Method for synthesizing tert-butylated hydroxyanisole through solid-liquid-phase reaction
A technology for tert-butyl hydroxyanisole and tert-butyl hydroxyanisole is applied in the field of synthesizing tert-butyl hydroxyanisole by solid-liquid phase reaction, which can solve the problems of high cost, influence on catalyst activity, complicated preparation process, etc., so as to improve production The effect of efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
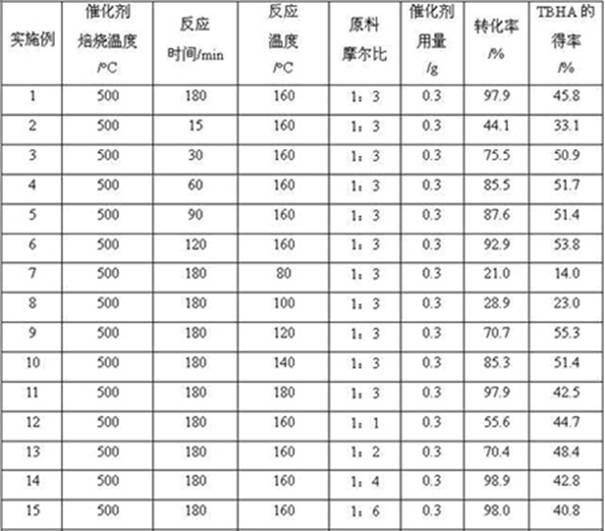
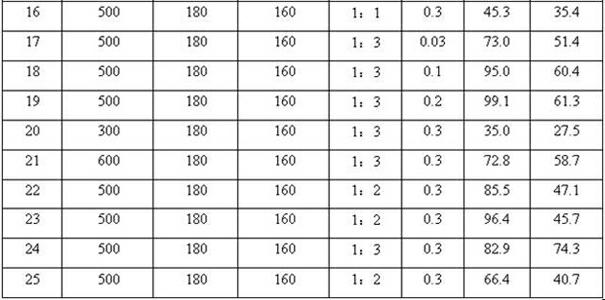
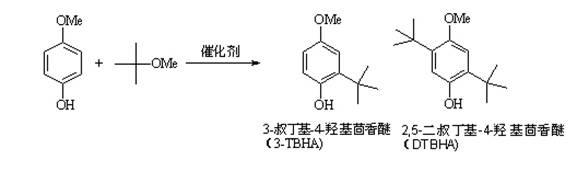
[0025] Add hydrogen-type Y molecular sieve catalyst (Si / Al molar ratio = 3:1, pre-treated by roasting at 500°C for 4 h), p-methoxyphenol, methyl tert-butyl ether and 10 mL solvent cyclohexane into a 20 mL autoclave The mixture of alkanes (the molar ratio of the three is 1:3:14.2), with N 2 The air in the reactor was replaced several times, and finally 0.3MPa of N 2 As a protective gas, keep the temperature at 160°C and react for 180 minutes. After the reaction, the reaction kettle is cooled rapidly, the reaction liquid and the catalyst are separated by suction filtration, and the content of p-methoxyphenol and tert-butylhydroxyanisole in the product is analyzed by gas chromatography. , to determine the conversion rate of p-methoxyphenol and the yield of tert-butylhydroxyanisole, the conversion rate of p-methoxyphenol was 97.9%, and the yield of tert-butylhydroxyanisole (TBHA) was 45.8%. The data are listed in Table 1.
Embodiment 2~6
[0027] Under the same conditions as in Example 1, the reaction time was changed (15 min, 30 min, 60 min, 90 min, 120 min respectively), and the results are listed in Table 1 (2-6).
Embodiment 7~11
[0029] Under the same conditions as in Example 1, the reaction temperatures were changed (80°C, 100°C, 120°C, 140°C, 180°C, respectively), and the reaction results were listed in Table 1 (7-11).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com