Patents
Literature
30 results about "Fumagillin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
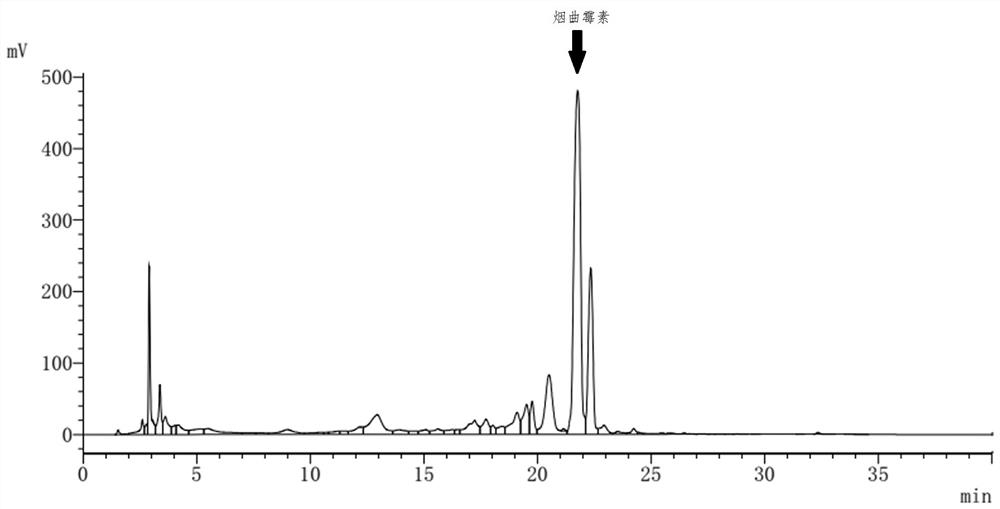
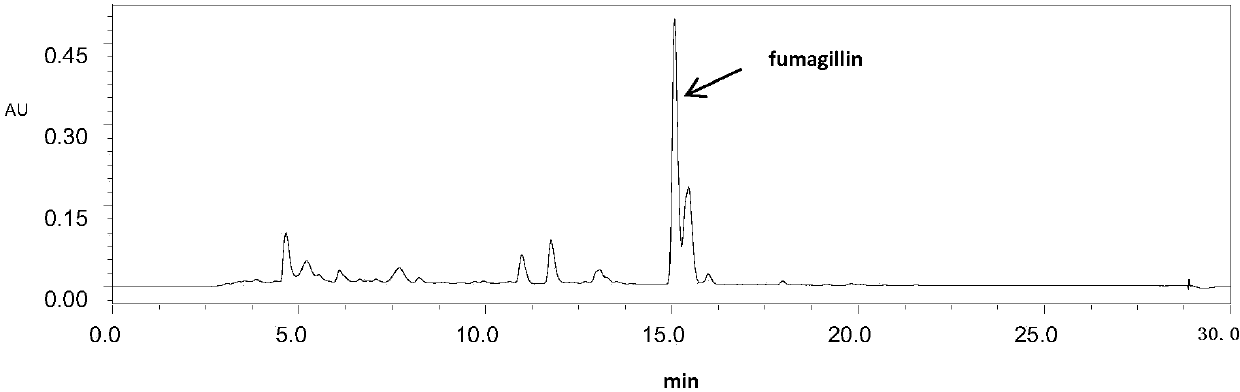
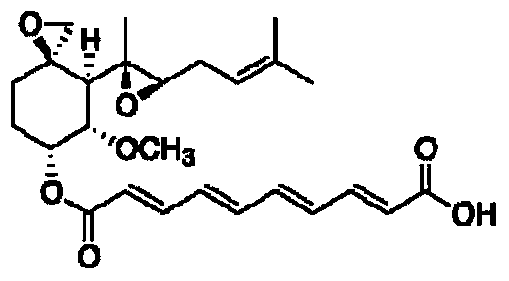
Fumagillin is a complex biomolecule and used as an antimicrobial agent. It was isolated in 1949 from the microbial organism Aspergillus fumigatus.
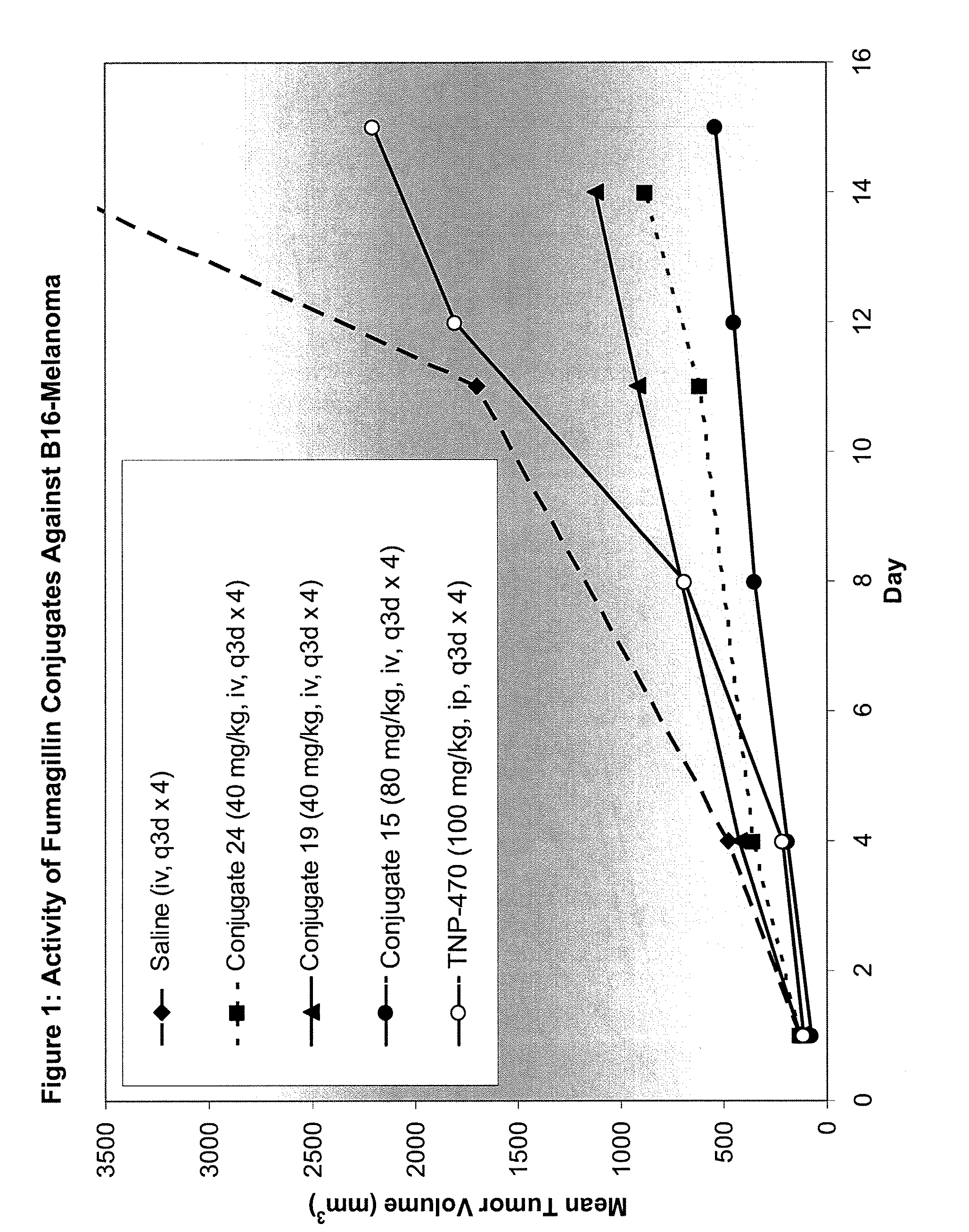
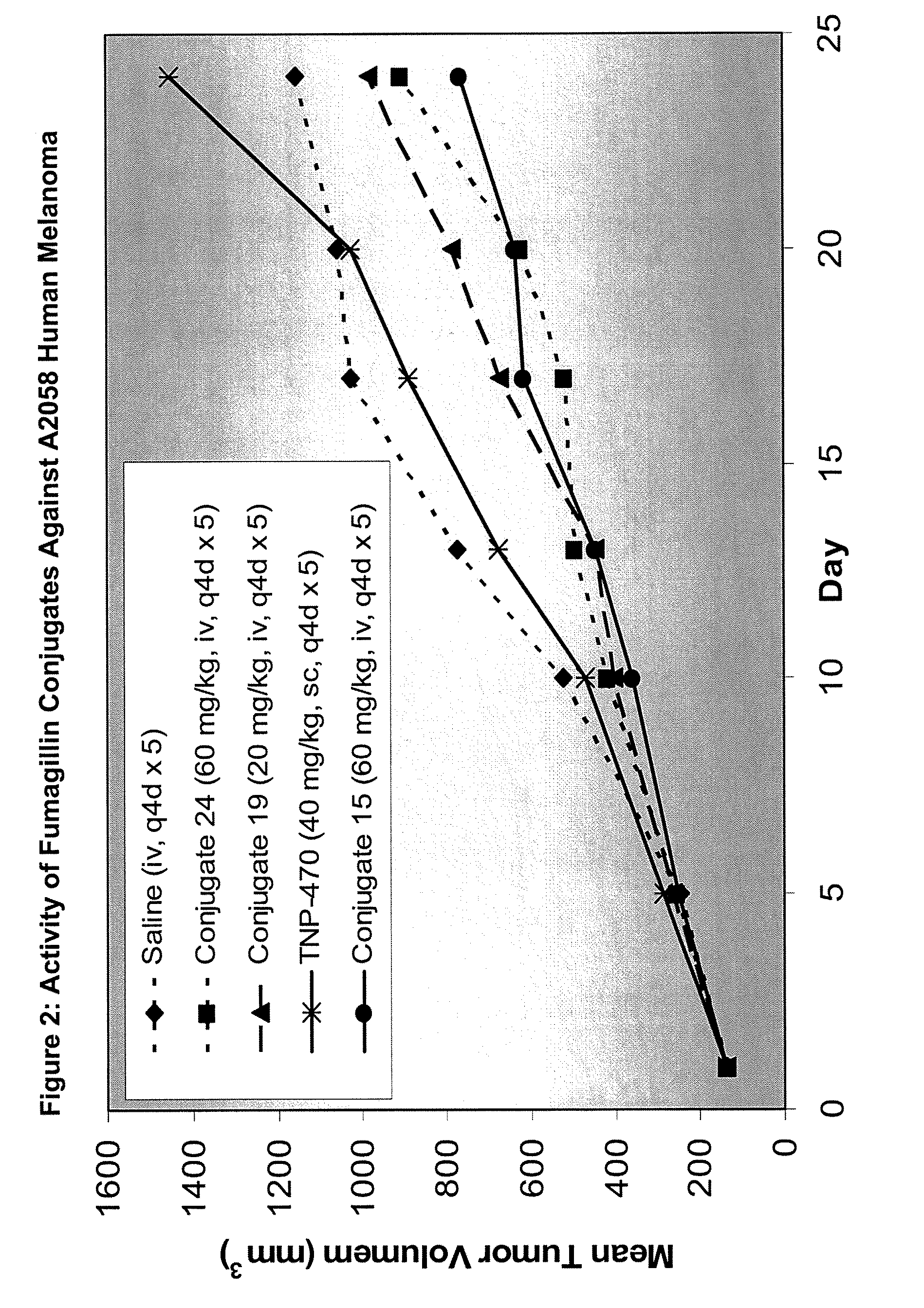
Biocompatible biodegradable fumagillin analog conjugates
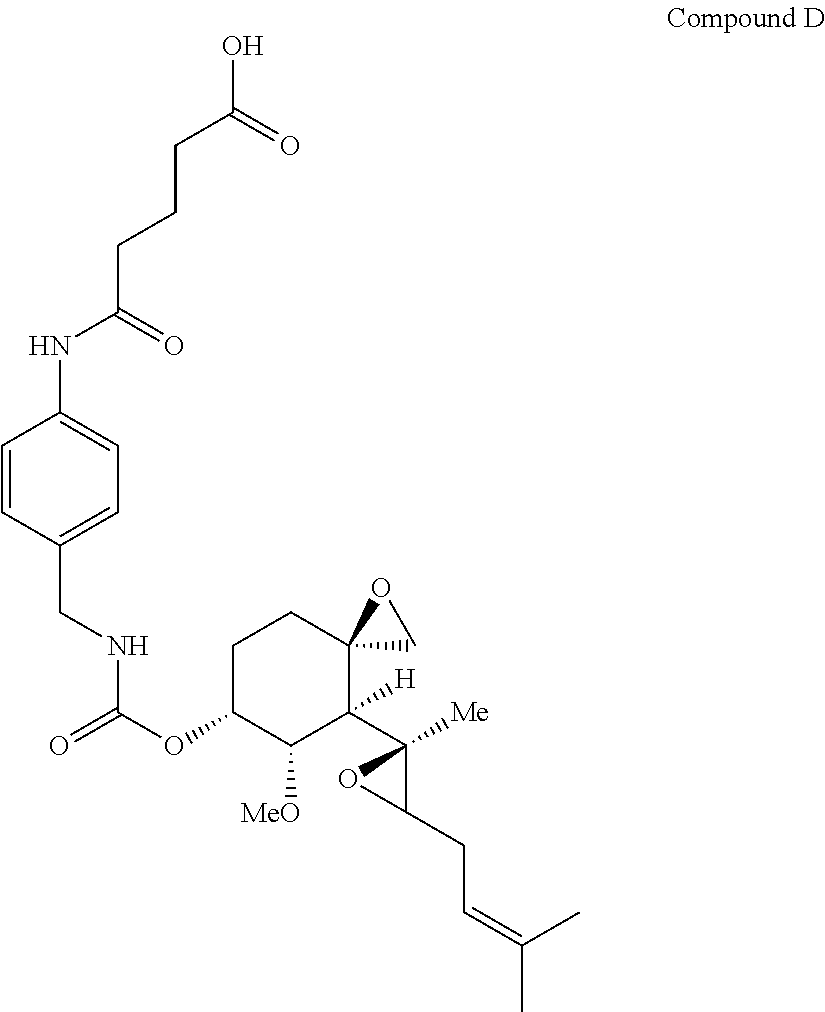
Fumagillin analog polymer conjugates, methods of making fumagillin analog polymer conjugates, compositions comprising a polymer conjugate of a fumagillin analog, and methods for treating cancer, or treating angiogenic diseases comprising administering to a subject in need thereof an effective amount of a polymer conjugate of a fumagillin analog, are described. Also described are novel fumagillin analogs, methods of making fumagillin analogs, compositions comprising at least one fumagillin analog, and methods for treating cancer, or treating angiogenic diseases comprising administering to a subject in need thereof an effective amount of a fumagillin analog.
Owner:MERSANA THERAPEUTICS INC
Biocompatible biodegradable fumagillin analog conjugates
Fumagillin analog polymer conjugates, methods of making fumagillin analog polymer conjugates, compositions comprising a polymer conjugate of a fumagillin analog, and methods for treating cancer, or treating angiogenic diseases comprising administering to a subject in need thereof an effective amount of a polymer conjugate of a fumagillin analog, are described. Also described are novel fumagillin analogs, methods of making fumagillin analogs, compositions comprising at least one fumagillin analog, and methods for treating cancer, or treating angiogenic diseases comprising administering to a subject in need thereof an effective amount of a fumagillin analog.
Owner:MERSANA THERAPEUTICS INC
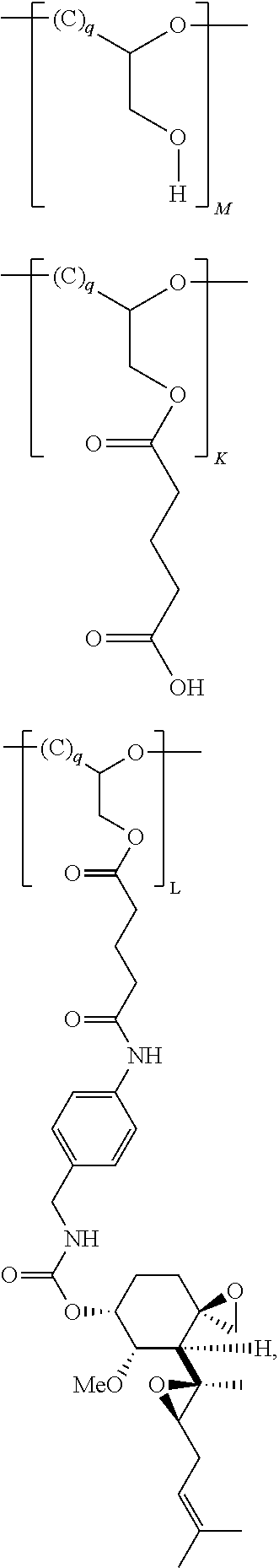
Pharmaceutical formulations for fumagillin derivative-phf conjugates
InactiveUS20130189218A1Effective treatmentOrganic chemistryDigestive systemPharmaceutical formulationPolymer
The invention described herein provides a mixture comprising polymer molecules or salts thereof, wherein a polymer molecule in the mixture comprises covalently bound subunits L, K, and M wherein the average molecular weight of the polymer molecules in the mixture is about 50 kDa to about 200 kDa, wherein the mole percentage of subunit M, K and L, relative to the total amount of subunits in the mixture, is about 90.5 to about 96 mol %, about 2.8 to about 7.3 mol %, and about 1.2 to about 2.2 mol %, respectively.
