A kind of tridentate carbene and its preparation method and application
A carbene complex and reaction technology, applied in the field of tridentate carbene and its preparation, can solve the problems that tridentate bicarbene ligands have not been reported, and achieve the effects of good catalytic activity, low cost and simple synthesis route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] The present embodiment prepares the compound shown in formula [a], comprises the following steps:
[0088] 1) 1,3-bis(1,3-bis(2,6-diisopropyl-benzene)-1,2,3-triazole)-dimethylamine salt (L 2 ) preparation
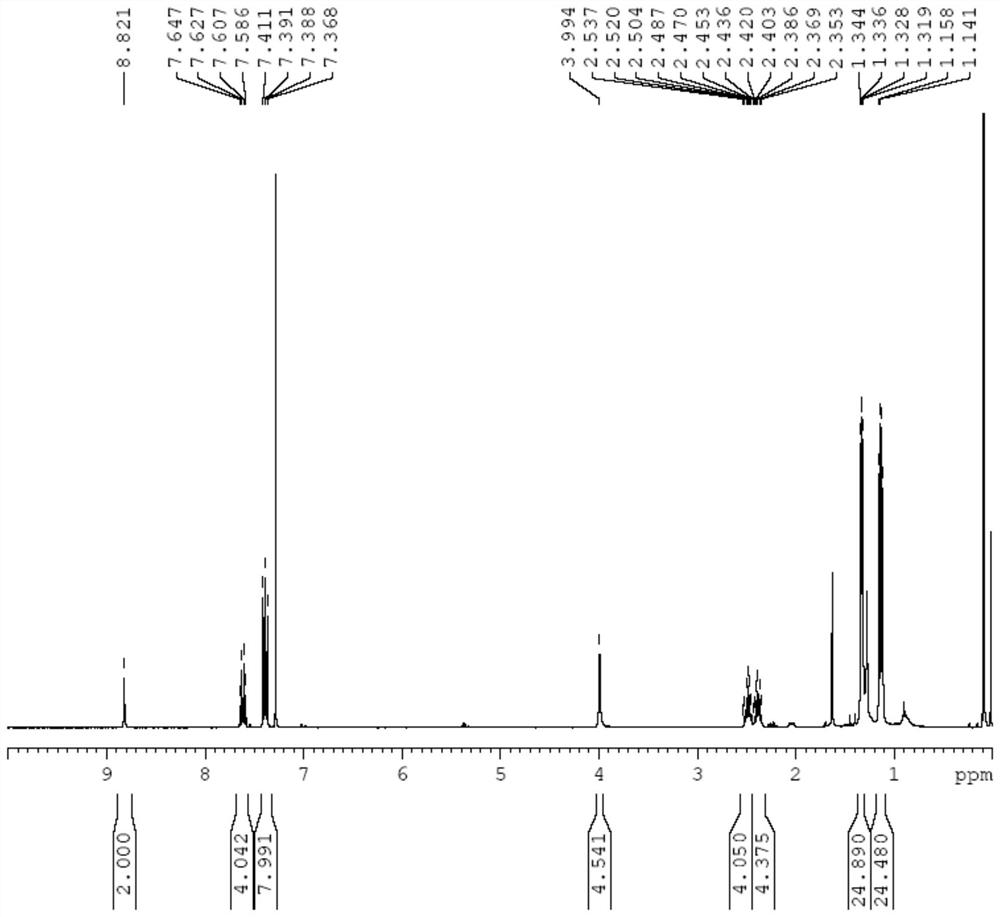
[0089] Add potassium hexafluorophosphate (1.9g, 10.4mmol) into a 250mL schlenk bottle, heat and pump for N 2 Several times, add 1,3-bis(2,6-diisopropyl-benzene)-triazene (5.0 g, 13.4 mmol) after the schelenk bottle is cooled, and ventilate three times, then cool down to -78 ° C, Add 50 mL of ultra-dry dichloromethane, add BOC-protected dipropynylamine (10 g, 5.2 mmol), and after the temperature is stabilized at -78 ° C, protect from light, add t BuOCl (1.30mL, 11.4mmol), heated by itself overnight. Suction filter in a ventilated place, wash the solid with dichloromethane, collect the filtrate, and remove the dichloromethane by rotary evaporation to obtain a white solid. Then add 100mL diethyl ether to wash to obtain white solid L 1 3.98 g, yield: 63%.
