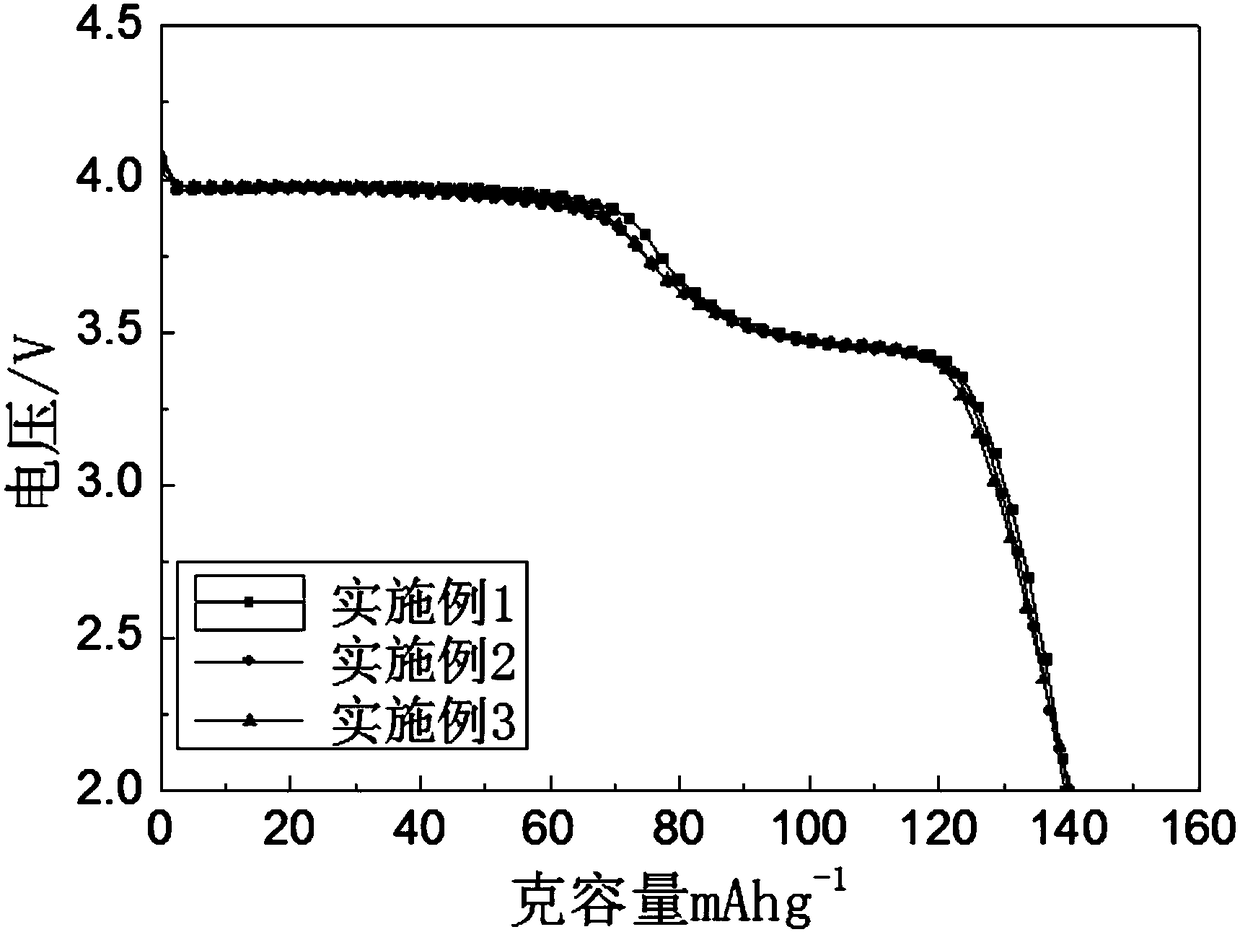
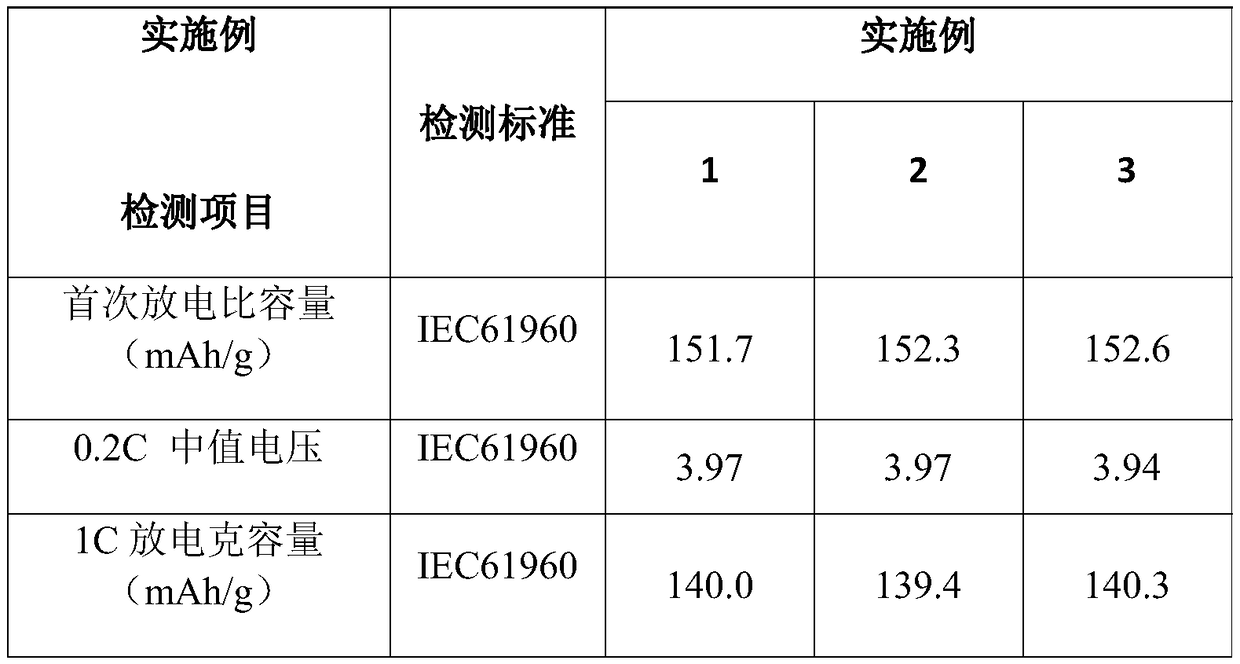
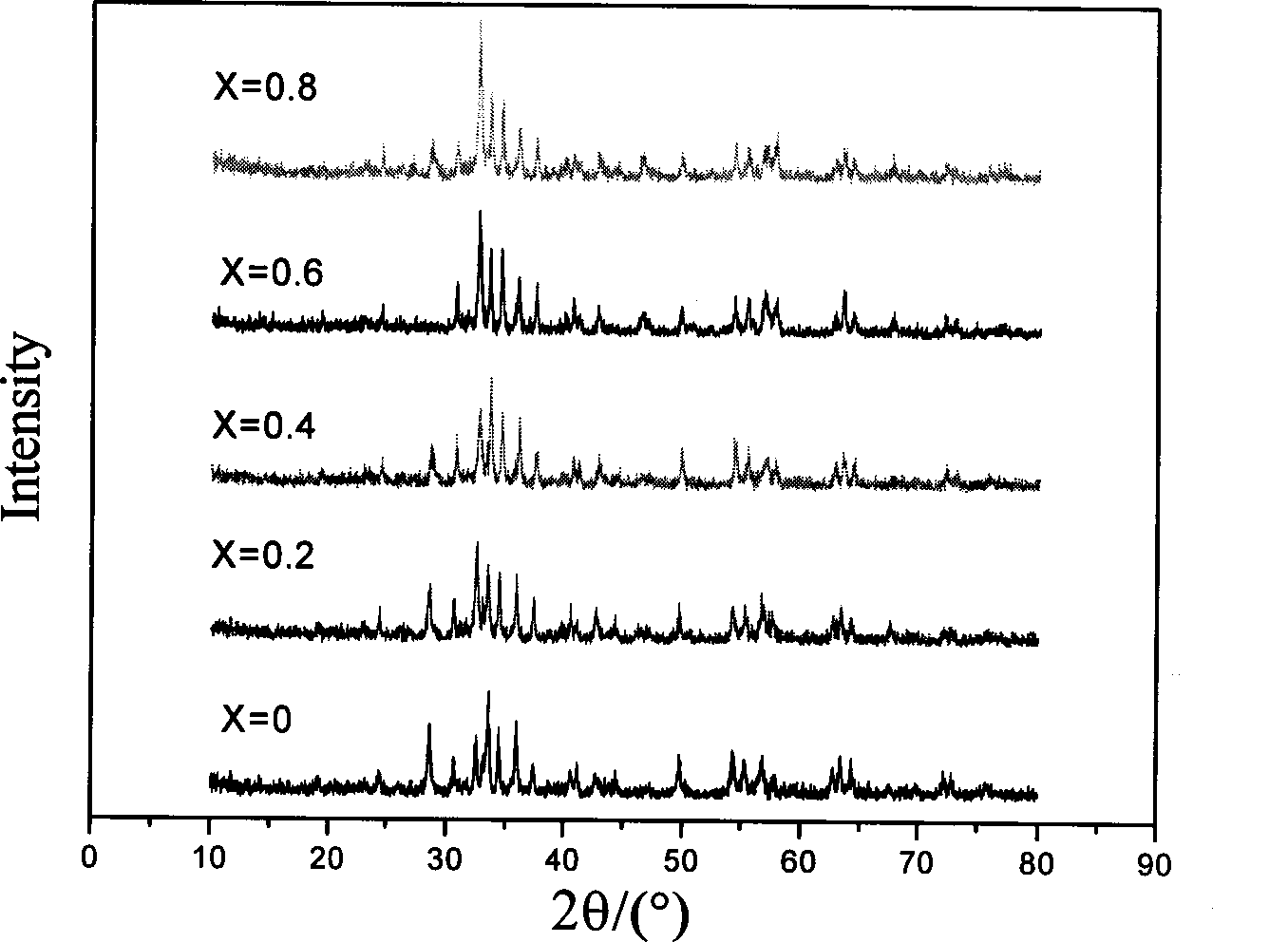
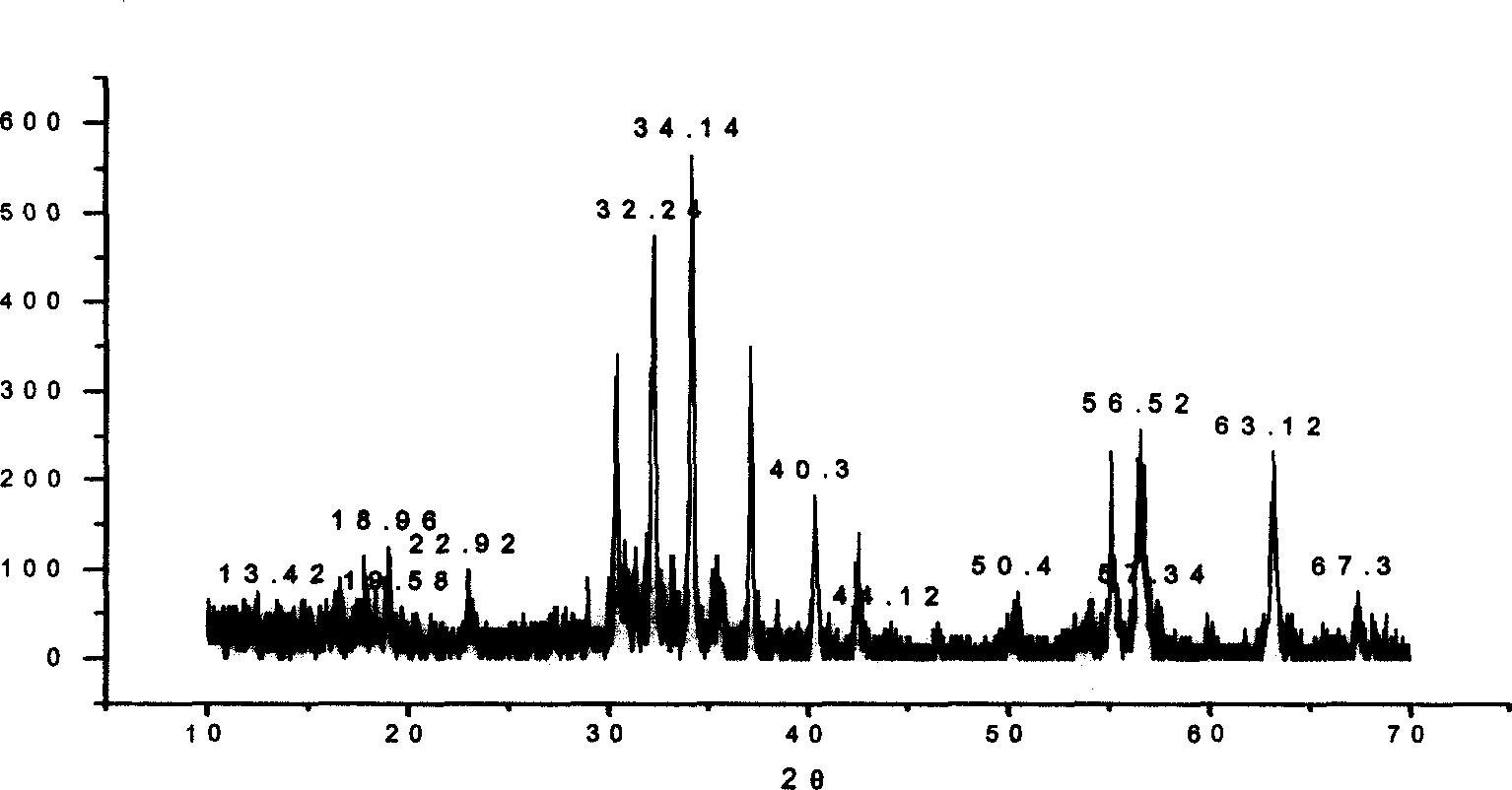
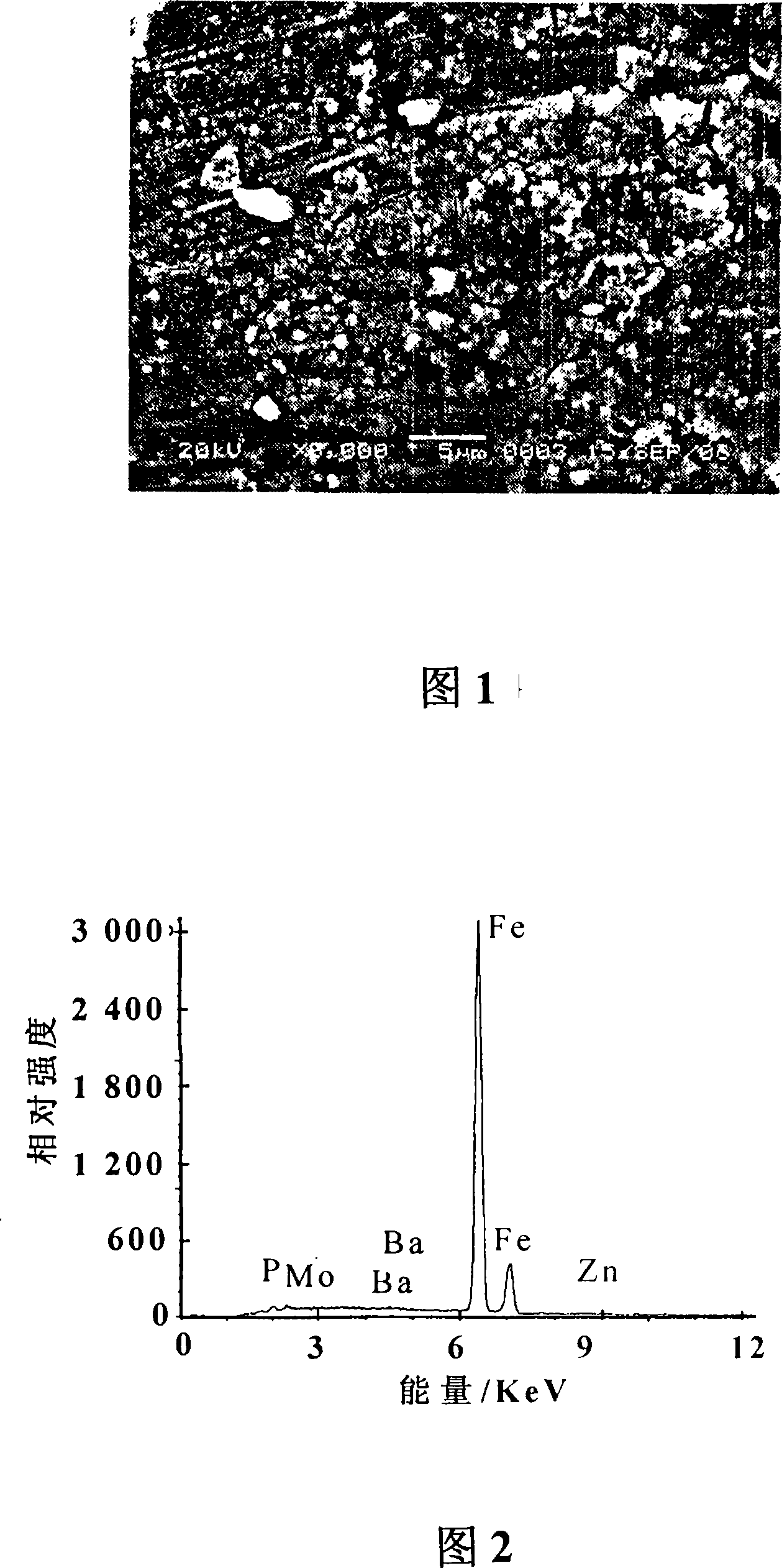
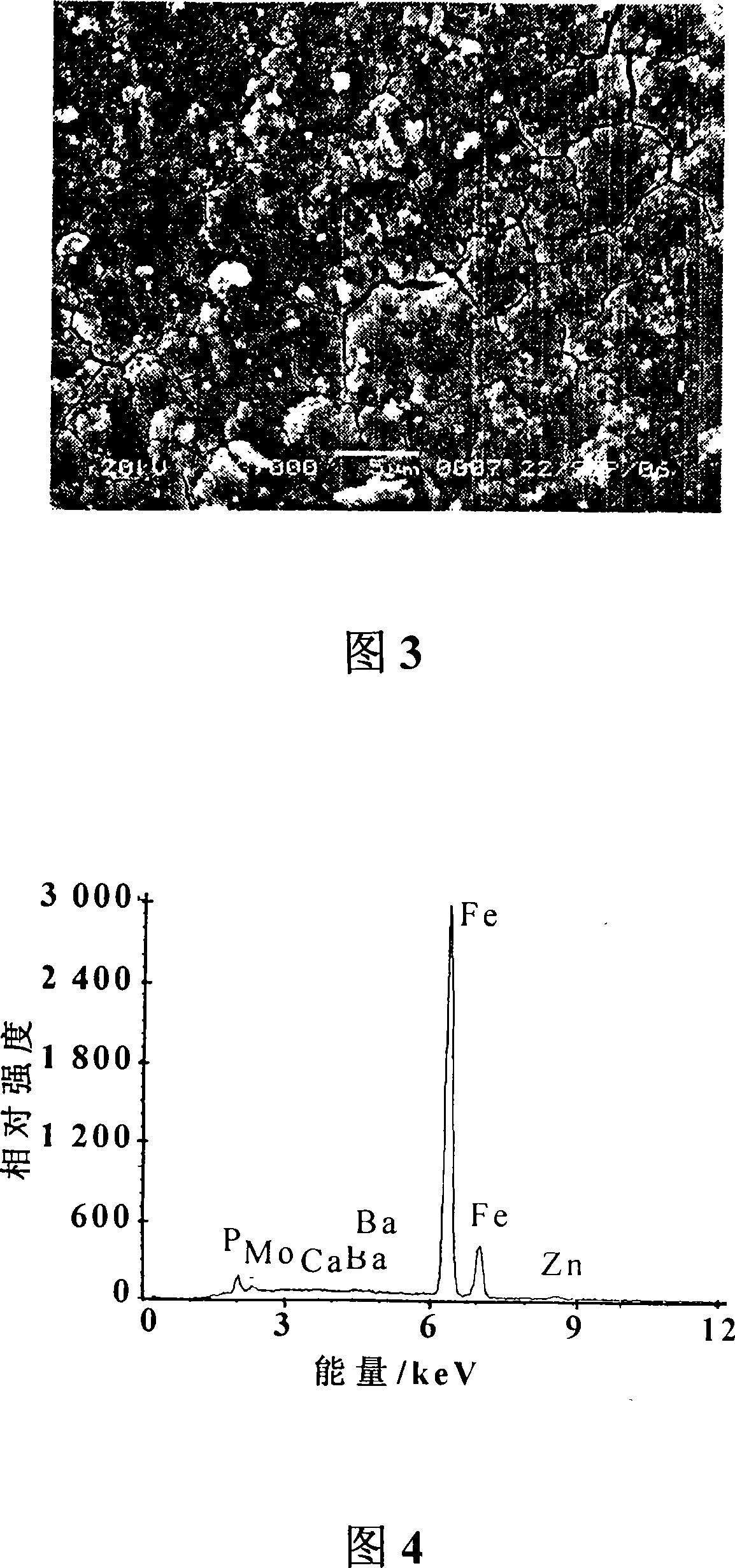
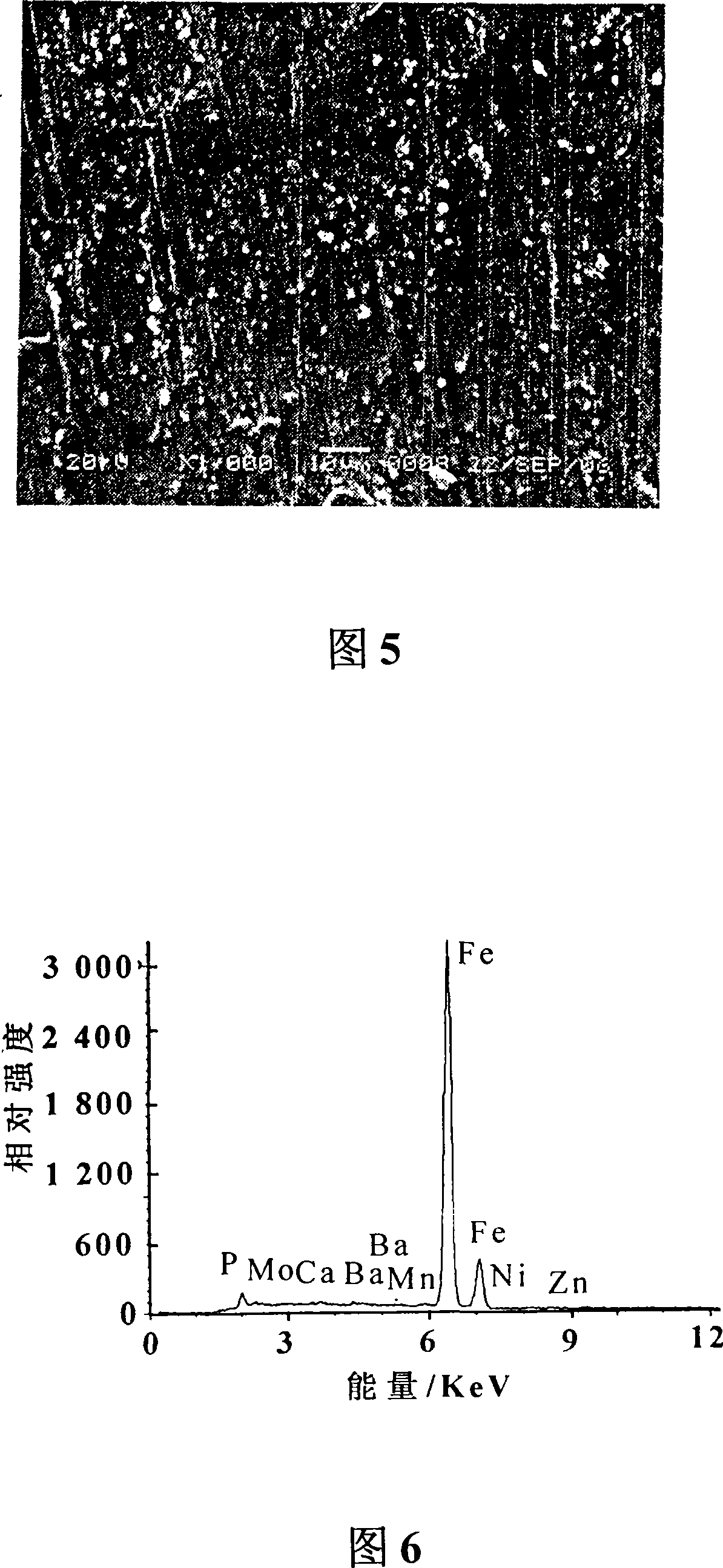
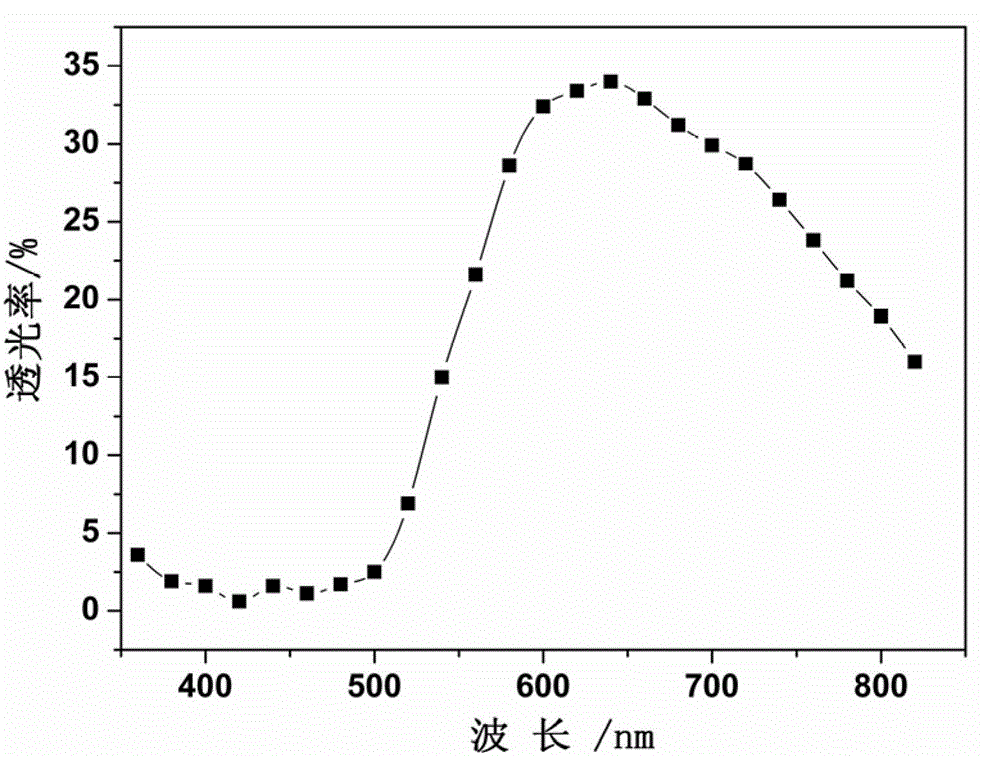
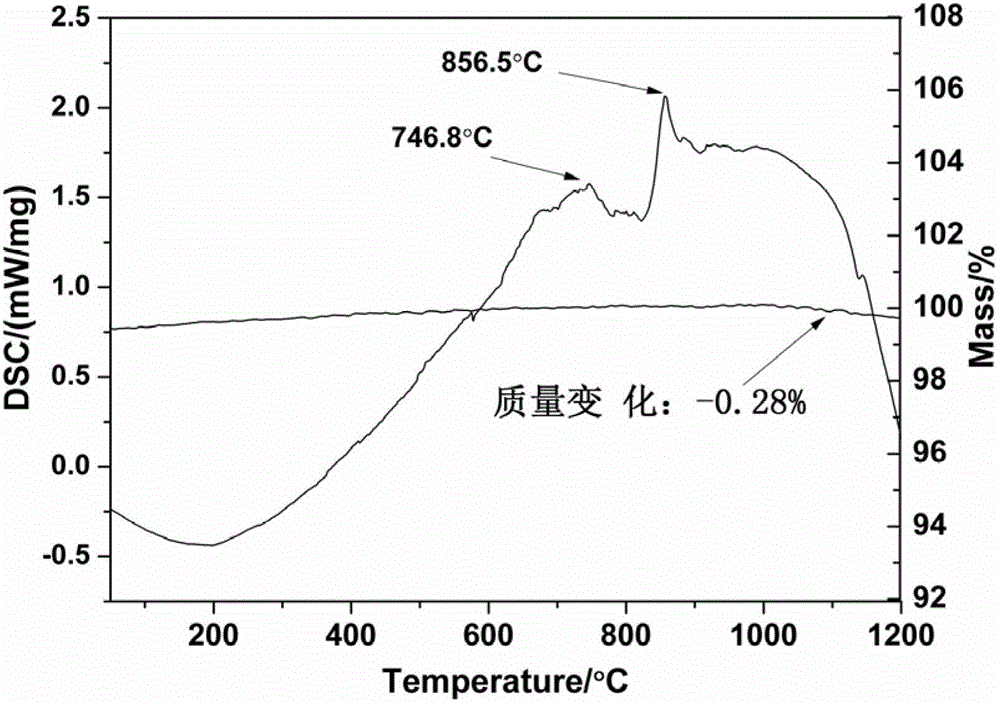
