Process for preparing ultrapure nitric acid
A preparation process, pure nitric acid technology, applied in the field of ultra-high-purity nitric acid preparation process, can solve the problems of unstable product quality, large fluctuations in purity, and high cost, and achieve the goal of overcoming unstable product quality, improving purity, and stabilizing quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
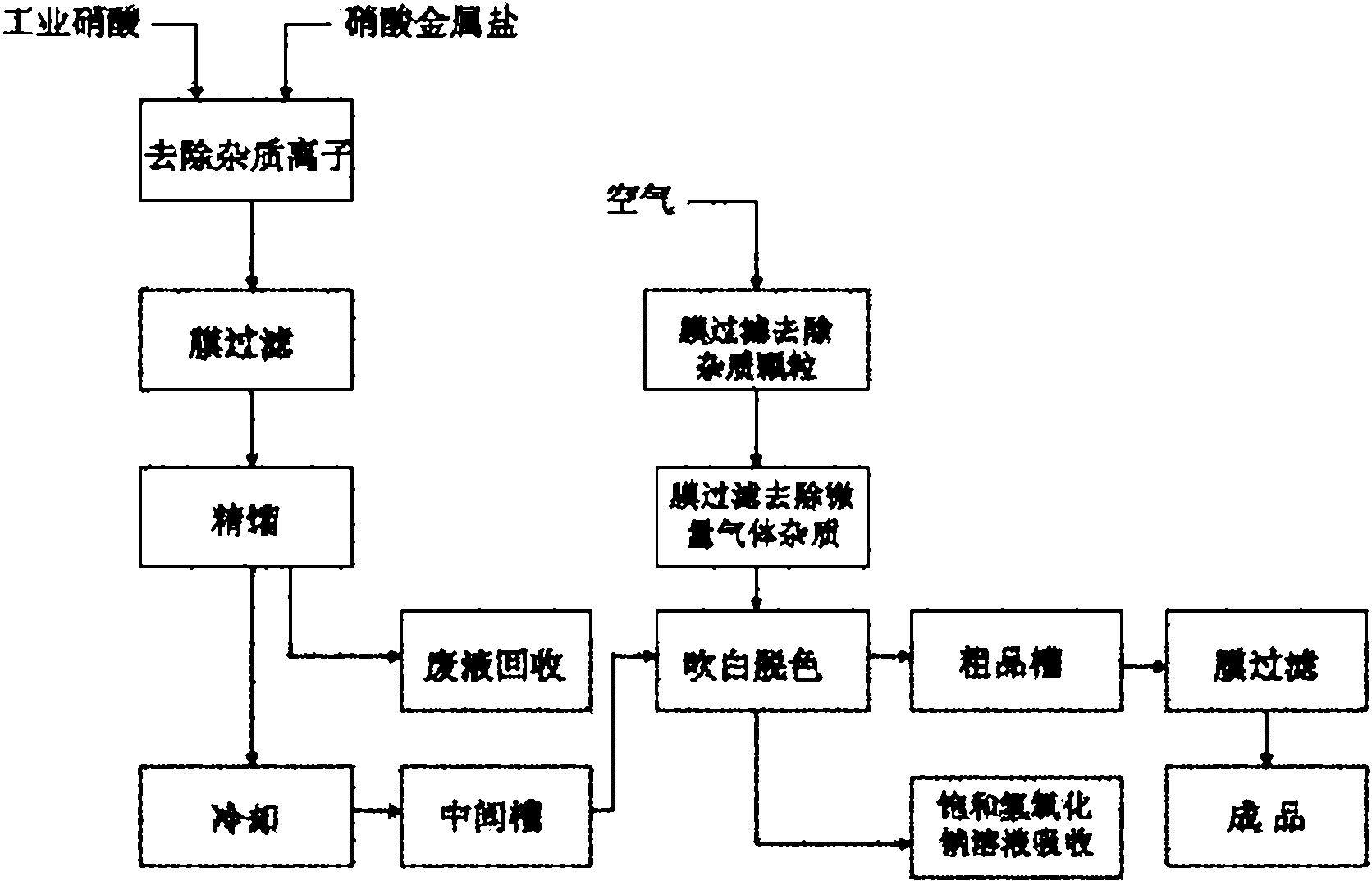
[0027] Industrial nitric acid pretreatment before rectifying of embodiment 1
[0028] The ultrapure water and the silver nitrate are respectively prepared into a 1-10wt% silver nitrate solution, and the barium nitrate is prepared into a 1-10wt% barium nitrate solution.
