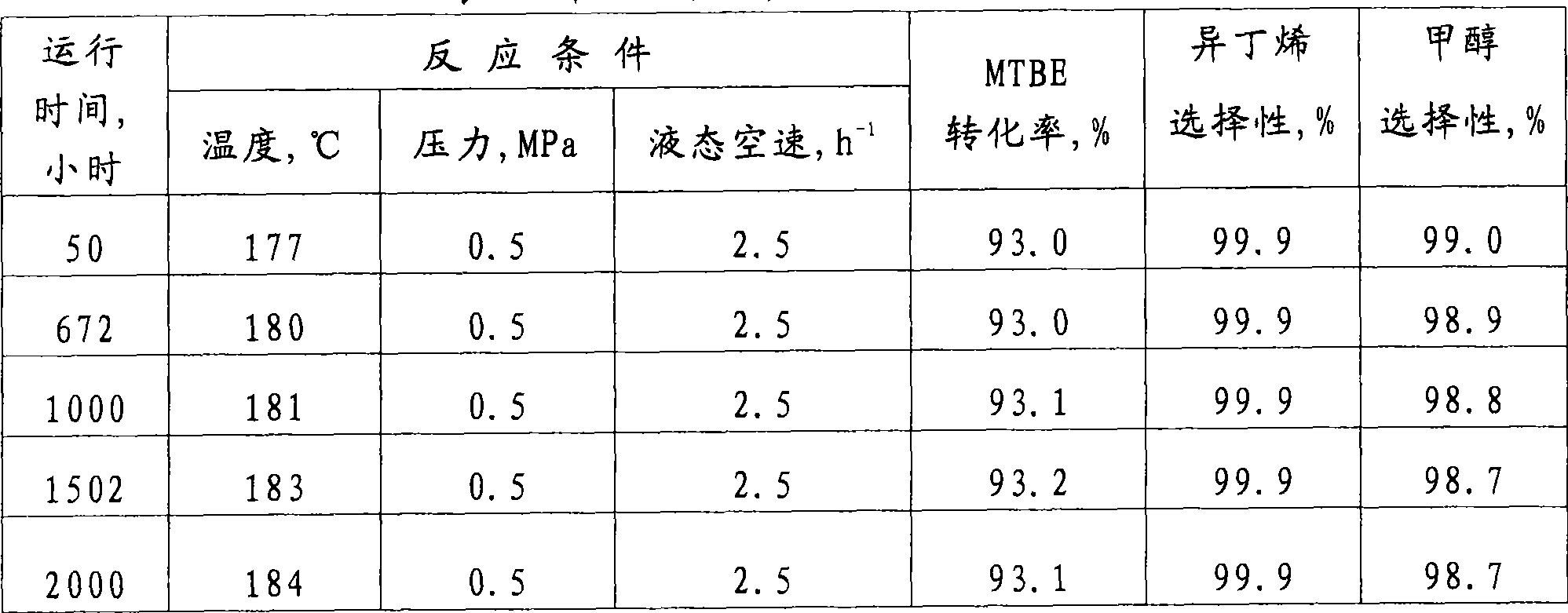
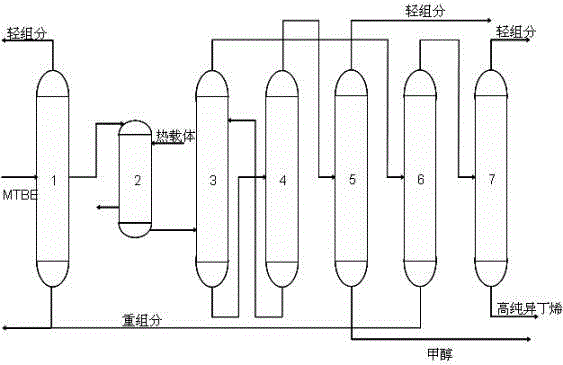
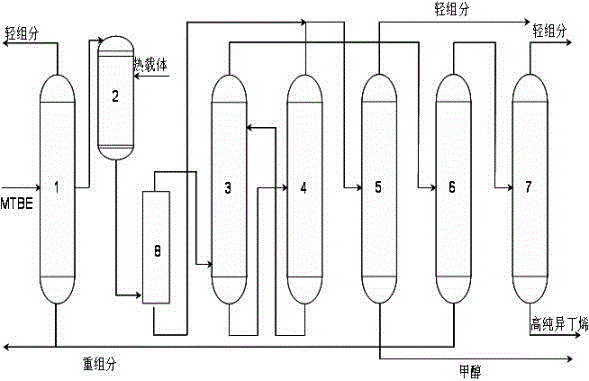
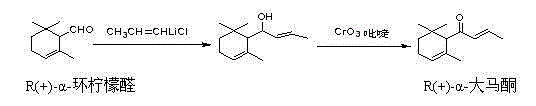
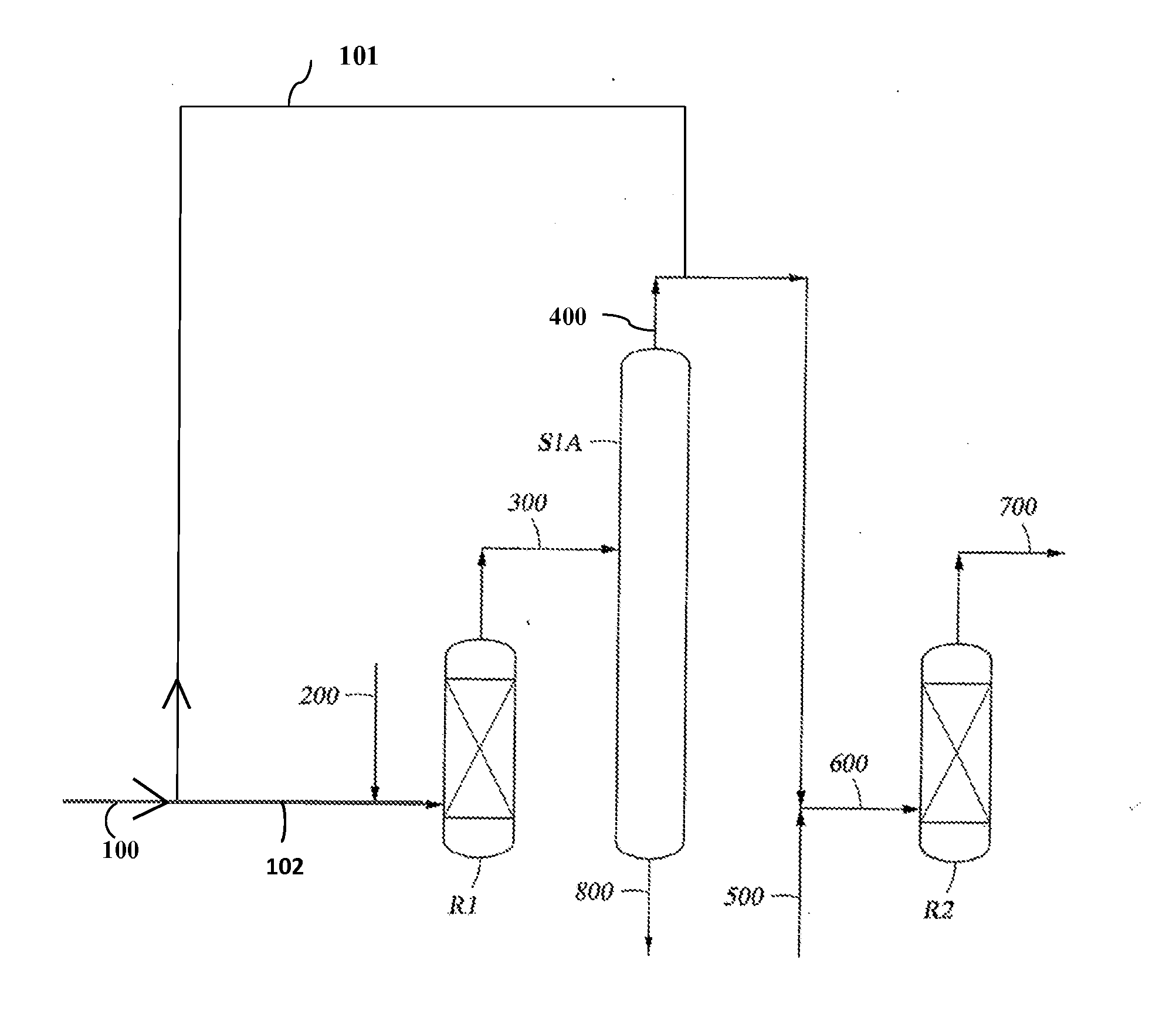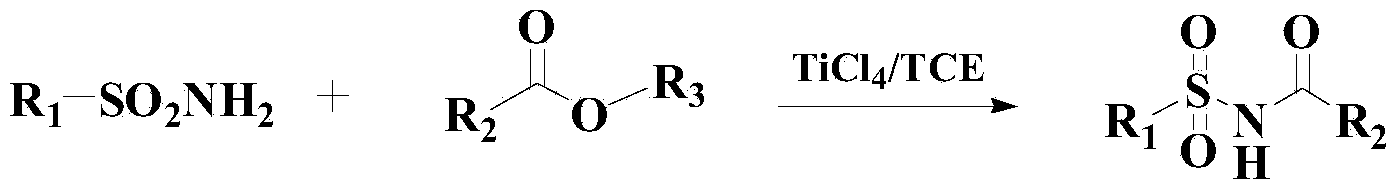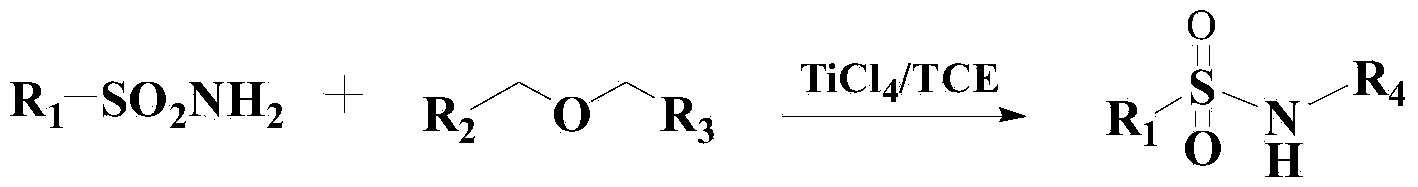Patents
Literature
181 results about "Methyl t-butyl ether" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
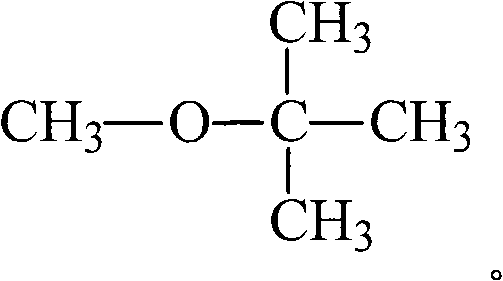
Methyl-tert-butyl ether, also known as tert-butyl methyl ether, methyl t-butyl ether or MTBE, is classified as a member of the dialkyl ethers. Dialkyl ethers are organic compounds containing the dialkyl ether functional group, with the formula ROR', where R and R' are alkyl groups.