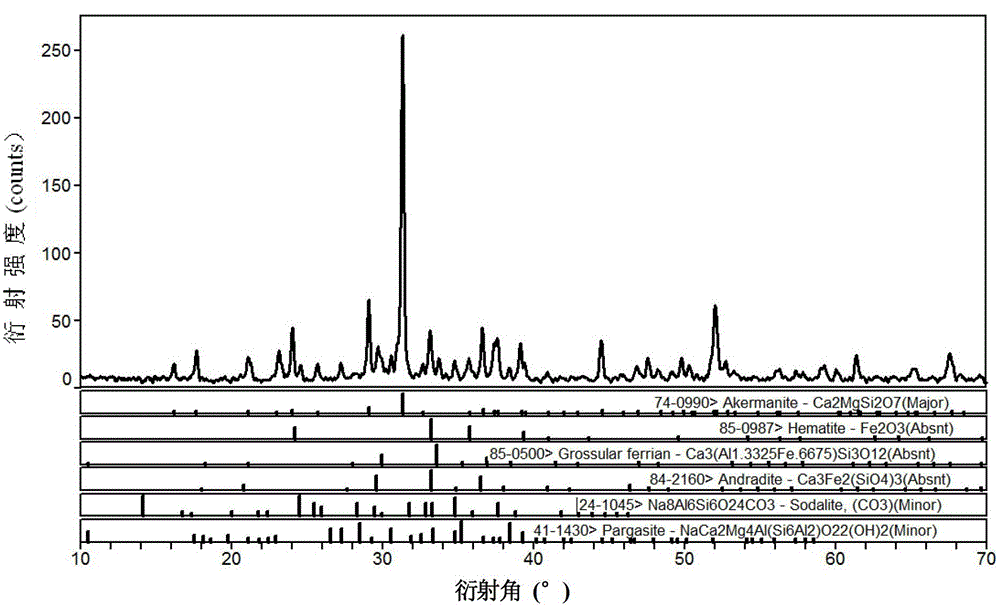
Patents
Literature
592 results about "Barium nitrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Barium nitrate is the inorganic compound with the chemical formula Ba(NO₃)₂. It, like most barium salts, is colorless, toxic, and water-soluble. It burns with a green flame and is an oxidizer; the compound is commonly used in pyrotechnics.
