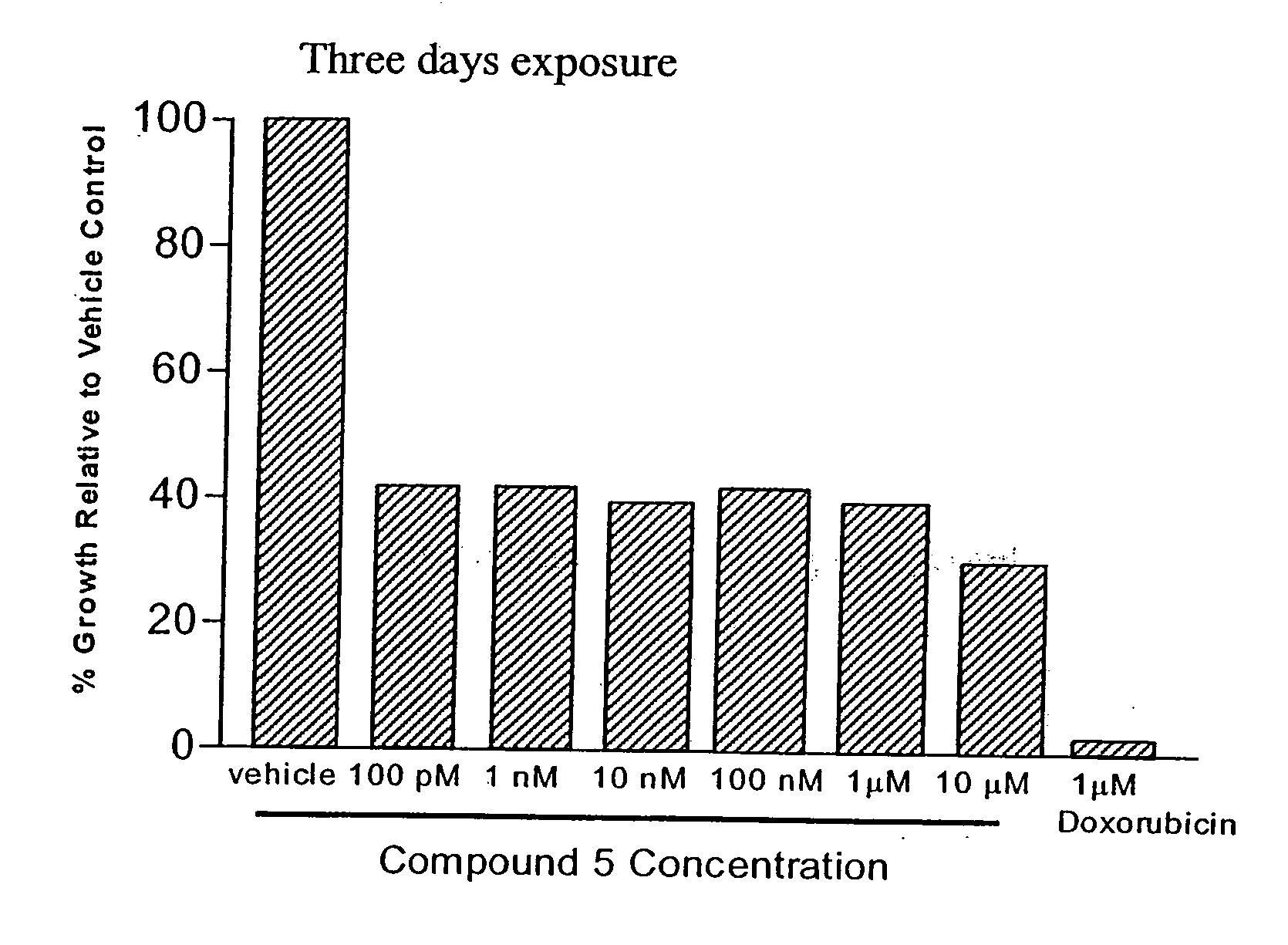
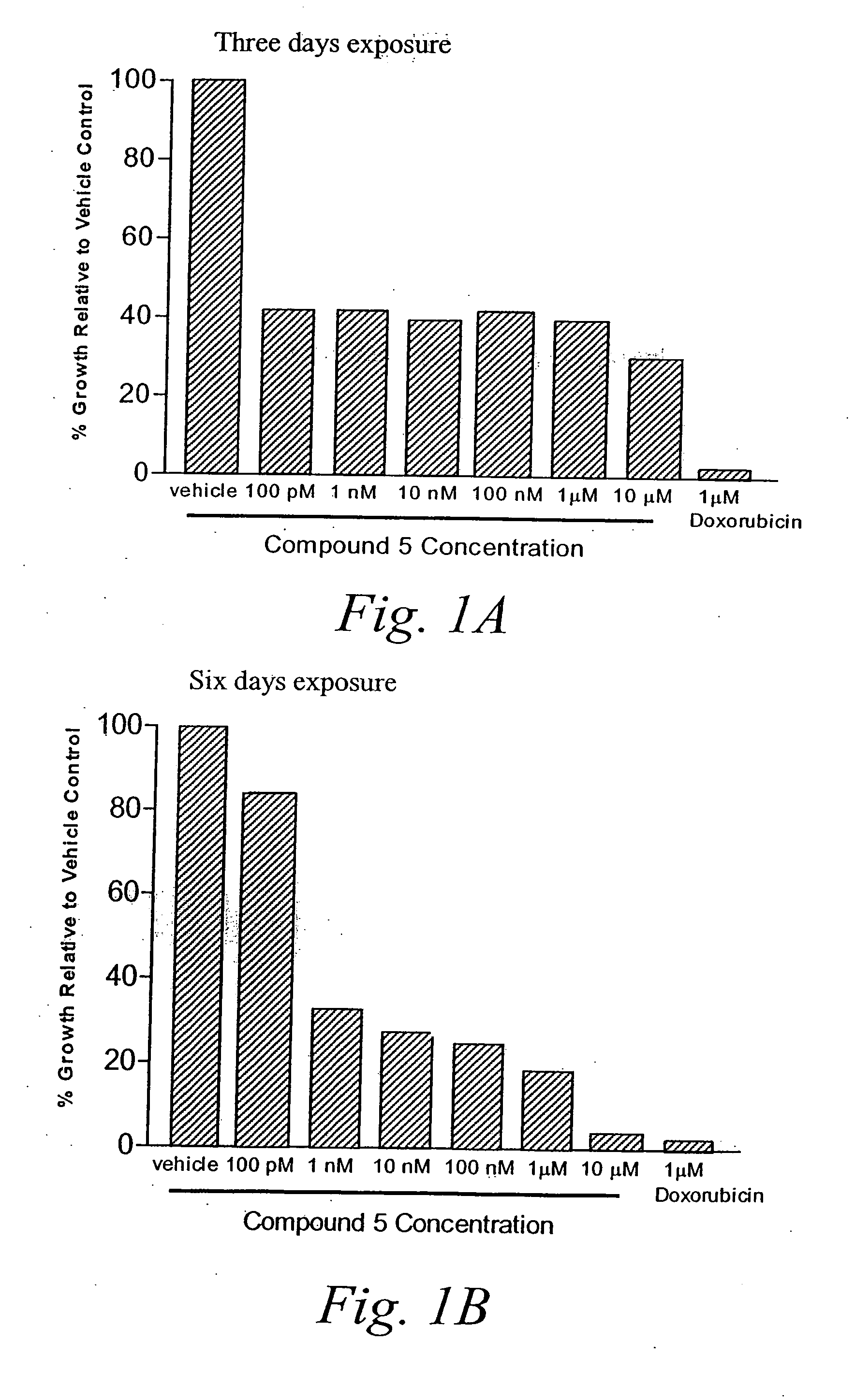
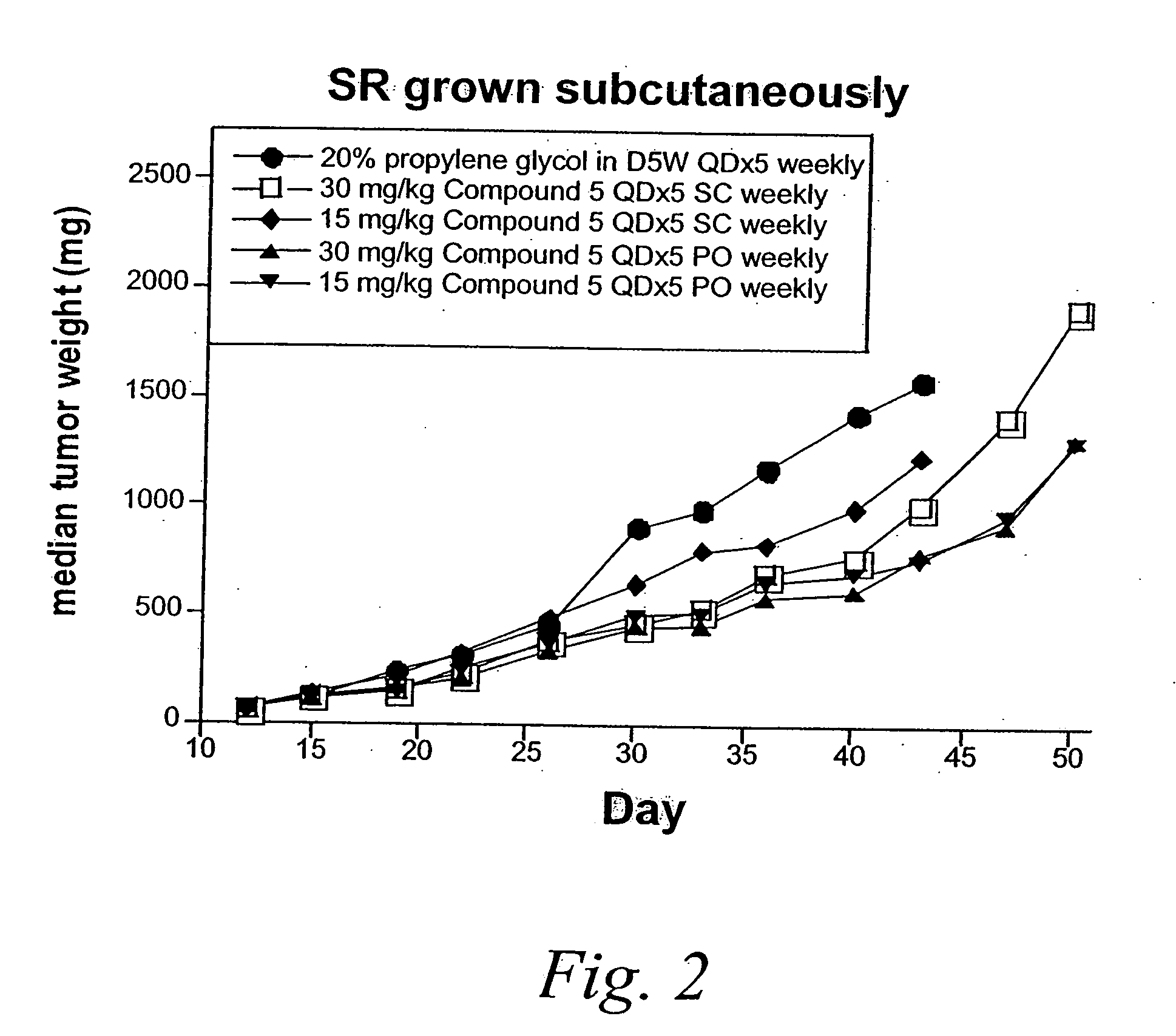
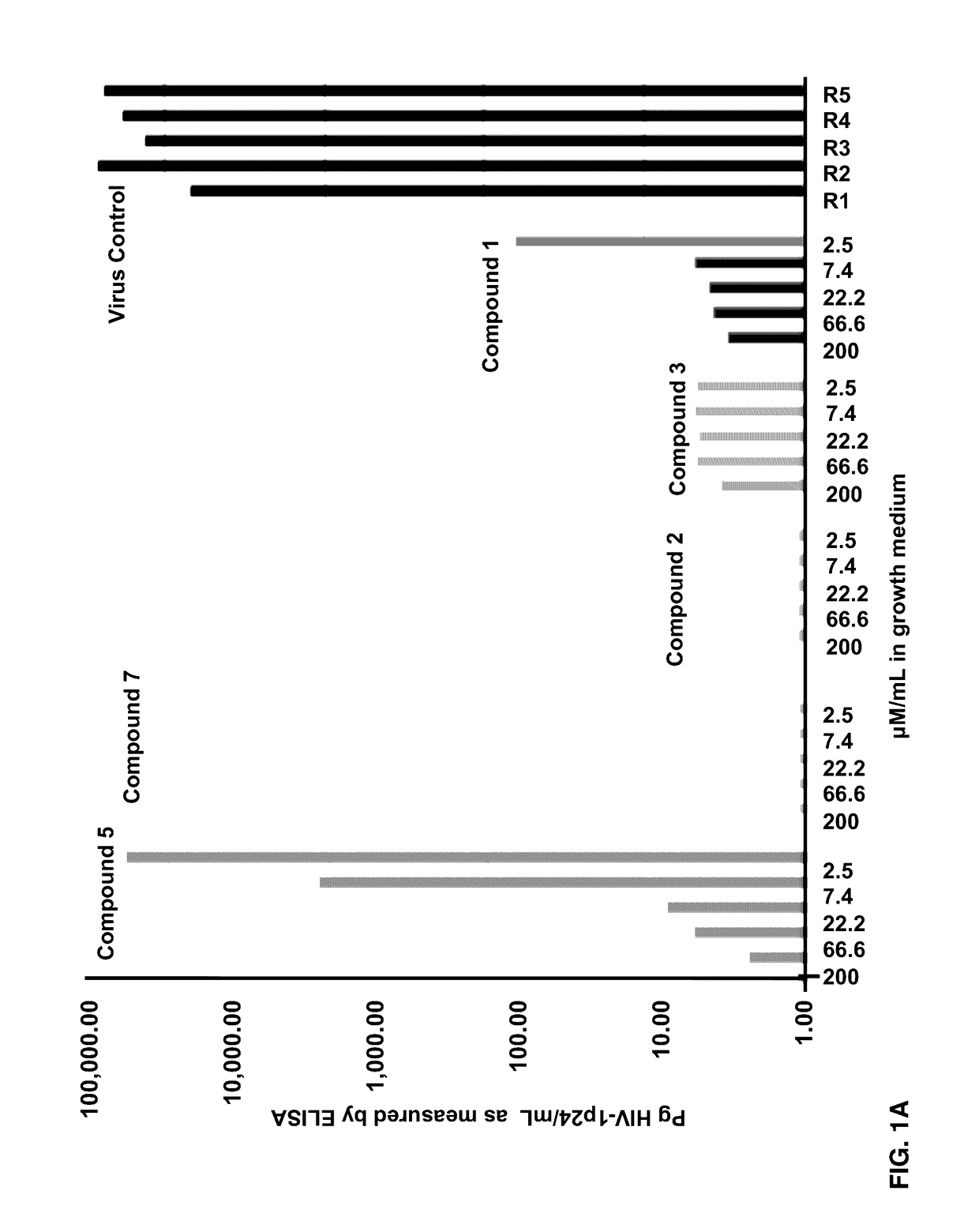
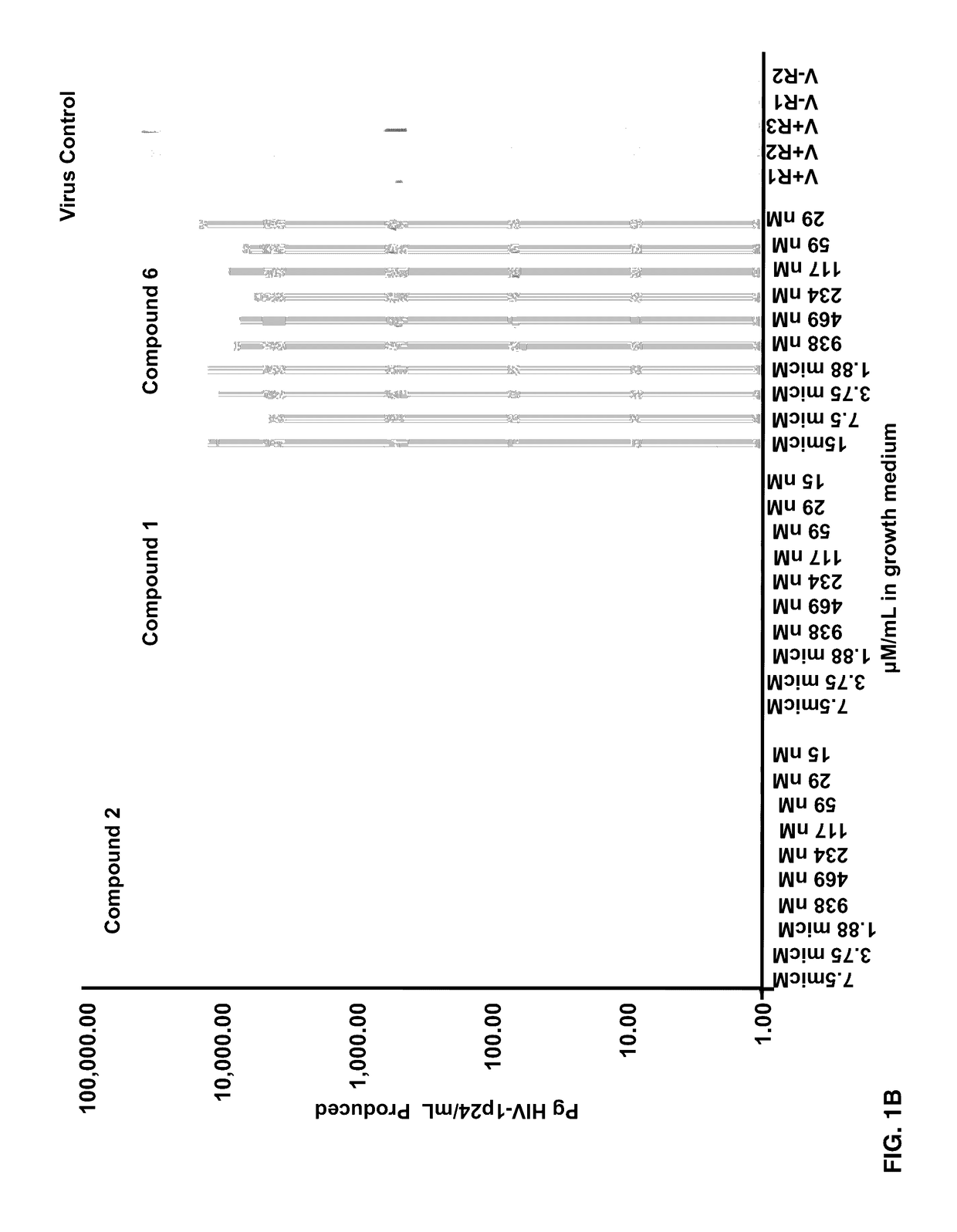
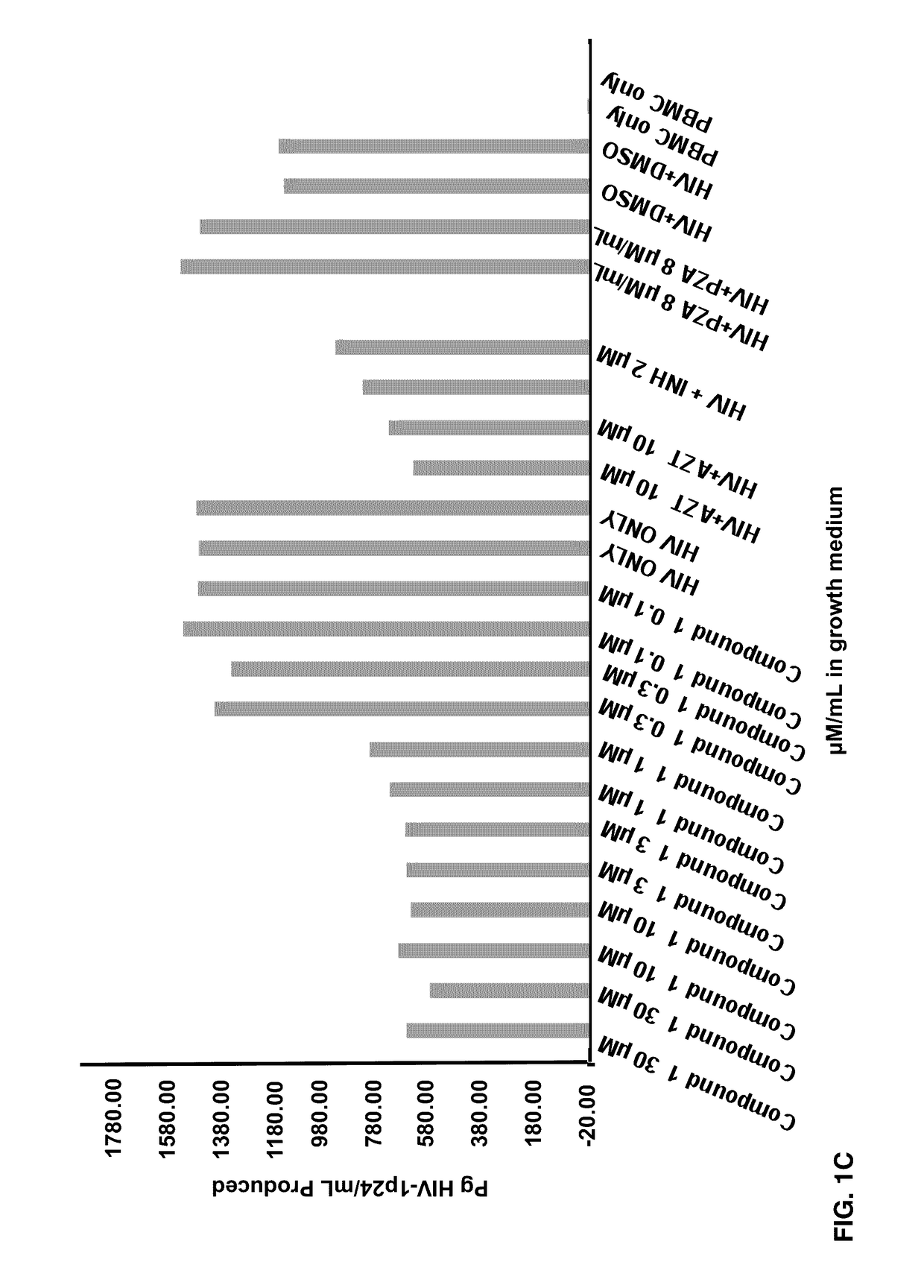
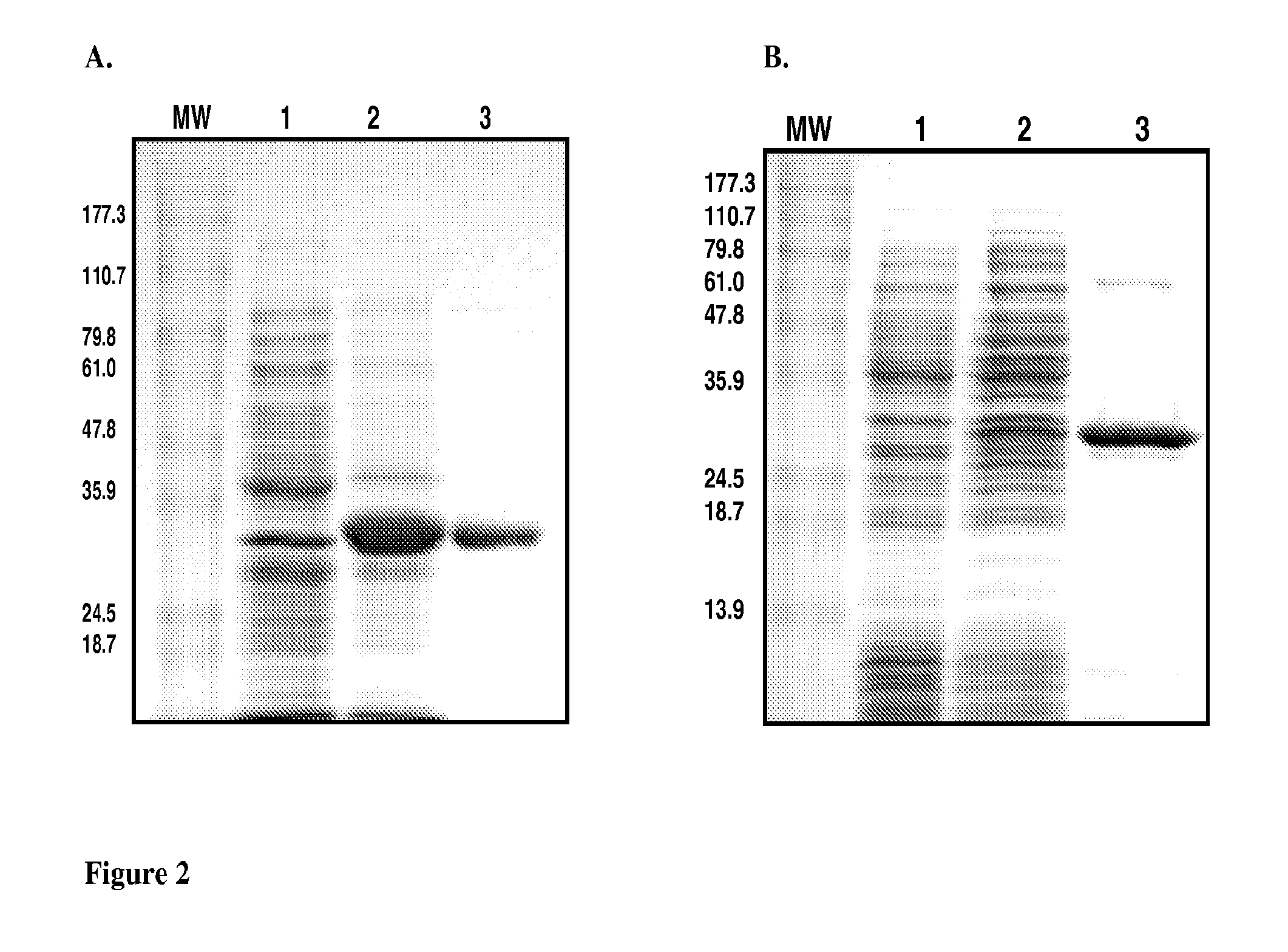
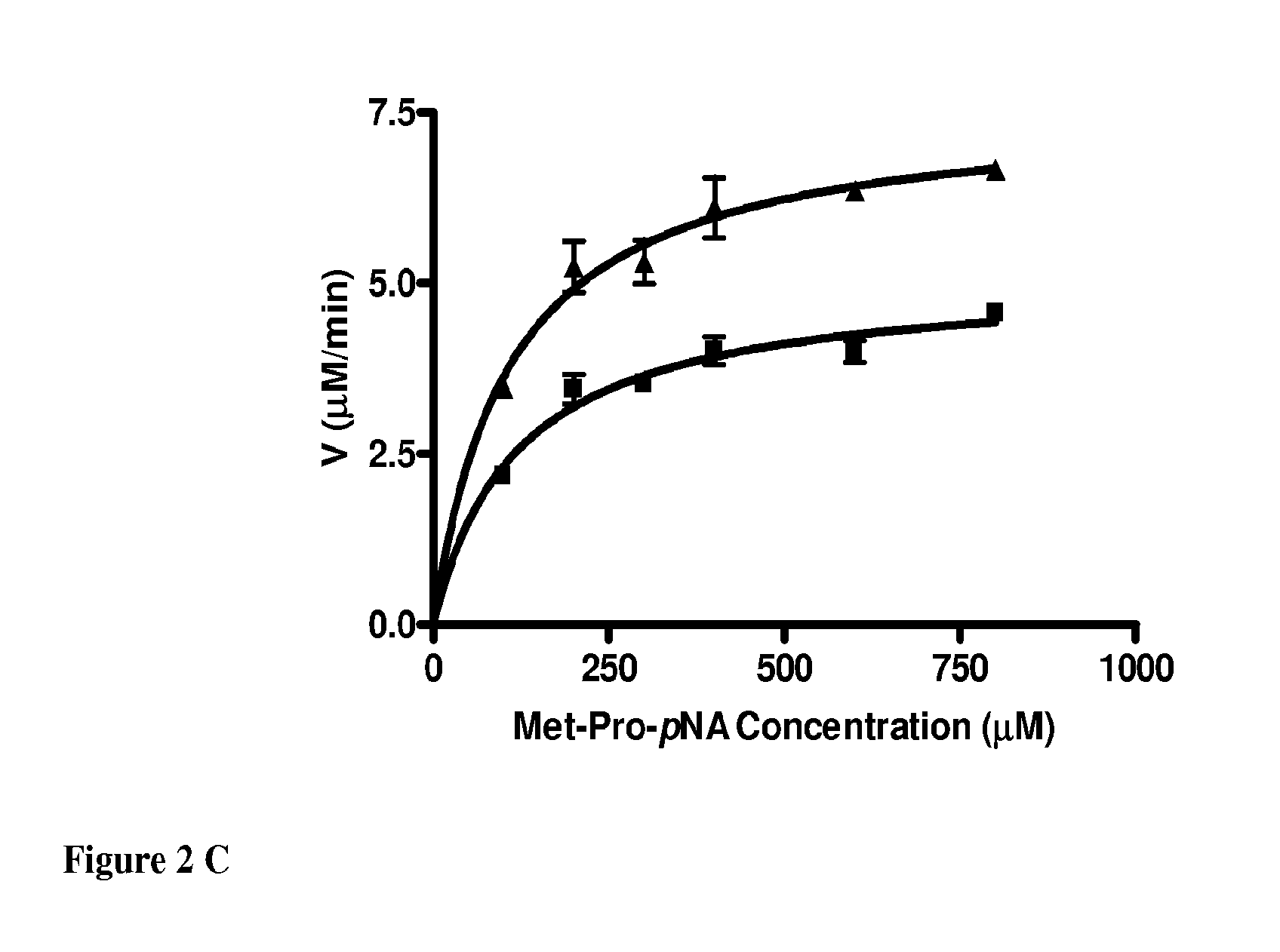
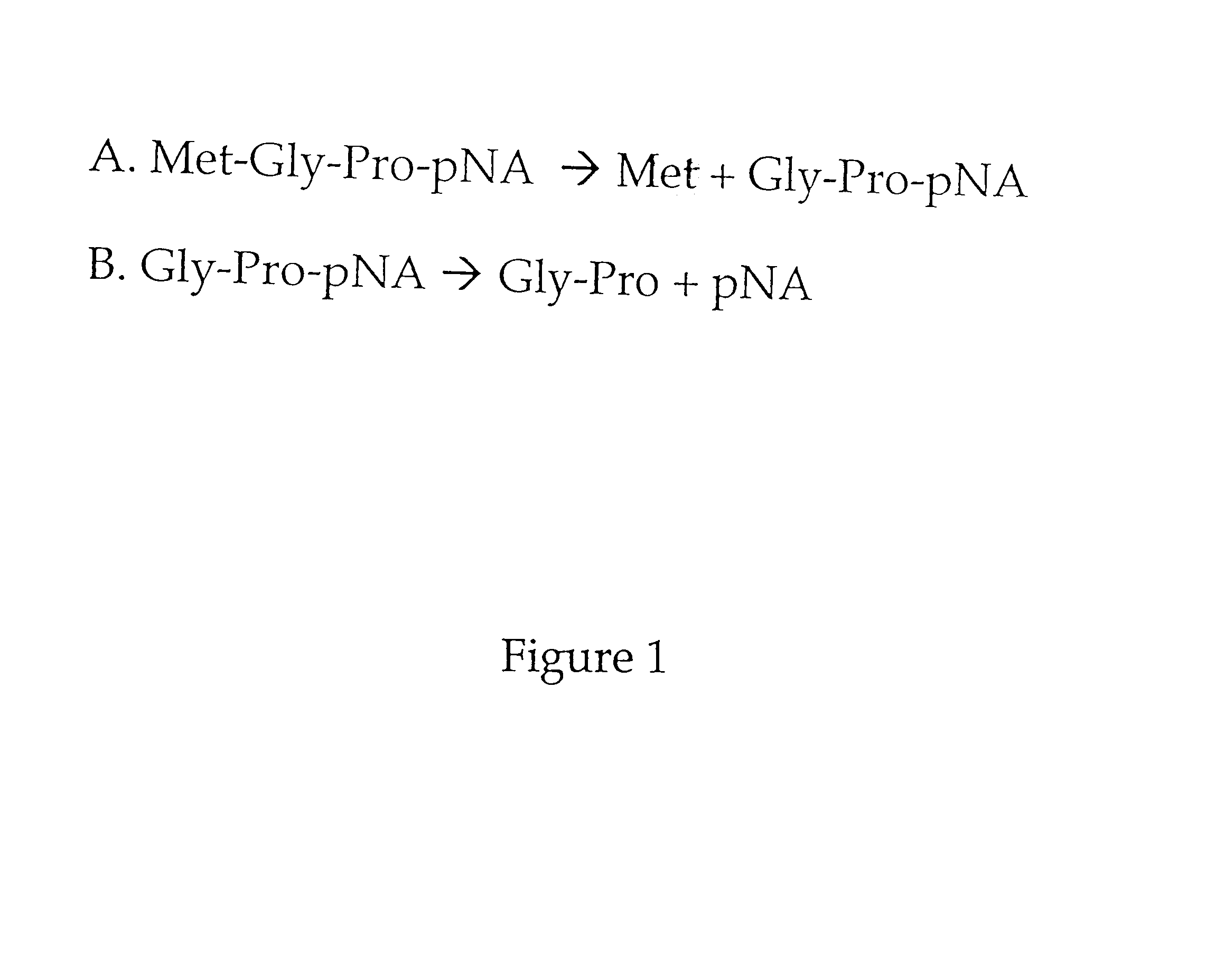Patents
Literature
39 results about "Methionine aminopeptidase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
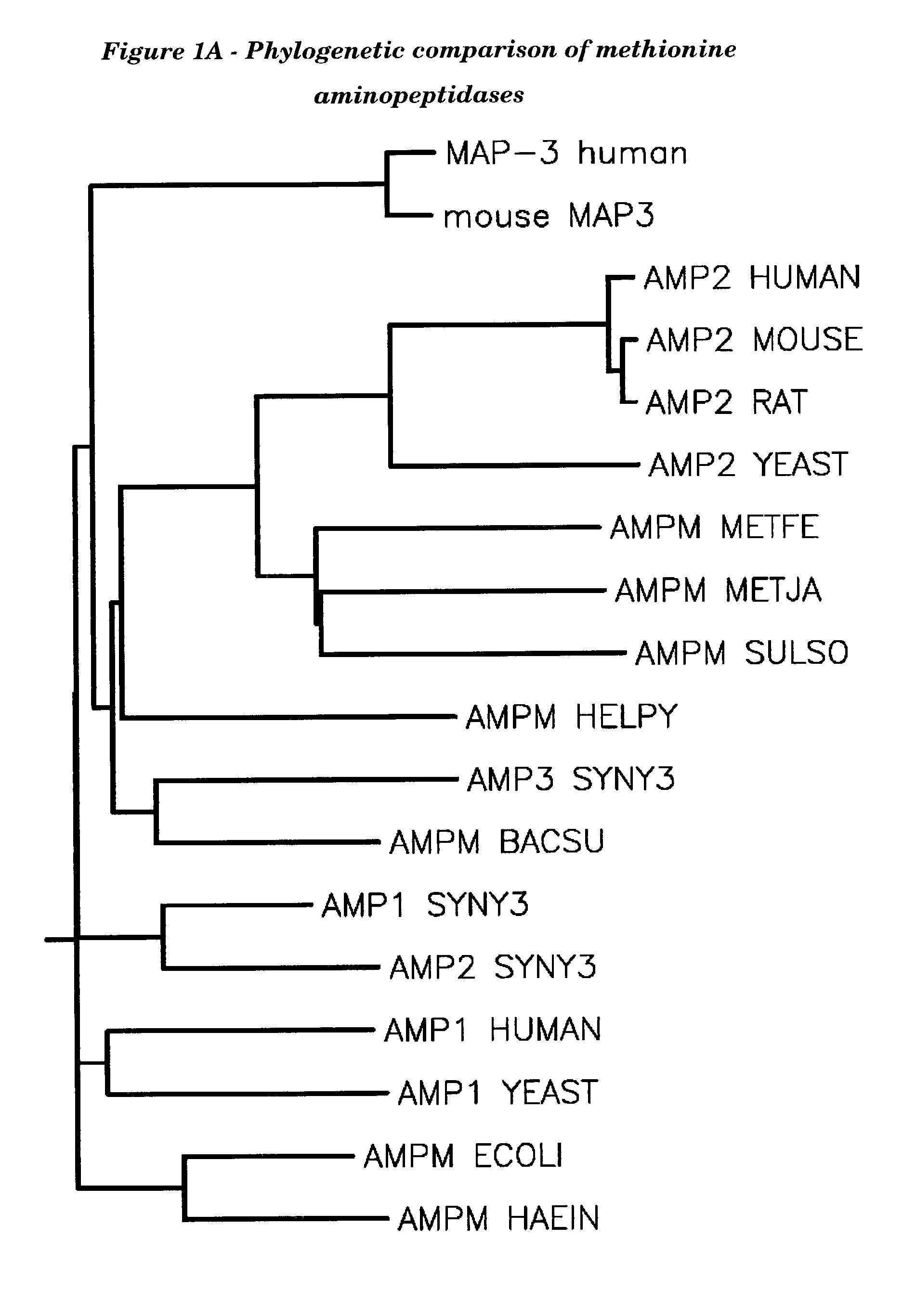
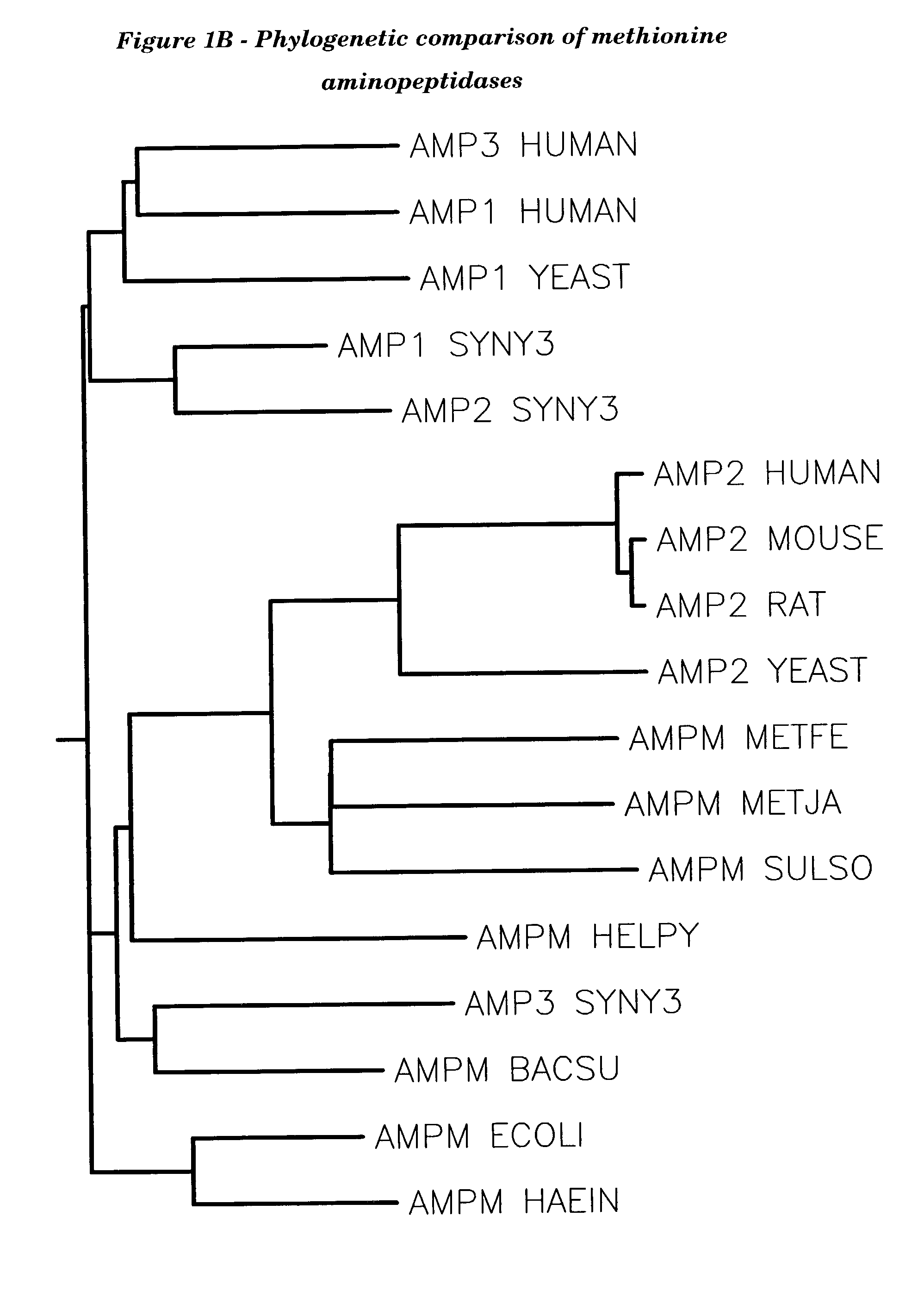
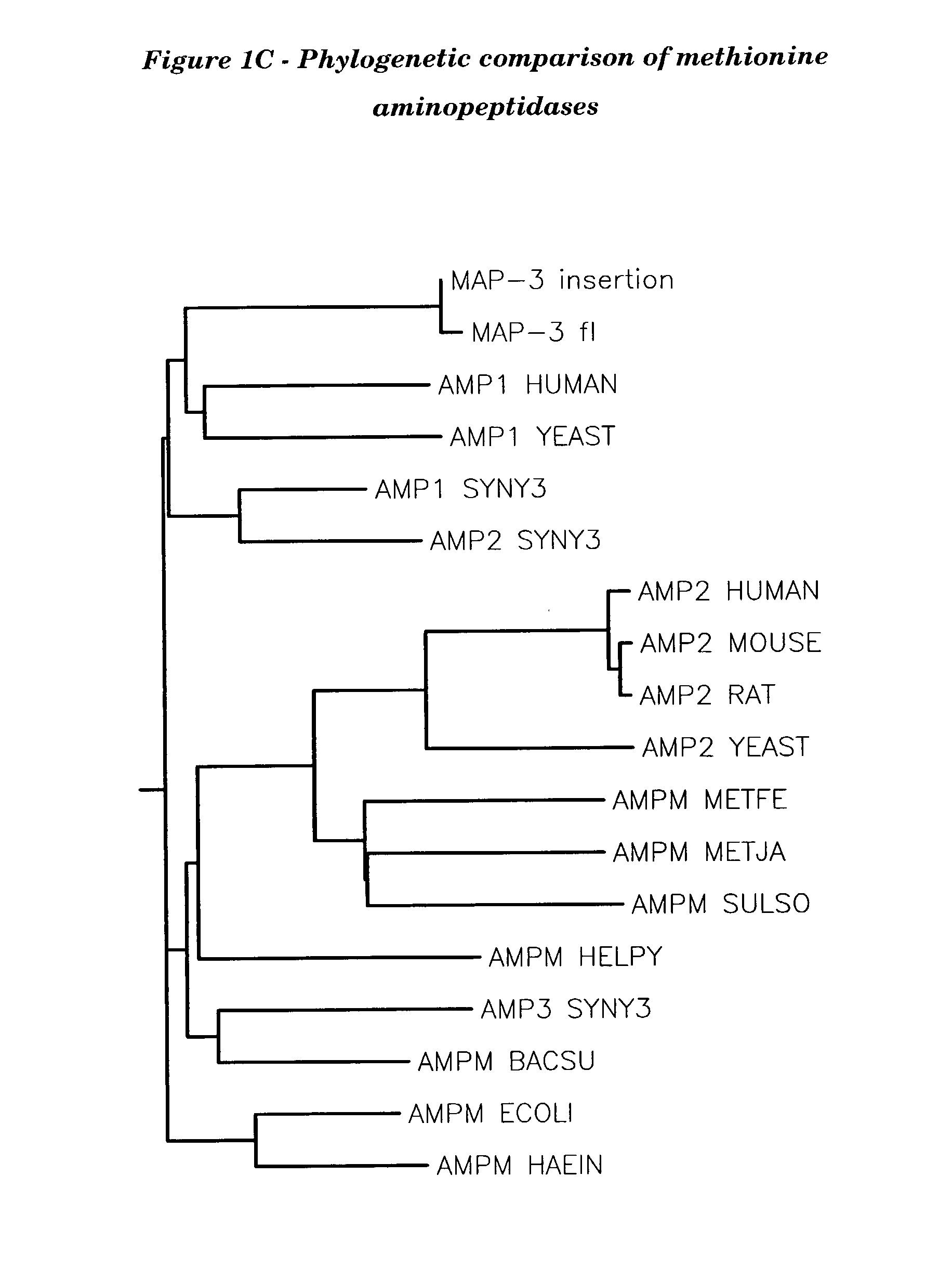
Methionyl aminopeptidase (EC 3.4.11.18, methionine aminopeptidase, peptidase M, L-methionine aminopeptidase, MAP) is an enzyme. This enzyme catalyses the following chemical reaction. This membrane-bound enzymatic activity is present in both prokaryotes and eukaryotes.
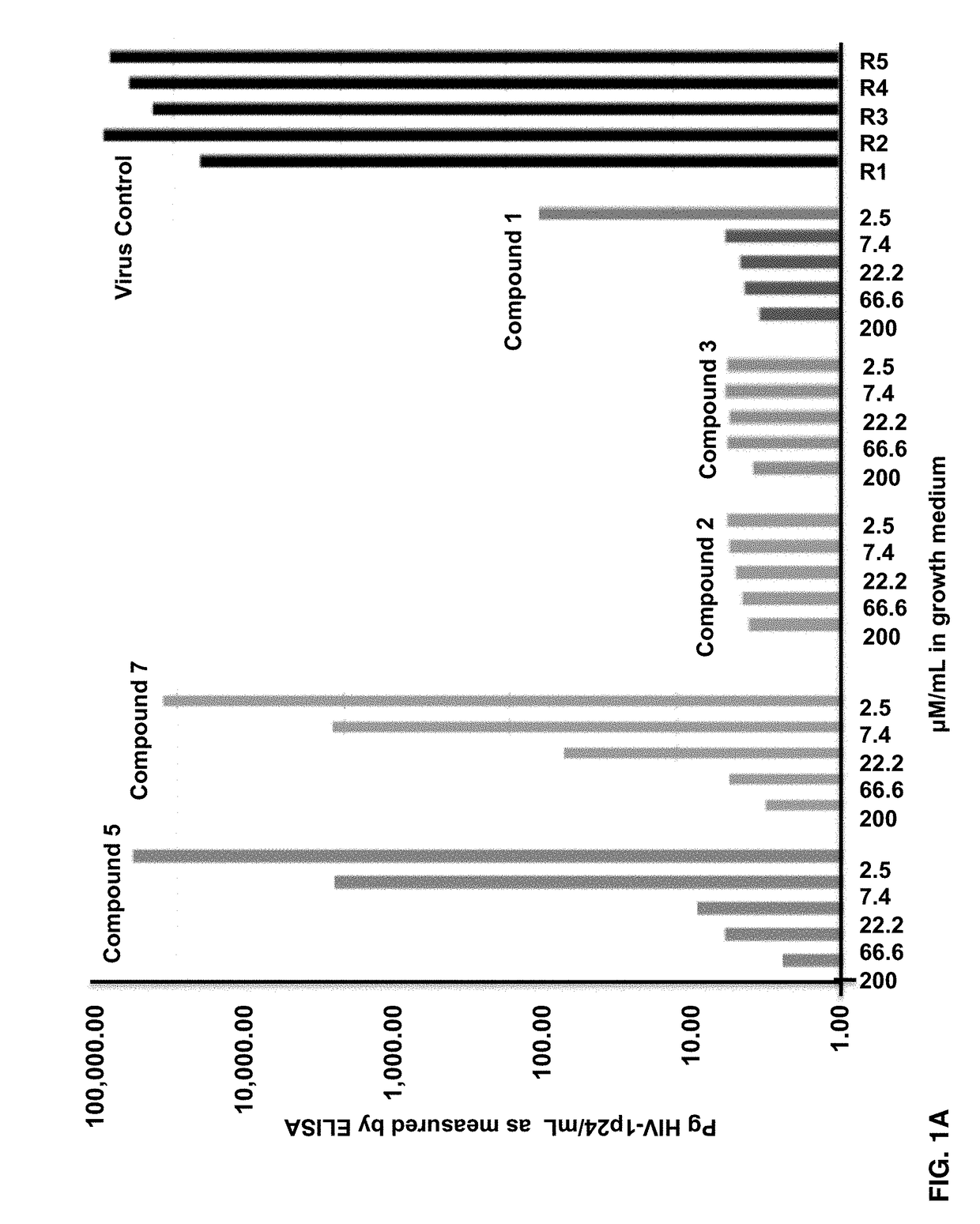
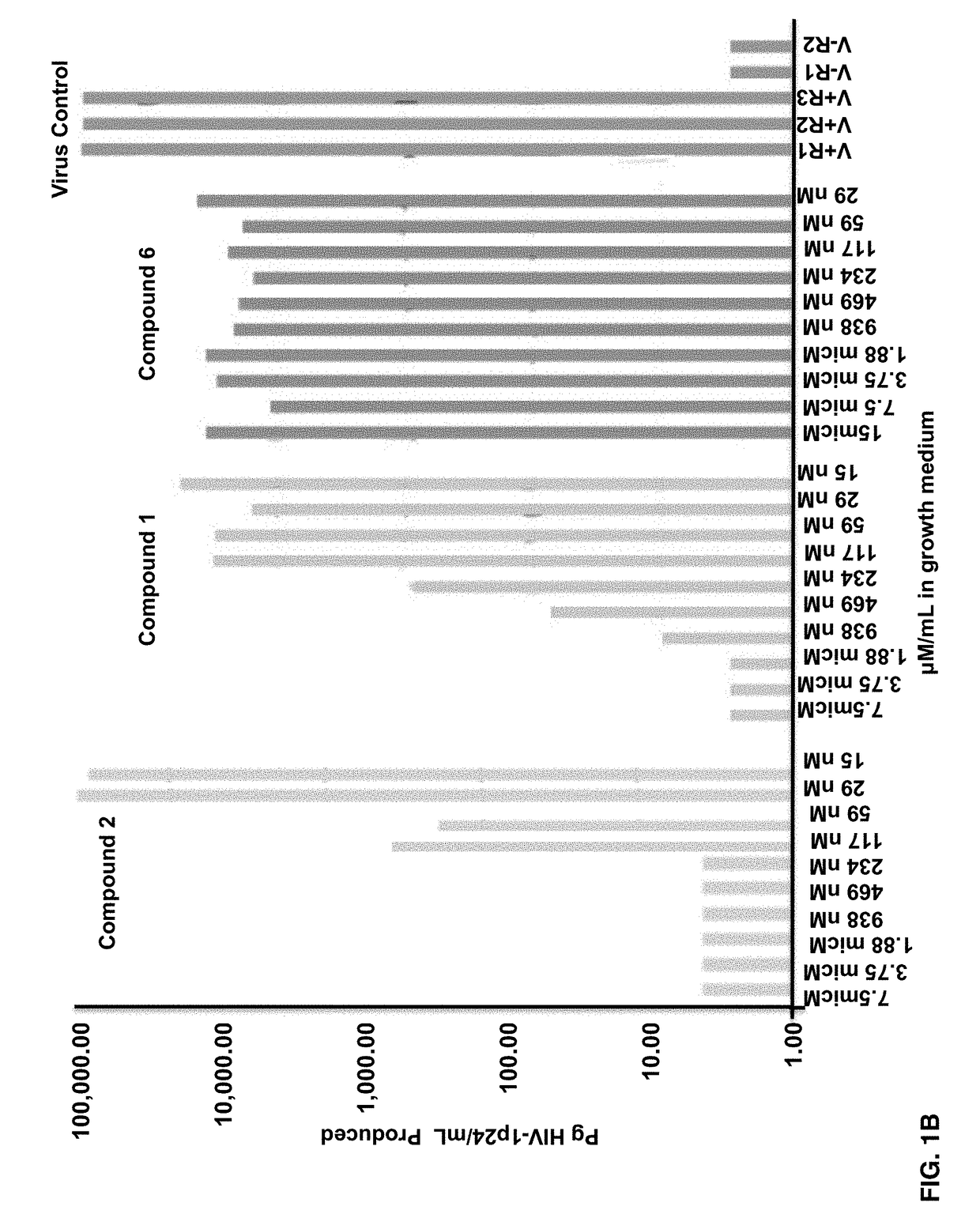
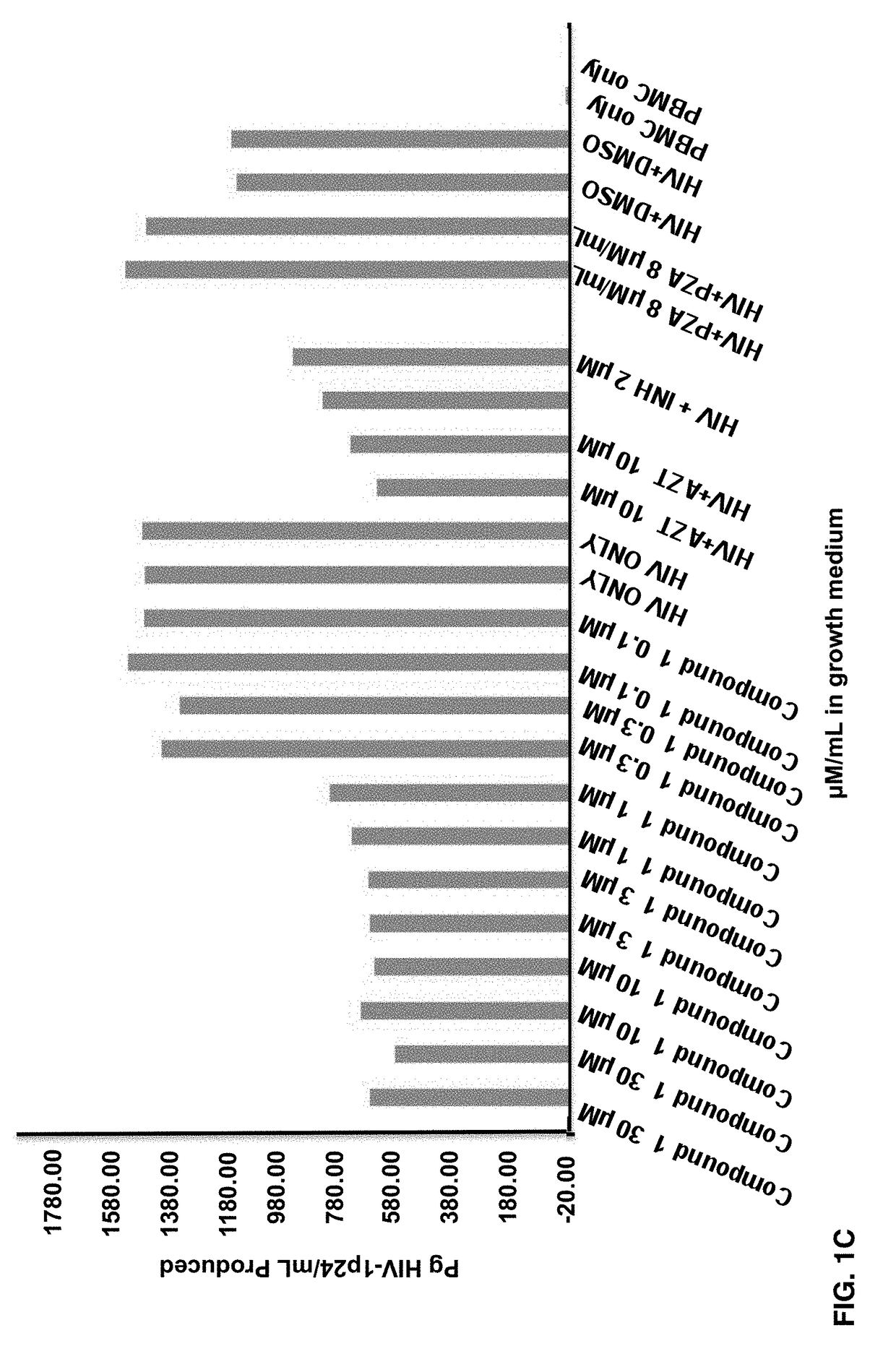
Fluorogenic or fluorescent reporter molecules and their applications for whole-cell fluorescence screening assays for caspases and other enzymes and the use thereof
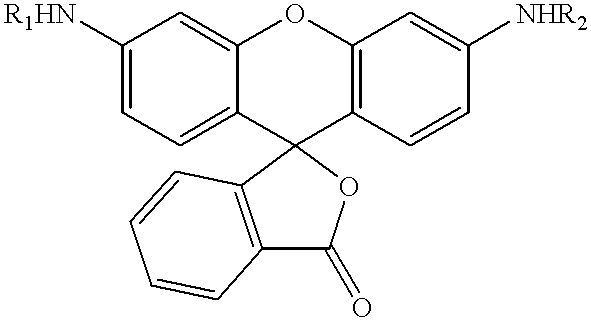
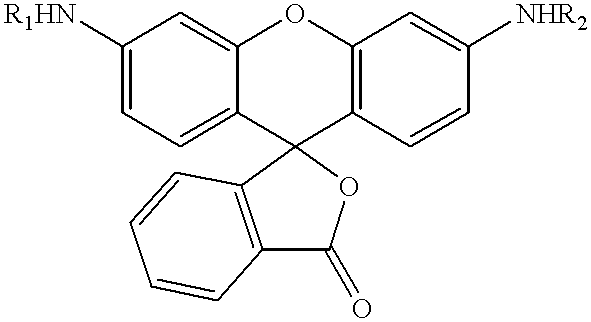
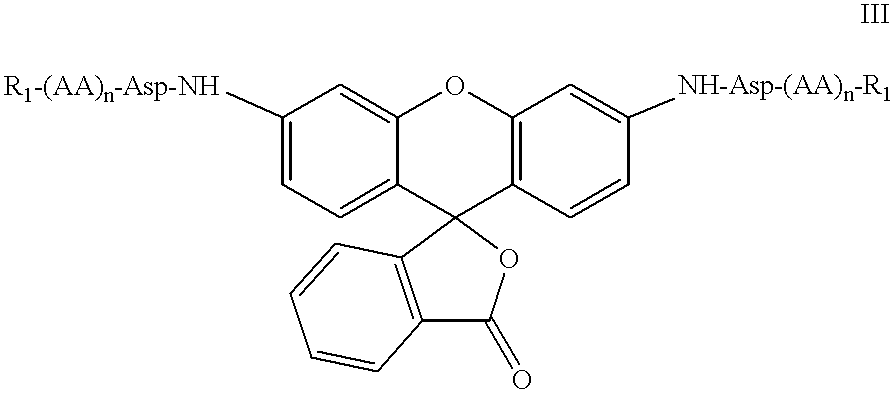
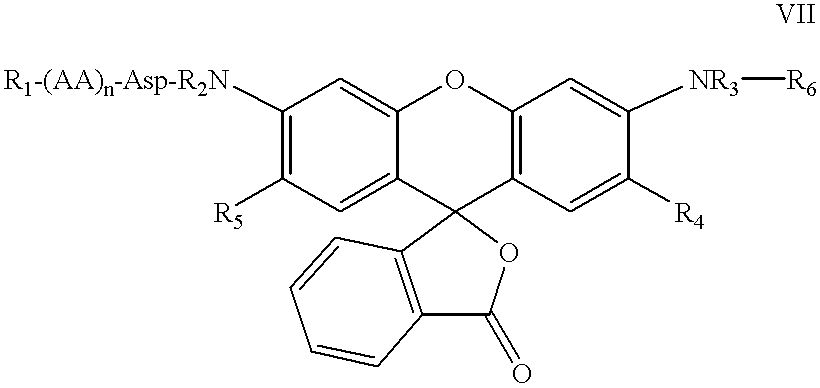
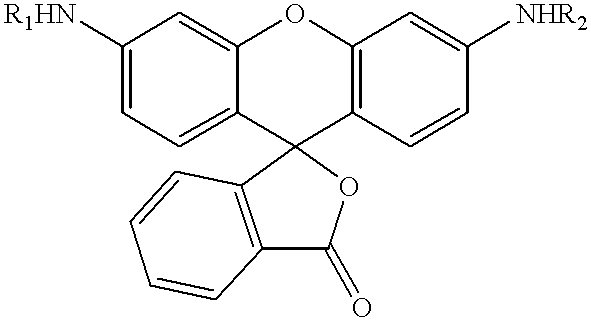