Owner:MERSANA THERAPEUTICS INC
Extraction method of fumagillin
InactiveCN105622593AAvoid the extraction processAvoid stripping and other processesOrganic chemistryResource utilizationSolvent
The invention provides an extraction method of fumagillin.The method comprises the steps that 1, the pH value of fumagillin fermentation liquor is adjusted to be 1-6.0 and adsorbed with macroporous resin; 2, the resin adsorbing the fermentation liquor is put into a chromatographic column and cleaned with ethanol water with the mass concentration of 15%-30%; 3, the resin is eluted with absolute ethyl alcohol or ethanol water with the mass concentration of 50%-98%, eluent is concentrated and dried, and a coarse fumagillin extraction product is obtained.According to the extraction method, the processes such as various solvent extraction and back extraction in a traditional technology are omitted, the operational reliability is high, simpleness and convenience are achieved, the production process is safe and free of pollution, the resource utilization rate is high, the production cost is low, environmental friendliness is facilitated, and the extraction method is a green and environment-friendly method for extracting the fumagillin.
Owner:BEE RES INST CHINESE ACAD OF AGRI SCI
Method for producing fumagillin by aspergillus fumigatus
ActiveCN106591389AIncrease productionOrganic active ingredientsAntipyreticSporeAspergillus fumigatus
The invention relates to a method for producing fumagillin by aspergillus fumigatus. The method comprises the following steps: inoculating aspergillus fumigatus into a GMM solid medium to activate strains, wherein the pH value of the GMM medium is 5 to 7; preparing activated strain spores into spore suspension, and then inoculating the spore suspension into a CYA solid medium, a CXMA solid medium or an LMM liquid medium, wherein the pH values of the CYA solid medium and the CXMA solid medium are natural, and the pH value of the LMM liquid medium is adjusted to be 5 to 7, and carrying out culturing at the temperature of 25 to 37 DEG C; collecting cultured aspergillus fumigatus to extract fumagillin and fumagillin derivatives. According to the invention, carbon and nitrogen sources and trace elements of fumagillin which affect the growth of aspergillus fumigatus are aimed to be screened, and the concentration of corresponding trace elements is optimized to obtain higher yield of fumagillin.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
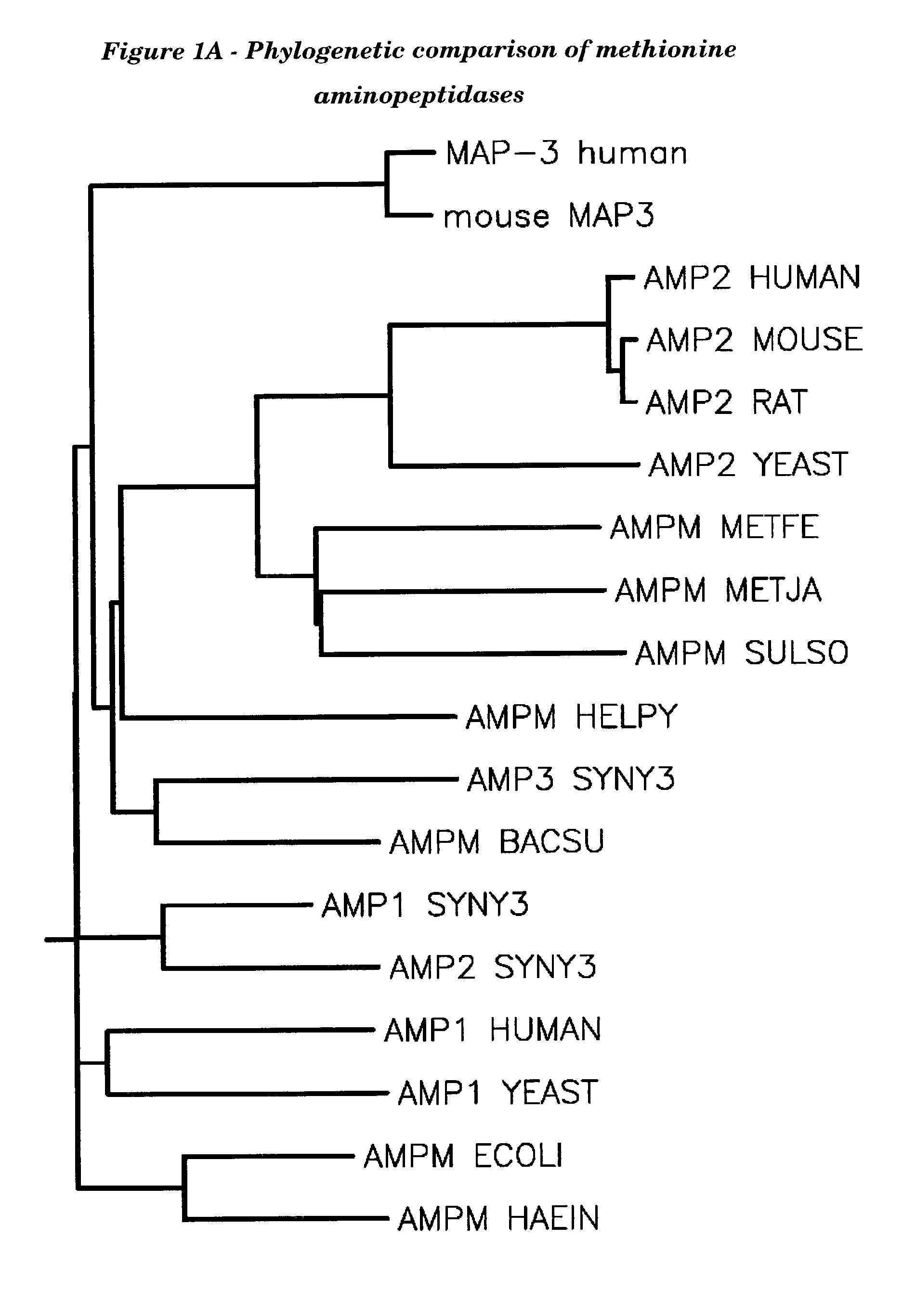
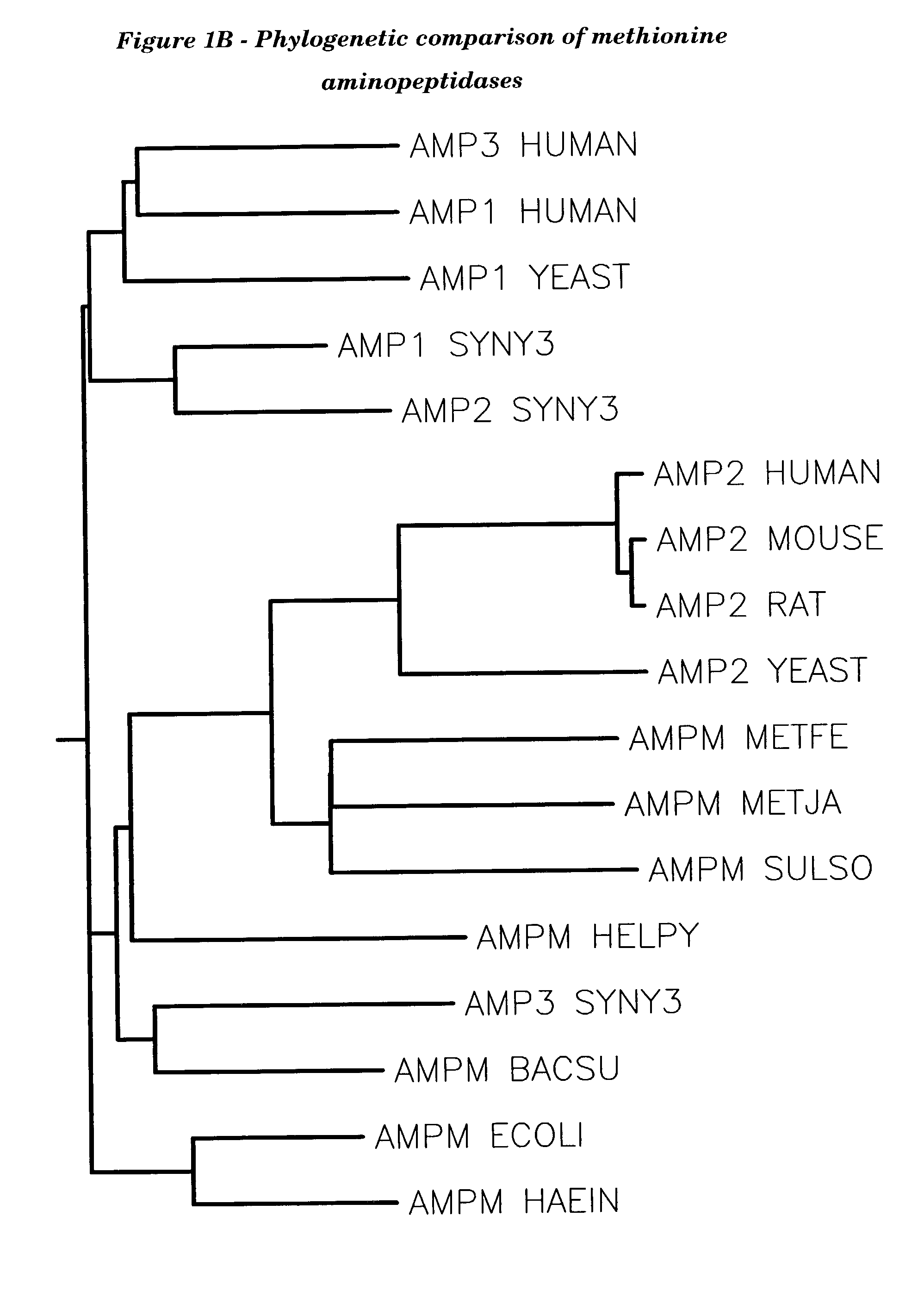
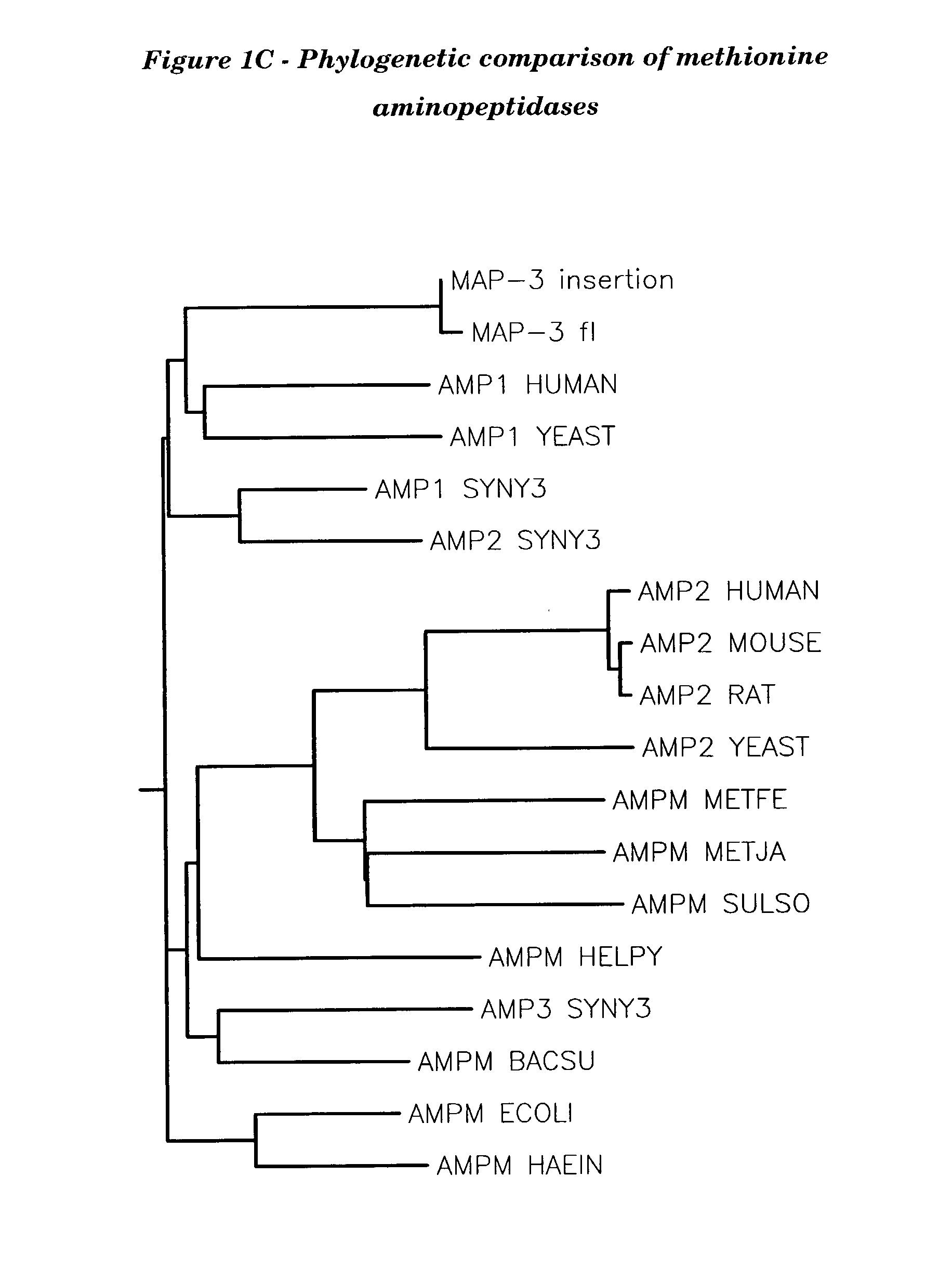
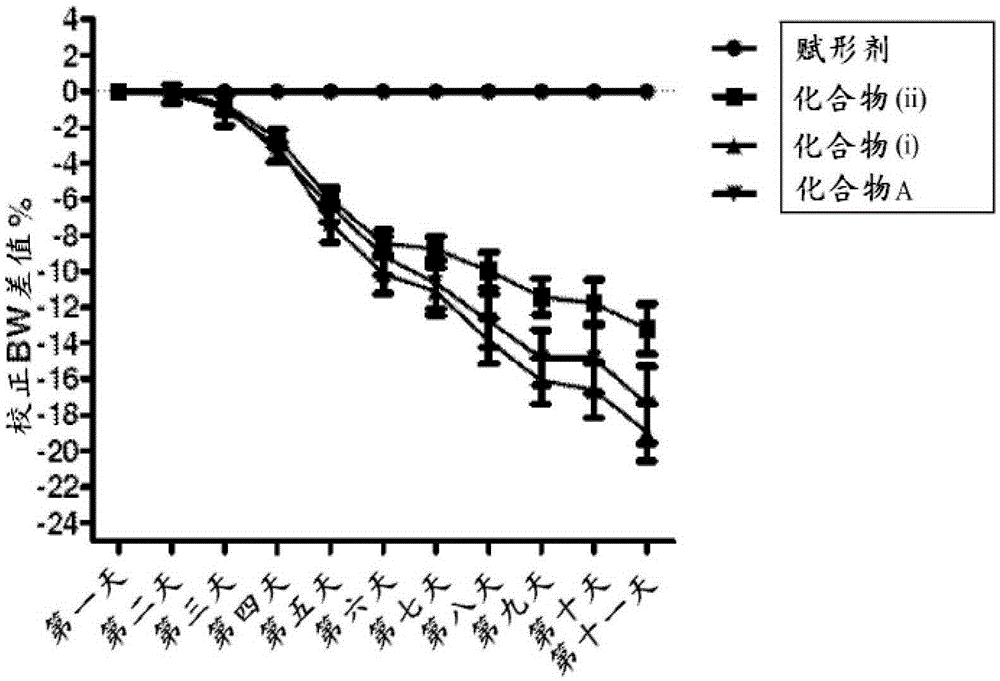
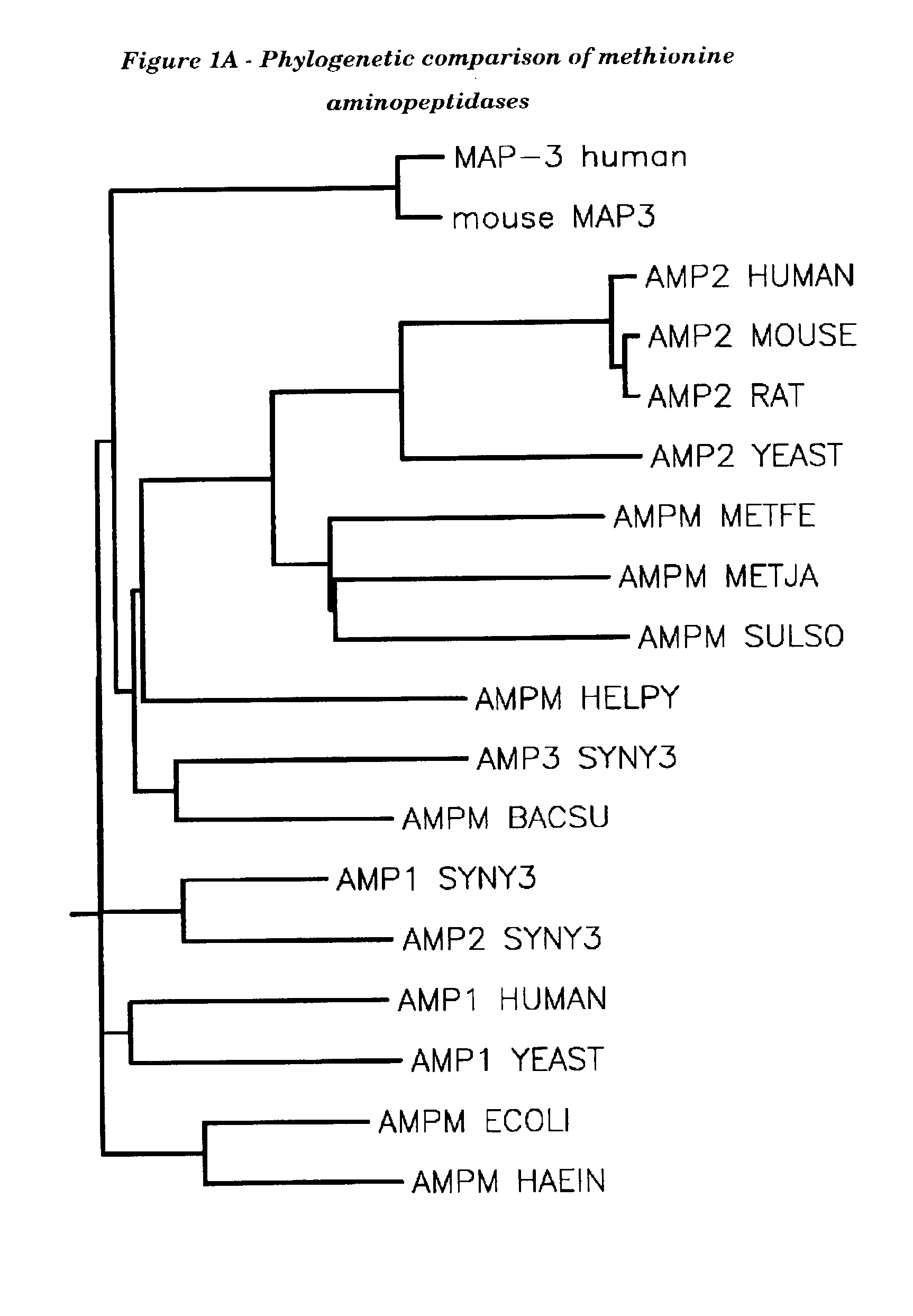