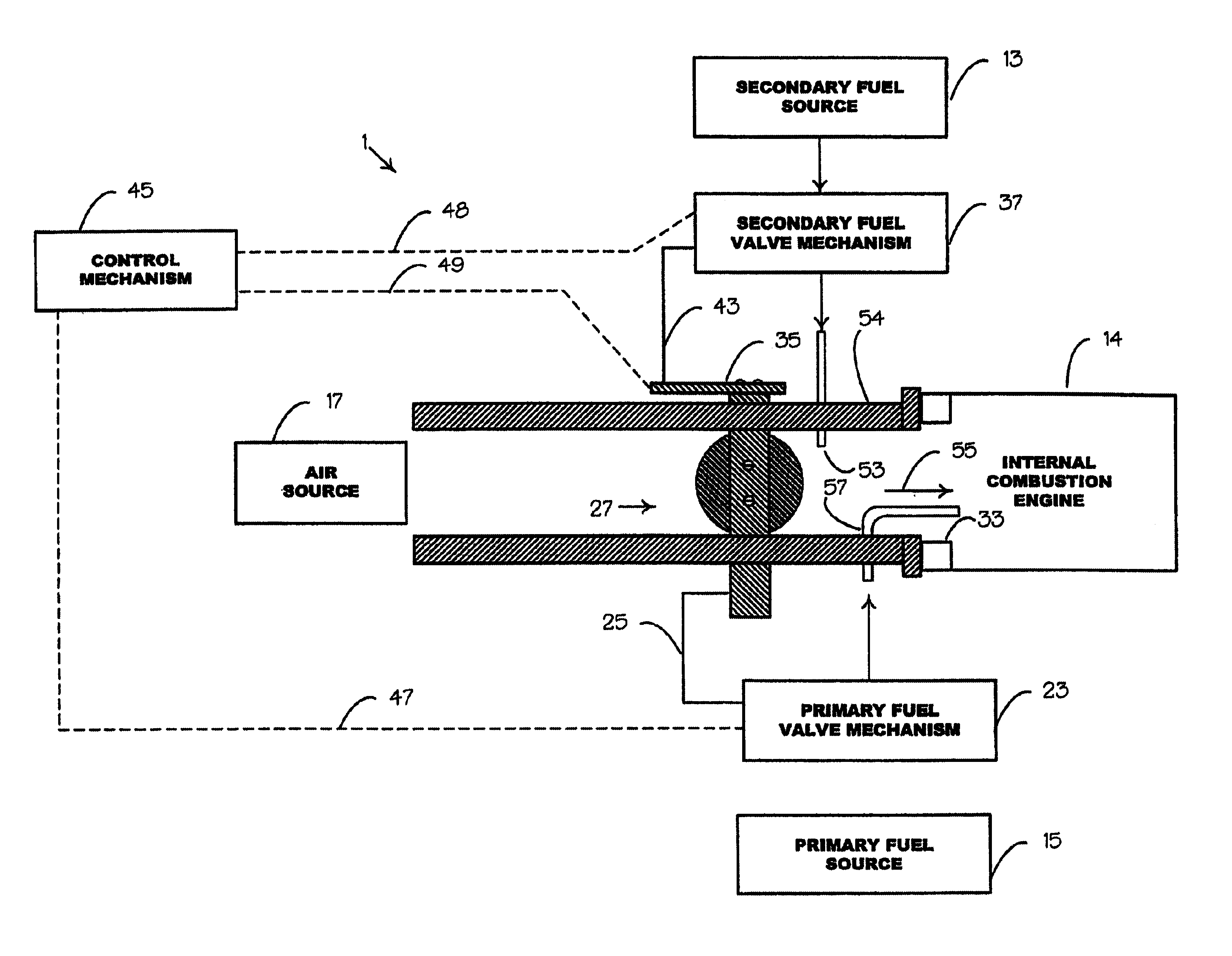
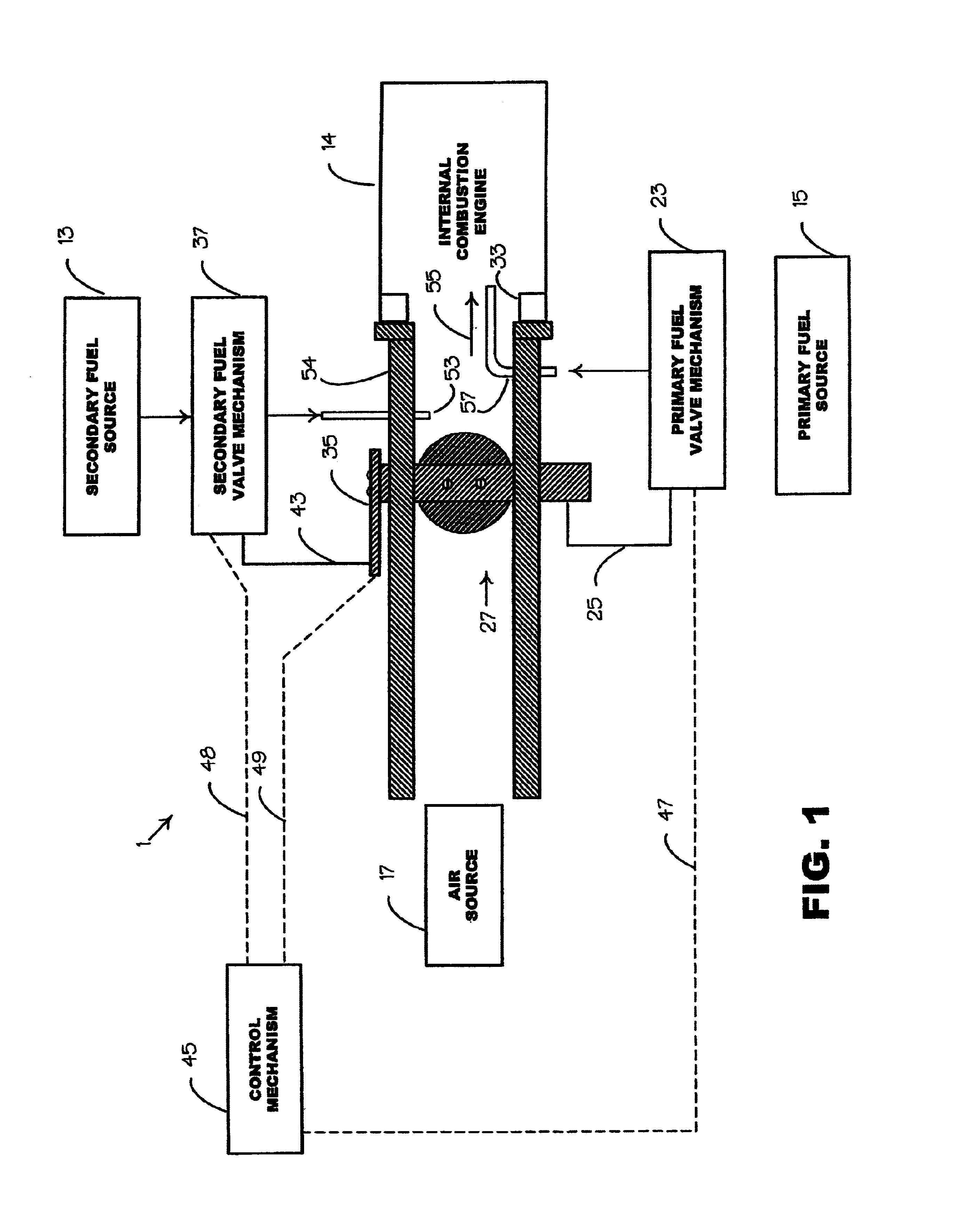
Internal combustion system using acetylene fuel
InactiveUS6076487AInternal combustion piston enginesNon-fuel substance addition to fuelCarbon chainInternal combustion engine
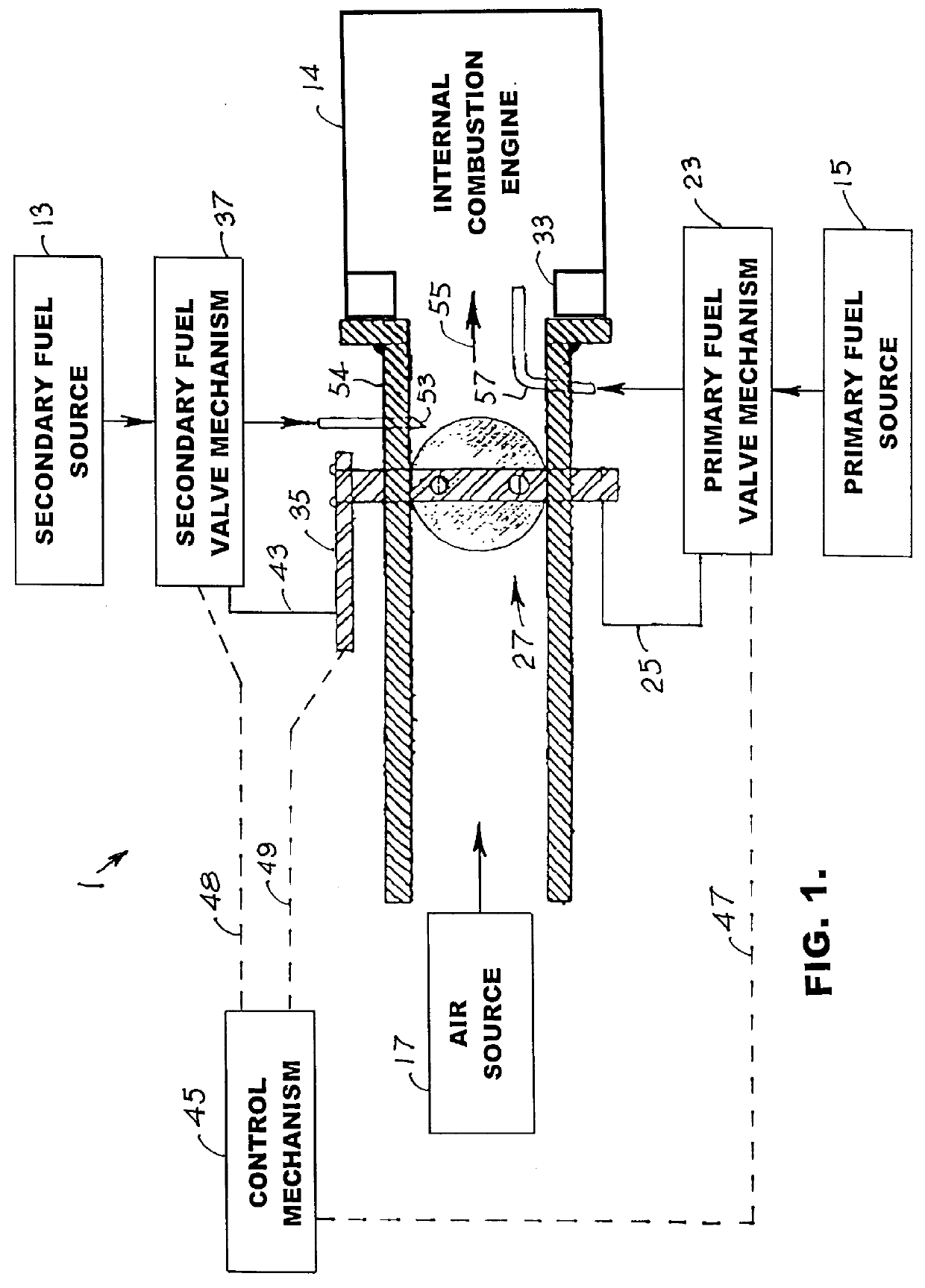
An environmentally clean dual fuel for an internal combustion engine, comprising acetylene as a primary fuel and a combustible fuel, such as one or more fluids selected from an alcohol such as ethanol, methanol or any other alcohol or alcohols from the group comprising C1-C20 carbon chains, ethers such as from the group comprising dimethyl ether, diethyl ether, methyl t-butyl ether, ethyl t-butyl ether, t-amyl methyl ether, di-isopropyl ether and the like, low-molecular-weight esters such as from the group comprising methyl formate, methyl acetate, ethyl acetate, methyl propionate, ethyl propionate and the like, or other suitable combustible fluid such as mineral spirits and the like, as a secondary fuel for operatively preventing early ignition and knock arising from the primary fuel. The dual fuel, internal combustion system, which generally utilizes a two-stage process for start-up and operation and can be operated with air- or liquid-cooling, is environmentally clean with hydrocarbon, CO, NOx, and SOx emissions substantially eliminated.
Owner:GOTEC
Internal combustion system adapted for use of a dual fuel composition including acetylene
InactiveUS6575147B2Easy to operateImprove performanceNon-fuel substance addition to fuelInternal combustion piston enginesCarbon chainMineral spirit
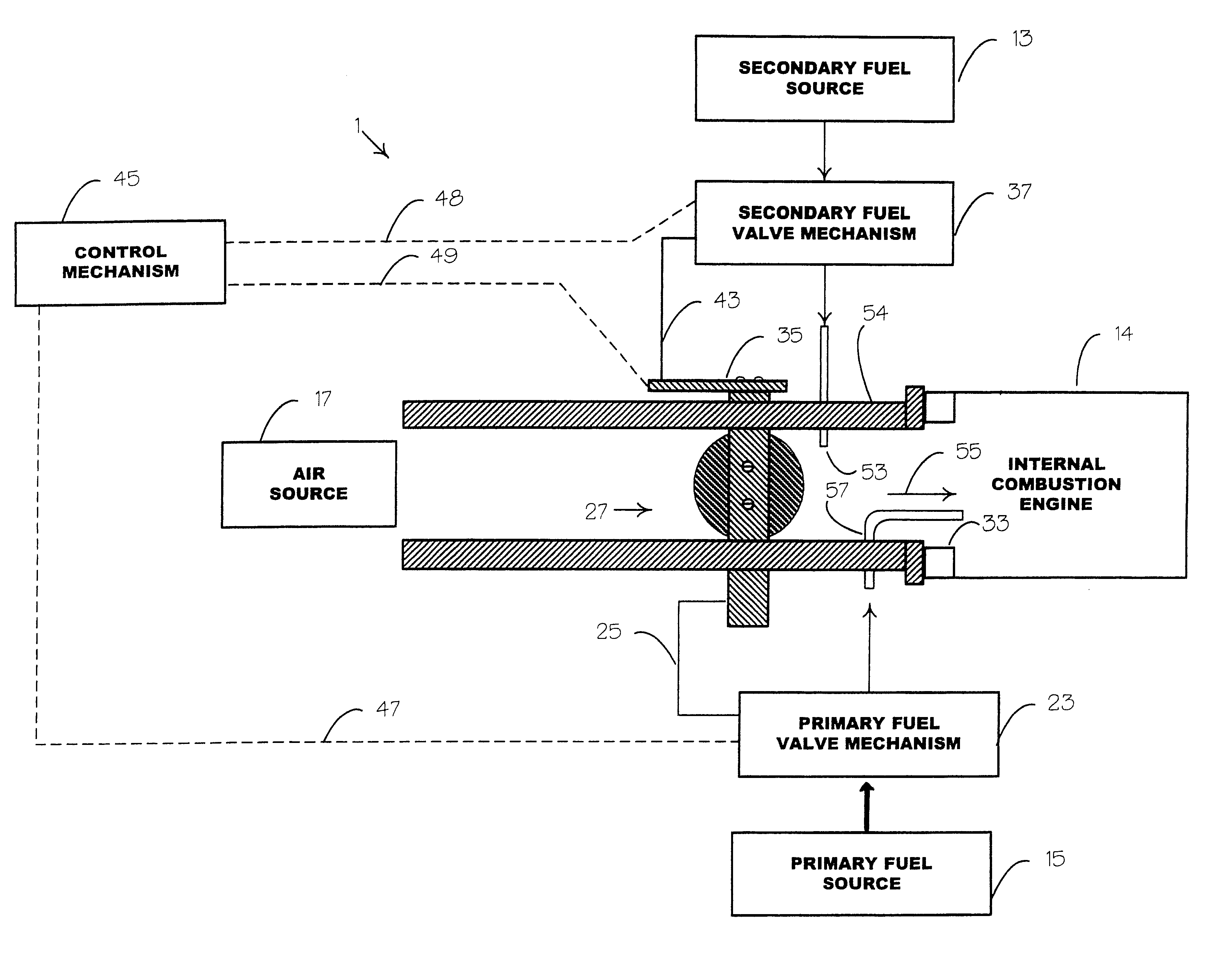
An internal combustion engine adapted to use an environmentally clean multi-fuel composition, comprising acetylene as a primary fuel and a combustible fuel, such as one or more fluids selected from an alcohol such as ethanol, methanol or any other alcohol or alcohols from the group comprising C1-C12 carbon chains, ethers such as from the group comprising dimethyl ether, diethyl ether, methyl t-butyl ether, ethyl t-butyl ether, t-amyl methyl ether, di-isopropyl ether and the like, low-molecular-weight esters such as from the group comprising methyl formate, methyl acetate, ethyl acetate, methyl propionate, ethyl propionate, ethyl malate, butyl malate, and the like, or other suitable combustible fluid such as mineral spirits and the like, as a secondary fuel for operatively preventing early ignition and knock arising from the primary fuel.
