Patents
Literature
37 results about "Methyl trifluoromethanesulfonate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
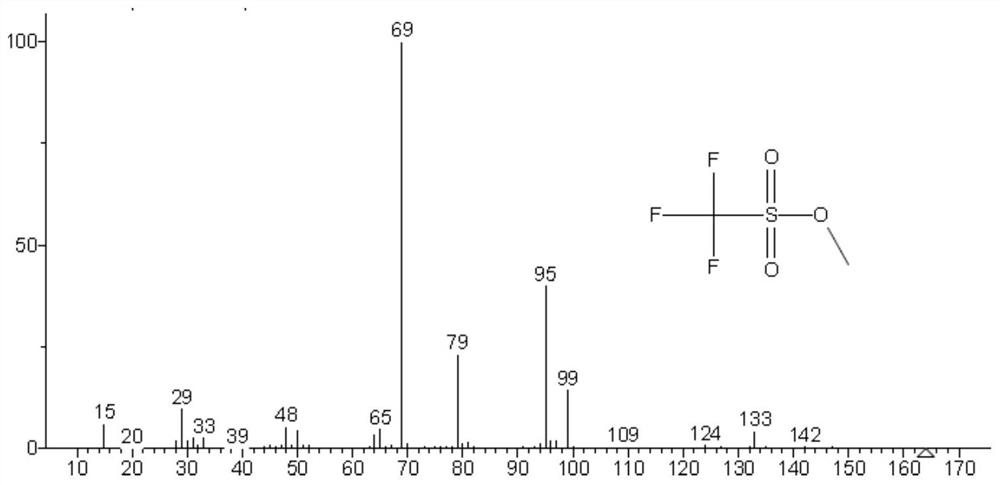
Methyl trifluoromethanesulfonate, also commonly called methyl triflate and abbreviated MeOTf, is the organic compound with the formula CF₃SO₂OCH₃. It is a colourless liquid which finds use in organic chemistry as a powerful methylating agent. The compound is closely related to methyl fluorosulfonate (FSO₂OCH₃). Although there has yet to be a reported human fatality, while several cases were reported for methyl fluorosulfonate (LC₅₀ (rat, 1 h) = 5 ppm), methyl triflate is expected to have similar toxicity based on available evidence.
Preparation method of cationic metal-organic framework membrane material
ActiveCN110563992AImprove adsorption capacityIngenious ideaWater/sewage treatment by sorptionIon exchangeMetal-organic framework
The invention provides a preparation method of a cationic metal-organic framework membrane material, and belongs to the field of metal-organic framework materials. A metal-organic framework material UiO-66-NH2 is prepared from aminoterephthalic acid and metalklic zirconium, and then a UiO-66-NH2 membrane is prepared from granular UiO-66-NH2 and polyvinylidene fluoride by adopting a mixed matrix process; and amino groups of the UiO-66-NH2 membrane are quaternized with methyl trifluoromethanesulfonate, and finally the quaternized UiO-66-NH2 membrane is acidized to form the cationic UiO-66-NMe<3+> membrane with an anion exchange group. The UiO-66-NMe<3+> material in the membrane form retains the efficient selective adsorbability of the metal-organic framework material, can be conveniently separated from an aqueous solution, and has a good application prospect in removal and enrichment of anionic pollutants in water.
Owner:QINGDAO TECHNOLOGICAL UNIVERSITY
Thick oil viscosity reducer, preparing method thereof and thick oil viscosity reducing method
ActiveCN105018062AImprove viscosity reduction efficiencySimple implementation of viscosity reductionDrilling compositionMethyl carbonatePotassium hydroxide
The invention discloses a thick oil viscosity reducer, a preparing method thereof and a thick oil viscosity reducing method. The total weight of the thick oil viscosity reducer serves as a benchmark, a component A accounts for 3-30% weight of the thick oil viscosity reducer, a component B accounts for 5-40% weight of the thick oil viscosity reducer, and a solvent accounts for 30-92% weight of the thick oil viscosity reducer. The component A is at least one of halogenate methane, p-toluenesulfonic acid methyl ester, dimethyl sulfate, methyl trifluoromethansulfonate and dimethyl carbonate. The component B is at least one of sodium hydroxide, potassium hydroxide, ammonia water, R1NOH and R2NX, wherein R1 and R2 are independently C1-C4 alkyl groups, and X is F or Cl or Br or I. The higher viscosity reducing efficiency can be achieved when the thick oil viscosity reducer is used for reducing the viscosity of the crude oil.
