Method and device for preparing nitric acid through metal nitrate pyrolysis
A metal nitrate and nitrate technology, which is applied in the direction of nitric acid, oxide/hydroxide preparation, nitrogen oxide/oxyacid, etc., can solve the problems of expensive equipment, high production cost, endangering human safety, etc., and achieve reduction Production cost, solution to recycling, high recovery rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
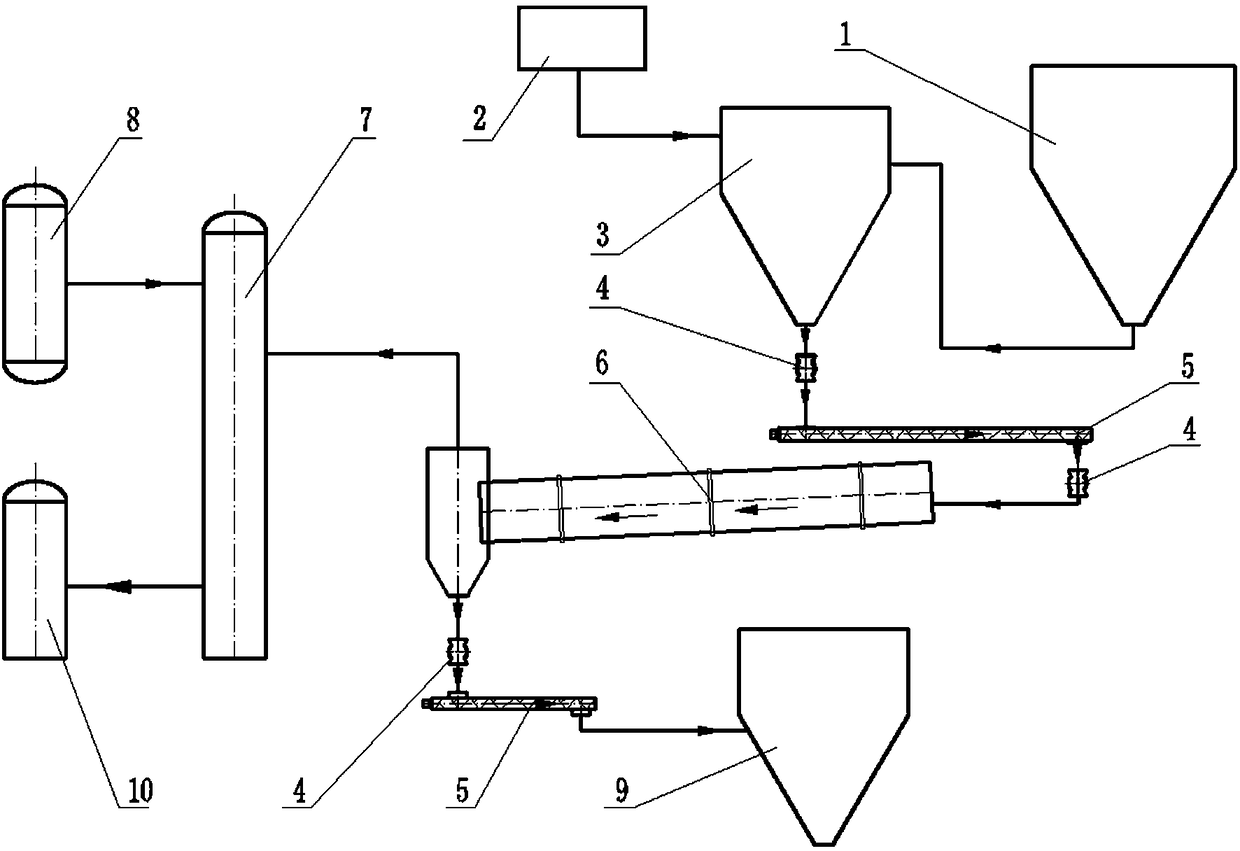
[0031] A method for preparing nitric acid by pyrolysis of metal nitrate, in which metal nitrate powder is pyrolyzed in a closed device to produce O2, NO2 and metal oxide powder, and the obtained O2 and NO2 are introduced into the absorption tower, After being circulated and absorbed by the absorption liquid provided in the absorption tower, nitric acid with the required concentration can be obtained.
Embodiment 2
[0033] A method for preparing nitric acid by pyrolysis of metal nitrate, in which metal nitrate powder is pyrolyzed in a closed device to produce O2, NO2 and metal oxide powder, and the obtained O2 and NO2 are introduced into the absorption tower, After being circulated and absorbed by the absorption liquid provided in the absorption tower, nitric acid with the required concentration can be obtained.
[0034] The metal nitrate is one or more of calcium nitrate, magnesium nitrate, sodium nitrate, potassium nitrate, copper nitrate and iron nitrate.
[0035] The pressure in the airtight device is kept positive.
[0036] The absorbing liquid is water.
[0037] The thermal decomposition of metal nitrate powder is carried out in the brick kiln.
Embodiment 3
[0039] A device for producing nitric acid by pyrolysis of metal nitrate, which includes a nitrate silo, a rotary kiln, and an absorption tower connected in sequence. The rotary kiln is made of high-temperature-resistant and corrosion-resistant materials, and the nitrate The pyrolysis is carried out in the brick kiln, and the above-mentioned devices are all closed devices.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com