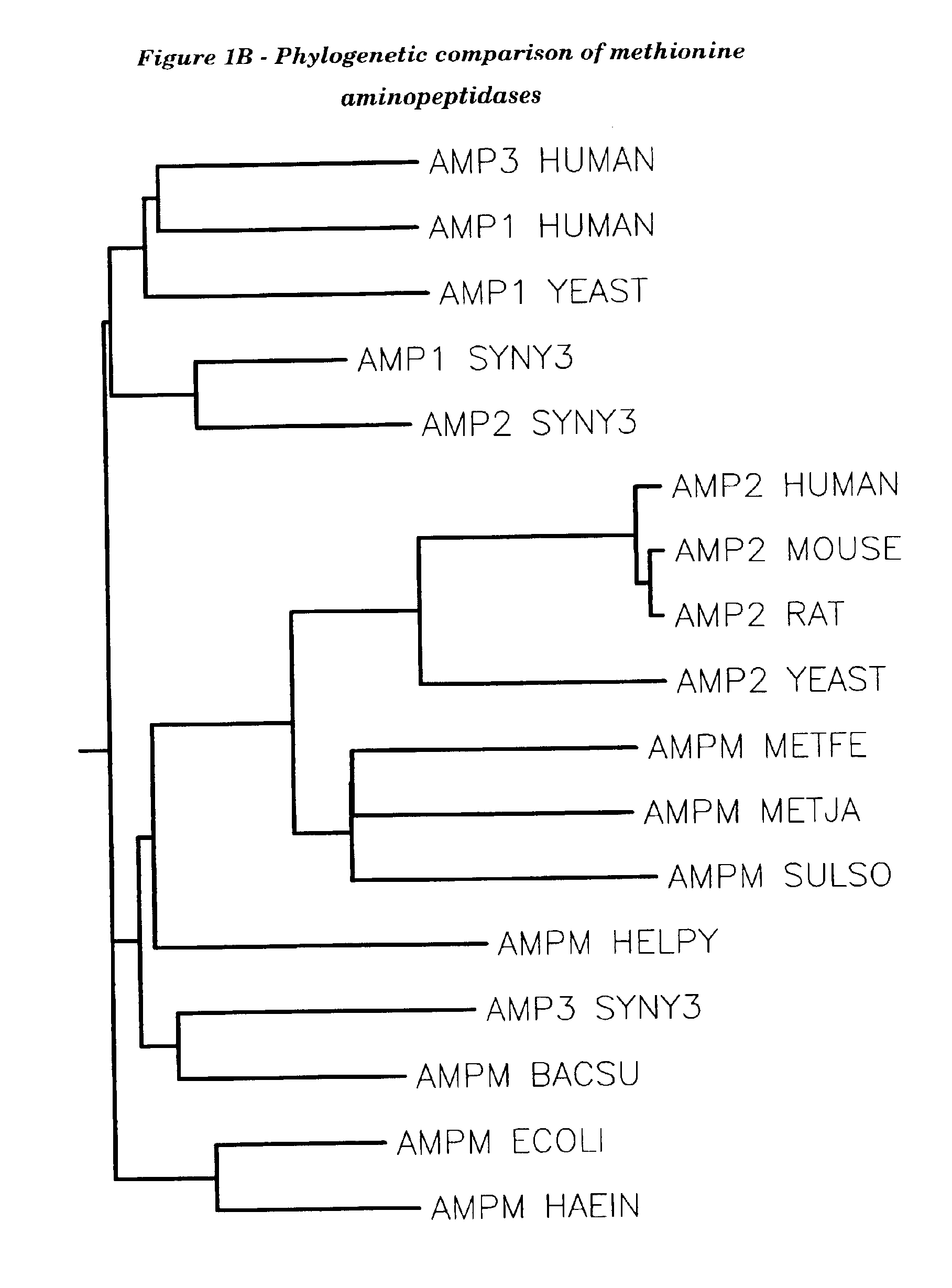
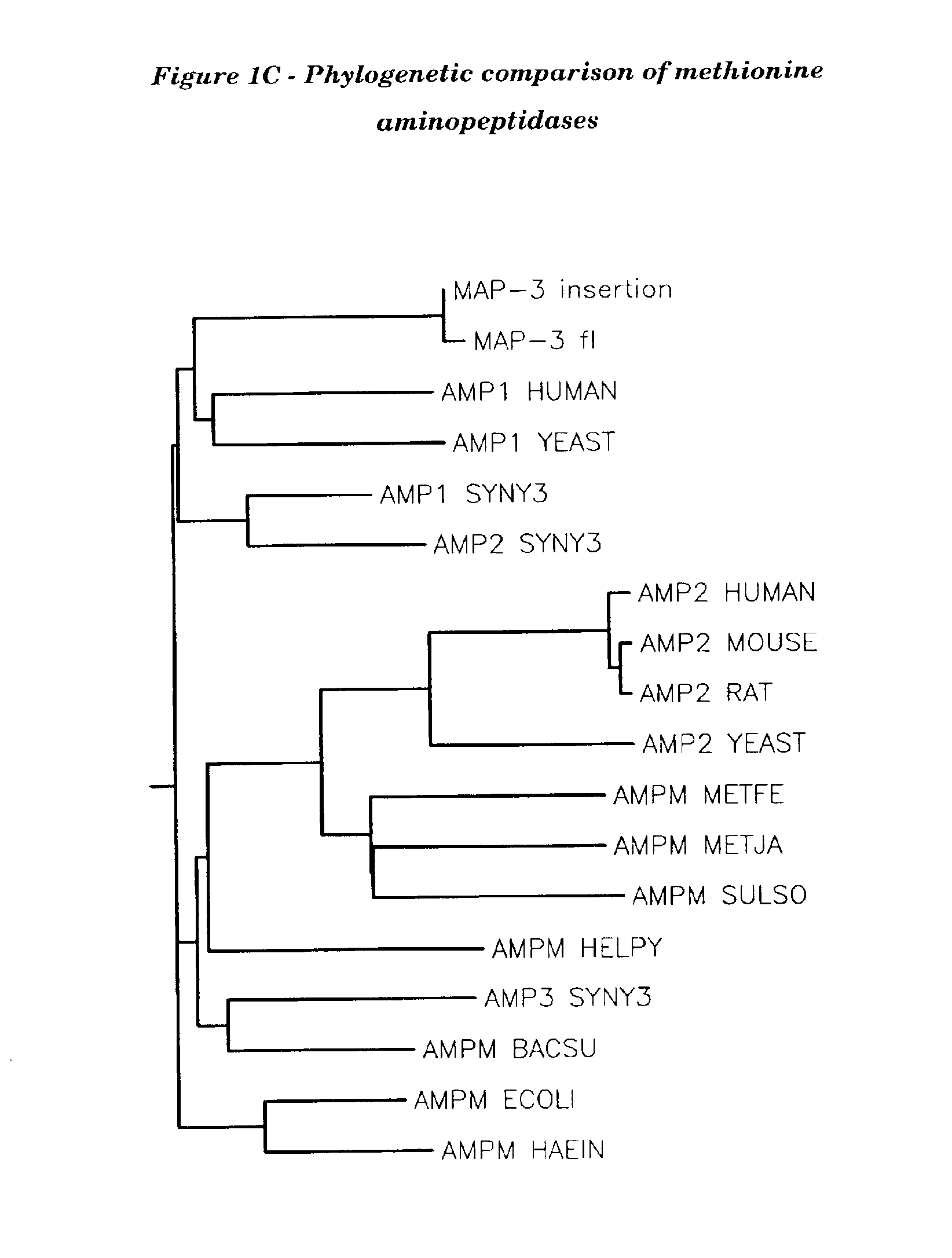
Human methionine aminopeptidase type 3
Methionine aminopeptidases catalyse the co-translational removal of amino terminal methionine residues from nascent polypeptide chains. A newly-discovered enzyme, designated methionine aminopeptidase type-3 (MetAP-3), has a substrate specificity which is similar to MetAP-1 and MetAP-2, although it is not inhibited by fumagillin, an irreversible inhibitor of MetAP-2. MetAP-3 also preferentially localizes to mitochondria, unlike MetAP-1 and MetAP-2, which accumulate in the cytoplasm. One embodiment of the present invention relates to human cDNAs encoding polypeptides comprising MetAP-3. Other embodiments of the invention relate to nucleic acid molecules derived from these cDNAs, including complements, homologues, and fragments thereof, and methods of using these nucleic acid molecules, to generate polypeptides and fragments thereof. Other embodiments of the invention relate to antibodies directed against polypeptides comprising MetAP-3, and methods to screen for compounds or compositions that preferentially or specifically effect the activity of polypeptides comprising MetAP-3.
Owner:PHARMACIA CORP
Sustained release anticancer agent carrying angiogenesis inhibitor and cytotoxic drug
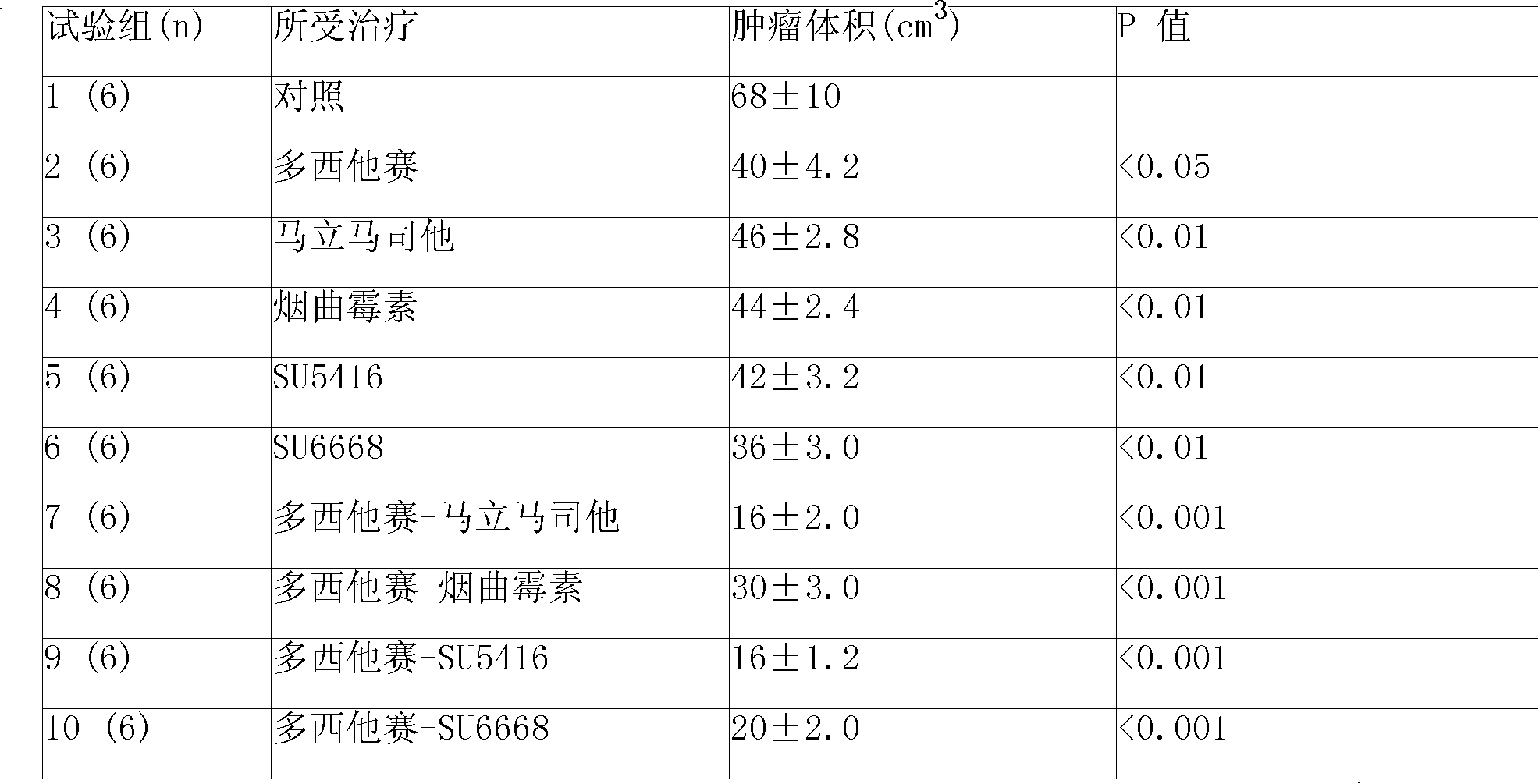
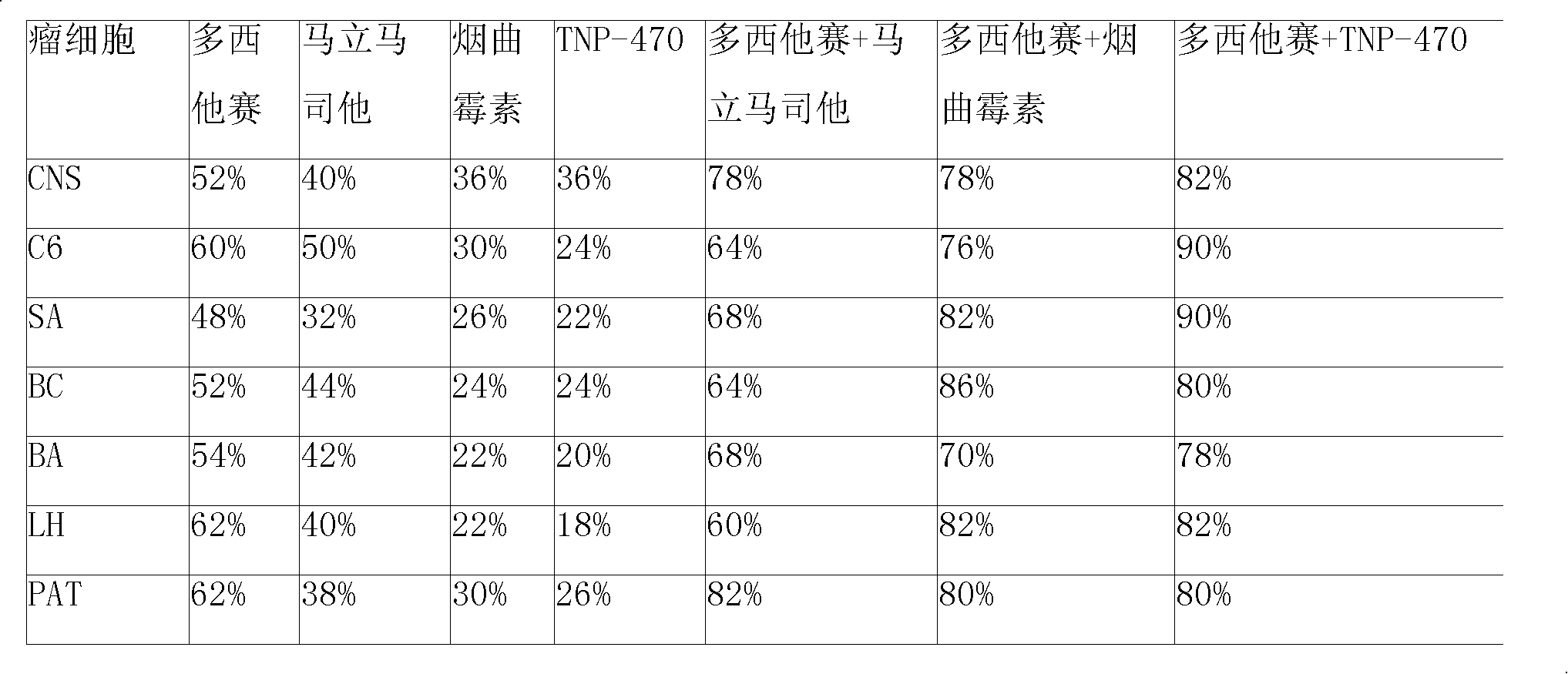
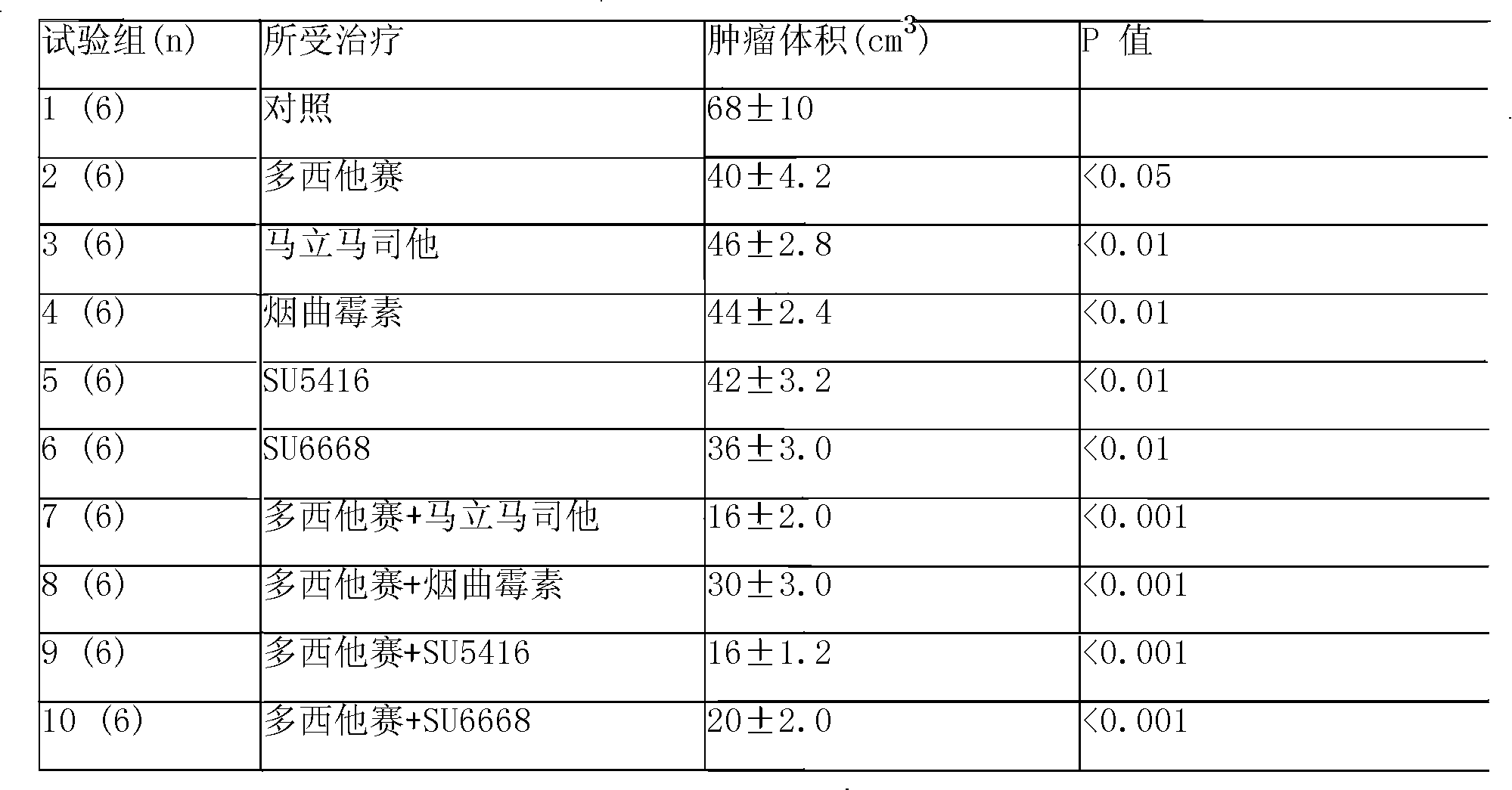
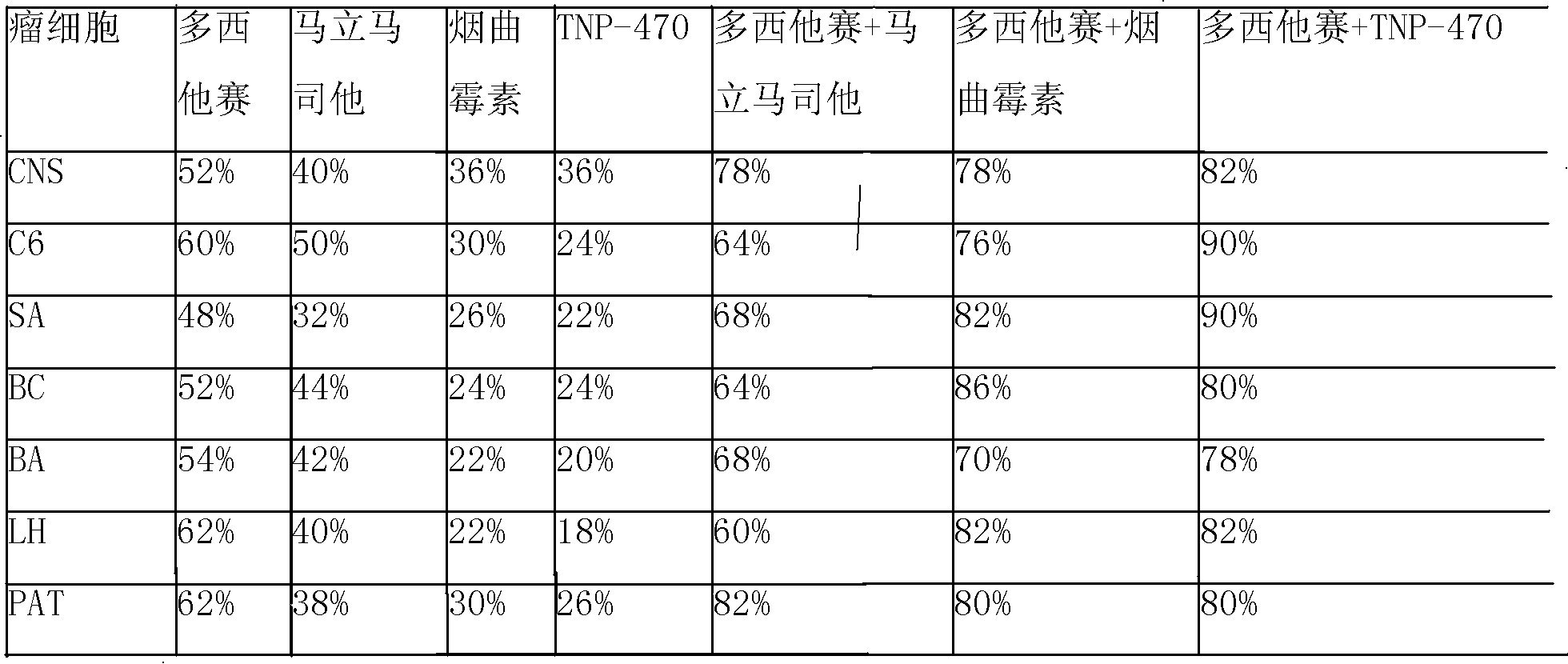
Disclosed is an anticancer slow release injection carrying both anti-angiogenesis agent and cytotoxic drugs, which comprises slow release micro-balloons and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. The anticancer active constituents being the combination of anti-angiogenesis agent selected from Marimastat, SU5416, SU6688, Fumagillin or TNP-470, with cytotoxic drugs selected from Eptaplatin, Nedaplatin, Melphalan, 4-hydroperoxycyclophosphamide, hydroxyl radical Paclitaxel, 10-desacetyltaxuyunnanin, 7-epi-taxol, Vinorelbine, Tamoxifen, Amethopterin, Adriamycin, pidorubicin, actinomycin D, Tallimustine, Atrimustine, Semustine or Ranimustine, the slow release auxiliary materials are selected from di-aliphatic acid and sebacylic acid copolymer, ethylene-vinylacetate copolymer or lactic acid polymer, the viscosity of the suspension adjuvant is 100-3000cp and is selected from sodium carboxymethylcellulose.
Owner:JINAN KANGQUAN PHARMA TECH
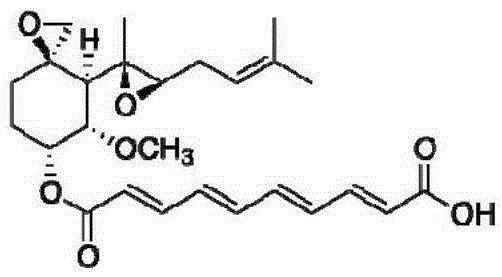
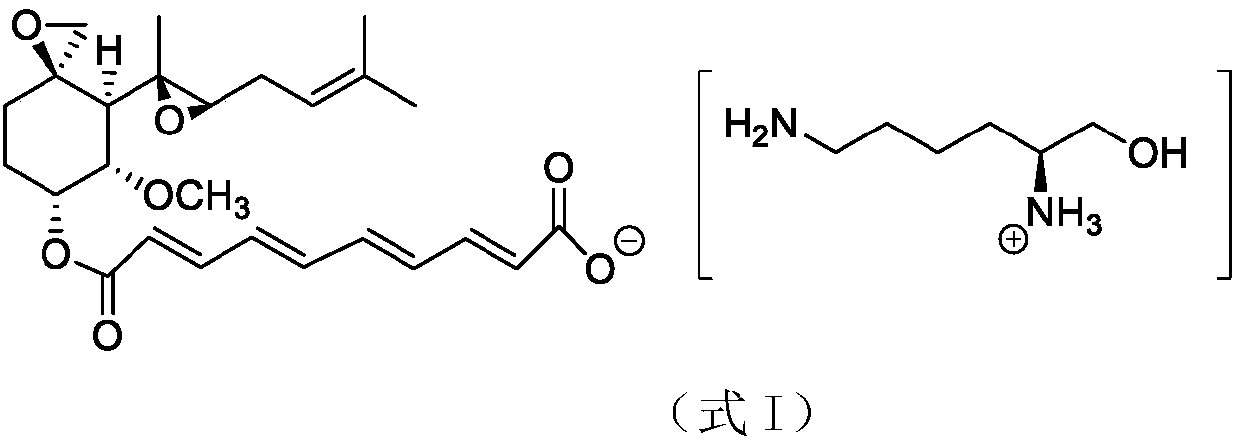
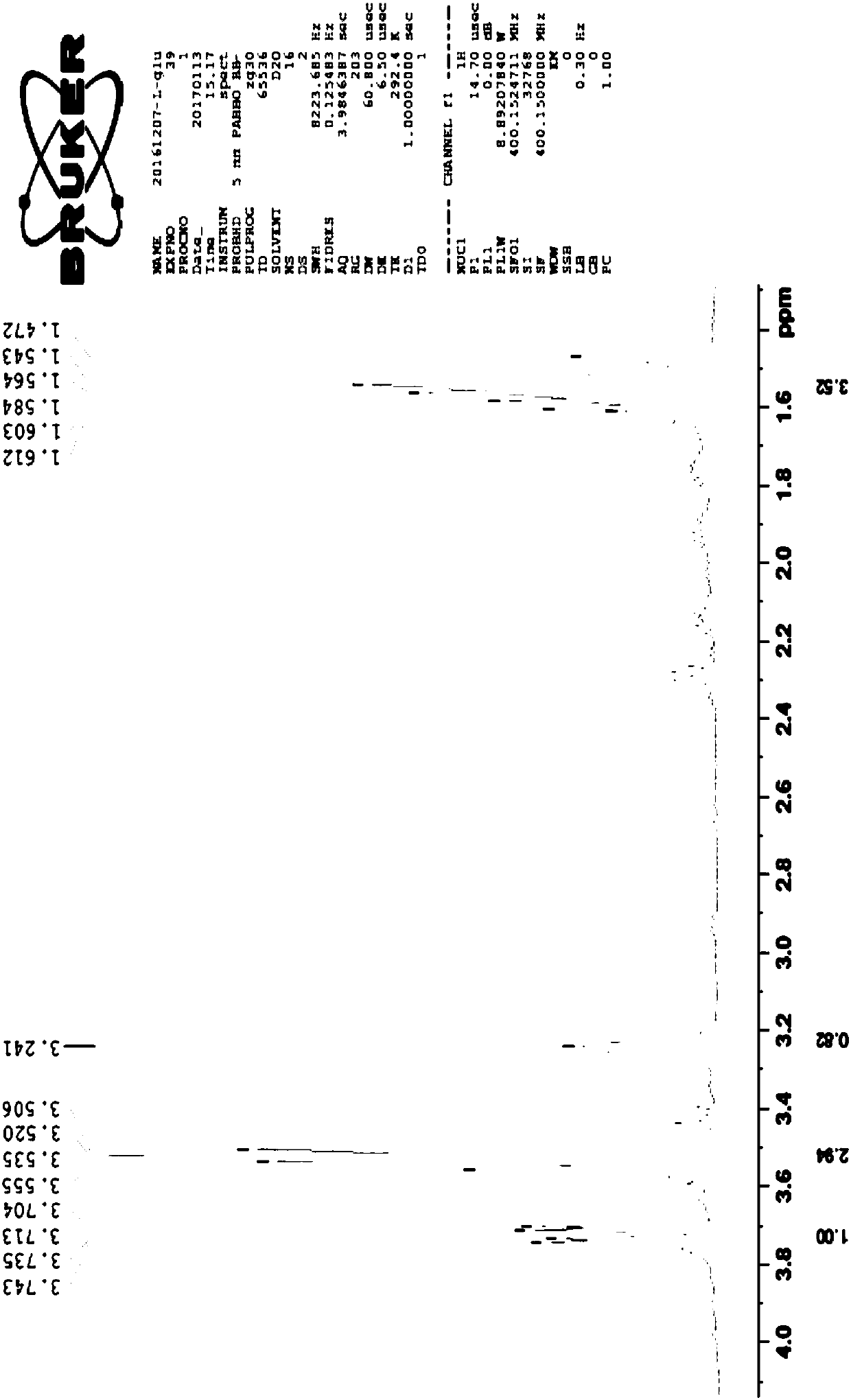
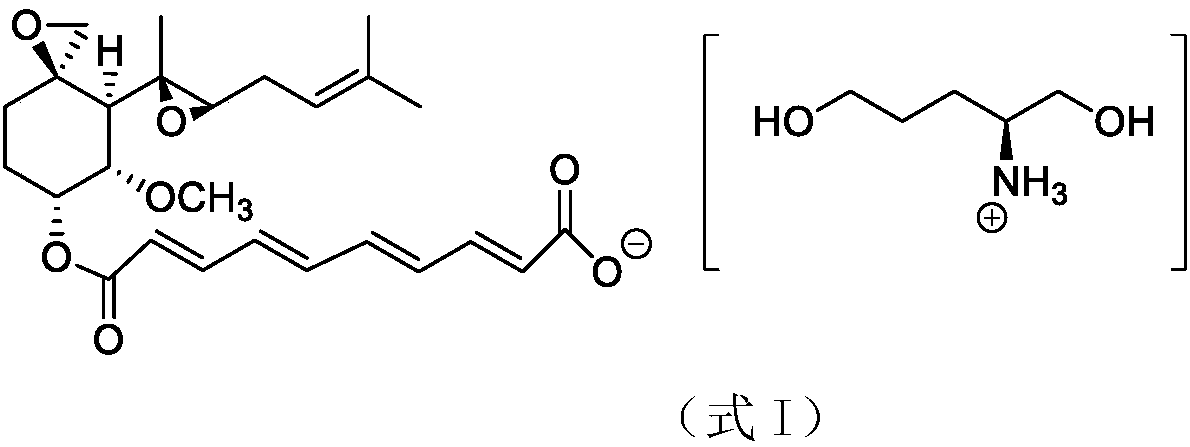
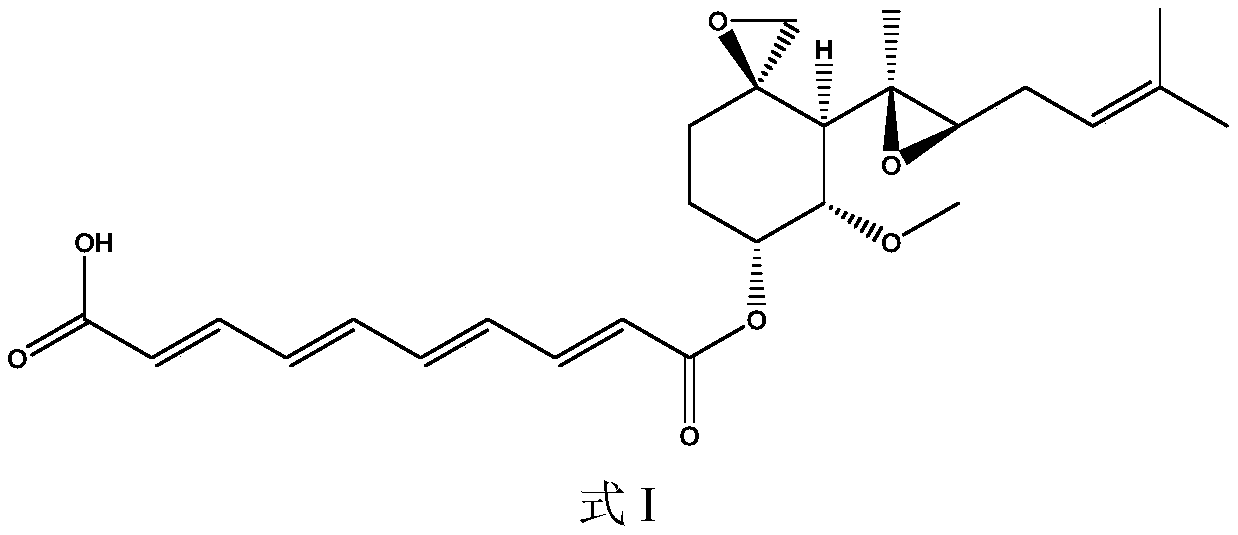
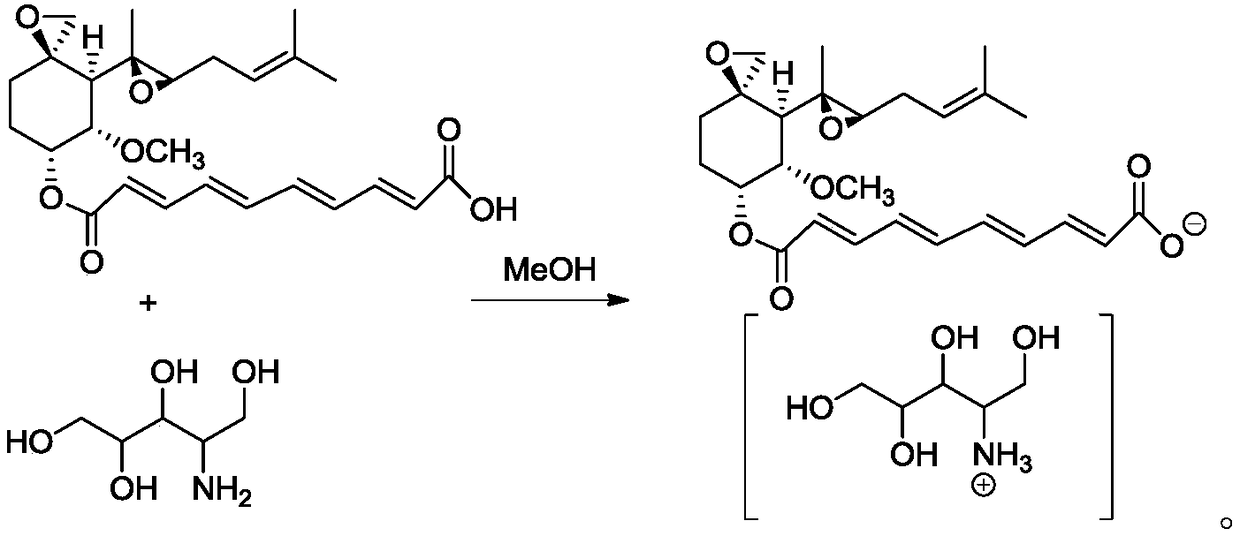
