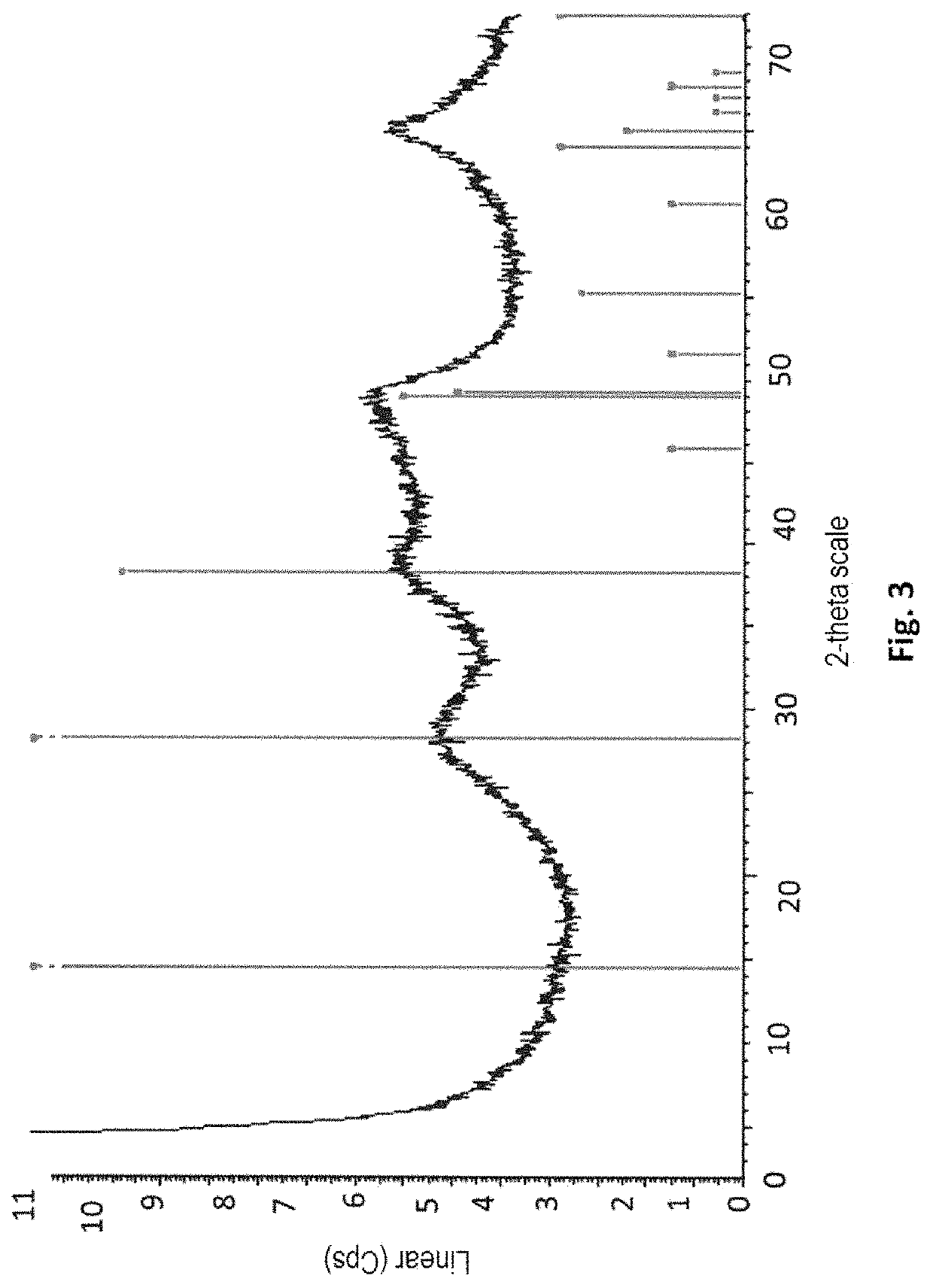
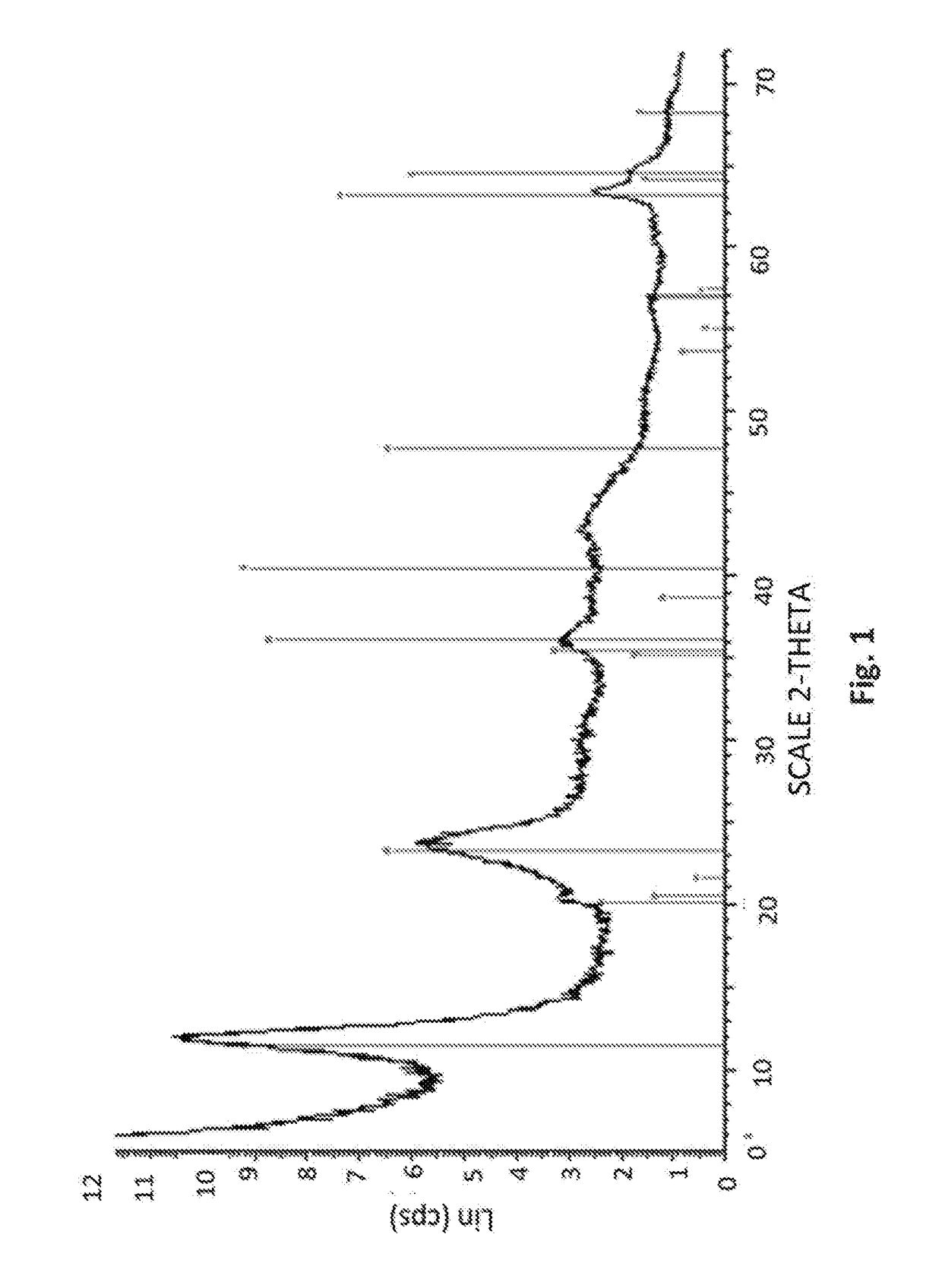
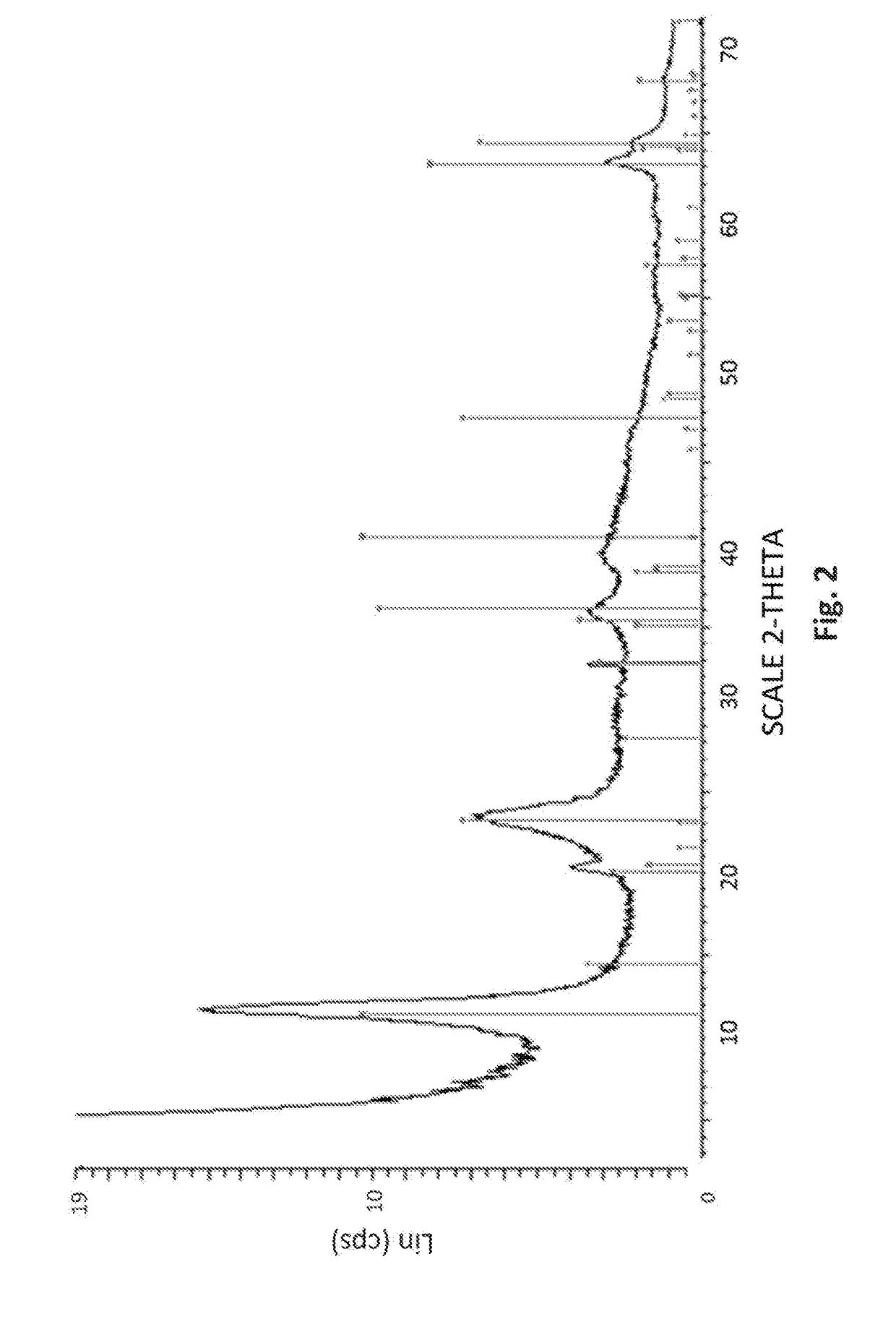
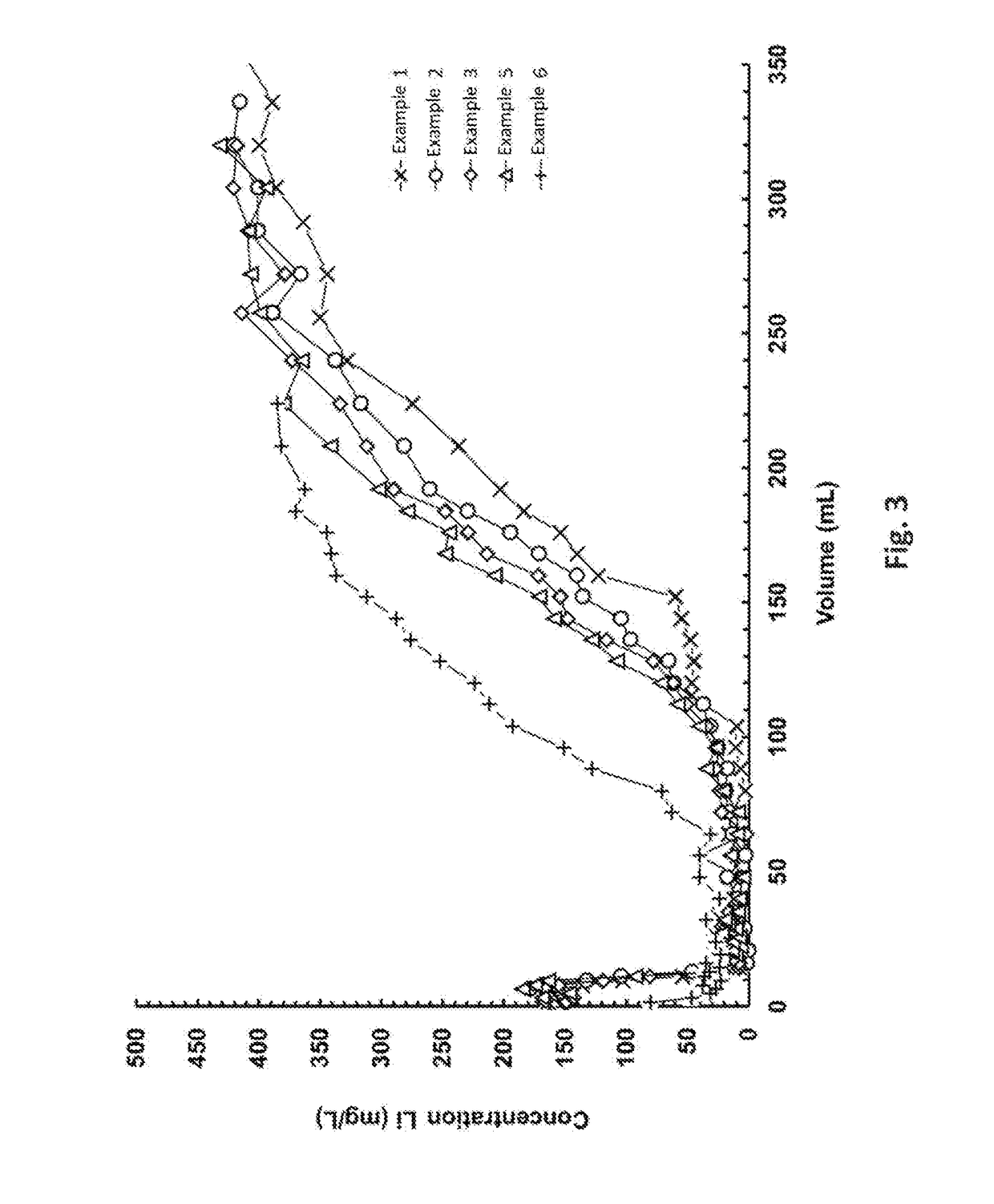
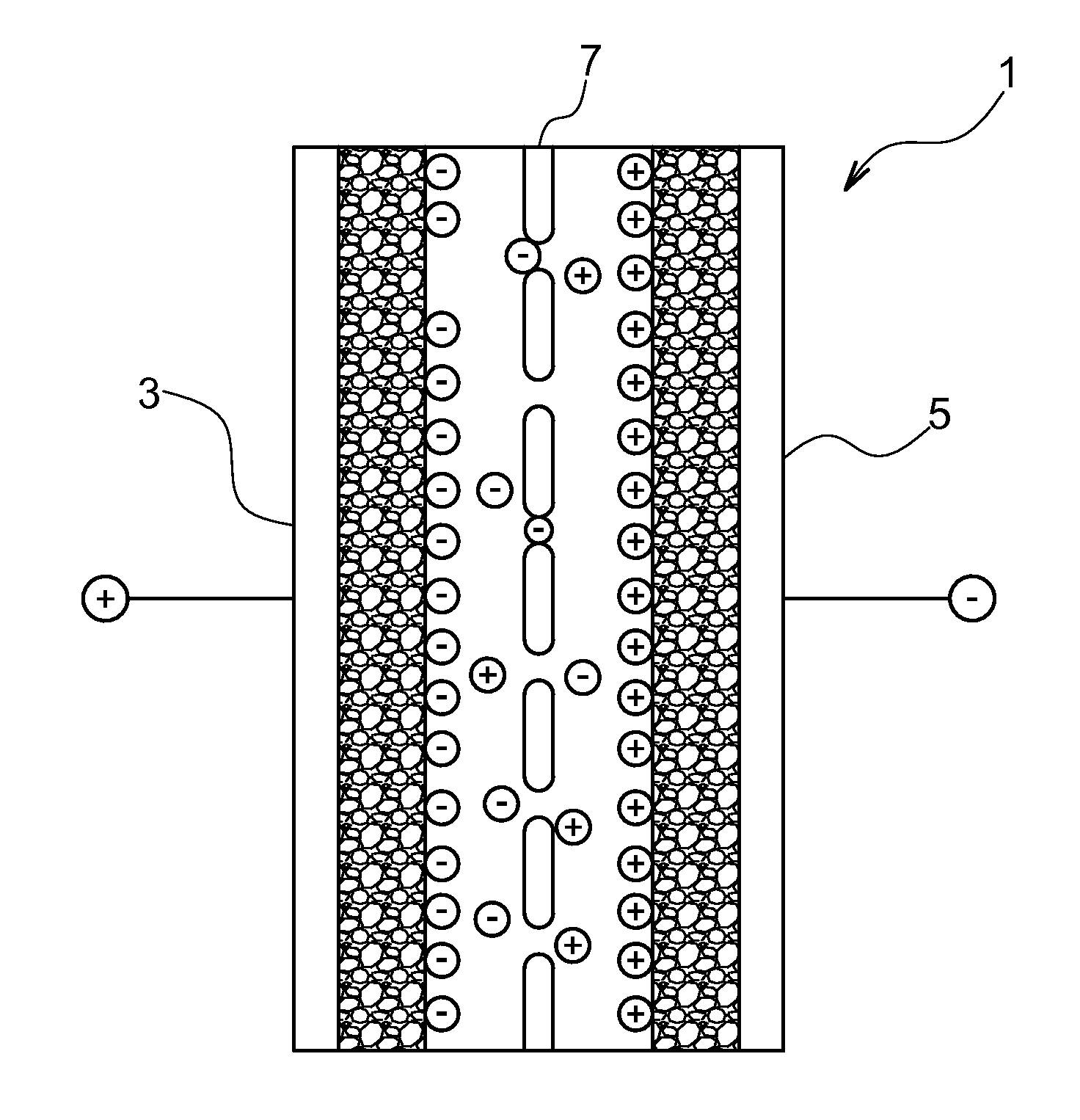
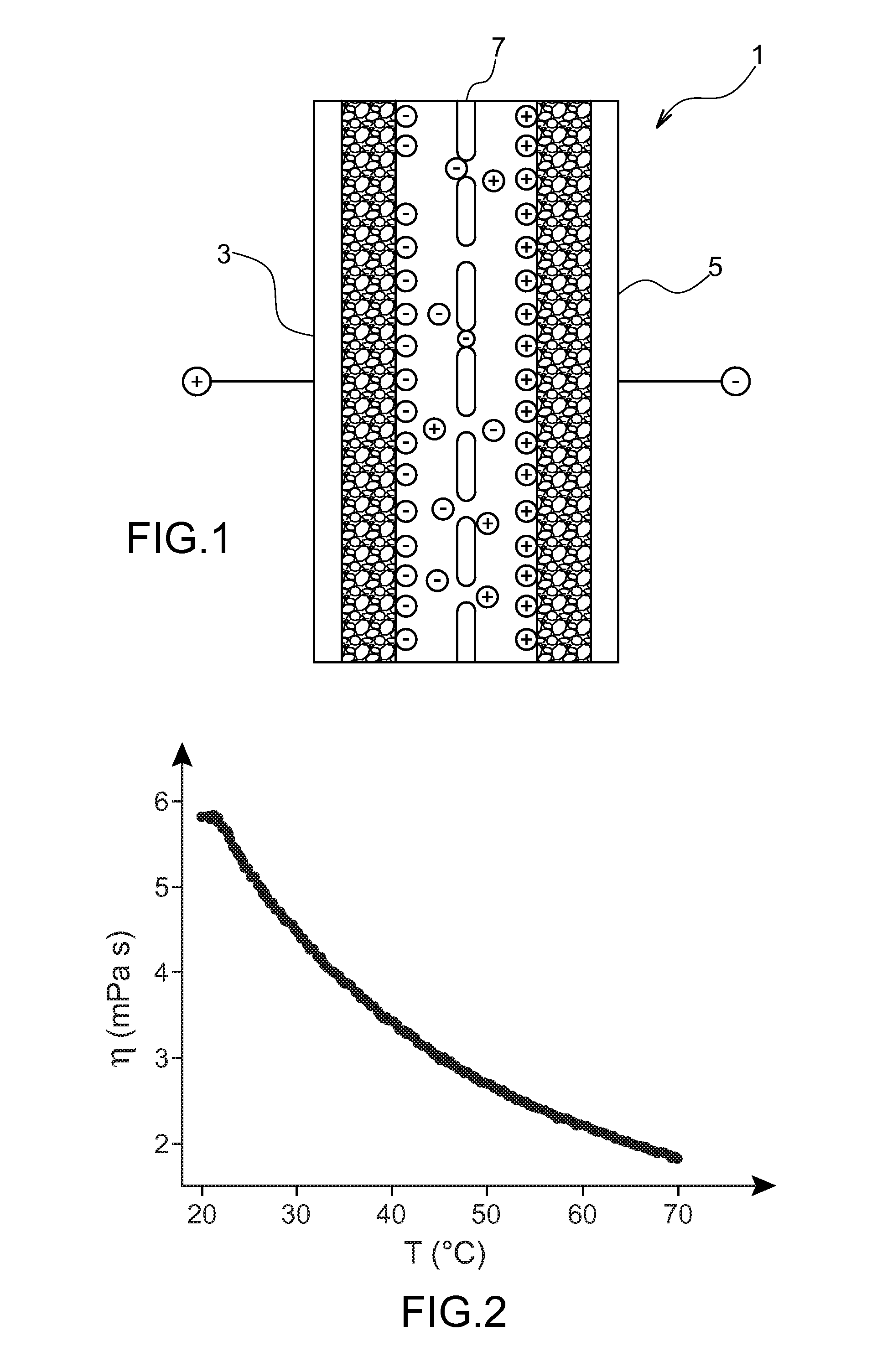
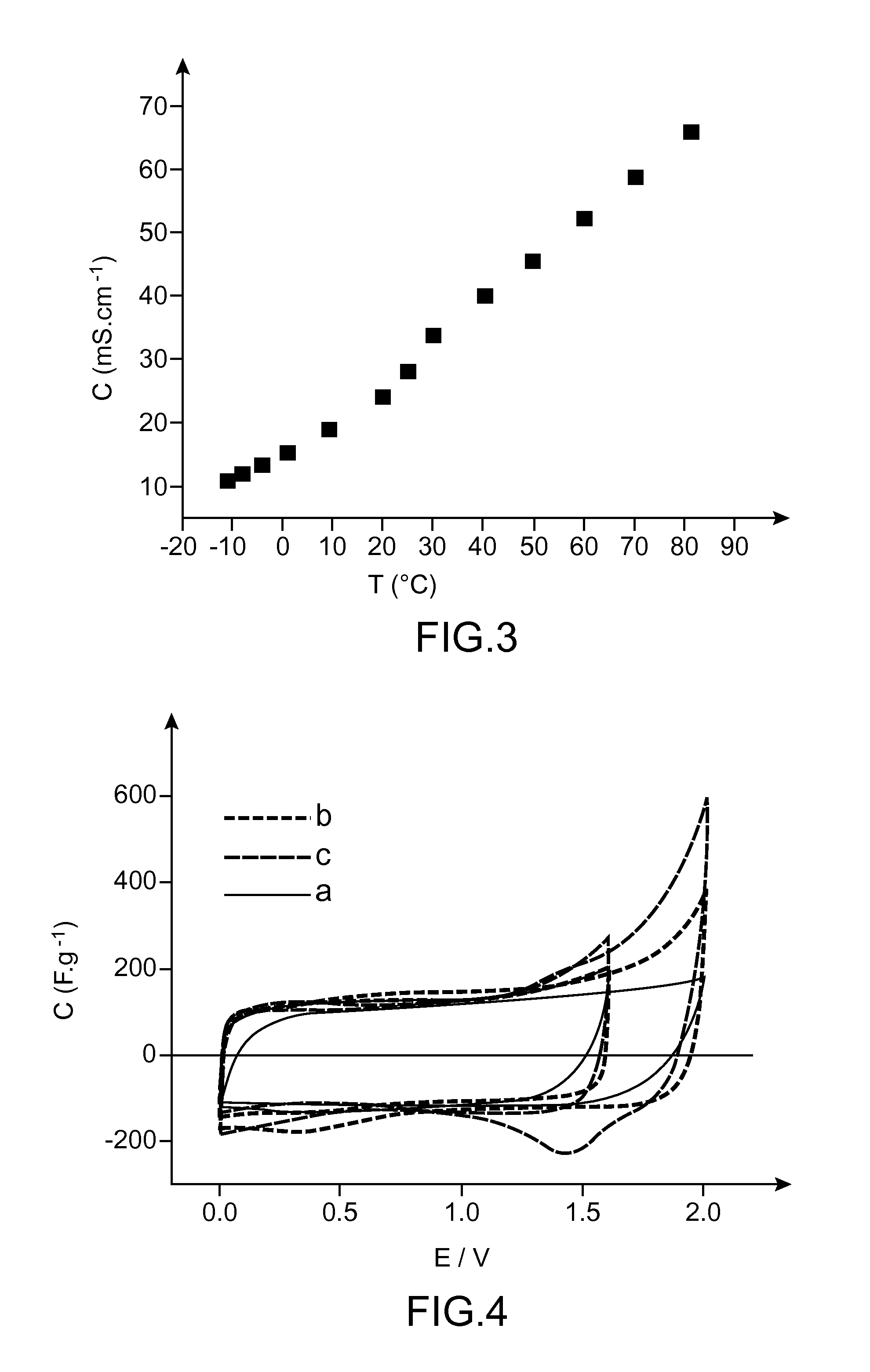
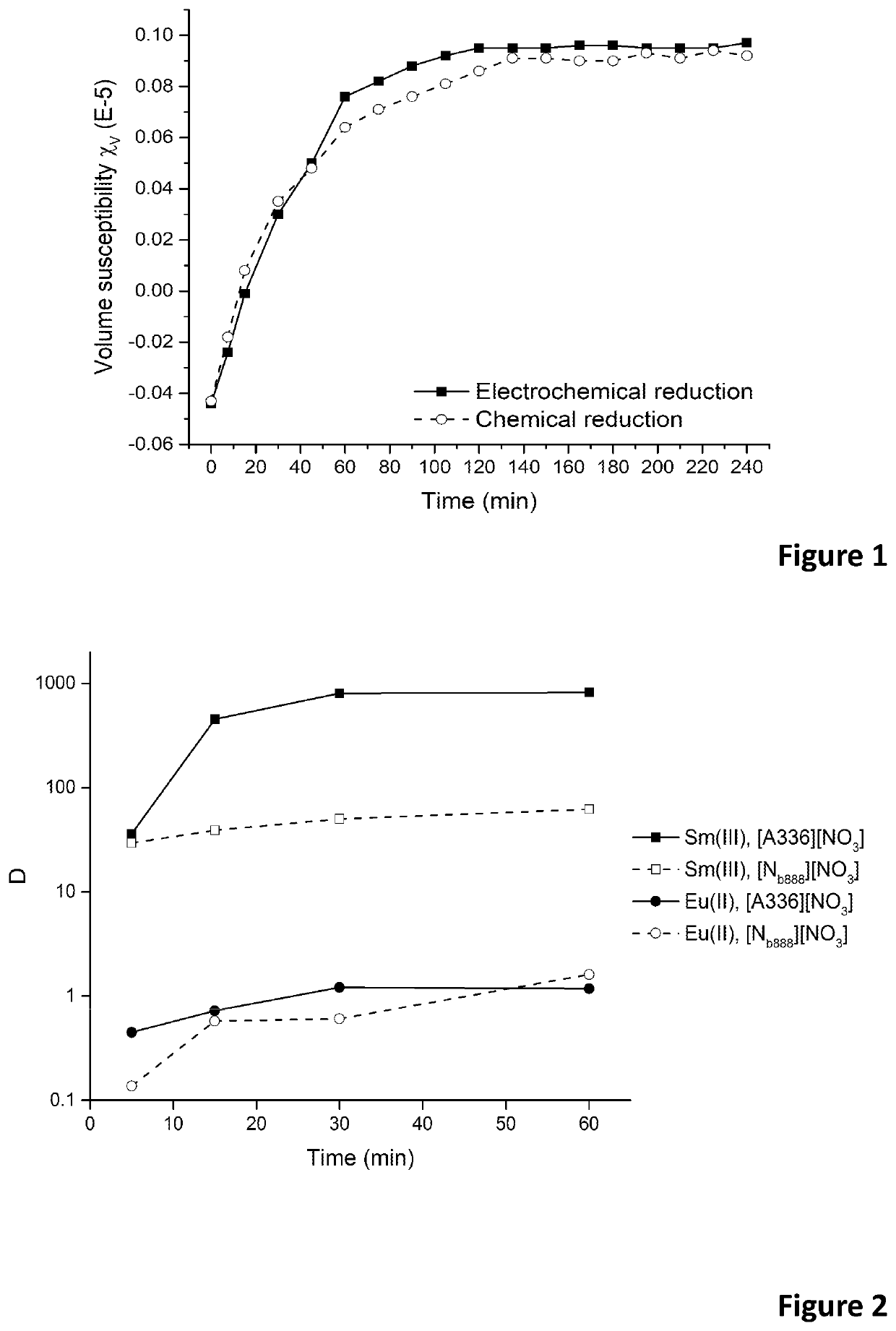
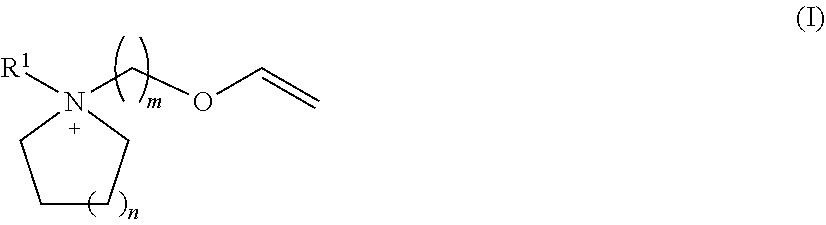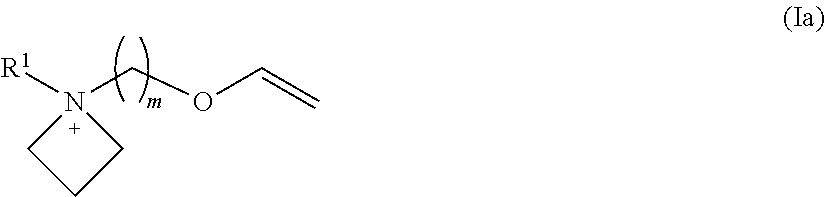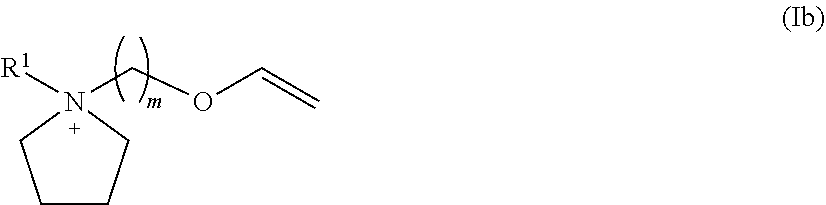Patents
Literature
36 results about "Nitrate anion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nitrate is a nitrogen oxoanion formed by loss of a proton from nitric acid. Principal species present at pH 7.3. It is a nitrogen oxoanion, a member of reactive nitrogen species and a monovalent inorganic anion. It is a conjugate base of a nitric acid.
