Patents
Literature
606results about "Nitric acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
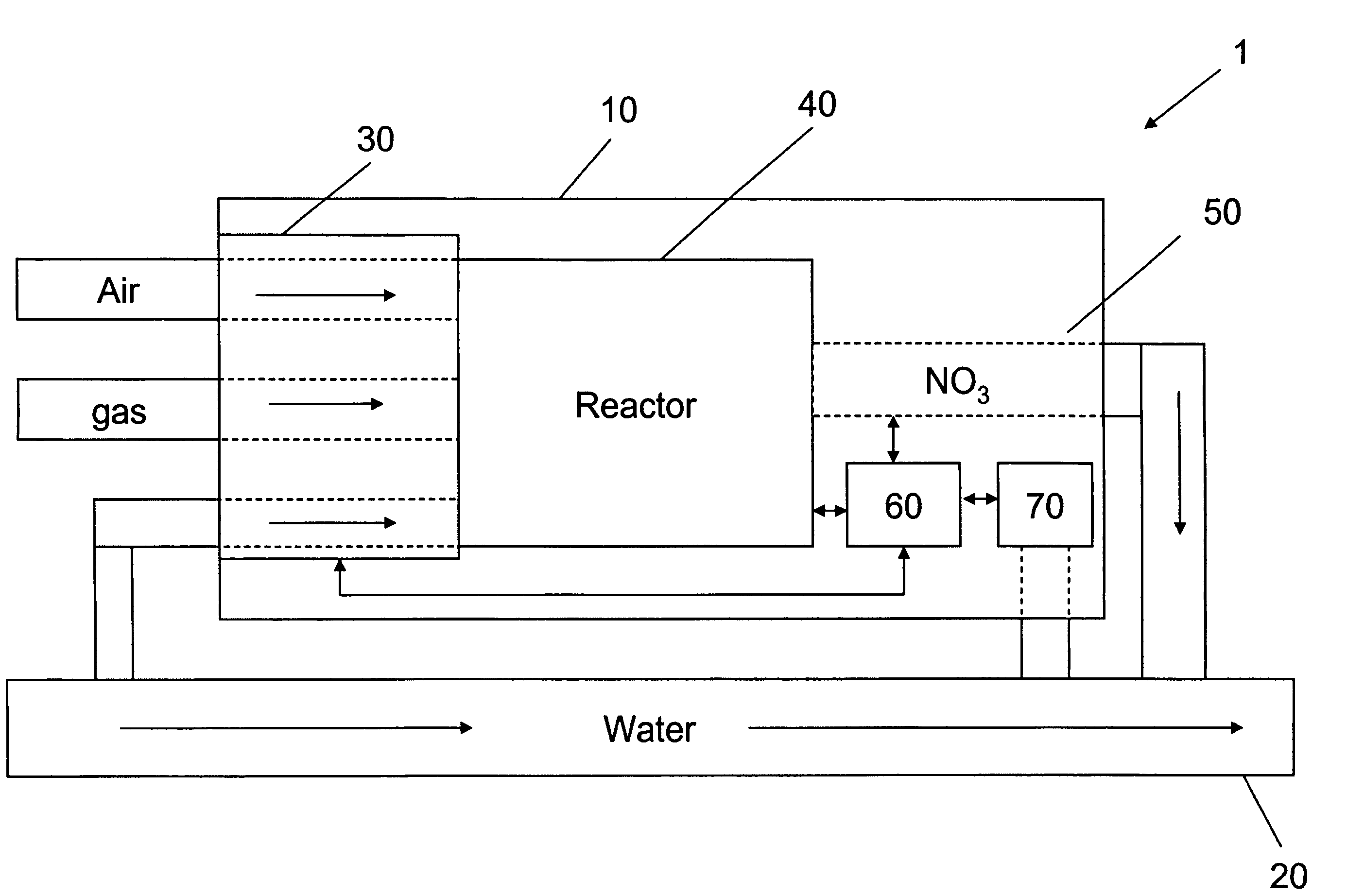
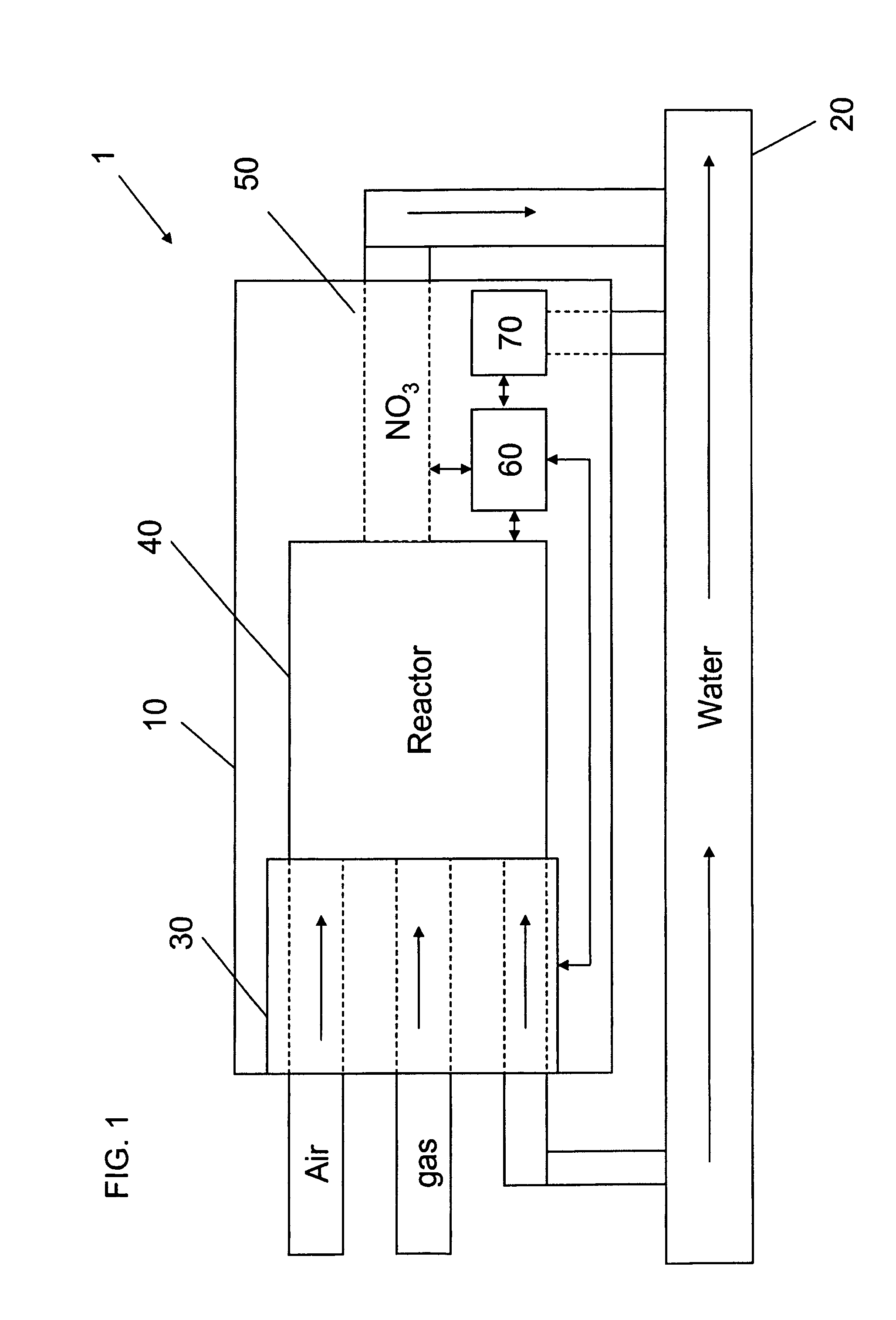
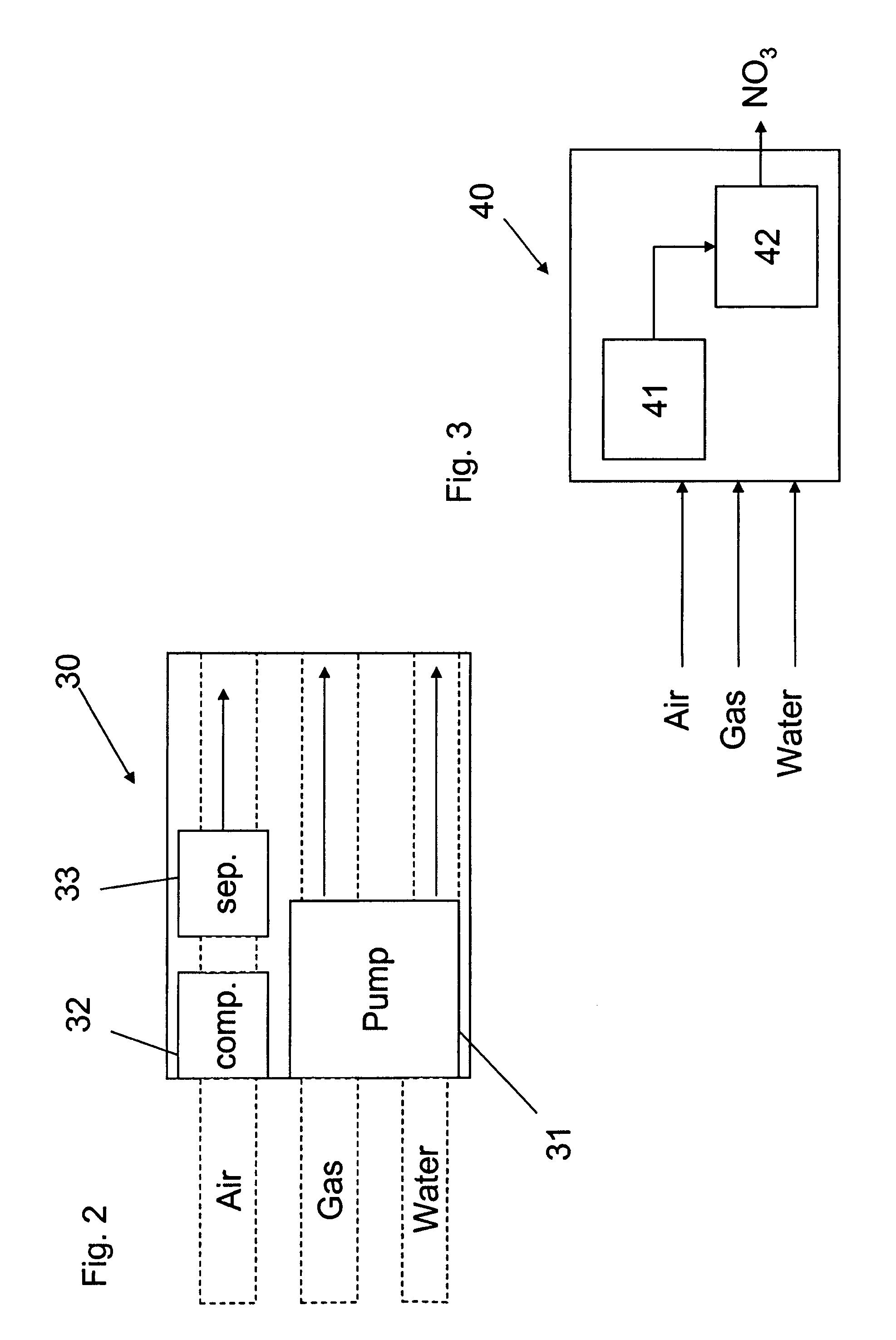
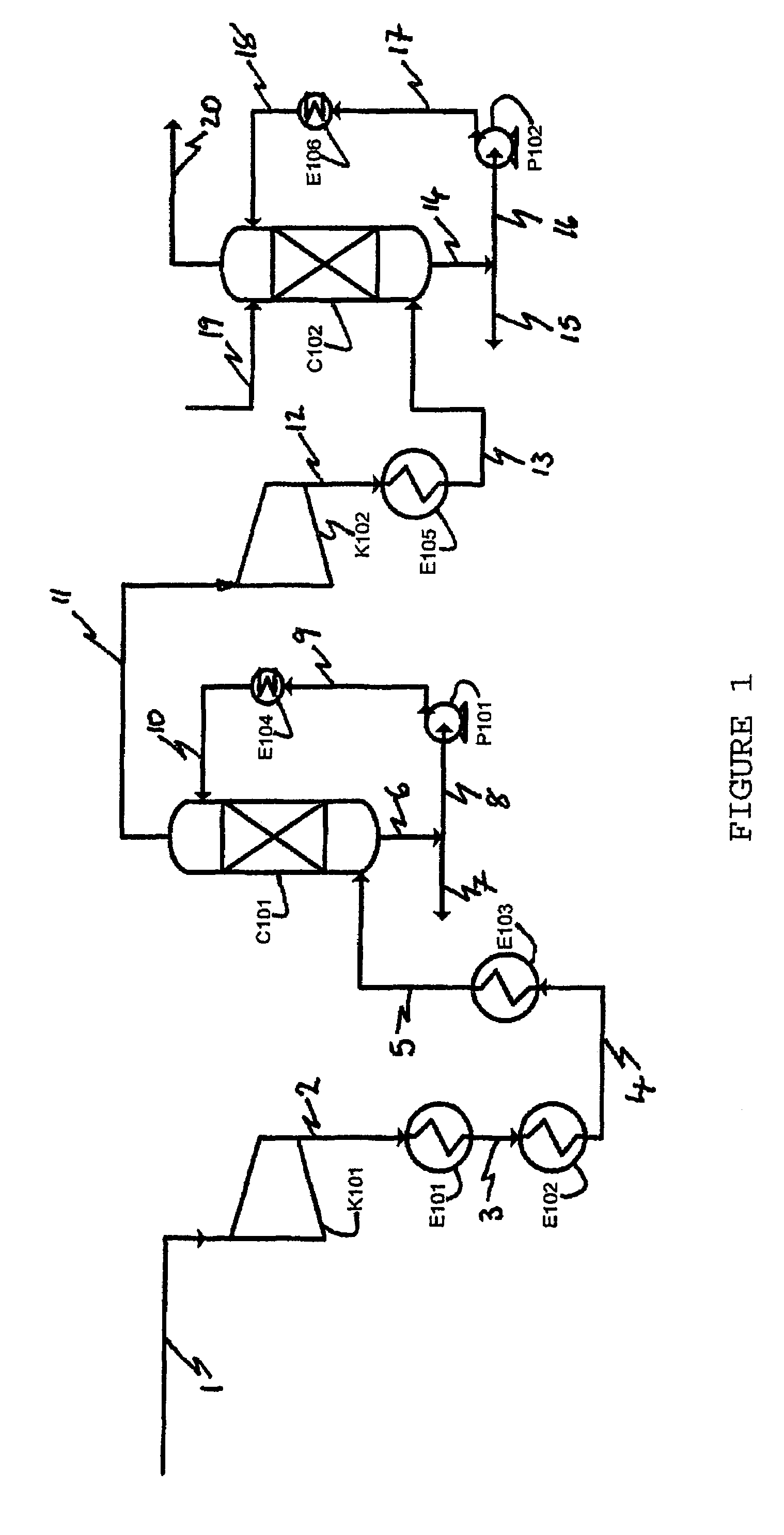
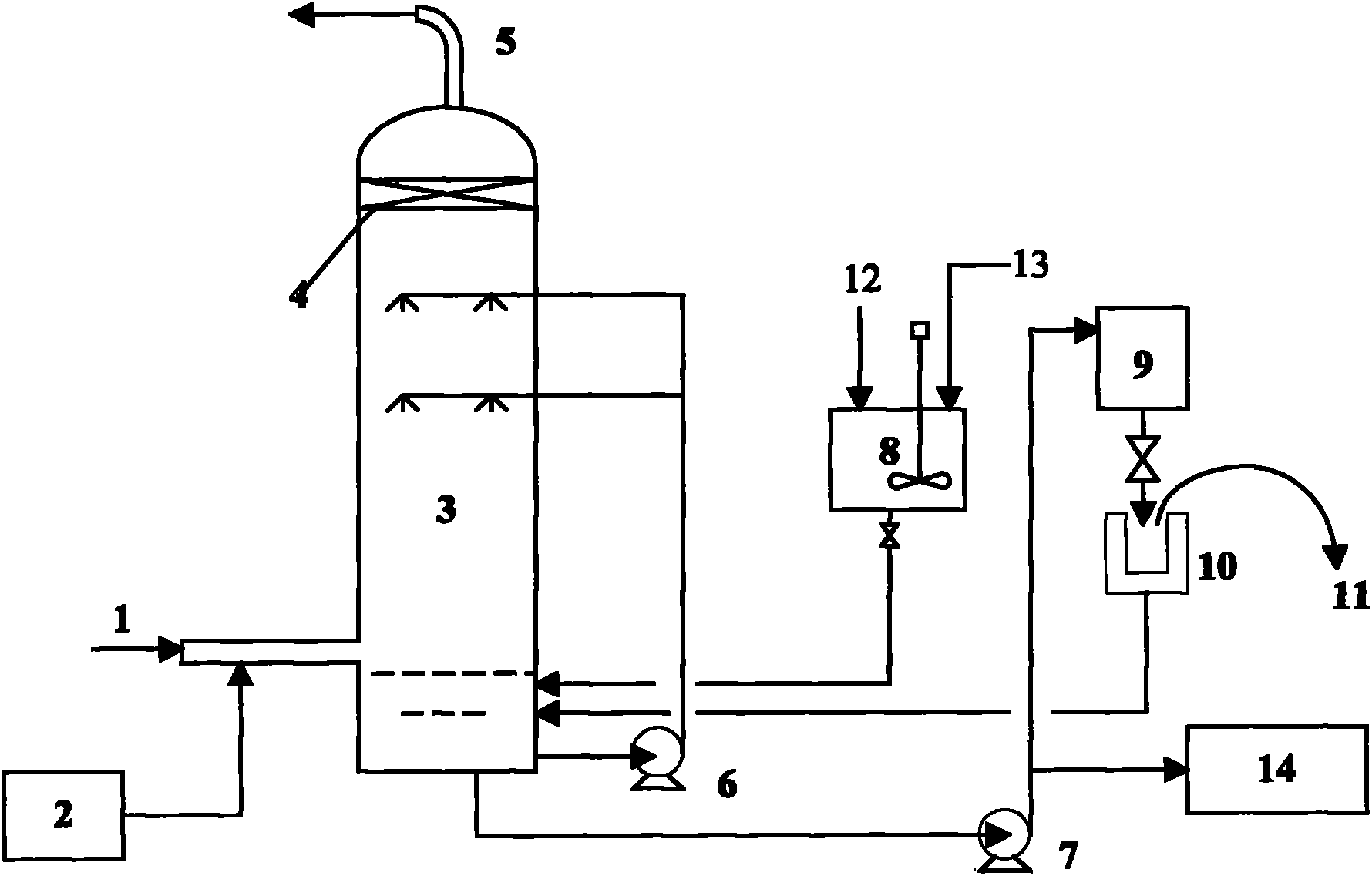
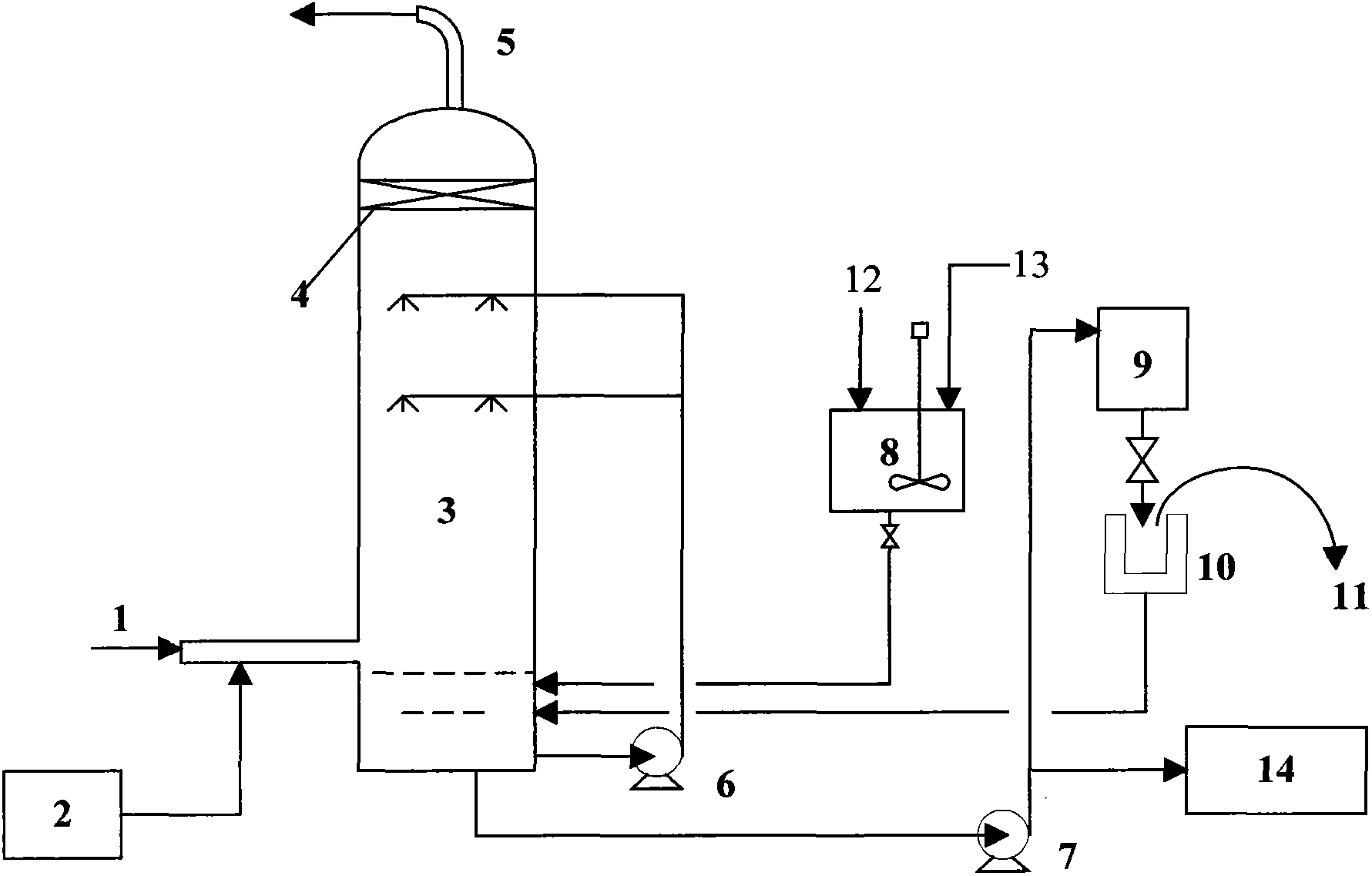
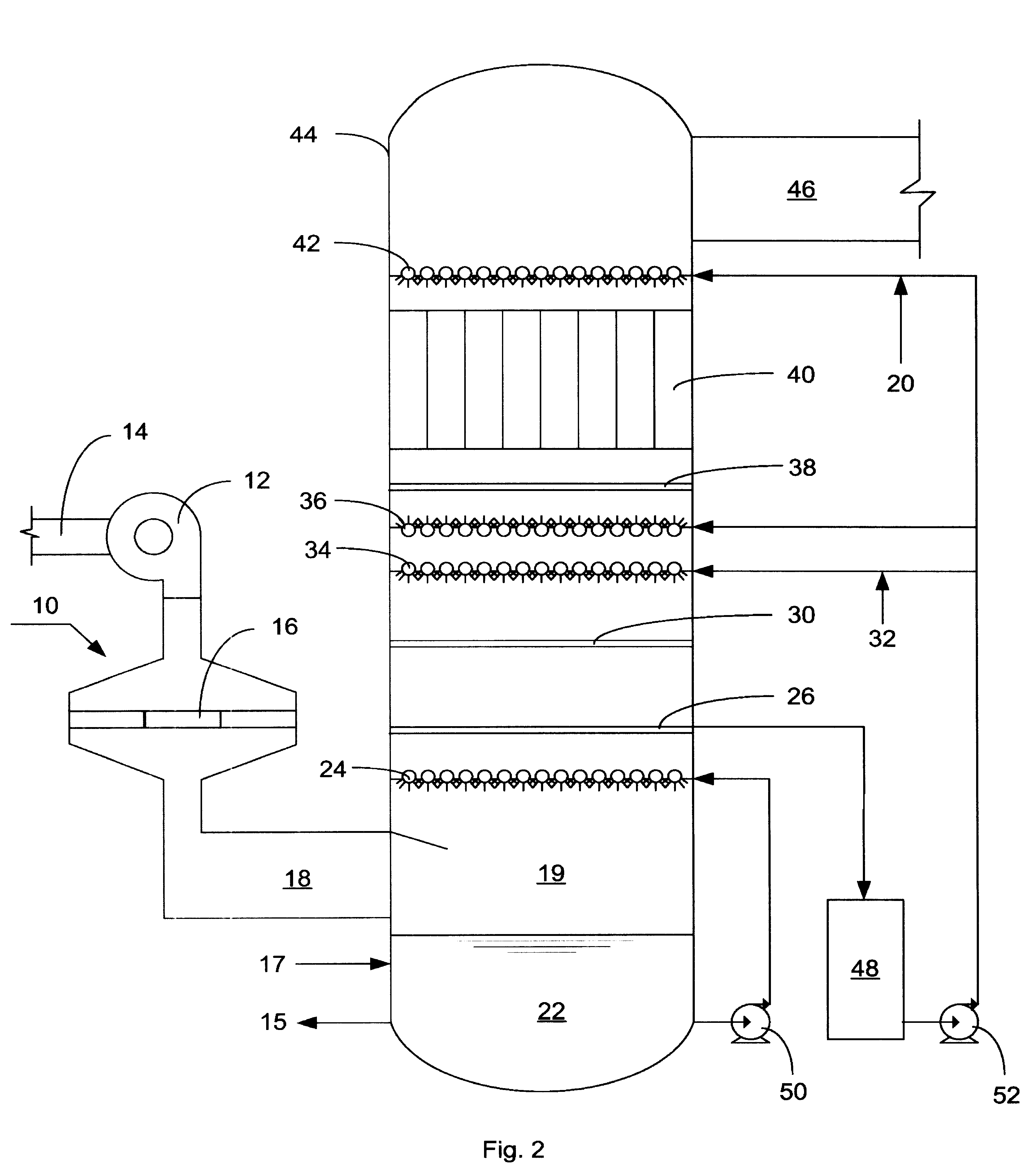
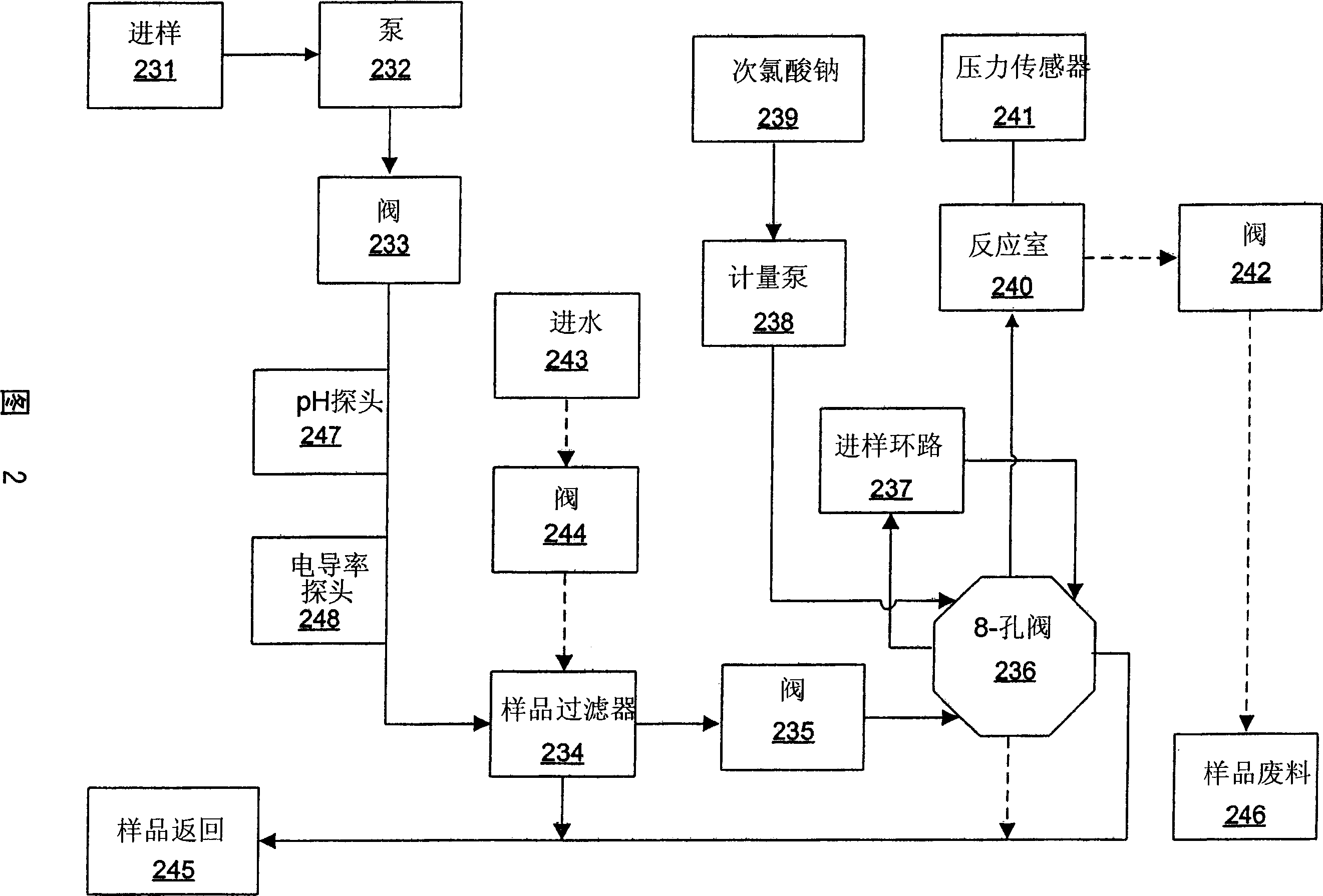
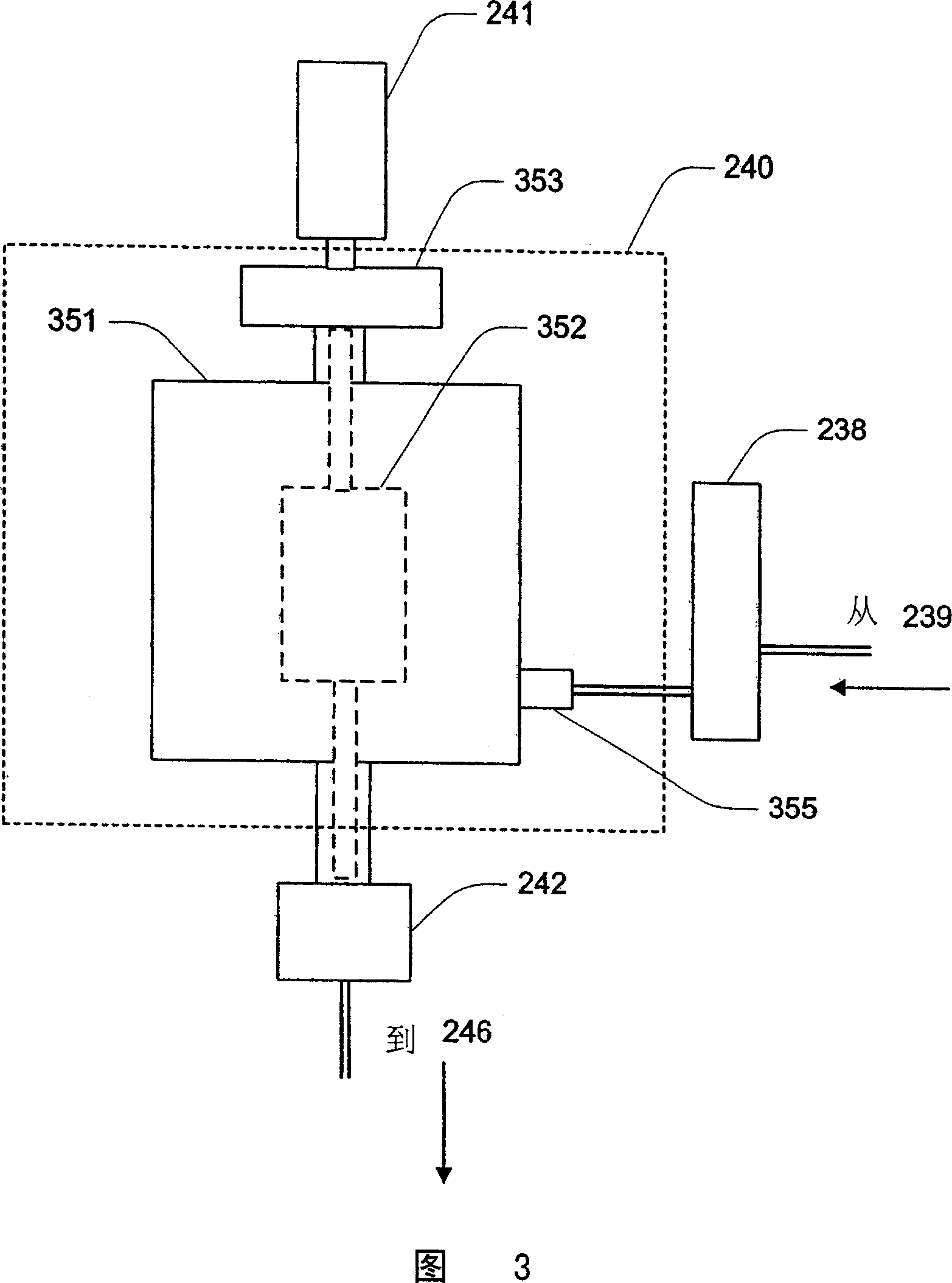
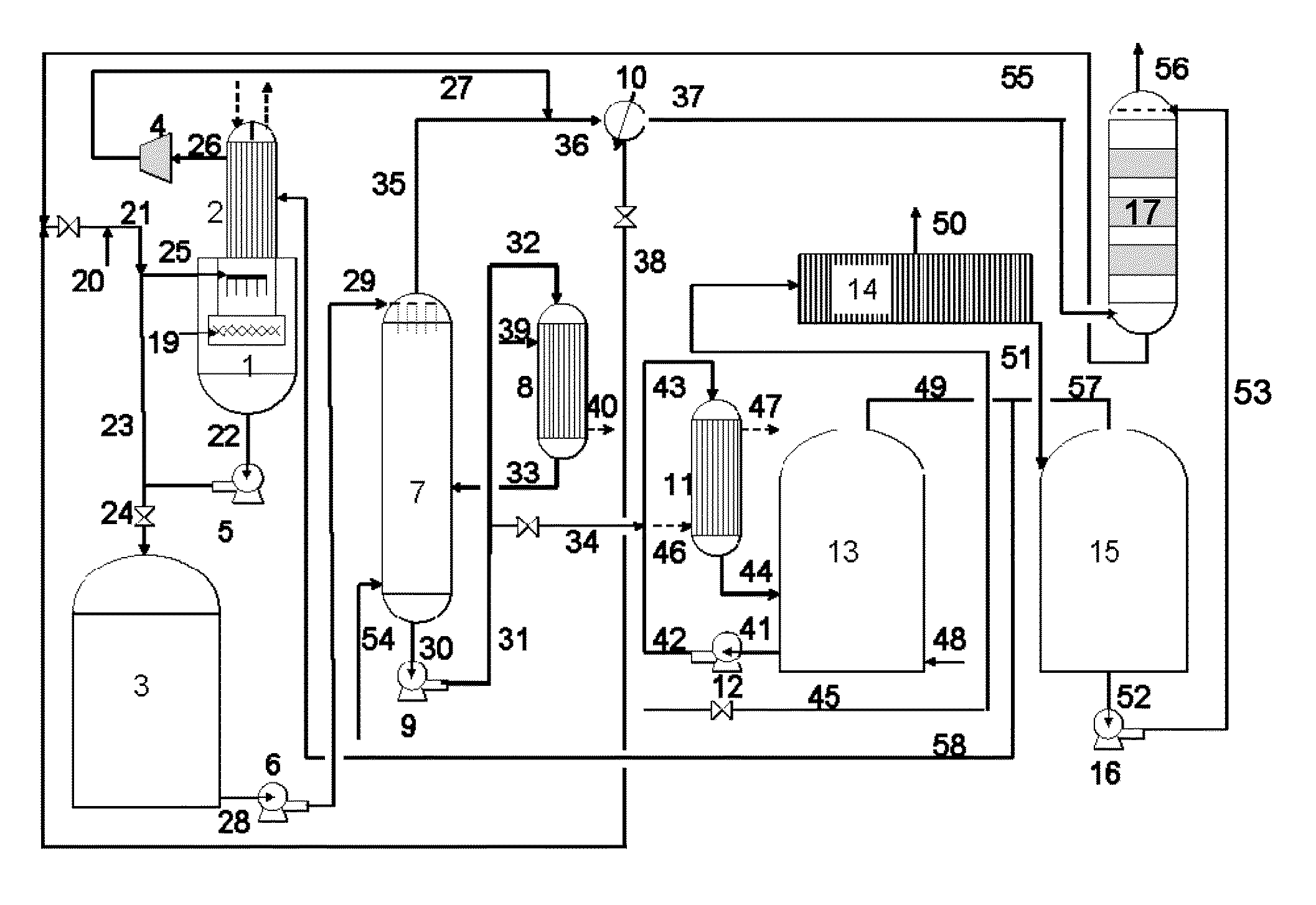
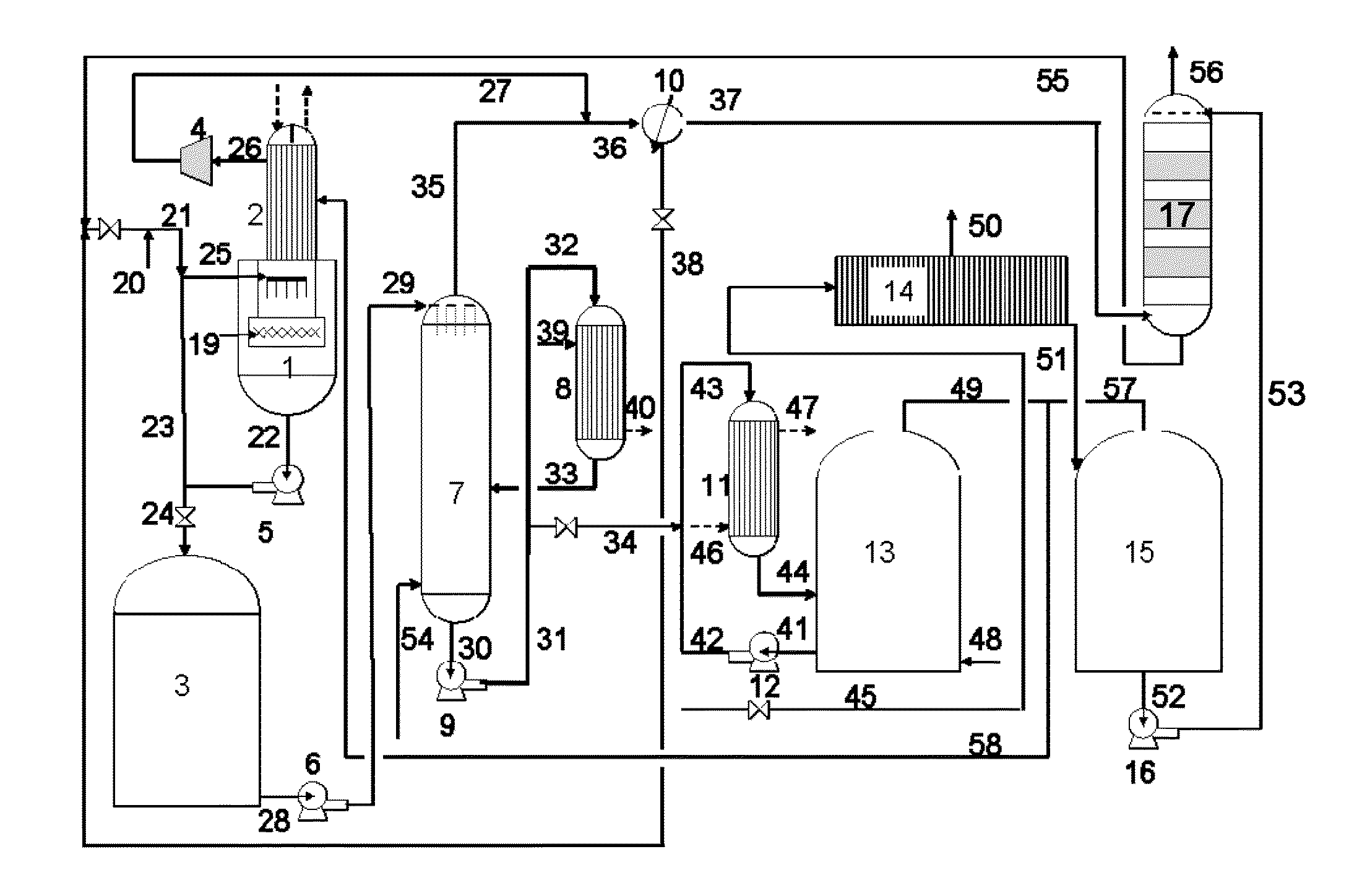
Apparatus for on-site production of nitrate ions
InactiveUS7514058B1Enhanced overall recoveryWater treatment parameter controlWater treatment compoundsMicrobial enhanced oil recoverySulfate-reducing bacteria
An apparatus and method produces nitrate ions on-site from water, natural gas and air extracted in proximity to the apparatus. The apparatus generates nitrate ions and brings the nitrate ions into contact with an aqueous system. Hydrogen sulfide present in the aqueous system is removed and the production of hydrogen sulfide by sulfate-reducing bacteria (SRB) is eliminated by introducing into the system nitrate ions, whereby denitrifying microorganisms, using nitrate, outcompete the sulfate-reducing bacteria for the available carbon nutrients, thus preventing the SRB from producing hydrogen sulfide. Nitrate ions generated by the apparatus and added to the aqueous system which contains the denitrifying microorganisms can enhance oil recovery by means of microbial enhanced oil recovery mechanisms.
Owner:NITRA GEN LLC
Purification of carbon dioxide
SO2 and / or NOx are removed from gaseous CO2 at elevated pressure(s) in the presence of molecular oxygen and water and, when SO2 is to be removed, NOx, to convert SO2 to sulfuric acid and / or NOx to nitric acid. The sulfuric acid and / or nitric acid is / are then removed from the gaseous carbon dioxide to produce SO2-free, NOx-lean carbon dioxide gas. The invention has particular application in the removal of SO2 and / or NOx from carbon dioxide flue gas produced in an oxyfuel combustion process, for example, in a pulverized coal fired power station.