A preparation method of fumagillin amino alcohol
InactiveCN109867637ALow toxicityGood water solubilityOrganic compound preparationAmino-hyroxy compound preparationSolubilityAlcohol
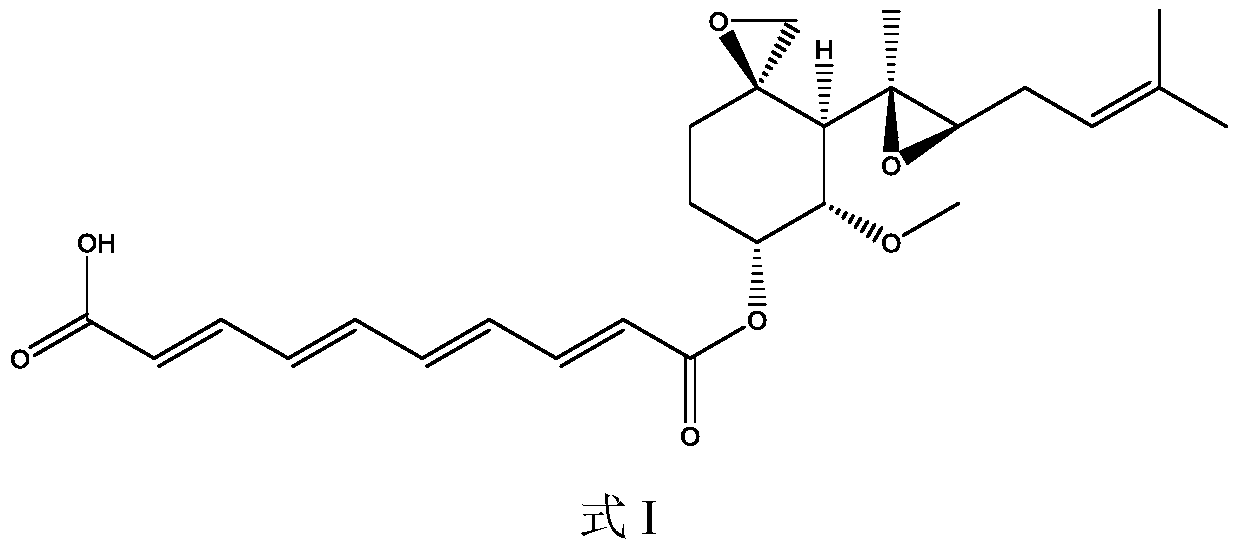
The invention discloses a preparation method of fumagillin amino alcohol shown as a formula I in the specification. The method comprises the following steps: (1) reducing amino acid shown in a formulaII to obtain a compound shown in a formula III; and (2) under the heating condition of a methanol solution, carrying out an ammonium salt synthesis reaction on fumagillin and the compound shown in the formula III to obtain fumagillin amino alcohol. The preparation method is short in reaction route and high in synthesis efficiency, the separation and purification steps are reduced, and mass production can be achieved. Meanwhile, the synthesized fumagillin amino alcohol is low in toxicity, can be dissolved and degraded in nature and is good in water solubility.
Owner:INST OF QUALITY STANDARD & TESTING TECH FOR AGRO PROD OF CAAS
A preparation method of fumagillin amino alcohol
InactiveCN109867640ALow toxicityGood water solubilityOrganic compound preparationAmino-hyroxy compound preparationSolubilityAlcohol
The invention discloses a preparation method of fumagillin amino alcohol shown as a formula I in the specification. The method comprises the following steps: (1) reducing amino acid shown in a formulaII to obtain a compound shown in a formula III; and (2) carrying out an ammonium salt synthesis reaction on fumagillin and the compound shown in the formula III to obtain fumagillin amino alcohol. The preparation method is short in reaction route and high in synthesis efficiency, the separation and purification steps are reduced, and mass production can be achieved. Meanwhile, the synthesized fumagillin amino alcohol is low in toxicity, can be dissolved and degraded in nature and is good in water solubility.
Owner:INST OF QUALITY STANDARD & TESTING TECH FOR AGRO PROD OF CAAS
A sustained release anticancer agent carrying angiogenesis inhibitor and clorfarabine
Disclosed is an anticancer slow release injection carrying both anti-angiogenesis agent and clofarabine, which comprises slow release micro-balloons and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents, slow release auxiliary materials, and specific dissolvent containing suspension adjuvant. The anti-angiogenesis agent is selected from Marimastat, Fumagillin, gefinitib, erlotinib, lapatinib, lapatinib, endothelium chalone, imatinib, Imatinib, Gasanib, Avastin, Cananib, sorafenib, sunitinib, oersteda or panitoma, the slow release auxiliary materials are selected from Polifeprosan, sebacylic acid copolymer, EVAc, polylactic acid and copolymer, The viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose.