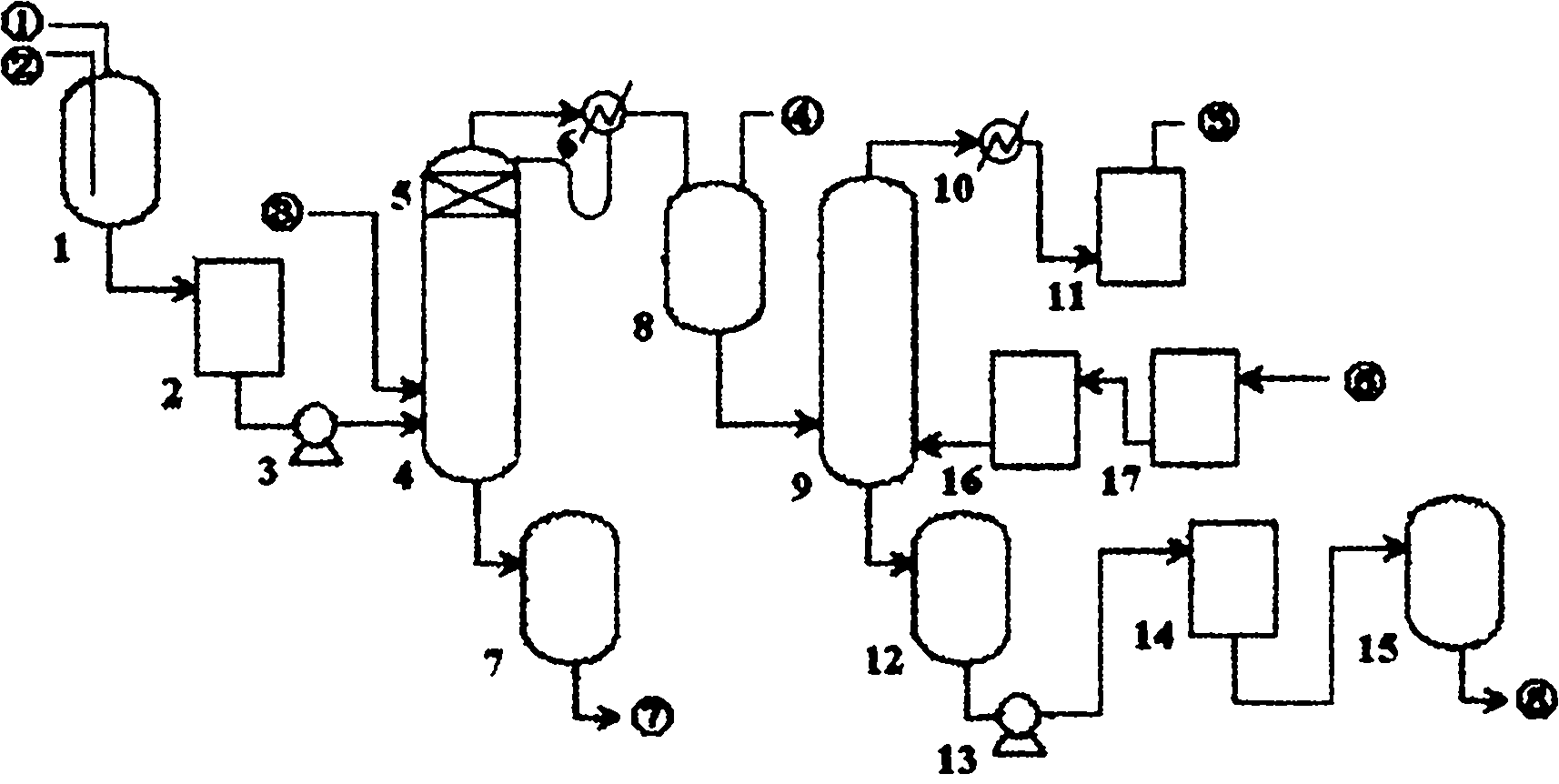
[0029] Add industrial nitric acid in the rectification preprocessor 1, at room temperature, drip 1-10wt% silver nitrate solution and 1-10wt% barium nitrate solution under stirring respectively, make the anion Cl in the nitric acid - and SO 4 2- React with silver nitrate and barium nitrate to precipitate silver chloride and barium sulfate, and use 0.1μm perfluorinated membrane filter 2 to perform membrane filtration to obtain the impurity-removing anion Cl - and SO 4 2- The pretreatment liquid is transported to the rectification tower 4 tower kettle with the delivery pump 3 to carry out the next process. The test results of the pretreatment solution are shown in Table 1.