Owner:GOTEC INC
Complex oxide catalysts and process for producing (meth) acrolein and (meth) acrylic acid
InactiveUS6383973B1Good reproducibilityHigh activityOrganic compound preparationOrganic chemistry methodsNitrate anionAlkaline earth metal
Complex oxide catalysts represented by the formula,(in which A is Ni or Co; B is Na, K, Rb, Cs or Tl; C is an alkaline earth metal; D is P, Te, Sb, Sn, Ce, Pb, Nb, Mn, As, B or Zn; E is Si, Al, Ti or Zr; and where a is 12, 0<=b<=10, 0<c<=10, 0<d<=10, 2<=e<=15, 0<f<=10, 0<=g<=10, 0<=h<=4 and 0<=i<=30)are provided. The catalysts are characterized in that the molar ratio of the total nitrate anions to the molybdenum at the time of catalyst preparation is more than 1 but not more than 1.8. When used in the reaction for producing (meth)acrolein and (meth)acrylic acid by vapor-phase oxidation of at least a compound selected from propylene, isobutylene, t-butanol and methyl-t-butyl ether, the catalysts exhibit excellent activity and selectivity and maintain stable performance over prolonged period.
Owner:NIPPON SHOKUBAI CO LTD
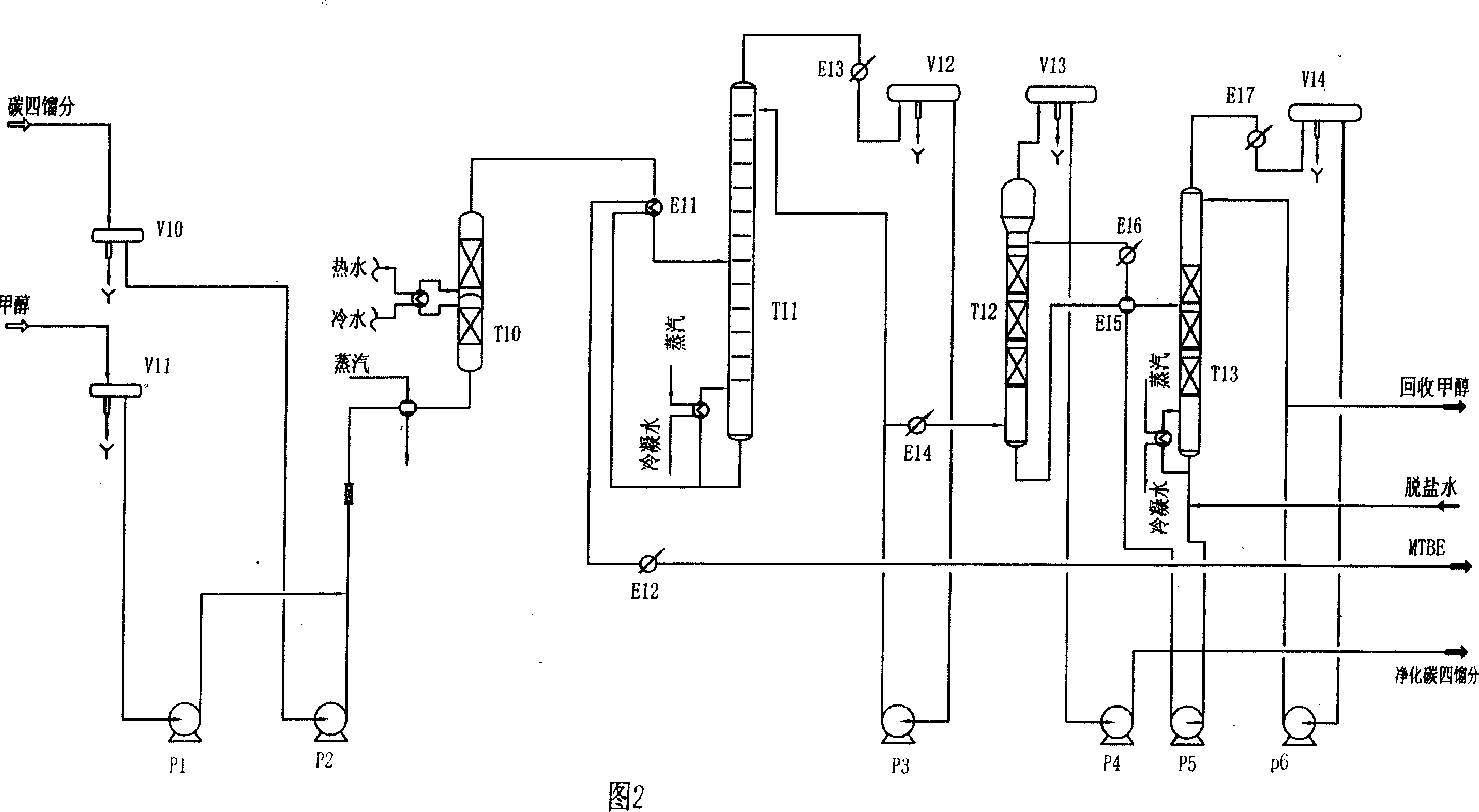
Phrcess of coproducing methyl tert-butyl ether and tert-butyl alcohol
InactiveCN101020622ASave energyLess investmentPreparation by hydroxy group additionEther preparation by compound additionMethanol waterMethyl t-butyl ether
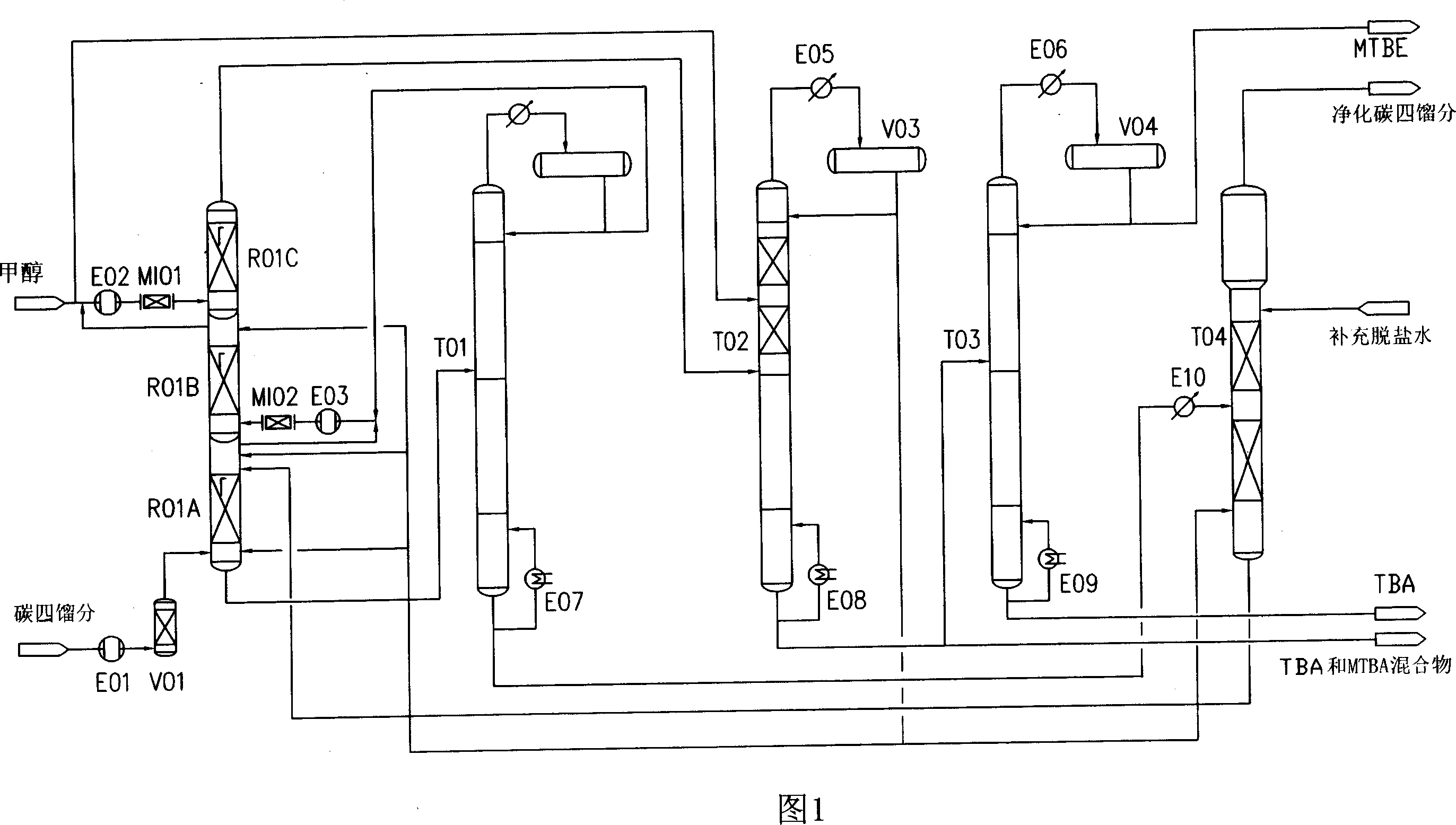
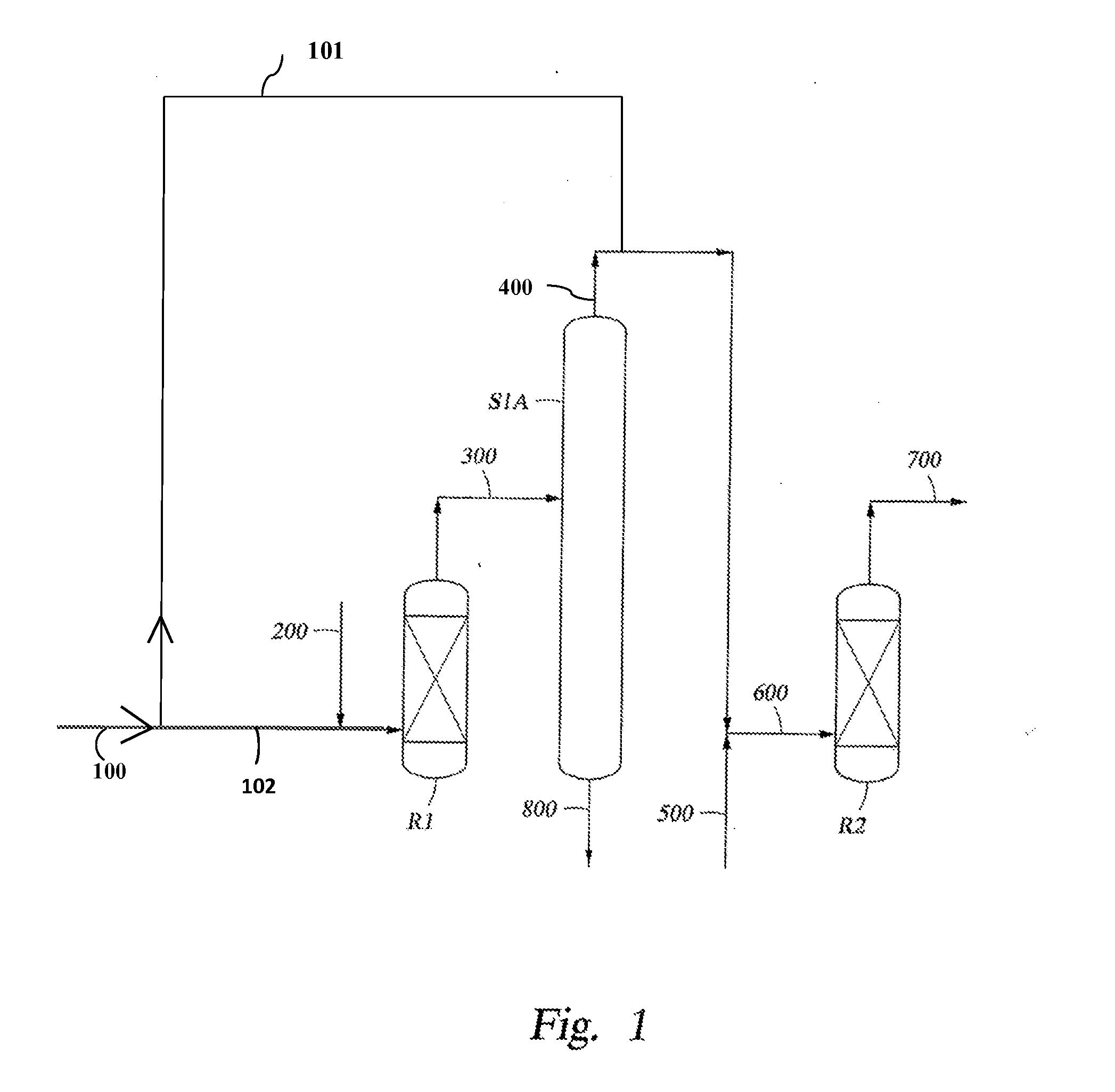
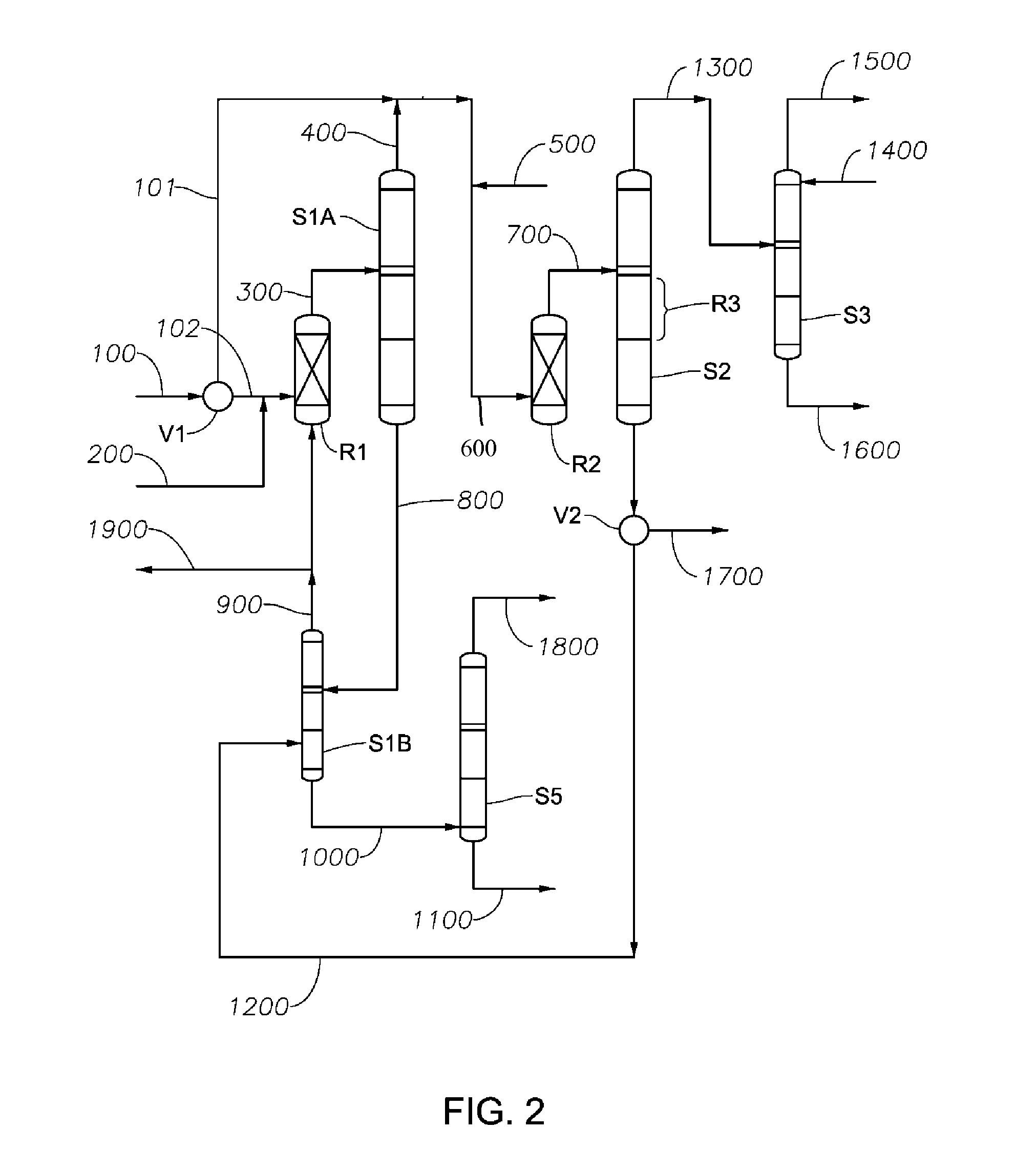
The present invention relates to process of co-producing methyl tert-butyl ether and tert-butyl alcohol with isobutene and methanol in C4 fraction. The present invention features that two kinds of product are produced in an identical equipment with reduced investment, and that separation is completed through several reactions, resulting in simplified equipment and facilitated separation.
Owner:西安道特石化工程有限公司
Catalysts for production of unsaturated aldehyde and unsaturated carboxylic acid and a process for producing unsaturated aldehyde and unsaturated carboxylic acid using the catalysts
InactiveUS6583316B1High yieldGuaranteed uptimeOrganic compound preparationOrganic chemistry methodsGas phaseCerium
Improved catalysts for use in vapor phase oxidation of at least one compound selected from the group consisting of propylene, isobutylene, t-butanol and methyl-t-butyl ether with molecular oxygen or a molecular oxygen-containing gas to produce the corresponding unsaturated aldehyde and unsaturated carboxylic acid are provided. The improved catalysts are compositions comprising (A) a complex oxide containing as essential components molybdenum, bismuth and iron, which is known per se as a catalyst for said reaction and (B) a complex oxide containing cerium and zirconium as the essential components. When the improved catalysts are used, the production operation of unsaturated aldehyde and unsaturated carboxylic acid can be continued stably for over prolonged period.