Owner:JINAN KANGQUAN PHARMA TECH
A method for preparing fumagillin amino alcohol
InactiveCN109867639ALow toxicityGood water solubilityOrganic compound preparationAmino-hyroxy compound preparationSolubilityAlcohol
The invention discloses a method for preparing fumagillin amino alcohol shown as a formula I in the specification. The method comprises the following steps: (1) reducing amino acid shown in a formulaII to obtain a compound shown in a formula III; and (2) under the heating condition of a methanol solution, carrying out an ammonium salt synthesis reaction on fumagillin and the compound shown in theformula III to obtain fumagillin amino alcohol. The method is short in reaction route and high in synthesis efficiency, the separation and purification steps are reduced, and mass production can be achieved. Meanwhile, the synthesized fumagillin amino alcohol is low in toxicity, can be dissolved and degraded in nature and is good in water solubility.
Owner:INST OF QUALITY STANDARD & TESTING TECH FOR AGRO PROD OF CAAS
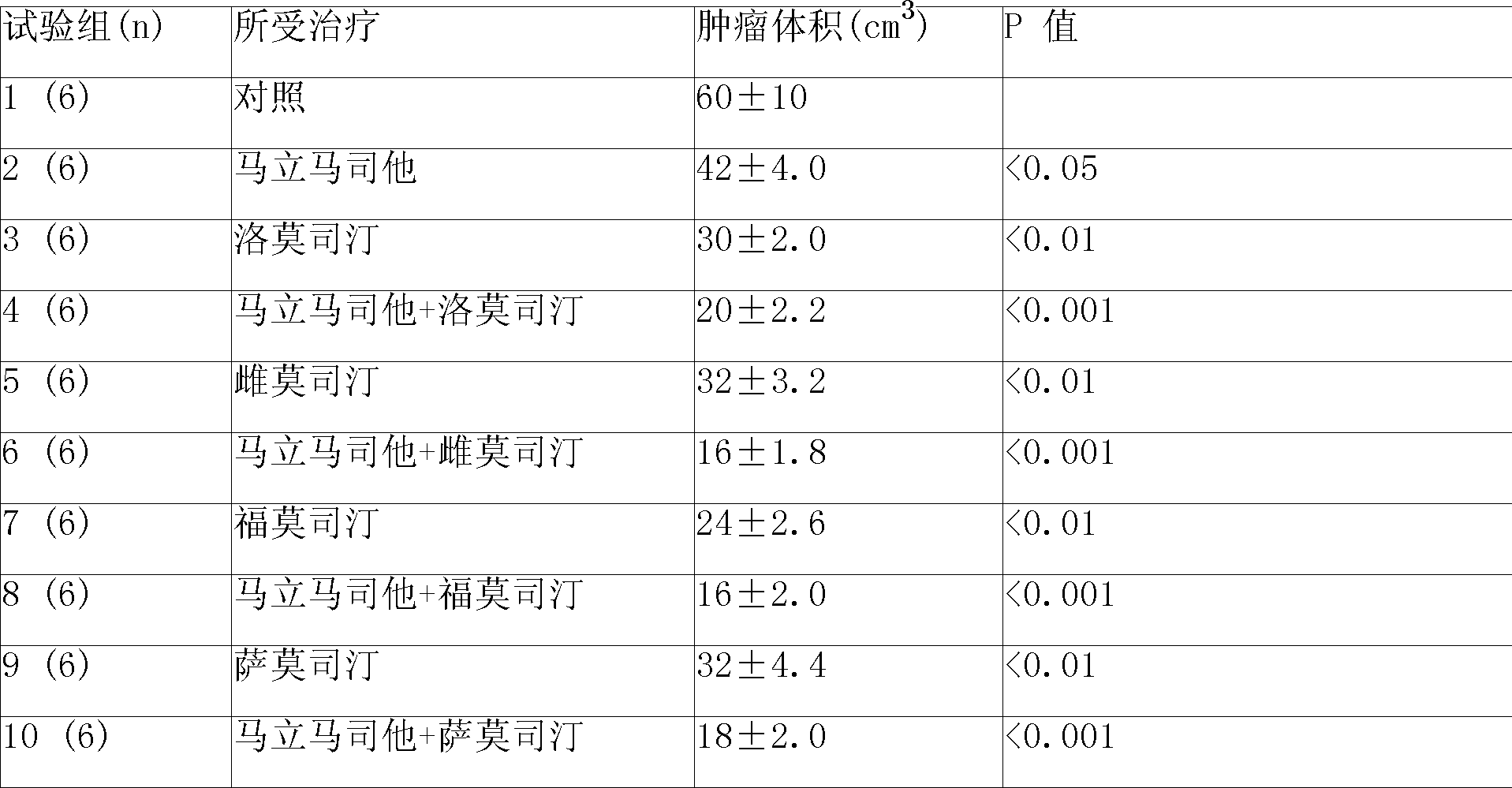
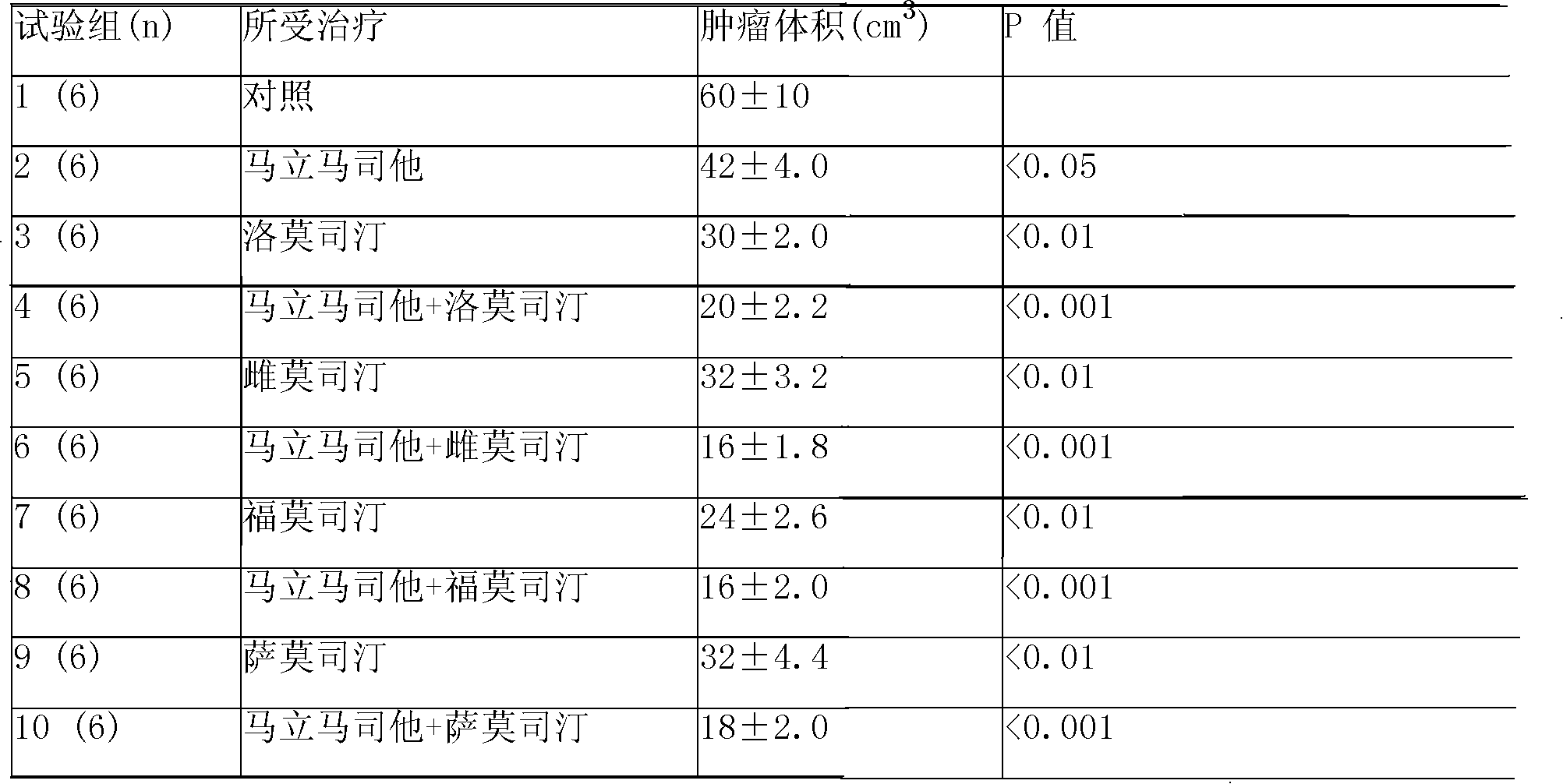
Compound sustained-released injection containing marimastat as neovascularization inhibitor
InactiveCN101336911APharmaceutical delivery mechanismPharmaceutical non-active ingredientsDepressantSuspending Agents
A compound sustained-released injection containing an angiogenesis inhibitor marimastat comprises sustained-released microspheres and a solvent. The sustained-released microspheres comprise a sustained-released adjuvant, an angiogenesis inhibitor selected from marimastat and fumagillin, and a cell toxicant selected from hydroxycamptothecin, mitozolomide, 4-carboxy temozolomide, docetaxel, oxaliplatin, sunplatinum, iphosphamide, lomustine, estramustine, fotemustine, semustine, etoposide, teniposide, vinblastine, anastrozole, fluorouracil and mitomycin c; and the solvent is a common solvent or a special solvent containing a suspending agent. The sustained-released adjuvant is selected from polifeprosan, poly(lactic acid), sebacic acid polymer such as poly(erucic acid dimmer-sebacic acid) and poly(fumaric acid-sebacic acid), EVAc, etc.; and the suspending agent has a viscosity of 100-3,000cp (20-30 DEG C) and is selected from sodium carboxymethyl cellulose, etc. The sustained-released microspheres can also be made into a sustained-released implant, which can enhance the curative effect of non-operative treatments such as chemotherapy and radiotherapy by intratumoral or peritumoral injection or placement.