Owner:AIR PROD & CHEM INC
Device and method for simultaneously desulfurizing and denitrifying flue gas by ozone catalytic oxidation process
InactiveCN102247750AEfficient oxidationDispersed particle separationSulfur-trioxide/sulfuric-acidCatalytic oxidationAbsorption of water
The invention relates to a flue gas pollutant treatment process and aims to provide a device and method for simultaneously desulfurizing and denitrifying a flue gas by ozone catalytic oxidation process. The device comprises a desulfurization and denitrification tower, an ozone generator, absorption liquid circulating equipment and desulfurization and denitrification by-product post-treatment equipment. Ozone enters from a flue or the lower part of the desulfurization and denitrification tower, a catalyst is added to an absorption liquid, and the absorption liquid is injected in from the upper part of the desulfurization and denitrification tower, so that SO2 and NO in the flue gas are oxidized by ozone with high efficiency under the action of the catalyst, and in combination with the absorption of water or alkaline substances, SO2 and NOx in the flue gas are recovered in the form of high value-added sulfuric acid and nitric acid products, or ammonium sulfate / ammonium nitrate mixed nitrogen fertilizers, potassium sulfate / potassium nitrate mixed potassium fertilizers or ammonium / potassium compound fertilizers respectively, thereby achieving resource recovery and value maximization of the desulfurization and denitrification process. The desulfurization and denitrification process provided by the invention has the advantages of simple structure, low investment and low operation cost. The desulfurization rate and the denitrification rate of the desulfurization and denitrification process provided by the invention can reach more than 96% and more than 90% respectively.