Owner:NIPPON SHOKUBAI CO LTD
Complex oxide catalysts and process for producing (meth) acrolein and (meth) acrylic acid
InactiveUS20020103077A1Good reproducibilityHigh activityOrganic compound preparationOther chemical processesNitrate anionMeth-
Complex oxide catalysts represented by the formula,<paragraph lvl="0"><in-line-formula>MoaWbBicFedAeBfCgDhEiOx< / in-line-formula>(in which A is Ni or Co; B is Na, K, Rb, Cs or Tl; C is an alkaline earth metal; D is P, Te, Sb, Sn, Ce, Pb, Nb, Mn, As, B or Zn; E is Si, Al, Ti or Zr; and where a is 12, 0<=b<=10, 0<c<=10, 0<d<=10, 2<=e<=15, 0<f<=10, 0<=g<=10, 0<=h<=4 and 0<=i<=30) are provided. The catalysts are characterized in that the molar ratio of the total nitrate anions to the molybdenum at the time of catalyst preparation is more than 1 but not more than 1.8. When used in the reaction for producing (meth)acrolein and (meth)acrylic acid by vapor-phase oxidation of at least a compound selected from propylene, isobutylene, t-butanol and methyl-t-butyl ether, the catalysts exhibit excellent activity and selectivity and maintain stable performance over prolonged period.
Owner:NIPPON SHOKUBAI CO LTD
Dual fuel composition including acetylene
InactiveUS7288127B1Easy to operateImprove performanceInternal combustion piston enginesNon-fuel substance addition to fuelCarbon chainDiethyl ether
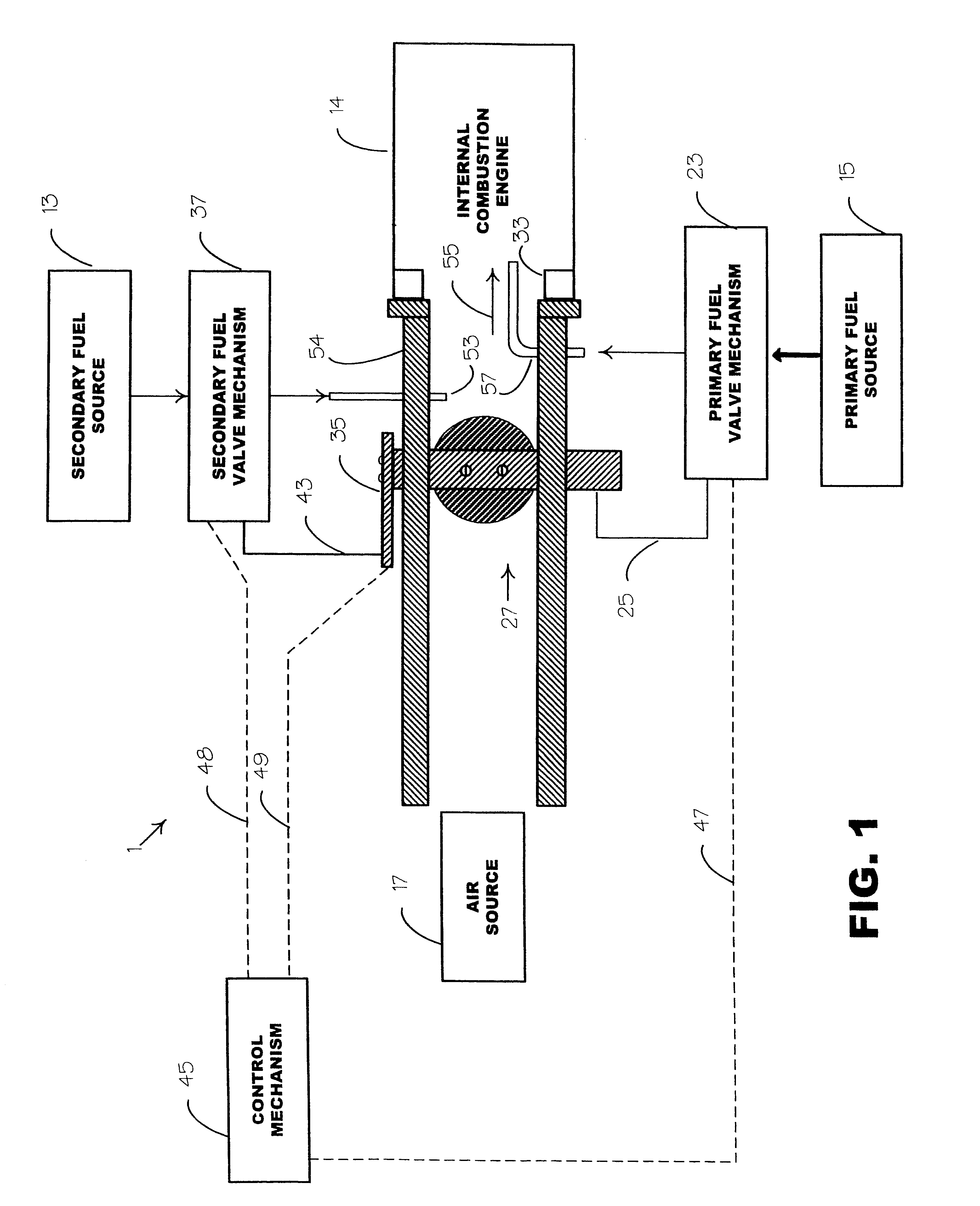
An environmentally clean dual fuel for use in an internal combustion engine, comprising acetylene as a primary fuel and a combustible fuel, such as one or more fluids selected from an alcohol such as ethanol, methanol or any other alcohol or alcohols from the group comprising C1-C20 carbon chains, ethers such as from the group comprising dimethyl ether, diethyl ether, methyl t-butyl ether, ethyl t-butyl ether, t-amyl methyl ether, di-isopropyl ether and the like, low-molecular-weight esters such as from the group comprising methyl formate, methyl acetate, ethyl acetate, methyl propionate, ethyl propionate, ethyl malate, and butyl malate, and the like, or other suitable combustible fluid such as mineral spirits and the like, as a secondary fuel for operatively preventing early ignition and knock arising from the primary fuel.