The present invention relates to novel fluorescent dyes, novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of caspases and other enzymes involved in apoptosis in whole cells, cell lines and tissue samples derived from any living organism or organ. The reporter molecules and assay processes can be used in drug screening procedures to identify compounds which act as inhibitors or inducers of the caspase cascade in whole cells or tissues. The reagents and assays described herein are also useful for determining the chemosensitivity of human cancer cells to treatment with chemotherapeutic drugs. The present invention also relates to novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of type 2 methionine aminopeptidase, dipeptidyl peptidase IV, calpain, aminopeptidase, HIV protease, adenovirus protease, HSV-1 protease, HCMV protease and HCV protease.
Owner:CYTOVIA INC
Fluorogenic or fluorescent reporter molecules and their applications for whole-cell fluorescence screening assays for capsases and other enzymes and the use thereof
InactiveUS6342611B1Organic chemistryMicrobiological testing/measurementScreening proceduresApoptosis
The present invention relates to novel fluorescent dyes, novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of caspases and other enzymes involved in apoptosis in whole cells, cell lines and tissue samples derived from any living organism or organ. The reporter molecules and assay processes can be used in drug screening procedures to identify compounds which act as inhibitors or inducers of the caspase cascade in whole cells or tissues. The reagents and assays described herein are also useful for determining the chemosensitivity of human cancer cells to treatment with chemotherapeutic drugs. The present invention also relates to novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of type 2 methionine aminopeptidase, dipeptidyl peptidase IV, calpain, aminopeptidase, HIV protease, adenovirus protease, HSV-1 protease, HCMV protease and HCV protease.
Owner:CYTOVIA INC
Fluorescence dyes and their applications for whole-cell fluorescence screening assays for caspases, peptidases, proteases and other enzymes and the use thereof
InactiveUS6248904B1Microbiological testing/measurementBiological testingScreening proceduresCancer cell
The present invention relates to novel fluorescent dyes, novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of caspases and other enzymes involved in apoptosis in whole cells, cell lines and tissue samples derived from any living organism or organ. The reporter molecules and assay processes can be used in drug screening procedures to identify compounds which act as inhibitors or inducers of the caspase cascade in whole cells or tissues. The reagents and assays described herein are also useful for determining the chemosensitivity of human cancer cells to treatment with chemotherapeutic drugs. The present invention also relates to novel fluorogenic and fluorescent reporter molecules and new enzyme assay processes that can be used to detect the activity of type 2 methionine aminopeptidase, HIV protease, adenovirus protease, HSV-1 protease, HCMV protease and HCV protease.