Owner:JINAN KANGQUAN PHARMA TECH
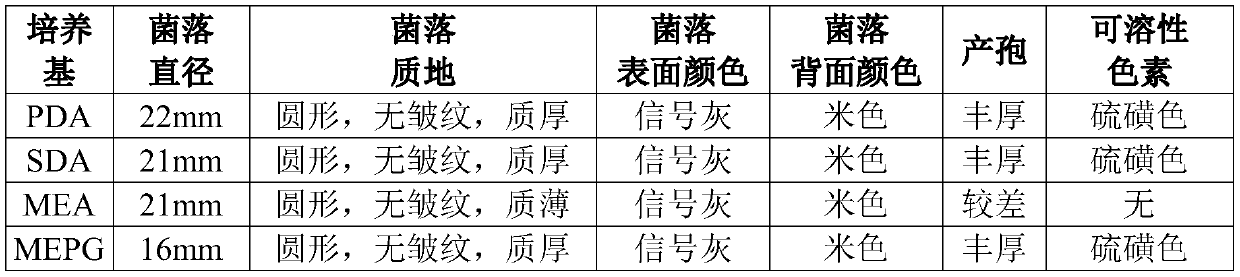
A kind of culture medium and culture method for aspergillus fumigatus fermentation producing fumagillin
ActiveCN108165590BAvoid it happening againNo need for separation and purificationFungiMicroorganism based processesBiotechnologyGlycerol
Owner:HENAN INST OF SCI & TECH +2
Compound sustained release injection containing newborn blood vessel inhibitor
InactiveCN101224191AOrganic active ingredientsPharmaceutical delivery mechanismDepressantSuspending Agents
The invention relates to a compound sustained-released injection containing a neovascularization inhibitor, which comprises sustained-release microspheres and menstruum; wherein, the sustained-release microspheres are composed of sustained-release excipients, the neovascularization inhibitor selected from marimastat or fumagillin, etc. and a cytotoxic drug selected from hydroxyl campto thecine, mitozolomide, 4-carboxyl temozolomide, docetaxel, oxaliplatin, hetaplatin, ifosfamide, lomustine, estramustine, fotemustine, samustine, etoposide, teniposide, vinblastine, anastrozole, fluorouracil or mitomycin C. The menstruum is special menstruum containing a suspending agent. The sustained-release excipients are selected from polifeprosan, polylactic acid, decanedioic acid polymer, such as poly (erucic acid dipolyme-decanedioic acid) and poly (allomaleic acid-decanedioic acid), etc. and EVAc, etc; the suspending agent, the viscosity of which is 100cp-3000cp (between 25 DEG C and 30 DEG C), is selected from carboxymethylcellulose sodium, etc. The sustained-release microspheres can also be prepared to be a sustained-release implant. The sustained-release implant is injected or deposited to the interior of tumour or around the tumour, which can improve the treatment effect of non-operative treatments, such as radio-chemotherapy, etc.
Owner:JINAN KANGQUAN PHARMA TECH
Production method of traditional Chinese medicine preparation toxin remover for preventing and treating mycotoxicosis
InactiveCN103599251BEliminate damageEnhance detoxification abilityAntinoxious agentsPlant ingredientsMycotoxicosisIndian-lilac
The invention discloses a production method of a traditional Chinese medicine preparation toxin remover for preventing and treating mycotoxicosis. The traditional Chinese medicine preparation toxin remover is characterized by comprising the following raw materials by weight percent: 7-9% of folium artemisiae argyi, 6-8% of mother chrysanthemum, 7-9% of dandelion, 7-9% of fordia cauliflora hemsl, 6-8% of oriental wormwood, 7-8% of sweet wormwood, 7-9% of derris eriocarpa how, 7-9% of sticktight, 5-7% of semen plantaginis, 6-8% of black nightshade, 6-7% of azadirachta indica and 8-9% of dried radix rehmanniae. The production method comprises the following steps: A, respectively cleaning, airing and crushing the above traditional Chinese medicine raw materials, sieving by a 300-mesh sieve, so as to obtain traditional Chinese medicine fine powders; B, respectively weighing traditional Chinese medicine fine powders according to the ratio, and mixing and stirring well; C, adding a proper amount of auxiliary material to dilute, and mixing well, so as to obtain a brown solid powdery product. The production method has the beneficial effects that the traditional Chinese medicine preparation toxin remover has specific adsorption capacity, can efficiently remove various mycotoxins in a target manner, and has a good effect on zearalenone, vomitoxin, aflatoxin, ochratoxin, fumagillin, T-2 toxin and the like in an oriented mode; the detoxification ability of the liver on the toxins can be improved; and the damage to an animal body caused by the toxins can be removed.
Owner:山东绿州动物药业有限公司
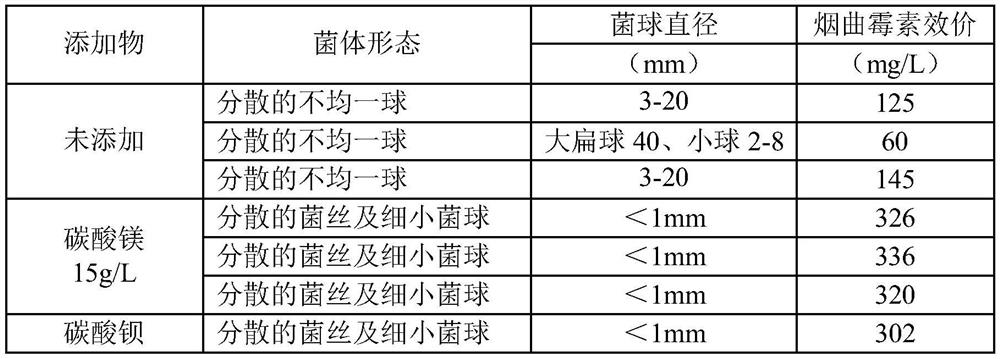
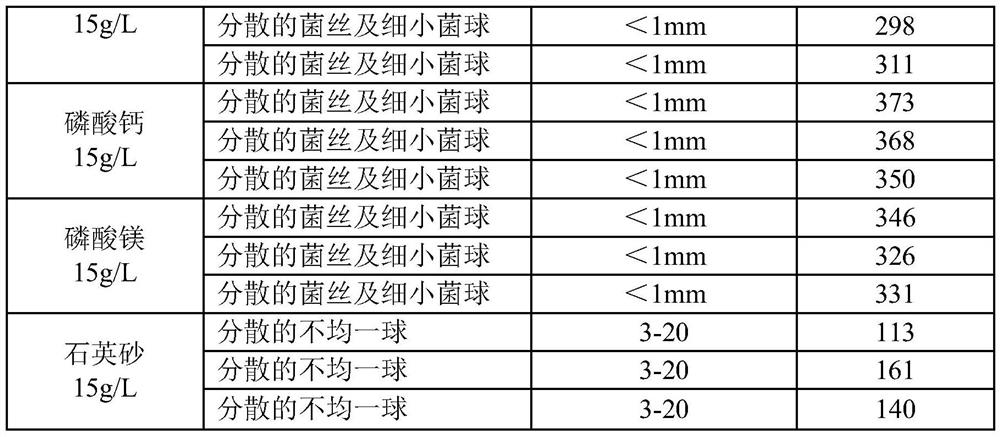
Liquid medium of aspergillus fumigatus
ActiveCN109722389AHave unexpected effectsRaise the initial pHFungiMicroorganism based processesAspergillus fumigatusLiquid medium
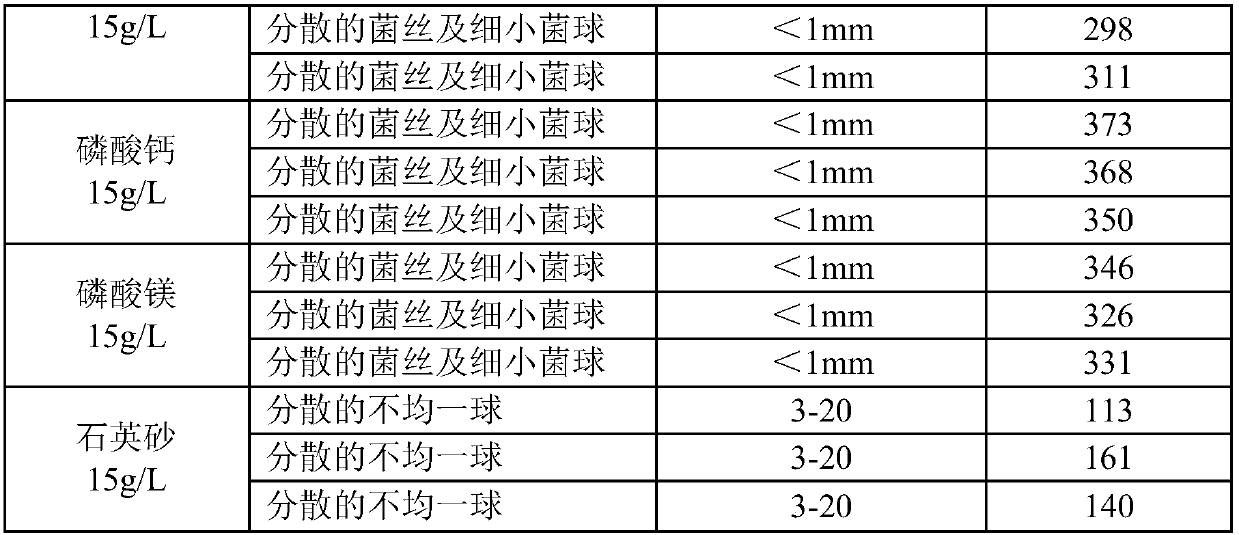
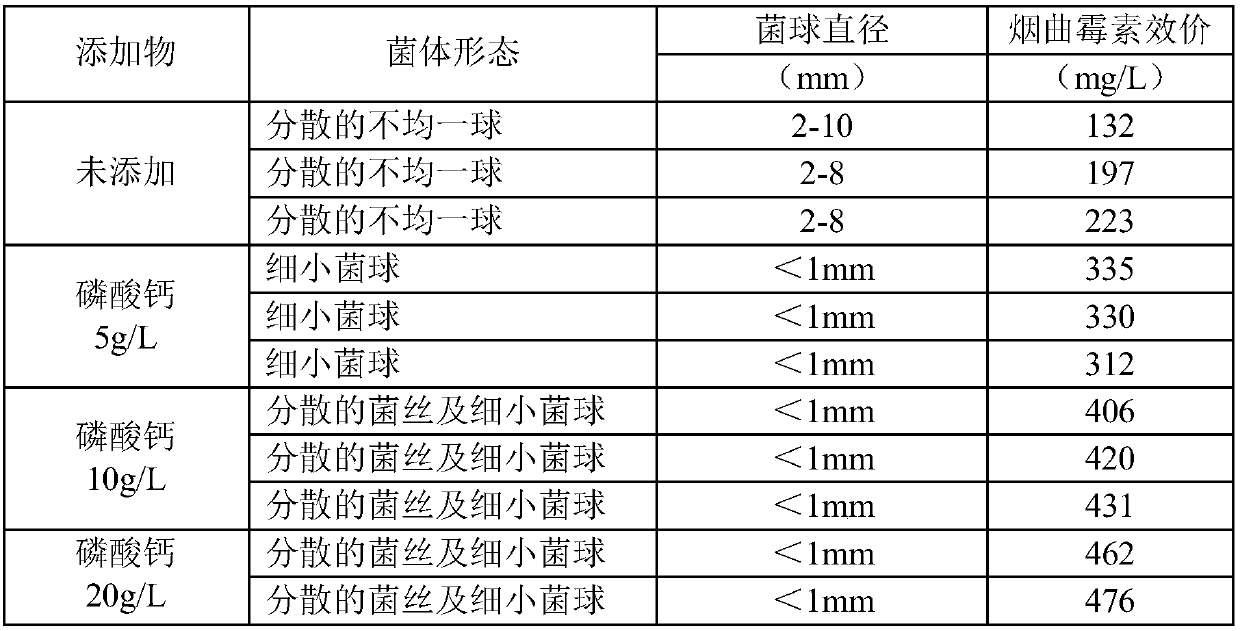
The invention belongs to the technical field of medicine, and provides a liquid medium of aspergillus fumigatus. Insoluble strong base weak acid salt powder is added to the liquid medium of aspergillus fumigatus NRRL2436, thereby reducing the formation of the bacterium balls during the liquid culture process of the aspergillus fumigatus. The bacteria are present in the form of fine bacterium ballsor dispersed hyphae, and the formation of bacteria of different sizes is reduced. Homogenization of the liquid culture of aspergillus fumigatus is achieved, and the fermentation titer of fumagillin is significantly improved.
Owner:鲁南新时代生物技术有限公司
A kind of penicillium and method for producing fumagillin
ActiveCN109182147BIncreased ability to produce fumagillinRealize industrial productionOrganic active ingredientsFungiBiotechnologyMicrobiology
The invention discloses a new Penicillium sp. HS‑NF‑684Z, the preservation number is: CGMCC No.14144; meanwhile, it discloses the preparation of fumagillin or a drug combination containing fumagillin by culturing it way of things.
Owner:ZHEJIANG HISUN PHARMA CO LTD
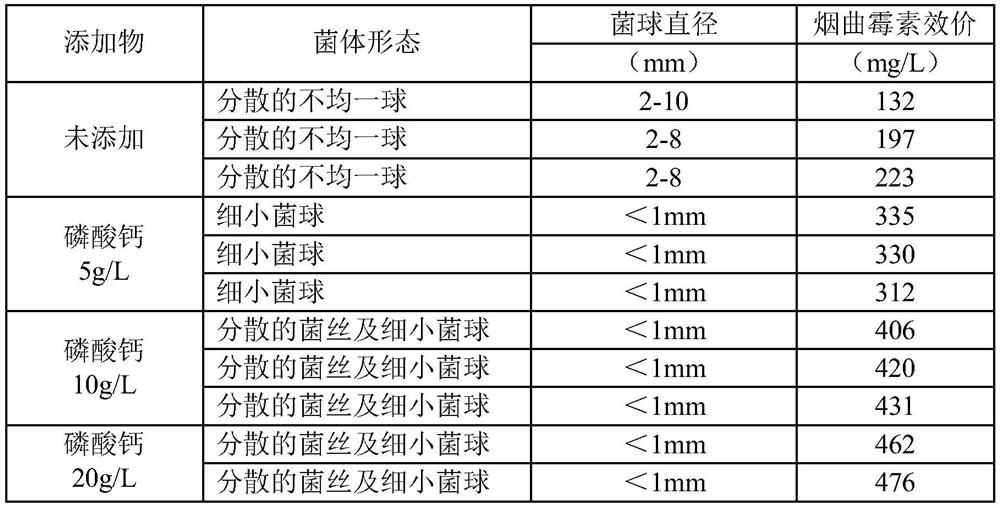
A kind of Aspergillus fumigatus liquid culture medium
ActiveCN109722389BRaise the initial pHEasy to shapeFungiMicroorganism based processesBiotechnologyAspergillus fumigatus
The invention belongs to the technical field of medicine, and provides a liquid culture medium of Aspergillus fumigatus, that is, adding insoluble strong base and weak acid salt powder to the liquid culture medium of Aspergillus fumigatus NRRL2436, which reduces the formation of bacterial balls in the liquid culture process of Aspergillus fumigatus, The bacterium exists in the form of tiny spheroids or dispersed hyphae, which reduces the formation of spheroids of different sizes, realizes the homogenization of liquid culture of Aspergillus fumigatus, and significantly improves the fermentation titer of fumagillin.
