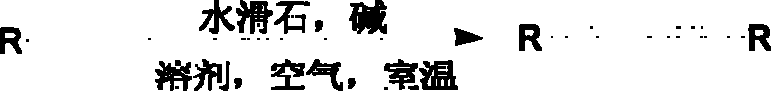Acetylene even-coupling method under temperate condition
A terminal alkyne and coupling technology, which is applied in the field of preparation and recovery of LDHs catalysts, can solve the problems of no literature reports and limited literature, and achieve good economic benefits, low requirements, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Taking the preparation of NiAl-LDH catalyst as an example, the preparation of hydrotalcite catalyst adopts coprecipitation method. Ni(NO 3 ) 2 6H 2 O and Al(NO 3 ) 3 9H 2 O by Ni + / Al 3+ The molar ratio is 3:1 to form a solution, and the 3M NaOH solution is used as a coprecipitant. The above two solutions are added dropwise to the stirring 0.5M NaOH solution at a rate of 1 drop per second. 2 CO 3 In the solution, the pH of the solution was controlled to be 10, and the solution was aged in an oil bath at 65° C. for 18 hours. The reaction solution was filtered, the solid was washed with distilled water, and then dried in an oven at 80° C. for 12 hours to prepare the corresponding NiAl-LDH catalyst.
[0027] The reaction formula with phenylacetylene as the reaction substrate is as follows:
[0028]
[0029] Weigh 0.204g (2.0mmol) of the reaction substrate phenylacetylene, 0.464g (4.0mmol) of TMEDA, and 0.56g of NiAl-LDH catalyst, and place them in a glass fla...
Embodiment 2
[0031] Weigh 0.204g (2.0mmol) of the reaction substrate phenylacetylene, 0.232g (2.0mmol) of TMEDA, and 0.36g of CuCr-LDH catalyst, and place them together in a glass flask, add 10mL of organic solvent ethyl acetate, and The reaction was stirred for 12 hours. After the reaction was completed, the reaction mixture solution in the flask was filtered. The filtered solid was washed 2-3 times with ethyl acetate, then dried at 80° C. for 6 hours, and directly used for the next catalytic cycle. The filtrate was combined with the washed solvent, concentrated with a rotary evaporator, and then separated through a silica gel column, and the eluent was n-hexane / ethyl acetate to obtain the yield of 1,4-diphenyl-1,3-butadiyne 16%.
Embodiment 3
[0033]Weigh 0.204g (2.0mmol) of the reaction substrate phenylacetylene, 0.24g (3.0mmol) of pyridine, and 0.15g of CuAl-LDH catalyst, and place them together in a glass flask, add 10mL of organic solvent acetone, and stir the reaction at normal temperature and pressure 10 hours. After stopping the reaction, the reaction mixture solution in the flask was filtered. The filtered solid was washed 2-3 times with acetone, then dried at 80° C. for 6 hours, and directly used for the next catalytic cycle. The filtrate was combined with the washed solvent, concentrated with a rotary evaporator, and then separated through a silica gel column, and the eluent was n-hexane / ethyl acetate to obtain the yield of 1,4-diphenyl-1,3-butadiyne was 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com