Owner:GOTEC
Method for analyzing distribution of sulfur in methyl tert-butyl ether, sulfide qualitative analysis database, and use of database
ActiveCN104807895AReduce dosageSimple methodComponent separationSpecial data processing applicationsAnalysis dataQualitative analysis
The invention provides a method for qualitatively / quantitatively analyzing distribution of sulfur in a methyl tert-butyl ether product through using a gas chromatography / sulfur chemiluminescence detector. The qualitative / quantitative analysis method can be used to identify most sulfur compounds with different structure types in the methyl tert-butyl ether product and quantitatively analyze all sulfides in order to make real time adoption of a suitable desulphurization means in the production field convenient and guarantee the quality of the product. The invention also provides a sulfide qualitative analysis database based on the gas chromatography / sulfur chemiluminescence detector, and an application thereof.
Owner:CHINA PETROLEUM & CHEM CORP
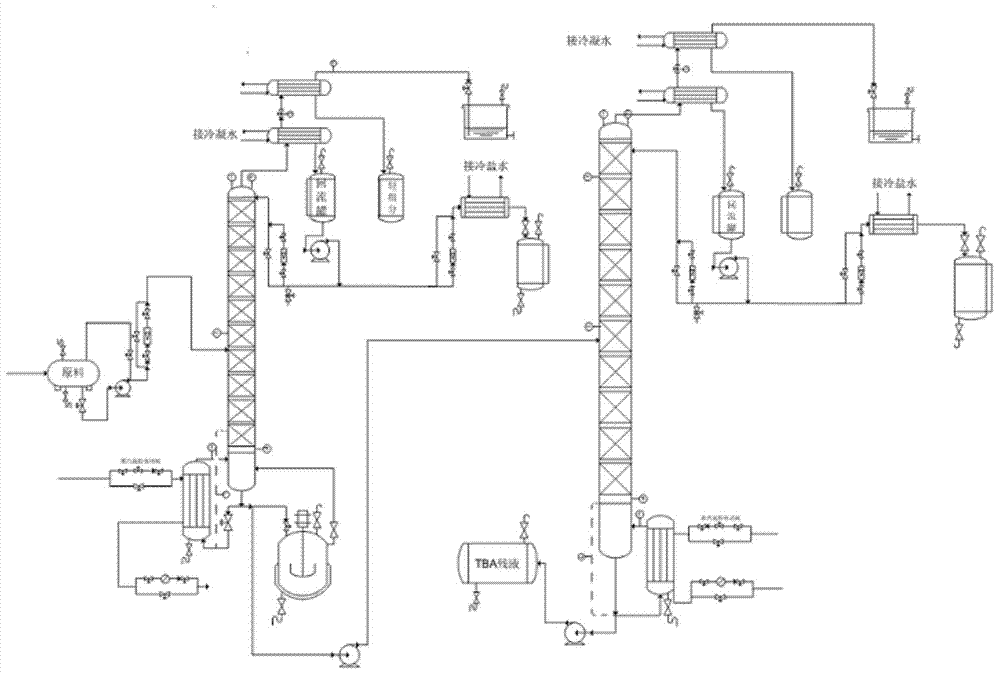
Preparation method and device of methyl tertiary-butyl ether (MTBE)
ActiveCN104250205AImprove conversion rateHigh yieldEther separation/purificationChemical industryWater flowCatalytic distillation
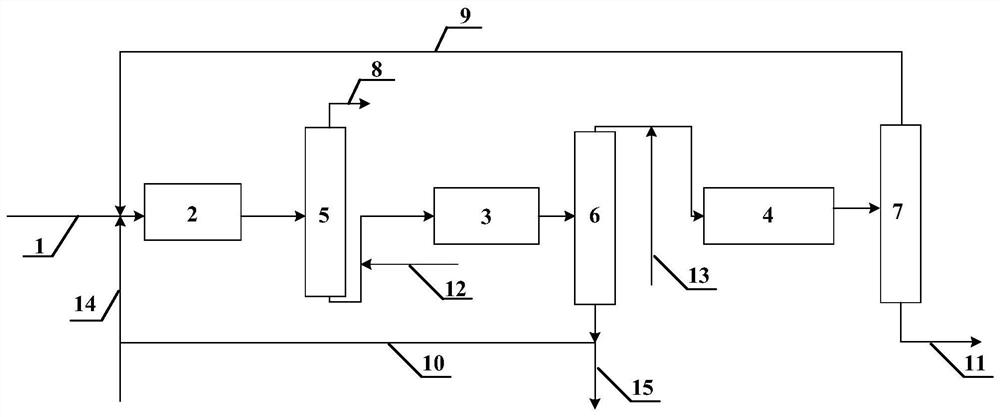
The invention relates to an ether production technology and especially relates to a method and production device for preparing methyl tertiary-butyl ether (MTBE) by a reaction of tertiary butanol and methanol. The method comprises that tertiary butanol and methanol are mixed and undergo a reaction to produce mixed gas, the mixed gas is fed into an extraction tower and is extracted, isobutene and MTBE are distilled off from the tower top, tertiary butanol, methanol, a trace amount of MTBE, water and an extractant flow out of the tower bottom, methanol is supplied for a tower top component of the extraction tower, the mixture is fed into a catalytic distillation tower, a methanol- and isobutene-containing tower top product of the catalytic distillation tower is fed back to a reactor and then undergoes a reaction, the MTBE is discharged from the bottom of the catalytic distillation tower, the materials discharged from the bottom of the catalytic distillation tower enters into a first extractant recovery tower, tertiary butanol, methanol and a trace amount of MTBE are distilled off from the top of the first extractant recovery tower, then are fed back to the reactor and then undergo a reaction, and the extractant and water flow out of the tower bottom, then enter into a second extractant recovery tower and then is recovered for recycle by reduced pressure distillation. The method and device solve the problem of azeotropy of tertiary butanol and water and improve a tertiary butanol one-step conversion rate to 87-94%.
Owner:CHINA PETROLEUM & CHEM CORP
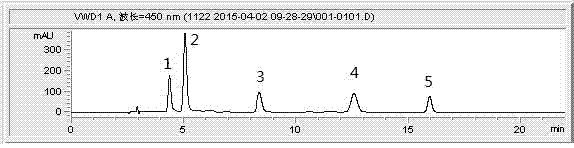
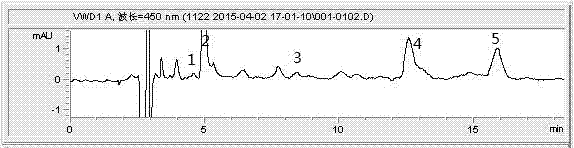
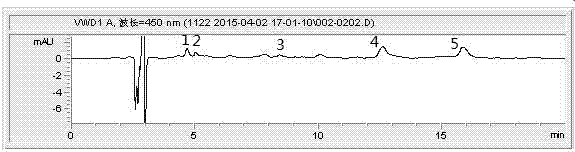
Method for efficiently extracting carotenoids in yellow peach fruits and determining carotenoids in yellow peach fruits by liquid phase
InactiveCN104749293AAccurate measurementEasy to operateComponent separationGradient elutionColumn temperature
The invention discloses a method for efficiently extracting carotenoids in yellow peach fruits and determining the carotenoids in the yellow peach fruits by a liquid phase. Extracting conditions are as follows: the material-liquid ratio of pulp to an extracting agent acetone is 1 to 6 (g / mL), and ultrasonic light-shielding assisted extraction is carried out for 1 hour; chromatographic conditions are as follows: a YMC-C30 type chromatographic column is adopted; a mobile phase comprises methyl tert-butyl ether methanol with a ratio being 30 to 70; an isocratic elution manner is adopted; a flow speed is equal to 1.0mL / min; the wavelength is 450nm; the column temperature is 25 DEG C; a sample feeding amount is 20 microliters; and the time lasts for 18 minutes. According to the method for efficiently extracting the carotenoids in the yellow peach fruits and determining the carotenoids in the yellow peach fruits by the liquid phase, the extraction material-liquid ratio is 1 to 6 (g / mL), the ultrasonic light-shielding assisted extraction is carried out for 1 hour and the methyl tert-butyl ether and the methanol with the ratio of 30 to 70 are used as the mobile phase for carrying out the isocratic elution; the three conditions are combined so that a plurality of types of carotenoids in the yellow peach fruits can be effectively and completely extracted and rapidly and accurately determined; and the method is easy to operate, free of saponification and gradient elution, good in repeatability of a determined result, high in accuracy and short in liquid phase time.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Hydrocarbon Conversion Process
The invention relates to methods and equipment for converting C3+ olefin to, e.g., one or more of di-C3+ olefin, oligomers and polymers of C3+ olefin, branched C4+-aldehydes, C4+-carboxylic acids, and C4+ oxygenates. The invention encompasses producing methyl tert-butyl ether and diisobutylene, and converting methyl tert-butyl ether to isobutylene.
Owner:EXXONMOBIL CHEM PAT INC
Device and method for deeply removing organic sulphides in methyl tertiary butyl ether (MTBE)
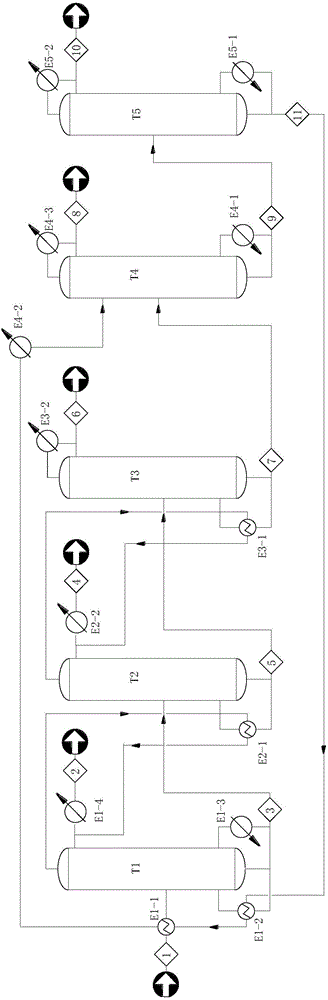
ActiveCN104829436AMTBE recovery increasedReduce process energy consumptionEther separation/purificationChemical industryReboilerMulti effect
The invention provides a device and a method for deeply removing organic sulphides in methyl tertiary butyl ether (MTBE). The device comprises a high-pressure multi-effect rectifying tower, a medium-pressure multi-effect rectifying tower, a normal-pressure multi-effect rectifying tower, an extraction rectifying tower and an extraction agent recycling tower connected in series; a condenser is arranged on the top of each tower; a reboiler is arranged at the bottom of each tower; a material outlet at the bottom of each tower is connected to a material inlet in the middle of a next tower through a pipeline; 90%-95% of qualified MTBE products can be distilled out through the tops of the multi-effect rectifying towers; the organic sulphides are enriched at the bottom of the extraction rectifying tower under the action of an extraction agent; remained MTBE distilled by the tops of the towers returns into a raw material tank; and the cycle repeats. By means of reasonable heat exchange network design, vapor from the tops of the towers and heat of the extraction agent are sufficiently utilized, so that energy consumption in a desulfurizing process is reduced; the total sulphur content of MTBE produced through a multi-effect rectifying and extraction rectifying coupling method is less than 10 ppm; deep sulphur removing requirements are satisfied; the total recovery rate of MTBE is up to 99.8%; and furthermore, the theoretical energy-saving efficiency is up to 53%.
Owner:CHINA CONSTR IND & ENERGY ENG GRP CO LTD +1
Alcohol hydrocarbons composite for cars and preparation method thereof
InactiveCN101319152AReduce pollutionIncrease the calorific value of combustionLiquid carbonaceous fuelsLiquid fuelSolvent
The present invention relates to the liquid fuel technical field, in particular to an alcohol hydrocarbon complex liquid fuel for a car and a preparation method thereof. The compositions in percentage by weight of the liquid fuel are: 20 to 40 percent of alcohol, 40 to 60 percent of light hydrocarbon, 8 to 12 percent of solvent naphtha, 10 to 15 percent of methyl tert-butyl ether, and 0.01 to 0.05 percent of functional additive, wherein the alcohol is methanol or ethanol, the light hydrocarbon is naphtha or light hydrocarbon between C6 and C12. The method comprises the following steps of: mixing the 20 to 40 percent of alcohol with the 0.01 to 0.05 percent of functional additive at a the temperature of between 0 and 20 DEG C; stirring for 10 minutes to obtain a mixed solution A; mixing the 40 to 60 percent of light hydrocarbon and the 8 to 12 percent of solvent naphtha, and then stirring for 10 minutes to obtain a mixed solution B; mixing the mixed solution A with the 10 to 15 percent of methyl tert-butyl ether, and then stirring for 10 minutes; mixing with the mixed solution B, and stirring for 10 minutes and sealing for 24 hours, and thus obtaining the product. Compared with the non-premium grade gasoline, the product is increased in oxygen content, sufficient in combustion, beneficial to environment protection, and can reduce the noxious gas of the automobile exhaust emission. Not only the cost is low and the material is easy to get, but also the required devices are less and the process is simple.