Owner:CYTOVIA INC
Inhibitors of methionine aminopeptidase-2 and uses thereof
InactiveUS20050239878A1Organic active ingredientsBiocideMethionine aminopeptidaseParasitic infection
The instant invention provides compositions and methods for treating a subject suffering from one of a number of conditions, including an angiogenic disease, such as cancer, an autoimmune disorder or a parasitic infection.
Owner:PRAECIS PHARM INC
Methods for identifying inhibitors of methionine aminopeptidases
InactiveUS6261794B1Microbiological testing/measurementPeptidesMethionine aminopeptidaseMethionine biosynthesis
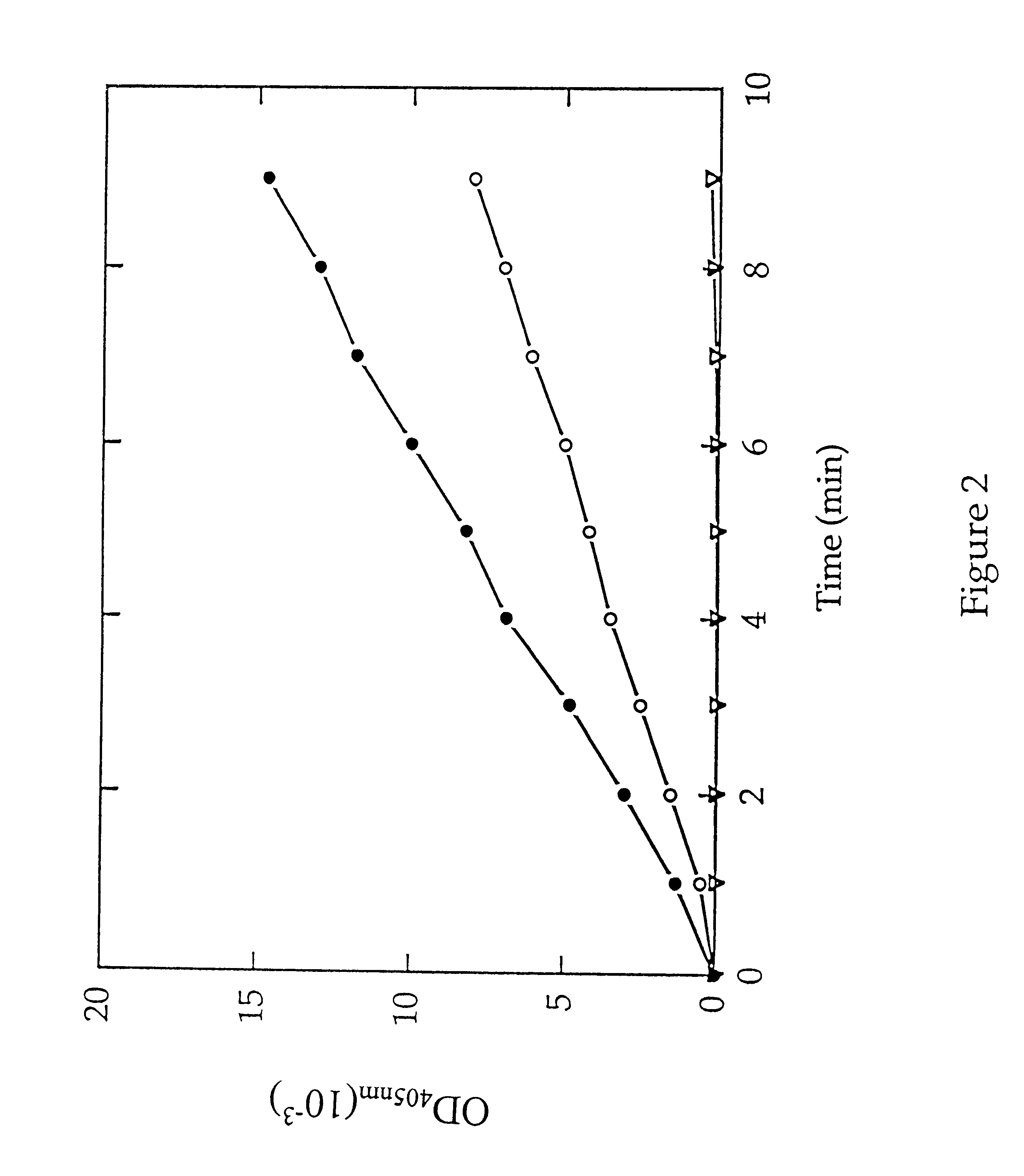
Methods are provided for detecting methionine aminopeptidase (MAP) activity and for detecting inhibitors of MAP. The methods utilize a peptide comprising an N-terminal methionine which can be cleaved from the peptide by MAP, and a C-terminal detection moiety which is released by a second peptidase only if the N-terminal methionine has been cleaved from the peptide. When the peptide is combined with MAP and the second peptidase, the detection moiety is released, while the addition of a MAP inhibitor will inhibit the release of the detection moiety. Reaction mixes, peptides, and kits which are useful for practicing the methods are also provided.