[0090]...
Embodiment 2
[0101] Compound shown in the present embodiment preparation formula [b]
[0102] In Example 1, 1,3-bis(2,6-diisopropyl-benzene)-triazene was replaced by 1,3-bis(2,4,6-trimethyl-benzene)-tri Nitrogen, the rest of the conditions are the same as in Example 1 to obtain the compound shown in formula [b].
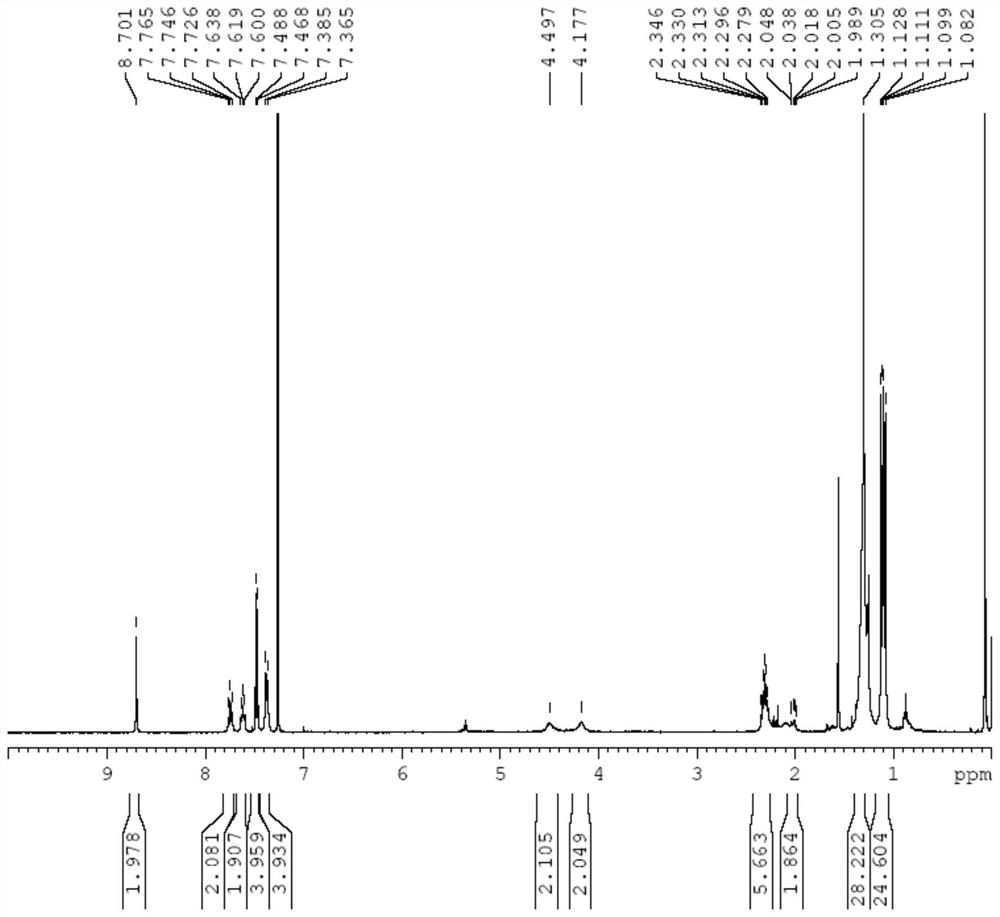
[0103] The compound shown in the formula [b] prepared in embodiment 2 can be passed through proton nuclear magnetic resonance spectrum ( 1 (HNMR) test to verify its structure, characterized as follows:
[0104] 1 HNMR (CDCl 3 ,400MHz,δppm)δ7.06(s,4H),7.05(s,4H),4.00(s,4H),2.36(d,J=2.4Hz,12H),2.15(s,12H),2.09(s ,12H).