Owner:孙昔铭 +1
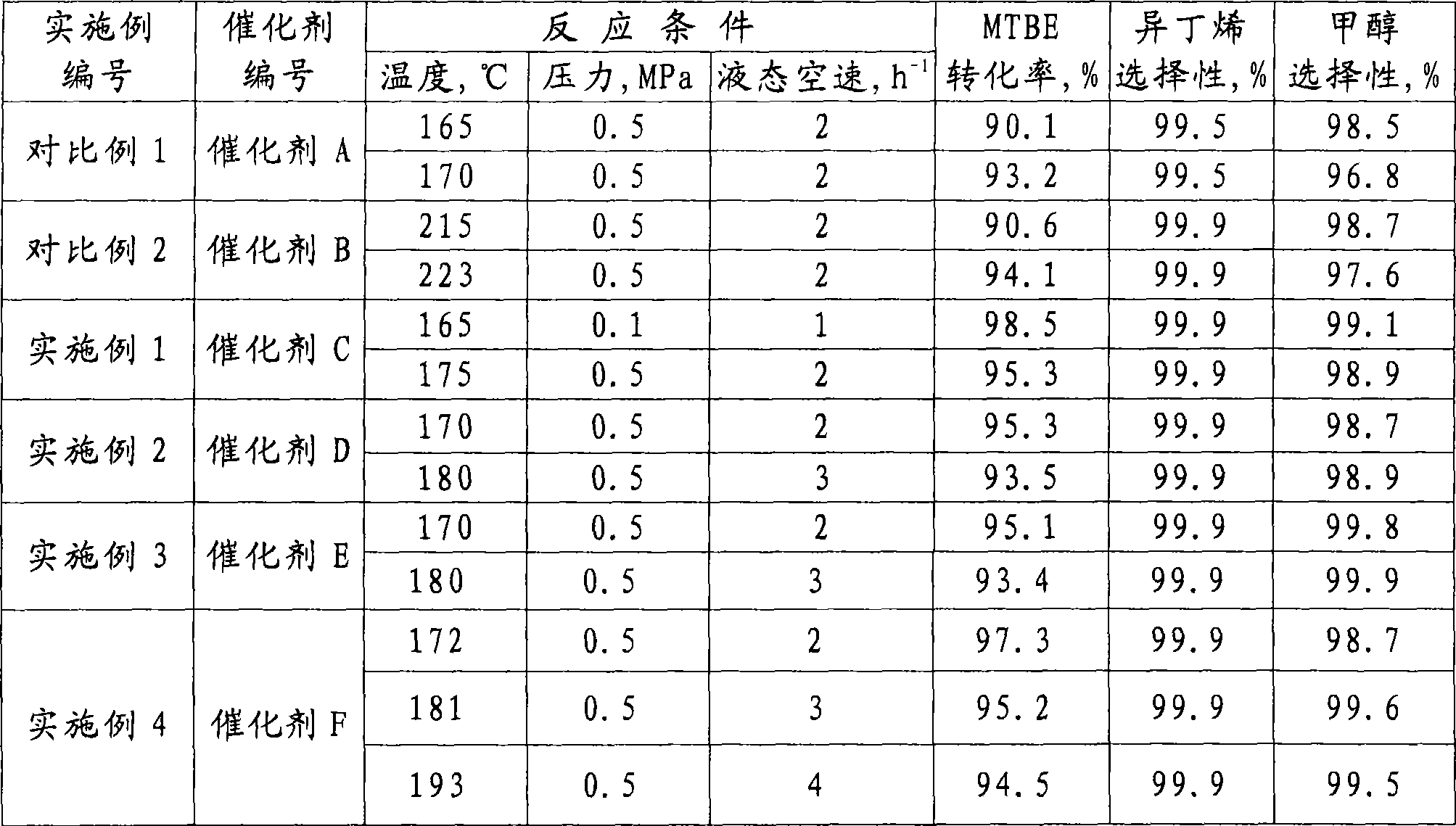
Catalyst for preparing tert-olefins by cracking tert-alkyl ethers, preparation method and application thereof
ActiveCN101530805AReduce consumptionHigh cracking conversionPhysical/chemical process catalystsHydrocarbon from oxygen organic compoundsButeneFiltration
The invention relates to a solid acid catalyst used in reactions for preparing tert-olefins by cracking tert-alkyl ethers, a preparation method and an application thereof, wherein the content of sulfides in the catalyst is calculated by sulfur and accounts for 0.1-15 percent by weight of the weight of alumina, and the content of fluorides is calculated by fluorine and accounts for 0.1-10 percent by weight of the weight of alumina. The catalyst uses sulfur compounds and solution containing fluorine compounds to process an alumina carrier and is prepared by filtration, drying and calcination. The catalyst is used in the reactions for preparing the tert-olefins by cracking the tert-alkyl ethers, for example, the reaction for preparing isobutene by cracking methyl tert-butyl ether, thereby having high conversion rate, high selectivity of tert-butene and joint product methanol, high product purity as well as good stability and being capable of being used under the condition of substantial increase of feed loads particularly.
Owner:CHINA PETROLEUM & CHEM CORP
Process for preparing isobutene through methyl tertiary-butyl ether cracking
ActiveCN104591944AOrganic compound preparationDistillation purification/separationMethyl t-butyl etherGas phase
The invention discloses a process method for preparing isobutene through methyl tertiary-butyl ether cracking. According to the invention, methyl tertiary-butyl ether is subjected to a cracking reaction in a reactor; an obtained cracking product is subjected to heat exchange cooling until the temperature is higher than 50 DEG C and no higher than 90 DEG C; an obtained cooling product is separated in a separator; a gas-phase product is discharged from the upper part of the separator, and enters a water washing tower; a product discharged from the upper part of the water washing tower sequentially enters an isobutene de-heavy tower and an isobutene de-light tower, such that an isobutene product is obtained. According to the method, water consumption of the water washing tower is reduced, and washing effect of the water washing tower is improved. Also, methanol recovery tower and methanol rectification tower work loads are greatly reduced, and energy consumption is reduced. The prepared isobutene can reach polymerization-grade requirements.
Owner:CHINA PETROLEUM & CHEM CORP +1
Synthesis method of (S)-(-)-alpha-damascenone
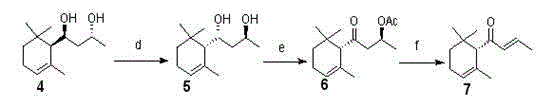
InactiveCN104630289ALoose requirementsHigh optical purityOrganic compound preparationOrganic chemistry methodsDamasconeDouble bond
The invention discloses a synthesis method of (S)-(-)-alpha-damascenone, which comprises the following steps: adding alpha-ionone into an alcohol or ether solvent, adding a hydrogen peroxide water solution and a sodium hydroxide water solution, stirring to perform double bond oxidization reaction to generate alpha,beta-epoxy-alpha-ionone, adding tetrahydrofuran, water and ethanol to generate beta-epoxy-alpha-ionone under the catalytic action of an aluminum-mercury mixture, carrying out ring-opening hydrogenation to generate 4-hydroxy-4-(2,6,6-trimethylcyclohexyl-2-alkenyl)-2-butanone, adding acetonitrile and an acetic acid solution to react with Me4NHB(OAc) to obtain a raw material diol, reacting the diol with vinyl acetate and methyl tert-butyl ether under the catalytic action of lipase while stirring to obtain an optical isomer diol, adding DMP (dimethylpyrazole) and CH2Cl2 to react to obtain propyl 1-methyl-3-oxo-3-(2,6,6-trimethyl-cyclohexyl-2-alkenyl)-acetate, and carrying out elimination reaction under the catalytic action of inorganic alkali to generate the (S)-(-)-alpha-damascenone dominant antipode. The method has the advantages of cheap and accessible raw materials, mild reaction conditions and higher conversion rate, is simple to operate, and can obtain the antipode with higher optical purity.
Owner:ANHUI HYEA AROMAS
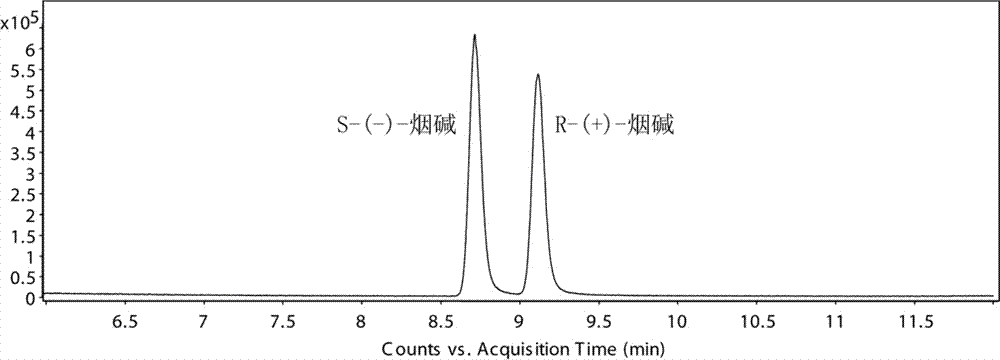
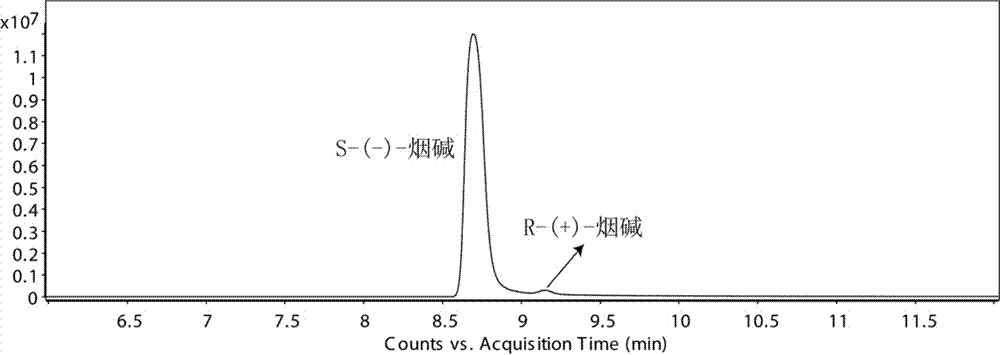
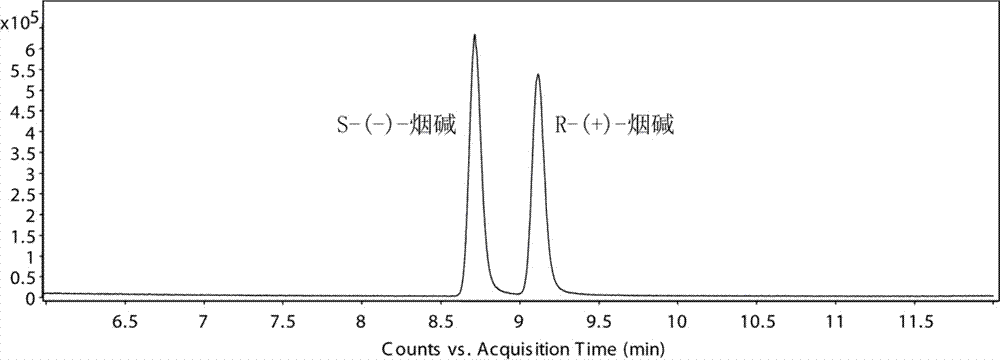
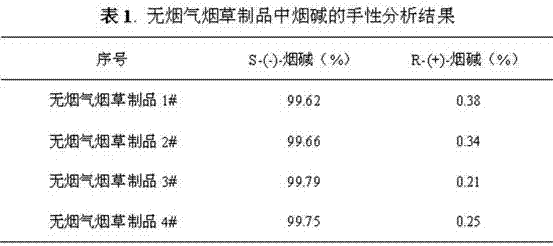
Chiral analysis bonded phase chromatography-tandem mass spectrometry method for nicotine in tobacco juice
ActiveCN106896173AChiral analysis meetsHigh sensitivityComponent separationPurification methodsSolid phase extraction
The invention discloses a chiral analysis bonded phase chromatography-tandem mass spectrometry method for nicotine in tobacco juice. The method is characterized by comprising the following steps: infiltrating an electronic tobacco juice sample with a sodium hydroxide solution, extracting by using a methyl tertiary butyl ether and methanol solution, performing matrix dispersion solid-phase extraction and purification on extract liquor, analyzing by using a tandem mass-bonded phase chromatography tandem mass spectrometry method with two chiral columns, and performing quantitative detection on the ratio of S-(-)-nicotine to R-(+)-nicotine in the electronic tobacco juice by using a peak area normalization method. By adopting the detection method disclosed by the invention, a sample extraction and purification method is simple and efficient, contamination of the sample liquid to chromatographic columns can be reduced, the bonded phase chromatography tandem mass spectrometry method is high in sensitivity, good in specificity and short in analysis time, false positive can be excluded, and chiral analysis on the nicotine in large scales of electronic tobacco juice samples can be met.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Filter medium, preparation method thereof, filter element, water purifying plant and water dispenser
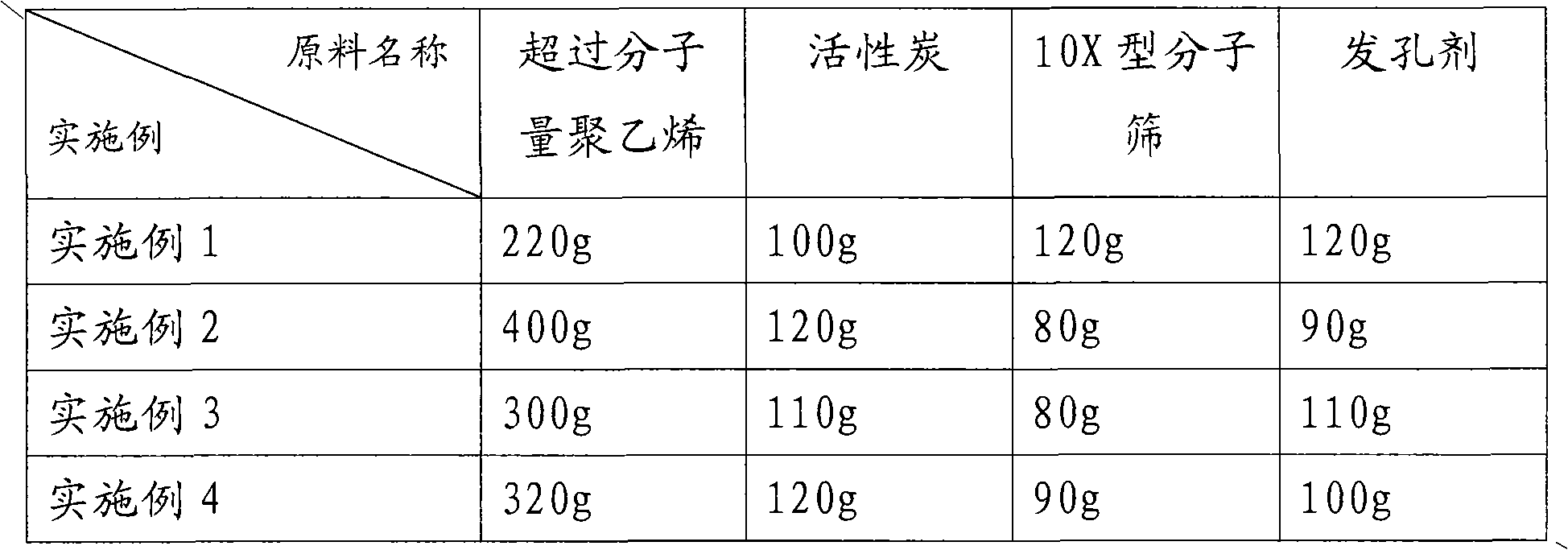
InactiveCN101898117AWide variety of sourcesImprove water qualityOther chemical processesWater/sewage treatment by sorptionMolecular sieveActivated carbon
The invention discloses a preparation method of a filter medium for removing methyl tertiary butyl ether in water, comprising the following steps: a) mixing raw materials containing ultra-high molecular weight polyethylene (PE), activated carbon, 10X-type molecular sieve powder and pore forming agent at the following weight ratio: 220-400 parts of the ultra-high molecular weight PE, 100-120 partsof the activated carbon, 80-120 parts of the 10X-type molecular sieve powder and 90-120 parts of the pore forming agent; and b) pressing, sintering and cooling the mixture obtained in step a) in a die. The invention also discloses a filter medium prepared by the preparation method, a filter element utilizing same, a water purifying plant and a water dispenser. Compared with the prior art, the invention has the advantages that the proposed technical scheme can remove the methyl tertiary butyl ether in water, and the removal rate is above 96%, so that water quality is improved. The method is simple, the raw materials for preparing filter medium have wide resources and low cost.
