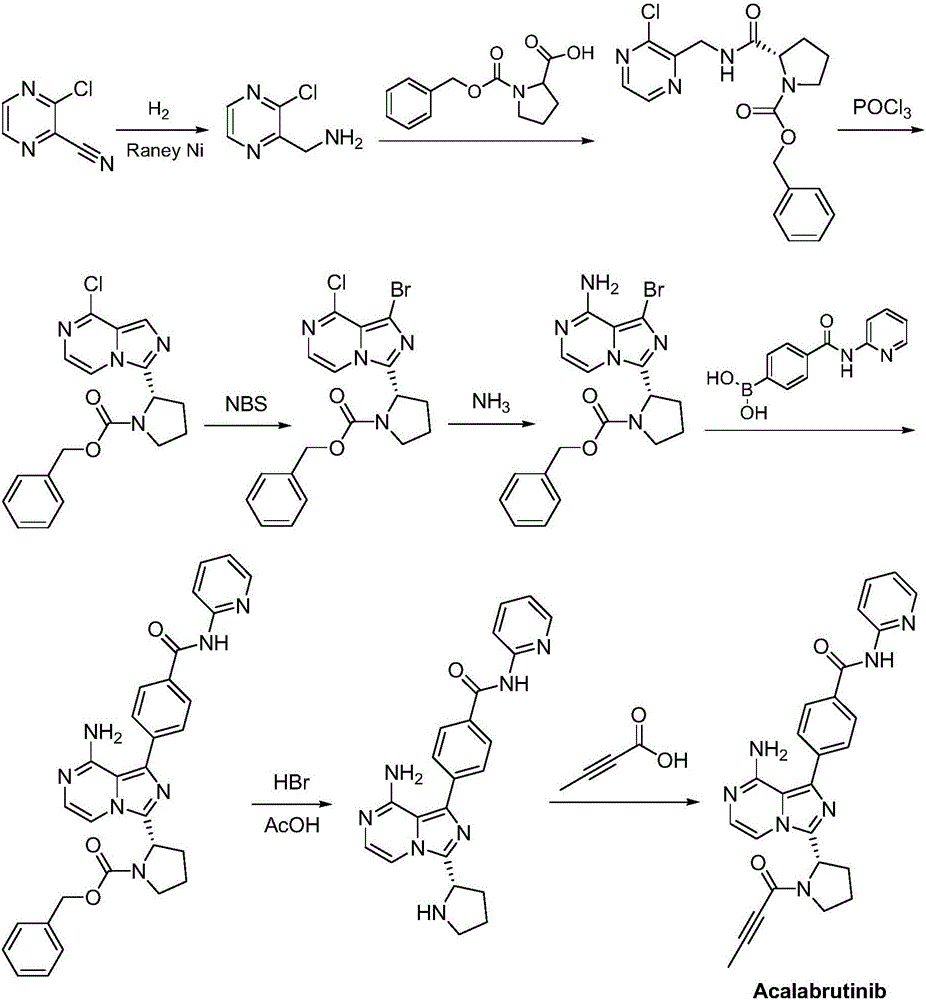
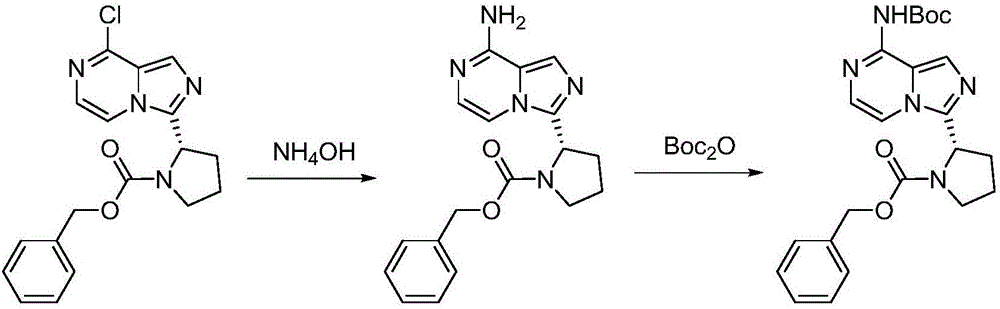
Synthesis method of BTK inhibitor Acalabrutinib for treating chronic lymphocytic leukemia
A technology of lymphocytes and synthetic methods, applied in the field of medicinal chemical synthesis, can solve problems such as complex and cumbersome operations, unfavorable scale-up production and industrial promotion, and many impurities, and achieve the effects of simplified process flow, simplified operation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of benzyl (S)-2-(8-aminoimidazo[1,5-a]pyrazin-3-yl)-1-pyrrolidinecarboxylate:
[0031] (S)-Benzyl 2-(8-chloroimidazo[1,5-a]pyrazin-3-yl)-1-pyrrolidinecarboxylate (10 g, 28 mmol) was dissolved in N-methylpyrrolidone (80 mL), Add ammonia water (168mmol) with a concentration of 28% by mass, and place the reaction mixture in a closed stainless steel reactor at 85°C and 2.5 atmospheric pressure to react for 6 hours. After the reaction is completed, cool to 40°C and release the pressure of the system, and slowly add water (50mL), cooled to 10°C, crystallized for 3h, filtered, and recrystallized from isopropanol to obtain (S)-2-(8-aminoimidazo[1,5-a]pyrazin-3-yl)-1 -benzyl pyrrolidinecarboxylate, off-white solid (8.5g), yield 90%, the reaction formula of this step is as follows:
[0032]
[0033] (2) Preparation of (S)-2-(8-tert-butoxycarbonylaminoimidazo[1,5-a]pyrazin-3-yl)-1-pyrrolidinecarboxylic acid benzyl ester:
[0034] (S)-2-(8-Aminoimidazo[1,5-a]py...
Embodiment 2
[0052] (1) Preparation of benzyl (S)-2-(8-aminoimidazo[1,5-a]pyrazin-3-yl)-1-pyrrolidinecarboxylate:
[0053] (S)-Benzyl 2-(8-chloroimidazo[1,5-a]pyrazin-3-yl)-1-pyrrolidinecarboxylate (15 g, 42 mmol) was dissolved in N-methylpyrrolidone (75 mL), Add ammonia water (273mmol) with a concentration of 28% by mass, and place the reaction mixture in a closed stainless steel reactor at 70°C and a pressure of 3 atmospheres to react for 8 hours. After the reaction is completed, cool to 40°C and release the pressure of the system, and slowly add water (50mL), cooled to 10°C, crystallized for 3h, filtered, and recrystallized from isopropanol to obtain (S)-2-(8-aminoimidazo[1,5-a]pyrazin-3-yl)-1 -benzyl pyrrolidinecarboxylate, off-white solid (12.9g), yield 91%, the reaction formula of this step is the same as that of Example 1.
[0054] (2) Preparation of (S)-2-(8-tert-butoxycarbonylaminoimidazo[1,5-a]pyrazin-3-yl)-1-pyrrolidinecarboxylic acid benzyl ester:
[0055] (S)-Benzyl 2-(8-ami...
Embodiment 3
[0067] (1) Preparation of benzyl (S)-2-(8-aminoimidazo[1,5-a]pyrazin-3-yl)-1-pyrrolidinecarboxylate:
[0068] (S)-Benzyl 2-(8-chloroimidazo[1,5-a]pyrazin-3-yl)-1-pyrrolidinecarboxylate (4.5g, 12.6mmol) was dissolved in N-methylpyrrolidone (70mL ), adding ammonia water (69.4mmol) with a concentration of 28% by mass percentage, the reaction mixture was placed in a closed stainless steel reactor and stirred for 4 hours at 90°C and 2 atmospheric pressure, and after the reaction was completed, it was cooled to 35°C and the system pressure was released. Slowly add water (50mL), cool to 10°C, crystallize for 3h, filter, and recrystallize from isopropanol to obtain (S)-2-(8-aminoimidazo[1,5-a]pyrazin-3-yl )-benzyl 1-pyrrolidinecarboxylate, off-white solid (3.9g), yield 92%, the reaction formula of this step is the same as that of Example 1.
[0069] (2) Preparation of (S)-2-(8-tert-butoxycarbonylaminoimidazo[1,5-a]pyrazin-3-yl)-1-pyrrolidinecarboxylic acid benzyl ester:
[0070] (S)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com