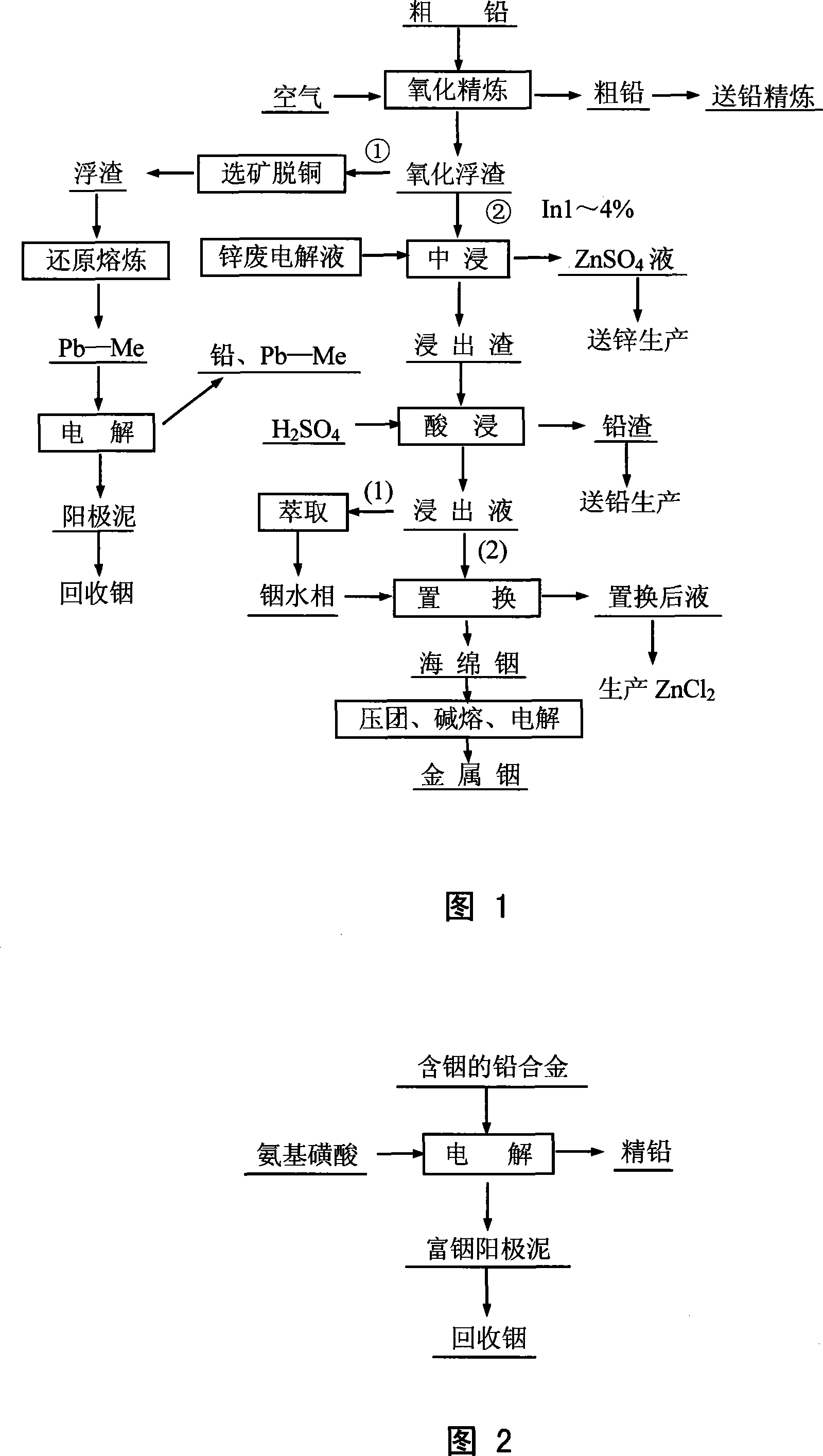
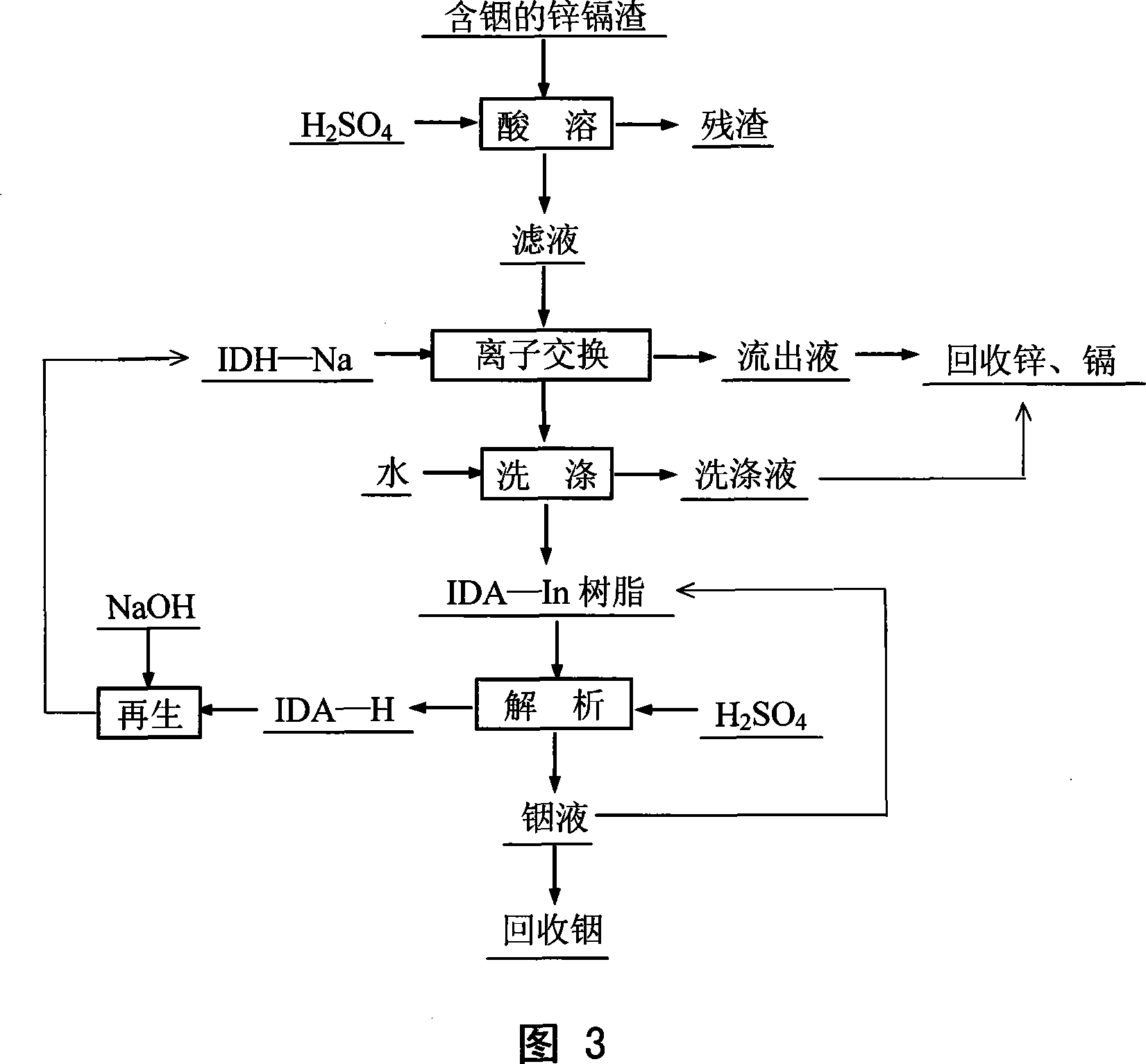
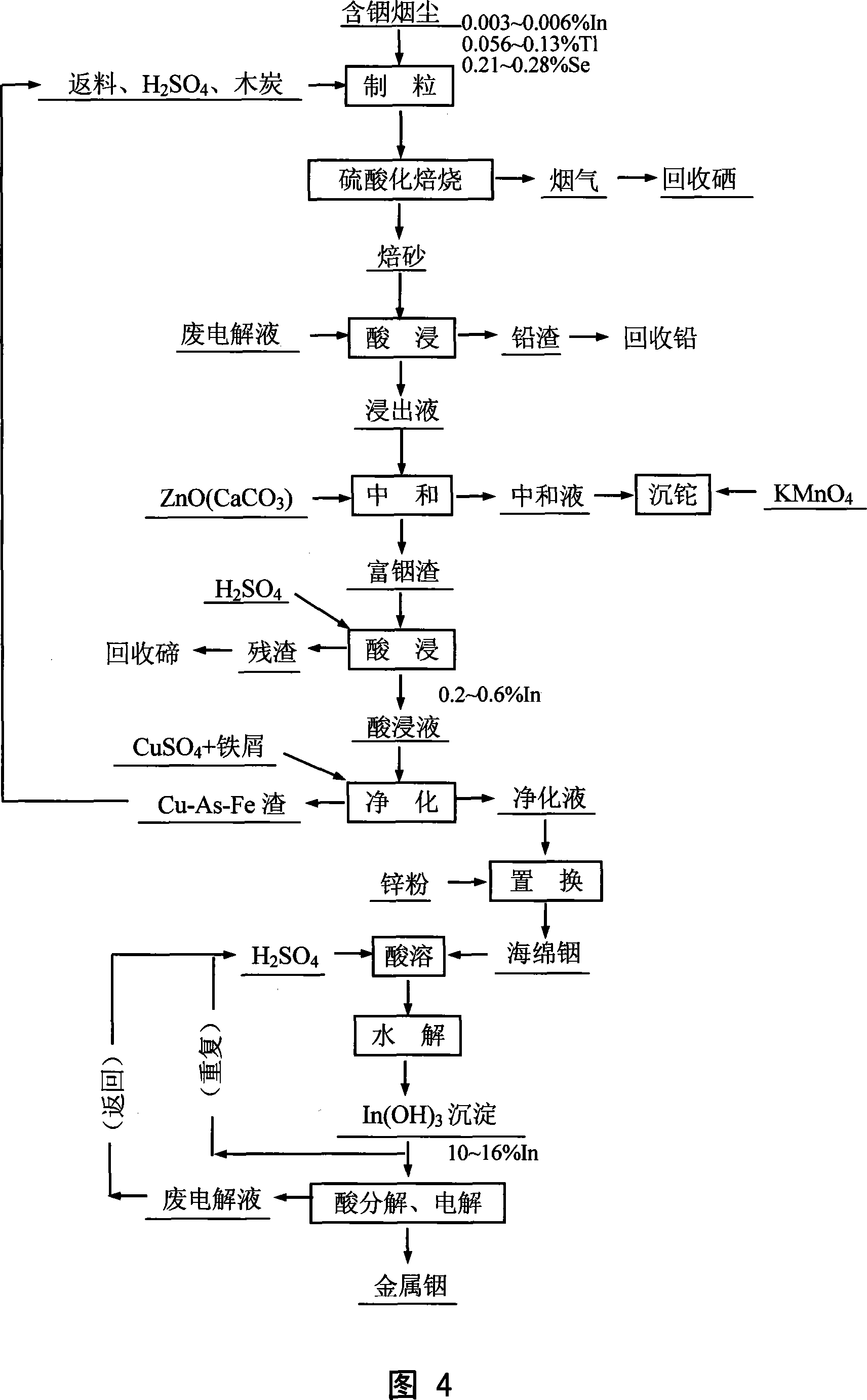
Method for leaching indium from indium sulfide concentrate
A technology for indium sulfide and indium concentrate is applied in the smelting field of extracting indium, which can solve the problems of large consumption of reagents, large environmental pollution, dispersion of valuable metals, etc., and achieve the effects of concentration of valuable metals, simplification of smelting process, and process enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0069] Example 1: At a temperature of 100°C and normal pressure:
[0070] For indium sulfide concentrate containing 4500 g / t of indium, 23% iron, 31% sulfur and nitric acid oxidant (HNO 3 content 60%) and concentrated sulfuric acid (H 2 SO 4 content 98%) aqueous solution is mixed, pumped into the reaction tank continuously with a pump, the temperature in the kettle is controlled at 100°C ± 1 with steam or tap water, the particle size of the concentrate is 200 mesh, H 2 SO 4 :In molar ratio is 600:1, HNO 3 The :In molar ratio is 