Owner:QIDI ELECTRIC GROUP
Process and adsorbent for separating ethanol and associated oxygenates from a biofermentation system
InactiveUS20130317260A1Oxygen-containing compound preparationOrganic compound preparationSorbent2,3-Butanediol
Disclosed is a process and an adsorbent for the separation of ethanol associated oxygenates from a dilute mixture of ethanol and associated oxygenates in water in the presence of organic compounds derived from a biofermentation process. After pretreatment, the separation is carried out in a simulated moving bed adsorption system employing an stationary phase adsorbent comprising fluorinated carbon or modified C18 silica gel selective for the adsorption of ethanol and associated oxygenates, such as 2,3-butanediol, with a mobile phase desorbent selected from the group consisting of methanol, ethanol, propanol, and methyl tertiary butyl ether. The process is useful for removing water from dilute aqueous mixtures of organic compounds comprising ethanol in dilute concentration in water and produced by fermentation, biomass extraction, biocatalytic and enzymatic processes which are not economically recoverable by conventional distillation methods.
Owner:OROCHEM TECH INC
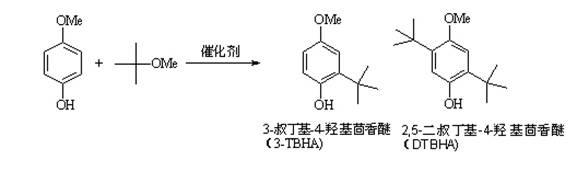
Method for synthesizing tert-butylated hydroxyanisole through solid-liquid-phase reaction
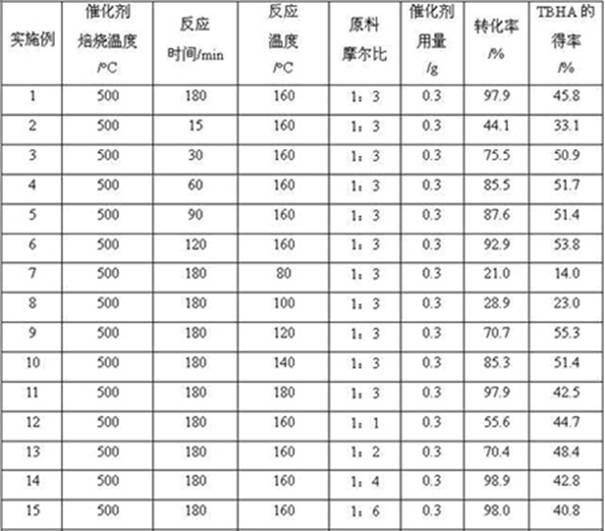
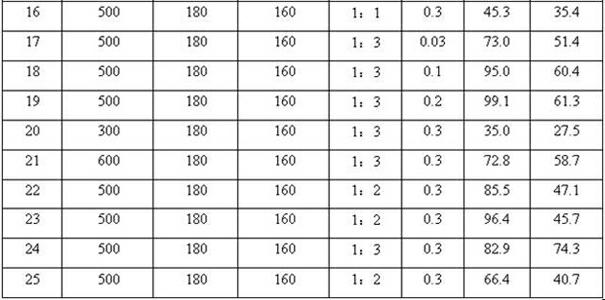
InactiveCN101973858AAvoid the problem of being unable to reuse and discharge large amounts of acidic wastewaterIncrease productivityOrganic chemistryMolecular sieve catalystsPtru catalystReaction temperature
The invention provides a method for synthesizing tert-butylated hydroxyanisole under the catalytic action of a hydrogen Y molecular sieve by using p-methoxy phenol and methyl tert-butyl ether as raw materials. In the method, the Y molecular sieve with a hydrogen Si / Al ratio of 2.6-7.6 is used, the reaction time is 15-180 min, the reaction temperature is 80-180 DEG C, the p-methoxy phenol conversion rate is 21-99 percent and the tert-butylated hydroxyanisole yield is 14-74 percent. The invention adopts a catalyst which has low cost and can be repeatedly used through simple solvent washing or roasting treatment, can solve the problems of unavailable catalyst recovery and great acidic wastewater discharge of a traditional tert-butylated hydroxyanisole production process through an F-C (Friedel-Crafts) reaction and has important industrial application prospects, and meanwhile, the catalyst and the reaction process, adopted in the method, benefits the improvement of the production efficiency.
Owner:ZHEJIANG UNIV
Catalyst for methyl tert-butyl ether cracking reaction to prepare isobutene and preparation method thereof
ActiveCN103433072AImprove temperature resistanceHigh selectivityOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbon from oxygen organic compoundsBoron trifluorideActive site
The invention provides a catalyst for methyl tert-butyl ether cracking reaction to prepare isobutene and a preparation method thereof. The catalyst is prepared through supporting boron trifluoride with perfluorinated sulfonic resin as a support. Perfluorinated sulfonic resin supplies a B-acidity active site to the catalyst, and boron trifluoride supplies an L-acidity active site to the catalyst, so that the temperature resistance of the catalyst is high, the catalyst can still keep relatively-high activity after long-term operation, and the service life of the catalyst is long.
Owner:KAIRUI ENVIRONMENTAL PROTECTION TECH
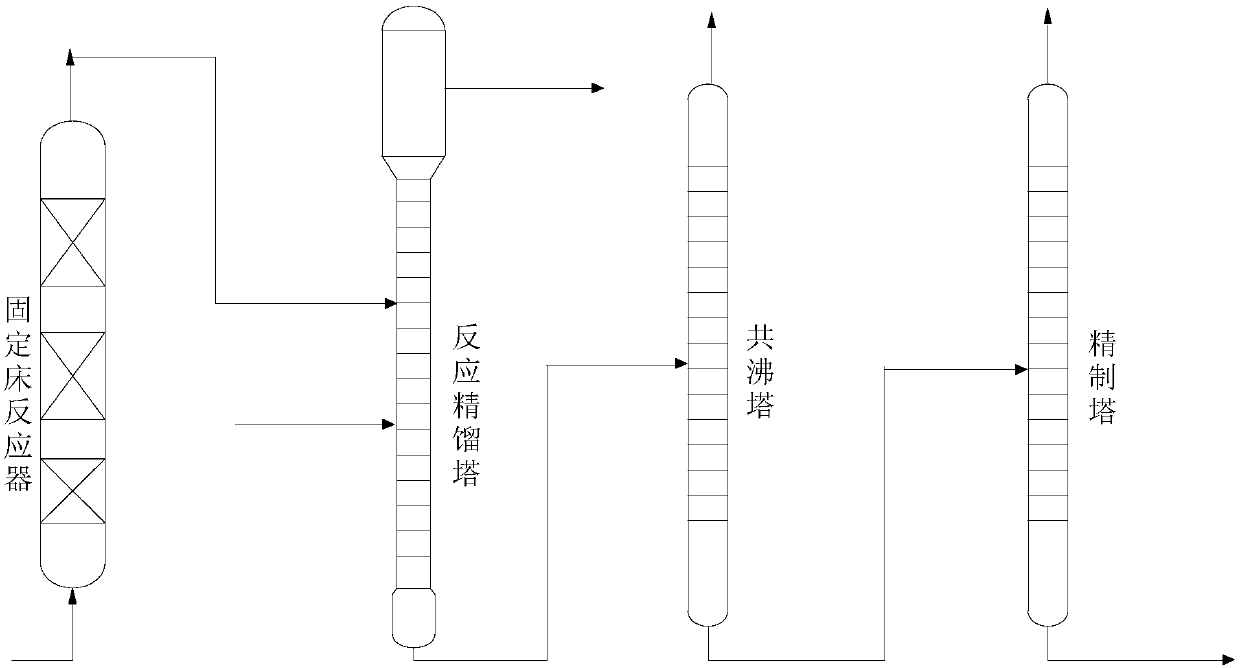
Double-rectifying-tower series separation and purification method for recovery of methyl tert-butyl ether-tetrahydrofuran in waste liquid in statins synthesis process
ActiveCN103706136AHigh purityDistillation regulation/controlFractional distillationPurification methodsMethyl t-butyl ether
The invention discloses a double-rectifying-tower series separation and purification method for recovery of methyl tert-butyl ether-tetrahydrofuran in waste liquid of a statins synthesis process, the method is as follows: leading the waste liquid of the statins synthesis process into a packed tower I, controlling the tower top temperature at 30-40 DEG C for distillation treatment to obtain an isoprene and water mixture from the tower top, and then obtaining isoprene by further layering treatment; leading a tower kettle discharging liquid of the packed tower I into a packed tower II, and controlling the tower top temperature at 50-65 DEG C for distillation treatment to obtain an methyl tert-butyl ether-tetrahydrofuran mixed solvent from the tower top of the packed tower II. According to the method, two packed distillation towers are in series for operation, the separation operation process is low in energy consumption and simple in process, and can improve the recovery rate of the solvent methyl tert-butyl ether-tetrahydrofuran, the economic benefit can be improved, and at the same time the environmental pollution and destruction can be reduced.