Preparation method of high-performance anti-corrosion repairing mortar
A preparation method of high-performance anti-corrosion repairing mortar including following components, by weight: 150-250 parts of cement, 280-725 parts of quartz sand, 50-200 parts of mineral powder, 50-150 parts of fly ash, 0-50 parts of silicon ash, 5-10 parts of barium nitrate, 0.1-1 part of an early strength agent, 1-4 parts of PP fibers, 0.5-20 parts of latex powder, 0.5-5 parts of cellulose ether, 0.5-2 parts of a water repellent agent, 0.001-0.1 parts of an air entraining agent, 0.1-2 parts of a water reducing agent, 0-1 part of a defoaming agent, and 100-250 parts of water. The invention, aiming to the characteristic of sulphate attack and the performance requirement of repairing mortar, discloses the repairing mortar which can repair the surface of damaged concrete with a protective film formed thereon to prevent the concrete form being corroded again. The repairing mortar is good in construction performance, can be used for coating the surface of the damaged concrete or filling damaged positions of the concrete. The repairing mortar is high in bonding strength and strength, is good in anti-permeability and flexibility, is simple in preparation process, is especially suitable for repairing the concrete subjected to sulphate attack, is great in market prospect and is good in practicability.
Owner:潍坊德霖建材科技有限公司
Chrysotile tailing microcrystal glass and preparation method thereof
InactiveCN101891389AImprove impact resistanceGood chemical resistance and anti-corrosion functionWear resistantQuenching
The invention discloses chrysotile tailing microcrystal glass and a preparation method thereof and belongs to the technical field of chrysotile tailing control and comprehensive utilization. The chrysotile tailing microcrystal glass comprises the following raw materials in percentage by weight: 30 to 50 percent of chrysotile tailing, 27 to 50 percent of fly ash, 9 to 10 percent of limestone, 0 to 4 percent of industrial barium nitrate, 0 to 4 percent of industrial fluorite and 4 to 6 percent of industry pure alkali. The preparation method comprises the following steps of: 1) performing pre-homogenizing treatment on the chrysotile tailing; 2) preparing a mixture; 3) melting glass liquid; 4) performing water quenching and preparing powder; 5) performing press forming; 6) performing crystallization and sintering; and 7) performing demoulding and post-processing. The microcrystal glass prepared by the method is applicable to building, chemical anticorrosion, wear resistant lining for mine and the like. The chrysotile tailing microcrystal glass and the preparation method have a reasonable formula, can effectively improve the comprehensive utilization rate of the chrysotile tailing and reduce environment pollution, and, through harmless phase inversion to the chrysotile tailing, lower the cost for treating chrysotile tailing waste and are beneficial for popularization and application.
Owner:SOUTHWEAT UNIV OF SCI & TECH
Retardation method for alkali-activated-carbonate/slag gel material
InactiveCN1699251AEasy to useThe process steps are simpleSolid waste managementBarium dichlorideBarium nitrate
The invention provides a retardation method for alkali-activated-carbonate / slag gel material which comprises, at normal temperature, calculated by mass portions, dissolving 4-10 portions of barium chloride or barium nitrate into 100 portions of water, thus obtaining retarder solution, then charging 200-300 parts of slag powder, agitating homogeneously, charging in turn 220-3100 parts of exciting agent, 200-3800 parts of marginal carbonate powdered ore, agitating homogeneously, finally obtaining the end product.
Owner:SOUTH CHINA UNIV OF TECH
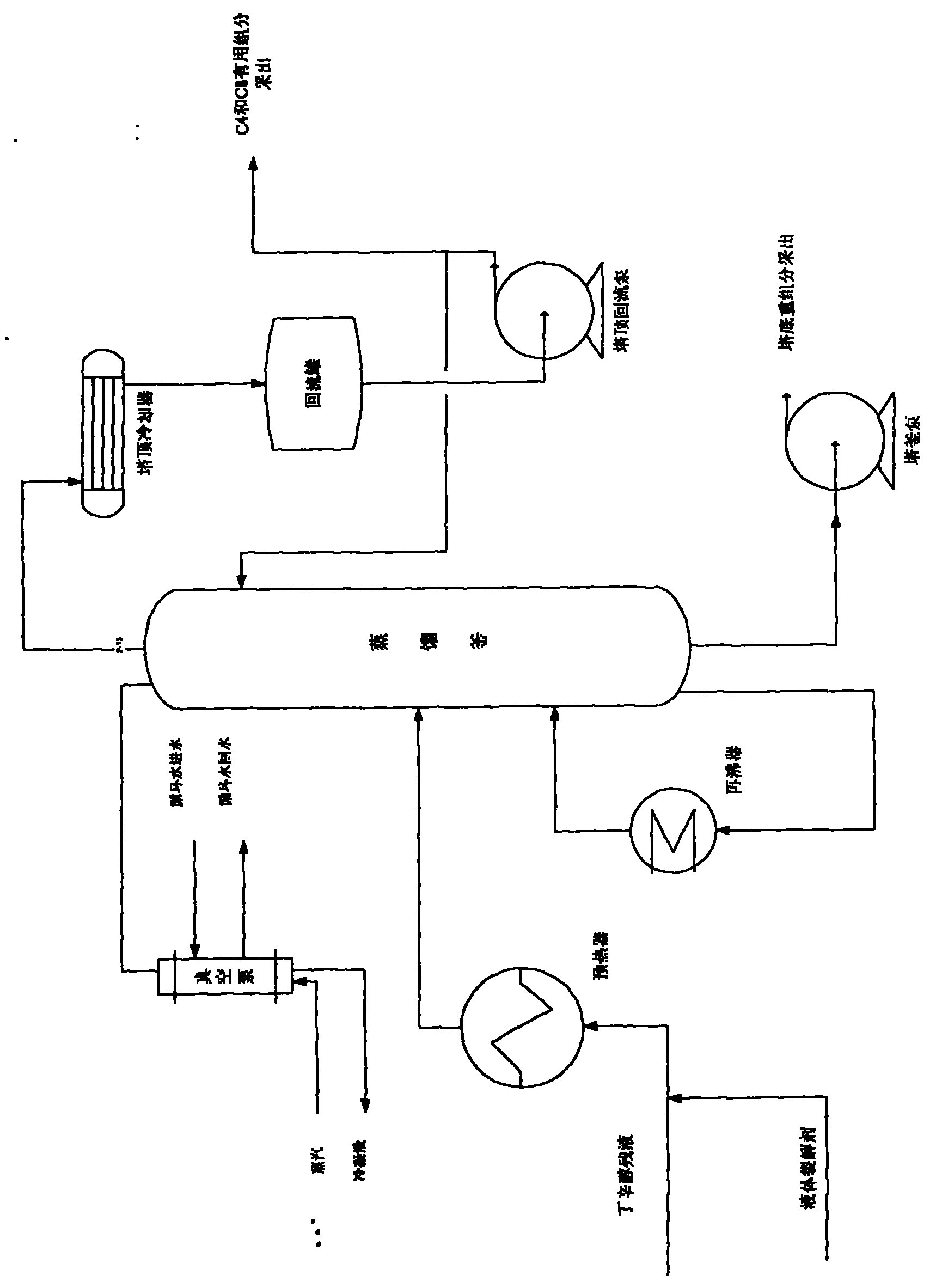
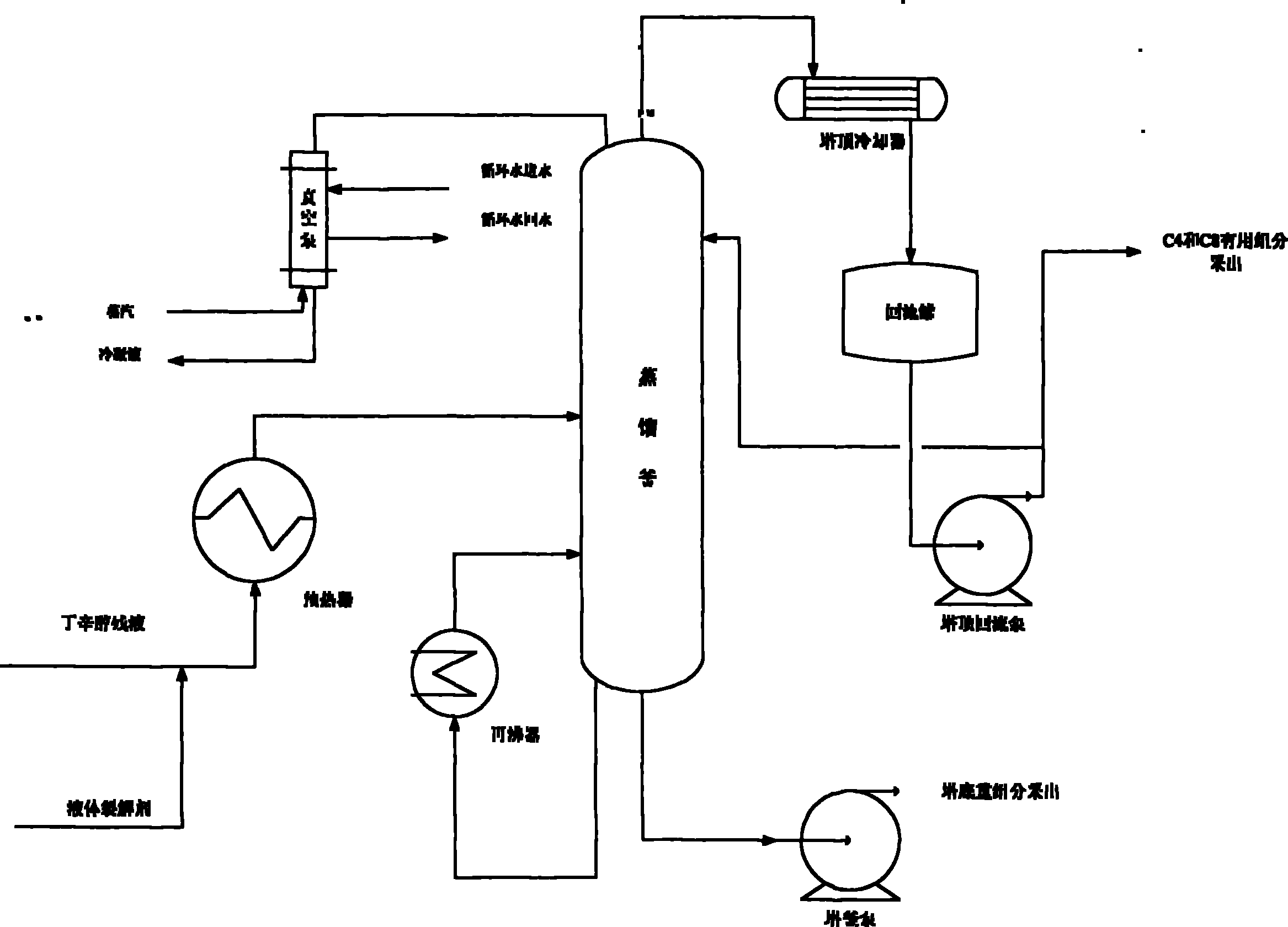
Production technology for cracking butyl octanol residual liquid into C4 and C8 by means of alkaline liquid cracking agent
ActiveCN101892066AReasonable useEmission reductionThermal non-catalytic crackingFractionationPotassium hydroxide
The invention relates to production technology for cracking butyl octanol residual liquid into C4 and C8 by means of an alkaline liquid cracking agent. The technology is characterized by comprising the following steps of: preparing the alkaline liquid cracking agent, wherein the alkaline liquid cracking agent consists of sodium hydroxide, potassium hydroxide, water, isopropanol, ethylene glycol, potassium permanganate, ethanol, barium nitrate and diboron trioxide; preheating the alkaline liquid cracking agent and the butyl octanol residual liquid through a preheater and adding into a distilling still, wherein the tower bottom temperature of the distilling still is between 200 and 280 DEG C, the tower top temperature is between 130 and 190 DEG C, and the tower top pressure is between 0.08 and 0.1Mpa below zero; and extracting the C4 and C8 generated by cracking from the tower top of the distilling still, pumping the C4 and C8 into a fractionating column at the downstream for fractionation, and extracting a small amount of uncracked heavy components from a tower bottom pump. The production technology has the advantages of high production capacity, high cracking selectivity, high content of the cracked useful components, low cost, simple process, and batch distillation or continuous distillation.