Owner:SAINT LOUIS UNIVERSITY
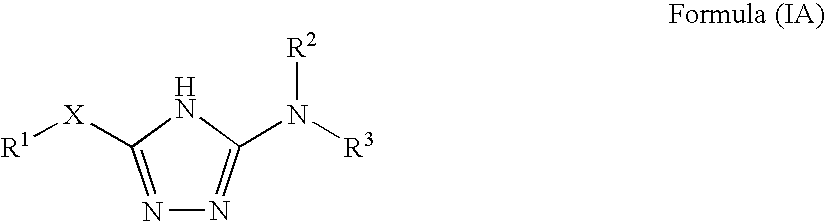
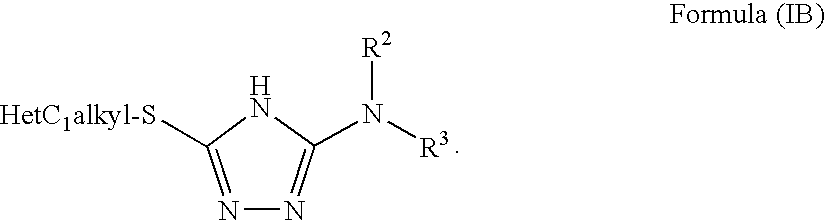
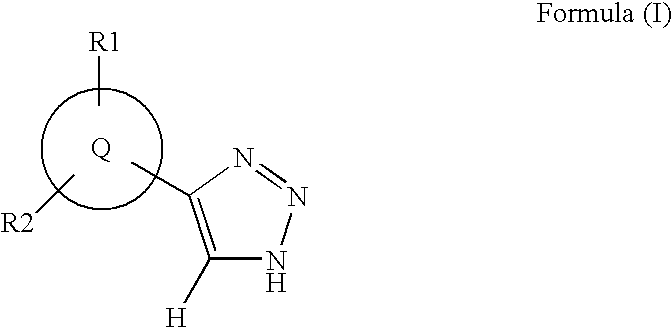
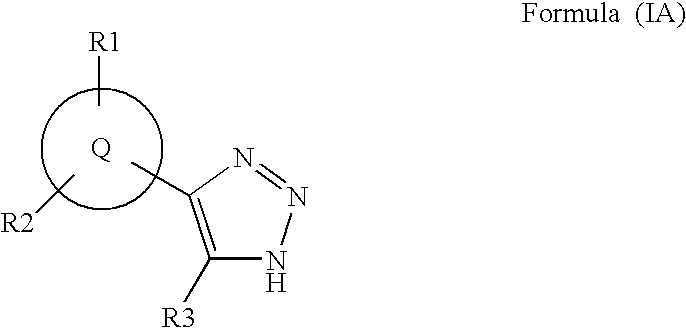
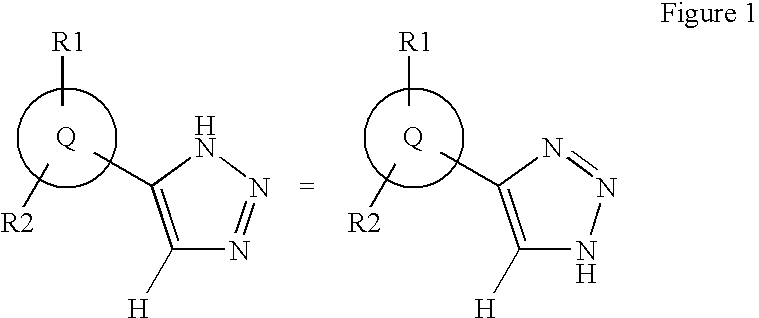
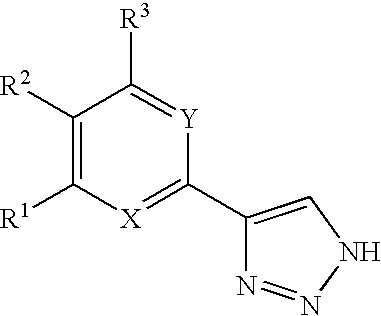
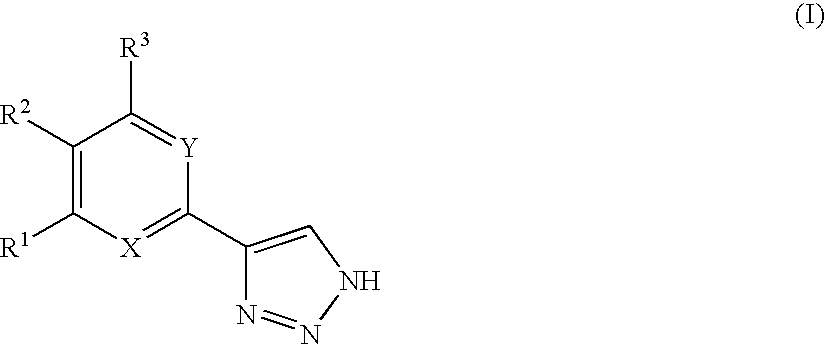
1,2,4-triazole derivatives, compositions, process of making and methods of use
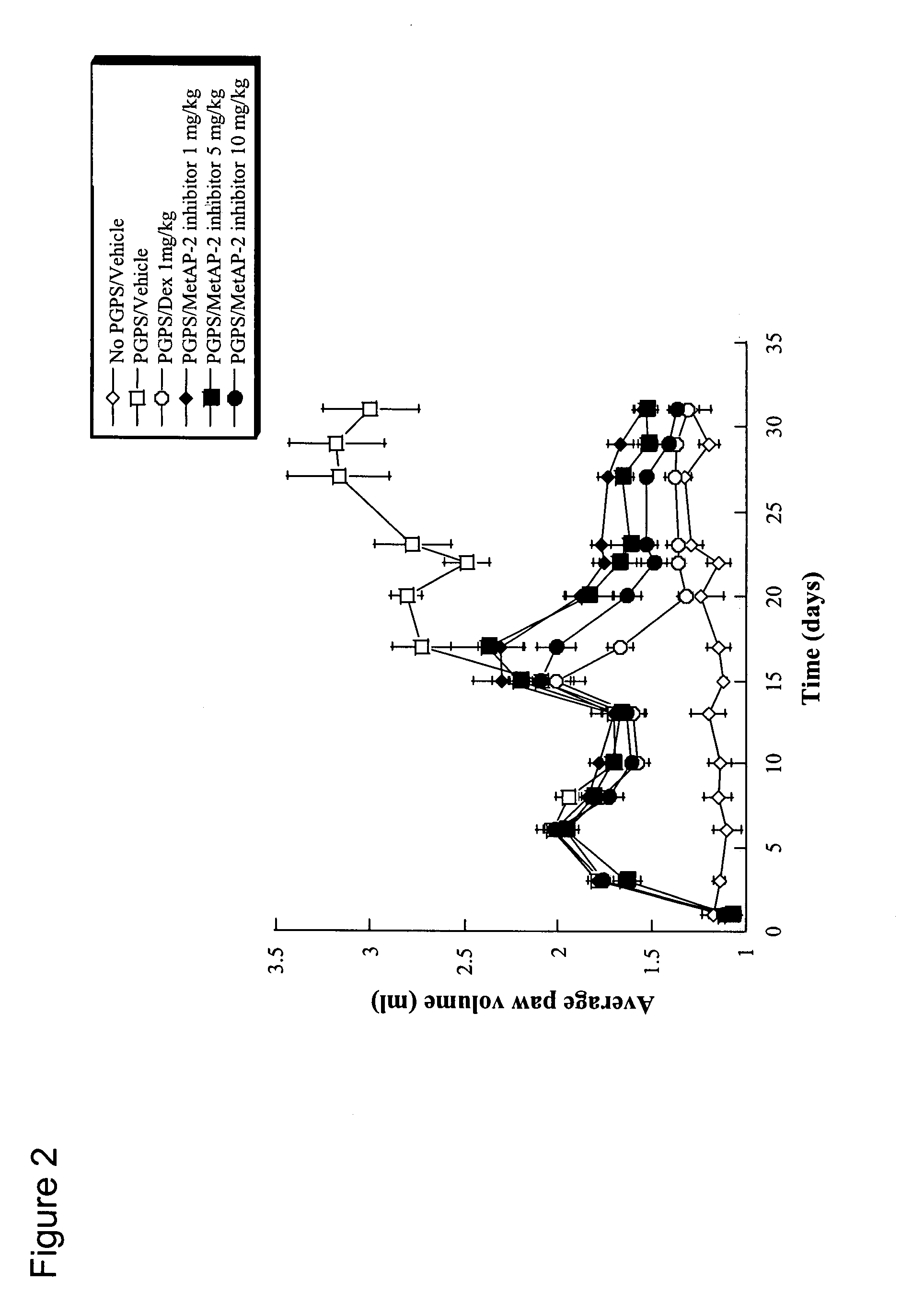
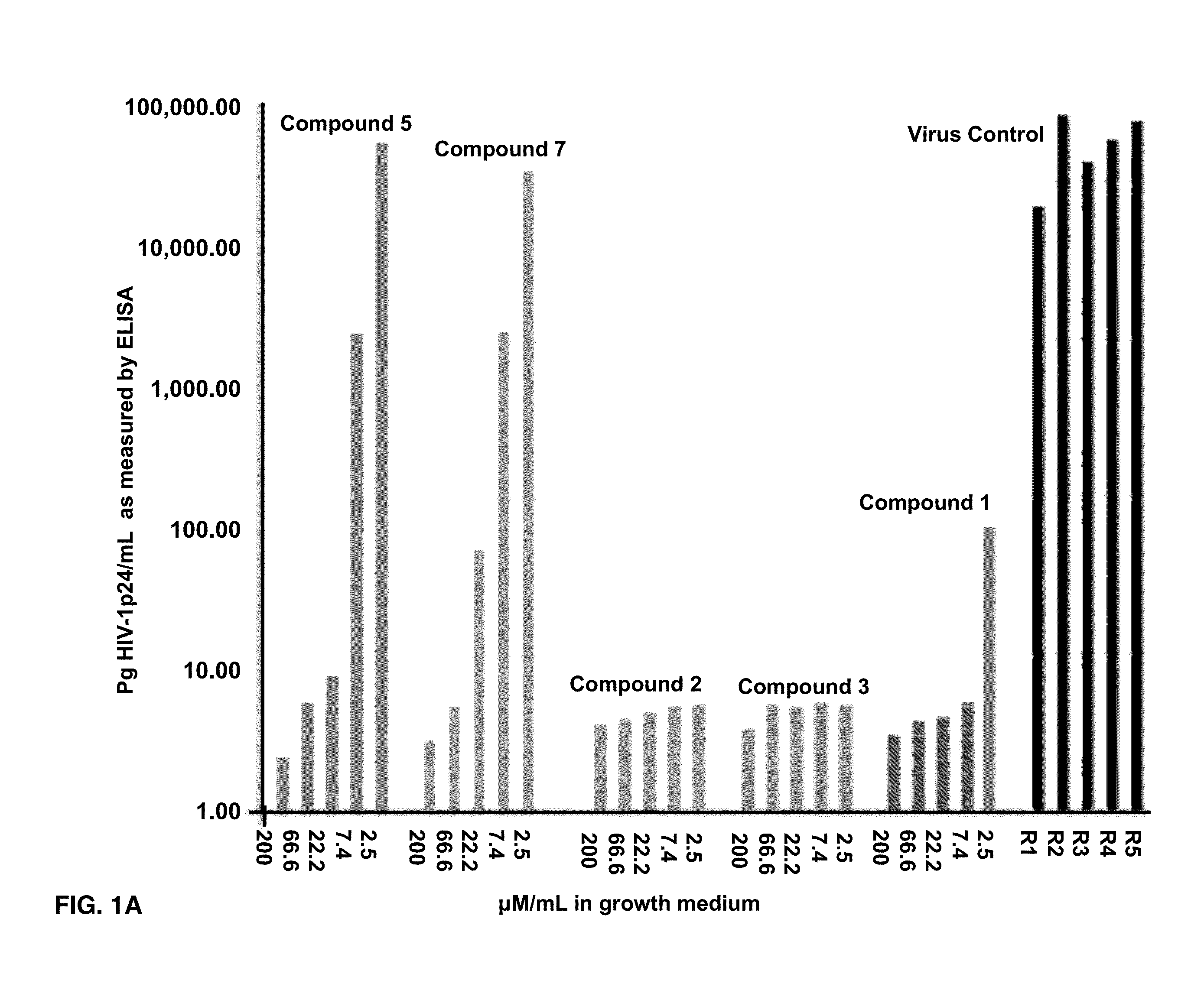
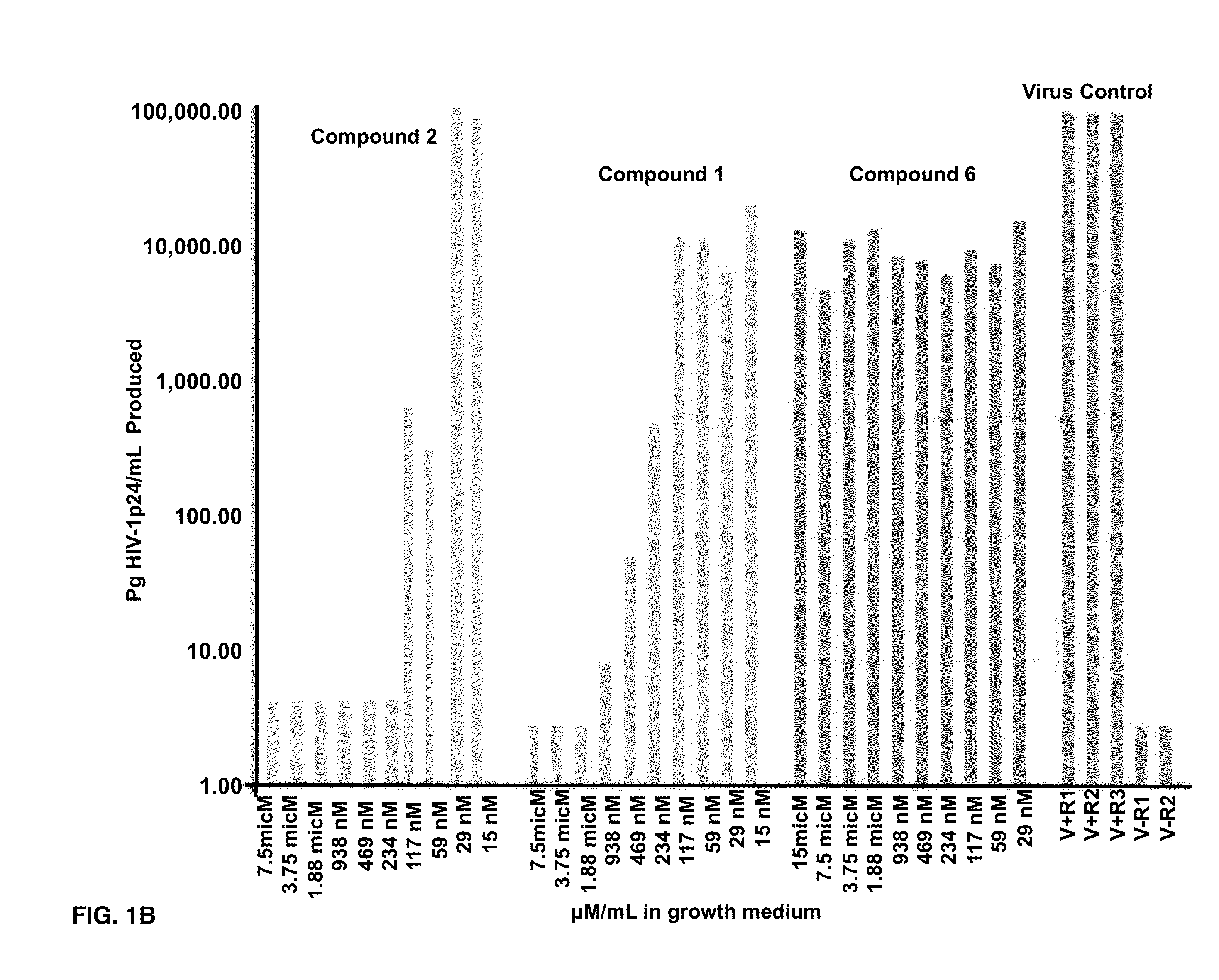
Compounds of this invention are non-peptide, reversible inhibitors of type 2 methionine aminopeptidase, useful in treating conditions mediated by angiogenesis, such as cancer, haemangioma, proliferative retinopathy, rheumatoid arthritis, atherosclerotic neovascularization, psoriasis, ocular neovascularization and obesity.
Owner:GLAXO SMITHKLINE LLC
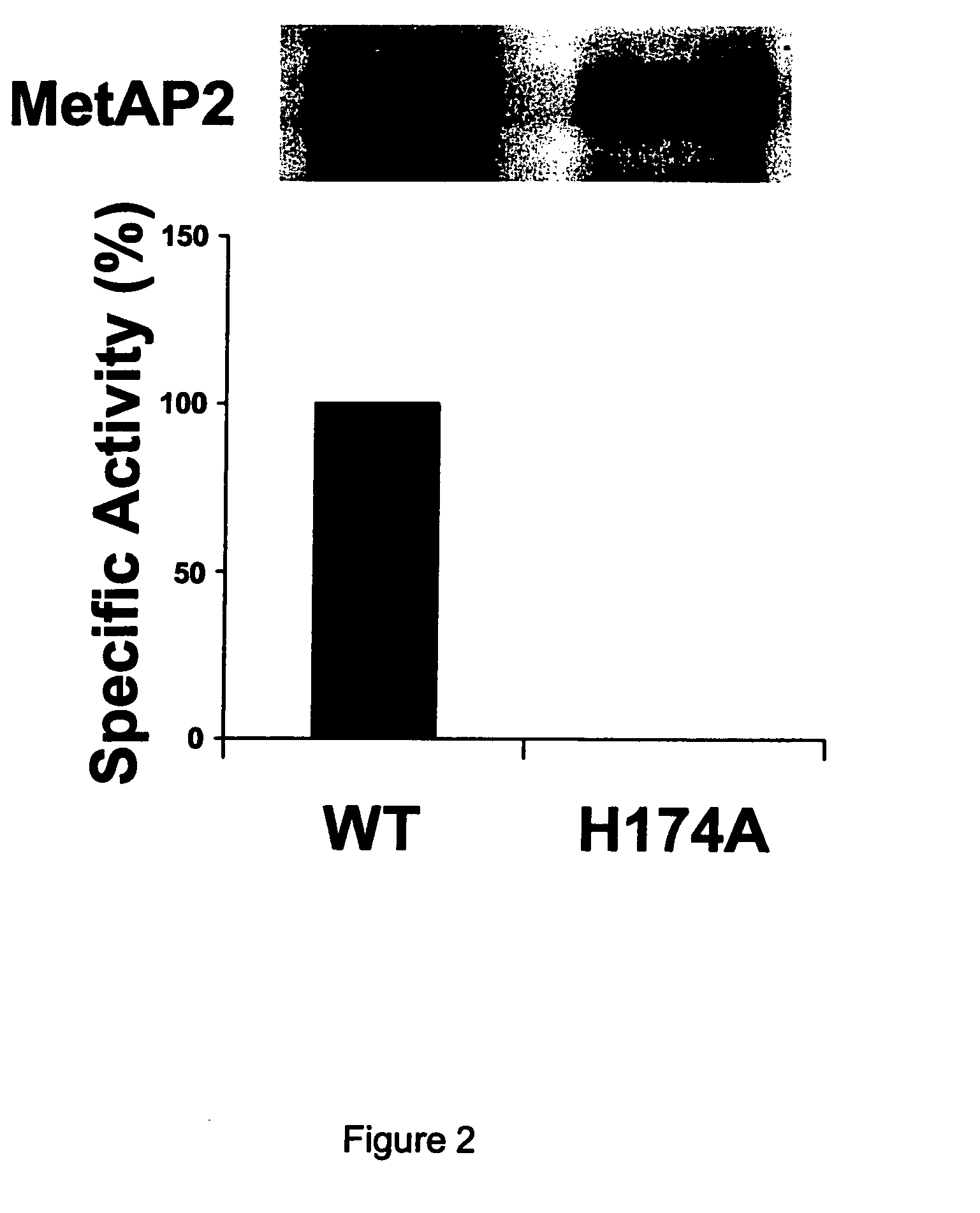
Dominant negative variants of methionine aminopeptidase 2 (MetAP2) and clinical uses thereof
InactiveUS20050032221A1Increase ratingsEliminate negative effectsBiocideCompound screeningMethionine aminopeptidaseMethionine biosynthesis
Inhibitors of type 2 methionine aminopeptidases (“MetAP2”), specifically dominant negative variants of MetAP2, both polypeptides and encoding polynucleotides, are provided. Also provided are methods of treating subjects suffering from cancer, diseases mediated by the immune system or opportunistic infections using inhibitors of MetAP2. Also provided are high through put screens and assays to detect and identify inhibitors of MetAP2 and downstream effectors of MetAP2.
Owner:CHANG YIE HWA +2
Methods for identifying inhibitors of methionine aminopeptidases
InactiveUS20010047078A1Microbiological testing/measurementTripeptide ingredientsMethionine aminopeptidaseEnzyme binding
Methods are provided for detecting methionine aminopeptidase (MAP) activity and for detecting inhibitors of MAP. The methods utilize a peptide comprising an N-terminal methionine which can be cleaved from the peptide by MAP, and a C-terminal detection moiety which is released by a second peptidase only if the N-terminal methionine has been cleaved from the peptide. When the peptide is combined with MAP and the second peptidase, the detection moiety is released, while the addition of a MAP inhibitor will inhibit the release of the detection moiety. Reaction mixes, peptides, and kits which are useful for practicing the methods are also provided.
Owner:SAINT LOUIS UNIVERSITY
Triazole inhibitors of type 2 methionine aminopeptidase
Disclosed are compounds which are non-peptide, reversible inhibitors of type 2 methionine aminopeptidase, useful in treating conditions mediated by angiogenesis, such as cancer, haemangioma, proliferative retinopathy, rheumatoid arthritis, atherosclerotic neovascularization, psoriasis, ocular neovascularization and obesity.