100:1, the liquid-solid ratio is 10:1, under normal pressure conditions, the chemical reaction of oxidation and dissolution of indium sulfide concentrate is carried out, and the reaction time is 240 minutes. The NO gas generated during the reaction is oxidized, and then generates nitric acid when it meets water, and the nitric acid catalyst is recycled.
[0071] Indium leaching rate is 95%.
example 2
[0072] Example 2: At a temperature of 60°C, under normal pressure conditions:
[0073] For indium sulfide concentrate containing 1500 g / t of indium, 35% iron, 29% sulfur and nitric acid oxidant (HNO 3 content 10%) and sulfuric acid (H 2 SO 4 content 50%) aqueous solution is mixed, pumped into the reaction tank continuously with a pump, the temperature in the kettle is controlled at 60°C ± 1 with steam or tap water, the concentrate particle size is 150 mesh, H 2 SO 4 :In molar ratio is 300:1, HNO 3 The :In molar ratio is 50:1, the liquid-solid ratio is 3.5:1, and the oxidation and dissolution chemical reaction of indium sulfide concentrate is carried out, and the reaction time is 10 minutes. The NO gas generated during the reaction is oxidized, and then generates nitric acid when it meets water, and the nitric acid catalyst is recycled.
[0074] Indium leaching rate is 65%.
example 3
[0075] Example 3: At a temperature of 85°C, under normal pressure conditions:
[0076] For indium sulfide concentrate containing 3000 g / t of indium, 28% iron, 38% sulfur and catalyst nitric acid (HNO 3 content 50%) and sulfuric acid (H 2 SO 4 content 65%) aqueous solution is mixed, pumped into the reaction tank continuously with a pump, the temperature in the kettle is controlled at 85°C ± 1 with steam or tap water, the concentrate particle size is 200 mesh, H 2 SO 4 :In molar ratio is 450:1, HNO 3 The :In molar ratio is 75:1, the liquid-solid ratio is 5.0:1, and the oxidation and dissolution chemical reaction of indium sulfide concentrate is carried out, and the reaction time is 120 minutes. The NO gas generated during the reaction is oxidized, and then generates nitric acid when it meets water, and the nitric acid catalyst is recycled.
[0077] Indium leaching rate is 91.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com