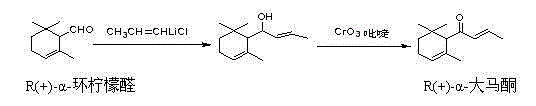
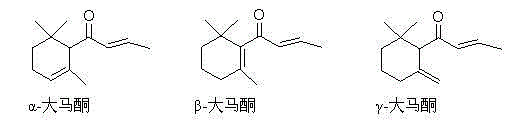
Synthesis method of (S)-(-)-alpha-damascenone
A synthetic method and technology of damascenone, which is applied in the field of synthesis of α-damascenone, can solve the problems of few specific synthesis studies and reports, difficult industrial production, complex and difficult synthetic methods, etc. The effect of high conversion rate and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
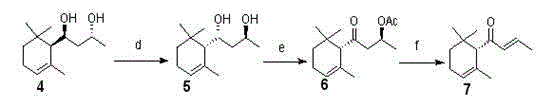
Embodiment 1
[0034] (1) Synthetic diols:
[0035] a. Add 50g of racemic α-ionone to 300ml of methanol, and continuously and slowly add 110ml of aqueous hydrogen peroxide solution with a mass concentration of 30% and 25ml of 6M hydrogen peroxide dropwise while the solution is cooled to 0°C and stirred. sodium solution. The mixed solution was stirred at 4°C for 6 days, and 50 ml of 30% hydrogen peroxide solution and 30 ml of methanol were additionally added dropwise every day. After the reaction, 300ml of water was added and extracted with ether. The organic phase was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The product α,β-epoxy-α-ionone was separated by column chromatography and recrystallized from hexane with a yield of about 81%.
[0036] b. Add 90ml of tetrahydrofuran, 30ml of water and 30ml of ethanol to the product obtained in the above step a, stir evenly at 0°C, then add a mixture of aluminum and mercury with a catalytic amount of 20mol% fo...
Embodiment 2
[0043] (1) Synthetic diols:
[0044] a. Add 70g of racemic α-ionone to 500ml of methanol, and continuously and slowly add 180ml of 30% hydrogen peroxide aqueous solution and 45ml of 6M sodium hydroxide aqueous solution dropwise while the solution is cooled to 0°C and stirred. The mixed solution was stirred at 4°C for 6 days, and 70 ml of 30% hydrogen peroxide solution and 50 ml of methanol were additionally added dropwise every day. After the reaction, 500ml of water was added and extracted with ether. The organic phase was dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. The product α,β-epoxy-α-ionone was separated by column chromatography and recrystallized from hexane with a yield of about 85%.
[0045] b. Add 150ml of tetrahydrofuran, 50ml of water and 50ml of ethanol to the product obtained in step a above, stir evenly at 0°C, then add a mixture of aluminum and mercury with a catalytic amount of 20 mol% for catalysis, and react at 0°C for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com