Catalytic system of nitrate anions for CO2/epoxide copolymerization
ActiveUS8598309B2Reduce manufacturing costEliminate needOrganic compound preparationGroup 8/9/10/18 element organic compoundsNitrate anionPolycarbonate
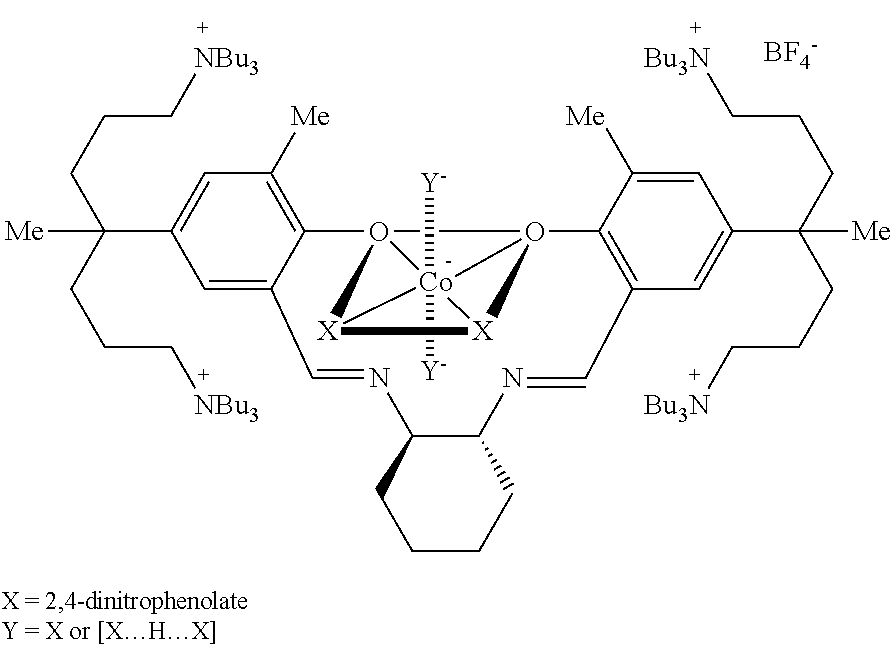
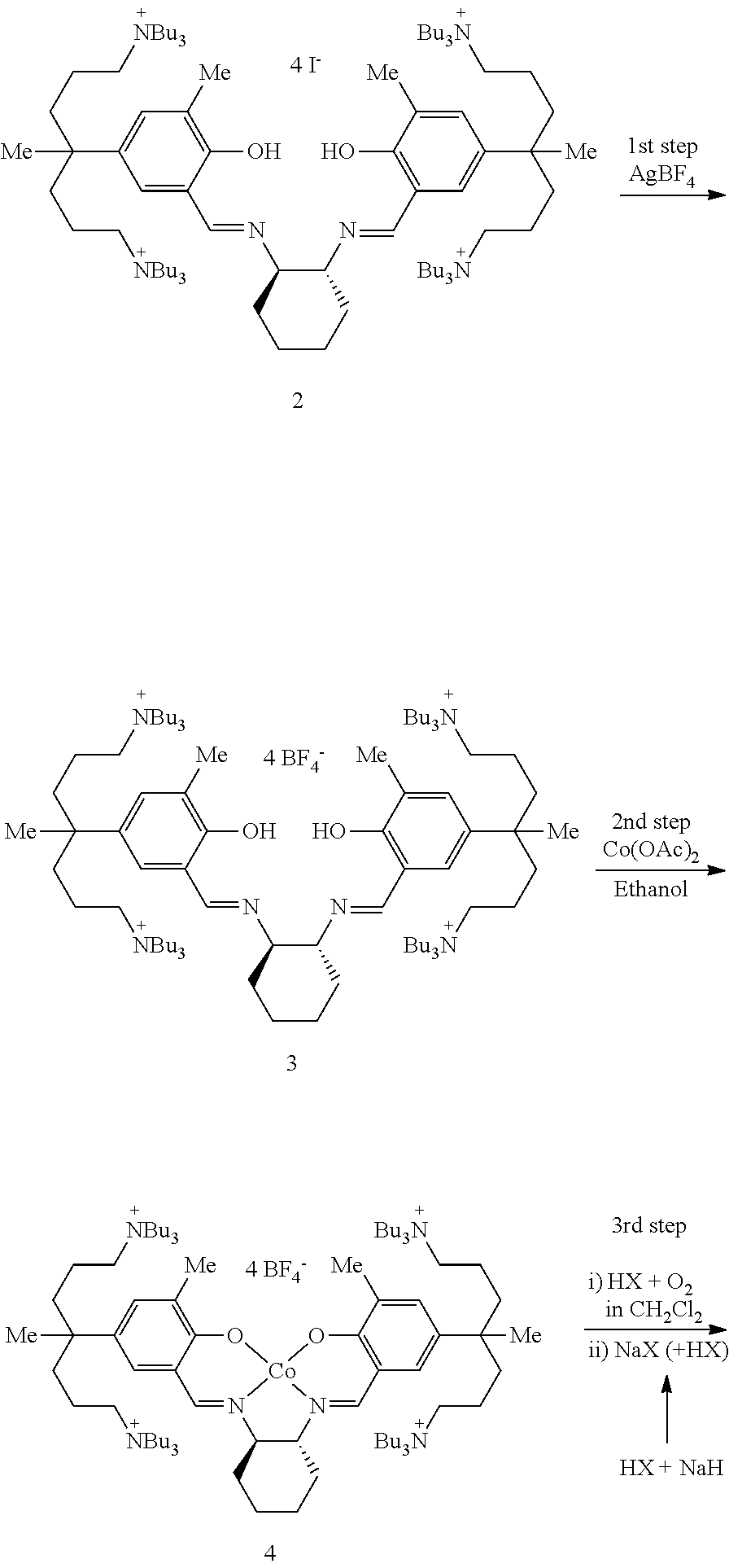
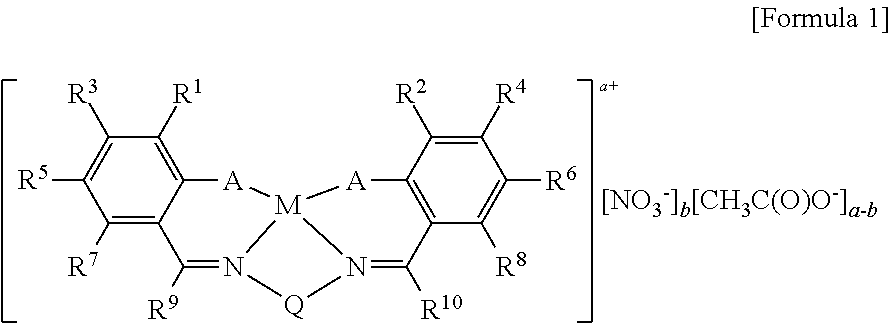
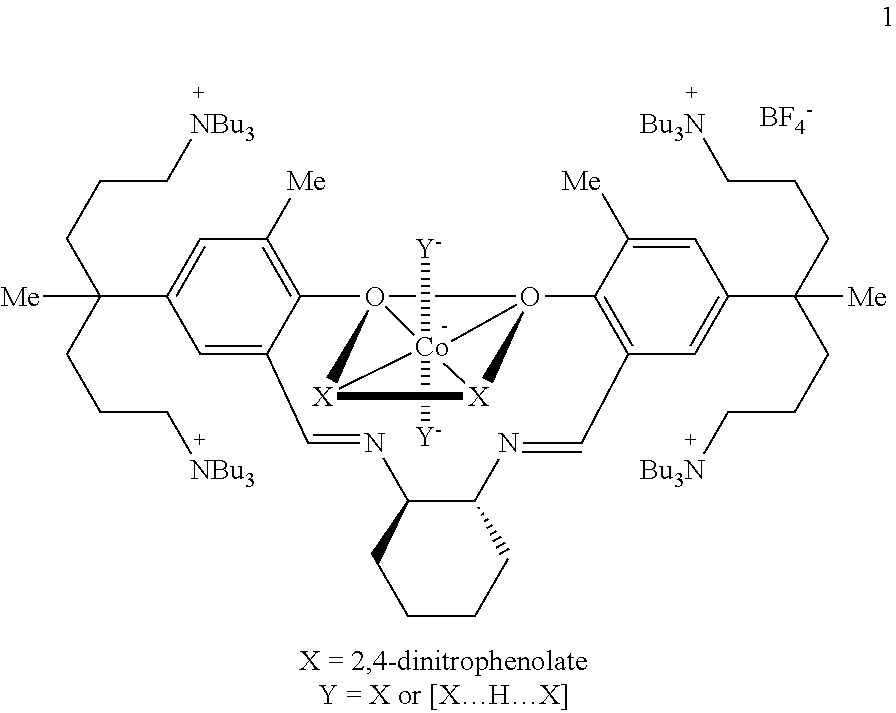
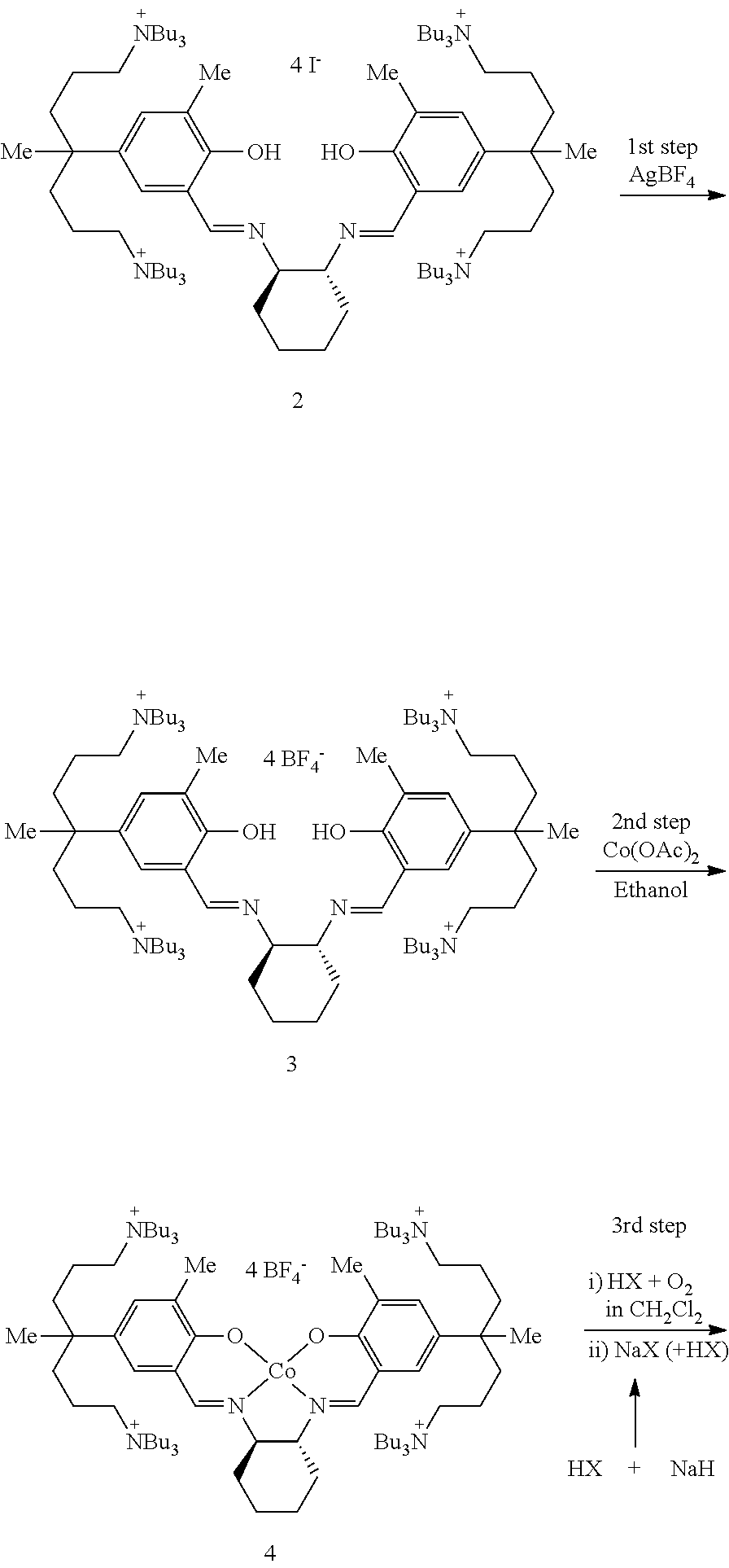
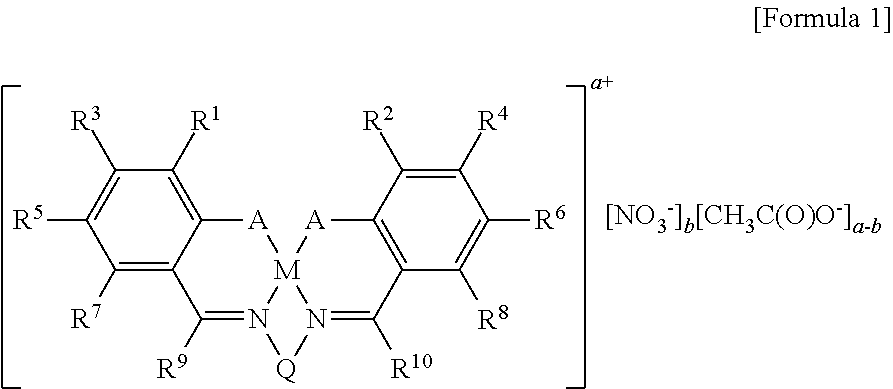
This invention relates to a Salen type ligand including three or more quaternary ammonium salts of nitrate anions, to a trivalent metal complex compound prepared from this ligand and a method of preparing the same, to a method of preparing polycarbonate by copolymerizing an epoxide compound and carbon dioxide using the complex compound as a catalyst, and to a method of separating and collecting the catalyst from the copolymer after copolymerization. This catalyst used to copolymerize an epoxide compound and carbon dioxide can be more simply prepared, and has lower catalyst preparation and recovery costs, and higher activity, compared to conventional catalysts.
Owner:SK INNOVATION CO LTD
Complex oxide catalysts and process for producing (meth) acrolein and (meth) acrylic acid
InactiveUS6383973B1Good reproducibilityHigh activityOrganic compound preparationOrganic chemistry methodsNitrate anionAlkaline earth metal
Complex oxide catalysts represented by the formula,(in which A is Ni or Co; B is Na, K, Rb, Cs or Tl; C is an alkaline earth metal; D is P, Te, Sb, Sn, Ce, Pb, Nb, Mn, As, B or Zn; E is Si, Al, Ti or Zr; and where a is 12, 0<=b<=10, 0<c<=10, 0<d<=10, 2<=e<=15, 0<f<=10, 0<=g<=10, 0<=h<=4 and 0<=i<=30)are provided. The catalysts are characterized in that the molar ratio of the total nitrate anions to the molybdenum at the time of catalyst preparation is more than 1 but not more than 1.8. When used in the reaction for producing (meth)acrolein and (meth)acrylic acid by vapor-phase oxidation of at least a compound selected from propylene, isobutylene, t-butanol and methyl-t-butyl ether, the catalysts exhibit excellent activity and selectivity and maintain stable performance over prolonged period.
Owner:NIPPON SHOKUBAI CO LTD
Catalytic System of Nitrate Anions for CO2/ Epoxide Copolymerization
ActiveUS20110207909A1Reduce manufacturing costAchieve mass productionOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsNitrate anionQuaternary ammonium cation
This invention relates to a Salen type ligand including three or more quaternary ammonium salts of nitrate anions, to a trivalent metal complex compound prepared from this ligand and a method of preparing the same, to a method of preparing polycarbonate by copolymerizing an epoxide compound and carbon dioxide using the complex compound as a catalyst, and to a method of separating and collecting the catalyst from the copolymer after copolymerization. This catalyst used to copolymerize an epoxide compound and carbon dioxide can be more simply prepared, and has lower catalyst preparation and recovery costs, and higher activity, compared to conventional catalysts.