Owner:山东瑞利尔石油装备有限责任公司
Sparkle beads producing cardamon green firework effect, and manufacturing method thereof
The invention relates to firework effect sparkle beads and a manufacturing method thereof, and specifically relates to sparkle beads producing a cardamon green firework effect, and a manufacturing method thereof. The sparkle beads comprises main components of, by weight: 50-60 parts of barium nitrate, 6-8 parts of phenolic resin, 18-22 parts of aluminum-magnesium alloy, 6-8 parts of polrvinyl chloride, 13-17 parts of potassium perchlorate, and 1-3 parts of cryolite. The manufacturing method of the sparkle beads comprises the steps of: agent weighing; raw material crushing; mixing and stirring; molding; drying; and packaging. With the sparkle beads provided by the invention, a novel firework color variety is provided. A light duration is 3.5 seconds, and a sparkle bead combustion residue rate is lower than 0.3%.
Owner:浏阳市五环烟花厂
A method for recovering and preparing a lithium-manganese-iron phosphate positive-electrode material covered with carbon from waste lithium iron phosphate batteries
InactiveCN108923090AHigh recovery rateHigh purityCell electrodesNanotechnologyLithium iron phosphateFiltration
The invention provides a method of recovery and reutilization of waste lithium iron phosphate batteries. The method includes (1) separating positive electrode material mixture from the waste lithium iron phosphate batteries; (2) fully dissolving the positive electrode material mixture with sulfuric acid, performing filtration to obtain a first filtrate, adding ammonia water into the filtrate understirring until the pH value of the system is 1.0-1.9, stirring the mixture, and performing filtration to obtain a second filtrate and iron phosphate precipitate; (3) adding barium hydroxide or bariumnitrate into the second filtrate and performing filtration to obtain a third filtrate; (4) adding the third filtrate, the iron phosphate precipitate, a manganese source, a phosphorus source and a carbon source according to the element ratio of a product to be prepared that is lithium-manganese-iron phosphate LiFe<1-x>Mn<x>PO4 to obtain a solution mixture; and (5) subjecting the solution mixture to ball milling, drying and crushing, pre-sintering a product at a first temperature in an inert atmosphere, and sintering the product at a second temperature to obtain the lithium-manganese-iron phosphate positive-electrode material covered with carbon. Through the method, all elements in the waste lithium iron phosphate batteries are recovered and reutilized.
Owner:SHENZHEN DYNANONIC
Formula and preparation process of concrete sulfate resistance agent
The invention relates to a formula of a concrete sulfate resistance agent, which comprises the following ingredients by weight percentage: one or more of 30% to 50% ferric nitrate, ferrous nitrate and magnesium nitrate, 20% to 40% barium nitrate, 0.5% to 10% sodium silicate, 5% to 10% lithium hydroxide, 1% to 5% sodium benzoate and 30% to 60% coal ash or grounded mineral powder. A preparation process of the concrete sulfate resistance agent comprises the following steps of: mixing one or more of 30% to 50% ferric nitrate, ferrous nitrate and magnesium nitrate, 20% to 40% barium nitrate, 0.5% to 10% sodium silicate, 5% to 10% lithium hydroxide, 1% to 5% sodium benzoate and 30% to 60% coal ash or grounded mineral powder in a mixer or a mixing tank for 5 to 30 minutes, discharging the materials, sealing and preserving. The sulfate resistance coefficient of the concrete sulfate resistance agent can reach 95% to 98%, so that the impervious and the freeze proof levels of the concrete are greatly improved, and the service durability of the concrete is greatly enhanced.
Owner:WATER RESOURCES RES INST OF SHANDONG PROVINCE
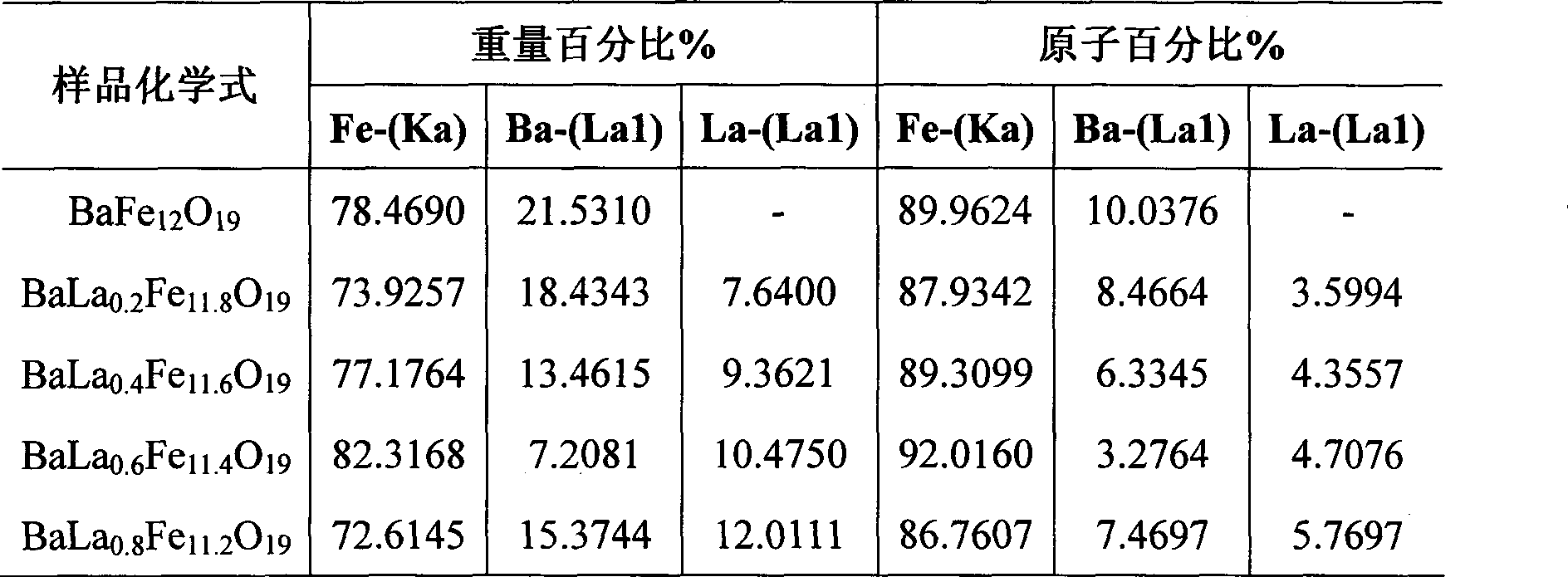
Lanthanum doped nano barium ferrite film and method of manufacturing the same
The invention relates to a lanthanum doping nanometer barium ferrite thin film and a preparation method thereof, which is technically characterized in that the prescription is as follows: 1.48 g / 100 ml to 1.60 g / 100 ml of glycol, 2.51 g / 100 ml to 2.71 g / 100 ml of citric acid, 1.74 g / 100 ml of iron nitrate, 0.125 g / 100 ml of barium nitrate and 0.03 g / 100 ml to 0.125 g / 100 ml lanthanum nitrate. The preparation method is that the iron nitrate, the barium nitrate, the lanthanum nitrate, and the like, serve as raw materials to prepare a forerunner body the forerunner body of the lanthanum doping nanometer barium ferrite in a sol-gel method; sol-gel method; clean silicon dioxide serves as a support base, the iron nitrate, the barium nitrate and the lanthanum nitrate serve as main salt, the citric acid serves as complexing agent, the glycol serves as complexing agent assist, and the soakage-drawing method is adopted to make the film. The method has the advantages of simple process flow and low cost; the method is convenient for preparing the thin film on bases with different shapes, the lanthanum doping nanometer barium ferrite thin film with high purity is obtained, and the thin film can be used for preparing magnetic recording materials and absorbing materials.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Sulfur-free ring stabilizing agent propellant powder
The invention discloses sulfur-free ring stabilizing agent propellant powder which consists of the following components in parts by weight: 70 parts of an oxidant, 34 parts of a burnable agent, 6 parts of an adhesive, 2 parts of a stabilizing agent, 2 parts of an anti-aging agent, 1 part of a catalyst and 2 parts of an antismoke agent which are uniformly mixed according to a general method, wherein the oxidant is a mixture consisting of potassium perchlorate, amine perchlorate and barium nitrate; the burnable agent is a mixture consisting of potassium hydrogen terephthalate, potassium acid phthalate, nitrocellulose, purified terephthalic acid and hemp stem carbon powder; the stabilizing agent adopts calcium stearate; the anti-aging agent adopts calcium phthalate; the catalyst adopts copper oxide; the antismoke agent adopts aluminum hydroxide; the adhesive is selected from one or a mixture of multiple of phenolic resin, strong adhesive powder, glutinous rice flour and polyvinyl alcohol according to any ratio. The sulfur-free ring stabilizing agent propellant powder is high in power capability and excellent in safety performance indexes and has excellent storage stability, and the environment-friendly performance is greatly improved.
Owner:HUNAN FEIYU NEW MATERIAL CO LTD
Combined chemical for fireworks and its prepn process
InactiveCN1810741AReasonable ratio of material selectionImprove thermal stabilityExplosivesSlagFireworks
The present invention is one kind of combined chemical for fireworks and its preparation process, and aims at providing one kind of combined chemical for fireworks possessing high heat stability, high mechanical sensitivity and high safety for compounding, producing, storing and transporting. It is ignited only in burning its fuse. The combined chemical consists of oxidant of barium nitrate and / or potassium nitrate; and inflammable including one or several of sulfur, aluminum powder, Al-Mg alloy powder, charcoal powder, titanium powder, coal powder, alumina slag, iron slag, carbon slag, resin and starch dextrin in the ratio of 100 to 10-120. It may also contain coloring agent of barium nitrate, strontium nitrate, copper carbonate and / or sodium oxalate; and effect chemical comprising sounder and color bead.
