Process for synthesizing barium ferrite micro powder by self combustion method
A technology of barium ferrite and self-combustion method, which is applied in the field of preparing barium ferrite micropowder, can solve the problems of difficulty in precipitation and washing, complicated equipment, and difficulty in industrialized production, and achieves the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
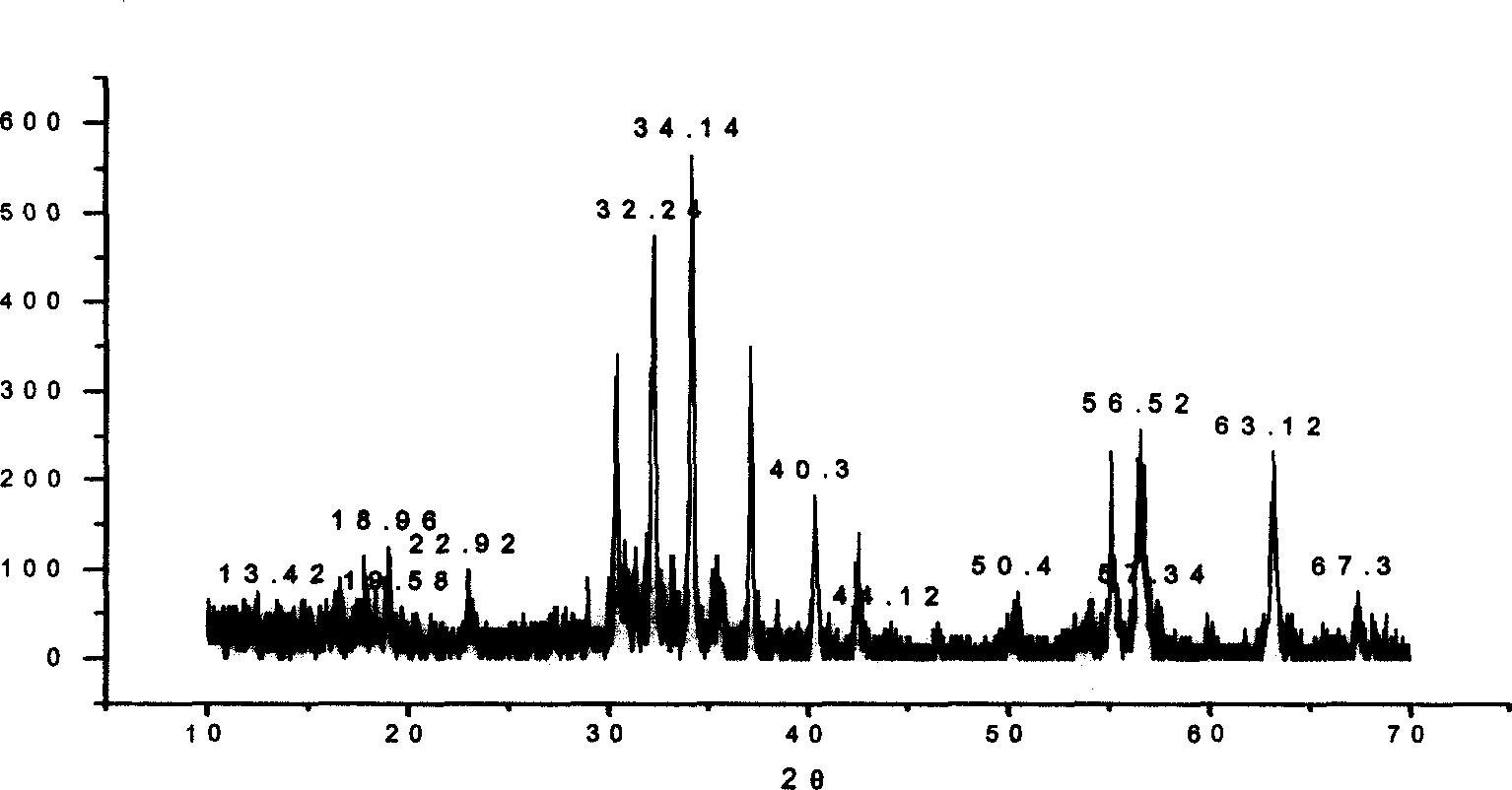
Examples
Embodiment 1
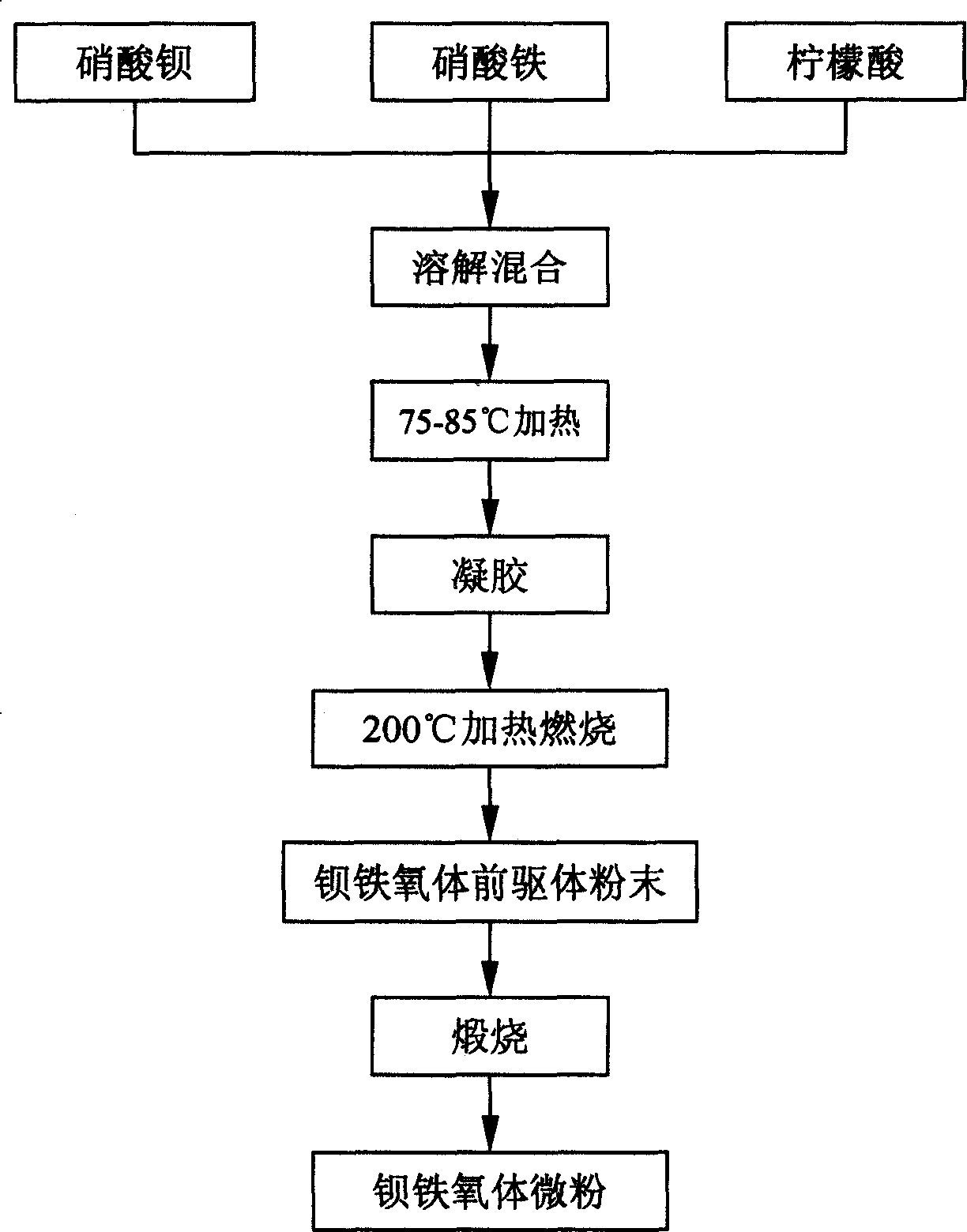
[0021] The method for synthesizing barium ferrite micropowder by self-combustion method, the concrete steps are: first in a reactor, adding 80ml concentration is 0.75M Fe(NO 3 ) 3 Solution, M is the molar concentration, and in another reactor, adding 20ml concentration is 0.25M Ba(NO 3 ) 2 solution. Under stirring conditions, the above two solutions were mixed to obtain a mixed solution A. Then add 0.19mol citric acid into the mixed solution A, and stir to obtain the mixed solution B. Adjust the pH value of the mixed solution B to 7.5 with ammonia water, heat while stirring at 75°C, and the following chemical reaction occurs:
[0022]
[0023]
[0024]
[0025] As the solvent evaporates, the viscosity of the solution increases, and the resulting complexes are linked by hydrogen bonds, forming a gel in 8-12 hours. After the gel is formed, move it to a constant temperature electric furnace at 200°C. The dry gel begins to expand, boil, and smoke in about...
Embodiment 2
[0032] The method for synthesizing barium ferrite micropowder by self-combustion method, the concrete steps are: first in a reactor, adding 80ml concentration is 0.75M Fe(NO 3 ) 3 solution, add 20ml concentration of 0.25M Ba(NO 3 ) 2 solution. Under stirring conditions, the above two solutions were mixed