Owner:SK INNOVATION CO LTD
Complex oxide catalysts and process for producing (meth) acrolein and (meth) acrylic acid
InactiveUS20020103077A1Good reproducibilityHigh activityOrganic compound preparationOther chemical processesNitrate anionMeth-
Complex oxide catalysts represented by the formula,<paragraph lvl="0"><in-line-formula>MoaWbBicFedAeBfCgDhEiOx< / in-line-formula>(in which A is Ni or Co; B is Na, K, Rb, Cs or Tl; C is an alkaline earth metal; D is P, Te, Sb, Sn, Ce, Pb, Nb, Mn, As, B or Zn; E is Si, Al, Ti or Zr; and where a is 12, 0<=b<=10, 0<c<=10, 0<d<=10, 2<=e<=15, 0<f<=10, 0<=g<=10, 0<=h<=4 and 0<=i<=30) are provided. The catalysts are characterized in that the molar ratio of the total nitrate anions to the molybdenum at the time of catalyst preparation is more than 1 but not more than 1.8. When used in the reaction for producing (meth)acrolein and (meth)acrylic acid by vapor-phase oxidation of at least a compound selected from propylene, isobutylene, t-butanol and methyl-t-butyl ether, the catalysts exhibit excellent activity and selectivity and maintain stable performance over prolonged period.
Owner:NIPPON SHOKUBAI CO LTD
Method for preparing emulsified nanometer grade zero valent iron and nanometer grade bimetal and use thereof
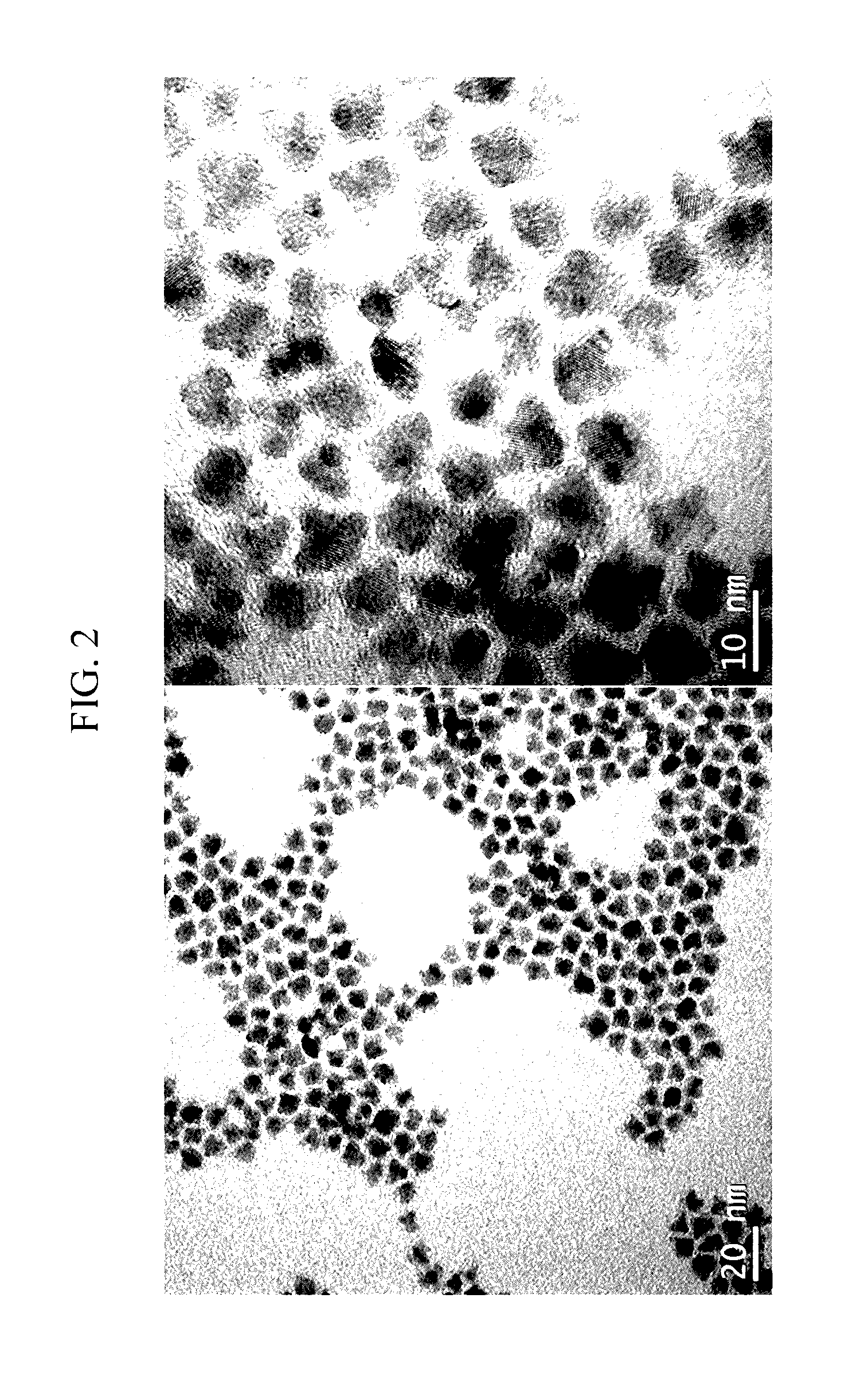
The invention discloses the method for preparation of emulsified nanometer zero valence iron and nanometer bimetallic. The method comprises the following steps: 1 adding the NaBH4 solution into the mixture solution of FeSO4.7H2O and stabilizing agent which is amylogen, getting the emulsified nanometer zero valence iron, the density of amylogen being 0.2-2%, the mole ratio of NaBH4 and FeSO4.7H2O solution being 2:1; 2 mixing emulsified nanometer zero valence iron and K2PdCl6 solution, carrying out reaction, entering nitrogen, when the color of solution changed from bistre to pea green, getting the nanometer bimetallic, the proportion by weight of Pd and Fe being 5-10:10000. The emulsified nanometer zero valence iron and nanometer bimetallic are used to restore the pollution water containing chlorine, nitrate anion and heavy metal. The method is simple, the specific surface area of emulsified bimetallic particle is large, the dispersity is good, and the chemical reactivity is high.
Owner:ZHEJIANG UNIV
Method for regenerating heteropolyacid catalyst and method for producing methacrylic acid
InactiveUS6673733B2High catalytic activityOther chemical processesOrganic compound preparationNitrate anionNitrogenous heterocyclic compound
The invention provides a method for regenerating with high efficiency a deteriorated catalyst of reduced activity, said catalyst originating from a heteropolyacid catalyst containing heteropolyacid formed of molybdophosphoric acid and / or molybdovanadophosphoric acid, or a salt thereof, to a heteropolyacid catalyst which exhibits approximately equivalent activity level to that of the fresh catalyst. Said method comprises mixing a deteriorated catalyst and a nitrogen-containing heterocyclic compound under the conditions whereunder ammonium ions and nitrate anions are present at such ratio that the amount of total ammonium ions per mol of total nitrate anions does not exceed 1.7 mols, drying the mixture and calcining the same.
Owner:SUMITOMO CHEM CO LTD
Method for preparing heteropolyacid catalyst and method for producing methacrylic acid
InactiveUS20010029233A1High yieldStrengthOther chemical processesOrganic compound preparationNitrate anionIon content
A novel method for preparing a heteropolyacid catalyst containing a heteropolyacid composed of molybdophosphoric acid and / or molybdovanadophosphoric acid, or a salt of the heteropolyacid, is provided. The method comprises preparing an aqueous solution or aqueous dispersion which (1) contains the nitrogen-containing heterocyclic compound, nitrate anions and ammonium ions, (2) the ammonium ion content not exceeding 1.7 mols per mol of the nitrate anion content, and (3) the ammonium ion content not exceeding 10 mols per 12 mols of the molybdenum atom content by mixing raw materials containing the catalyst-constituting elements with the nitrogen-containing heterocyclic compound in the presence of water, drying and calcining the same. This heteropolyacid catalyst excels over conventional catalysts in performance, life and strength.
Owner:SUMITOMO CHEM CO LTD
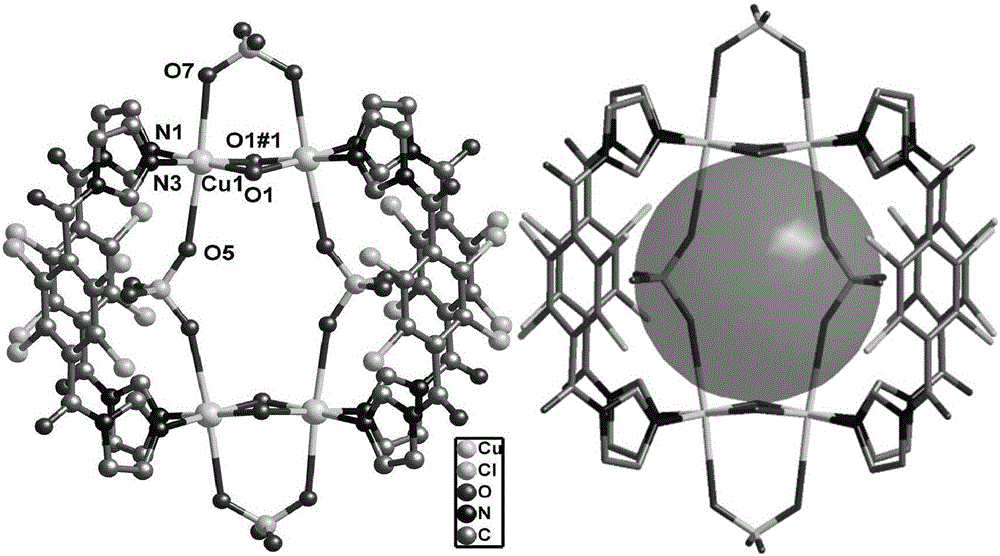
Thermal stable copper metal organic framework material and preparation method and application thereof
ActiveCN105820182AEasy to manufactureSimple processOrganic-compounds/hydrides/coordination-complexes catalystsCopper organic compoundsNitrate anionGas phase