Owner:EAST CHINA UNIV OF SCI & TECH
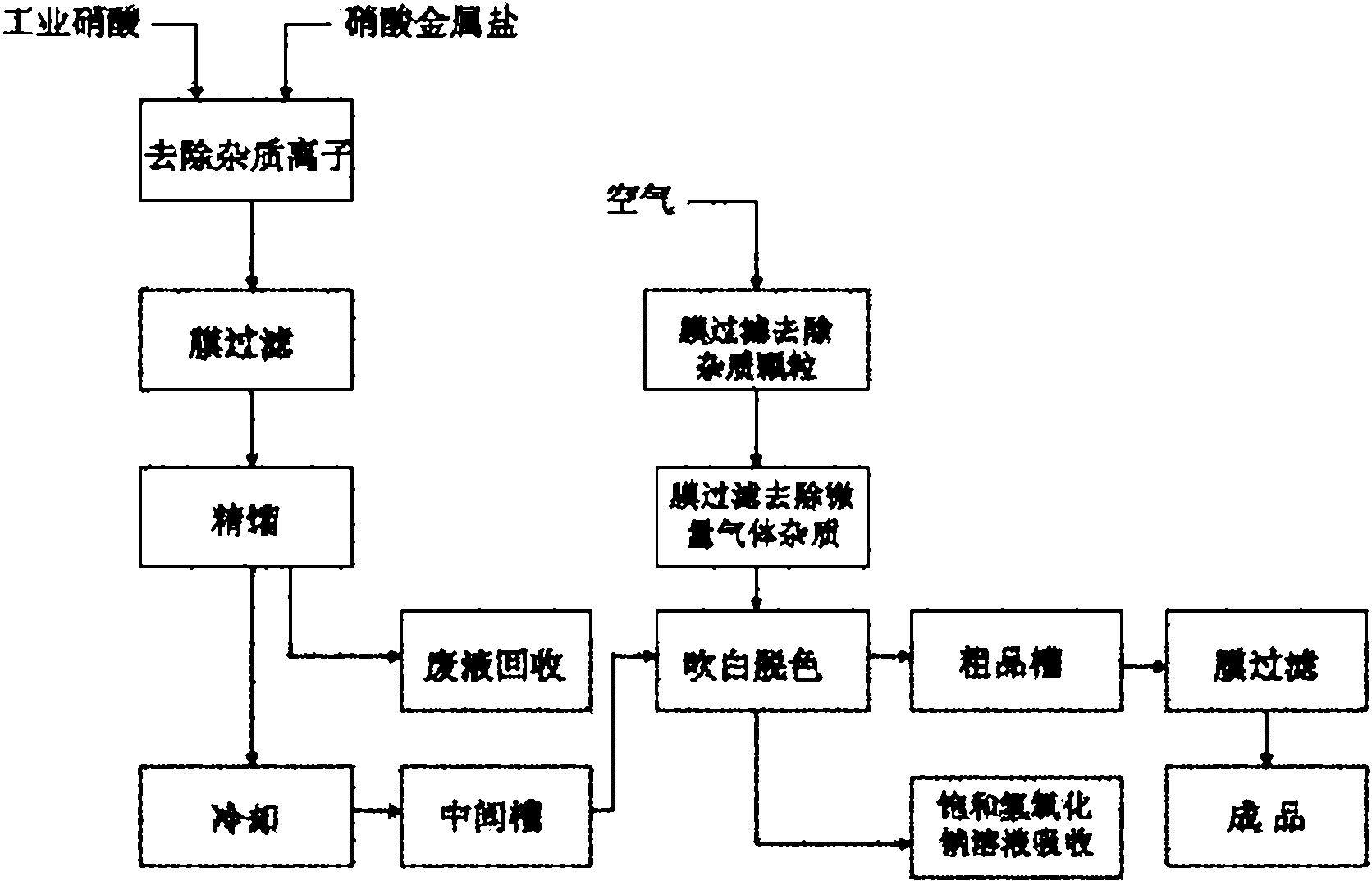
Process for preparing ultrapure nitric acid
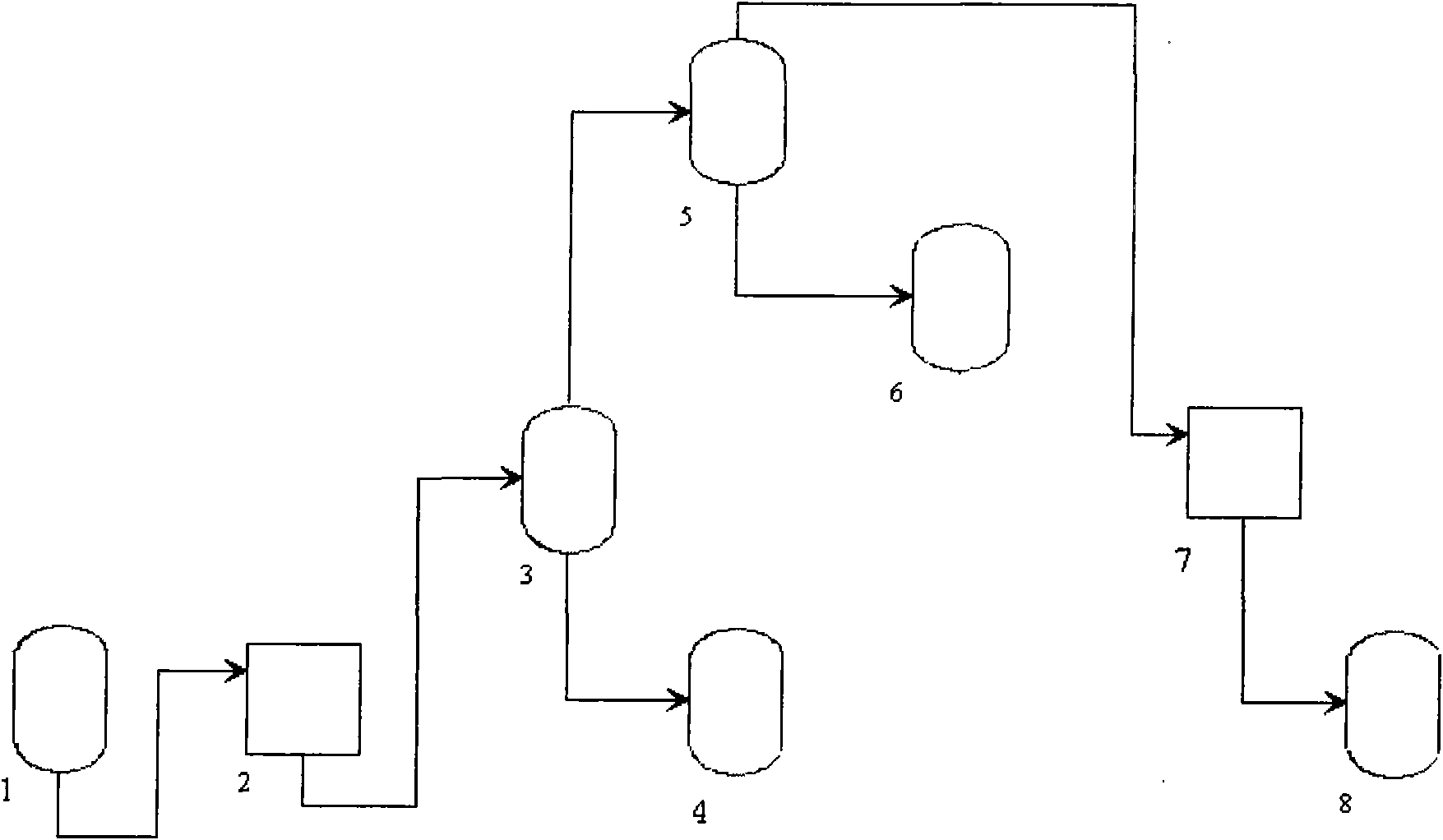
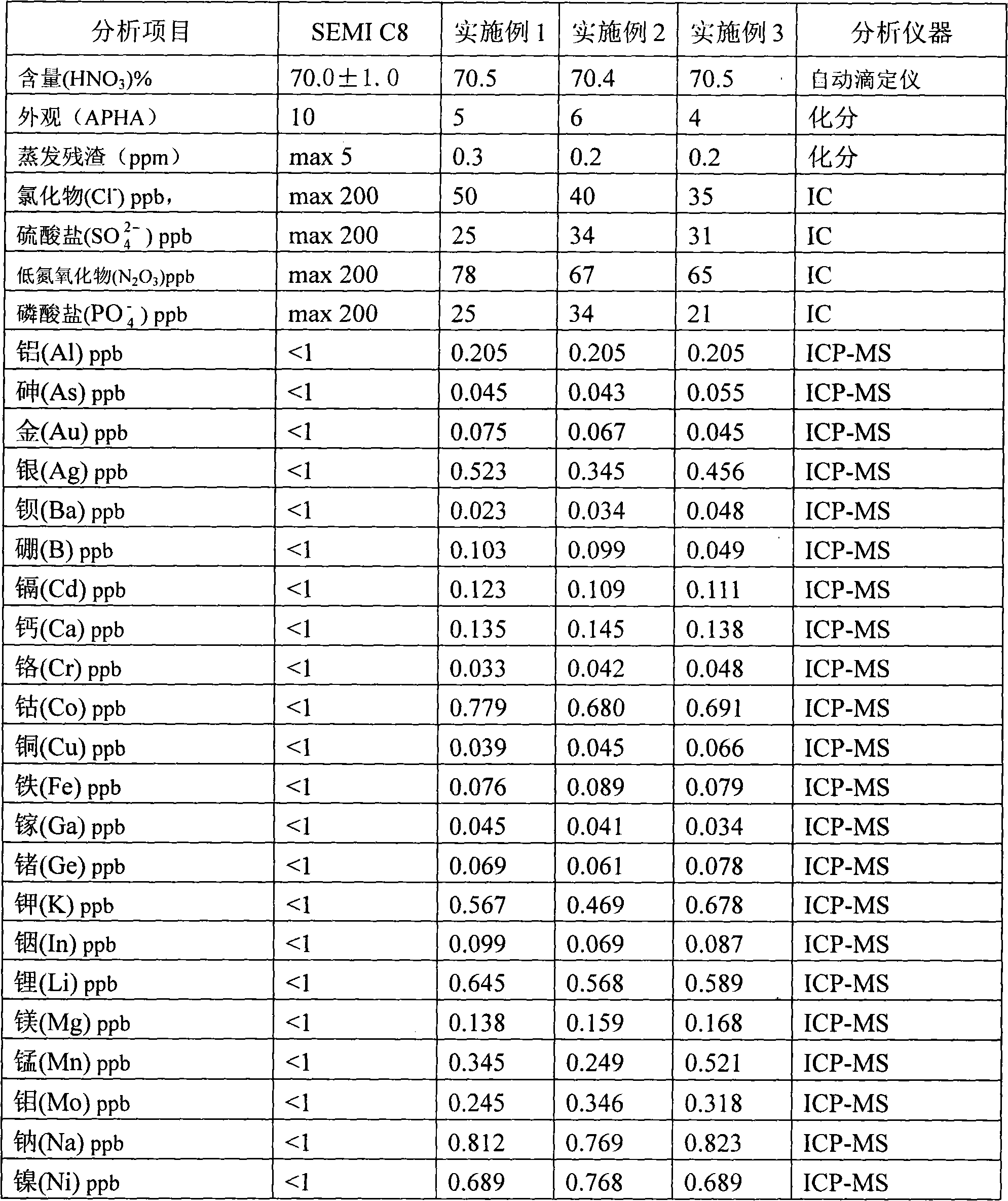
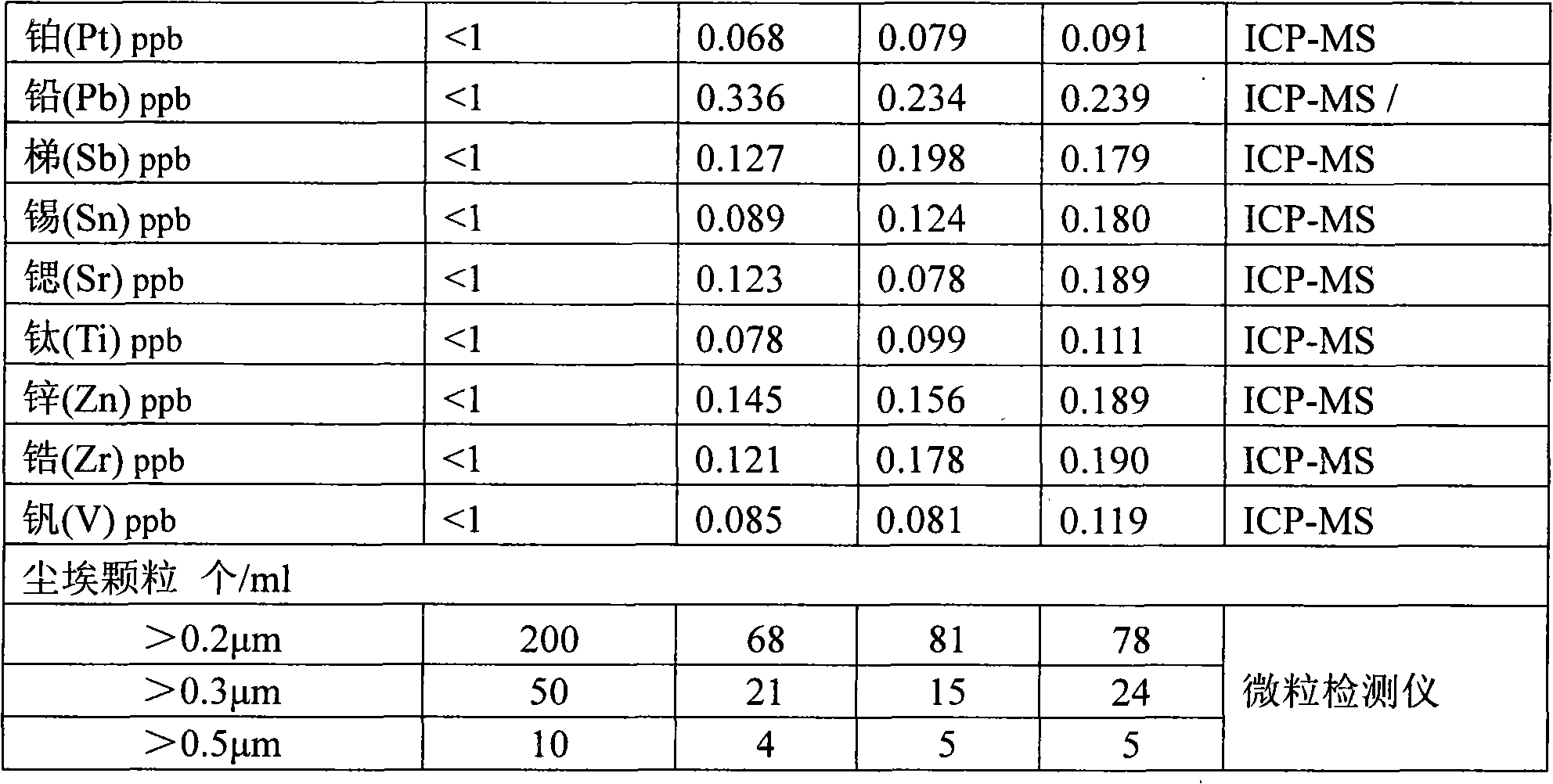
InactiveCN102001635AEfficient removalSolve the problem of high content of impurity ionsNitric acidInorganic saltsNitrogen oxides
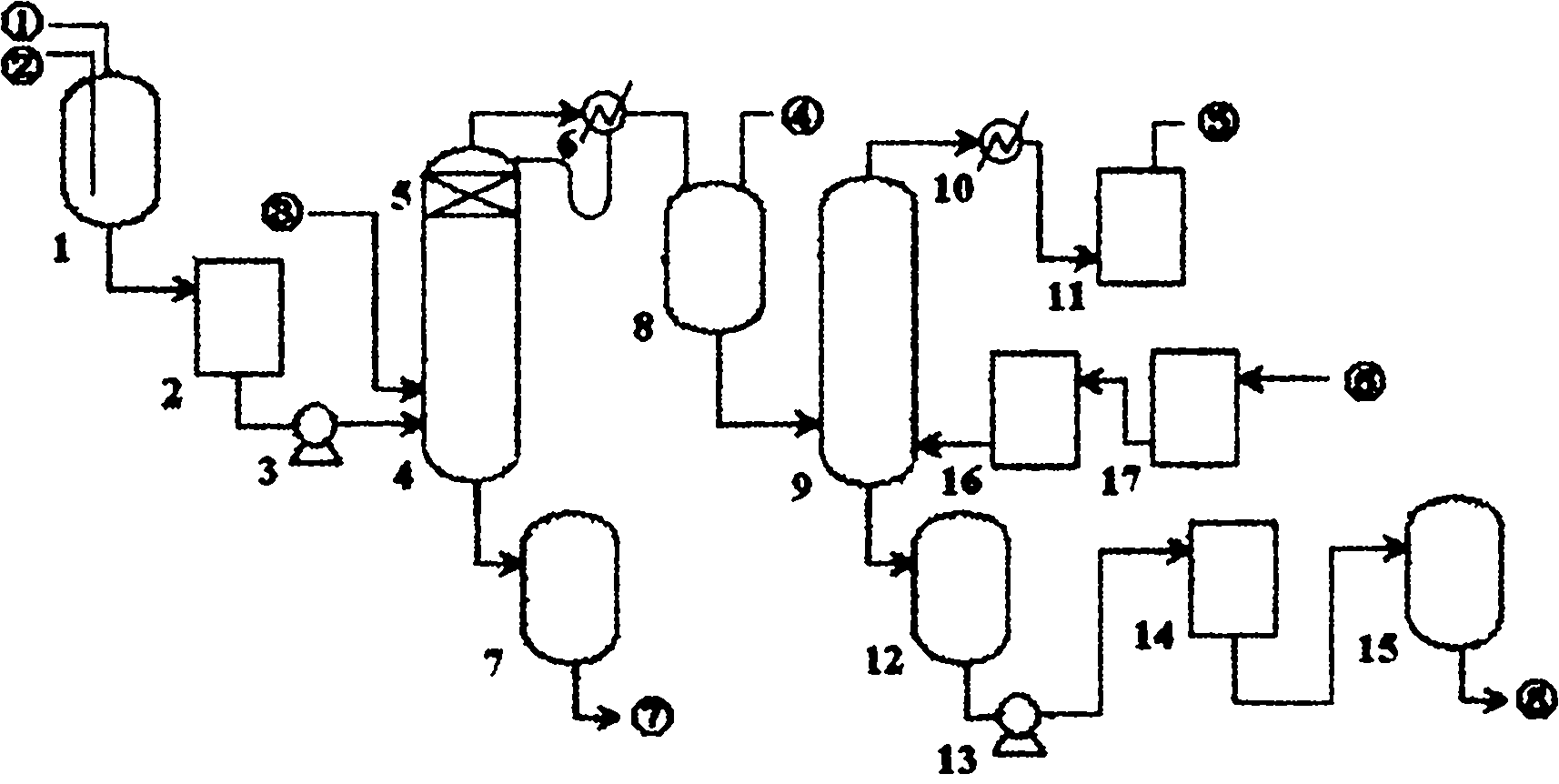
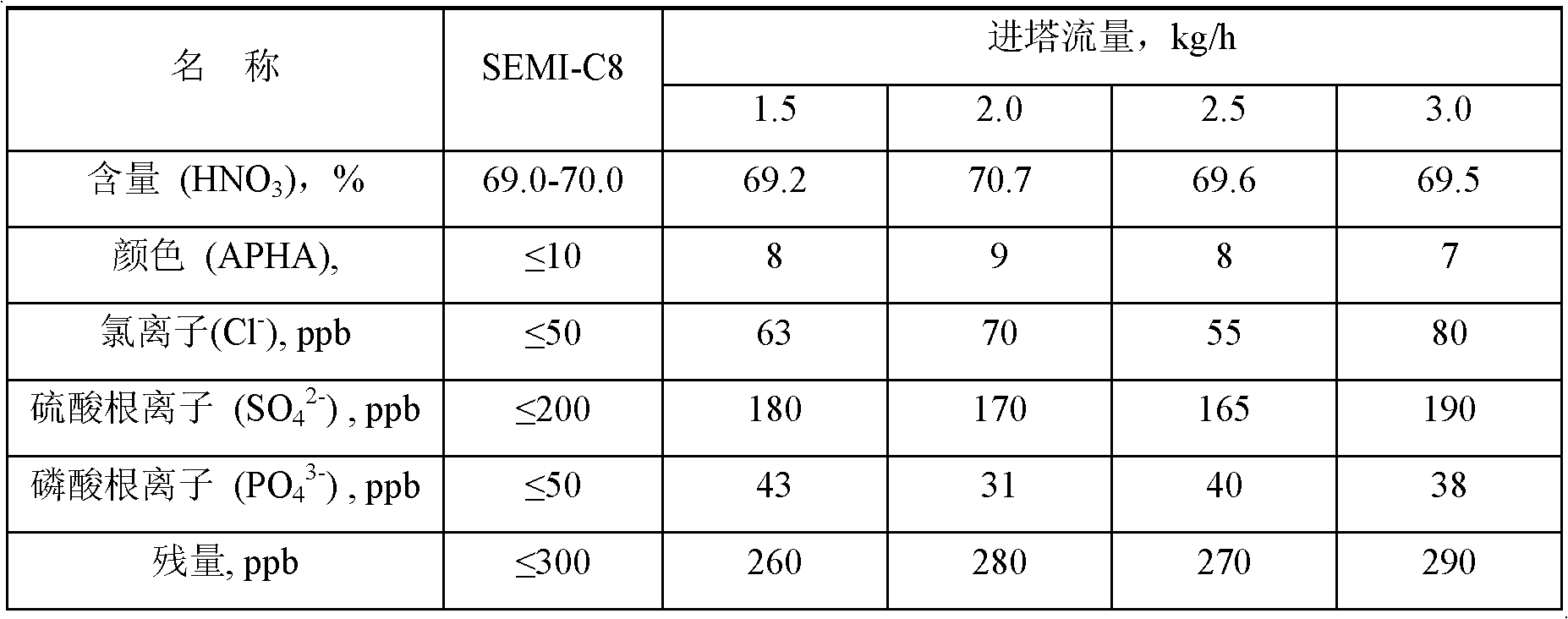
The invention discloses a process for preparing ultrapure nitric acid. The method comprises the following steps of: adding nitric acid metal salt to react with impurity anions, namely Cl<-> and SO4<2-> in industrial nitric acid serving as a raw material, precipitating, performing membrane filtration purification, rectifying to remove inorganic salt, regulating rectification liquid to form 69 to 70 weight percent nitric acid by using ultrapure water, white-blowing by using purified air in a white-blowing tower, absorbing blown nitrogen oxide by using saturated aqueous solution of sodium hydroxide, adding white-blowing liquid into a crude product groove, and performing membrane filtration to obtain the ultrapure nitric acid. The detection shows that: the content of the ultrapure nitric acidprepared by the process reaches 69 to 70 weight percent and various indexes of the product all meet the semiconductor equipment and material international (SEMI) C8 standard. The rectification is performed and waste acid is collected simultaneously, and the waste acid is treated and recycled, so that the quality and productivity of the product are improved; and the product has stable quality, lowimpurity content and high purity and is suitable for large-scale and continuous production.