Owner:JIANGSU ALPHA PHARM CO LTD
Production process of medicinal phloroglucinol
ActiveCN103012069ARaw materials are easy to getStable reaction conditionsOrganic chemistryOrganic compound preparationHollow fibreChemical industry
The invention discloses a production process of medicinal phloroglucinol, and belongs to the field of medicine chemical industry. The production process comprises the following steps of: uniformly mixing 1,3,5-trimethoxy benzene with concentrated hydrochloric acid, adding phosphoric acid, and adding a catalyst; stirring for 2-4 hours at room temperature; adding in an ice bath, adding Na2CO3 in different batches while stirring; regulating the pH value to 2-3; filtering; extracting a filtrate for three times by methyl tert-butyl ether; combining the extracting liquids; recovering the methyl tert-butyl ether to obtain a yellow solid, namely coarse phloroglucinol; adding proper amount of pure water into the coarse product to dissolve the coarse product; heating to 80 DEG C, stirring to dissolve the coarse product; adding 0.50% of active carbon, preserving the heat for 45minutes; filtering by a filter membrane of 0.45 microns, and filtering a filtrate by a hollow fiber; and cooling the obtained filtrate to room temperature, crystallizing for 4hours, centrifuging, reserving a white crystal substance, and drying the white crystal substance under a vacuum environment to obtain the product. The production process disclosed by the invention is simple in process, low in cost, high in yield being greater than 85% and high in product purity being greater than 99.8%.
Owner:HUNAN ER KANG PHARMA
Preparation method of ethylene glycol mono-tert-butyl ether
ActiveCN110759817ALow reaction pressureSimple processEther separation/purificationChemical industryPolymer sciencePtru catalyst
The invention relates to a preparation method of ethylene glycol mono-tert-butyl ether, and belongs to the technical field of organic chemical engineering. The preparation method of the ethylene glycol mono-tert-butyl ether provided by the invention uses ethylene glycol and methyl tert-butyl ether as raw materials, uses a solid acid catalyst, and uses a fixed bed reaction and a reactive distillation process to prepare the ethylene glycol mono-tert-butyl ether. The method has low reaction pressure, a simple process flow and low production costs, and belongs to environment-friendly processes.
Owner:CHINA PETROLEUM & CHEM CORP
Pretreatment method, detection method and kit for simultaneously detecting multiple steroid hormones in blood sample
ActiveCN112198265AHigh separation purityThe separation method is simpleComponent separationBulk chemical productionPretreatment methodMethyl t-butyl ether
The invention relates to a pretreatment method, a detection method and a kit for simultaneously detecting multiple steroid hormones in a blood sample. The pretreatment method comprises the following steps: 1), preliminarily extracting the steroid hormone in a serum sample by using methyl tert-butyl ether; 2), performing centrifuging, taking supernatant, and carrying out sufficient alkali washing with an alkaline solution; and 3), performing centrifuging after alkali washing, taking supernate, performing freezing and blow-drying, and redissolving the obtained solid components to obtain a to-be-detected solution for simultaneously detecting various steroid hormones. According to the method, at least five steroid hormones of 17-hydroxy progesterone, androstenedione, cortisol, 21-deoxycortisoland 11-deoxycortisol can be separated at the same time, the separation effect is good, and the operation is simple and convenient.
Owner:GUANGZHOU KINGMED DIAGNOSTICS CENT
Method for synthesizing benzene sulfonamide compounds
InactiveCN103819369AEfficient responseMild responseOrganic-compounds/hydrides/coordination-complexes catalystsSulfonic acid amide preparationBenzeneMethyl tertiary butyl ether
The invention relates to a method for preparing benzene sulfonamide compounds. In the method, a ternary catalyzing system of ethyltriphenylphosphonium bromide-silver compounds-porphyrin is adopted; the method for preparing N-tert-Butylbenzenesulfenamide from the reaction of methyl tertiary butyl ether with a weak reactivity and benzene sulfonamide compounds is realized; remarkably technical effects of preferable reaction temperature, high yield and good universality are achieved; moreover, as appropriate additives are added in the reaction, the collision between molecules is promoted and the reaction time is shortened; the method has favorable industrialization perspective and industrialized production value.
Owner:甘肃皓骏药业有限公司
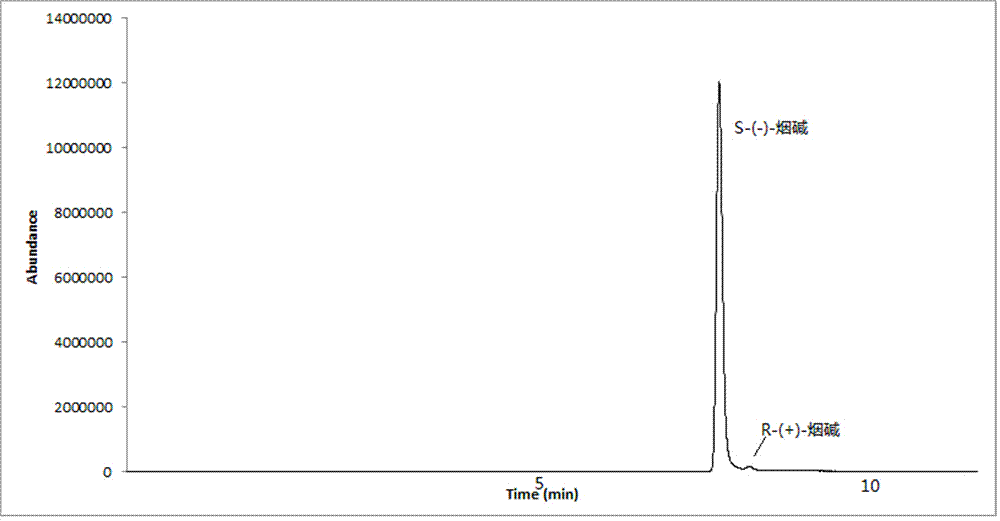
Chiral analysis bonded phase chromatography-tandem mass spectrometry for nicotine in smokeless tobacco product
The invention discloses a chiral analysis bonded phase chromatography-tandem mass spectrometry for nicotine in a smokeless tobacco product. The chiral analysis bonded phase chromatography-tandem mass spectrometry comprises the following steps: drying, crushing and sieving a sample of a smokeless tobacco product; soaking the sample with a sodium hydroxide solution; extracting with methyl tert-butyl ether and methanol solution to obtain an extract; performing matrix solid-phase extraction and purification on the extract; analyzing with two chiral columns tandem bonded phase chromatography-tandem mass spectrometry; and quantitatively detecting S-(-)- nicotine and R-(+)- nicotine in the smokeless tobacco product by a peak area normalization method. A detection method provided by the invention adopts a simple and efficient sample extraction and purification method, and can reduce pollution of sample liquid on a chromatographic column; the bonded phase chromatography-tandem mass spectrometry detection method is high in sensitivity, good in specificity, capable of excluding false positive, short in analysis time, and capable of meeting chiral analysis for nicotine in mass samples of smokeless tobacco product.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Catalyst for preparing isoamylene by splitting decomposition of t-amyl-methyl ether and preparation method and application thereof
ActiveCN103041834AHigh selectivityLow reaction temperaturePhysical/chemical process catalystsOrganic compound preparationDecompositionChemistry
The invention discloses a multicomponent (X / Z2O3 / YO4 / Al2O3) catalyst for preparing isoamylene and a preparation method thereof. According to the method, the catalyst comprises the following components by weight percent: 20% to 95% of Al2O3, 0.1% to 20% of Z2O3, 0.1% to 50% of YO4 and 0.1% to 40% of X. The catalyst can take a reaction to prepare the isoamylene by the splitting decomposition of t-amyl-methyl ethe at a temperature lower than that of the existing catalyst of the same type. Under the condition that the high selection on the isobutene and the methanol and the high conversion rate of the splitting decomposition of the t-amyl-methyl ether are guaranteed, as compared with the existing catalyst of the same type, the catalyst provided by the invention has the effects that the space velocity of liquids in the reaction is improved exponentially, and water and other inert substances are not required to be added into a splitting decomposition reaction system of the t-amyl-methyl ether. Therefore, under the condition of unchanging the existing reaction equipment, the energy consumption in the industrial production is lowered, and the equipment utilization rate is improved exponentially.
Owner:EAST CHINA UNIV OF SCI & TECH
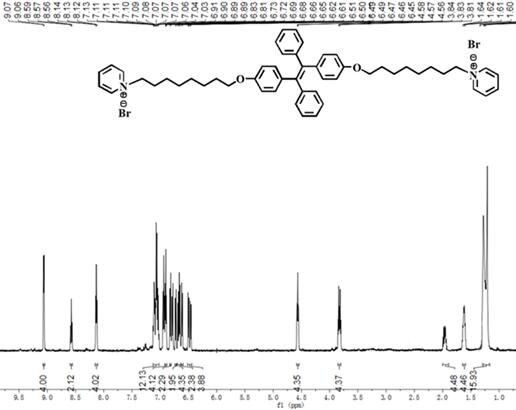
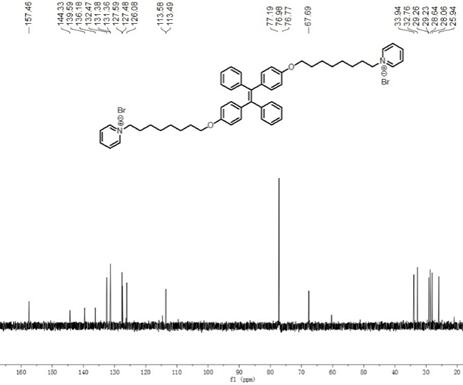
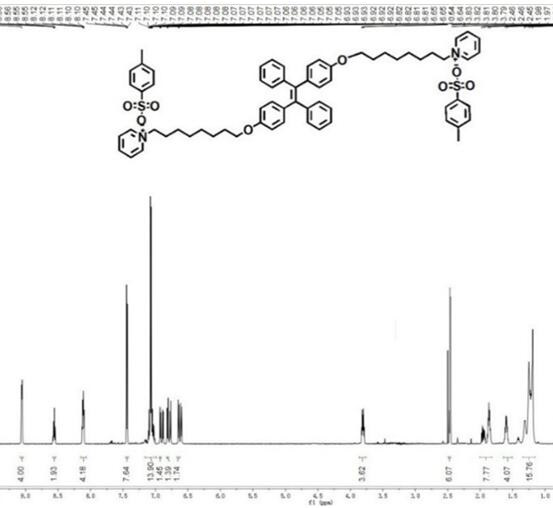
Preparation method of nano-assembly with AIE effect
The invention provides a preparation method of a nano-assembly with an AIE effect. The preparation method comprises the following steps: adding TPE-2Br and anhydrous pyridine into an anhydrous chloroform solution, carrying out a reflux reaction at 65-70 DEG C under the protection of nitrogen, conducting cooling to room temperature, performing filtering, precipitating the obtained solution in methyl tert-butyl ether, performing centrifuging, removing a supernatant, and carrying out drying to obtain a one-dimensional nano-assembly TPE-ON; and dissolving TPE-ON in methanol, adding a sodium methylbenzene sulfonate solution, carrying out a stirring reaction at room temperature, performing extracting with dichloromethane, successively carrying out washing, drying and filtering, carrying out spin-drying on the obtained filtrate, and carrying out column chromatography separation and purification to obtain the two-dimensional nano-assembly TPE-ONS. By changing counter ions in molecules, conversion from the one-dimensional fiber assembly with AIE properties to the two-dimensional sheet assembly is realized. The fluorescence intensity of the TPE-ONS is 2-2.5 times the fluorescence intensityof the TPE-ON. In a mixed solution of TPE-ON and TPE-ONS, the fluorescence intensity and a TPE-ONS content show a certain correlation, namely Y is equal to 2113.71 + 5163.56x-2966.07X<2>; and the relative content of TPE-ON and TPE-ONS can be quantitatively detected and the exchange process of TPE-ON and TPE-ONS can be monitored through the correlation.
Owner:NORTHWEST NORMAL UNIVERSITY
Method for producing n-butene from isobutene
ActiveCN112441866ALow isobutylene contentHydrocarbon by isomerisationChemical industryMethyl t-butyl etherN-butylidenephthalide
The invention relates to a method for producing n-butene from isobutene, which comprises the following steps of: (1) feeding mixed C4 hydrocarbon containing isobutene into an etherification reactor for pre-etherification to enable most isobutene in the C4 hydrocarbon raw material to react with methanol to generate methyl tert-butyl ether, feeding a pre-etherification product into a catalytic distillation tower to enable unreacted isobutene to be completely etherified, and rectifying to separate an etherification product so as to obtain methyl tert-butyl ether and a C4 hydrocarbon product without isobutene; (2) cracking the methyl tert-butyl ether to generate isobutene and methanol; and (3) feeding the isobutene obtained by cracking into a normalizing unit, performing normalizing reaction under the conditions of 280-480 DEG C and 0.05-1.0 MPa, removing C5<+> heavy components from the normalizing reaction product, and returning the normalizing reaction product and the methanol obtained in the step (2) to the etherification reactor in the step (1). According to the method, isobutene in the raw materials can be converted into a C4 product which is rich in n-butene and hardly contain isobutene, and an effective way is provided for utilization of isobutene and MTBE.
Owner:CHINA PETROLEUM & CHEM CORP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com