The invention discloses a thermal stable copper metal organic framework material and a preparation method and application thereof, and relates to the field of pyrrolidine catalysts. A catalyst is the thermal stable copper metal organic framework material, and the chemical formula of the thermal stable copper metal organic framework material is Cu(L)(NO3)2, wherein L represents a 2,3,5,6-tetrafluoro-1,4-bis(1,2,4-triazol-1-methyl)benzene ligand, and NO3 is nitrate anion. Cupric nitrate tetrahydrate and the organic ligand 2,3,5,6-tetrafluoro-1,4-bis(1,2,4-triazol-1-methyl)benzene are subjected to a hydrothermal reaction under an airtight condition, and the copper metal organic framework material with a two-dimensional layered structure is obtained. The synthetic method is high in yield and good in reproducibility; the obtained crystal is high in purity and thermal stability. The copper metal organic framework material has better catalytic activity on catalyzing of tetrahydrofuran gas phase amination, the conversion rate of tetrahydrofuran reaches 72%, and the selectivity of pyrrolidine reaches 90%.
Owner:CHANGZHOU UNIV
Aerobic nitritation of ammonia and integrated anammox processes
ActiveUS10584047B2Emission reductionImprove toleranceWater treatment parameter controlWater treatment compoundsNitrate anionHydrophilic polymers
Processes are disclosed for the microbial nitritation of ammonia that attenuate the production of at least one of nitrate anion and nitrous oxide. The processes use an ME biocatalyst having a highly porous, hydrophilic polymeric structure with ammonia-oxidizing microorganisms substantially irreversibly retained therein. The processes are particularly useful for integration with anammox processes.
Owner:MICROVI BIOTECH
Methods of the purification and use of moderately saline water
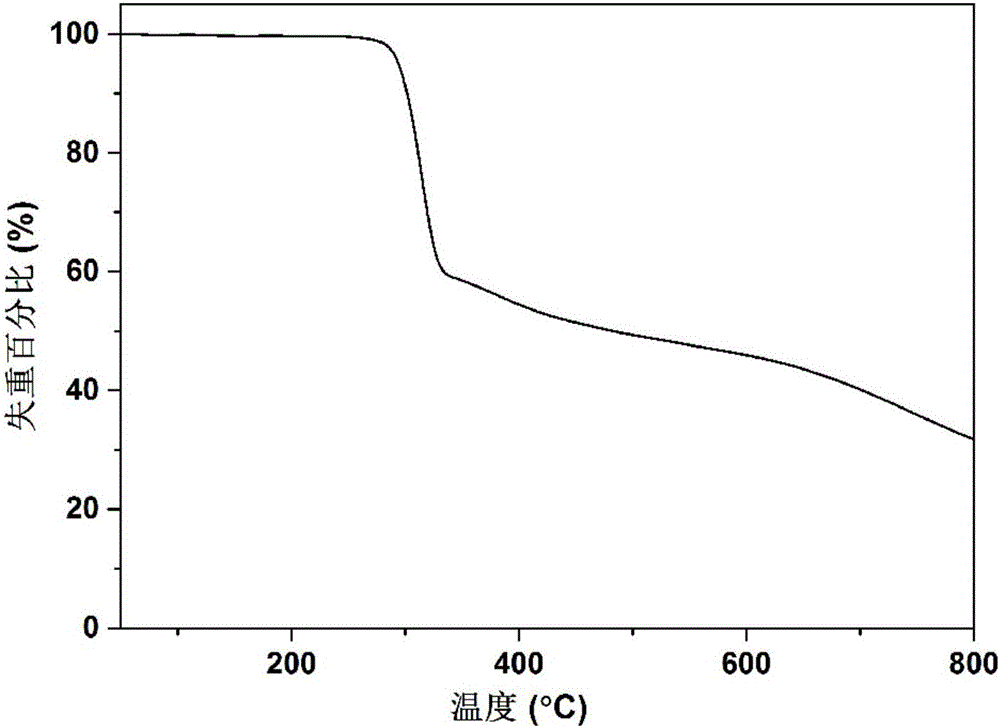
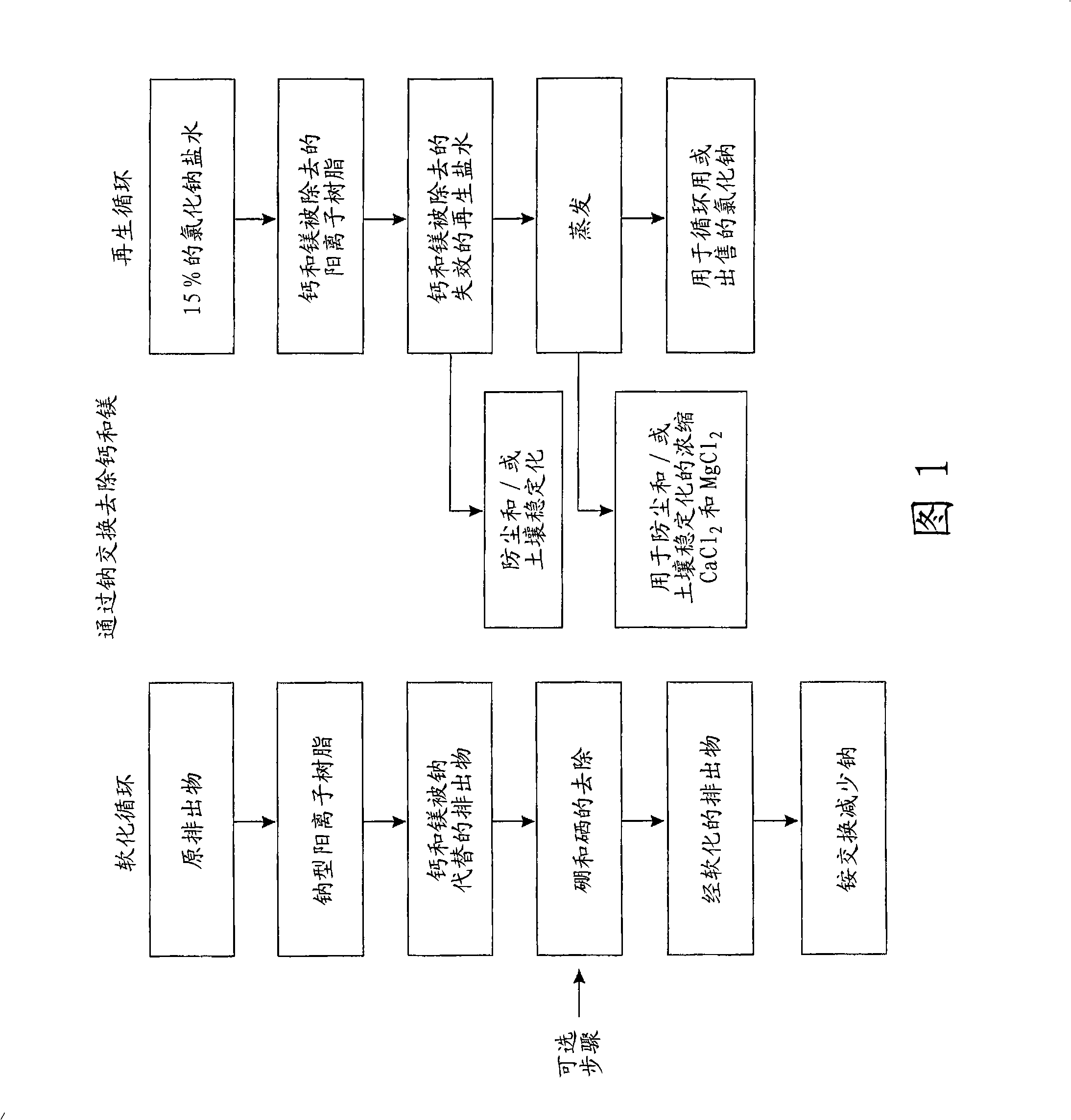
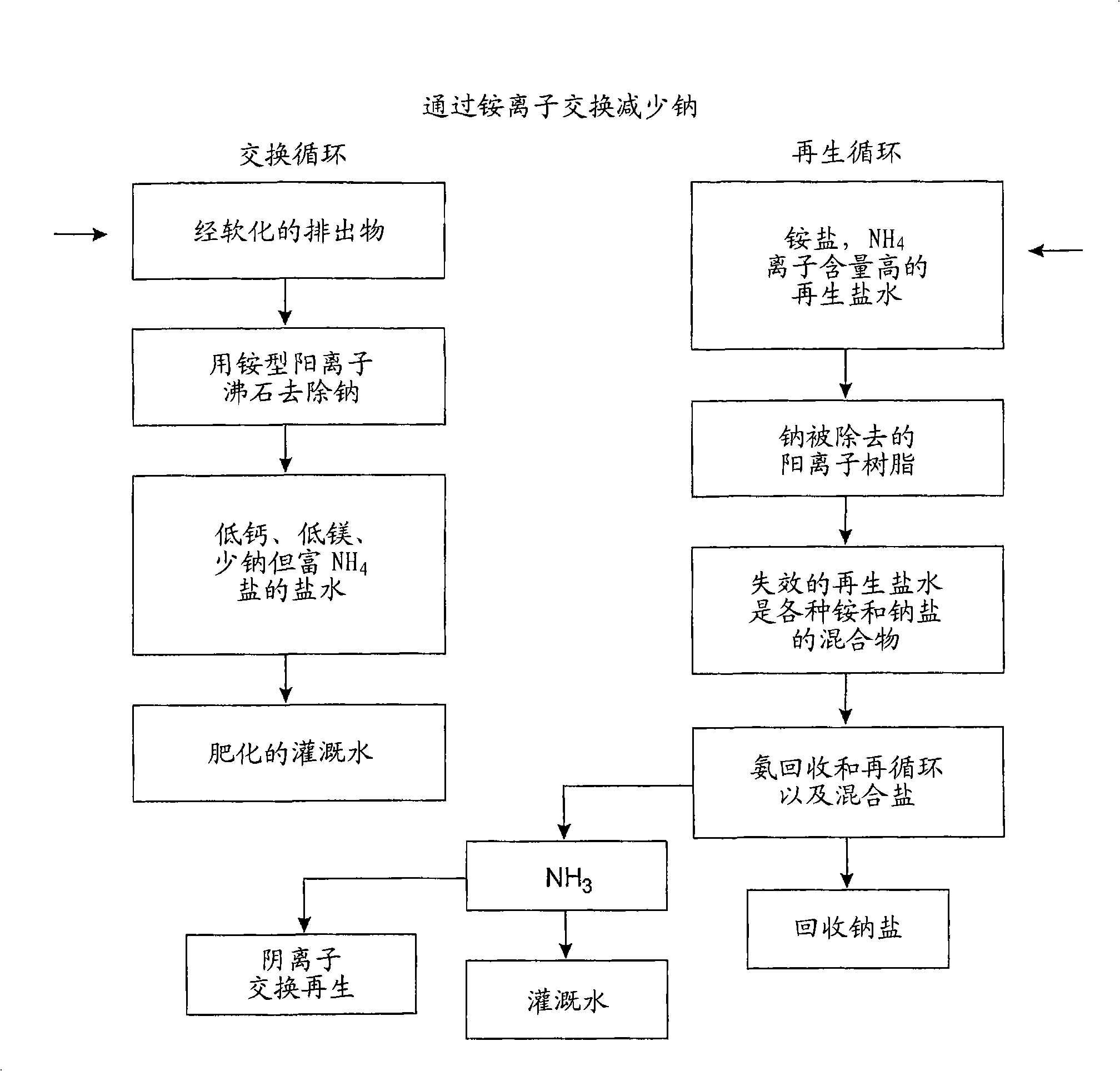
InactiveCN101257976AIon-exchanger regenerationSolid sorbent liquid separationNitrate anionIon exchange
The invention relates to purifying unwanted moderately saline water. The methods of the present invention including passing moderately saline water through an ion exchange media saturated with ammonium salts to produce fertilizer water. In addition, the present invention relates to a method of passing moderately saline water through a dual bed cation and anion exchange process for producing purified water. The first cation exchange media is saturated with acids of hydrochloric, nitric or sulfuric acids. Meanwhile, the second ion exchange media is saturated with ammonium hydroxide. Passing the moderately saline water through the first ion exchange media creates an acid rich water which is then passed through the second ion exchange media to remove chloride, sulfate, nitrate, and nitrite anions. Through a regenerative cycle, a fertilizer water is produced which is rich in ammonium chloride, ammonium nitrate or ammonium sulfate.
Owner:杰拉尔德·J·格罗特
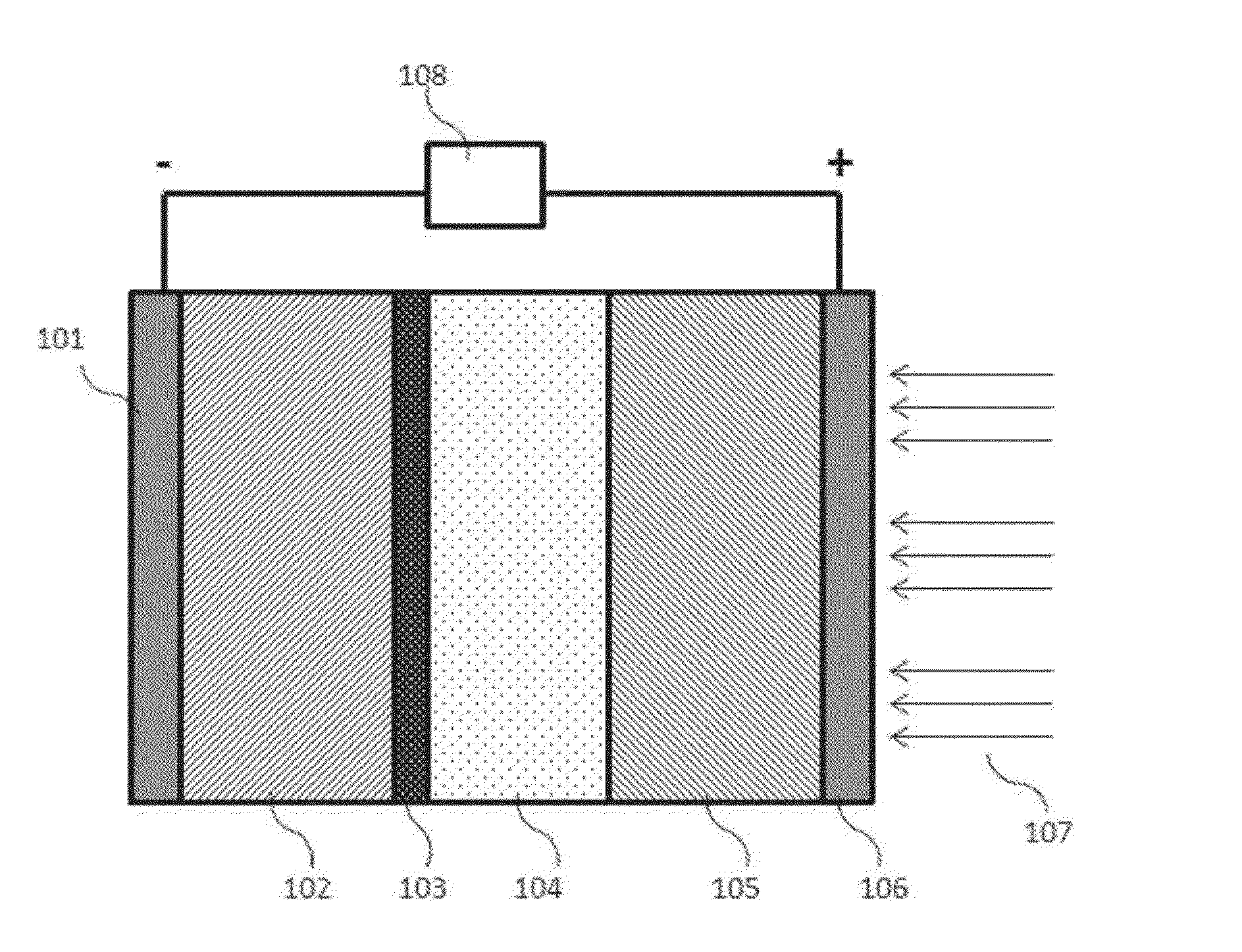
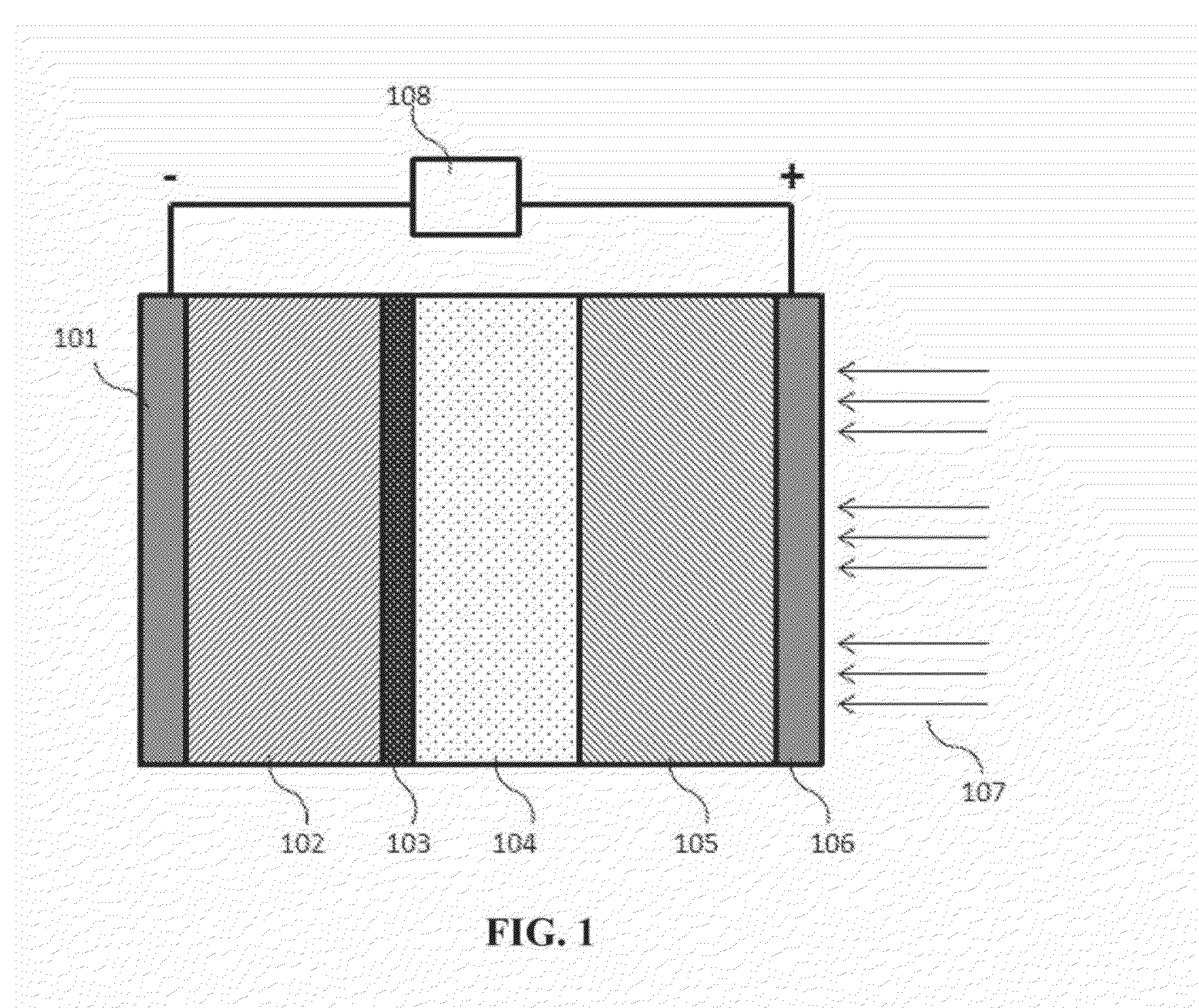
Intermediate temperature alkali metal/oxygen batteries employing molten nitrate electrolytes
InactiveUS20160049707A1Avoid contactFuel and primary cellsFuel and secondary cellsNitrate anionNitrate
High capacity alkali metal / oxygen batteries, e.g. Li / O2 batteries, employing molten salt electrolytes comprising alkali metal cations and nitrate anions are disclosed. Batteries of the present invention operate at an intermediate temperature ranging from. 80° C. to 250° C. Molten alkali metal nitrate electrolytes employed in O2 electrodes within this temperature range provide alkali metal / oxygen batteries having significantly improved efficiency and rechargeability compared to prior art systems.
Owner:LIOX POWER
Process for preparing an adsorbing material comprising a precipitating step of boehmite according to specific conditions and process for extracting lithium from saline solutions using this material
ActiveUS20180353932A1Improve adsorption capacitySolve the lack of cohesionOther chemical processesAluminium hydroxide preparationNitrate anionPrecipitation
The present invention relates to the field of solid materials for adsorption of lithium. In particular, the present invention relates to a novel method for preparing a crystallized and shaped solid material, preferably as extrudates, of formula LiXx.2Al(OH)3, nH2O with n being comprised between 0.01 and 10, x being equal to 1 when X is an anion selected from among chloride, hydroxide and nitrate anions, and x being equal to 0.5 when X is an anion selected from among sulfate and carbonate anions, comprising a step a) for precipitation of boehmite under specific temperature and pH conditions, at least one shaping step, preferably by extrusion, said method also comprising a final hydrothermal treatment step, the whole giving the possibility of increasing the adsorption capacity for lithium as well as the adsorption kinetics of the materials obtained as compared with the materials of the prior art when the latter is used in a method for extracting the lithium from saline solutions.
Owner:ERAMET +1
Processes for removing co-produced oxygenated organics from anaerobic fermentation broths for the bioconversion of syngas to product oxygenated organic compound
Processes are disclosed for economically and effectively removing co-produced oxygenated organic compound from an anaerobic, aqueous fermentation broth used for the bioconversion of syngas to product oxygenated organic compound. Nitrate anion is added to the broth and the broth is contacted with denitrifying microorganisms that bioconvert the nitrate and organic compounds in the broth to reduced nitrogen compound and carbon dioxide.