Owner:SHANGHAI CHEM REAGENT RES INST
NOx, Hg, and SO2 removal using ammonia
InactiveUS6991771B2Raise the ratioSmall sizeInternal combustion piston enginesExhaust apparatusFlue gasAmmonia
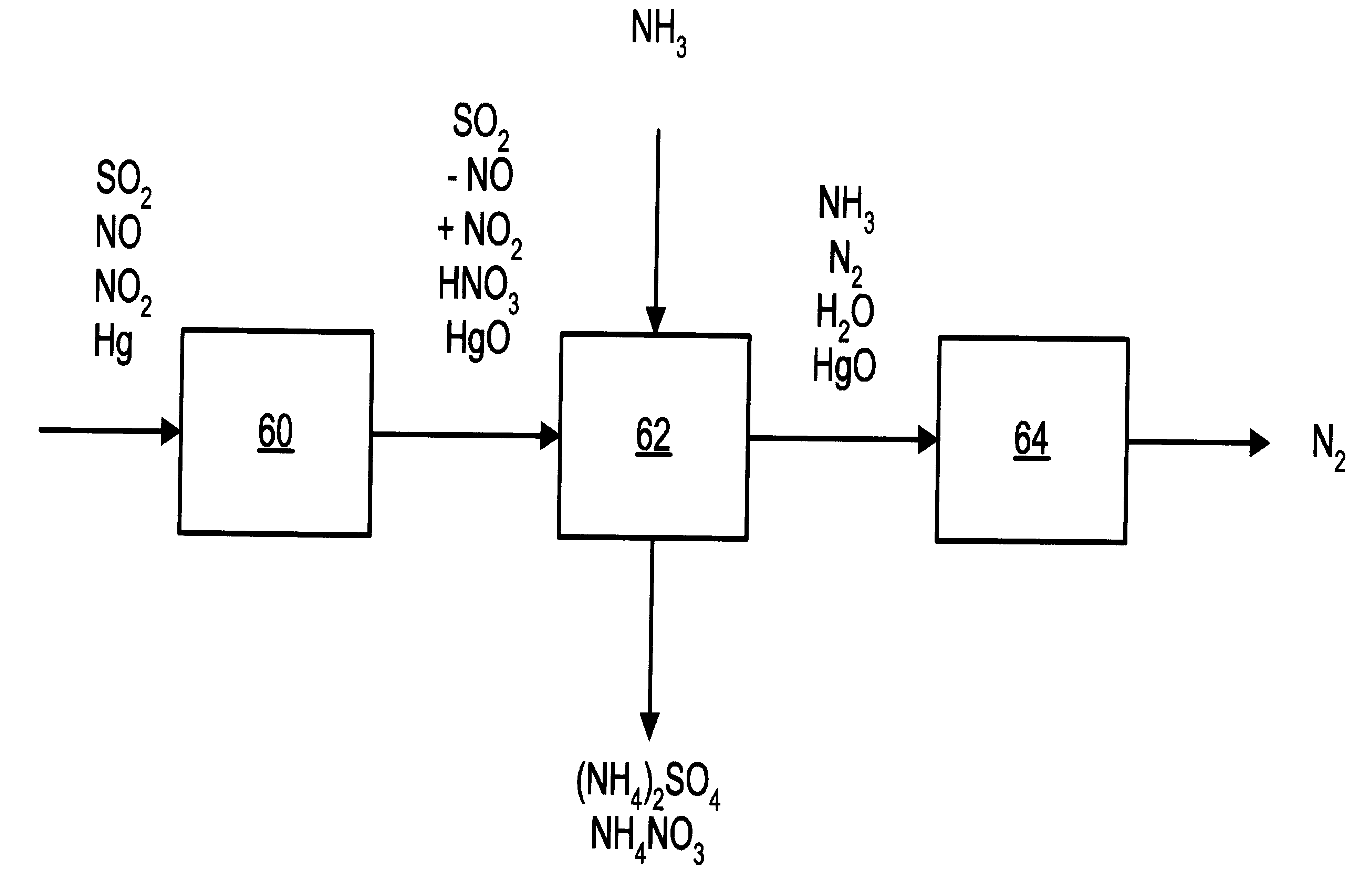
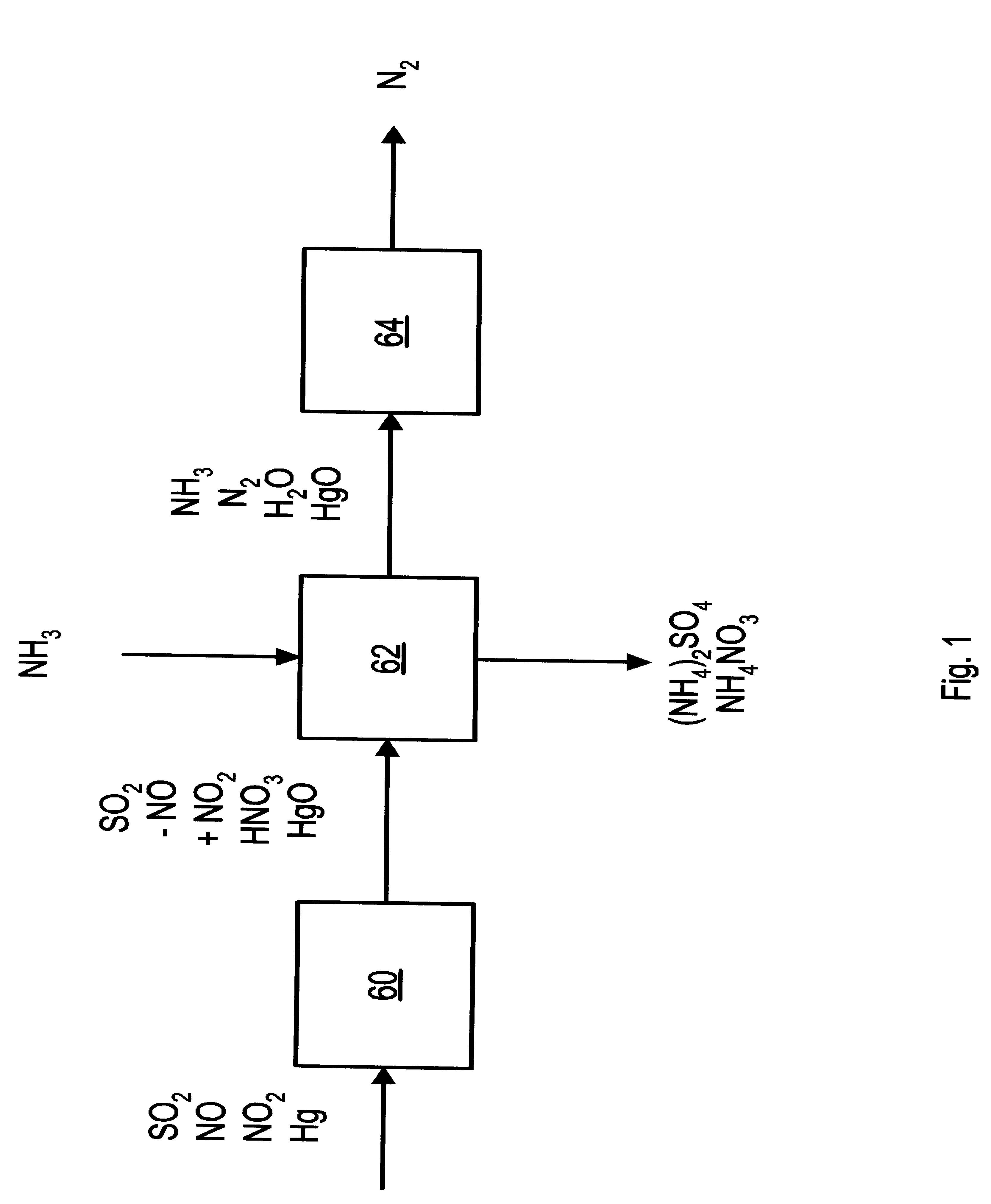
A process and apparatus for removing SO2, NO, and NO2 from a gas stream having the steps of oxidizing a portion of the NO in the flue gas stream to NO2, scrubbing the SO2, NO, and NO2 with an ammonia scrubbing solution, and removing any ammonia aerosols generated by the scrubbing in a wet electrostatic precipitator. The process can also remove Hg by oxidizing it to HgO and removing it in the wet electrostatic precipitator. Ammonium sulfate, a valuable fertilizer, can be withdrawn from the scrubbing solution.
Owner:POWERSPAN CORP
Nitric acid production and recycle
InactiveUS6264909B1Effectively and economicallyRate efficient and rapidUsing liquid separation agentProcess efficiency improvementRadiochemistryNOx
An improved process for either the manufacturing of nitric acid, recycling of nitric acid, or recovering of nitric acid, comprising the steps of: providing a source of NOx; reacting NO from the source of NOx with HNO3 in the presence of NO2- to produce a resulting product; and reacting the resulting product with O2 and H2O to produce nitric acid.
Owner:DRINKARD METALOX
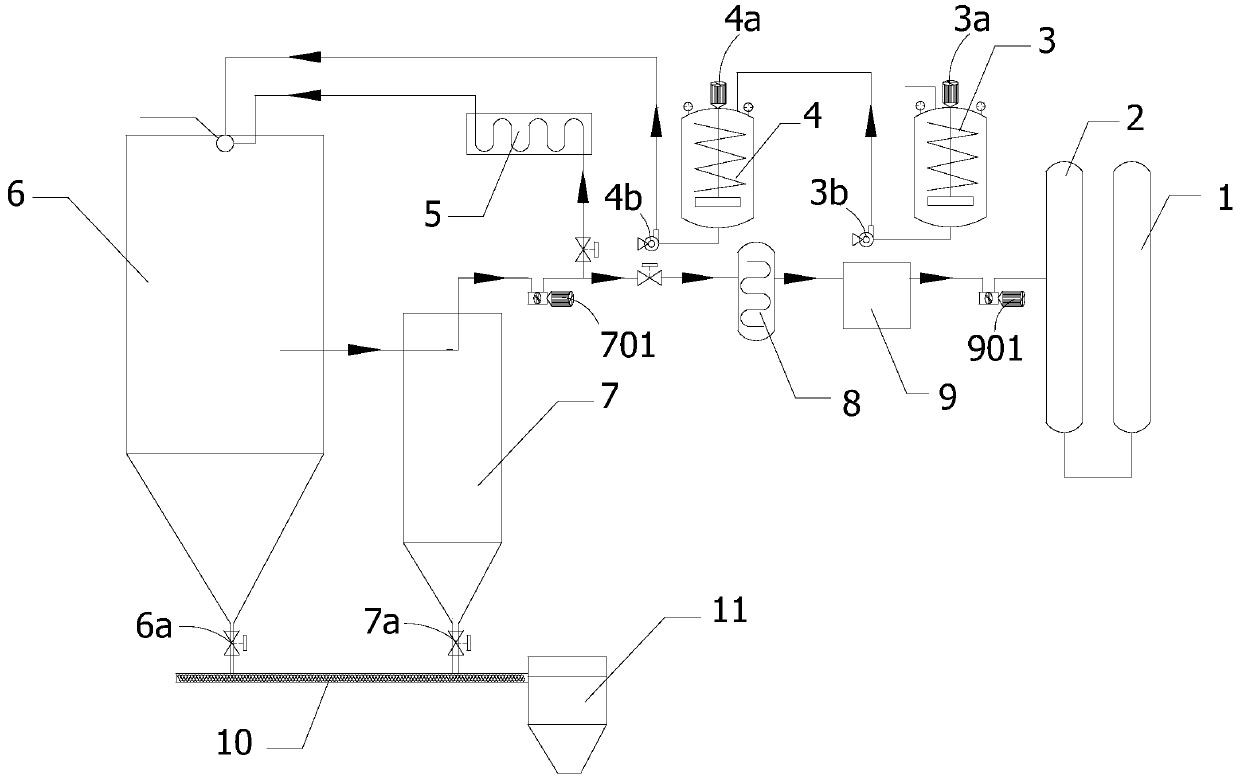
Method and device system for recovering nitric acid through pyrolyzing nitrate
ActiveCN109721038AImprove recycling ratesFull atomization heating decompositionOxide/hydroxide preparationChemical industryNitrateInternal temperature
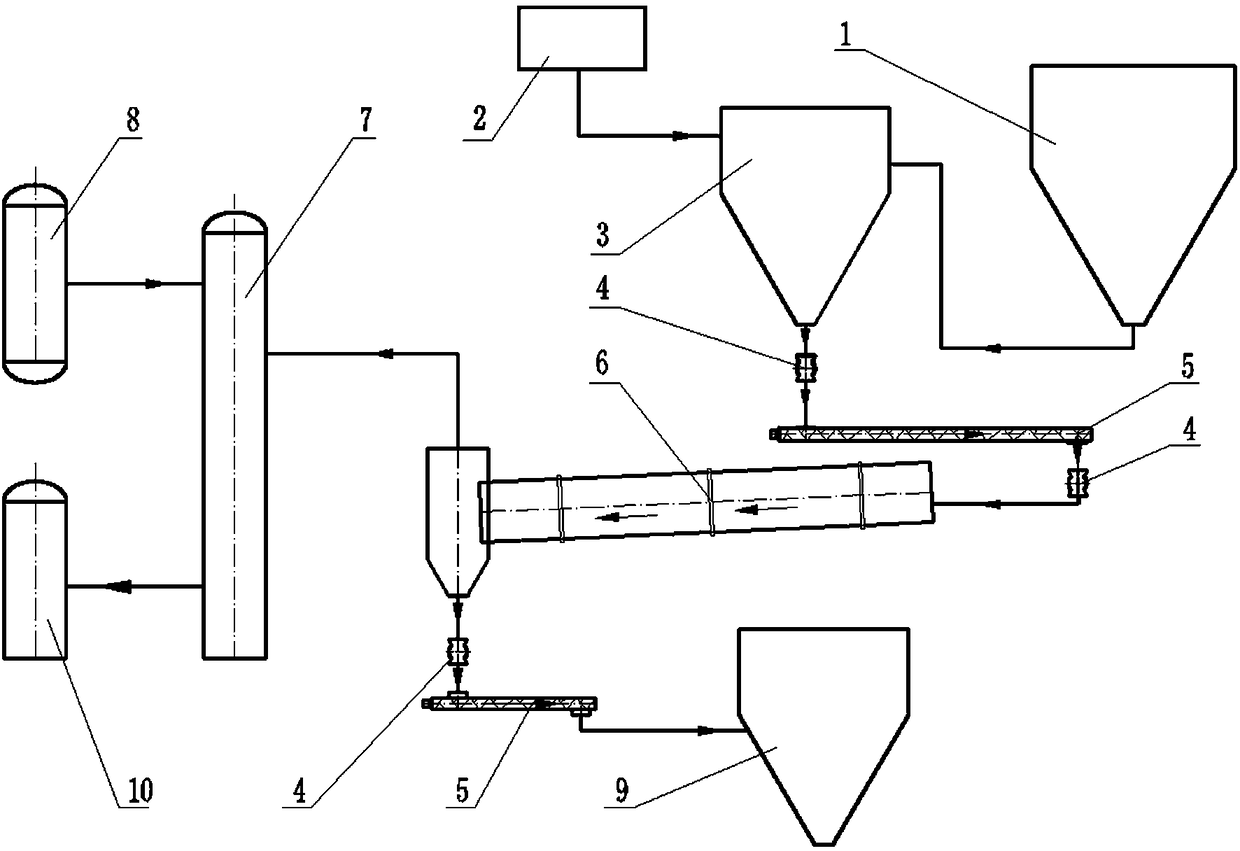
The invention discloses a method for recovering nitric acid by pyrolyzing nitrate. The method comprises the following steps: (1) conveying the nitrate into at least two preheaters, heating the nitrateto firstly liquefy the nitrate, and then heating the nitrate to a temperature less than decomposition temperature in order to obtain a nitrate hot fluid; (2) conveying the nitrate hot fluid into a decomposer, and heating the nitrate hot fluid with a high temperature gas to maintain the internal temperature of the decomposer at 500-800 DEG C in order to decompose the nitrate into a mixed gas and asolid powder; and (3) separating mixed gas and the solid powder, conveying a part of the mixed gas into a nitric acid recovery tank, heating the remaining mixed gas to 500-800 DEG C, and then returning the heated remaining mixed gas into the decomposer to heat the nitrate hot fluid in order to thermally decompose the nitrate hot fluid. The method for recovering nitric acid has the advantages of less corrosion damages to devices, no introduction of other impurity components and no interference in the heating process, good decomposition speed and decomposition rate of the nitrate, and high recycling rate of the nitric acid.
Owner:MEISHAN SHUNYING POWER BATTERY MATERIALS CO LTD
Method and device for preparing high-concentration dilute nitric acid
The invention discloses a method and device for preparing high-concentration dilute nitric acid. The method comprises the following steps: carrying out ammonia-air mixing, ammoxidation, condensation separation and fine separation on air subjected to impurity removal and dehumidification and liquid ammonia subjected to impurity removal, oil removal and evaporation, absorbing in an absorption tower in which 40 tower plates are arranged, meanwhile, carrying out cooling heat exchange by a three-stage water circulation technique to obtain the high-concentration dilute nitric acid of which the concentration is not lower than 68-70%. The method and device effectively enhance the nitric acid absorption efficiency, enhance the concentration of the dilute nitric acid, and can better satisfy the demands of modern industry for high-concentration dilute nitric acid.
Owner:TIANJI COAL CHEM IND GROUP
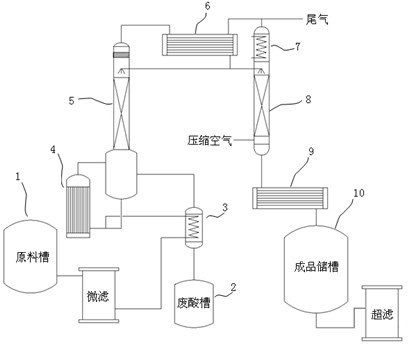
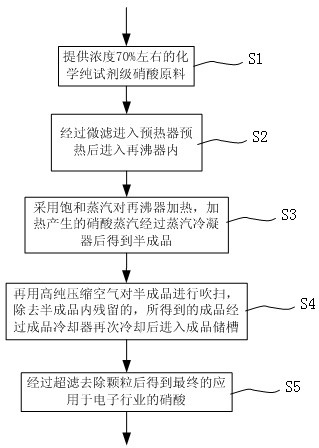
Method for producing electronic-grade nitric acid
The invention discloses a method for producing electronic-grade nitric acid. The method sequentially comprises the following steps of: a) providing a chemically pure reagent-grade nitric acid raw material of which the concentration is about 70 percent; b) performing microfiltration on the nitric acid raw material, adding the nitric acid raw material into a pre-heater for preheating, and adding into a reboiler; c) heating the reboiler with saturated vapor, and making nitric acid vapor generated by heating pass through a vapor condenser to obtain a semi-finished product; d) blowing the semi-finished product with high-purity compressed air to remove residual vapor in the semi-finished product, cooling the obtained finished product again with a finished product cooler, and adding the finishedproduct into a finished product storage tank; and e) performing ultrafiltration on the finished product to remove particles so as to finally obtain the nitric acid applied in the electronic industry.In the method for producing the electronic-grade nitric acid, the normal chemically pure reagent-grade nitric acid raw material of which the concentration is about 70 percent is directly rectified and then the rectified product is blown with the high-purity compressed air. The used raw material is safe; the pressure for the subsequent tail gas treatment is greatly reduced; and the production costis reduced.
