Catalyst for preparing tert-olefins by cracking tert-alkyl ethers, preparation method and application thereof
A tertiary alkyl ether and catalyst technology, which is applied in the field of preparation of the catalyst, can solve the problems of increased device operation, increased energy consumption, pipeline blockage, etc., and achieves low raw material consumption, high feed liquid space velocity, and low equipment investment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
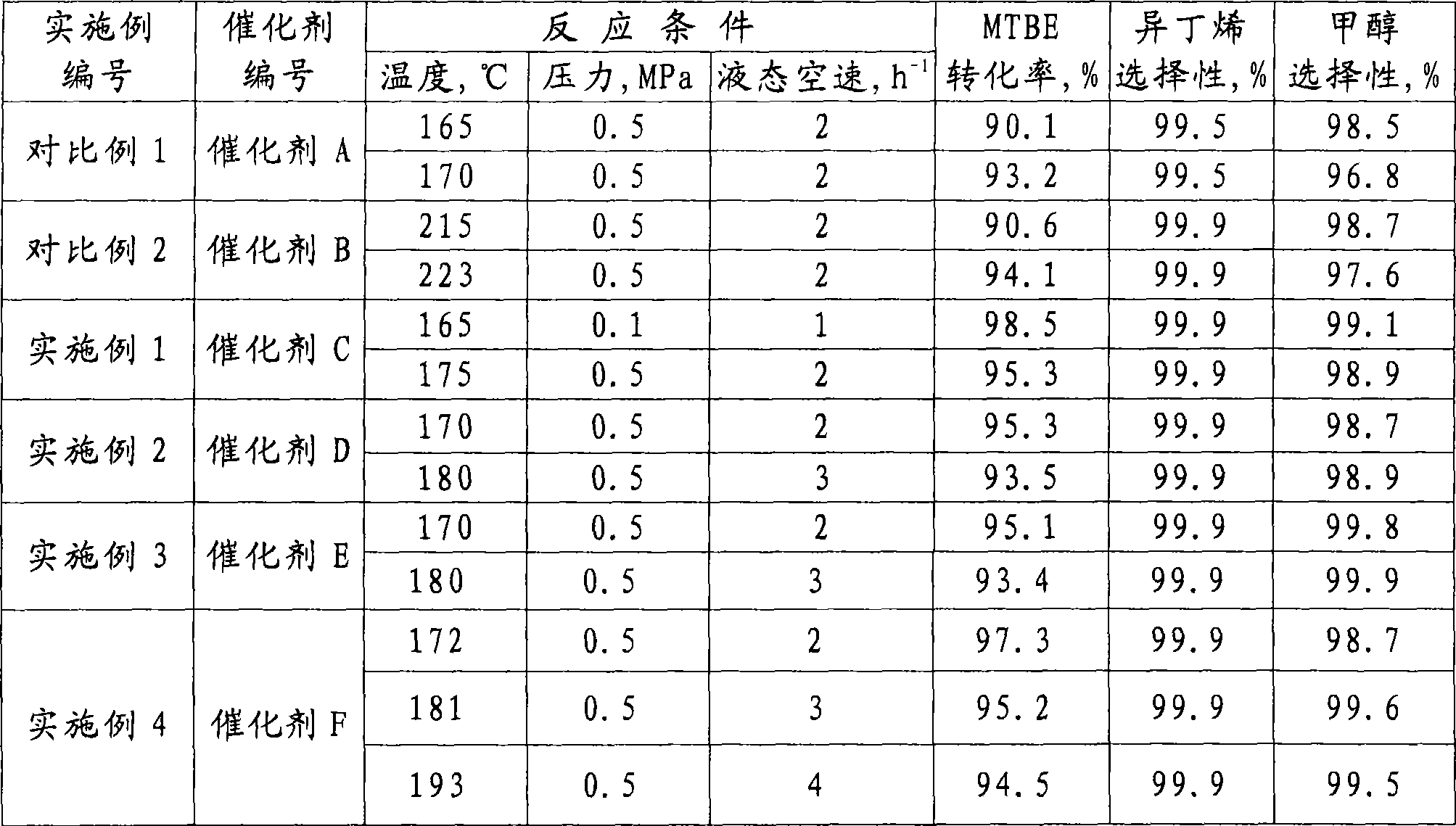
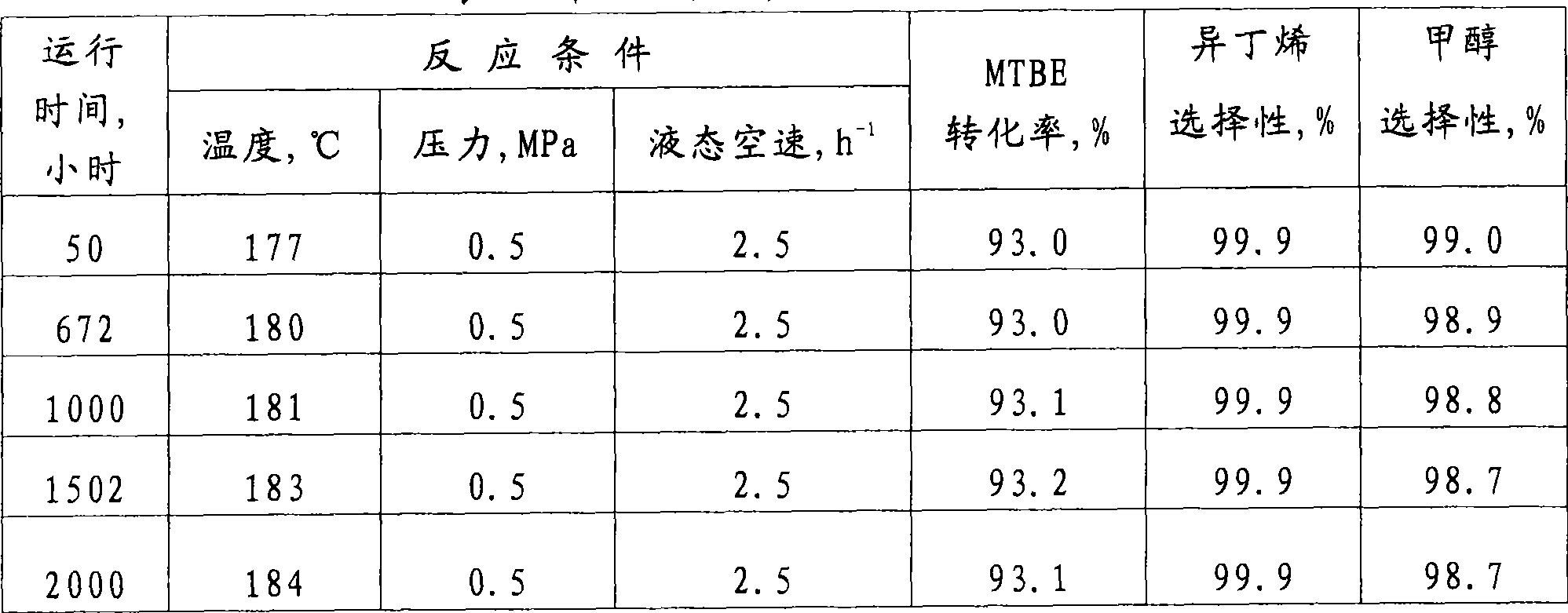
Examples
Embodiment 1
[0036] In the first step, 45.3g of ammonium sulfate was dissolved in 100ml of water, then 100ml of the same aluminum oxide used in Comparative Example 1 was put into the resulting ammonium sulfate aqueous solution and soaked for 2 hours at room temperature, then dried at 120°C for 2 hours after leaching, Then bake at 550°C for 4 hours in an air atmosphere to obtain sulfide-modified alumina; in the second step, dissolve 12.7g of ammonium fluoride in 100ml of water, and then dissolve 100ml of the sulfide-modified alumina obtained in the first step Alumina was immersed in the obtained ammonium fluoride aqueous solution at room temperature for 1.5 hours, dried at 120°C for 2 hours after leaching, and then calcined at 500°C for 3 hours in an air atmosphere to obtain catalyst C of the present invention. The content of sulfide in catalyst C was 5% by weight calculated as sulfur based on the weight of alumina, and the content of fluoride calculated as fluorine was 3% by weight based on...
Embodiment 2