Owner:邵兴宾
Lead-free primers
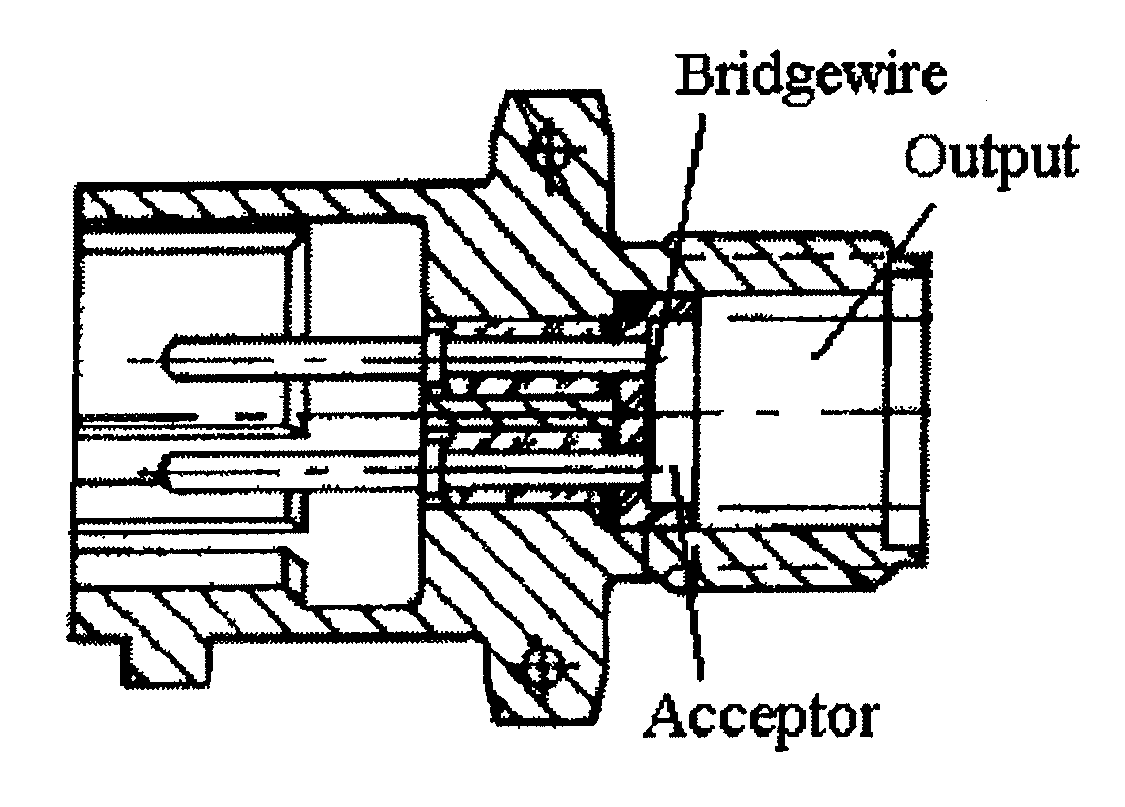
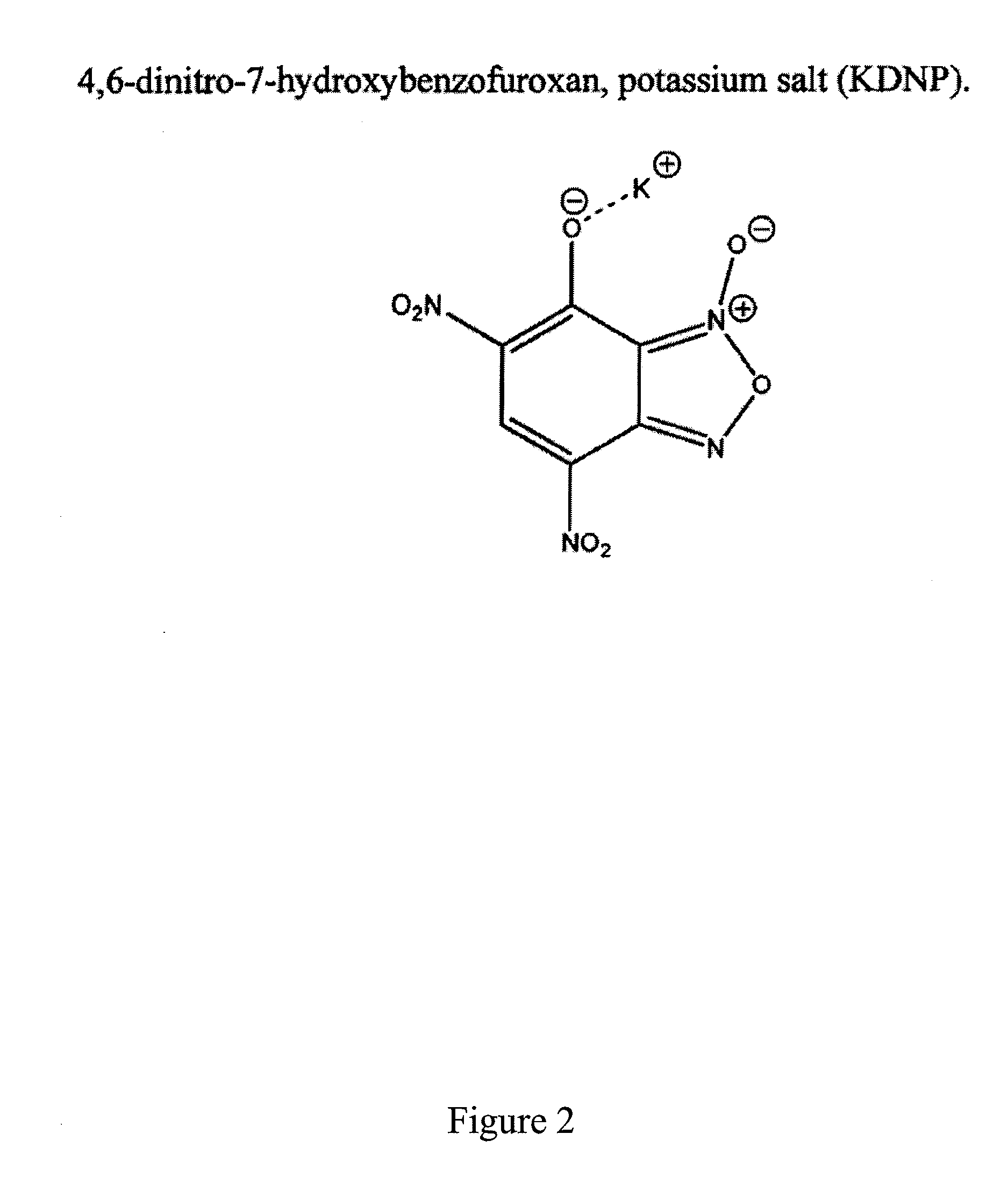
Embodiments of the present subject matter provide an improved percussion primer composition and improved hot-wire igniter acceptor, wherein lead styphnate is replaced with a lead-free material, 4,6-dinitro-7-hydroxybenzofuroxan, potassium salt (KDNP). Embodiments of the percussion primer composition include KDNP, a sensitizer, an oxidizer, calcium silicide, a fuel, and a binder. Sensitizers may include tetracene. Oxidizers may include alkali or alkaline earth nitrates, oxides, or peroxides (such as barium nitrate). Fuel materials may include metals, metal sulfides, or other non-metallic materials. Common binders may include nitrocellulose based shellacs, gum arabic / poly vinyl alcohol mixtures, and guar gum / poly vinyl alcohol mixtures. Embodiments of the hot-wire igniter device include a bridgewire, an acceptor, and an output, where KDNP is the acceptor. Power supply may be in the form of constant current / voltage or current flow from a capacitor discharge. Certain embodiments utilize a variety of output formulations, such as BKNO3, black powder, and Red Dot double base propellant.
Owner:PACIFIC SCI ENERGETIC MATERIALS +1
Active carbon supported ruthenium-based ammonia synthetic catalyst and preparation thereof
InactiveCN101322947AImproved size distributionSimple processOrganic-compounds/hydrides/coordination-complexes catalystsBulk chemical productionPotassium hydroxideRuthenium chloride
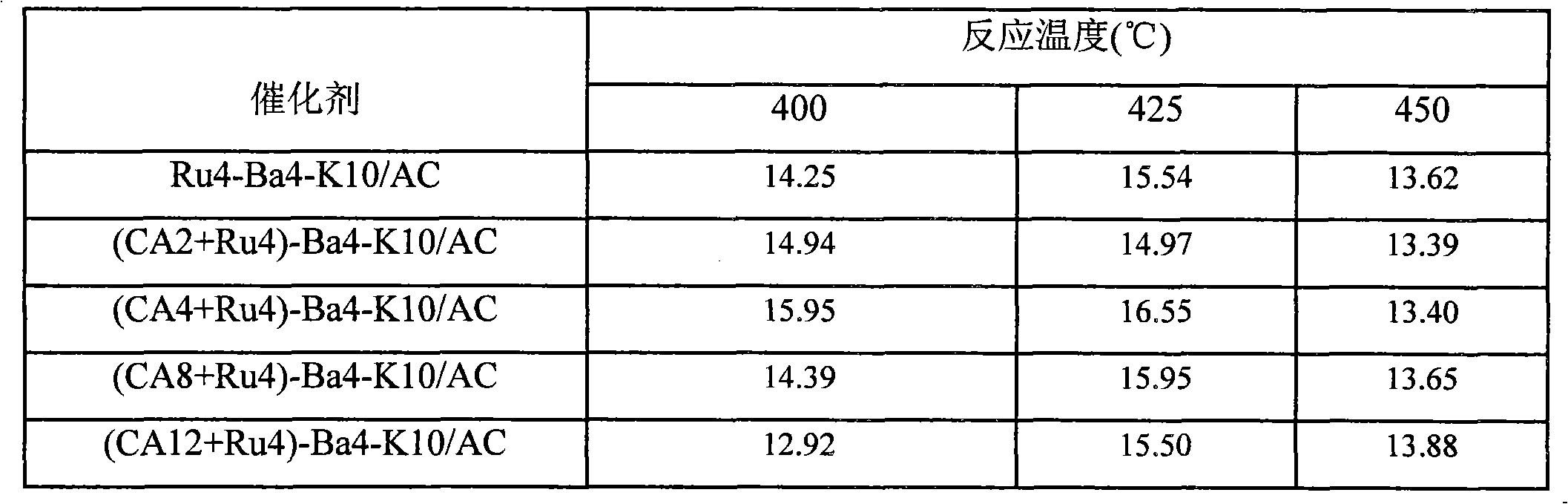
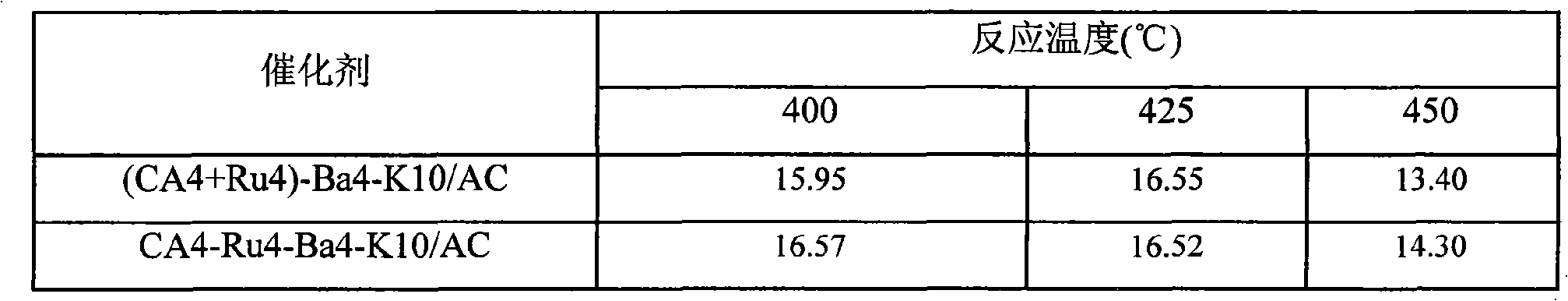
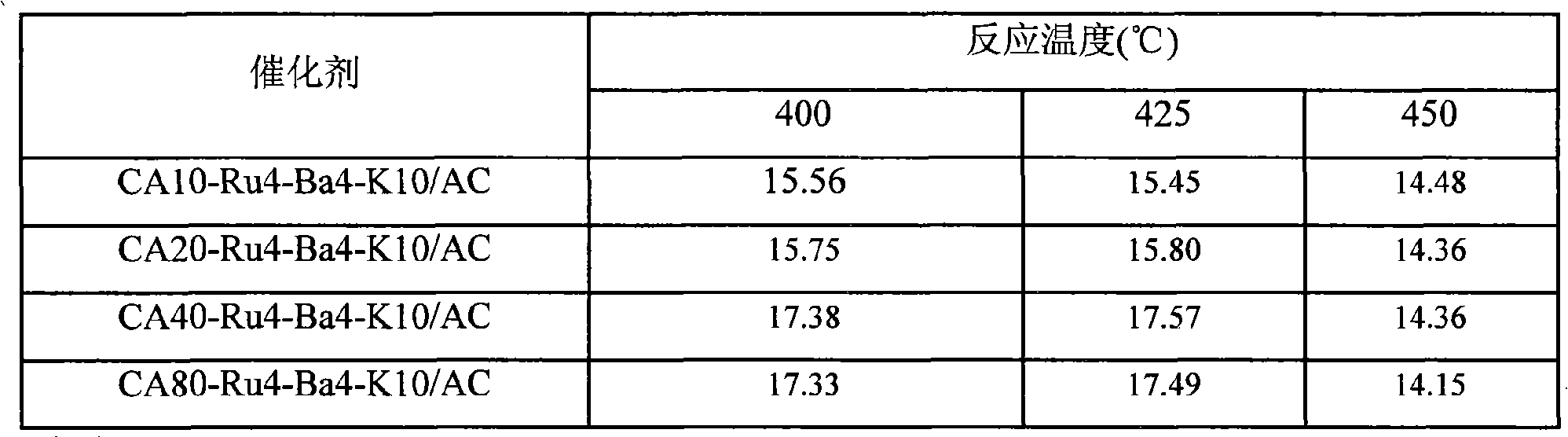
The invention provides an active carbon-supported ruthenium ammonia synthesis catalyst and a preparation method thereof. The catalyst takes active carbon as a carrier and takes ruthenium as an active component; the components of the catalyst are (CA+Ru)-Ba-K / AC and CA-Ru-Ba-K / AC, wherein, CA represents citric acid and AC represents the active carbon; in preparation of the catalyst, the citric acid is selected to carry out the pretreatment of the active carbon and ruthenium chloride; one or more of barium nitrate, potassium hydroxide or potassium nitrate is or are selected as an assistant; the equivalent-volumetric impregnation method is adopted in the preparation method for preparing the catalyst. Compared with the sol-gel method or the oxidation method in the prior art, the invention adopts the citric acid method and has the advantages of simple technique, high preparation efficiency, little energy consumption, cheap raw material and less material consumption, thus being beneficial to greatly lowering the production cost. The devices required by the invention are simple, are easy to be operated, do not need to change the original devices and are easy to realize mass production. The catalyst prepared by the invention has good performance and strong catalytic activity.
Owner:FUZHOU UNIV
Bright green cold firework composition and production method thereof
The invention discloses a bright green cold firework composition and a preparation method thereof. The composition comprises the following ingredients by weight percentage: 25-40% of boron powder, 5-15% of magnalium powder, 6-12% of titanium powder, 8-15% of barium nitrate, 6-10% of 52# chlorinated paraffin, 15-20% of nitrocotton and 15-20% of ammonium perchlorate. The preparation method of the composition comprises the steps of fuel mixing, fuel granulation, fuel grain drying and composition mixing. A cold firework produced by the composition can emit bright green light, the color variety of the cold firework is enriched, and the enjoyment effect of the cold firework is improved.