Owner:SYNATA BIO INC
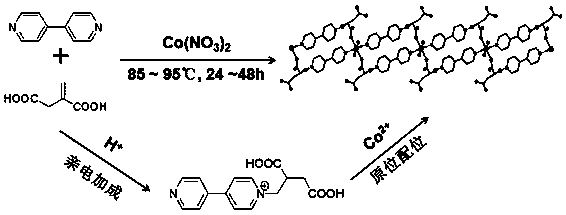
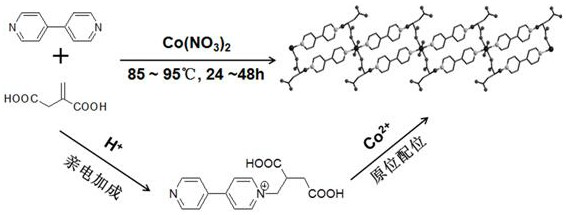
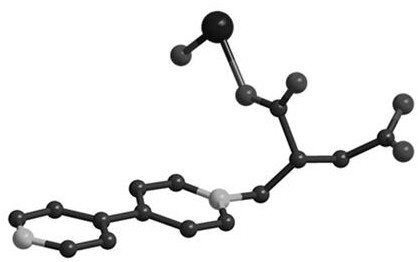
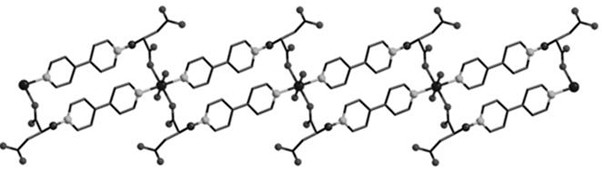
Magnetic cobalt (II) complex based on 4,4'-bipyridine-itaconic acid derivative ligand and preparation method of magnetic cobalt (II) complex
ActiveCN108727251AImprove stabilityRich coordination modeOrganic chemistryOrganic/organic-metallic materials magnetismNitrate anionElectrophilic addition
The invention discloses a magnetic cobalt (II) complex based on a 4,4'-bipyridine-itaconic acid derivative ligand and a preparation method of the magnetic cobalt (II) complex. The molecular formula ofthe magnetic cobalt (II) complex is [[Co(HL)2(H2O)2].2NO3]n, wherein HL represents the 4,4'-bipyridine-itaconic acid derivative ligand with one hydrogen atom removed from carboxylic acid, a single-crystal structure has a one-dimensional chain structure, and an asymmetric construction unit of the single-crystal structure contains half cobalt (II) ion, one 4,4'-bipyridine-itaconic acid derivative ligand, one end coordination water molecule and one free nitrate anion; pyridine nitrogen in 4,4'-bipyridineis subjected to an electrophilic addition reaction with double bonds in itaconic acid, a semi-rigid carboxylate ligand containing N(+)-C bonds is synthesized, and the ligand and the cobalt (II) ion are subjected to an in-situ coordination reaction, and the cobalt (II) complex is synthesized;the cobalt (II) complex has slow magnetic relaxation phenomenon at the low temperature and serves as a molecule-based magnetic material to be applied to the fields of information storage, quantum computation and the like.
Owner:SHANGQIU NORMAL UNIVERSITY
Process for preparing an adsorbing material comprising a precipitating step of boehmite according to specific conditions and process for extracting lithium from saline solutions using this material
ActiveUS10786802B2Improve adsorption capacityImproved adsorption kineticsOther chemical processesCombustible gas purificationNitrate anionSulfate radicals
Owner:ERAMET +1
Method for preparing an adsorbent material comprising a step of basic mixing, and method for extracting lithium from saline solutions using said material
ActiveUS20180345244A1Increase capacityImprove adsorption capacityOther chemical processesLithium compoundsNitrate anionLithium
The present invention relates to the field of solid materials for the adsorption of lithium. In particular, the present invention relates to a new method for the preparation of a crystallized and shaped solid material, preferably in extruded form, of formula LiXx.2Al(OH)3,nH2O, wherein n is between 0.01 and 10, x is 1 when X is an anion selected from among chloride, hydroxide and nitrate anions, and x is 0.5 when X is an anion selected from among sulfate and carbonate anions, comprising a boehmite precipitation step a) under specific temperature and pH conditions, at least one basic mixing shaping step, wherein the method also comprises a final hydrothermal treatment step, all to increase the lithium adsorption capacity and the kinetics of adsorption of the materials obtained, compared with the materials of the prior art when it is used in a method for lithium extraction from saline solutions.
Owner:ERAMET +1
Novel ionic liquids resulting from the association of a specific cation and a specific anion
PendingUS20200079733A1Group 4/14 element organic compoundsHybrid capacitor separatorsImideNitrate anion
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Processes for removing co-produced oxygenated organics from anaerobic fermentation broths for the bioconversion of syngas to product oxygenated organic compound
ActiveUS9663802B2Loss of downtimeLost production of product oxygenated organic compoundBiofuelsFermentationSyngasNitrate anion
Processes are disclosed for economically and effectively removing co-produced oxygenated organic compound from an anaerobic, aqueous fermentation broth used for the bioconversion of syngas to product oxygenated organic compound. Nitrate anion is added to the broth and the broth is contacted with denitrifying microorganisms that bioconvert the nitrate and organic compounds in the broth to reduced nitrogen compound and carbon dioxide.
Owner:SYNATA BIO INC
Composition comprising a specific ionic liquid
InactiveUS20150187514A1Low ion conductivityHigh viscosityHybrid capacitor separatorsHybrid capacitor electrolytesNitrate anionSolvent
The invention relates to a composition comprising an ionic liquid consisting in the combination of a pyrrolidinium cation and a nitrate anion and comprising a solvent selected from lactone solvents, carbonate solvents, nitrile solvents and mixtures thereof. The composition may be used as an electrolyte, for example, for such applications as energy storage devices.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES +1
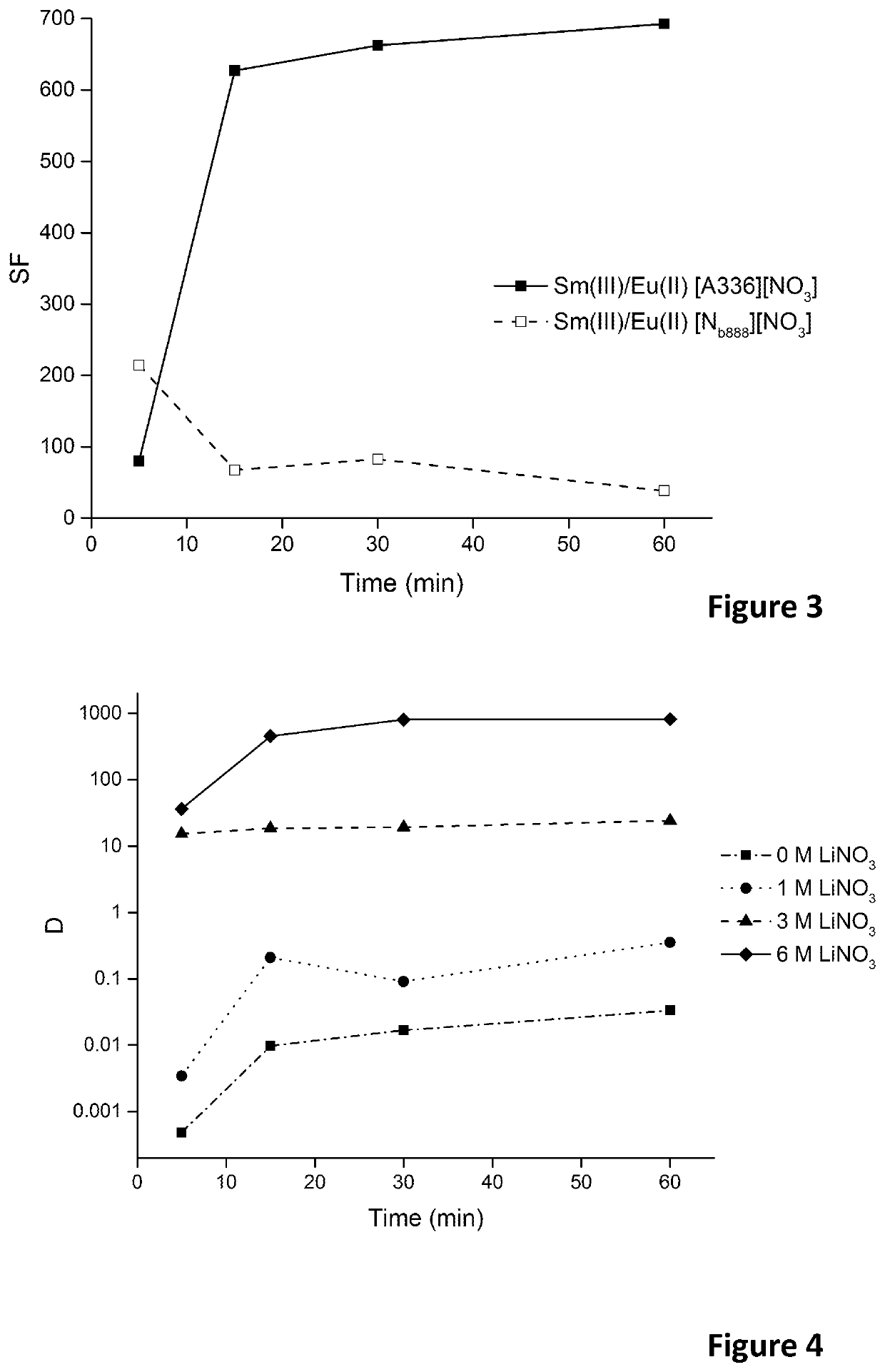
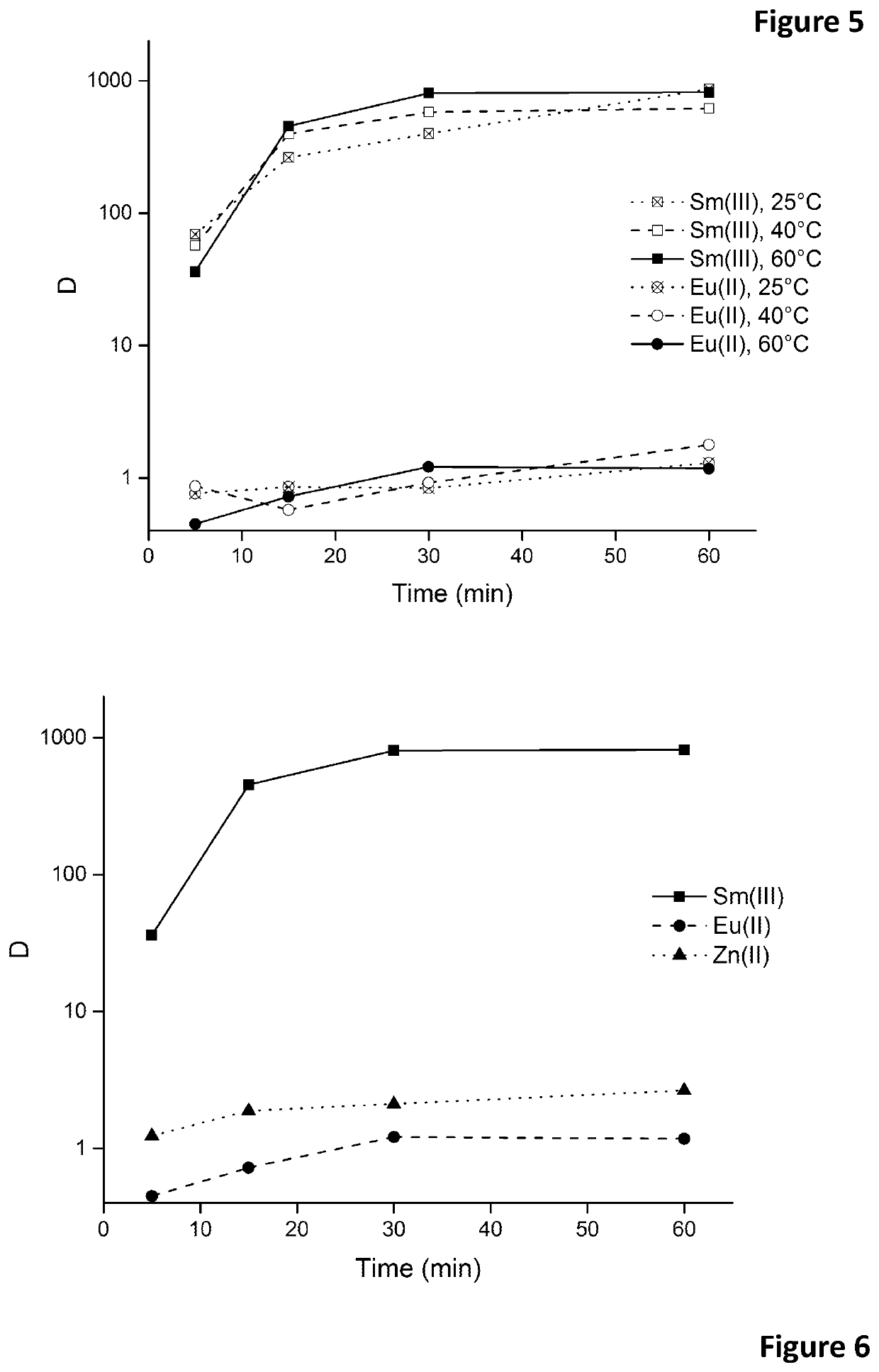
Removal of europium impurities from samarium-153 in nitrate media using ionic liquids
InactiveUS20210070628A1Easy to useMore efficiencyCation exchanger materialsSolvent extractionNitrate anionNitrate salts
A process of isolating samarium from a hydrophilic composition comprises nitrate ions, europium and samarium, by reducing europium(III) to europium(II) in this hydrophilic composition, and by extracting the samarium with a water-immiscible organic phase comprising an ionic liquid comprising nitrate anions.