Embodiment 3
[0106] The present embodiment prepares the compound shown in formula [c], comprises the following steps:
[0107] 1) L 4 preparation of
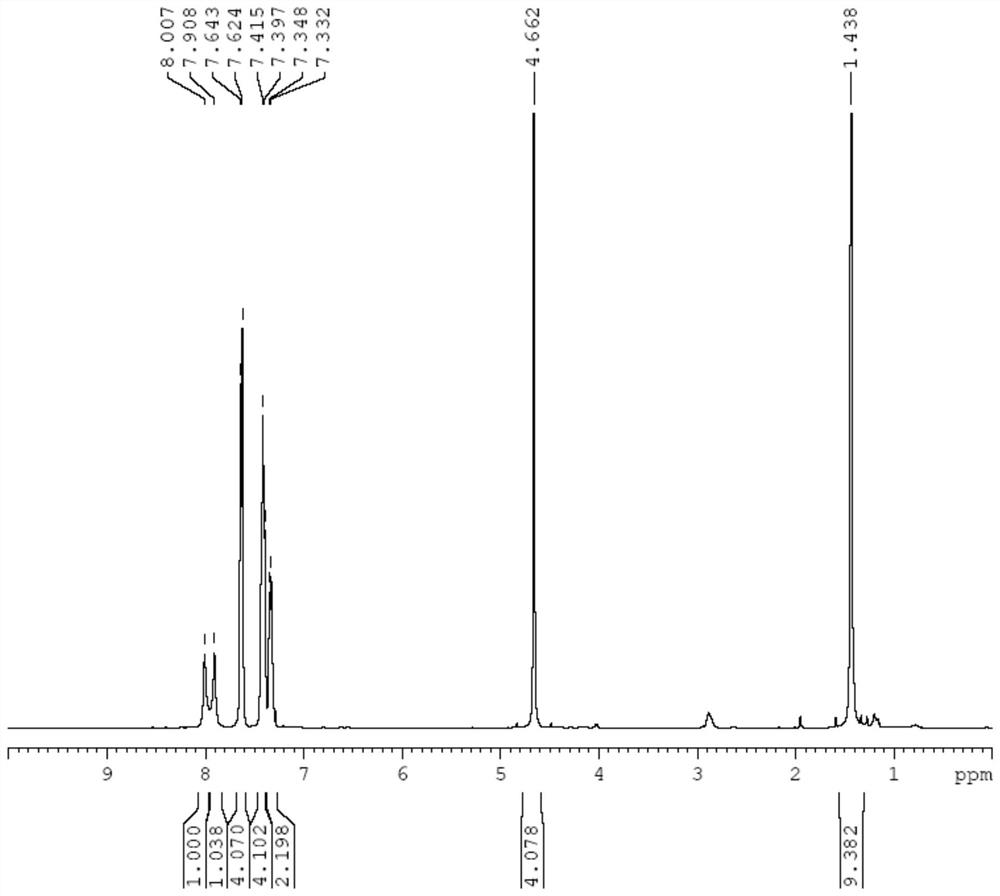
[0108] DMSO:H in an oven-dried 100 mL schlenk bottle 2 O (9:1) mixed solution was added with 1-phenyl-azidobenzene (0.68g, 5.7mmol) and cuprous iodide (0.099g, 0.5mmol) and stirred for 10 minutes. BOC-protected dipropynylamine (0.5 g, 2.6 mmol) was added and stirred for another 24 hours, the reaction mixture was added to ice water, a solid precipitated out, the solvent was filtered off, the precipitate was washed with water and acetone, and dried under vacuum to obtain a white solid L 3 0.98g, Yield: 87%.
[0109] Add L to the dried 100mL schlenk bottle 3 (86.3mg, 0.2mmol), and added 5mL of ultra-dry dichloromethane, cooled to -78°C, then added methyl trifluoromethanesulfonate (72.2mg, 0.44mmol), when the reaction rose to room temperature, added trifluoromethane Sulfonic acid (75.0mg, 0.50mmol) was extracted with dichloromethane after ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com