Owner:NANJING UNIV OF SCI & TECH
Sulfur-free and pearlite-free firecracker reagent and preparation method thereof
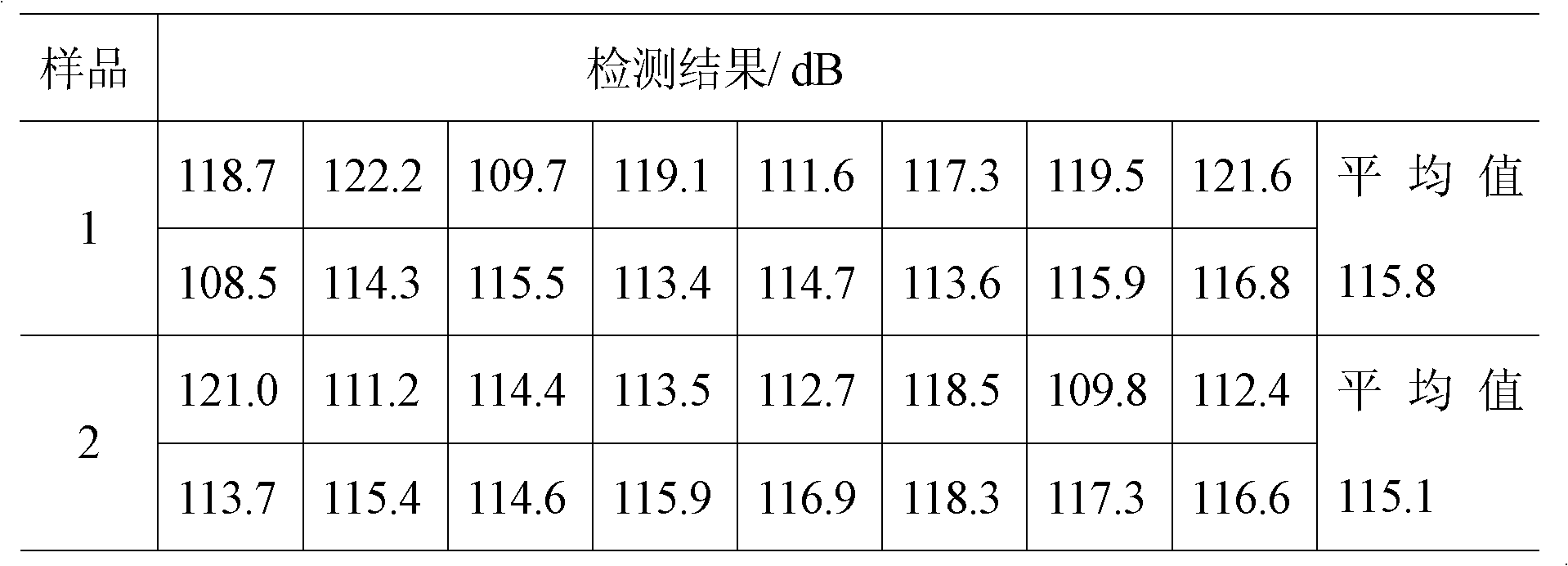
The invention discloses a sulfur-free and pearlite-free firecracker reagent and a preparation method thereof. The reagent comprises the following mass components: 35-65 percent of A component and 35-65 percent of B component, wherein the A component comprises the mass compositions: a mixture formed by 20-50 percent of potassium perchlorate, 0-30 percent of potassium nitrate and 30-50 percent of barium nitrate and a complexing agent occupying 1-5 percent of the mixture, and the B component comprises the mass compositions: 50-80 percent of aluminum powder, 10-25 percent of charcoal powder and 10-25 percent of organic foaming powder. The burning rate of a firecracker prepared from the reagent is more than 98 percent, the sound level value is no less than 100dB and is no more than 140dB, the time the gas product pressure rises from 0.69MPa to 2.07MPa is 1.74ms, a pH value of the pyrotechnic composition is 5-9, the moisture is no more than 1.5 percent, the hygroscopicity is no more than 2.0 percent, the thermal stability is 75 DEG C plus / minus 2 DEG C, the firecracker has no decomposition phenomenon under the condition of 48h, the friction sensitivity is no more than 40 percent, and the impact sensitivity of the pyrotechnic composition is no more than 0 percent. The key is that the reagent reduces the environmental pollution without harm to human bodies and has great application prospect.
Owner:HUNAN VOCATIONAL INST OF SAFETY TECH +1
Combustion assisting agent with aeration combustion-supporting and coal saving function
The invention offer strong oxygen combustion supporting synergy coal saving combustion adjuvant. Its main technique feature is proportioning the following each preparation by weight part ratio according to coal quality: strong oxidant: potassium permanganate, potassium chlorate, potassium nitrate, hydrogen peroxide, hexamethylene tetramine, potassium perchlorate; strong catalyst: manganese dioxide, magnesia; scale remover: calcium oxide dolomite fines, calcium carbonate; fume clearing agent: sodium carbonate, active magnesia, light magnesium oxide; desulfurizer: alumina, magnesium silicate, barium nitrate; leavening agent: industry using sodium chloride; diatomite: pumice, boric sludge; ferric oxide is very fit for low grade coal. The advantages of the invention are that it can make low grade coal burn fully, increase thermal efficiency, save energy, no pollution; and it can save fund for corporation; it can save 100-250 thousand yuan per ten thousand ton coal; and the coal saving rate can reach 15-25%.
Owner:迟万斌
Sulfur-smoke-free powder composition and preparation method thereof
InactiveCN103214322ADisadvantages of Avoiding Cryogenic DecompositionSolve the disadvantage of easy moisture absorptionExplosivesNitro compoundBarium nitrate
The invention discloses a sulfur-smoke-free firework powder composition and a preparation method thereof. According to the powder composition, potassium perchlorate and barium nitrate are used as oxidants, and a nitro-compound is used as a flammable agent, so that the safety and environment-friendliness of the firework production are greatly improved, the production cost of the firework powder is lowered, and various indexes are excellent through the detection. The preparation method of the sulfur-smoke-free firework powder composition is simple and mainly comprises the simple processes of mixing, pelletizing, drying and screening. According to the sulfur-smoke-free firework powder composition and the preparation method thereof disclosed by the invention, the problems that the conventional black powder is poor in safety, easy to absorb moisture, large in production risk and the like are solved. The sulfur-smoke-free firework powder composition has the advantages of having an emission effect better than that of black powder, achieving zero-exhaustion of sulfur dioxide and the like. The sulfur-smoke-free firework powder composition is extensively applicable to preparing firework propellant powder and 3-8-inch powder of large festive fireworks.
Owner:LIUYANG HELI HIGH TECH DEV CO LTD
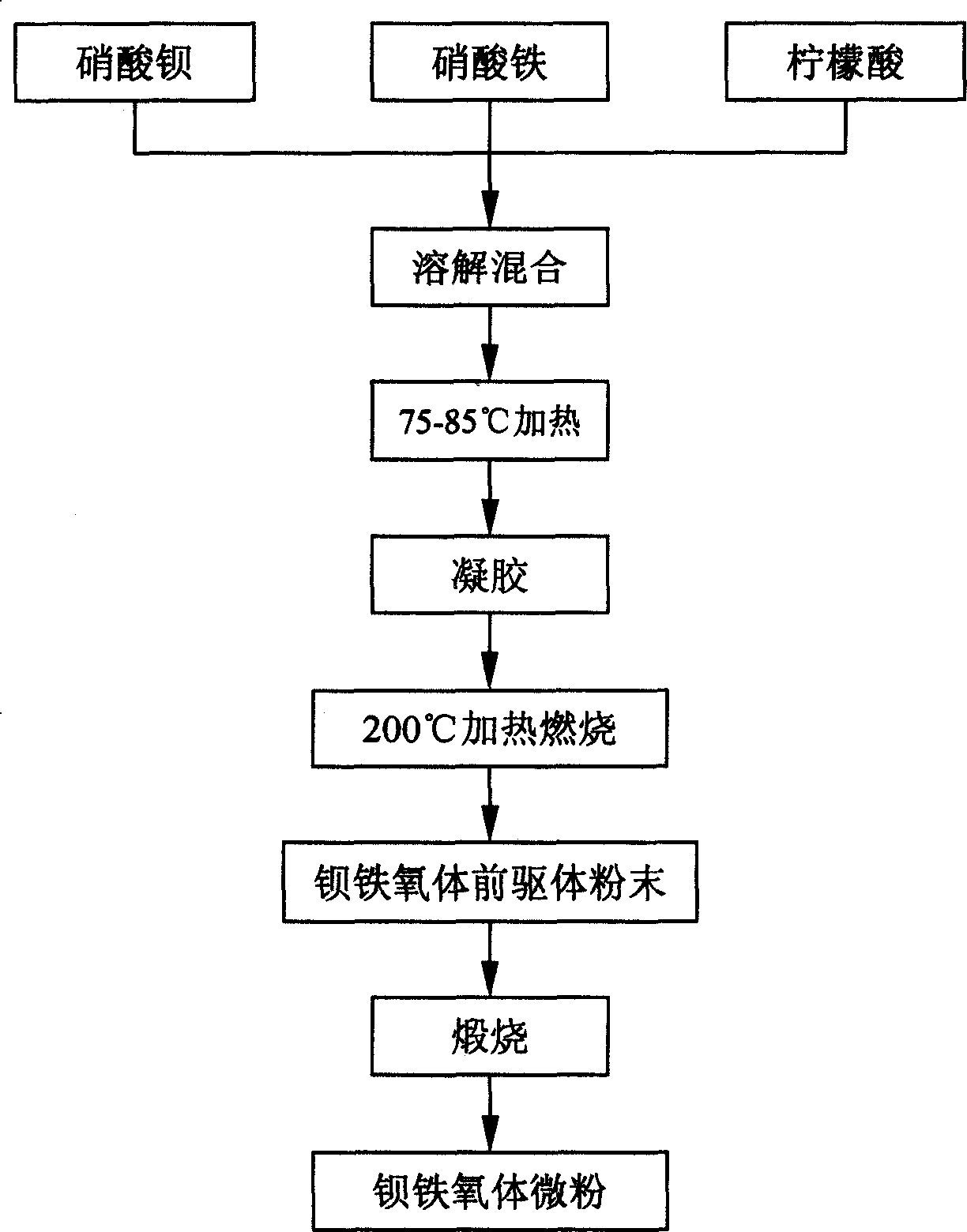
Process for synthesizing barium ferrite micro powder by self combustion method
The self-combustion process of synthesizing fine barium ferrite powder includes the following steps: 1) compounding 0.125-0.25 M barium nitrate solution, compounding 0.125-1.0 M ferric nitrate solution, and mixing via stirring to obtain mixed solution an of molar Fe / Ba ratio in 12; 2) mixing citric acid with mixed solution A to obtain mixed solution B with the molar ratio between nitrate radical and citric acid of 1 to 1-3 through stirring, regulating pH value with acid or alkali to 7.5-8.5, heating at 75-85 deg.c to evaporate for 8-12 hr to form gel; 3) evaporating water of the gel and self-spreading combustion in electric furnace at 200 deg.c to form powder precursor; and 4) calcining the powder precursor at 600-700 deg.c for 2-4 hr to obtain the fine barium ferrite powder. The present invention has the features of low synthesis temperature, high product purity and small average grain size.
Owner:WUHAN UNIV OF TECH
Normal temperature barium salt modified phosphorizing liquid capable of avoiding water wash after phosphorized
InactiveCN101109081AQuality improvementImprove corrosion resistanceMetallic material coating processesEpoxyBarium nitrate
The invention relates to a phosphating washing-free phosphating solution modified with barium salt at ambient temperature. The phosphating solution comprises a phosphoric acid, a nitric acid, and Zn2+, Ca2+, Mn2+, Ni2 plus salt thereof, ammonium molybdate,ammonium tungstate, phytate, H2O2 and barium nitrate or barium hydroxide. The pH value ranges from 1.6 to 4.2. The invention can be phosphated under soak, rinse, brush or the combined way thereof at 3 DEG C.to 45 DEG C.. The phosphating solution is stable and capable of automatically precipating and removing the SO42- inside the phosphating solution and therefore eliminating the influence of SO42- on quality; the phosphorizating membrane is continues, uniform and compact, the membrane weight is between 0.5g is multiplied by m and 3.0g is multiplied by m minus 2 and the iron-red epoxy primer is sprayed and the adhesion thereof can be 1 grade.