Owner:SCK CEN STUDIECENTRUM VOOR KERNENERGIE CENT DETUDE DE LENERGIE NUCLEAIRE
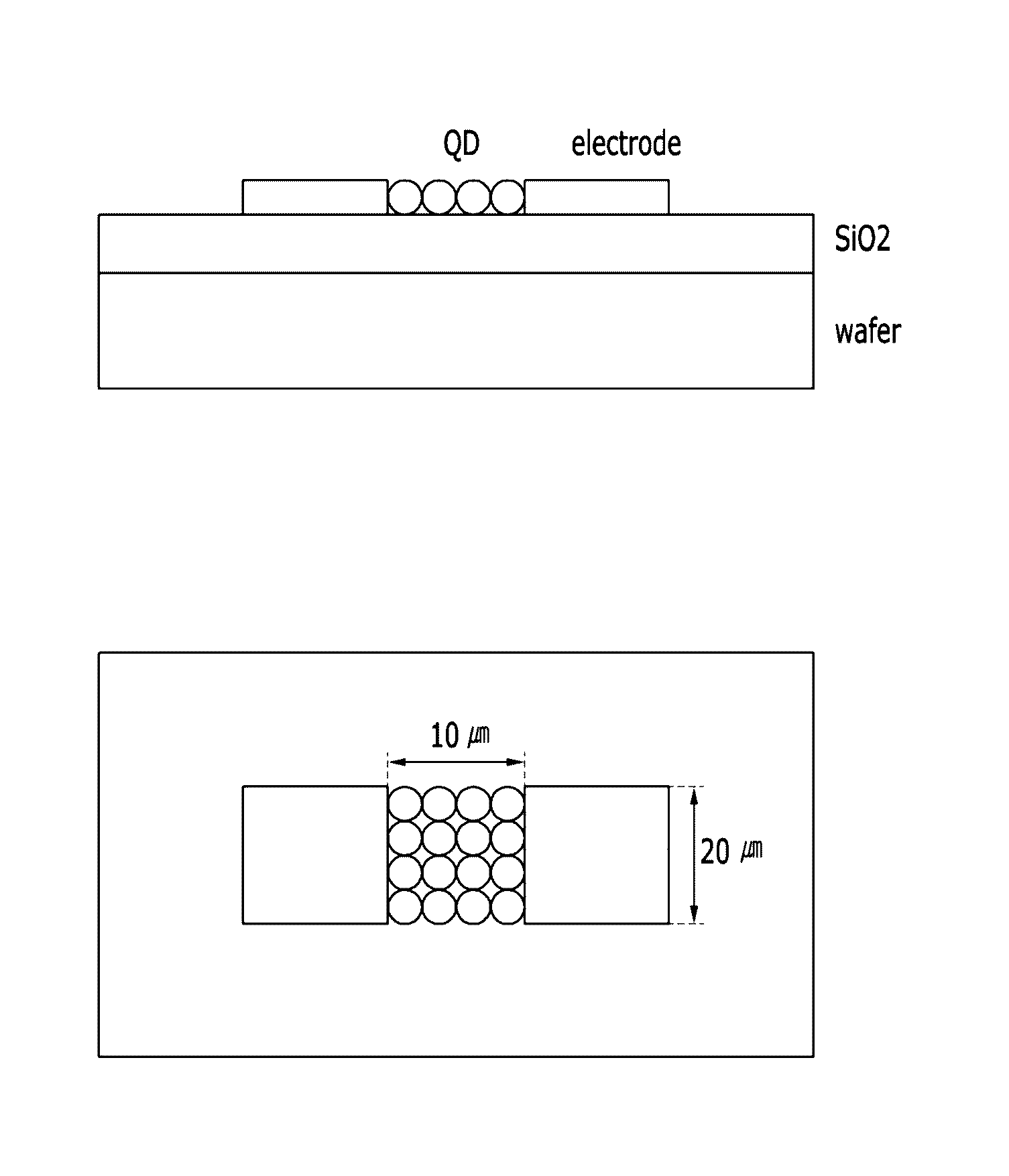
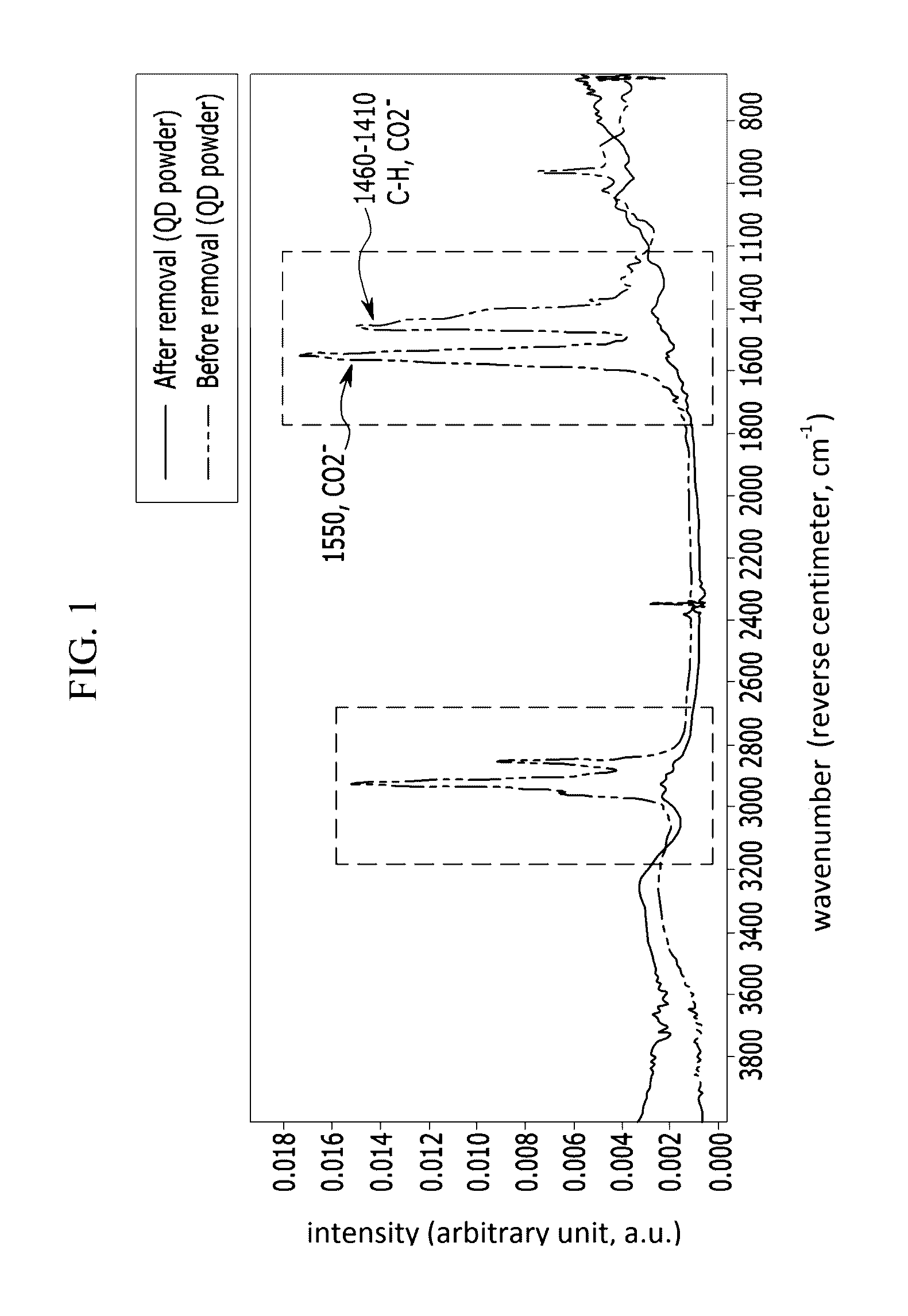
Methods of removing surface ligand compounds
ActiveUS9492840B2Easy to disassembleLiquid surface applicatorsMetal-working apparatusCompound aNitrate anion
A method for removing an organic ligand from a surface of a particle including:obtaining a particle having an organic ligand disposed on a surface thereof;contacting the particle with an alkylammonium salt represented by Chemical Formula 1:NR′4+A− Chemical Formula 1wherein groups R′ are the same or different and are each independently hydrogen or a C1 to C20 alkyl group, provided that at least one group R′ is an alkyl group, and A is a hydroxide anion, a halide anion, a borohydride anion, a nitrate anion, a phosphate anion, or a sulfate anion; andheat-treating the particle to carry out a reaction between the alkylammonium salt and the organic ligand.
Owner:SAMSUNG ELECTRONICS CO LTD
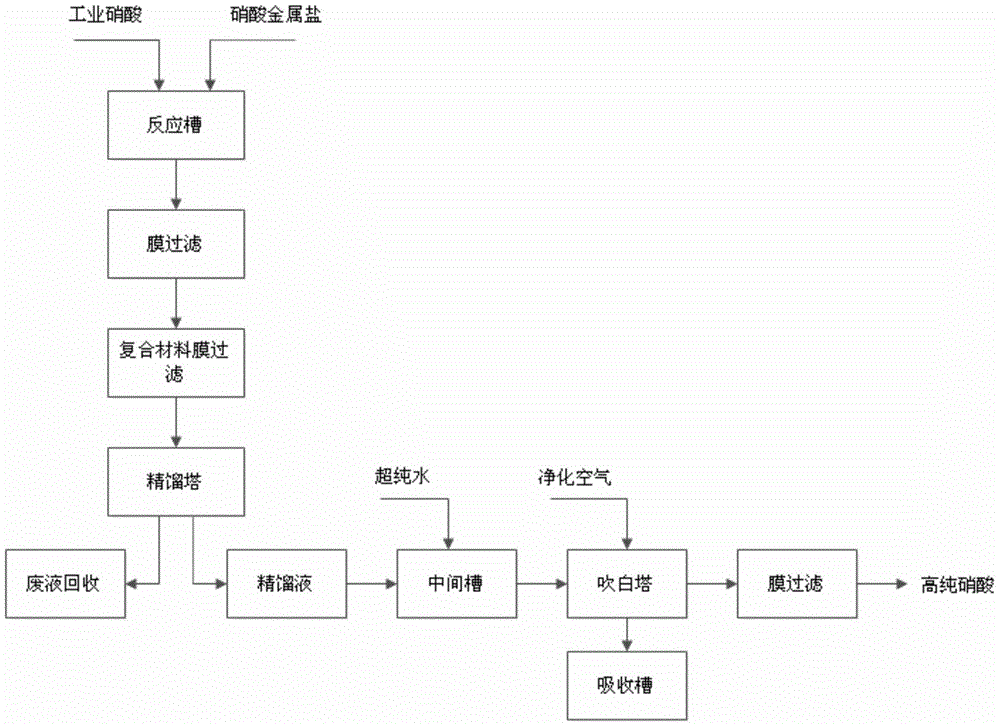
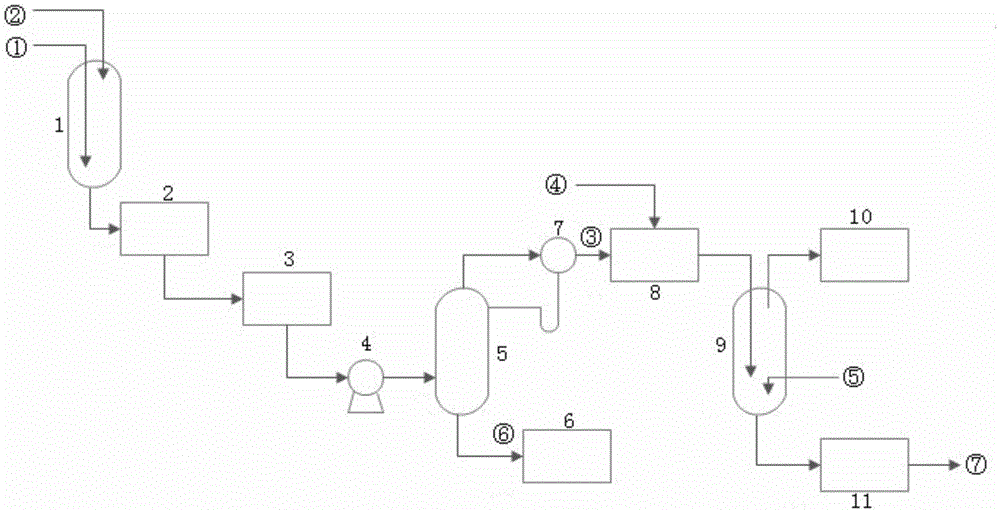
A kind of preparation method of high-purity nitric acid
Owner:SHANGHAI CHEM REAGENT RES INST
Tetranuclear copper complex, preparation method and application of tetranuclear copper complex in gas-phase amination catalysis of tetrahydrofuran
ActiveCN105732667AEasy to manufactureSimple processGroup 1/11 organic compounds without C-metal linkagesOrganic-compounds/hydrides/coordination-complexes catalystsNitrate anionGas phase
The invention discloses a tetranuclear copper complex, a preparation method and application of the tetranuclear copper complex in gas-phase amination catalysis of tetrahydrofuran, and relates to the field of pyrrolidine catalysts.The chemical formula of the tetranuclear copper complex is [Cu(L)(ClO4)(H2O)](dioxane)1.5, wherein L represents a 2,3,5,6-tetrachloro-1,4-di(imidazole-1-carbonyl)benzene ligand, ClO4 represents a nitrate anion, and dioxane represents 1,4-dioxane.The copper complex with a tetranuclear cage-shaped structure is obtained by adopting copperperchlorate hydrate and the organic ligand 2,3,5,6-tetrachloro-1,4-di(imidazole-1-carbonyl)benzene in blend solvent of 1,4-dioxane and methyl alcohol under the closed condition through a thermal reaction.The tetranuclear copper complex is applied to ammoniation of tetrahydrofuran and ammonia to prepare pyrrolidine.Accordingly, the technological process is simple; a catalyst is convenient to prepare, and the reproducibility is good; the conversion rate of tetrahydrofuran reaches 83%, and the selectivity of pyrrolidine reaches 95%.
Owner:CHANGZHOU UNIV
Method for preparing emulsified nanometer grade zero valent iron and nanometer grade bimetal and use thereof
The invention discloses the method for preparation of emulsified nanometer zero valence iron and nanometer bimetallic. The method comprises the following steps: 1 adding the NaBH4 solution into the mixture solution of FeSO4.7H2O and stabilizing agent which is amylogen, getting the emulsified nanometer zero valence iron, the density of amylogen being 0.2-2%, the mole ratio of NaBH4 and FeSO4.7H2O solution being 2:1; 2 mixing emulsified nanometer zero valence iron and K2PdCl6 solution, carrying out reaction, entering nitrogen, when the color of solution changed from bistre to pea green, getting the nanometer bimetallic, the proportion by weight of Pd and Fe being 5-10:10000. The emulsified nanometer zero valence iron and nanometer bimetallic are used to restore the pollution water containing chlorine, nitrate anion and heavy metal. The method is simple, the specific surface area of emulsified bimetallic particle is large, the dispersity is good, and the chemical reactivity is high.
Owner:ZHEJIANG UNIV
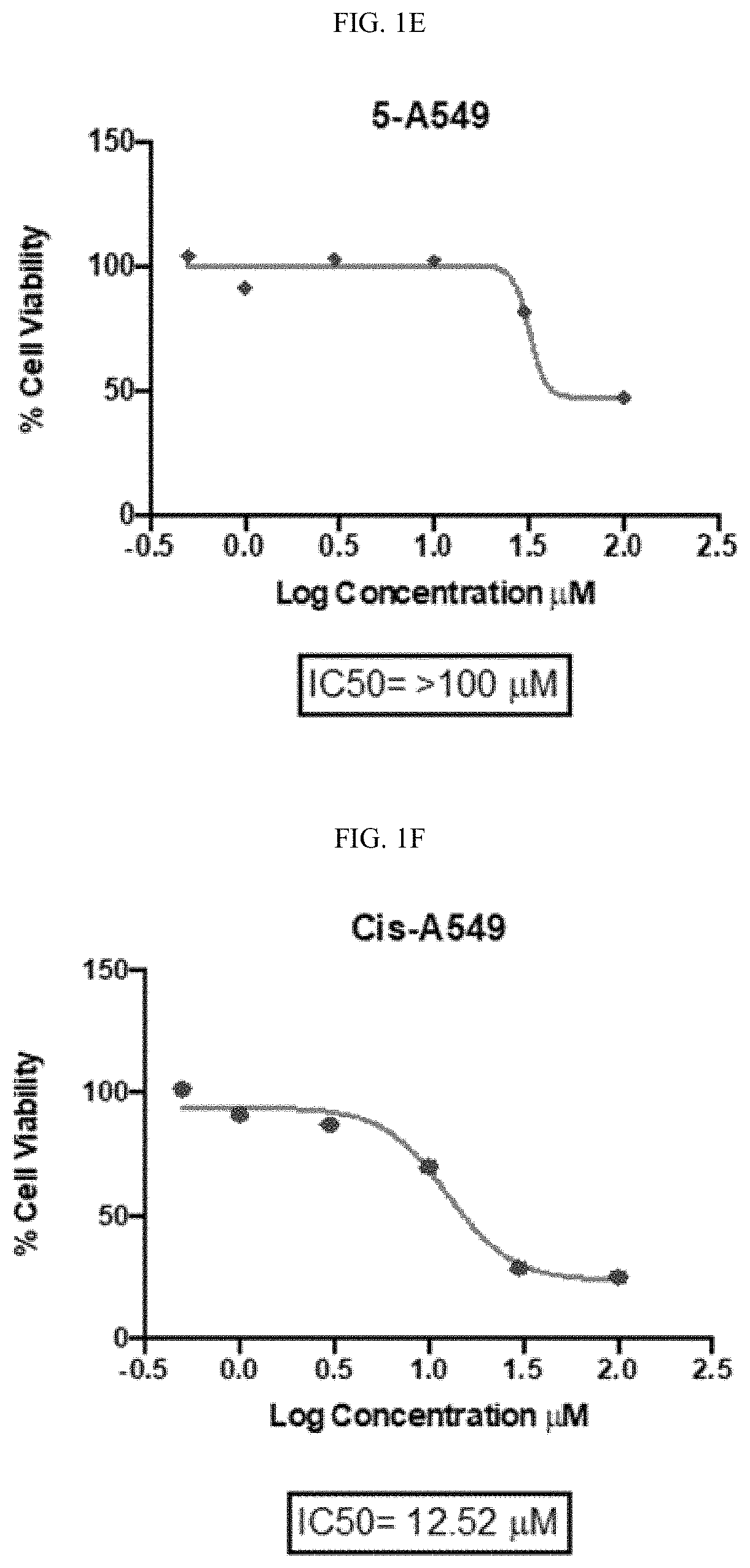
Platinum(II) ammine selenourea complexes and methods of treating cancer
A platinum(II) complex of formula (I),or a pharmaceutically acceptable solvate or tautomer thereof, wherein R1 and R2 are each independently a hydrogen, an optionally substituted alkyl, an optionally substituted cycloalkyl, an optionally substituted arylalkyl, or an optionally substituted aryl; or wherein R1 and R2 together form a five-, six-, or seven-membered ring with the nitrogen atoms to which they are attached; and X is a nitrate anion, a hexafluorophosphate anion, a hexafluoroantimonate anion, a trifluoromethanesulfonate anion, a tetrafluoroborate anion, a perchlorate anion, or a halide anion. A pharmaceutical composition containing the platinum(II) complex of formula (I), and a method of treating cancer are also disclosed.