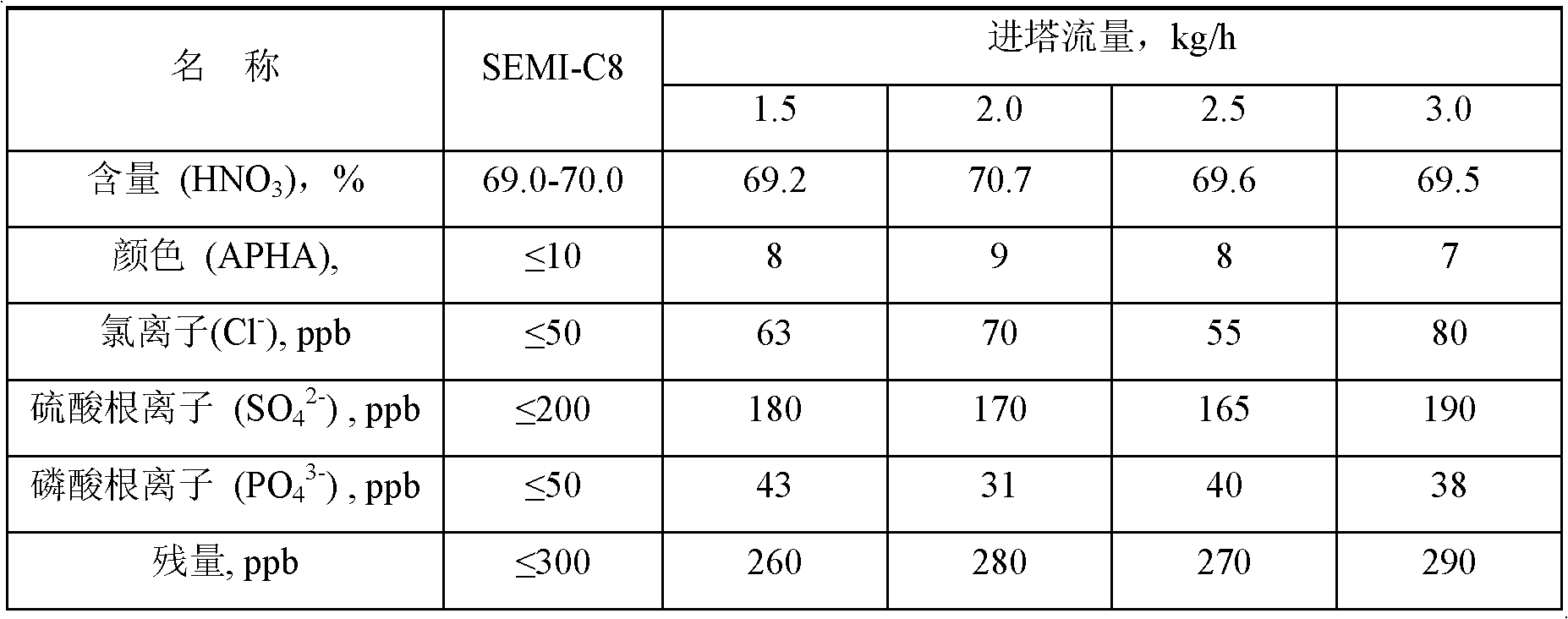
[0030] Table 1 Industrial nitric...
Embodiment 3
[0040] Influence of the hydrophilic microporous membrane demister 5 composed of perfluorosulfonic acid resin on the rectification result of embodiment 3
[0041] The rectification is carried out under the condition that the temperature of the 4th column of the rectification tower is 125-127 °C, and the flow rate of the pretreatment liquid into the tower is 2.0kg / h. The microporous membrane demister 5 prevents metal ions or solid particles from mixing into the steam to remove mist impurity particles in the steam. The test results are shown in Table 3.
[0042] Table 3 The impact of the hydrophilic microporous membrane demister on the top of the tower on the rectification result
[0043]
[0044]
[0045] As can be seen from Table 3, whether there is a hydrophilic microporous membrane eliminator 5 composed of perfluorosulfonic acid resin at the top of the rectification tower has a significant impact on the quality of the ultra-high-purity nitric acid product. During recti...
Embodiment 4
[0046] Embodiment 4 air cleaning and nitric acid blowing white
[0047] The unpurified air is filtered through the secondary membrane respectively, that is, the mist impurity particles in the gas are removed through the hydrophilic microporous membrane filter 17 composed of perfluorosulfonic acid resin, the oxygen concentration is increased, and then passed through the polyethersulfone ( PES) hollow fiber gas separation membrane membrane filter 16 removes trace acid gas SO in gas 2 , CO 2 、H 2 S and other impurities, that is, to obtain the purified air required for whitening. The test results before and after air purification are shown in Table 4.
[0048] Table 4 Test results before and after air purification
[0049] name
[0050] It can be seen from Table 4 that the oxygen concentration before and after air purification is increased from 21.8% to 36.5%, which is beneficial to the nitric acid bleaching process; the number of particles larger than 0.5 μm is redu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com