Owner:SHANGHAI ZHENGFAN TECH
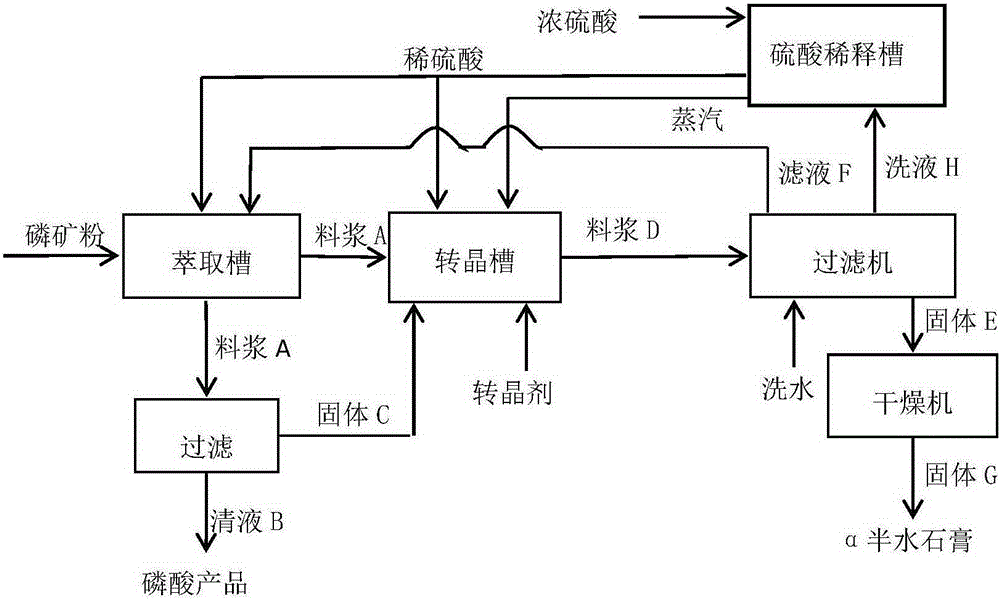
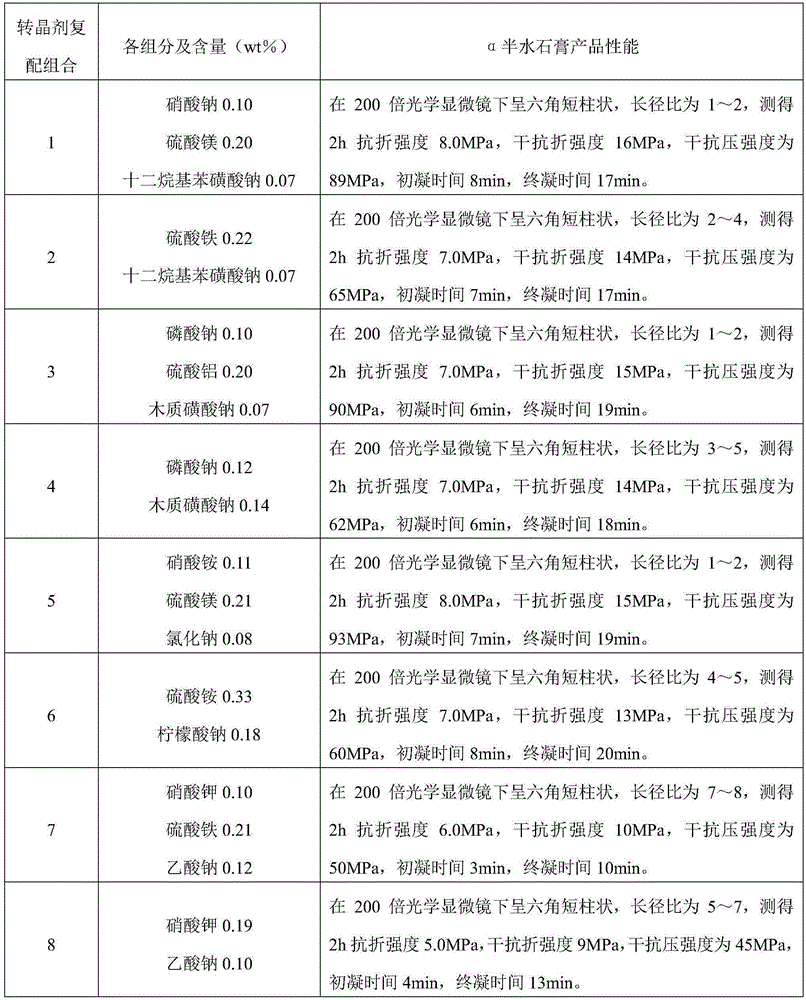
Production method for by-product alpha-hemihydrate gypsum of wet-process phosphoric acid
ActiveCN105253867ACrystalline form does not changeForm won't changeCalcium/strontium/barium sulfatesPhosphorus compoundsPhosphatePhosphoric acid
The invention discloses a production method for a by-product alpha-hemihydrate gypsum of wet-process phosphoric acid. The method comprises the following steps: adding ground phosphate rock and a part of dilute sulfuric acid into an extraction tank, carrying out an extraction reaction, separating a clear liquid from obtained mixed slurry, using the clear liquid as a finished product phosphoric acid, sending the phosphoric acid into an acid pool, and transferring a separated solid and residual mixed slurry into a crystal transformation tank together; adding sulfuric acid and a crystal transformation agent into the crystal transformation tank, carrying out a crystal transformation reaction at 60 to 130 DEG C for 1.5 to 7.5 h, subjecting obtained mixed acid slurry to solid-liquid separation, and subjecting the obtained solid to drying so as to obtain gypsum powder or adding water into the solid without drying so as to prepare gypsum products like gypsum boards, gypsum building blocks and gypsum members. The production method provided by the invention improves traditional technology and the prior art, eliminates discharge of phosphogypsum and acid non-soluble substances, reduces the content of phosphorus in gypsum, improves the utilization rate of phosphorus, makes full use of dilution heat of concentrated sulfuric acid and realizes energy saving and emission reduction.
Owner:贵州正磷科技有限公司
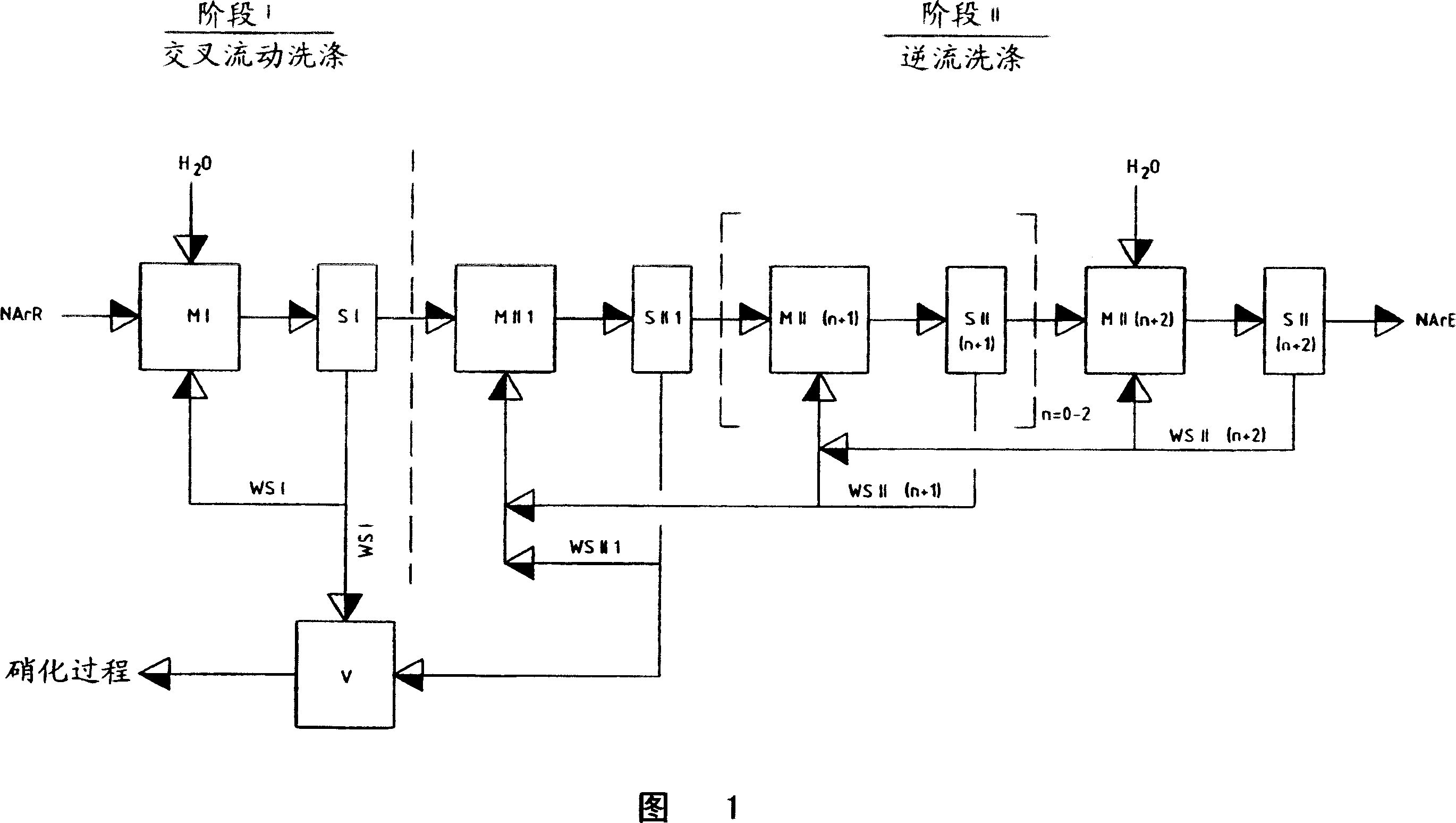
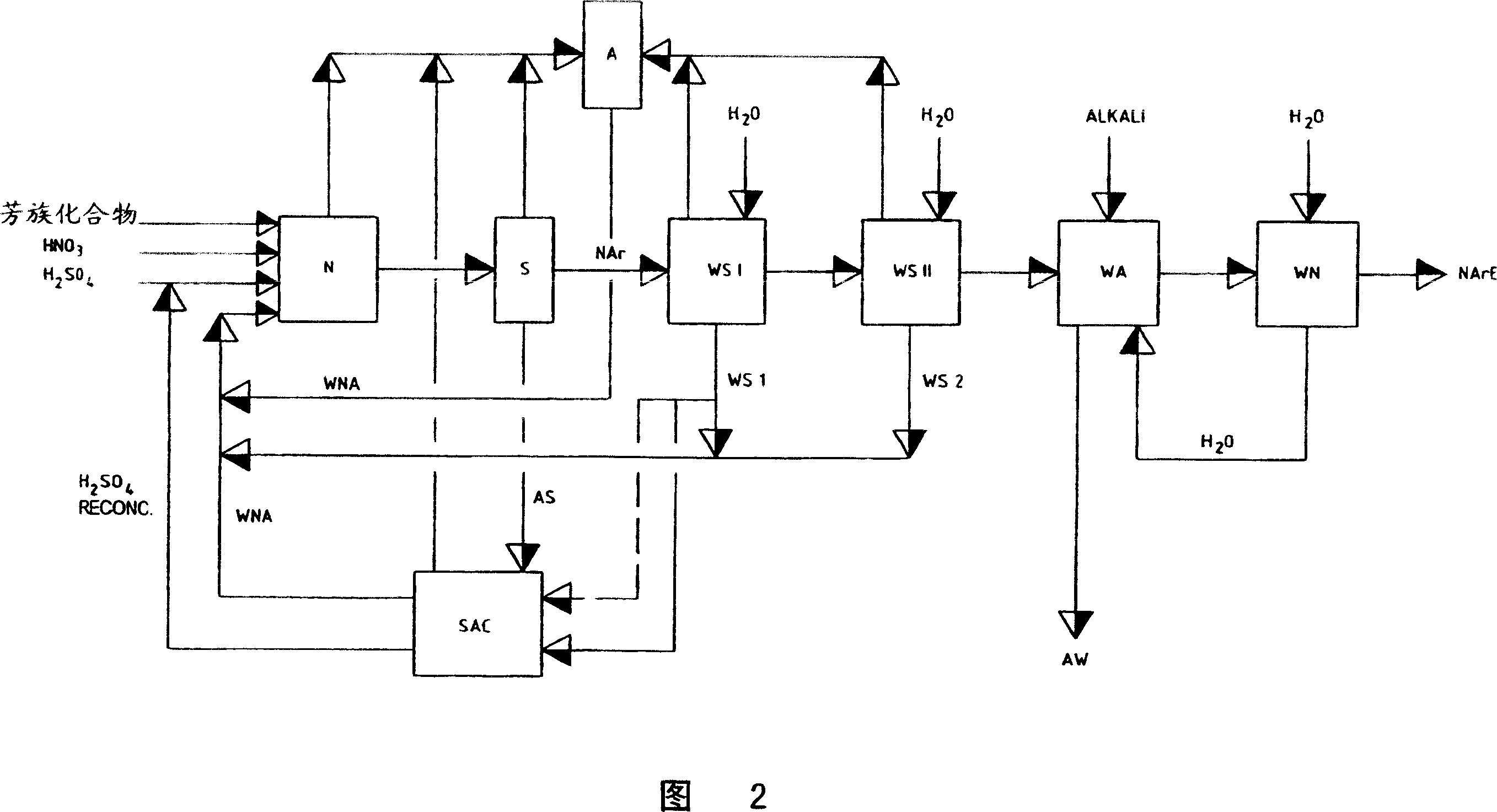
Recovery of the nitration acid mixtures from nitration processes
ActiveCN1951802AAvoid repetitionOrganic compound preparationSulfur-trioxide/sulfuric-acidProduction lineNitration
Removing and recovering nitrating acid mixtures of nitric acid, sulphuric acid and oxides of nitrogen, from the nitrated crude products occurring in the nitration of nitratable aromatic compounds, after the nitrating acid has been separated off, by a multistage extraction process involves one stage of a cross-flow extraction and another stage of a countercurrent extraction. An independent claim is also included for production plant for nitrating nitratable aromatic compounds with subsequent purification of the nitrated products, including removal and recovery of nitrating acid mixtures of sulphuric acid, nitric acid and oxides of nitrogen, comprises: (a) a nitrating unit for nitrating nitratable aromatic compounds, having at least one appropriate reaction containers for carrying out the nitration reactions; and (b) arranged in the production line downstream of the nitrating unit, a unit for carrying out acidic scrubbing by units of extraction and is comprising a cross-flow extraction unit for carrying out acidic scrubbing of nitrated crude products by units of cross-flow extraction, and in the production line downstream of the cross-flow extraction unit, a countercurrent extraction unit for carrying out acidic scrubbing of the nitrated crude products, scrubbed beforehand by units of cross-flow extraction, by units of countercurrent extraction.
Owner:JOSEF MEISSNER
Joint production method and production device of dilute nitric acid and concentrated nitric acid
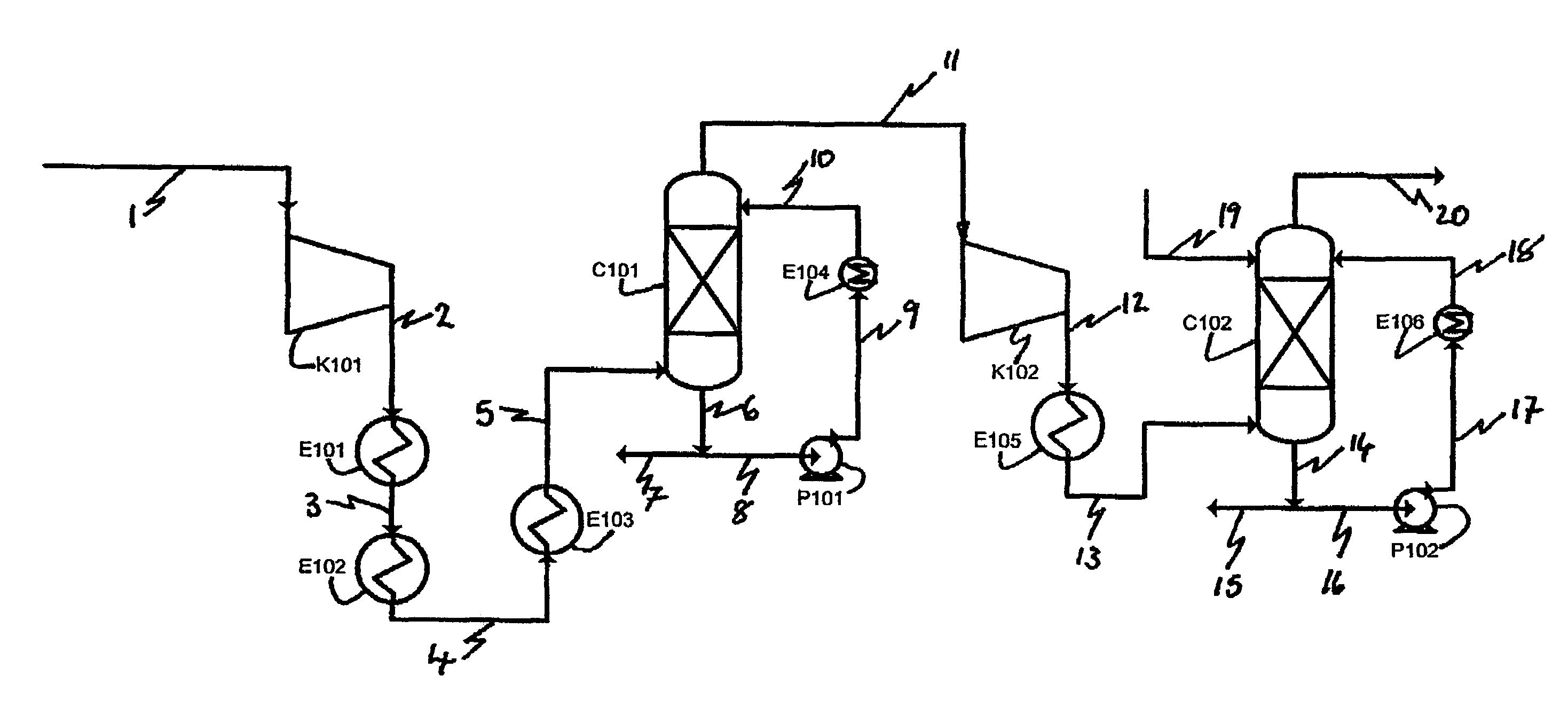
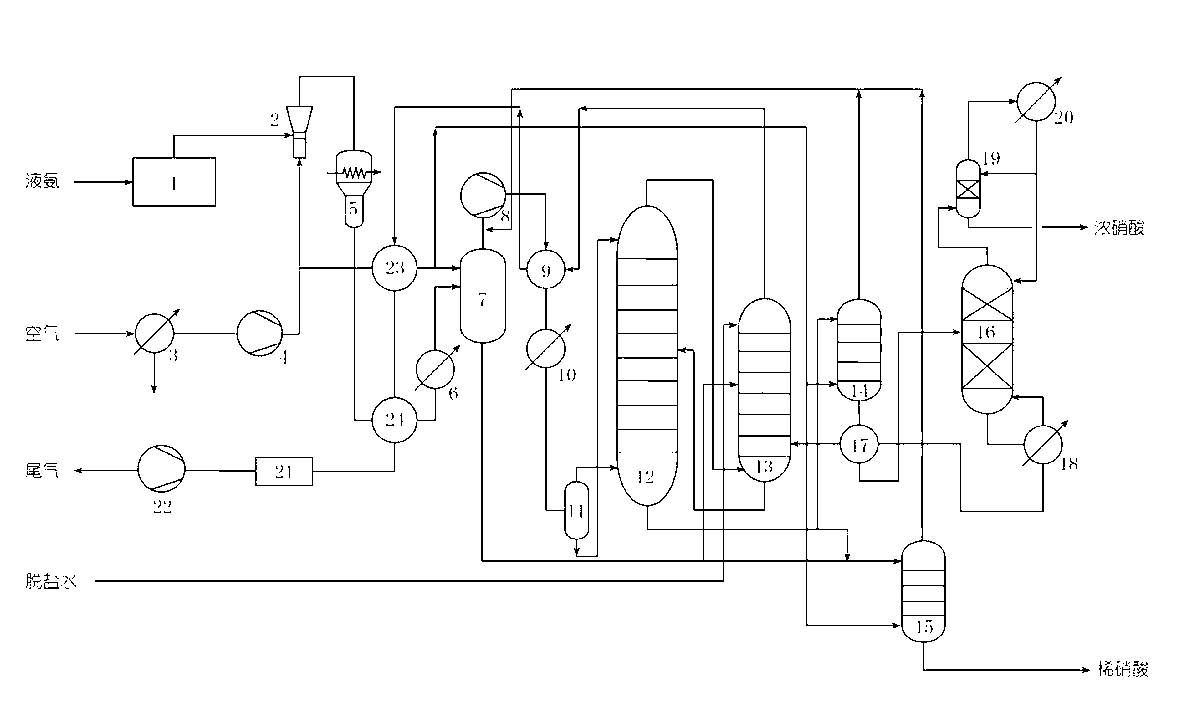
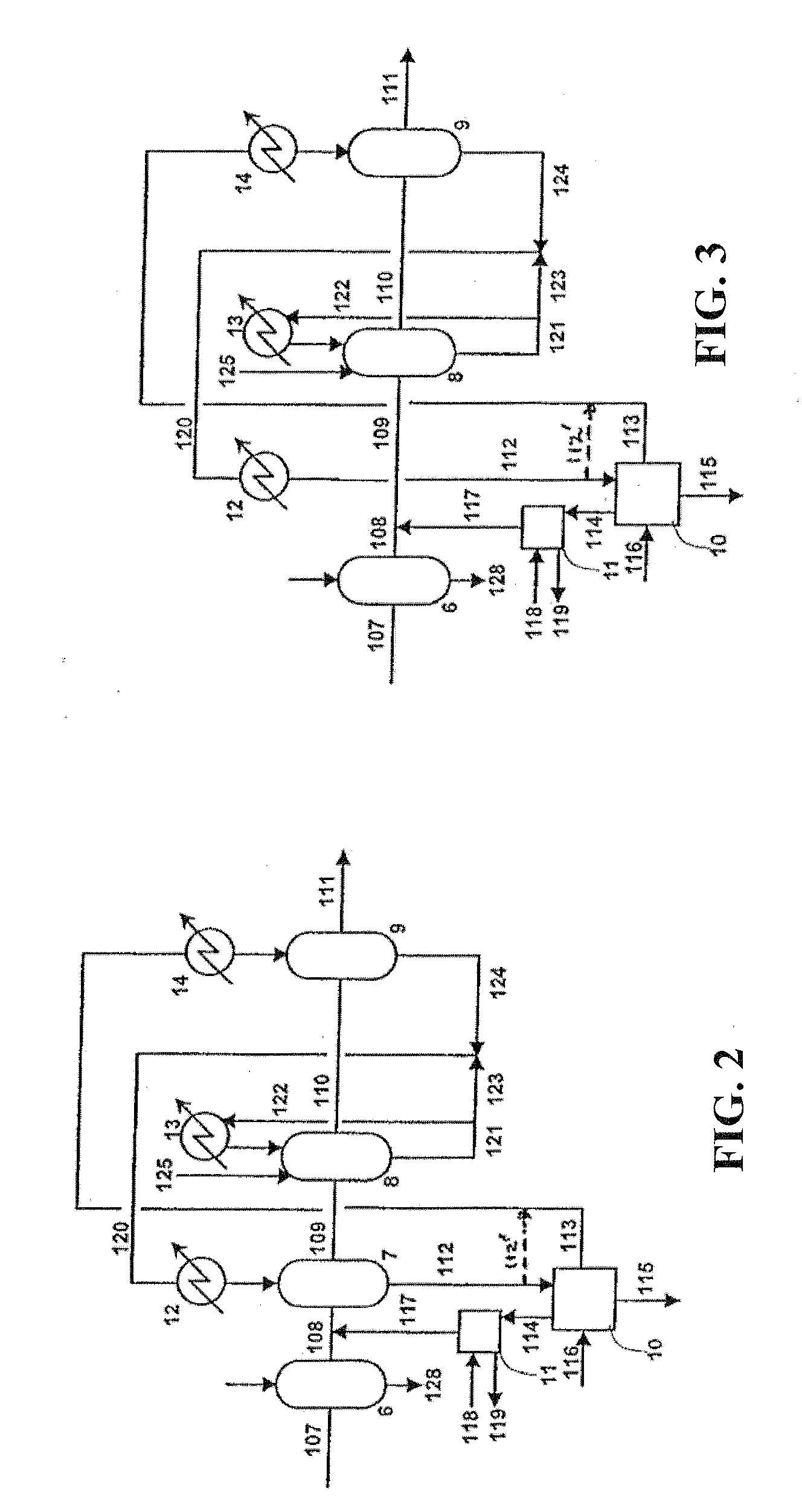
A joint production method of dilute nitric acid and concentrated nitric acid comprises the following steps: superazeotropic nitric acid with the mass concentration greater than 68.4% is produced by a dual-pressure dilute nitric acid production device, then concentrated nitric acid with the mass concentration greater than 98% is produced by a superazeotropic nitric acid distillation and rectification technology, and dilute nitric acid with the mass concentration greater than 60% is produced at the same time by matching. According to the production method, ammonia and air are employed to produce concentrated nitric acid, ammonia is oxidized at medium pressure, nitrogen oxides are absorbed at high pressure, the content of NOx in the exhaust gas after absorption is less than and equal to 30ppm after ammonia reduction reaction, the problem of environmental pollution is solved, and dilute nitric acid and concentrated nitric acid in any proportion and any concentration can be simultaneously produced without pure oxygen and any other dehydrating agent.