[0038] In the first step, 6.4 g of ammonium fluoride was dissolved in 100 ml of water, and then 100 ml of the same aluminum oxide used in Comparative Example 1 was put into the resulting ammonium fluoride aqueous solution for immersion at room temperature for 1.5 hours, and then dried at 120° C. for 2 hours after leaching. hours, and then roasted at 450°C for 4 hours in a nitrogen atmosphere to obtain fluoride-modified alumina; in the second step, 45.3g of ammonium sulfate was dissolved in 100ml of water, and then 100ml of the fluoride-modified alumina obtained in the first step The aluminum oxide was put into the obtained ammonium sulfate aqueous solution and immersed at room temperature for 2 hours, dried at 120° C. for 2 hours after leaching, and then calcined at 500° C. for 4 hours in a nitrogen atmosphere to obtain catalyst D of the present invention. The content of sulfide in catalyst D was 5% by weight calculated as sulfur based on the weight of alumina and the content o...
Embodiment 3
[0040] In the first step, 45.3g of ammonium sulfate was dissolved in 100ml of water, then 100ml of the same aluminum oxide used in Comparative Example 1 was put into the resulting ammonium sulfate aqueous solution and soaked for 1.5 hours at room temperature, and then dried at 120°C for 2 hours after leaching. Then roast at 600°C for 7.5 hours in an air atmosphere to obtain sulfide-modified alumina; in the second step, dissolve 19.1g of ammonium fluoride in 100ml of water, and then dissolve 100ml of the sulfide-modified alumina obtained in the first step Alumina was immersed in the obtained ammonium fluoride aqueous solution at room temperature for 2 hours, dried at 120°C for 3 hours after leaching, and then calcined at 550°C for 2.5 hours in an air atmosphere to obtain catalyst E of the present invention. The content of sulfide in catalyst E was 5% by weight calculated as sulfur based on the weight of alumina and the content of fluoride calculated as fluorine was 4.5% by weigh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Specific surface | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com