Owner:SMITHKLINE BECKMAN CORP
Methionine aminopeptidase-2 inhibitors and methods of use thereof
InactiveUS7348307B2Low toxicityInhibit methionine aminopeptidase 2BiocideDipeptide ingredientsMethionine aminopeptidaseBiochemistry
Owner:GLAXO SMITHKLINE LLC
Compounds and methods
InactiveUS20050143578A1Useful in preparationOrganic active ingredientsSenses disorderOcular neovascularizationMethionine aminopeptidase
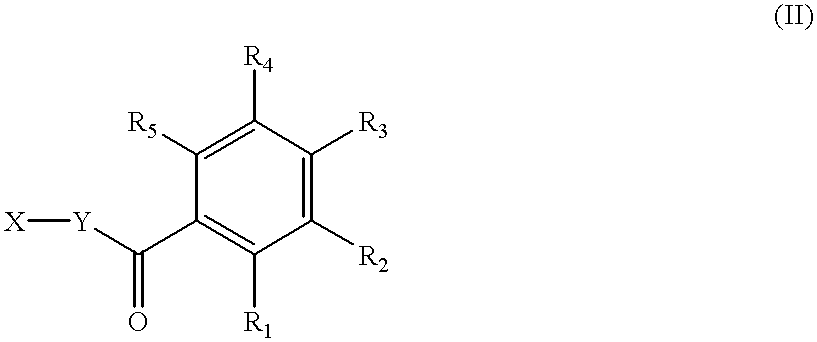
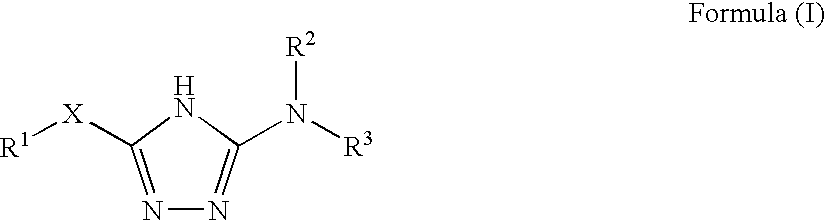
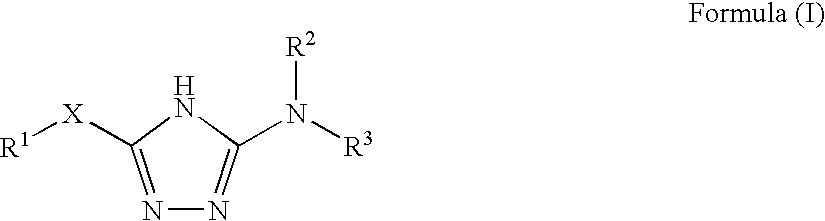
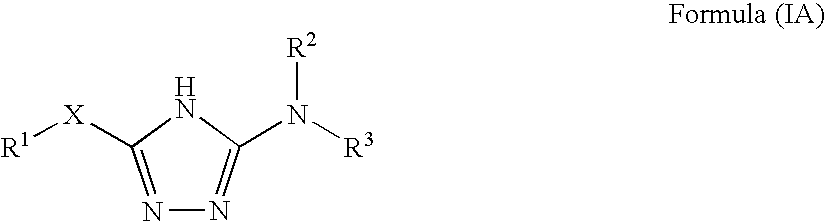
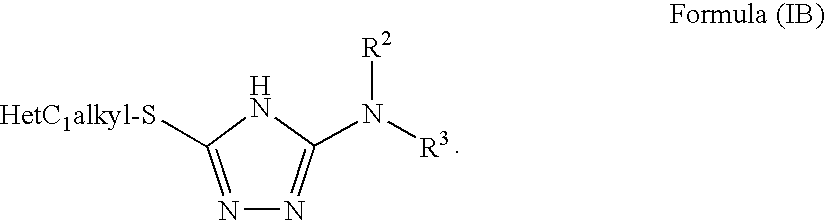
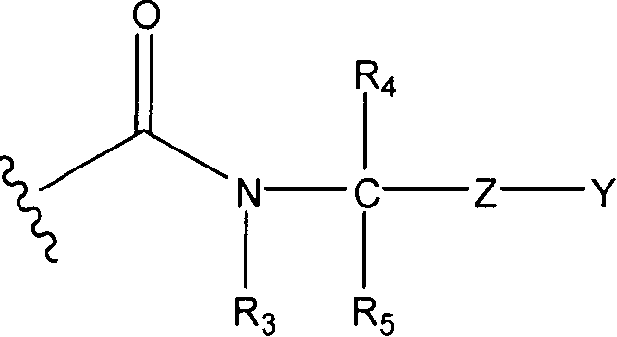
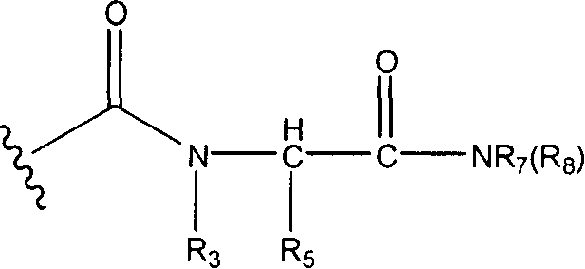
Disclosed are compounds of the formula: wherein the formula variables are as defined herein. The compounds of this invention are non-peptide, reversible inhibitors of type 2 methionine aminopeptidase. Also disclosed is the use of such compounds in treating conditions mediated by angiogenesis, such as cancer, haemangioma, proliferative retinopathy, rheumatoid arthritis, atherosclerotic neovascularization, psoriasis, ocular neovascularization and obesity.
Owner:SMITHKLINE BECKMAN CORP
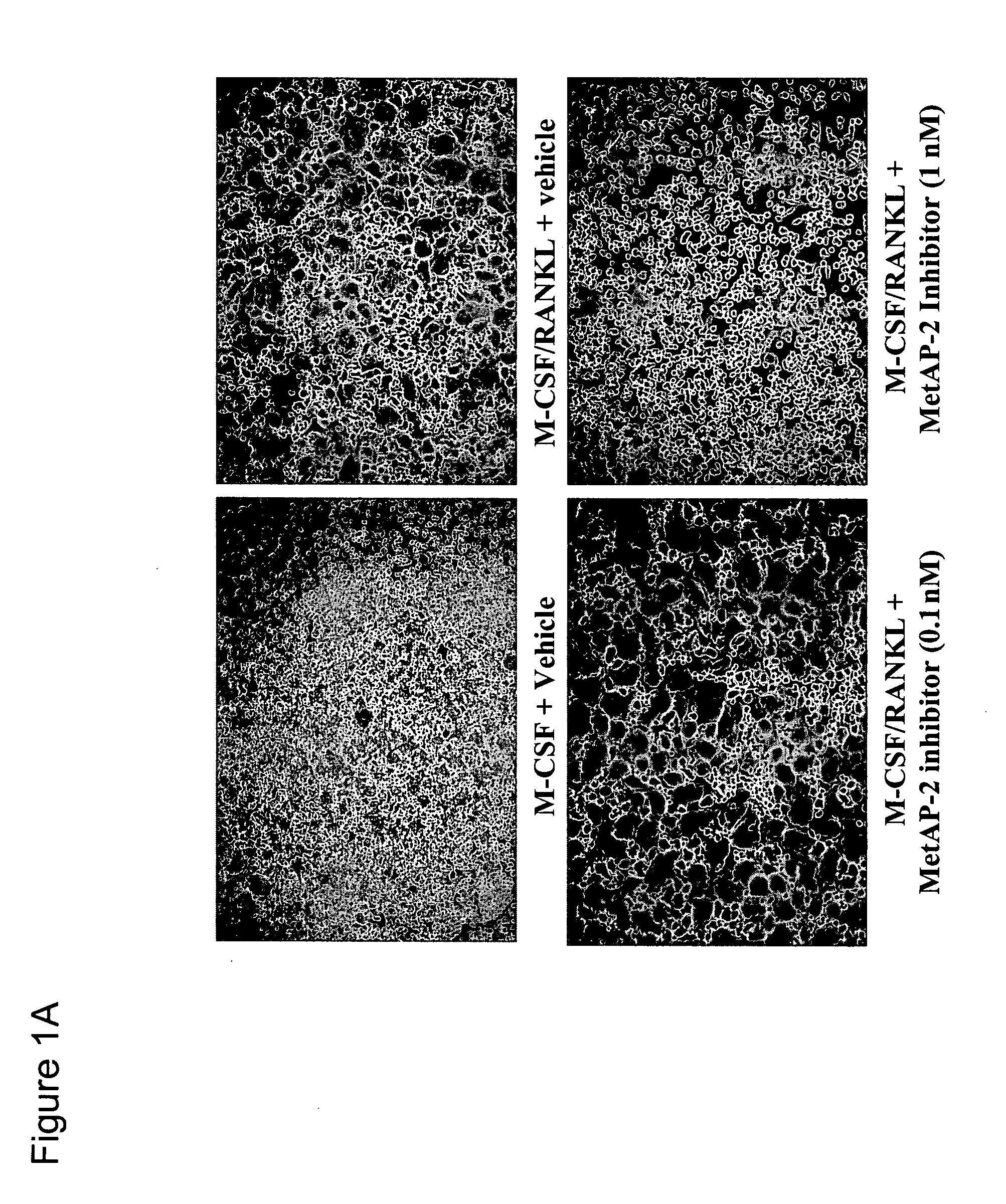
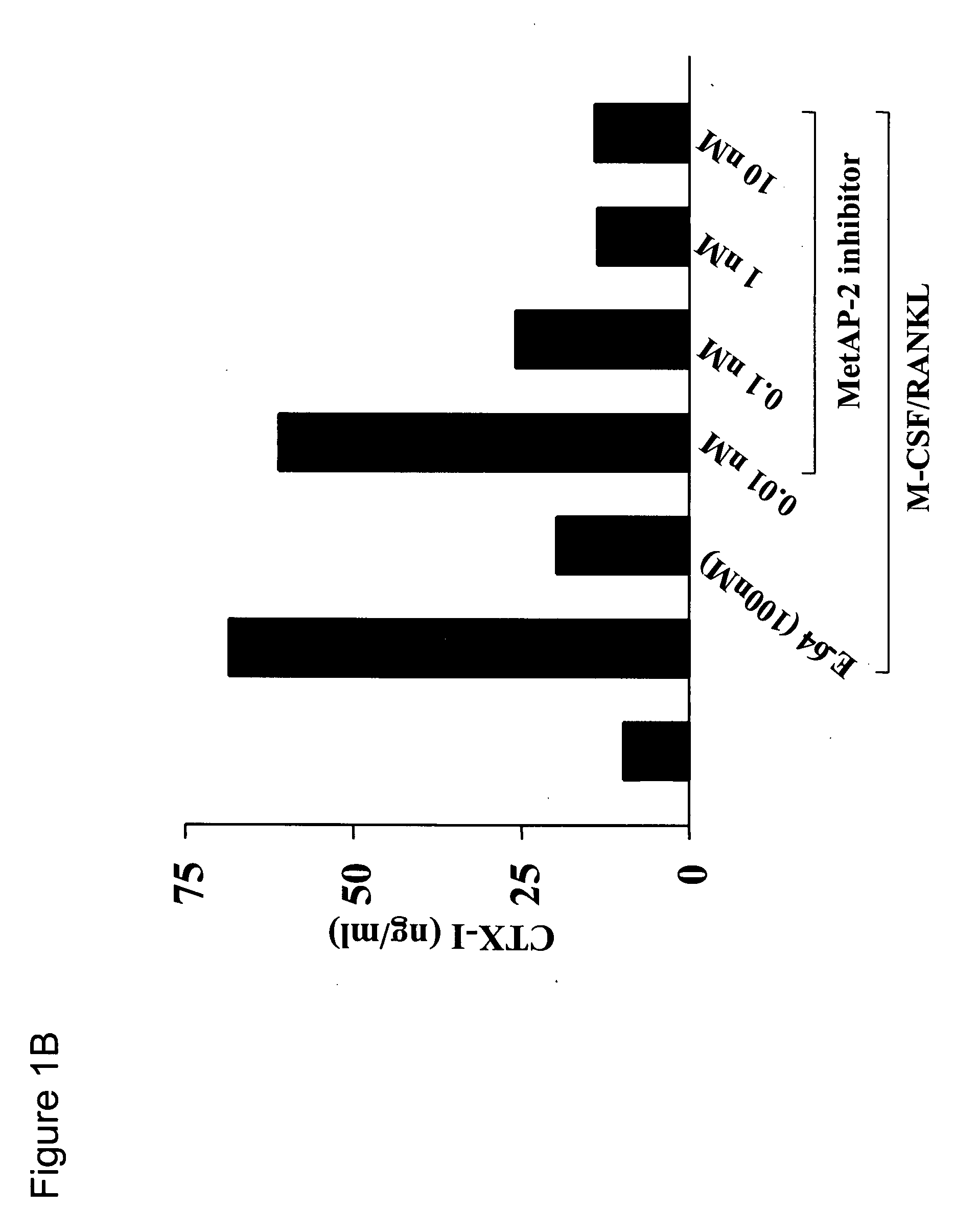
Methods for treating bone associated diseases by the use of methionine aminopeptidase-2 inhibitors
The instant invention provides methods and compositions for treating a subject suffering from bone associated diseases, such as osteoporosis.
Owner:PRAECIS PHARM INC
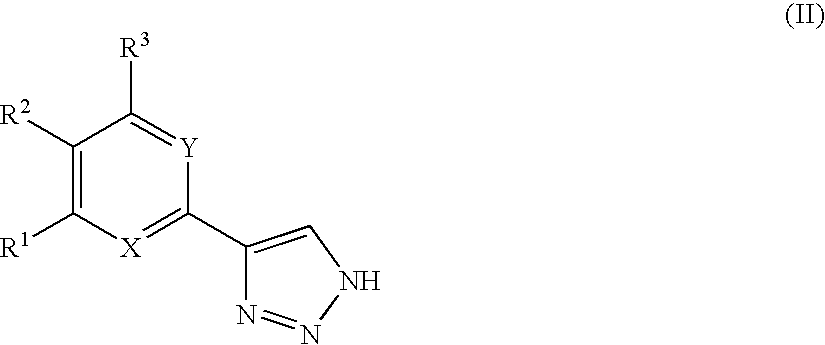
1,2,4-triazole derivatives, compositions, process of making and methods of use
Compounds of this invention are non-peptide, reversible inhibitors of type 2 methionine aminopeptidase, useful in treating conditions mediated by angiogenesis, such as cancer, haemangioma, proliferative retinopathy, rheumatoid arthritis, atherosclerotic neovascularization, psoriasis, ocular neovascularization and obesity.
Owner:GLAXO SMITHKLINE LLC
Methionine aminopeptidase-2 inhibitors and methods of use thereof
InactiveUS20070010452A1Low toxicityInhibit methionine aminopeptidase 2Dipeptide ingredientsTetrapeptide ingredientsMethionine aminopeptidaseMethionine biosynthesis
Owner:GLAXO SMITHKLINE LLC
Methionine Aminopeptidase Inhibitors for Treating Infectious Diseases
The present invention relates to methods for treating an infectious disease in a subject in need thereof via administration of a therapeutically effective amount of compounds described herein. The methods may utilize particular compounds, for example, a quinoline, a hydrazone, a quinone, or a pyrimidine derivative thereof or a pharmaceutical salt thereof.
Owner:TEXAS SOUTHERN UNIVERSITY +1
Substituted beta-amino acid inhibitors of methionine aminopeptidase-2
A class of substituted beta -amino acids are potent inhibitors of methionine aminopeptidase type 2 (MetAP2) and are thus useful in inhibiting angiogenesis and disease conditions which depend upon angiogenesis for their development such as diabetic retinopathy, tumor growth, and conditions of inflammation. Pharmaceutical compounds containing the compounds and methods of inhibiting methionine aminopeptidase-2, and angiogenesis are also disclosed.
Owner:ABBOTT LAB INC
Inhibitors of methionine aminopeptidases and methods of treating disorders
ActiveUS20120196852A1Inhibiting and reducingAntibacterial agentsBiocideMedicineMethionine aminopeptidase
The invention is directed towards novel naphthoquinone and naphthothiazole compounds, and methods of treating disorders related to MetAP, including tuberculosis and bacterial infection, using various naphthoquinone, hydroxyquinonline, and naphthothiazole compounds.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Methionine aminopeptidase-2 inhibitors and methods of use thereof
InactiveUS20070161570A1Better pharmacokinetic profileLimit CNS side effectOrganic active ingredientsBiocideMedicineMethionine aminopeptidase
The present invention provides angiogenesis inhibitor compounds comprising a MetAP-2 inhibitory core coupled to a peptide, as well as pharmaceutical compositions comprising the angiogenesis inhibitor compounds and a pharmaceutically acceptable carrier. The present invention also provides methods of inducing an immunosupressed condition and / or treating chronic allograft vasculopathy in a subject undergoing or who has undergone a transplant, by administering to the subject a therapeutically effective amount of one or more of the compounds of the invention.
Owner:PRAECIS PHARM INC
Compounds and methods
Compounds of this invention are non-peptide, reversible inhibitors of type 2 methionine aminopeptidase, useful in treating conditions mediated by angiogenesis, such as cancer, haemangioma, proliferative retinopathy, rheumatoid arthritis, atherosclerotic neovascularization, psoriasis, ocular neovascularization and obesity.