Owner:浙江金富春实业有限公司
Multi-stage process for purifying carbon dioxide and producing acid
Carbon dioxide is purified by processes employing NOx-rich sulfuric acid that can be formed by removal of SO2 from the carbon dioxide.
Owner:PRAXAIR TECH INC
Recycling process of tin-removing waste liquid
InactiveCN104894599APrecipitation will notWill not cause secondary pollutionPhotography auxillary processesProcess efficiency improvementTin dioxideLiquid waste
The invention discloses a recycling process of tin-removing waste liquid. The method includes the steps of S1, feeding the tin-removing waste liquid into a distilling device, and performing pressure reduction distillation under the temperature of 65-80 DEG C and the pressure of (0.2-0.5)*1.03*10<5>Pa to recycle nitric acid; S2, feeding distillation residual liquid into an electrolytic tank with an ion exchange membrane for electrolysis, controlling current density and cathode potential to recycle copper in the residual liquid through electrolysis, acquiring tin dioxide at the anode of the electrolytic tank, and acquiring metal copper at the cathode of the electrolytic tank; S3, controlling current density to be unchanged after metal copper is recycled, and changing cathode potential to recycle tin through electrolysis, constantly adding sulfuric acid solution in a cathode chamber during the electrolysis process until the electrolysis is ended, acquiring the tin dioxide at the anode of the electrolytic tank, and acquiring metal tin at the cathode of the electrolytic tank, wherein the current density is 100-200A / m<2>. The recycling process has the advantages that no chemical reagent is added, and secondary pollution is avoided.
Owner:CHENGDU HONGHUA ENVIRONMENTAL SCI & TECH CO LTD
Method and device for preparing nitric acid through metal nitrate pyrolysis
InactiveCN108862218AHigh recovery rateLow production costOxide/hydroxide preparationNitric acidPositive pressureToxic industrial waste
The invention relates to the field of industrial waste treatment, in particular to the field of hydrometallurgical production which requires nitric acid as an oxidant, and discloses a method and a device for preparing the nitric acid through metal nitrate pyrolysis. The method includes the steps of pyrolyzing metal nitrate powder in a closed device to generate O2, NO2 and metal oxide powder, injecting the obtained O2 and the obtained NO2 into a absorption tower, and circularly absorbing the O2 and the NO2 by absorption liquid in the absorption tower to obtain the nitric acid with the requiredconcentration. The device and the method have the advantages that the whole system is sealed with positive pressure, nitrate is fully pyrolyzed in a rotary kiln, the gases generated in the process arecompletely absorbed by the liquid in the absorption tower, and almost no waste gas and waste water are discharged; the nitric acid concentration can meet hydrometallurgical production requirements, high recovery of the nitric acid is realized, the production cost of the nitric acid is reduced greatly, and recycling of metal nitrate is achieved effectively at the same time.
Owner:MEISHAN SHUNYING POWER BATTERY MATERIALS CO LTD
Continuous producing technique for ultra-high pure nitric acid
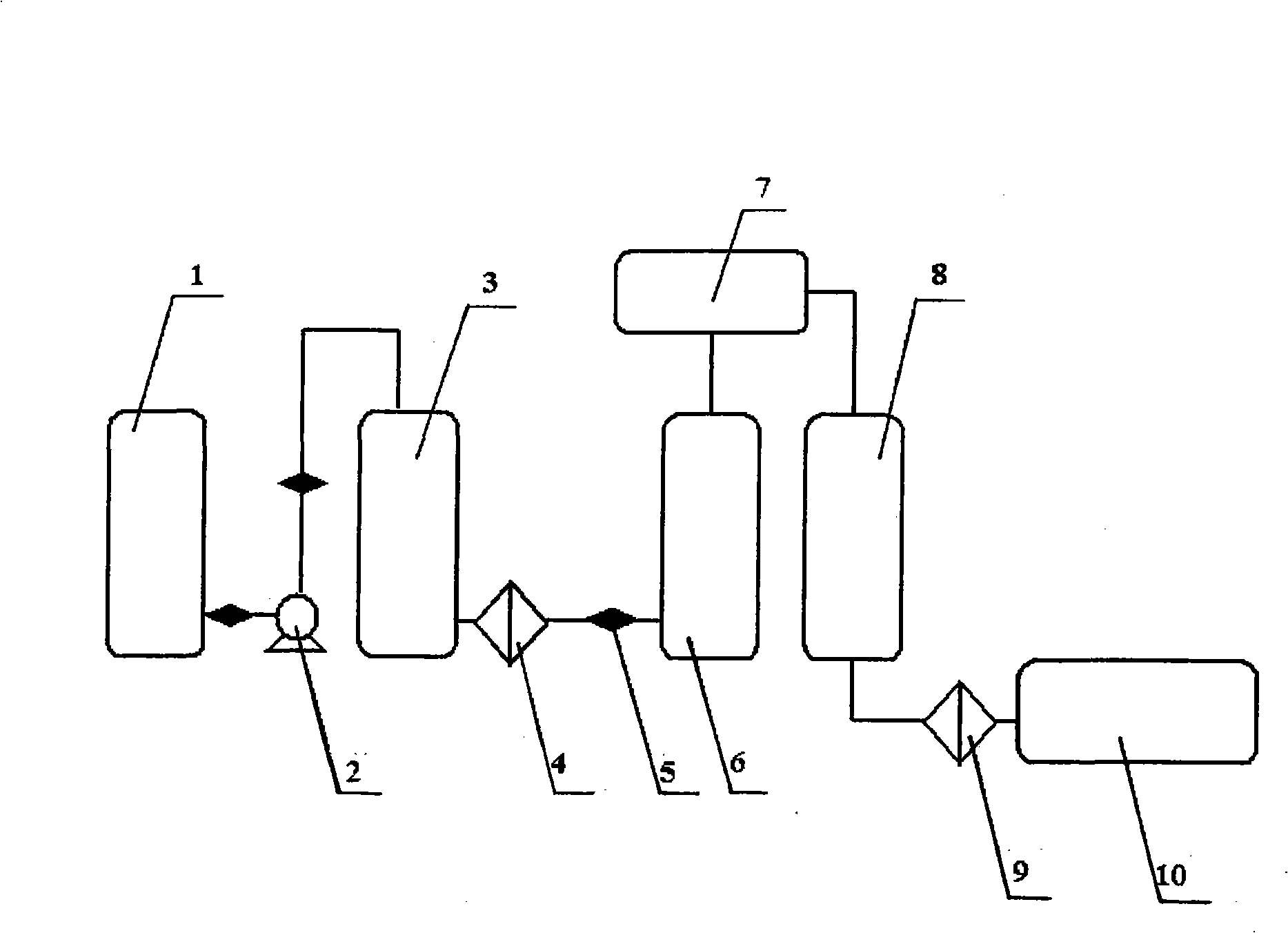
The invention relates to a process for continuously producing extra high purity nitric acid, which comprises the following steps: first, industrial grade 80-90% nitric acid raw material and double allyl 18-crown-6 ether organosilicon macromolecular complexing agent accounting for 0.2-2% of the weight of nitric acid raw material are mixed in a pretreater, then, the mixture is filtered by a micro-filtration membrane under a working pressure of 0.1-0.2 Mpa, the filtrate enters a rectification tower, and the semi-finished product going through the rectification tower is diluted by ultra pure water in a dilution device; after the dilution is finished, the dissociative NO2 is expelled by high pure nitrogen in a whitening device, and the obtained finished product is filtered by a nanometer filter membrane and enters a finished product receiver under the working pressure of 0.5-0.8 Mpa; wherein, the aperture of the micro filtration membrane is 0.2 to 0.8 Mu m, and the aperture of the nanometer filter membrane is 0.5 to 1.5 nm. In the prepared ultra high purity nitric acid, the content of single cation is lower than 1 ppb, the content of single anion is lower than 100 ppb, and the content of the dust particle larger than 0.5 Mu m is lower than 5 per milliliter. The production process for extra high purity nitric acid has the advantages of simple technique, low production costs, high purity of products, low content of impurity ions, and applicability for large-scale mass production.
Owner:JIANGYIN RUNMA ELECTRONICS MATERIAL
Low temperature oxidation of ammonia in nitric acid production
InactiveUS20120183467A1Low costHeterogenous catalyst chemical elementsCatalyst activation/preparationCatalytic oxidationNitrogen
Ammonia in a gas stream comprising oxygen and nitrogen may be effectively completely oxidized to a mixture of NO and NO2 for further processing to nitric acid. The gas stream is flowed over fine particles of La1-xSrxCoO3 and / or La1-xSrxMnO3, and / or La1-xSrxFeO3 where x=about 0.1, 0.2, or 0.3. The particles are supported as catalyst layers on gas stream-contacting surfaces of a flow-through catalyzed oxidation reactor. These relatively inexpensive perovskite-type materials may be used to promote oxidation of ammonia at temperatures below about 450° C. to about 500° C. to selectively produce a mixture of NO and NO2. This mixture is suitable for further oxidation to NO2 for adsorption into water to make nitric acid.
Owner:GM GLOBAL TECH OPERATIONS LLC
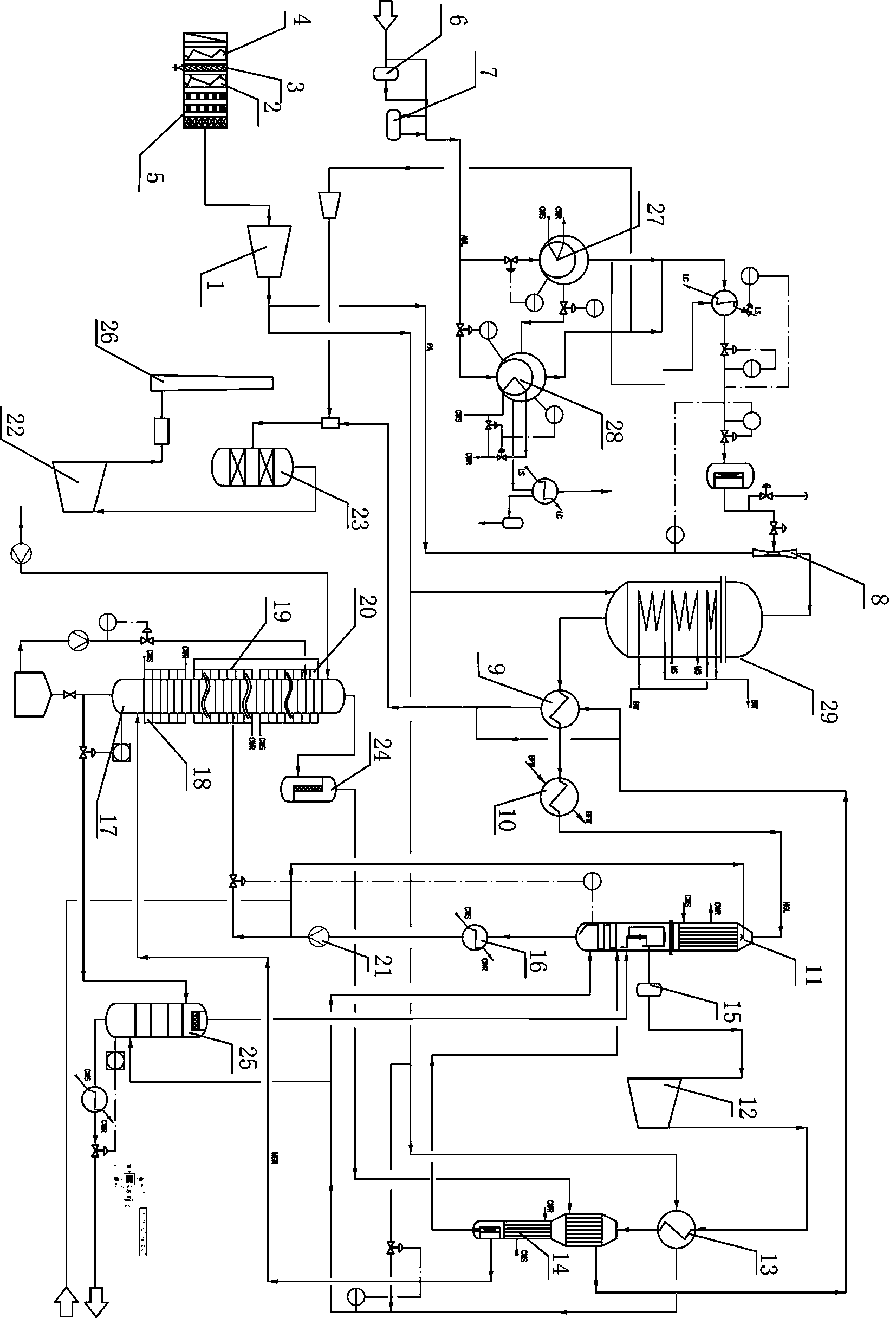
Nitric acid production technology with double-pressurized method
InactiveCN105366653AIncrease profitImprove absorption rateEnergy inputNitric acidHigh concentrationPlatinum
The invention discloses a nitric acid production technology with a double-pressurized method. The technology includes the steps of: (1) mixing of raw materials; (2) operation of an ammoxidation reaction; (3) thermal recovery and cooling; (4) absorption; (5) bleaching; and (6) treatment of tail gas. The production technology is reasonable and simple, and has high ammonia utilization rate, low platinum consumption, high absorptivity, high concentration of finished acid, and low content of NOx in the tail gas. Moreover, the production technology avoids wasting of energy resources, is suitable for promotion in various nitric acid manufactures, and has good thermal recovery so that the steam is more than self-sufficient.
Owner:蒋小华
Production method of oxygen-containing beta aluminum fluoride for aluminum electrolysis bath by using aluminium scruff ash
ActiveCN101177292AHigh activityImprove solubilityAluminium compoundsAmmonia preparation/separationSolubilityHigh activation
The invention relates to a method utilizing the primary aluminum lime to produce the fluoride-bearing Beta alumina used on the aluminum electrolytic bath. The invention is characterized in that the process is to add the primary aluminum lime into the water with a weight of 1 to 3 times of the aluminum lime for immersion cleaning, filtering and dehydration; vapor the solution after the filtering and dehydration to obtain the mixture crystallization of the NaCl, the KCl and the NaF; add the dehydrated aluminum lime into the dilute hydrochloric acid solution for sufficient mixing; stir the solution for 3 to 5 hours at a temperature of 80 to 100 DEG C to generate the NH3 and the Al(OH)3; calcine the Al(OH)3 generated after the hydrolysis to prepare the fluoride-bearing Beta alumina. The invention has the advantages that the produced fluoride-bearing alumina has higher activation and can dissolve in the electrolyte very well and be used for electrolysis production; not only the environment pollution generated by the aluminum lime during the preparation of the primary aluminum can be solved, but also the industrial waste can be transformed to the resource for full utilization.
Owner:GUIZHOU BRANCH CHINA ALUMINUM IND
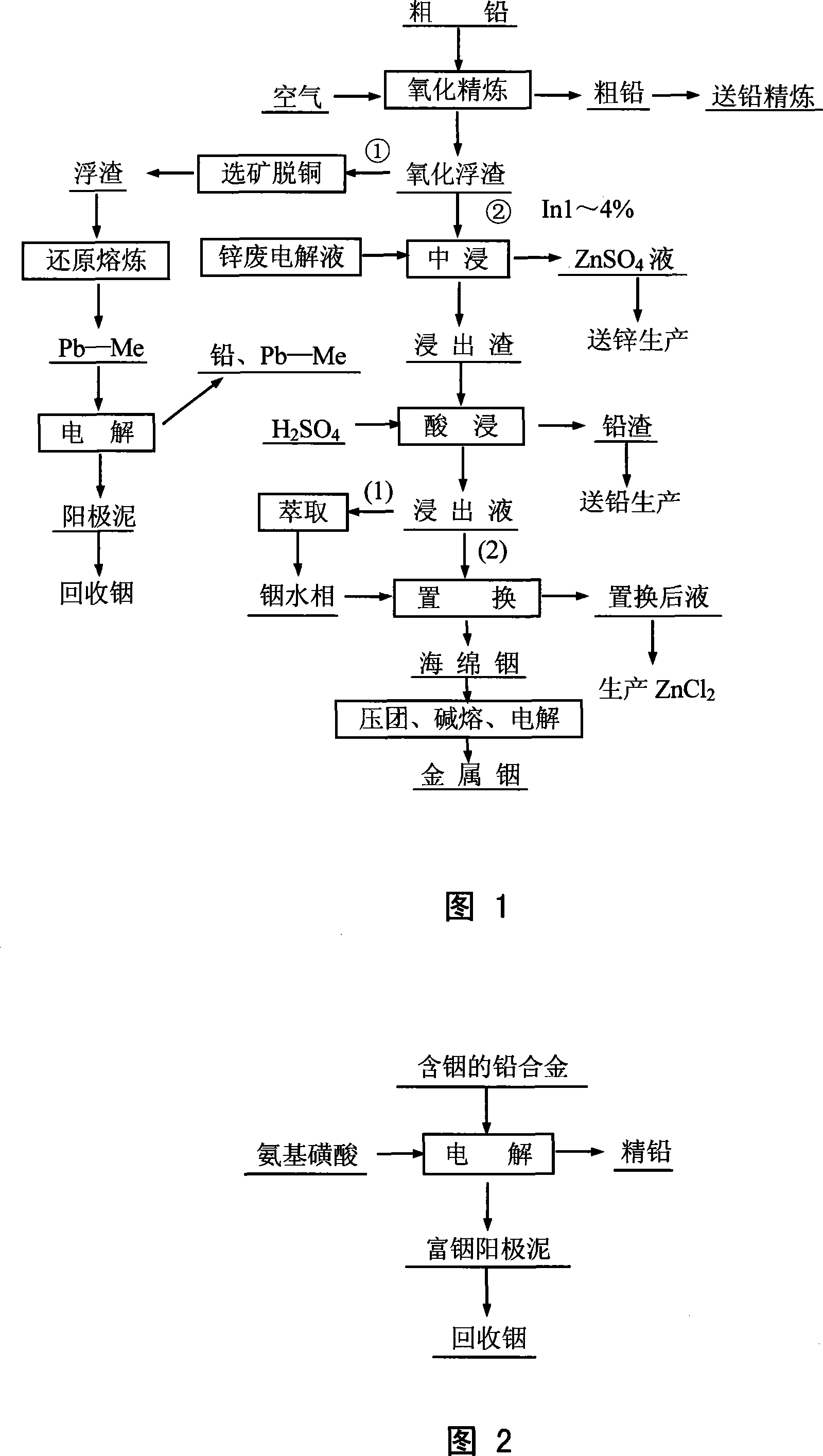
Method for leaching indium from indium sulfide concentrate
InactiveCN101113490AReduce consumptionSimple smelting processChemical recyclingProcess efficiency improvementIndiumSlag
An indium leaching method from indium sulfide concentrate ore pertains to the smelting technique of indium extracting, in particular to the smelting technique of recovery and utilization to the independent indium sulfide concentrate ore. The method has steps that: the indium sulfide concentrate ore, sulfuric acid solution and nitric acid solution are added into a reactor container to do oxidation and dissolution and obtained indium sulfide is added into liquid; then indium is distilled and separated out through extraction, stripping, replacement technologies; meanwhile sulfur and iron concentrating in leaching slag are recovered after reaction. The invention can directly recover and utilize the independent indium sulfide concentrate ore, can simplify the smelting technology and strengthen the process, and the invention is characterized by high metal recovery rate, easy indium separation, low reagent consumption and valuable metals centralization and low pollution.