Owner:KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS +1
A magnetic cobalt(ii) complex based on 4,4'-bipyridine-itaconic acid derivative ligand and its preparation method
ActiveCN108727251BImprove stabilityRich coordination modeOrganic chemistryOrganic/organic-metallic materials magnetismNitrate anionElectrophilic addition
The invention discloses a magnetic cobalt (II) complex based on a ligand derived from 4,4'-bipyridine-itaconic acid and a preparation method thereof. The molecular formula is {[Co(HL) 2 (H 2 O) 2 ]·2NO 3} n , HL represents a ligand derived from 4,4'-bipyridine-itaconic acid that removes a carboxylic acid hydrogen, the single crystal structure has a one-dimensional chain structure, and its asymmetric building block contains half a cobalt(II) ion, a 4,4'-bipyridine-itaconic acid-derived ligand, a terminal coordinated water molecule and a free nitrate anion; the pyridine nitrogen of 4,4'-bipyridine and the double bond in itaconic The addition reaction synthesizes a semi-rigid carboxylic acid ligand containing N(+)-C bonds, and the ligand reacts with cobalt(II) ions to form a cobalt(II) complex in situ; the cobalt(II) complex is in It has slow magnetic relaxation phenomenon at low temperature, and is used as a molecular-based magnetic material in information storage and quantum computing.
Owner:SHANGQIU NORMAL UNIVERSITY
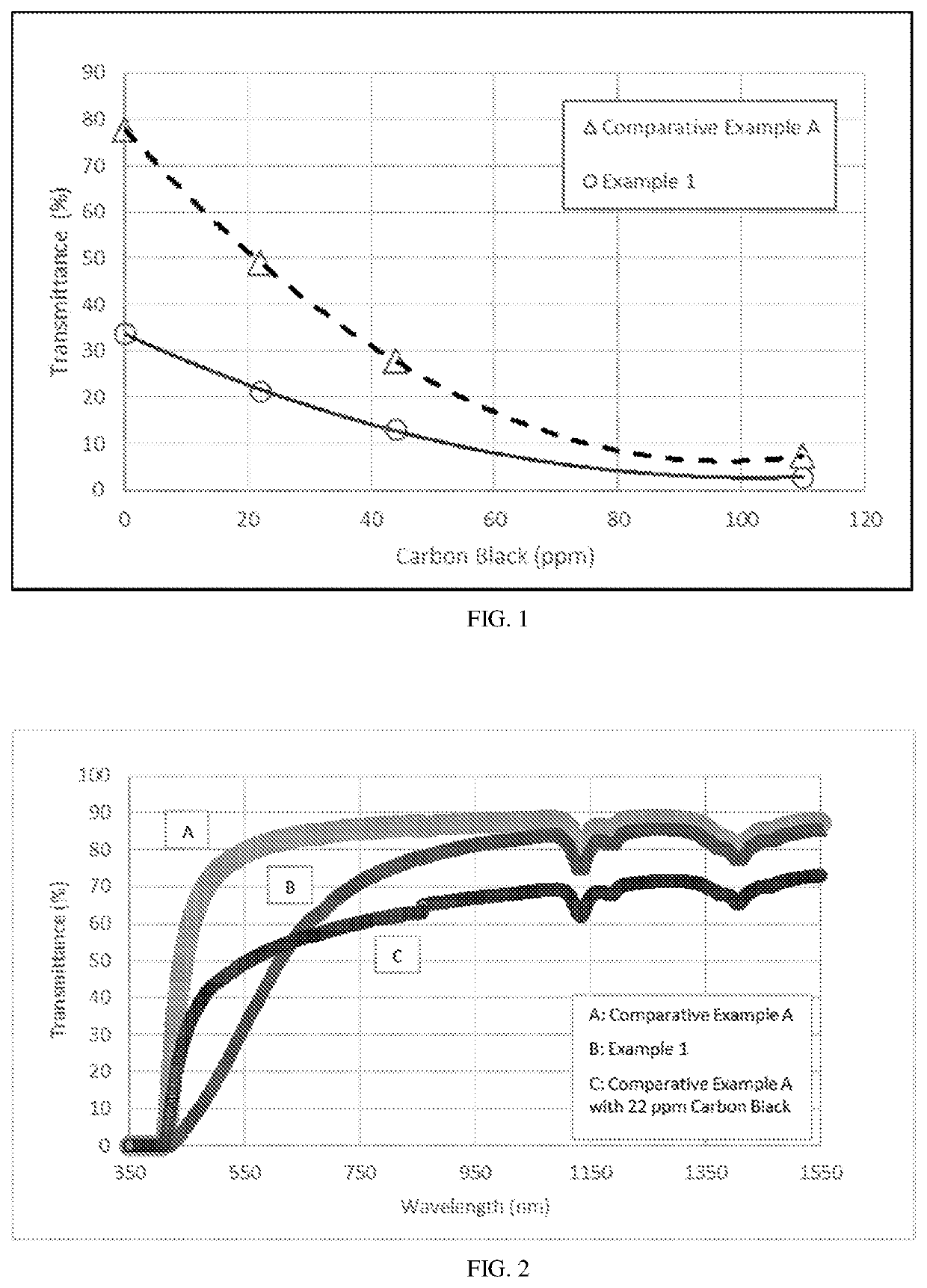
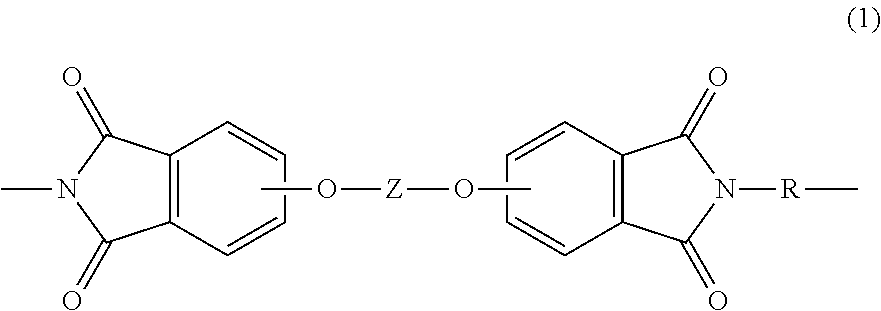
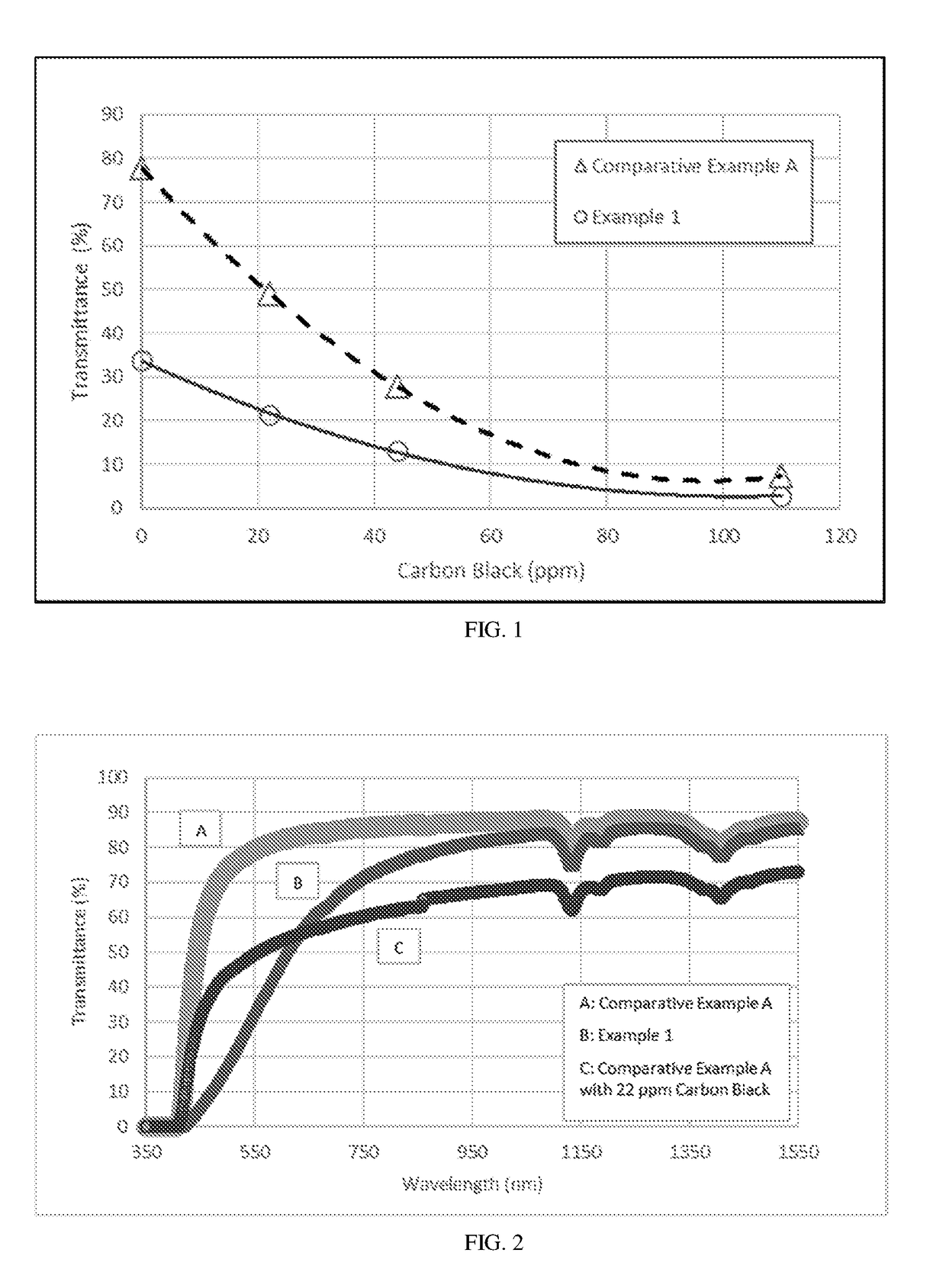
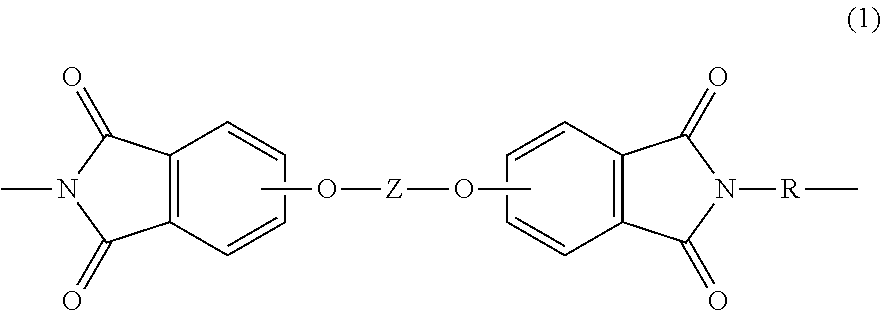
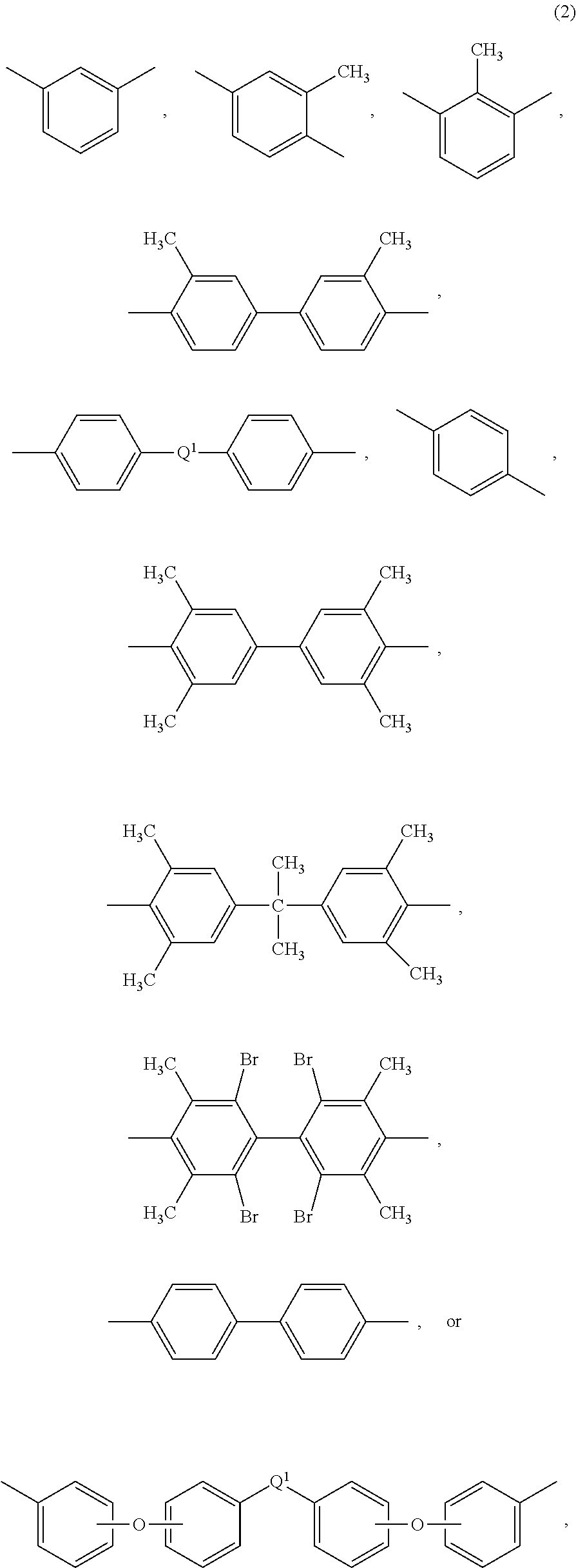
Polyetherimide compositions, methods of manufacture, and articles prepared therefrom
A polyetherimide composition includes a polyetherimide and two or more of a residual metal content, a sulfate anion, a phosphate anion, a nitrite anion, a nitrate anion, or a combination including at least one of the foregoing, a residual solvent content, a phosphorus-containing stabilizer, and alkali metal halide, alkaline earth metal halide, alkali metal carbonate, or a combination including at least one of the foregoing, wherein each of the aforementioned components, when present, is included in the composition in a particular amount. The resulting polyetherimide composition exhibits two or more useful properties. The polyetherimide composition can optionally further be combined with a polymer different from the polyetherimide to provide a thermoplastic composition. Methods of making the polyetherimide composition and articles including the polyetherimide composition are also described.
Owner:SHPP GLOBAL TECH BV
Acetylene even-coupling method under temperate condition
InactiveCN100400481CUnique catalytic effectHigh catalytic activityHydrocarbonsMetal/metal-oxides/metal-hydroxide catalystsNitrate anionCatalytic effect
The invention discloses the method of end-alkynes coupling, using end-alkynes as raw material, and using hydrotalcite as accelerating agent. The accelerating agent uses double hydroxy compound of divalent transient metal ion and tervalent transient metal ion as plywood, and uses carbonate or nitrate anion as layer-to-layer negative ion. The reaction condition is normal temperature and normal pressure, the product is 1, 3-alkynyl compound, and the divalent transient metal positive ion possesses the particular accelerating effect. The method has the advantages of good catalytic activity, short reaction time, mild reaction condition, simple operation, low cost and no environmental pollution.
Owner:ZHEJIANG UNIV
Bimetallic salt catalyst system and its uses
InactiveCN100430130CHigh activityHigh selectivityMetal/metal-oxides/metal-hydroxide catalystsPreparation by hydrolysisNitrate anionSulfate radicals
The bimetal salt catalyst system consists of bimetal salt catalyst NM and NM dissolving solvent NS, and has the general expression of NM[NY(I) +MO(II)]+NS(III), where, NM is bimetal salt catalyst, N is one or several of IA, IIA, IB and IIB metals, Y is one or several of halogen, (bi)carbonate radical, (bi)sulfate radical and (bi)phosphate radical, M in MO is one or several of RE metal ions, O is oxygen, carbonate radical and nitrate radical, and NS is organic solvent or water and possesses boiling point of bimetal salt catalyst NM solution higher than 190 deg.c. The bimetal salt catalyst system has high activity, high selectivity and good circular use performance.
Owner:NANJING TECH UNIV
Polyetherimide compositions, methods of manufacture, and articles prepared therefrom
A polyetherimide composition includes a polyetherimide and two or more of a residual metal content, a sulfate anion, a phosphate anion, a nitrite anion, a nitrate anion, or a combination including at least one of the foregoing, a residual solvent content, a phosphorus-containing stabilizer, and alkali metal halide, alkaline earth metal halide, alkali metal carbonate, or a combination including at least one of the foregoing, wherein each of the aforementioned components, when present, is included in the composition in a particular amount. The resulting polyetherimide composition exhibits two or more useful properties. The polyetherimide composition can optionally further be combined with a polymer different from the polyetherimide to provide a thermoplastic composition. Methods of making the polyetherimide composition and articles including the polyetherimide composition are also described.
Owner:SHPP GLOBAL TECH BV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com