Owner:鲁南新时代生物技术有限公司
A kind of method utilizing Aspergillus fumigatus to produce fumagillin
ActiveCN106591389BIncrease productionOrganic active ingredientsAntipyreticAspergillus fumigatusSpore
The invention relates to a method for producing fumagillin by aspergillus fumigatus. The method comprises the following steps: inoculating aspergillus fumigatus into a GMM solid medium to activate strains, wherein the pH value of the GMM medium is 5 to 7; preparing activated strain spores into spore suspension, and then inoculating the spore suspension into a CYA solid medium, a CXMA solid medium or an LMM liquid medium, wherein the pH values of the CYA solid medium and the CXMA solid medium are natural, and the pH value of the LMM liquid medium is adjusted to be 5 to 7, and carrying out culturing at the temperature of 25 to 37 DEG C; collecting cultured aspergillus fumigatus to extract fumagillin and fumagillin derivatives. According to the invention, carbon and nitrogen sources and trace elements of fumagillin which affect the growth of aspergillus fumigatus are aimed to be screened, and the concentration of corresponding trace elements is optimized to obtain higher yield of fumagillin.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Anticancer slow release agent loading both newborn blood vessel and tetrazole violet
InactiveCN100594886CSolution deliveryPharmaceutical non-active ingredientsDepressantTherapeutic effect
Disclosed is an anticancer slow release injection carrying both anti-angiogenesis agent and tetrazole Ionone, which comprises slow release micro-balloons and dissolvent, the slow release micro-balloons include anticancer active constituents, slow release auxiliary materials and specific dissolvent containing suspension agent. The anti-angiogenesis agent is selected from Marimastat, Fumagillin, gefinitib, erlotinib, lapatinib, pelinib, Thalidomide, Ranolazine, endostatin, imatinib, Avastin, sorafenib, sunitinib, the slow release auxiliary materials are selected from Polifeprosan, sebacylic acidcopolymer, EVAc, polylactic acid and their mixture of copolymer, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C), and is selected from sodium carboxymethylcellulose. The agentcan also be prepared into implanting agent for injection or placement in or around tumor with the effects of selectively increasing local concentration, lowering general reaction of the drugs, suppressing growth of tumor cells and blood vessel, and improving the treatment effect of the non-operative treatment methods such as chemotherapy.
Owner:SHANDONG LANJIN PHARMA
Compound sustained release injection containing newborn blood vessel inhibitor fumagillin
InactiveCN101224190AOrganic active ingredientsPharmaceutical delivery mechanismDepressantTherapeutic effect
The invention relates to a compound sustained-released injection containing a neovascularization inhibitor, which comprises sustained-release microspheres and menstruum; wherein, the sustained-release microspheres are composed of sustained-release excipients, the neovascularization inhibitor selected from marimastat or fumagillin, etc. and a cytotoxic drug selected from hydroxyl campto thecine, mitozolomide, 4-carboxyl temozolomide, docetaxel, oxaliplatin, hetaplatin, ifosfamide, lomustine, estramustine, fotemustine, samustine, etoposide, teniposide, vinblastine, anastrozole, fluorouracil or mitomycin C. The menstruum is special menstruum containing a suspending agent. The sustained-release excipients are selected from polifeprosan, polylactic acid, decanedioic acid polymer, such as poly (erucic acid dipolyme-decanedioic acid) and poly (allomaleic acid-decanedioic acid), etc. and EVAc, etc; the suspending agent, the viscosity of which is 100cp-3000cp (between 25 DEG C and 30 DEG C), is selected from carboxymethylcellulose sodium, etc. The sustained-release microspheres can also be prepared to be a sustained-release implant. The sustained-release implant is injected or deposited to the interior of tumour or around the tumour, which can improve the treatment effect of non-operative treatments, such as radio-chemotherapy, etc.
Owner:JINAN KANGQUAN PHARMA TECH
Compound sustained-released injection containing neonatal blood vessel restraining agent
InactiveCN101305992APharmaceutical delivery mechanismPharmaceutical non-active ingredientsCatharanthineDepressant
The invention relates to a compound sustained-release injection containing neovascularization inhibitors, which comprises sustained-release microspheres and a solvent; wherein, the sustained-release microspheres comprise sustained-release auxiliary materials, neovascularization inhibitors which are selected from Marimastat or fumagillin, etc., and cytotoxic drugs which are selected from hydroxycamptothecine, Mitozolomide, 4-carboxy temozolomide, docetaxel, oxaliplatin, hetaplatin, isophosphamide, lomustine, estramustine, fotemustine, samustine, etoposide, teniposide, catharanthine, anastrozole, fluorouracil, or mitomycin C; the solvent is a special solvent containing a suspending agent. The sustained-release auxiliary materials are selected from decanedioic acid copolymers such as Polifeprosan, polylactic acid, poly (erucic acid dimmer-decanedioic acid) and poly (fumaric acid-decanedioic acid) and so on, EVAc, etc.; the viscosity of the suspending agent ranges from 100cp to 3000cp (when the temperature is 25 to 30 DEG C), and the suspending agent is selected from sodium carboxymethyl cellulose, etc. The sustained-release microspheres can also be made into a sustained-release implanting agent, and can increase the curative effects of the non-operative treatments such as radiotherapy and chemotherapy, etc. when being injected or put in or around tumors.
Owner:JINAN KANGQUAN PHARMA TECH
An anticancer sustained release agent carrying angiogenesis inhibitor and cytotoxic drug
Disclosed is an anticancer slow release injection carrying both anti-angiogenesis agent and cytotoxic drugs, which comprises slow release micro-balloons and dissolvent, wherein the slow release microballoons comprise anti-cancer active constituents and slow release auxiliary materials, the dissolvent being specific dissolvent containing suspension adjuvant. the anti-angiogenesis agent is selected from Marimastat, SU5416, SU6688, Fumagillin and / or TNP-470, the cytotoxic drugs are selected from anti-taxone, alkylating agent, Topo enzyme inhibitor and / or plant alkaloid, The slow release auxiliary materials are selected from Polifeprosan, di-aliphatic acid and sebacylic acid copolymer, poly(erucic aciddipolymer-sebacylic acid), poly(fumaric acid-sebacylic acid), ethylene-vinylacetate copolymer, the viscosity of the suspension adjuvant is 100-3000cp (at 25-30 deg C).
Owner:JINAN KANGQUAN PHARMA TECH
Human methionine aminopeptidase type 3
Methionine aminopeptidases catalyse the co-translational removal of amino terminal methionine residues from nascent polypeptide chains. A newly-discovered enzyme, designated methionine aminopeptidase type-3 (MetAP-3), has a substrate specificity which is similar to MetAP-1 and MetAP-2, although it is not inhibited by fumagillin, an irreversible inhibitor of MetAP-2. MetAP-3 also preferentially localizes to mitochondria, unlike MetAP-1 and MetAP-2, which accumulate in the cytoplasm. One embodiment of the present invention relates to human cDNAs encoding polypeptides comprising MetAP-3. Other embodiments of the invention relate to nucleic acid molecules derived from these cDNAs, including complements, homologues, and fragments thereof, and methods of using these nucleic acid molecules, to generate polypeptides and fragments thereof. Other embodiments of the invention relate to antibodies directed against polypeptides comprising MetAP-3, and methods to screen for compounds or compositions that preferentially or specifically effect the activity of polypeptides comprising MetAP-3.
Owner:PHARMACIA CORP
A kind of radiation degradation treatment method of fumonisin and t-2 toxin
InactiveCN103698440BPositive effectGood environmental effectComponent separationPreparing sample for investigationEnvironmental resistanceHigh concentration
The invention relates to an irradiation degradation processing method of fumitremorgin and T-2 toxin. The irradiation degradation processing method comprises the steps of (1) preparing standard solutions for standard substances of fumitremorgin FB1 and the T-2 toxin, carrying out irradiation processing with the irradiation range of 0-200kGy on the standard solutions, and measuring the contents of the FB1 and T-2 through a liquid chromatogram-tandem mass spectrometry; (2) carrying out preprocessing on samples containing poison after irradiation with the irradiation range of 0-9kGy, and measuring the FB1 and T-2 degrading effects through a liquid chromatogram-tandem mass spectrometry; (3) selecting the FB1 and T-2 standard solutions with highest concentrations as the samples to carry out irradiation processing with the irradiation range of 0-200kGy, and then detecting and analyzing degradation products through the liquid chromatogram-tandem mass spectrometry. The irradiation degradation processing method provided by the invention has the advantages of realizing research and environment-friendly application of the FB1 and T-2 degradation product which lacks of irradiation degradation, the degradation effect is good, and the environment-friendly detoxification processing of mildew agricultural products waste is realized.
Owner:JIANGSU ACAD OF AGRI SCI
Compound sustained-released injection containing neonatal blood vessel restraining agent
InactiveCN101305991APharmaceutical delivery mechanismPharmaceutical non-active ingredientsCatharanthineDepressant
The invention relates to a compound sustained-release injection containing neovascularization inhibitors, which comprises sustained-release microspheres and a solvent; wherein, the sustained-release microspheres comprise sustained-release auxiliary materials, neovascularization inhibitors which are selected from Marimastat or fumagillin, etc., and cytotoxic drugs which are selected from hydroxycamptothecine, Mitozolomide, 4-carboxy temozolomide, docetaxel, oxaliplatin, hetaplatin, isophosphamide, lomustine, estramustine, fotemustine, samustine, etoposide, teniposide, catharanthine, anastrozole, fluorouracil, or mitomycin C; the solvent is a special solvent containing a suspending agent. The sustained-release auxiliary materials are selected from decanedioic acid copolymers such as Polifeprosan, polylactic acid, poly (erucic acid dimmer-decanedioic acid) and poly (fumaric acid-decanedioic acid) and so on, EVAc, etc.; the viscosity of the suspending agent ranges from 100cp to 3000cp (when the temperature is 25 to 30 DEG C), and the suspending agent is selected from sodium carboxymethyl cellulose, etc. The sustained-release microspheres can also be made into a sustained-release implanting agent, and can increase the curative effects of the non-operative treatments such as radiotherapy and chemotherapy, etc. when being injected or put in or around tumors.
Owner:JINAN KANGQUAN PHARMA TECH
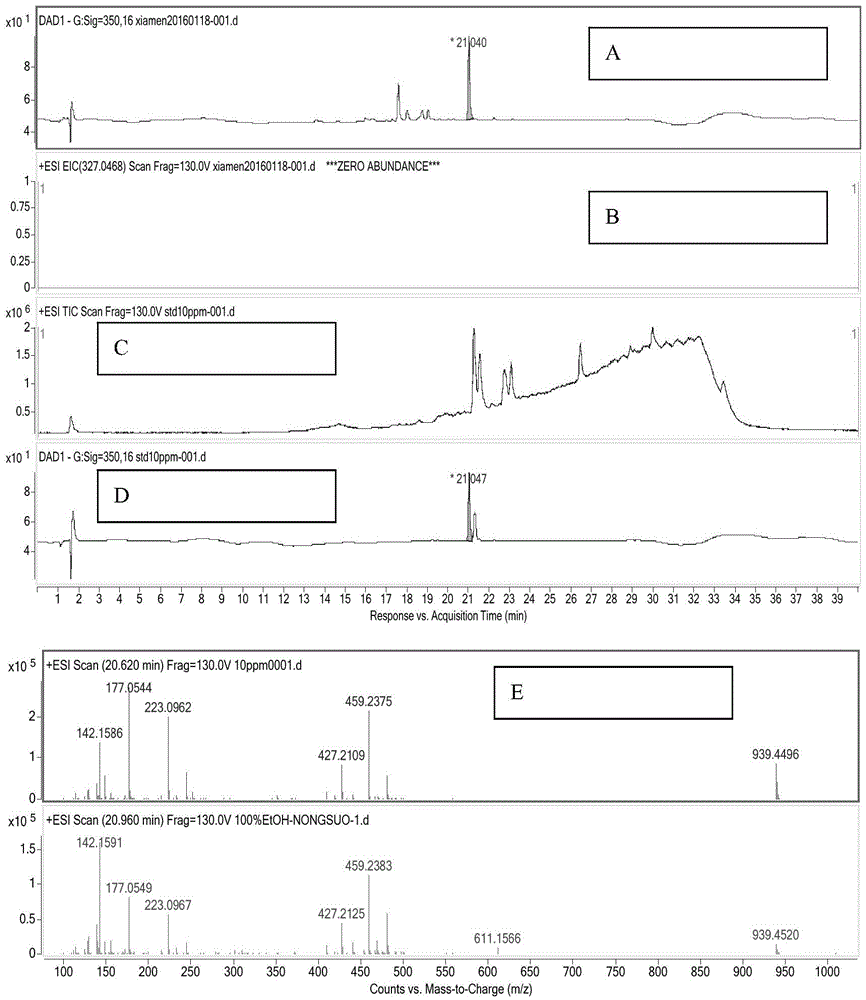
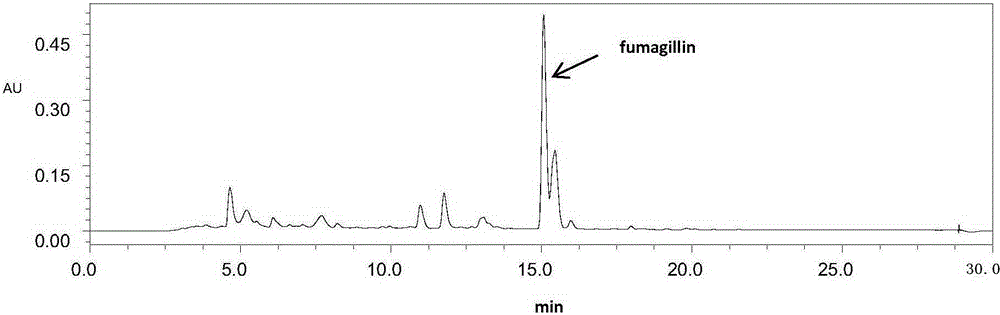
A kind of extraction and purification method of fumagillin
ActiveCN109053638BIncrease contentLarge-scale industrial productionOrganic chemistryBiotechnologyMethyl t-butyl ether
Provided is a fumagillin extraction and purification method, which comprises the steps of extraction with a methyl t-butyl ether (MTBE) or ethyl t-butyl ether (ETBE) fermentation liquid, MTBE or ETBE precipitation, crystallization and purification, etc. The method is simple to operate, has a high yield, a low cost, can be industrialized on a large scale, and has great a significance for the industrialized production of fumagillin and the later development of a fumagillin derivative.
Owner:ZHEJIANG HISUN PHARMA CO LTD
A kind of extraction method of fumagillin
InactiveCN105622593BImprove protectionImprove resource utilizationOrganic chemistryResource utilizationSolvent
The invention provides an extraction method of fumagillin.The method comprises the steps that 1, the pH value of fumagillin fermentation liquor is adjusted to be 1-6.0 and adsorbed with macroporous resin; 2, the resin adsorbing the fermentation liquor is put into a chromatographic column and cleaned with ethanol water with the mass concentration of 15%-30%; 3, the resin is eluted with absolute ethyl alcohol or ethanol water with the mass concentration of 50%-98%, eluent is concentrated and dried, and a coarse fumagillin extraction product is obtained.According to the extraction method, the processes such as various solvent extraction and back extraction in a traditional technology are omitted, the operational reliability is high, simpleness and convenience are achieved, the production process is safe and free of pollution, the resource utilization rate is high, the production cost is low, environmental friendliness is facilitated, and the extraction method is a green and environment-friendly method for extracting the fumagillin.