Owner:CROSS MATCH TECH +1
Human methionine aminopeptidase type 3
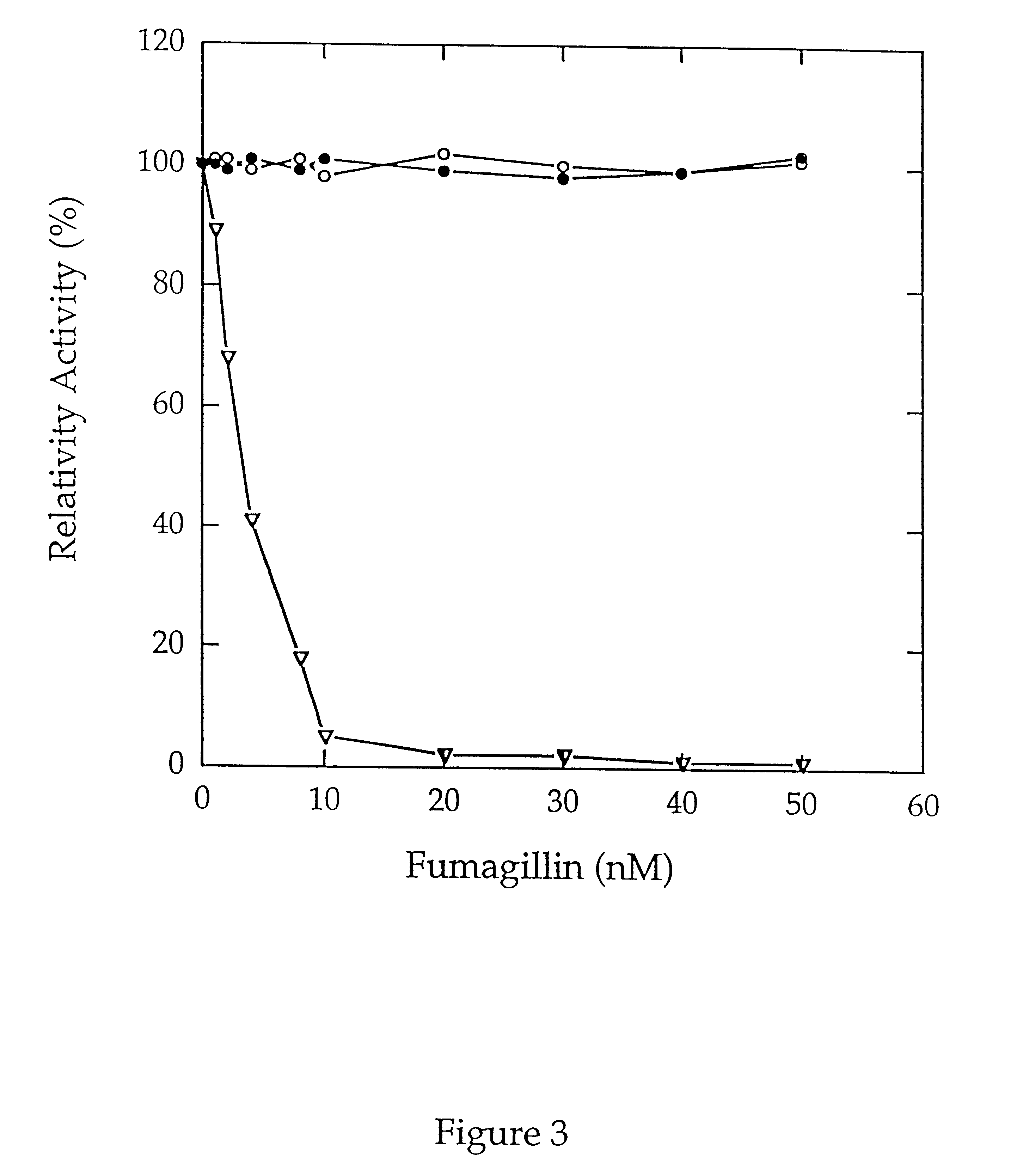
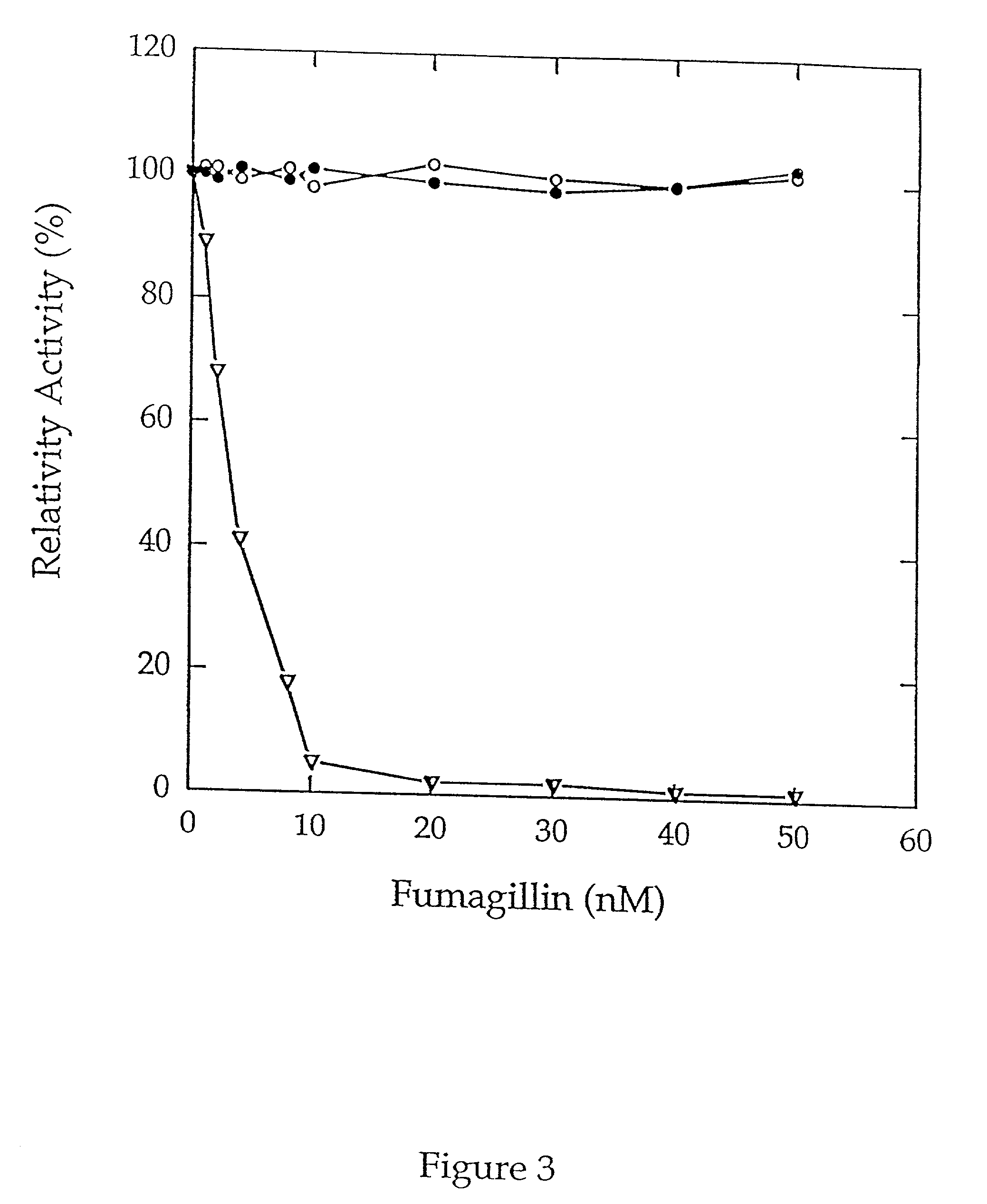
Methionine aminopeptidases catalyse the co-translational removal of amino terminal methionine residues from nascent polypeptide chains. A newly-discovered enzyme, designated methionine aminopeptidase type-3 (MetAP-3), has a substrate specificity which is similar to MetAP-1 and MetAP-2, although it is not inhibited by fumagillin, an irreversible inhibitor of MetAP-2. MetAP-3 also preferentially localizes to mitochondria, unlike MetAP-1 and MetAP-2, which accumulate in the cytoplasm. One embodiment of the present invention relates to human cDNAs encoding polypeptides comprising MetAP-3. Other embodiments of the invention relate to nucleic acid molecules derived from these cDNAs, including complements, homologues, and fragments thereof, and methods of using these nucleic acid molecules, to generate polypeptides and fragments thereof. Other embodiments of the invention relate to antibodies directed against polypeptides comprising MetAP-3, and methods to screen for compounds or compositions that preferentially or specifically effect the activity of polypeptides comprising MetAP-3.
Owner:PHARMACIA CORP
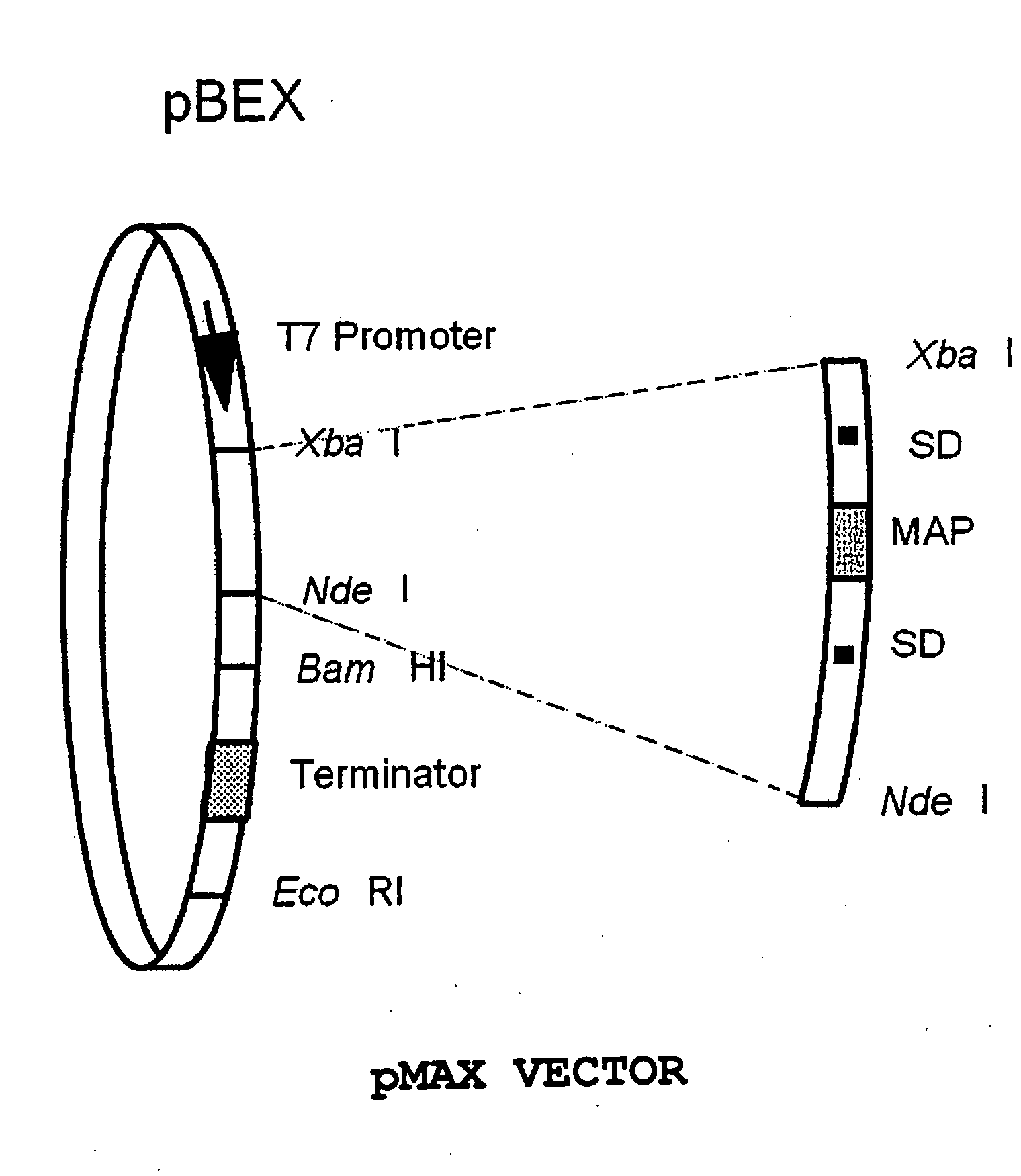
High-Copy-Number, High-Expression Vector Having Methionine Aminopeptidase Gene
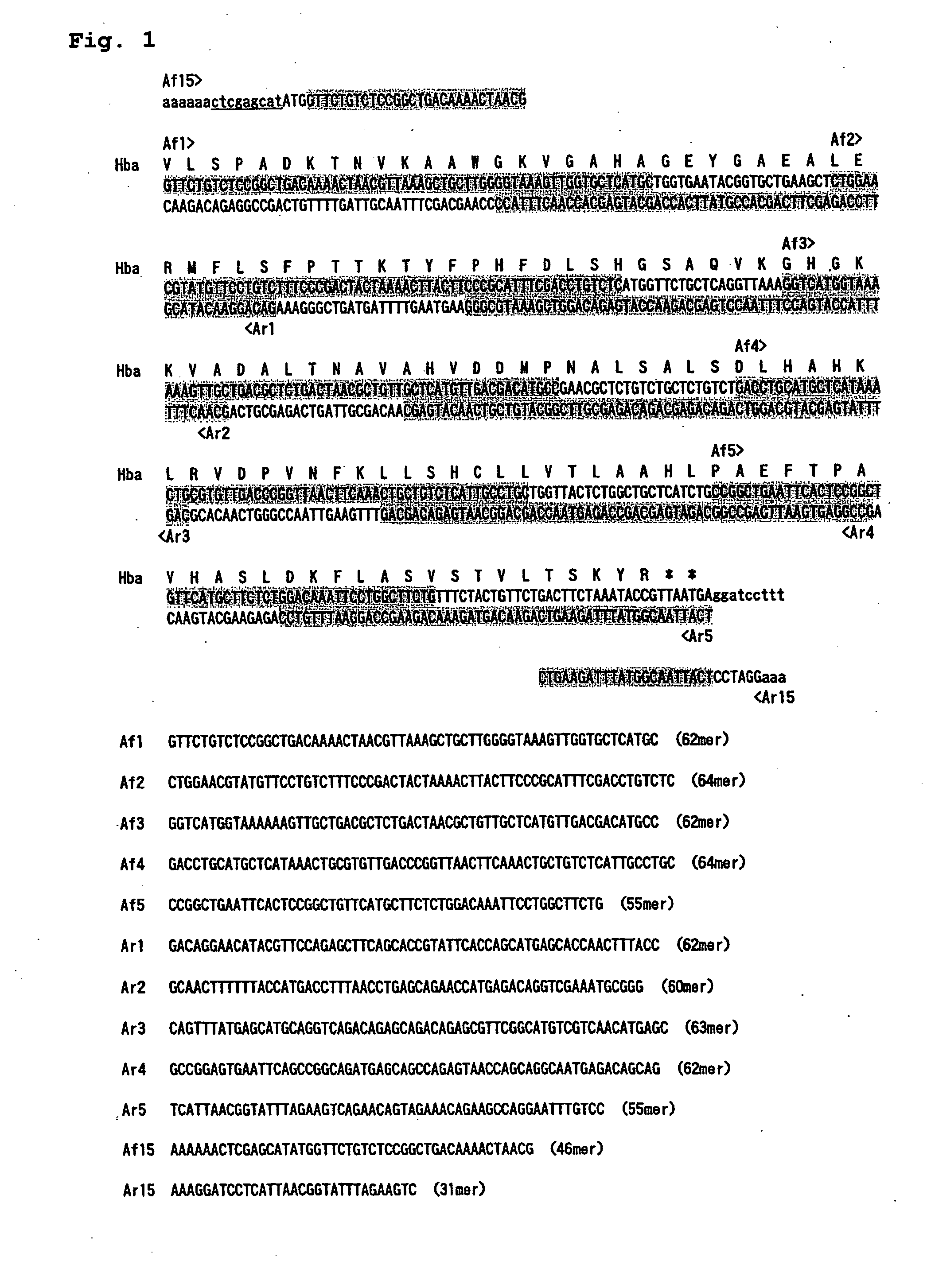
InactiveUS20080166769A1Simple waySatisfactory function/activityBacteriaHaemoglobins/myoglobinsMethionine aminopeptidaseCloning Site
Provided is a high-copy-number, high-expression vector capable of producing a protein having satisfactory functions and activity of the same level as that of a natural form of the protein, in a large quantity and in a simple manner. Also provided is a vector including: (A) a target gene or a cloning site of the target gene, (B) a sequence element necessary for the high copying of the target gene, (C) a sequence element necessary for the expression of the target gene and a methionine aminopeptidase gene; a method of producing the vector; a transformant having the vector introduced therein; and a process for producing a protein using the transformant.
Owner:NIPRO CORP
Formulations of Methionine Aminopeptidase Inhibitors for Treating Infectious Diseases
InactiveUS20170304288A1Organic chemistryComponent separationMethionine aminopeptidaseInfective disorder
Provided herein are formulations and co-solvent formulations and methods for treating an infectious disease utilizing the same. The formulations and co-solvent formulations may comprise a hydroxyquinoline analog or its pharmaceutically acceptable salt, a solvent and at least two surfactants. Also provided are methods of quantitating a hydroxyquinoline analog in a sample via chromatographic / spectrometric measurements.
Owner:TEXAS SOUTHERN UNIVERSITY
Methionine aminopeptidase-2 inhibitors and methods of use thereof
InactiveUS20090156624A1Low toxicityInhibit methionine aminopeptidase 2BiocidePeptide/protein ingredientsMethionine aminopeptidaseCancer research
Owner:PRAECIS PHARM INC
Inhibitors of methionine aminopeptidase-2 and uses thereof
InactiveCN1902215AOrganic active ingredientsNervous disorderAutoimmune conditionMethionine aminopeptidase
The present invention provides compositions and methods for treating a patient suffering from one of a variety of diseases, including angiogenic diseases, such as cancer, autoimmune disease or parasitic infection.
Owner:PRAECIS PHARM INC
Methionine Aminopeptidase Inhibitors for Treating Infectious Diseases
The present invention relates to methods for treating an infectious disease in a subject in need thereof via administration of a therapeutically effective amount of compounds described herein. The methods may utilize particular compounds, for example, a quinoline, a hydrazone, a quinone, or a pyrimidine derivatives thereof or a pharmaceutical salts thereof.