Owner:KUNMING UNIV OF SCI & TECH
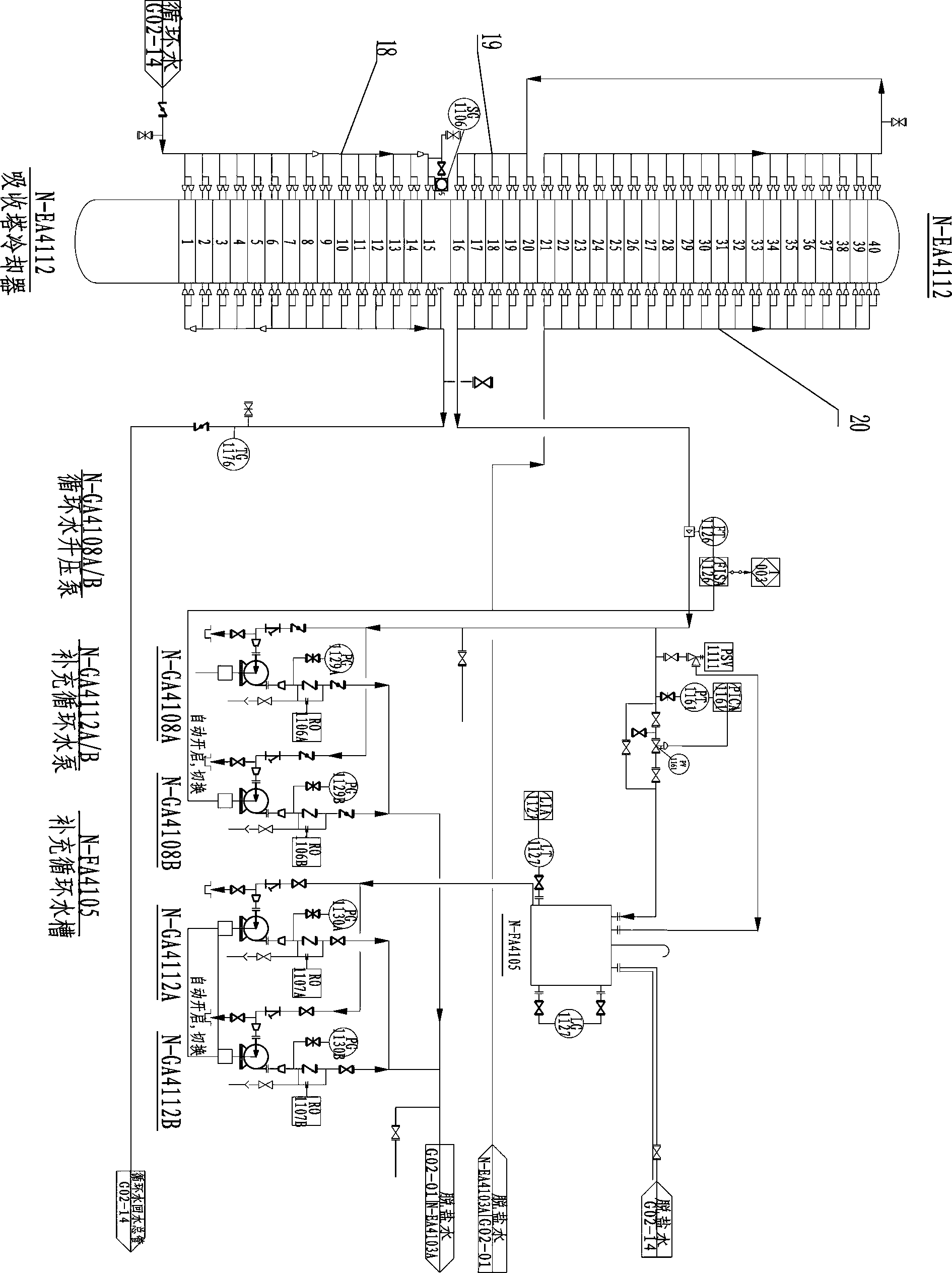
Medium-pressure nitric acid production process and equipment
PendingCN110540178AReduce ammonia consumptionCompact processChemical industryNitric oxideMolecular sieveDecomposition
The invention discloses a medium-pressure nitric acid production process. The medium-pressure nitric acid production process is characterized in that the ammoxidation and absorption pressure is 0.5-0.6 MPa; tail gas of an absorption tower passes through a carbon molecular sieve temperature swing adsorption (TSA) treatment device, so that the content of nitrogen oxides in the tail gas is reduced tobe less than 100 mg / Nm<3>; air compressor process air is used as regeneration desorption air of the carbon molecular sieve TSA treatment device, and the regeneration desorption air containing the nitrogen oxides can be returned to an oxidation reactor for reuse; a layer of N2O decomposition catalyst is added in the oxidation reactor to reduce the content of the N2O to 50-100 PPM through reaction;and a nitric acid bleaching tower is arranged at the bottom of the absorption tower, and the nitric acid bleaching tower and the absorption tower are integrated, so that the process is shortened, andthe equipment investment is reduced.
Owner:CHINA CHENGDA ENG
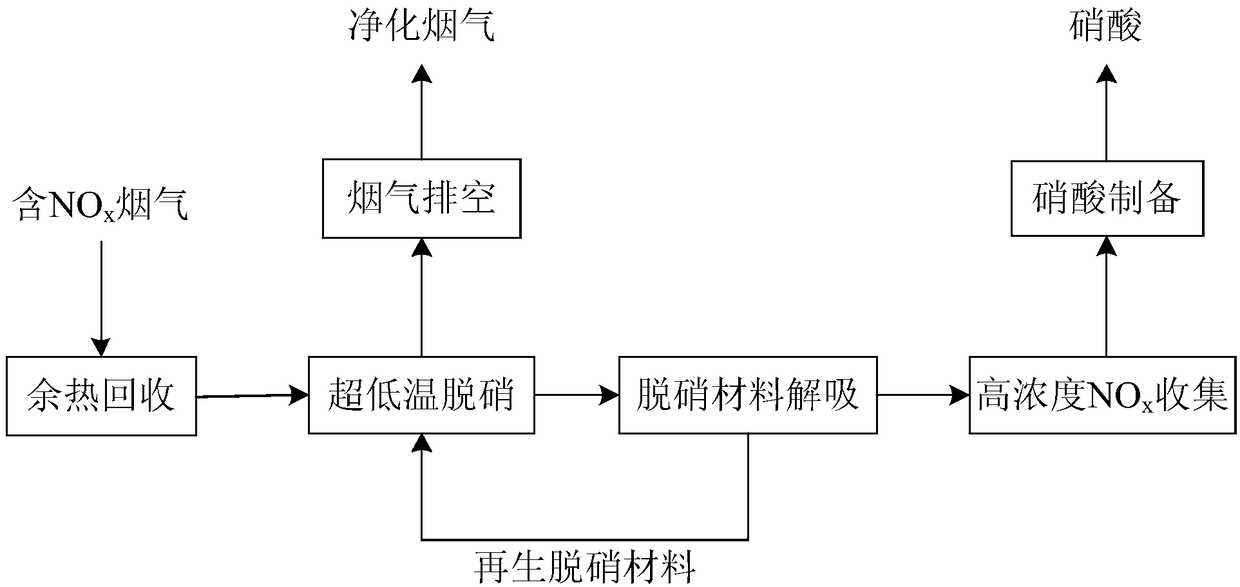
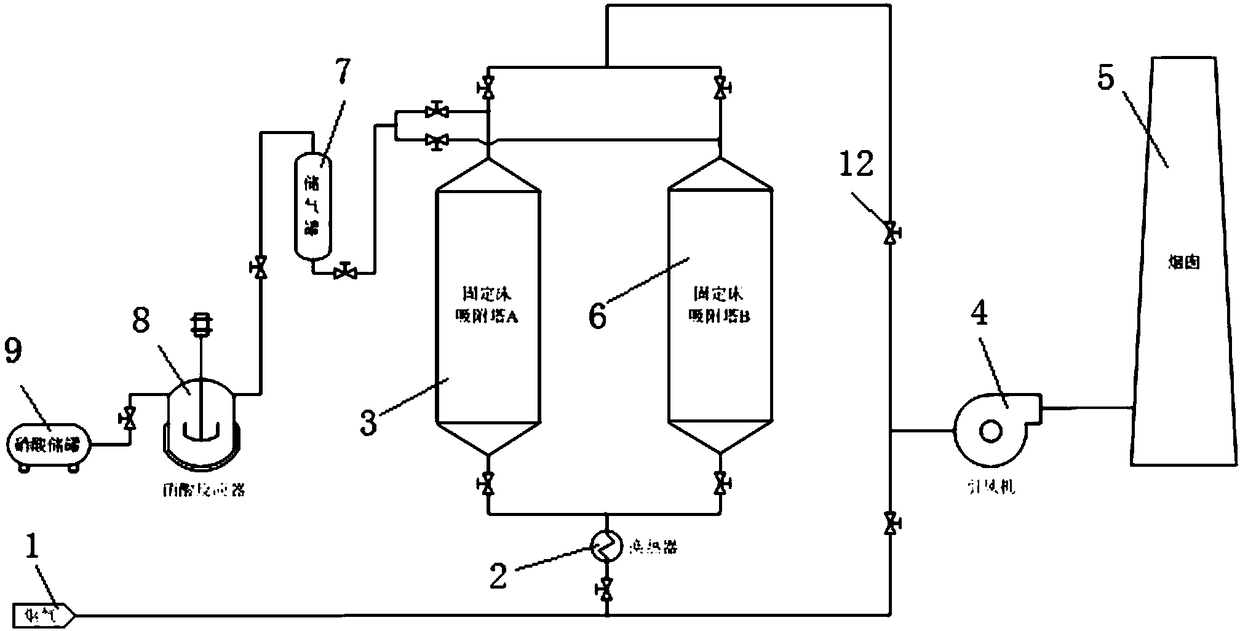
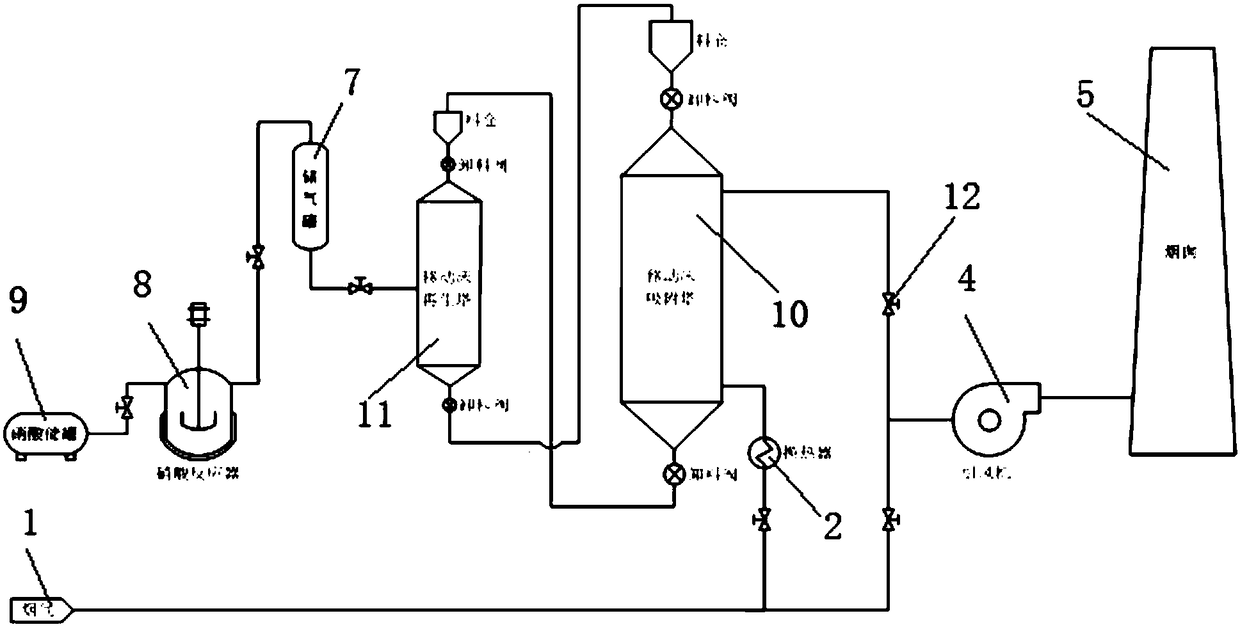
Method and technological system for achieving high-efficiency removal and resource utilization of NOx in ultralow temperature smoke
InactiveCN108310968ARealize resource utilizationWide temperature window for denitrificationGas treatmentDispersed particle separationHigh concentrationTechnological system
The invention discloses a method and a technological system for achieving high-efficiency removal and resource utilization of NOx in ultralow temperature smoke. The method comprises the following steps: step 1, a temperature of smoke is reduced to 25 to 75 DEG C after the smoke from an upstream work section passes through a waste heat recovery system; step 2, the cooled smoke is treated, and in the process, the smoke is denitrified at first to make NOx in the smoke to meet a standard, the smoke meeting the standard is discharged, the denitrified material is regenerated to obtain NOx with a higher concentration, and the regenerated denitrified material is repeatedly applied to smoke denitrification; step 3, the NOx with the higher concentration obtained in the step 2 is utilized to preparea nitric acid product. By means of the method disclosed by the invention, high-efficiency removal of the NOx in the smoke can be achieved under the condition lower than 100 DEG C; furthermore, resource utilization of the NOx is achieved; compared with the prior art, a technology of the method has the advantages of obvious advancement and economical efficiency.
Owner:SHAANXI COAL & CHEM TECH INST
Method for reclaiming nitric acid from nitrified waste acid
The invention belongs to the technical field of organic chemical nitrification reactions, and relates to a method for reclaiming nitric acid from nitrified waste acid. The method comprises the following steps of: adding a nitrified raw material into the nitrified waste acid, starting to stir, heating to a reaction temperature, and maintaining the reaction until the nitric acid in the nitrified waste acid is completely transformed; standing, and separating organic matters and the waste acid; and adding the organic matters into mixed acid for secondary nitrification, raising the temperature and maintaining the temperature until the reaction is completely performed. The method is used to remove oxynitride in the waste acid by recycling the nitric acid in the nitrified waste acid, so that corrosion of equipment is reduced; and the consumption of the nitric acid in the nitrifying process is reduced, and the production cost is reduced. According to the invention, the dose of the nitric acid in the nitrification reaction is reduced by sufficiently utilizing the nitric acid component in the nitrified waste acid; and the waste acid is concentrated after the nitric acid in the waste acid is reclaimed, and the corrosion of the oxynitride generated in the waste acid reclaiming process to reclaim equipment is reduced.
Owner:CHINA PETROLEUM & CHEM CORP +1
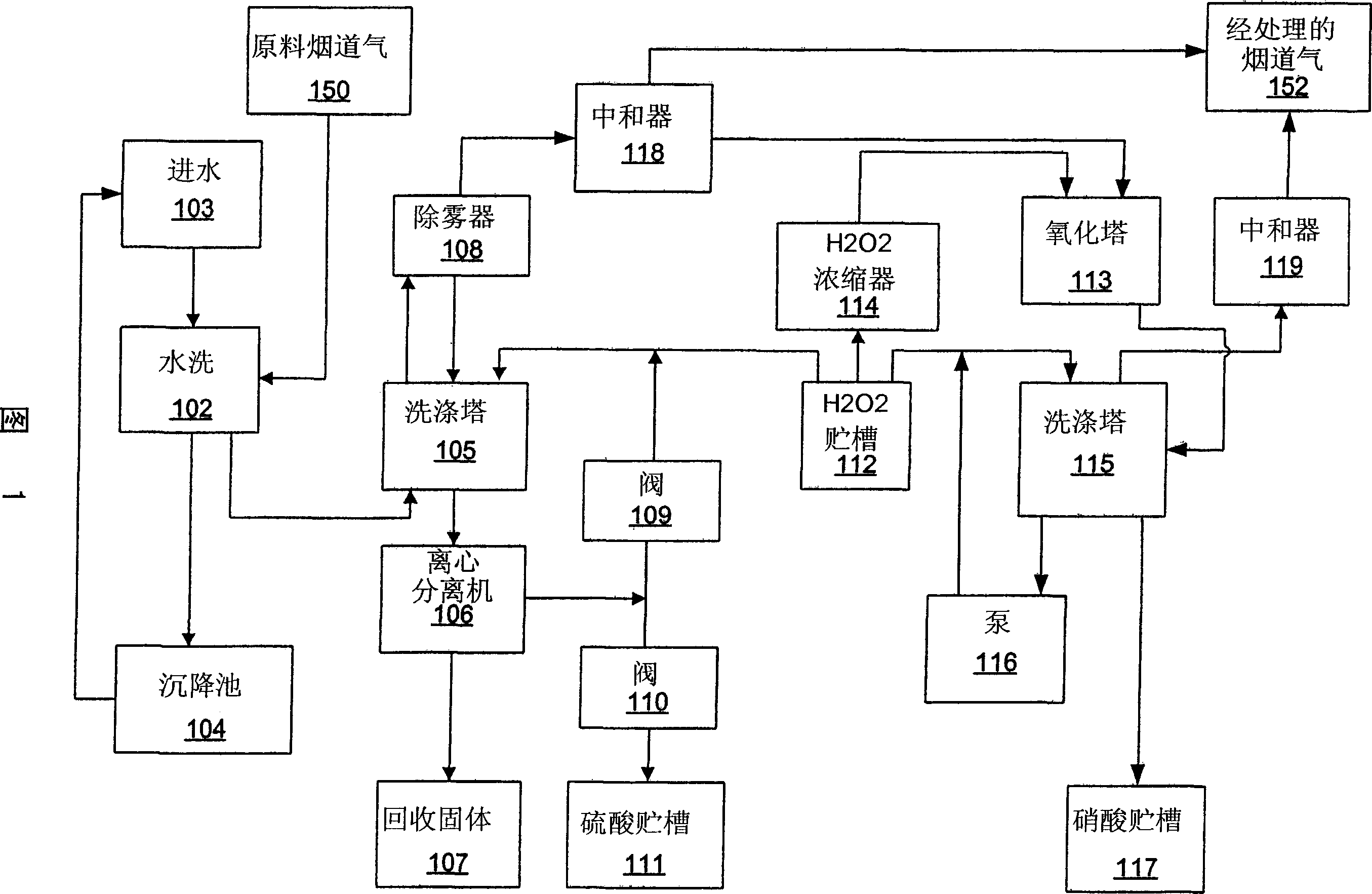
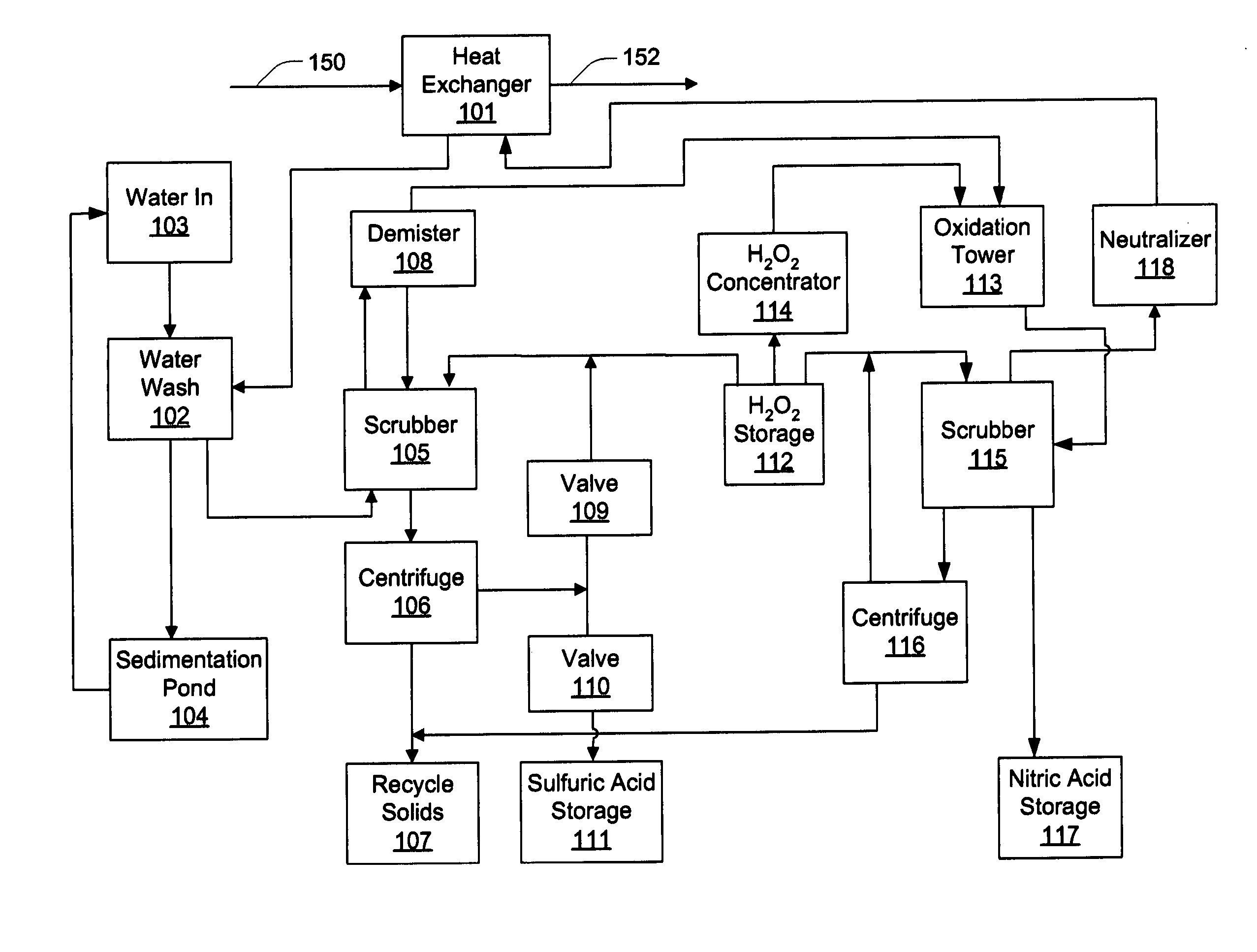
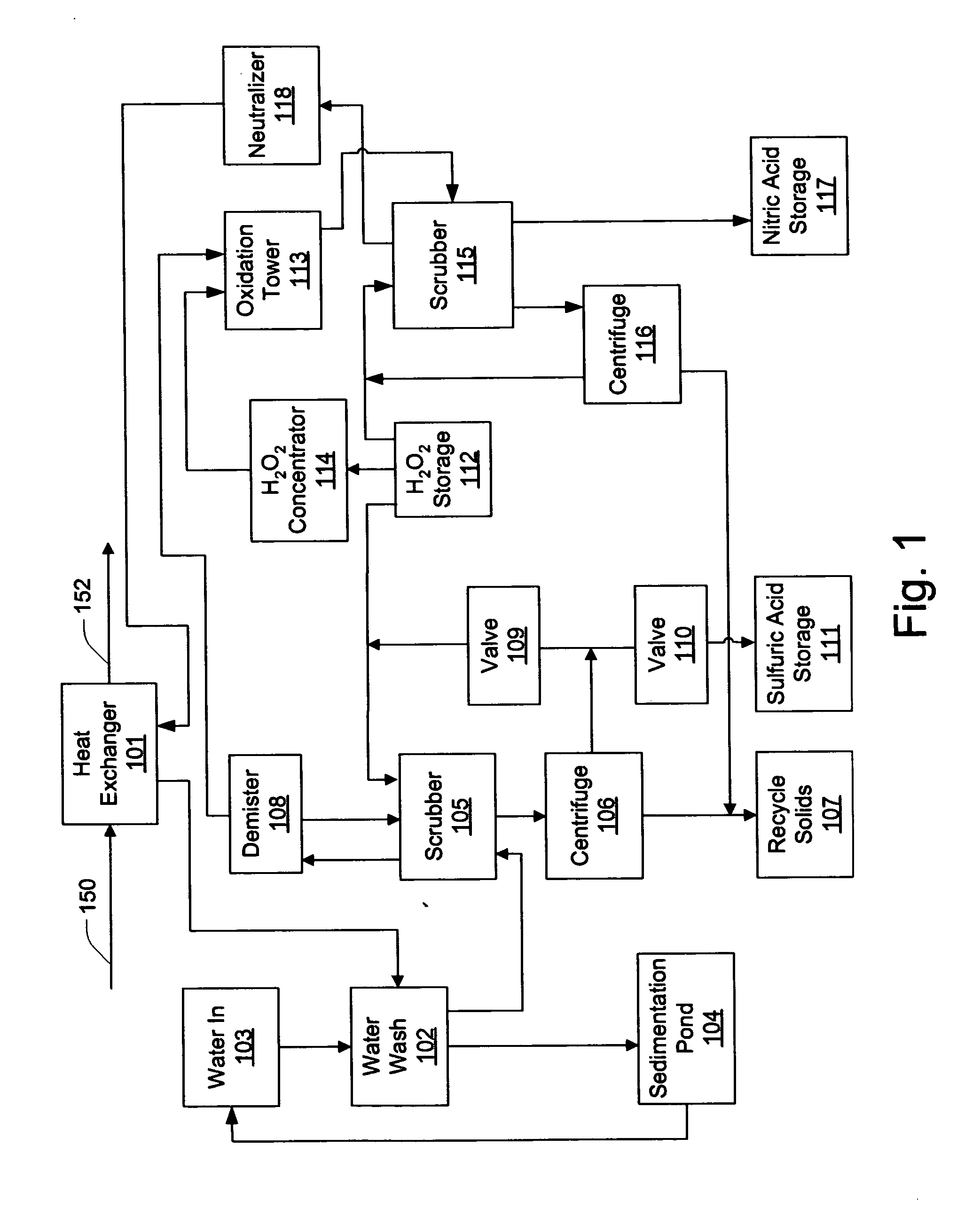
Method and apparatus utilising hydrogen peroxyde to reduce SOx, NOx and heavy metal emissions
Methods and apparatus utilizing hydrogen peroxide are useful to reduce SOx and mercury (or other heavy metal) emissions from combustion flue gas streams. The methods and apparatus may further be modified to reduce NOx emissions. Continuous concentration of hydrogen peroxide to levels approaching or exceeding propellant-grade hydrogen peroxide facilitates increased system efficiency. In this manner, combustion flue gas streams can be treated for the removal of SOx and heavy metals, while isolating useful by-products streams of sulfuric acid as well as solids for the recovery of the heavy metals. Where removal of NOx emissions is included, nitric acid may also be isolated for use in fertilizer or other industrial applications.