to obtain a mixed solution A. Then add 0.19mol citric acid into the mixed solution A, and stir to obtain the mixed solution B. Use ammonia water to adjust the pH value of the mixed solution B to 8.0, heat it while stirring at 80°C, and the following chemical reaction occurs:
[0033]
[0034]
[0035]
[0036] As the solvent evaporates, the viscosity of the solution increases, and the resulting complexes are linked by hydrogen bonds, forming a gel in 8-12 hours. After the gel is formed, move it to a constant temperature electric furnace at 200°C. The dry gel begins to expand, boil, and smoke in about 1 minute, and then generates a flame and discharges...
Embodiment 3
[0043] The method for synthesizing barium ferrite micropowder by self-combustion method, concrete steps are: first add 80ml concentration in a reactor and be 1.0M Fe(NO 3 ) 3 solution, add 20ml concentration of 0.125M Ba(NO 3 ) 2 solution. Under stirring conditions, the above two solutions were mixed to obtain a mixed solution A. Then add 0.57mol citric acid into the mixed solution A, and stir to obtain the mixed solution B. Use ammonia water to adjust the pH value of the mixed solution B to 8.5, heat while stirring at 85°C, and the following chemical reactions occur:
[0044]
[0045]
[0046]
[0047] As the solvent evaporates, the viscosity of the solution increases, and the resulting complexes are linked by hydrogen bonds, forming a gel in 8-12 hours. After the gel is formed, move it to a constant temperature electric furnace at 200°C. The dry gel begins to expand, boil, and smoke in about 2 minutes, and then generates a flame and emits a large am...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Saturation magnetization | aaaaa | aaaaa |
| Coercivity | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com