Owner:TEXAS SOUTHERN UNIVERSITY +1
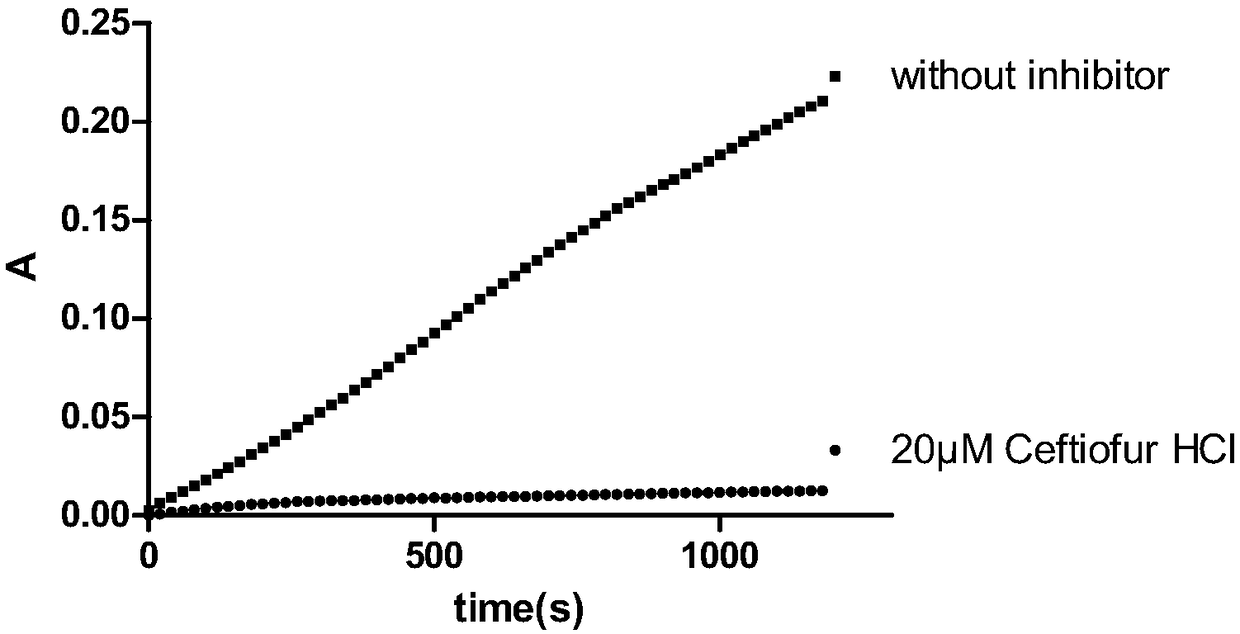
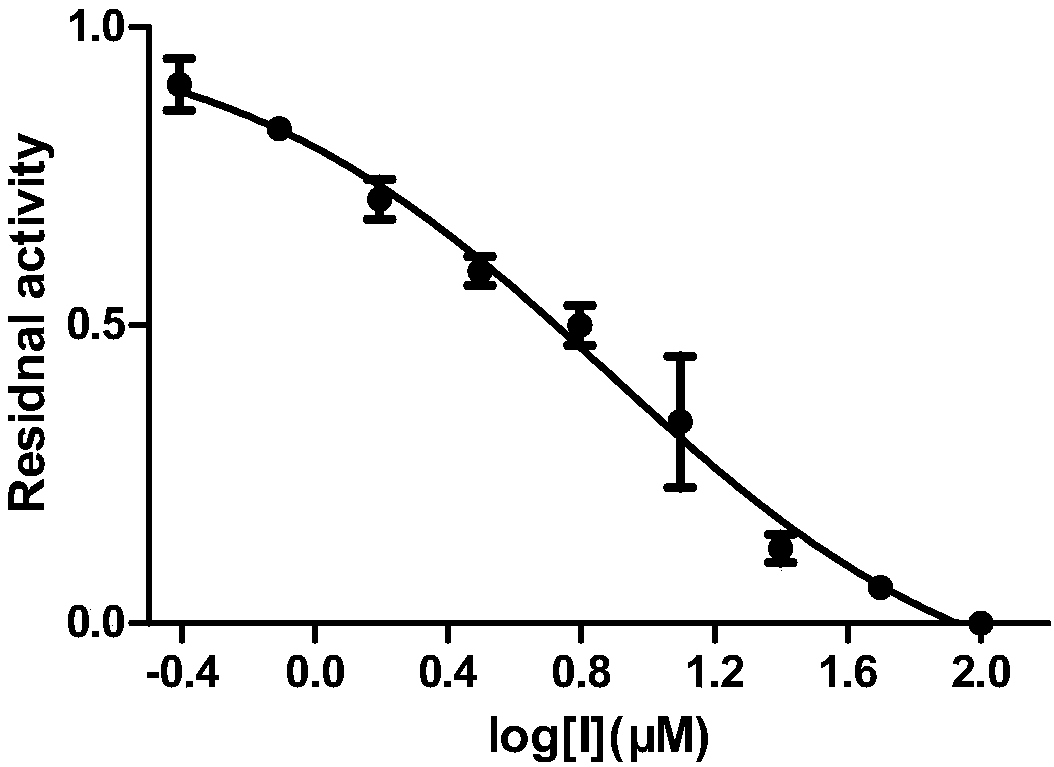
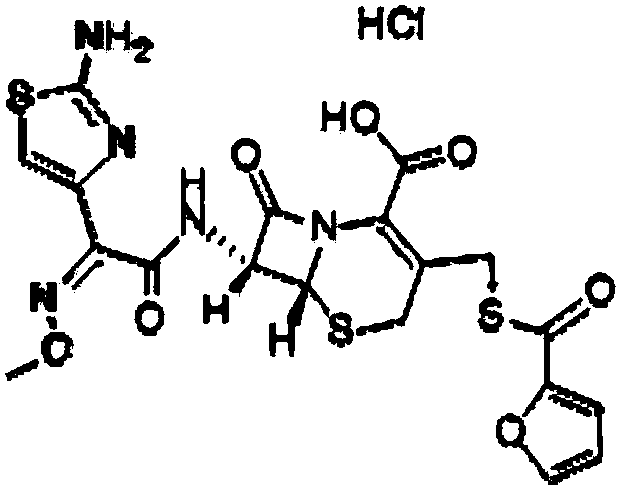
Applications of ceftiofur hydrochloride in prevention of mycobacterium tuberculosis infection
InactiveCN108125957AEnhanced inhibitory effectAntibacterial agentsOrganic active ingredientsMethionine aminopeptidaseMicrobiology
Owner:TIANJIN INT JOINT ACADEMY OF BIOTECH & MEDICINE
Preparation method for recombinant human interferon alpha-2b free of methionine
ActiveCN102367441APrecise positioningImprove stabilityFermentationInterferonsMethionine aminopeptidaseMethionine biosynthesis
The invention specifically relates to a preparation method for recombinant human interferon alpha-2b free of methionine, which belongs to the field of preparation of interferon. According to the method, primers are designed to amplify gene segments of methionine aminopeptidase, the gene segments of methionine aminopeptidase are cloned into the expression plasmid of pACYCDuct-1 to obtain the recombinant expression plasmid of pACYCDuct-1-MetAP which is introduced into the recombinant expression plasmid of pJW2-rhIFN-alpha-2b containing human interferon alpha-2b genes, 0.1 mu M IPTG is utilized to induce methionine aminopeptidase to express, human interferon alpha-2b protein is induced to express at a temperature of 40 DEG C, and methionine at N terminal of the human interferon alpha-2b protein is resected in the process of expression. The preparation method provided in the invention enables unnecessary Met residue at the N terminal of human interferon alpha-2b to be removed, stability and antiviral activity of human interferon alpha-2b to be improved, immunogenicity of human interferon alpha-2b to be reduced and an incidence rate of adverse reactions during utilization of human interferon alpha-2b by patients to be reduced.
Owner:ANHUI ANKE BIOTECHNOLOGY (GRP) CO LTD
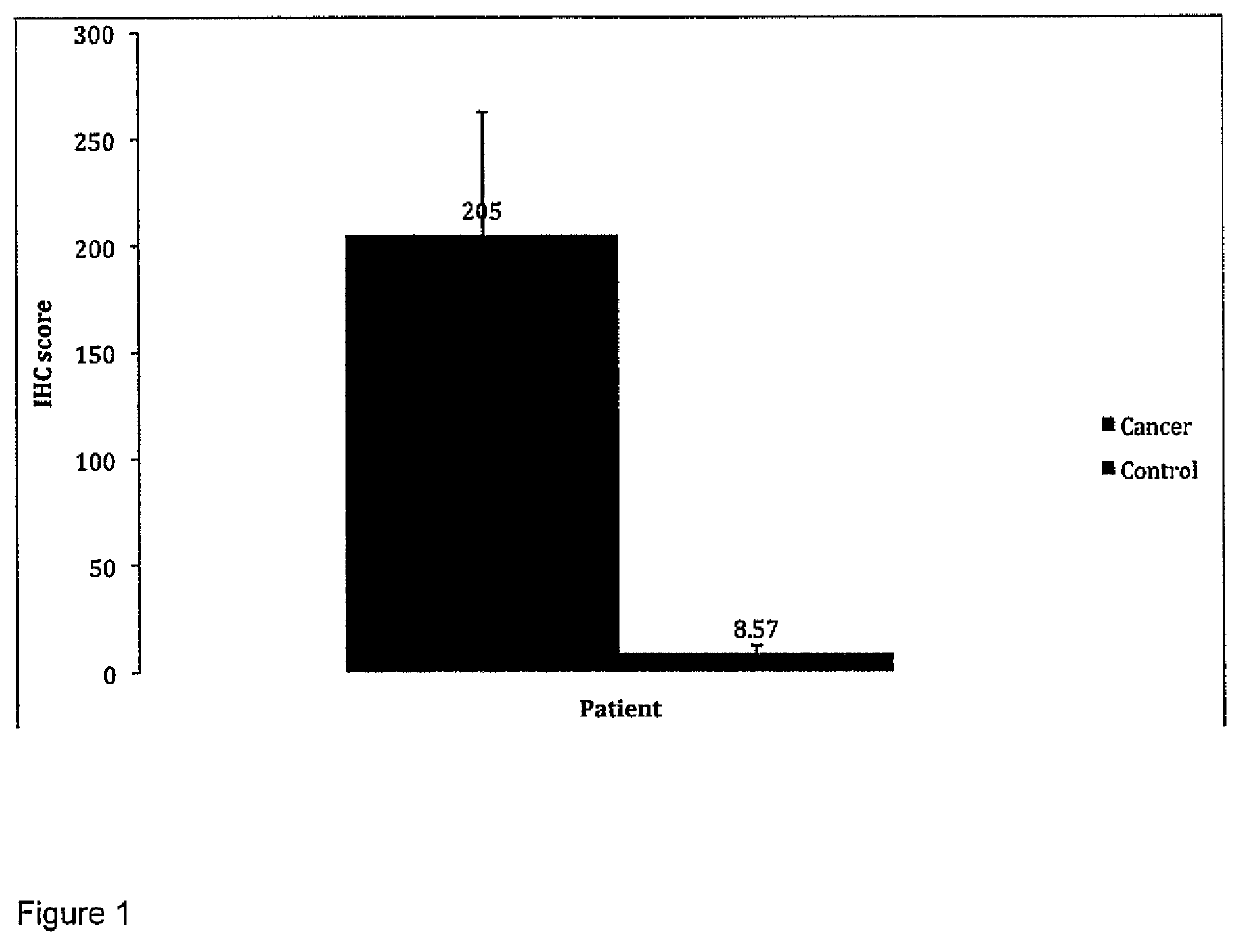
Methionine aminopeptidase overexpression in the peripheral blood and peripheral blood mononuclear cells is a marker for colorectal cancer screening, diagnosis and prognosis
ActiveUS11506663B2Health-index calculationMicrobiological testing/measurementPeripheral blood mononuclear cellMethionine aminopeptidase
Owner:VASTCON
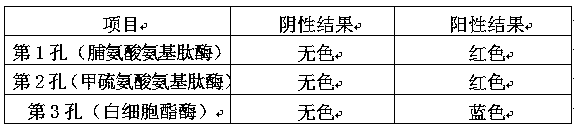
Kit for detecting mycoplasma genitalium
ActiveCN109576337AReduce testing costsReduce financial burdenMicrobiological testing/measurementMicroorganism based processesMethionine aminopeptidaseBiology
The invention discloses a kit for detecting mycoplasma genitalium. The invention provides two schemes; in a scheme I, the kit comprises two reagents, namely methionine aminopeptidase and leucocyte esterase, wherein an enzyme substrate for detecting the activity of the methionine aminopeptidase is a methionine derivative containing a chromogen, and an enzyme substrate for detecting the activity ofthe leucocyte esterase is an ester derivative containing the chromogen; in a scheme II, the kit comprises three reagents, i.e., the kit is also comprises a proline aminopeptidase reagent on the basisof the scheme I, and an enzyme substrate for detecting the activity of proline aminopeptidase is a proline derivative containing the chromogen. According to the kit disclosed by the invention, autosynthetic enzyme or enzyme generated by stimulating parasitic cells during a growth process of the Mg can be detected through a dry chemical enzyme method, the purpose of detecting the mycoplasma genitalium can be achieved, and the kit has the advantages of quickness, simpleness, accuracy and the like. By adopting the kit for detecting the mycoplasma genitalium, the sensitivity and the specificity are higher, properties of the kit are similar to properties of existing like detection reagents, and clinical test needs can be completely met.
Owner:AUTOBIO DIAGNOSTICS CO LTD
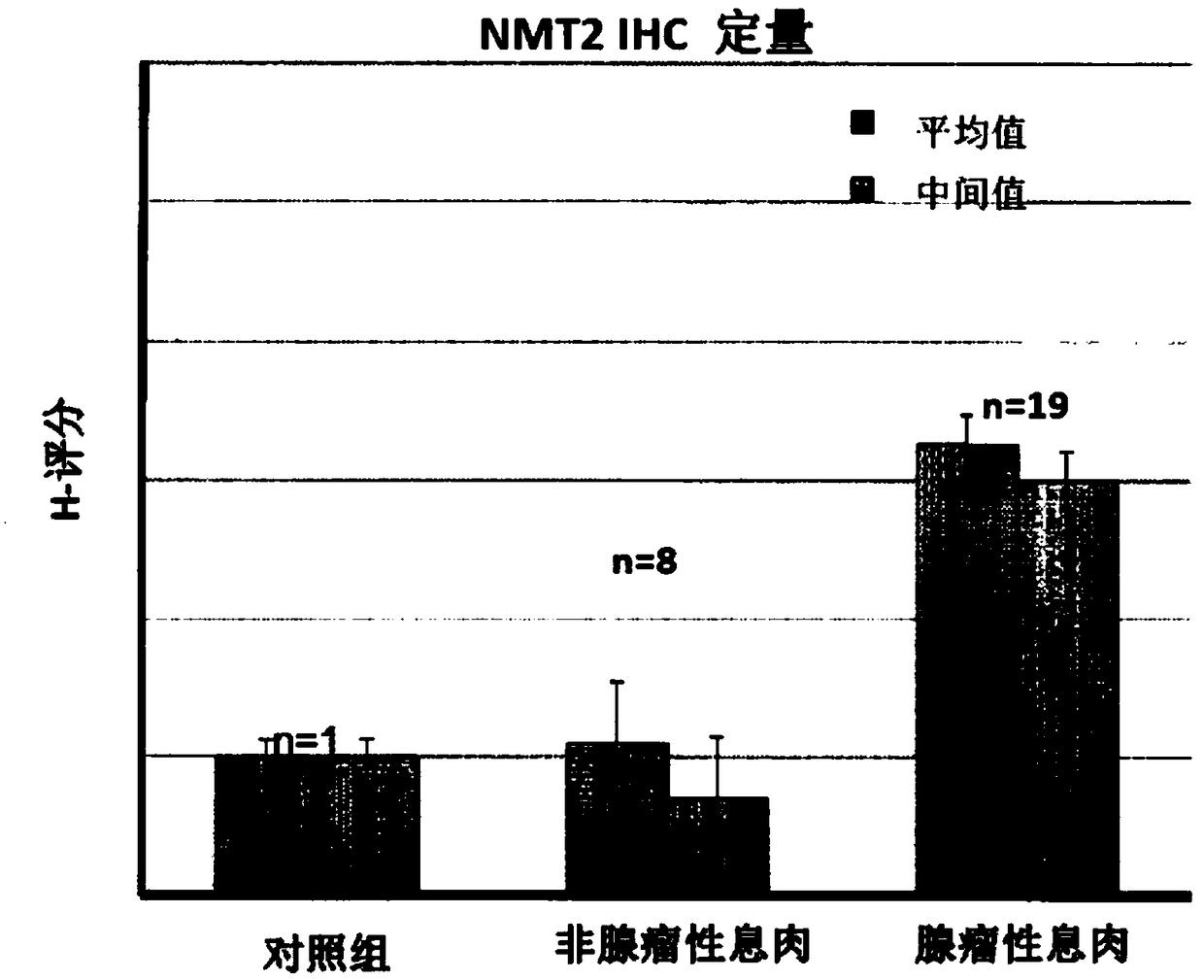
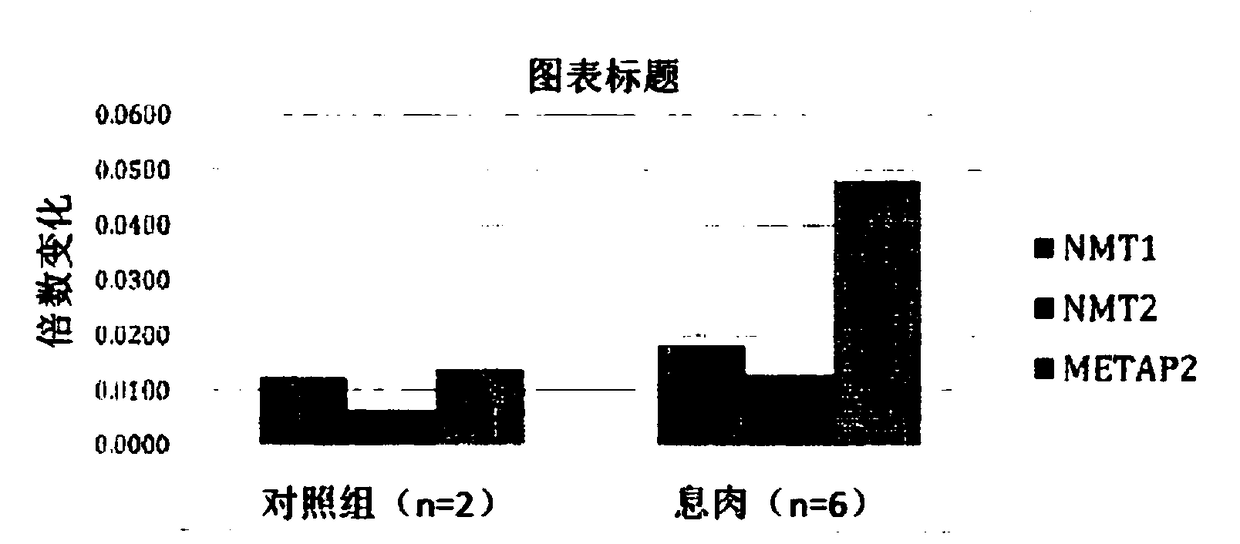
N-myristoyltransferase (NMT)1, nmt2 and methionine aminopeptidase 2 overexpression in peripheral blood and peripheral blood mononuclear cells is a marker for adenomatous polyps and early detection of colorectal cancer
InactiveCN109379896AMicrobiological testing/measurementDisease diagnosisPeripheral blood mononuclear cellMethionine aminopeptidase
Described herein is the identification of the NMT1, NMT2 and metAP2 genes, mRNA overexpressed in PBMCs of patients with adenomatous polyps in comparison with patients with non-adenomatous polyps and healthy controls. We also discovered that NMT2 levels are higher in the PBMCs of patients adenomatous polyps in comparison with patients with non-adenomatous polyps and healthy control subjects.
Owner:VASTCON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com