Owner:BEE RES INST CHINESE ACAD OF AGRI SCI
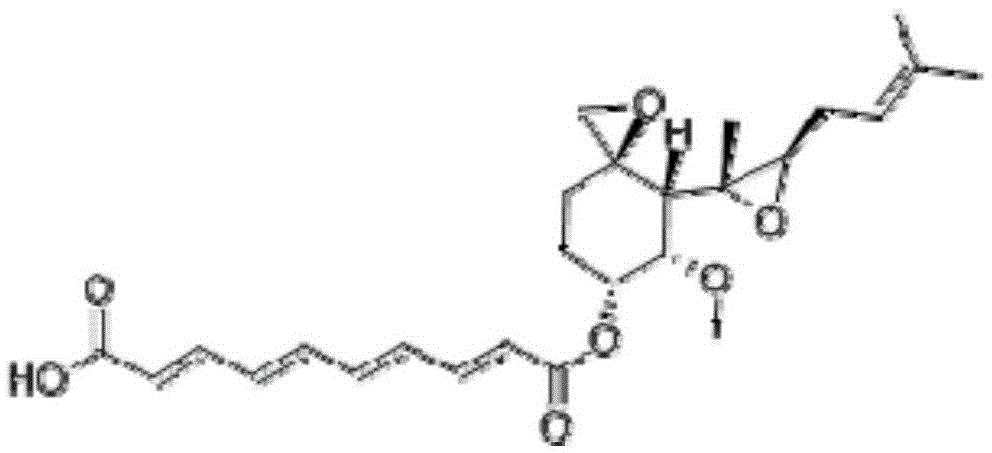
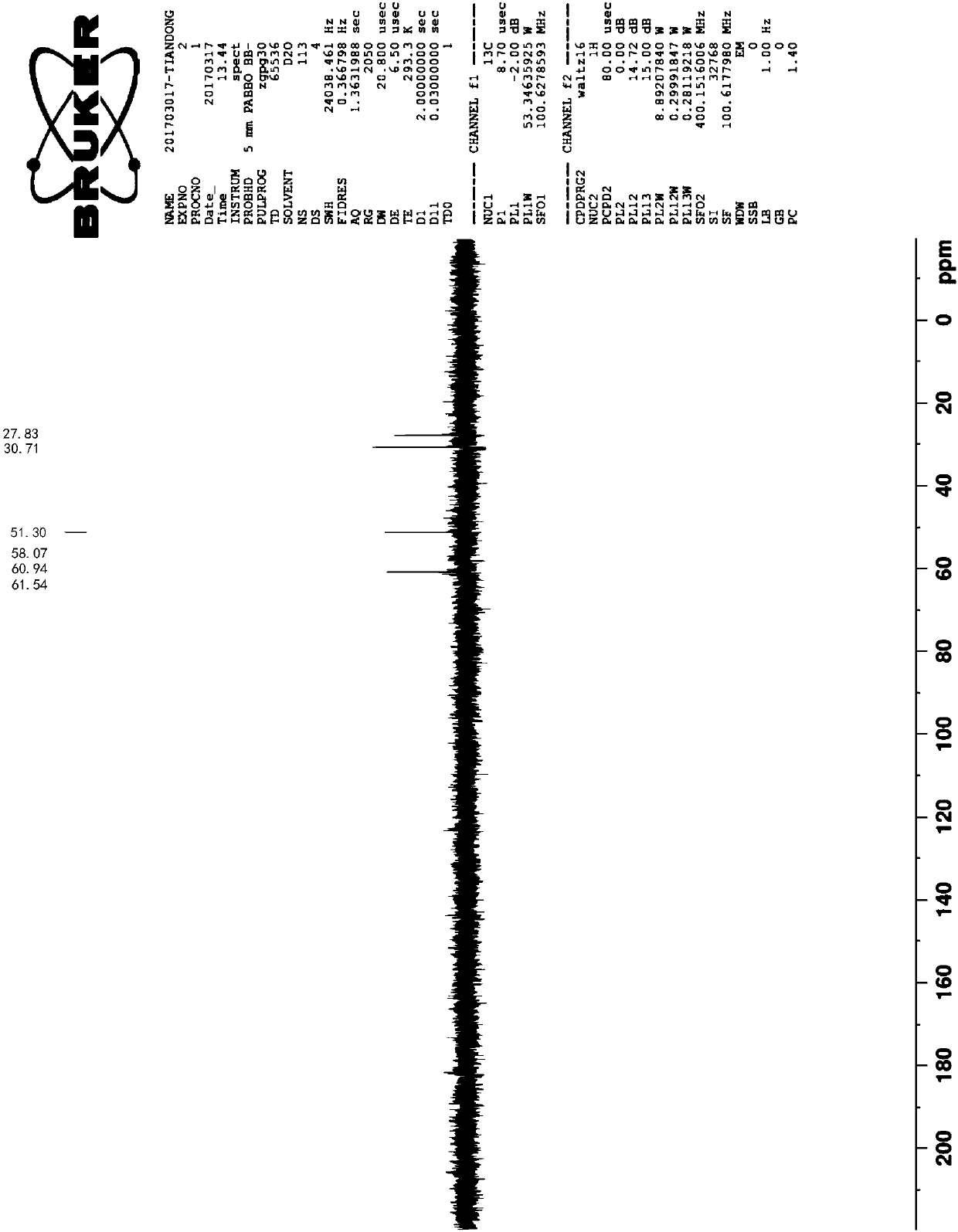
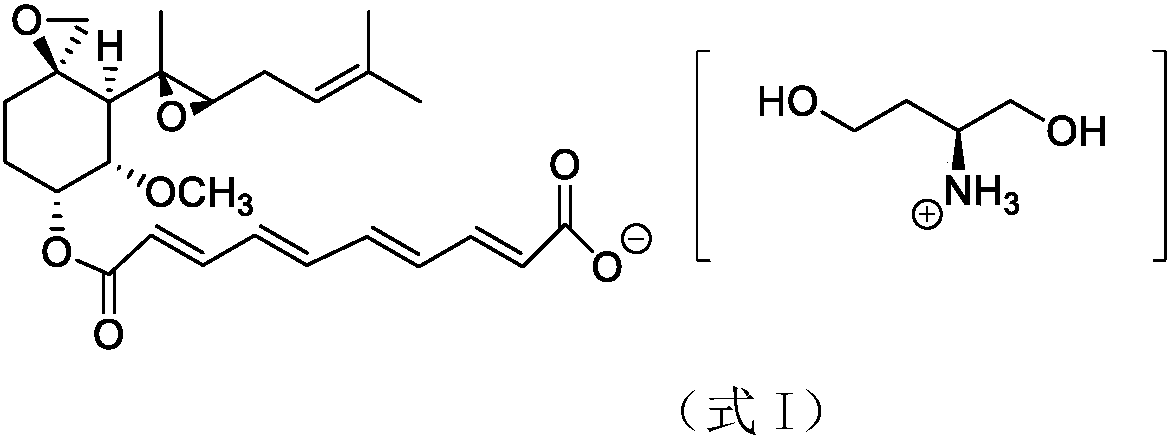
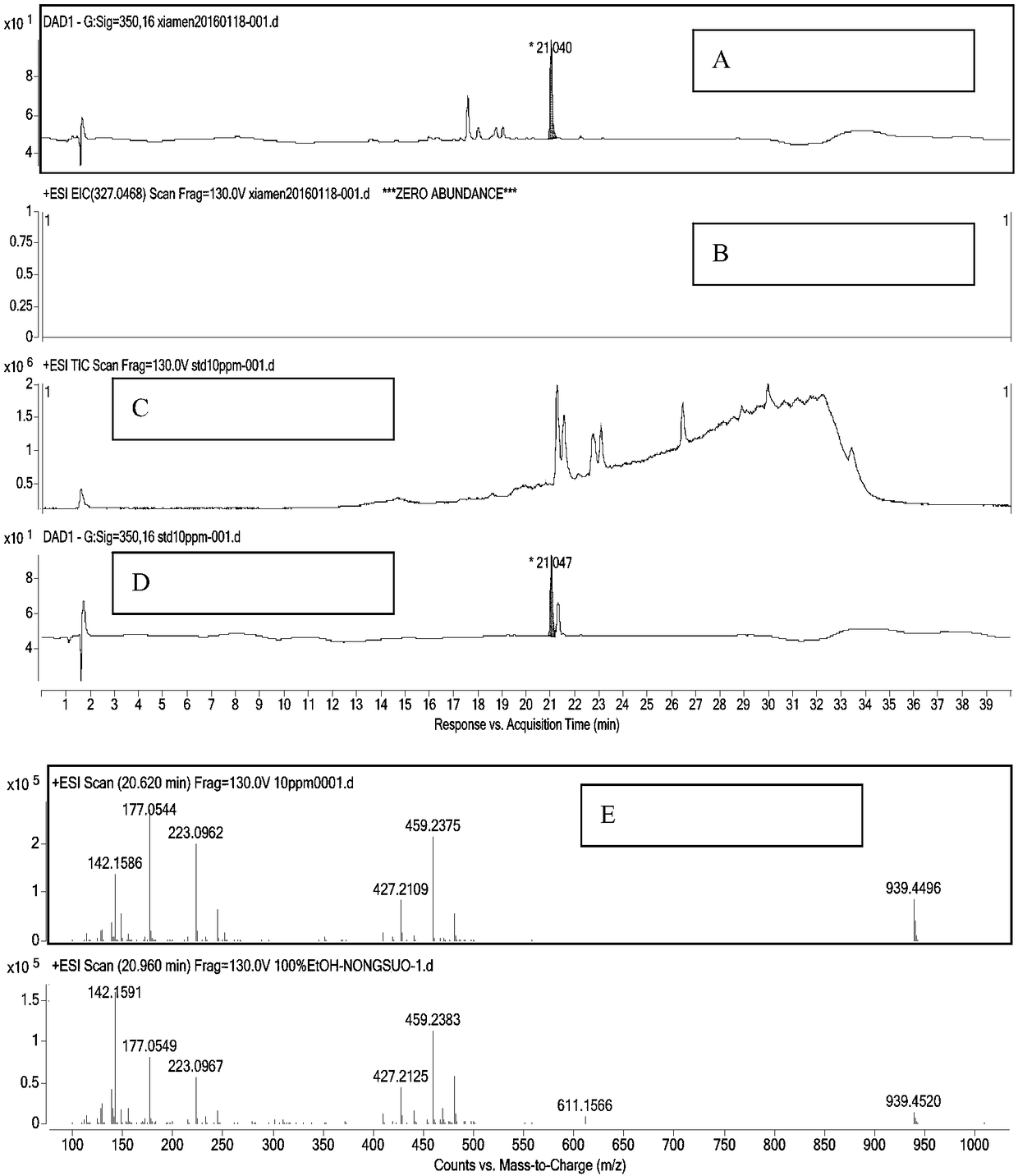
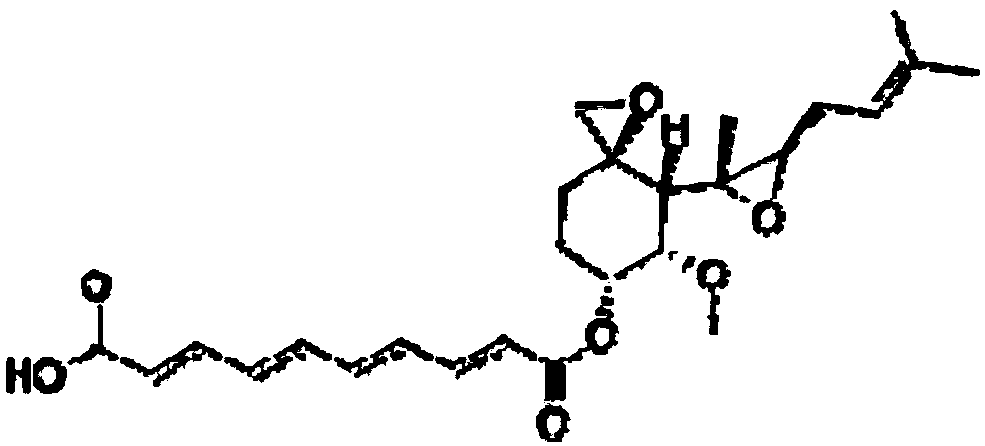
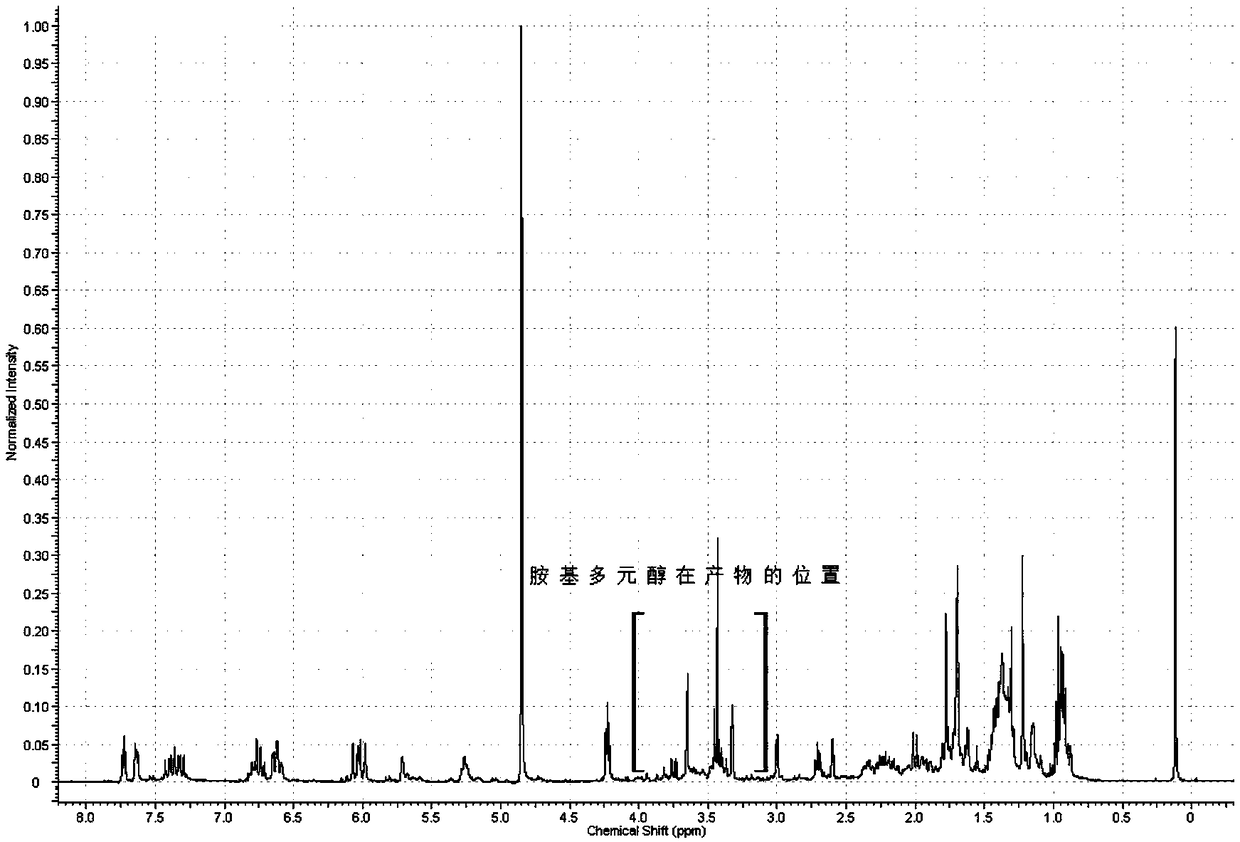
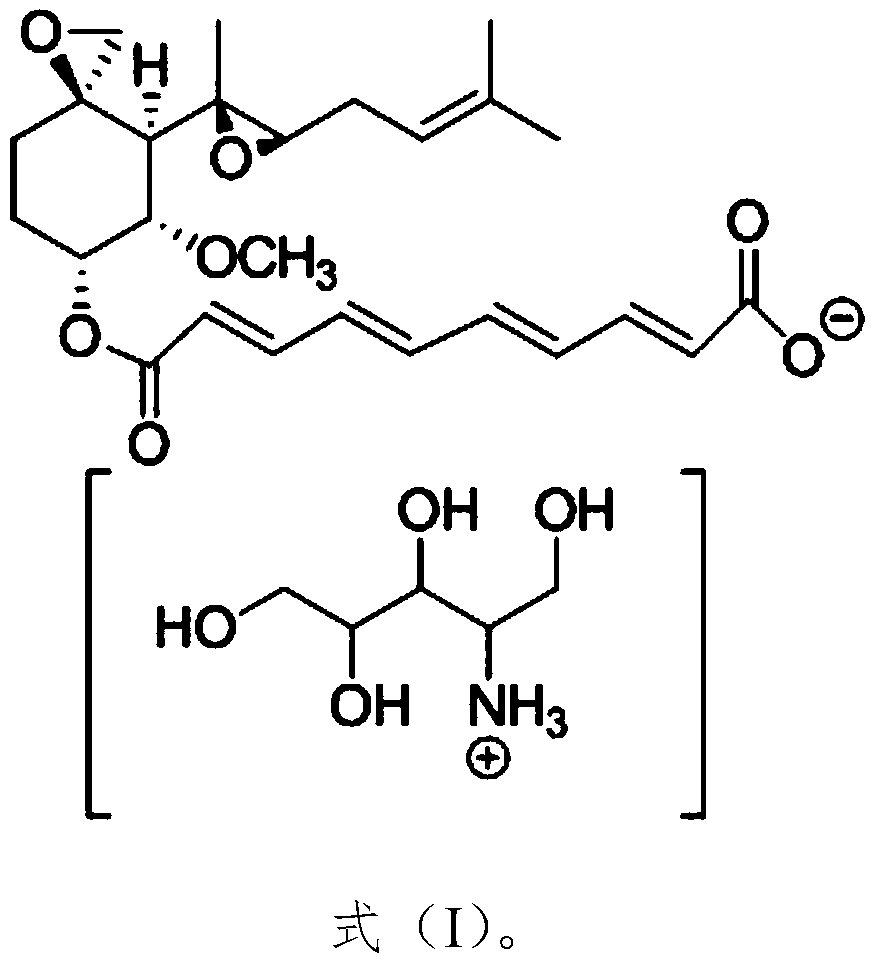
A kind of amino polyol fumagillin and its synthesis method and application
InactiveCN106831662BReduce health risksImprove solubilityOrganic active ingredientsOrganic chemistrySolubilityTreatment effect
Owner:BEE RES INST CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com