Owner:NASA
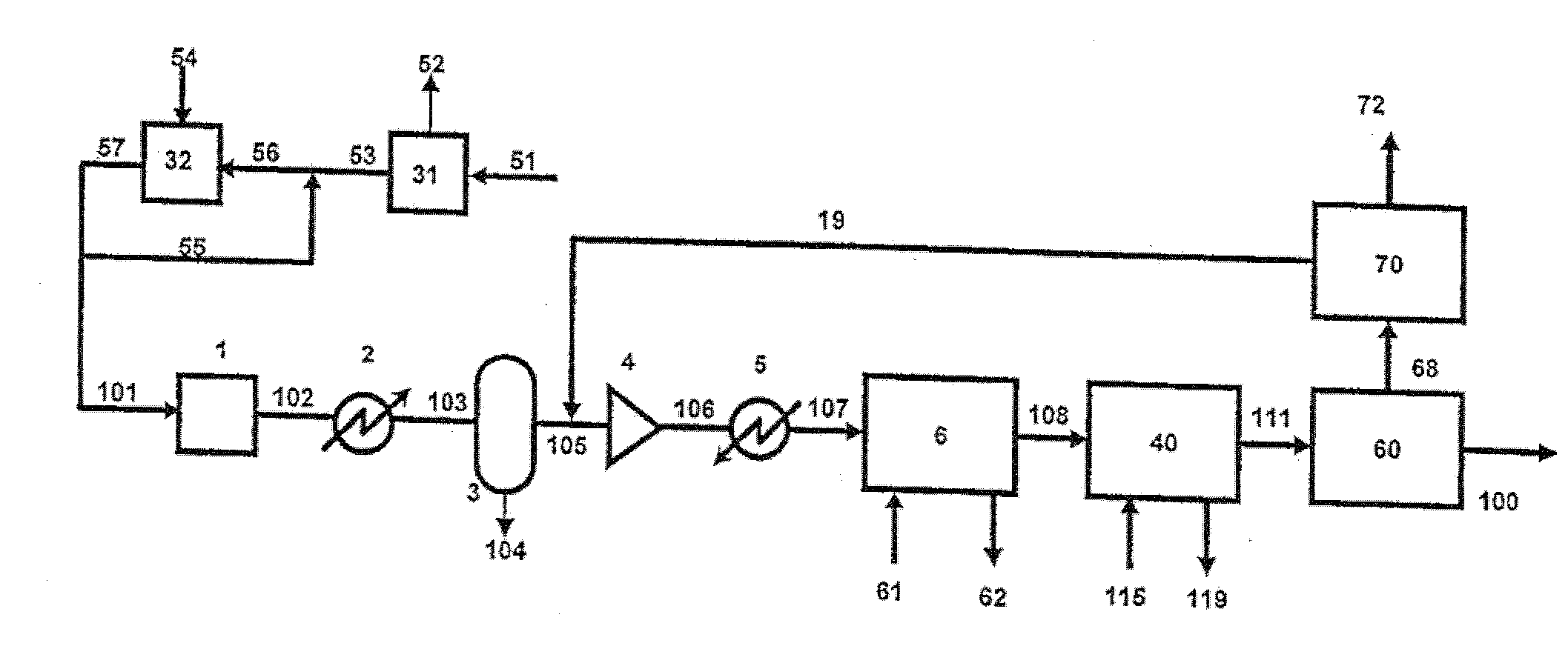
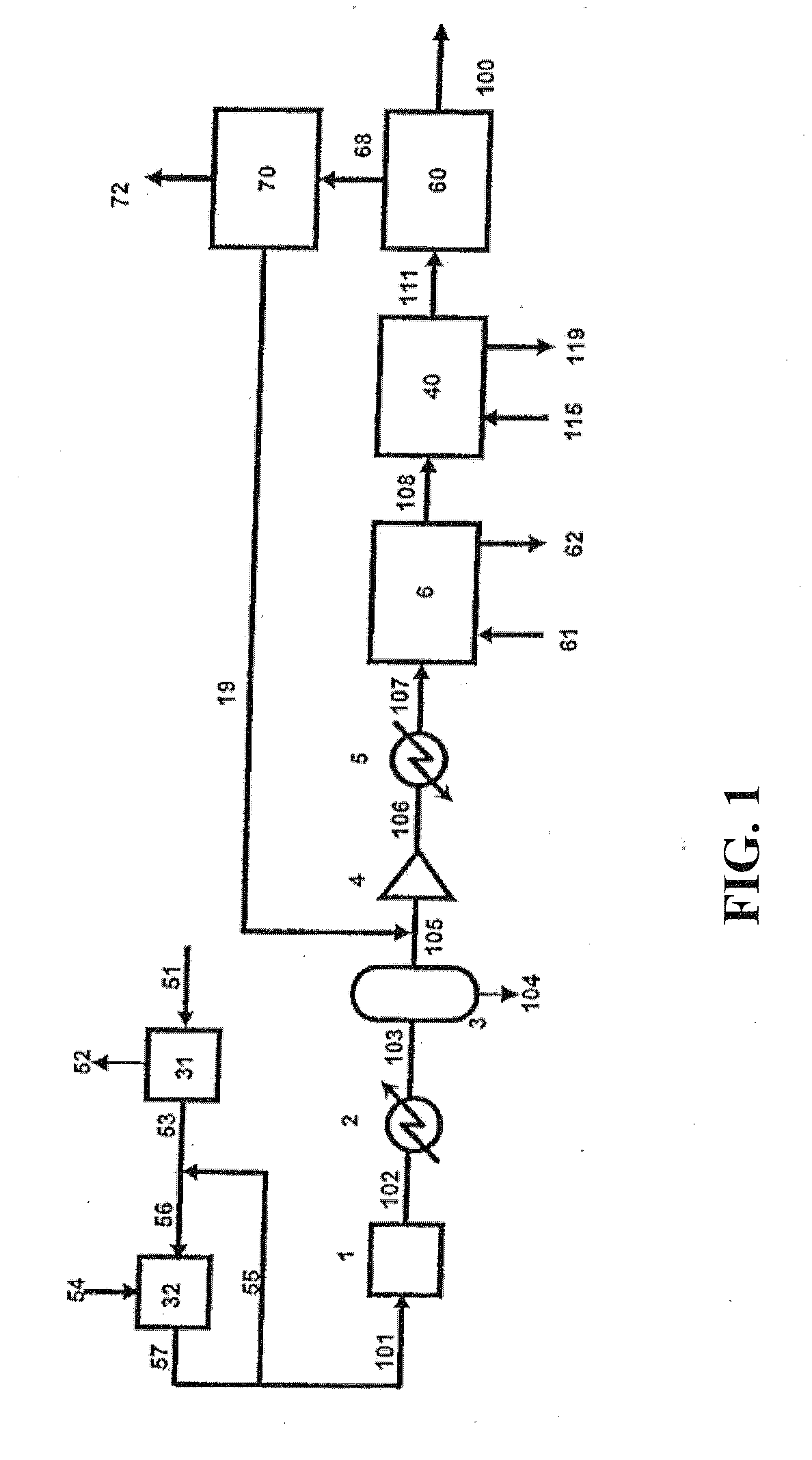
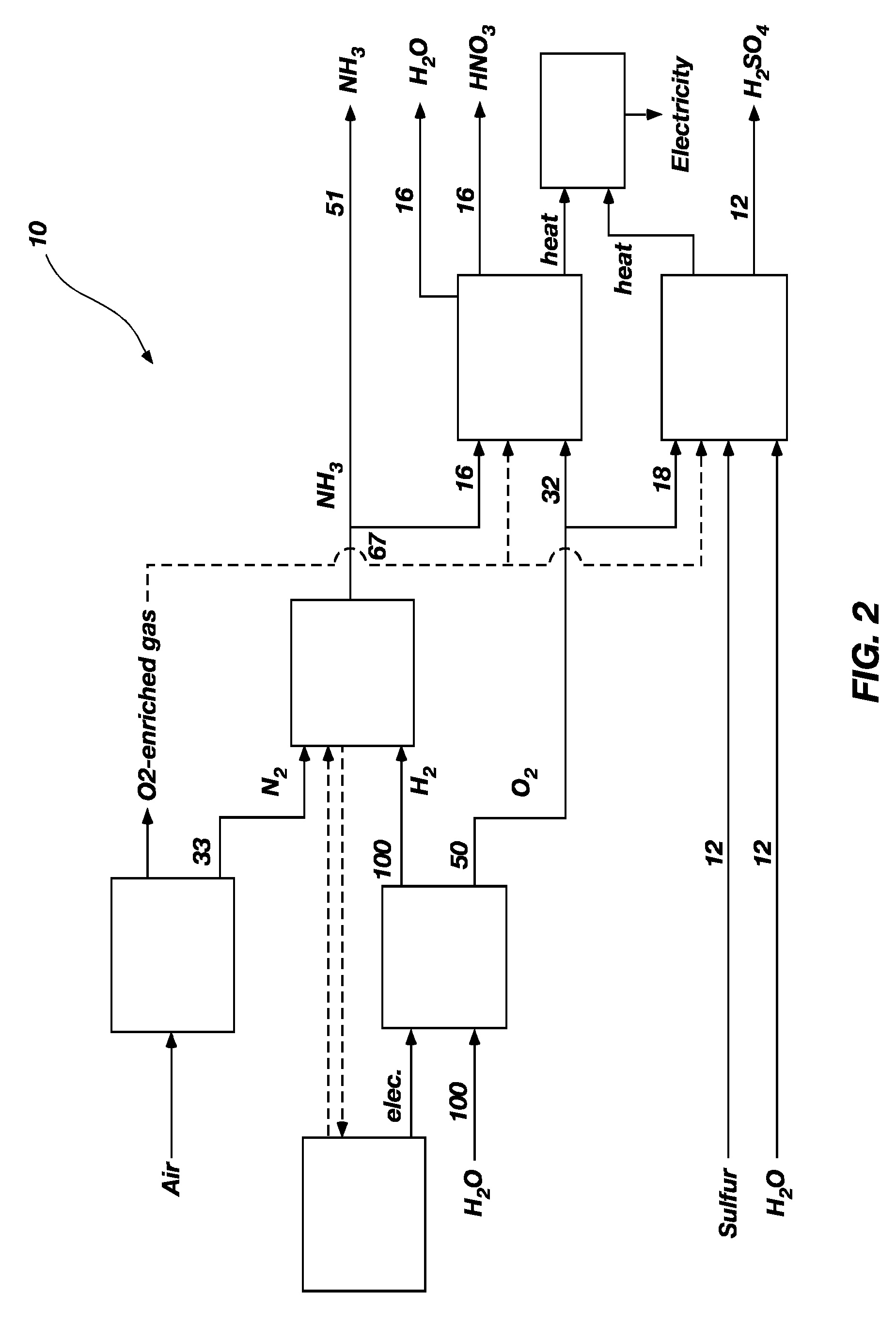
Integration of a water-splitting process with production of fertilizer precursors
Methods and apparatus for the integration of a water splitting process with the production of fertilizer precursors such as ammonia, nitric acid, and sulfuric acid are provided. At least one of heat and electricity from a power plant are used to split water into hydrogen gas and oxygen gas. Nitrogen gas is provided by air separation. The hydrogen gas and nitrogen gas are used to produce ammonia. The ammonia and oxygen gas are used to produce nitric acid. The oxygen gas, water, and sulfur are used to produce sulfuric acid. Further disclosed is an apparatus for the production of nitric acid comprising a power plant and an apparatus for the production of nitric acid. Also disclosed is an apparatus for the production of sulfuric acid comprising a power plant and an apparatus for the production of sulfuric acid.
Owner:BATTELLE ENERGY ALLIANCE LLC
Emission control system
InactiveUS20050255019A1Reduce NOxImprove system efficiencyCombination devicesExhaust apparatusCombustionFlue gas
Methods and apparatus utilizing hydrogen peroxide are useful to reduce NOx, SOx and mercury (or other heavy metal) emissions from combustion flue gas streams. Continuous concentration of hydrogen peroxide to levels approaching or exceeding propellant-grade hydrogen peroxide facilitates increased system efficiency. In this manner, combustion flue gas streams can be treated for the removal of NOx, SOx and heavy metals, while isolating useful by-products streams of sulfuric acid and nitric acid as well as solids for the recovery of the heavy metals.
Owner:ADMINISTATOR OF NAT AERONAUTICS & SPACE ADMINISTATION U S GOVERNMENT AS REPRESENTED BY THE
Method for continuously preparing metal oxides catalyst and apparatus thereof
ActiveUS9012351B2Low costContinuous productionOxygen/ozone/oxide/hydroxideCatalyst activation/preparationWater vaporSlurry
A method for continuously preparing a metal oxides catalyst comprises the following steps: dissolving metal materials using nitric acid solution to produce a metal nitrate solution, and also to produce NOx and water vapor; hydrolyzing the metal nitrate solution by introducing pressurized superheated water vapor into the metal nitrate solution to obtain a slurry of the hydrates of metal oxides as well as acidic gas, the main components of the acidic gas are NO2, NO, O2 and water vapor; filtrating and drying the slurry to obtain the hydrates of metal oxides and / or metal oxides; and then utilizing the obtained hydrates of metal oxides and / or metal oxides as raw materials and preparing the metal oxides catalyst by the conventional method for preparing a catalyst. The NOx gas produced can be absorbed to produce nitric acid which can be reused. An apparatus used for preparing metal oxides comprises a metal salt solution preparation system, a metal salt solution hydrolysis system, a product preparation system and a nitric acid preparation and recycling use system. The method and the apparatus can achieve continuous production, enclosed circulation and zero release in the whole process and can reduce the cost of production.
Owner:SYNFUELS CHINA TECH CO LTD
Recovery method of benzene series nitration waste acid
ActiveCN105858627AIncrease added valueProcess environmental protectionChemical industrySulfate/bisulfate preparationRecovery methodPotassium hydroxide
The invention provides a recovery method of benzene series nitration waste acid. The recovery method includes: mixing waste acid from a benzene series nitration reaction process with activated carbon for decolorizing, adding decolorized waste acid and solid potassium nitrate into an intermittent reactive distillation column bottom sequentially, heating to 60-70 DEG C with stirring for 0.5-3 hours, performing rectification operation while reaction is conducted, collecting overhead distillate, namely recovery liquid, adding water into the intermittent reactive distillation column bottom, heating with stirring to dissolve residues in the bottom, regulating a pH value of 1.4-2.1 by a potassium hydroxide water solution, filtering immediately, naturally cooling filtrate to separate out solids, filtering to obtain potassium bisulfate and recycling mother liquor. The recovery method of the benzene series nitration waste acid has the advantages that the waste acid is utilized effectively to obtain nitric acid required by the reaction and the high-added-value potassium bisulfate, so that the whole technological process is more environment friendly.
Owner:QUZHOU UNIV
Low-consumption and low-emission nitric acid production method and production equipment thereof
InactiveCN102491292AReduce consumptionEmission reductionEnergy inputNitric acidDecompositionNitric oxide gas
The invention discloses a low-consumption and low-emission nitric acid production method and production equipment thereof. The method and the equipment adopts the same low-pressure level for oxidation and absorption, the double-pressurization high-pressure level is omitted, a molecular sieve absorption device is adopted for tail gas treatment of a nitric acid absorption tower, in addition, secondary air is used for taking reaction with nitric oxide absorbed in the molecular sieve absorption device to form regenerated gas, the regenerated gas is sent into the nitric acid absorption tower to be recovered and reutilized, the nitrogen oxide content in the tail gas is lower than 100mg / Nm<3>, in addition, the yield of the nitric acid can also be improved through the recovery and the utilization of regeneration gas, a layer of silk screen is arranged on a sieve plate tower disc of an oxidation section of the nitric acid absorption tower, the oxidation speed at high pressure and low pressure can be effectively accelerated, and an N20 decomposition catalyst layer is also additionally arranged in an oxidation reaction vessel, so the N20 content in the nitric oxide gas in the oxidation reaction vessel can be reduced to about 100PPM. The nitric acid production method does not need to adopt two pressure levels, no nitric oxide compressor is adopted, no tail gas ammonification re-treatment device is adopted, the investment is greatly saved, and the consumption is reduced.
Owner:CHINA CHENGDA ENG
Method for preparing super-pure nitric acid
InactiveCN101870460AEfficient removalSolve the problem of high content of impurity ionsNitric acidFiltrationDust particles
The invention discloses a method for preparing super-pure nitric acid. The method comprises the following steps: taking industrial nitric acid as a raw material; performing first stage filtration by a membrane filter consisting of dibenzo-18-crown-6 and solid-phase carrier composite membrane; performing second stage serial continuous rectification on the filtrate; collecting heavy waste acid and light waste acid respectively; and performing second stage filtration with the membrane filter to obtain the super-pure nitric acid serving as the target product. Upon analysis and detection, the content of each metal ion impurity is less than 1ppb, the dust particles bigger than 0.5microns are less than 5 / ml and the SEMI C8 standard is met. By performing the second stage filtration with the membrane filter consisting of the dibenzo-18-crown-6 and the solid-phase carrier composite membrane, the method overcomes the disadvantage of instable quality of the product in an existing method; the method effectively improves the purity of the super-pure nitric acid product by using the second stage serial continuous rectification technology; and the super-pure nitric acid product obtained by the method has stable quality and high purity and is suitable for scale continuous production.
Owner:SHANGAI HUAYI MICROELECTRONICS MATERIAL +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com