Owner:余取民 +3
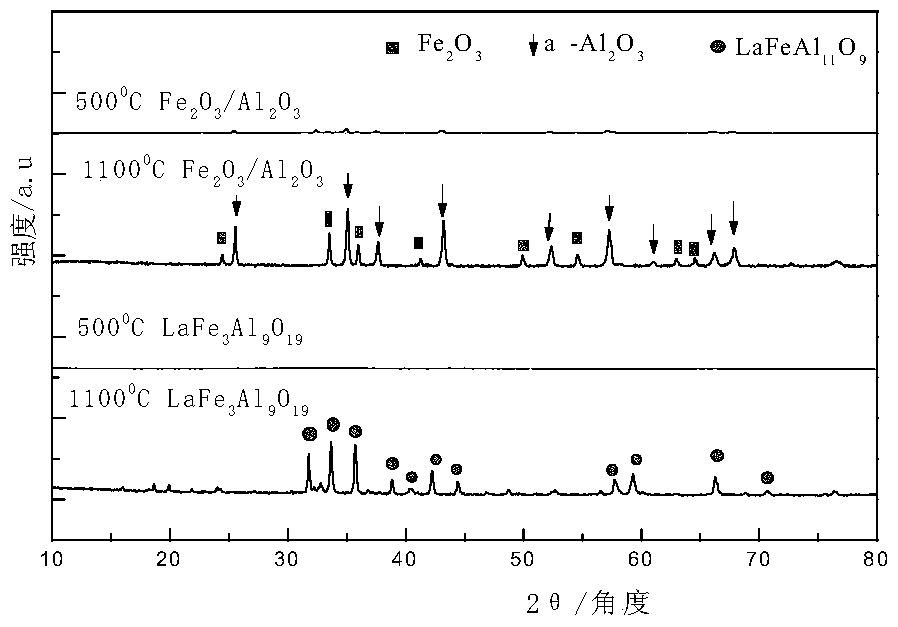
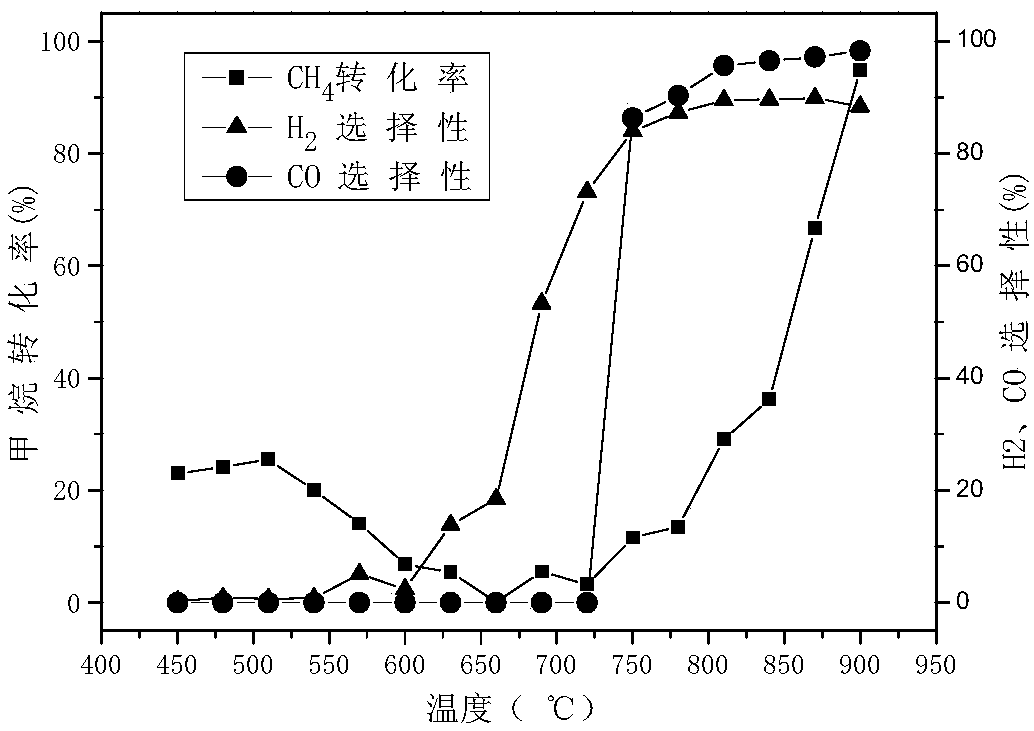
Oxygen carrier for chemical cycle dry gas reforming, and preparation method and application of oxygen carrier
ActiveCN105056955AEasy to prepareEase of industrial productionHydrogenMetal/metal-oxides/metal-hydroxide catalystsFiltrationRare earth
The invention provides an oxygen carrier for chemical looping dry gas reforming, and a preparation method and application of the oxygen carrier. The oxygen carrier is a composite metal oxide adopting a hexaaluminate structure, and has the general formula of AMxAl(12-x)O19, wherein A is rare earth metal lanthanum and / or barium, M is transition metal Fe, and x is greater than 0 or smaller than 5. The temperature of the oxygen carrier in a fuel reactor is 750-1000 DEG C, and the temperature of the oxygen carrier in an oxidation reactor is 750-1000 DEG C, and both of the reaction pressures are normal pressure. The preparation method comprises the following steps: using iron nitrate, aluminum nitrate, lanthanum nitrate and / or barium nitrate as a precursor to prepare a nitrate solution; adding ammonium carbonate as a precipitant for coprecipitation; performing suction filtration, washing, drying and roasting to obtain the oxygen carrier. The oxygen carrier has the advantages of being larger in oxygen-carrying rate, higher in reactivity, excellent in shock-resistant mechanical property and high temperature stability, environmental-friendly, low in cost and easy to prepare.
Owner:NORTHWEST UNIV(CN)
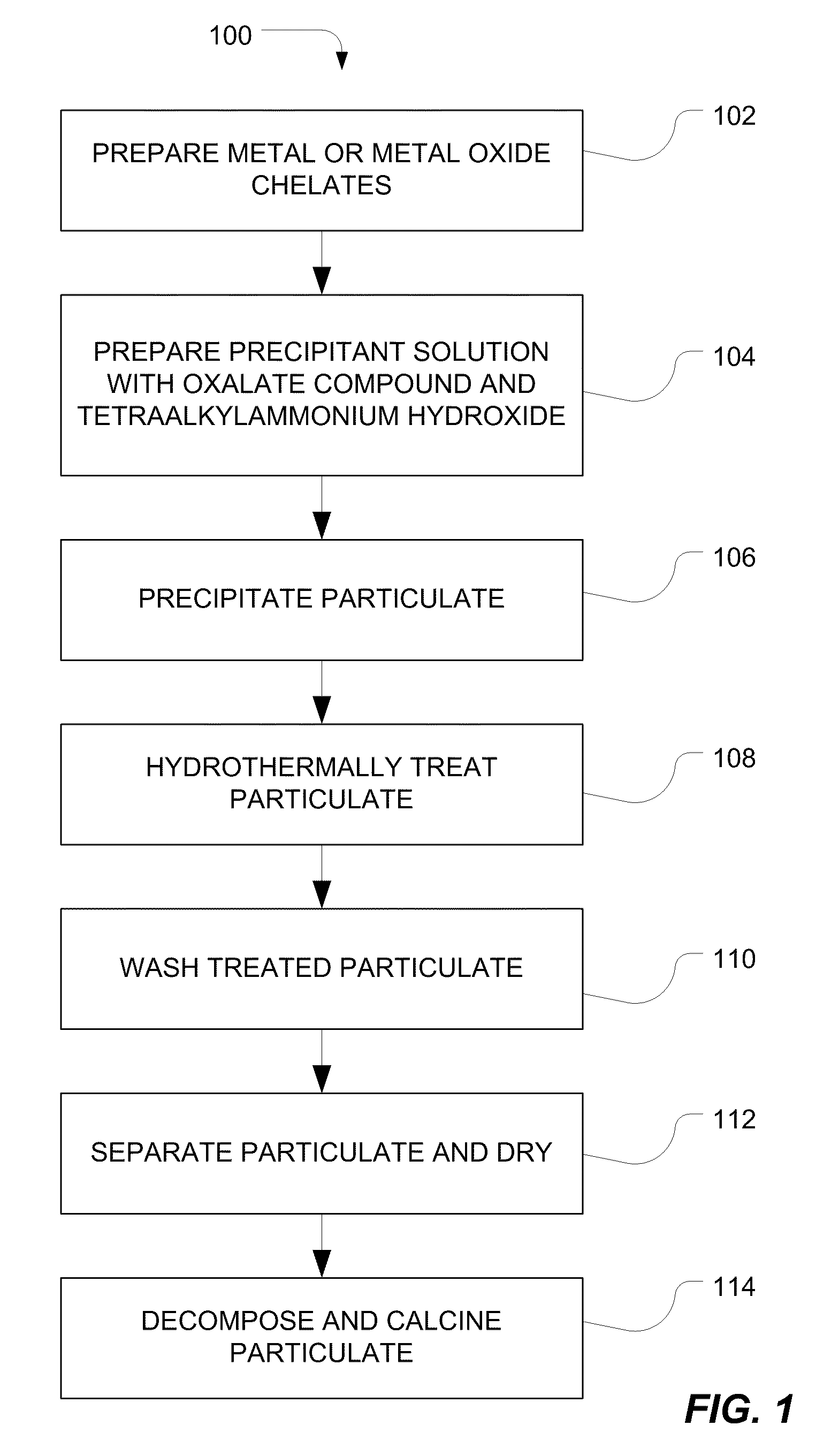
Method of Preparing Ceramic Powders
A method of forming composition-modified barium titanate ceramic particulate includes mixing a plurality of precursor materials and a precipitant solution to form an aqueous suspension. The plurality of precursors include barium nitrate, titanium chelate, and a metal or oxometal chelate. The precipitant solution includes tetraalkylammonium hydroxide and tetraalkylammonium oxalate. The method further includes treating the aqueous suspension at a temperature of at least 150° C. and a pressure of at least 200 psi, and separating particulate from the aqueous suspension after treating.
Owner:EESTOR
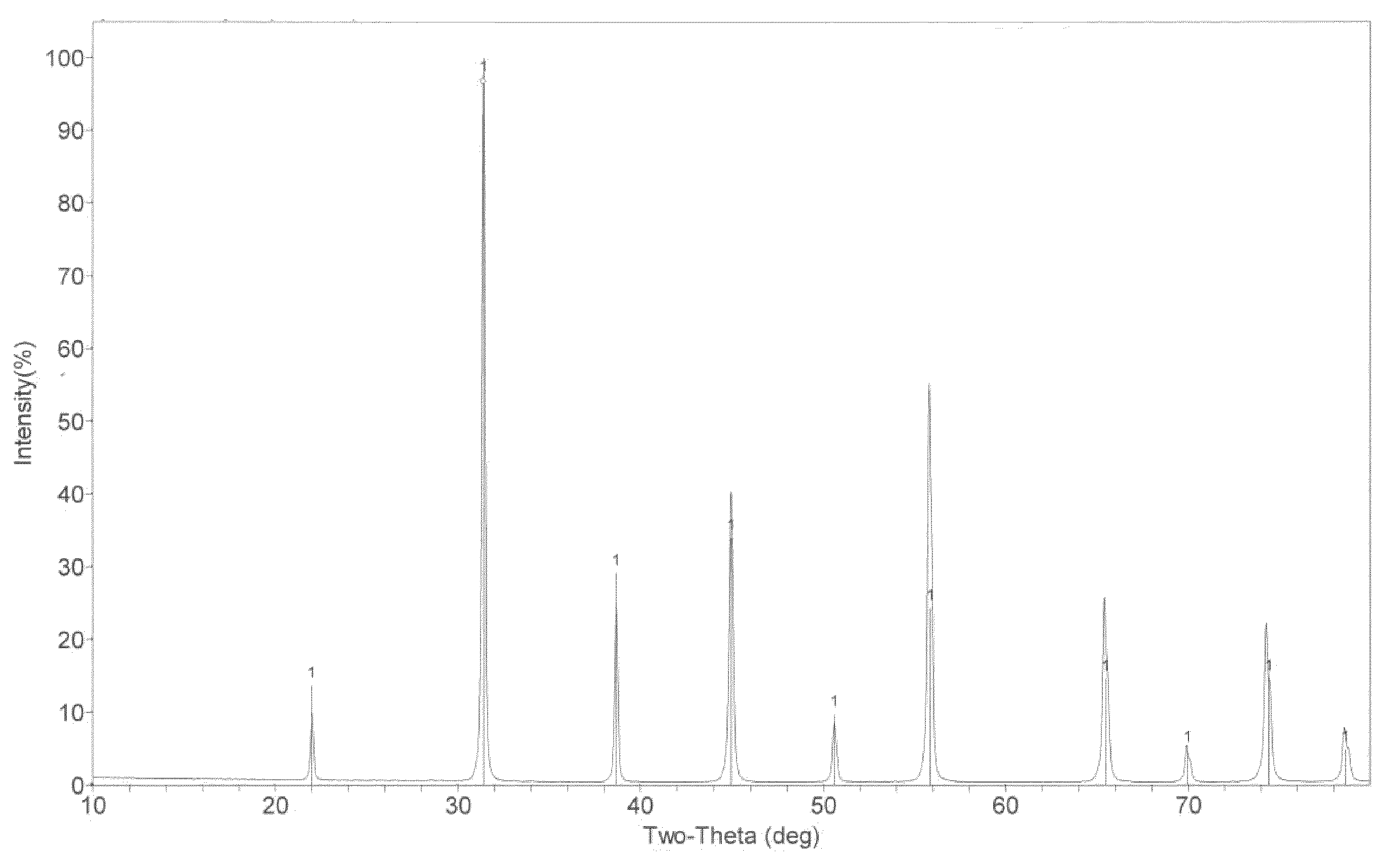
Zirconium-doped barium ferrite wave-absorbing material and preparation method thereof
ActiveCN104030667AApplication frequency band wideningMatching Thickness ReductionOther chemical processesBarium nitrateElectromagnetic shielding
The invention discloses a zirconium-doped barium ferrite wave-absorbing material having a chemical formula of BaFe12-xZrxO19, wherein x is 0.3-0.5, zirconium-doped barium ferrite is a polycrystalline powder, and Fe<3+> and Fe<2+> exist in the barium ferrite simultaneously. A preparation method comprises the preparation steps: mixing barium nitrate, iron nitrate and zirconium nitrate, adding deionized water, and dissolving into a nitrate solution; placing EDTA in deionized water, and dissolving into an EDTA solution; adding the nitrate solution into the EDTA solution, heating, drying, and thus obtaining a dry gel; and sintering the dry gel to obtain a zirconium-doped barium ferrite powder, then grinding, and thus obtaining the zirconium-doped barium ferrite wave-absorbing material. The wave-absorbing material has the characteristics of thin matching thickness and wide wave-absorbing frequency band, can be used for a wave-absorbing coating layer, and can have wide applications in the electromagnetic shielding and stealth fields.
Owner:ZHEJIANG UNIV
Cerium-doped nanometer barium ferrite thin film and method for making same
InactiveCN101452756AImprove absorbing performanceAdjust the magneticMagnetic layersCerium nitrateCerium nitrate hexahydrate
The invention relates to a cerium-doped nanometer barium ferrite film and a preparation method thereof. The film is characterized in that the formula comprises 1.48 to 1.60g / 100ml of glycol, 2.51 to 2.71g / 100ml of citric acid, 1.74g / 100ml of ferric nitrate, 0.125g / 100ml of barium nitrate, and 0.04 to 0.125g / 100ml of cerium nitrate. The preparation method comprises: a crystalline nanometer barium ferrite film is prepared on a quartz substrate; the cerium-doped nanometer barium ferrite film with high purity is obtained through the optimization of preparation technology; and the film can be used for preparing magnetic recording materials and wave-absorbing materials. The method has the advantages that the method has simple process flow and low cost, is convenient for preparing films on various substrates with different shapes, is easy to obtain an uniform and multi-component oxide film, is easy for quantitative doping, and can effectively control the constituents and a microscopic structure of the film.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Preparation method of graphene oxide/barium ferrite wave-absorbing material
InactiveCN104449561AHeating evenlyAvoid high temperature calcination processOther chemical processesBarium nitratePhysical chemistry
The invention relates to a novel material for absorbing electromagnetic waves, and in particular relates to a preparation method of a graphene oxide / barium ferrite wave-absorbing material. The preparation method comprises the following steps: forming a sol from analytically pure barium nitrate Ba(NO3)2 and ferric nitrate Fe(NO3)3 with a graphene oxide water solution by taking citric acid and ethylene glycol as a compound complexing agent; continuously heating and stirring the sol to form a gel; and igniting the gel by virtue of microwave-assisted auto-combustion to further prepare the graphene oxide / barium ferrite wave-absorbing material. The preparation method disclosed by the invention is simple and controllable in process, uniform in temperature distribution of the entire system through microwave field heating, and meanwhile, the preparation method can be used for greatly shortening reaction time and greatly improving the wave-absorbing performance of a barium ferrite material with the doping of the graphene oxide.
Owner:SHANGHAI YUEDA NEW MATERIAL TECH
Composite cerium-zirconium-barium oxides and preparing method thereof
The invention provides a kind of super tiny cerium-zirconium-barium compound oxide and the manufacturing method. The molecular formula is CexZr1-xBayOz, the mol proportion of cerium, zirconium and barium is: Ce=X(X=0.1í½1) : Xr=1-X: Ba=Y(Y=0.1í½0.5), it uses metal nitrate such as cerium nitrate, zirconium nitrate, barium nitrate as materials, through polyglycol high molecular surface decorating agent, uses butyl alcohol azeotropy distillation. The baked temperature is 600 deg.C í½700 deg.C, the time is 3í½4 hours.
Owner:ZHEJIANG UNIV
Formula of gunpowder for timing firing cable of display shell
InactiveCN102249824ASafe storagePromote safe productionInorganic oxygen-halogen salt explosive compositionsNon-explosive/non-thermic compositionsPotassium nitrateCombustion
The invention relates to a formula of gunpowder for a timing firing cable of a display shell. According to the invention, because in the formula provided by the invention, potassium perchlorate is used as a main component, thereby completely avoiding the use of a high-risk potassium nitrate product and effectively reducing the generation of risk accidents caused by a firing cable; barium nitrate in the components has combustion and speed regulation effects, so that the combustion speed of the firing cable meets the timing ignition requirement of the display shell, thereby effectively achieving the timing effect; and the combustion color of the firing cable is golden or silvery through using larch carbon powder, a yellow light agent or titanium sponge in the components.
Owner:徐敏
Composite wave-absorbing material of zinc oxide-coated barium ferrite and preparation method thereof
InactiveCN102504759AImprove absorbing performanceStable structureOther chemical processesDistillationBarium nitrate
The invention discloses a composite wave-absorbing material of zinc oxide-coated barium ferrite and a preparation method thereof, and belongs to the technical field of wave-absorbing materials. The composite wave-absorbing material comprises zinc oxide-coated barium ferrite particles. The preparation method for the composite wave-absorbing material comprises the following steps: preparing ferric nitrate, barium nitrate and citric acid into a solution in molar ratio; regulating the pH value of the solution; performing drying by distillation until gel is generated; then heating for an auto-igniting process reaction; roasting to obtain the barium ferrite particles; dissolving zinc acetate into diethylene glycol, and adding a polyvinylpyrrolidone surface active agent to prepare a solution 1; adding a barium ferrite powder to the solution 1 in ferrum-zinc molar ratio; and reacting at certain temperature to obtain a zinc oxide-coated barium ferrite powder. The invention has the advantages that the preparation process is simple, and the production cost is low. The prepared composite wave-absorbing material of the zinc oxide-coated barium ferrite has excellent wave-absorbing performance, a stable structure and good dispersion property.
Owner:TIANJIN UNIV
Safety oxidant for firecrackers
The invention is a firecracker safety oxidant, including the following components in weight ratio: barium nitrate, potassium perchlorate and perlite powder. Its advantages: it has better effect than potassium chlorate and potassium perchlorate oxidants; for adopting barium nitrate and adopting perlite powder as flame retardant and dampproofer, its safety is by far the higher than that of potassium chlorate and potassium perchlorate oxidants, and simultaneously its cost is lower than potassium perchlorate oxidant and only 5% higher than potassium chlorat oxidant. It has a wide prospect in market.
Owner:雍和平
Method for preparing nano barium-strontium titanate powder by adopting hydrothermal method
InactiveCN101786887AUniform particle size distributionAvoid grain growthBarium strontium titanateBarium titanate
The invention discloses a method for preparing nano barium-strontium titanate powder by adopting a hydrothermal method, which is used for solving the technical problem of large granularity of the barium-strontium titanate powder prepared by a method of the prior art. The method comprises the following steps of: adding strontium nitrate, barium nitrate, anatase phase titanium oxide and a mineralizer into a high-pressure kettle; then adding deionized water and stirring; later on, raising the temperature to a hydrothermal temperature according to a certain speed and preserving heat; after heat preservation time arrives, furnace cooling to room temperature; and then repeatedly washing and filtering by using deionized water and drying. Because the barium-strontium titanate powder is prepared by adopting the hydrothermal method, the barium-strontium titanate powder with uniform size distribution is obtained through controlling different barium-titanium proportions, and moreover, the defects of grain growth, impurity mixing, and the like easily caused in a sintering process are overcome.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Method for preparing microcrystalline glass by utilizing blast furnace steel slag
The invention relates to a method for preparing a microcrystalline glass by utilizing steel slag. The method comprises the following steps of: firstly, carrying out drying and ball milling on the steel slag and removing iron contained in the steel slag to prepare powder of 100 meshes; then mixing the steel slag powder, quartz sand, sodium nitrate, barium nitrate, zinc oxide and cerium dioxide into a compound material; placing the mixture obtained from the former step into a crucible, and smelting at 1250-1380 DEG C to form a transparent steel slag glass; and finally, crystallizing the transparent steel slag glass to form the steel slag microcrystalline glass containing calcium akermanite as a main crystal phase. The method disclosed by the invention utilizes the blast furnace steel slag as a main raw material and achieves the utilization amount of the steel slag at 96%; and the microcrystalline glass prepared according to the method has the advantages of high structural strength, low water absorption, high crystalline degree and various color. The preparation process disclosed by the invention is simple in operation process and low in cost, can effectively reduce the production cost of the microcrystalline glass, protects the environment and changes the wastes into valuables, is suitable for industrialized large-scale production and has obvious economic benefit and environmental benefit.
Owner:承德远通钢铁设备制造有限公司
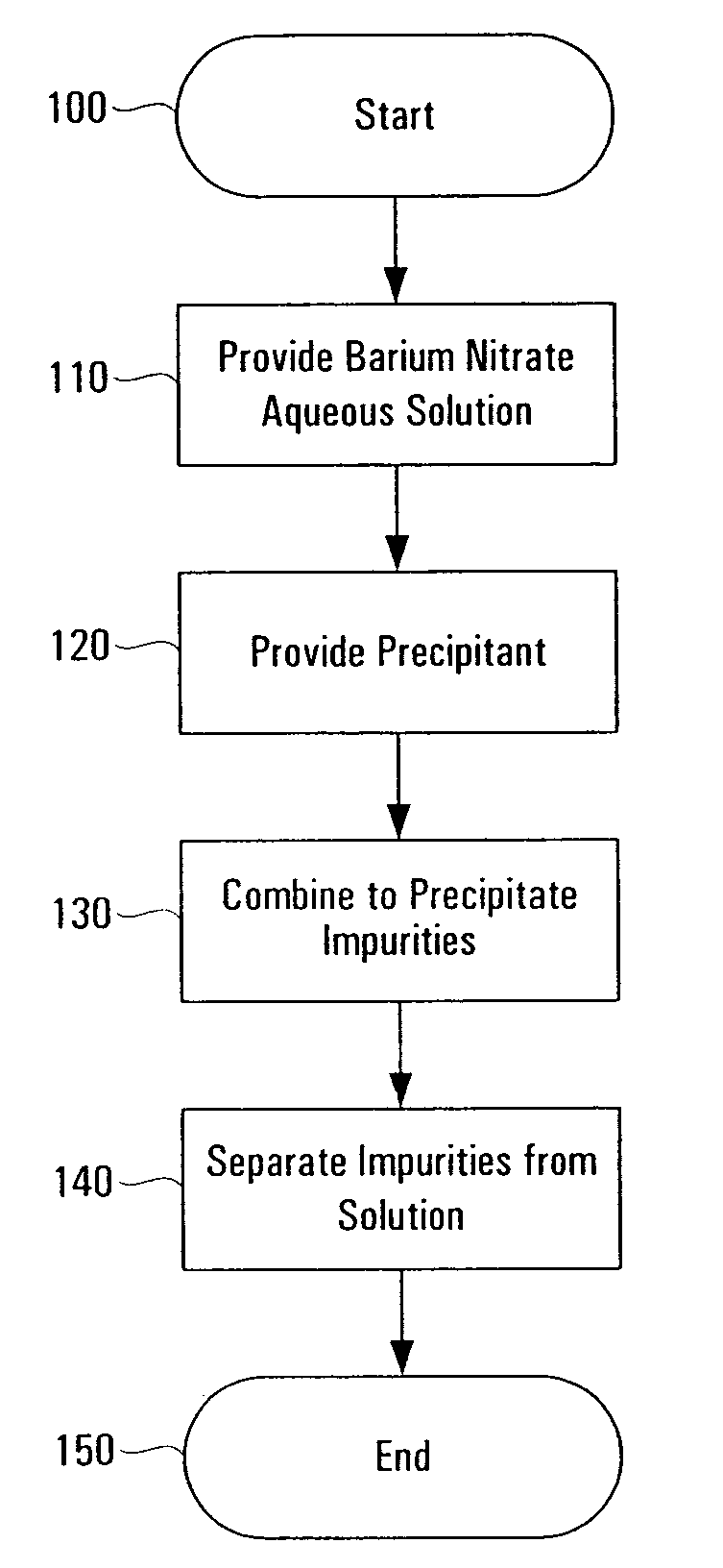
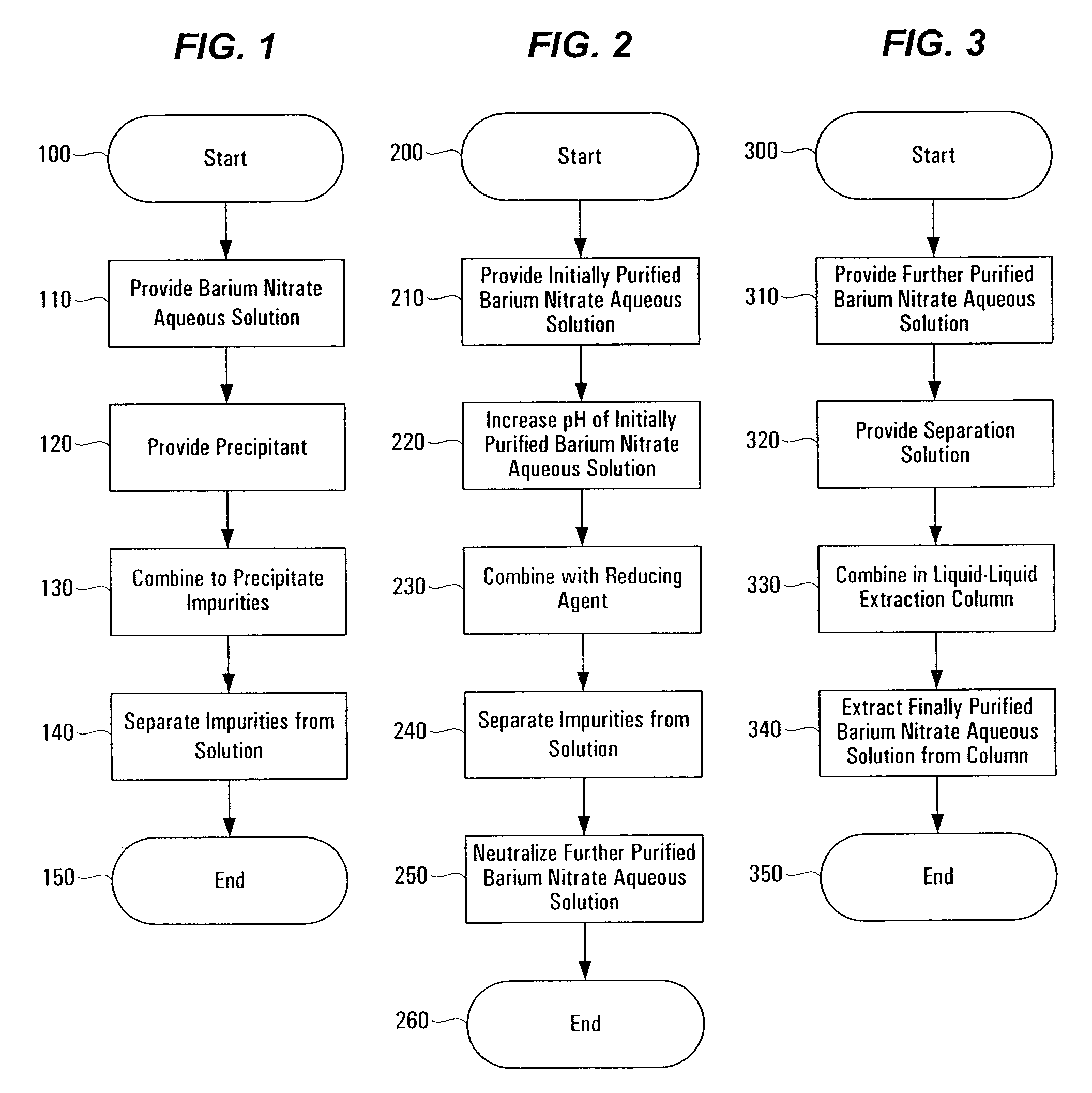
Method of purifying barium nitrate aqueous solution
InactiveUS7648687B1Solid sorbent liquid separationLiquid solutions solvent extractionBarium nitrateOxidation state
Purification techniques have been developed for ceramic powder precursors, e.g., barium nitrate. These techniques can be performed using one or more of the following operations: (1) removal of impurities by precipitation or coprecipitation and separation using a nonmetallic-ion-containing strong base, e.g., tetraalkylammonium hydroxides; (2) reduction of higher oxidation-state-number oxymetal ions and subsequent precipitation as hydroxides that are separated from the solution; and (3) use of liquid-liquid exchange extraction procedures to separate certain impurities.
Owner:EESTOR
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com