Owner:CHINA PETROLEUM & CHEM CORP +1
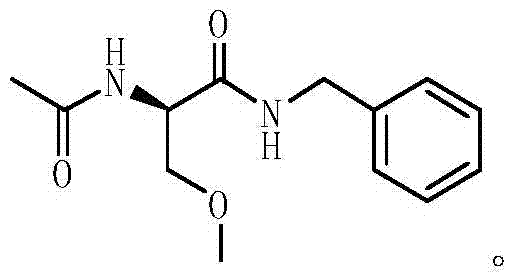
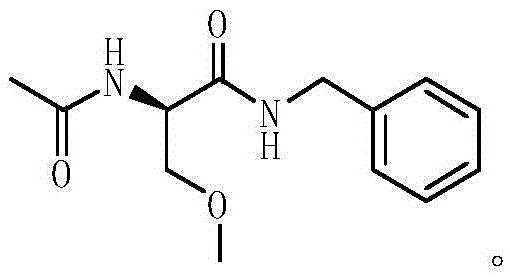
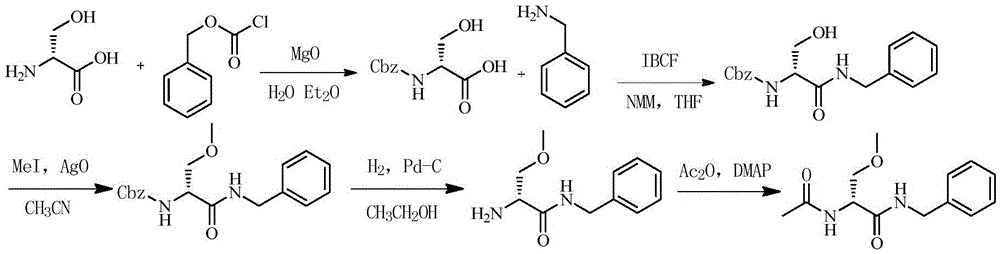
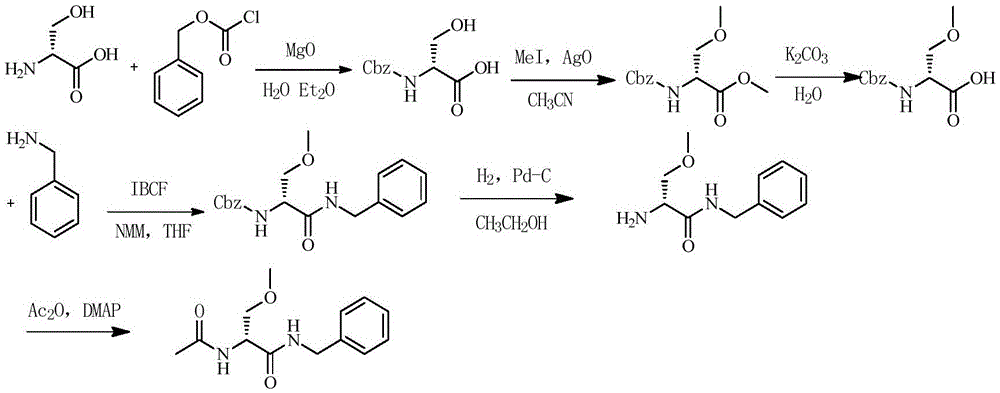
Method for preparing Lacosamide by one-pot method
ActiveCN105523957AReduce usageReduce generationOrganic compound preparationCarboxylic acid amides preparationAcetylationBenzylamine
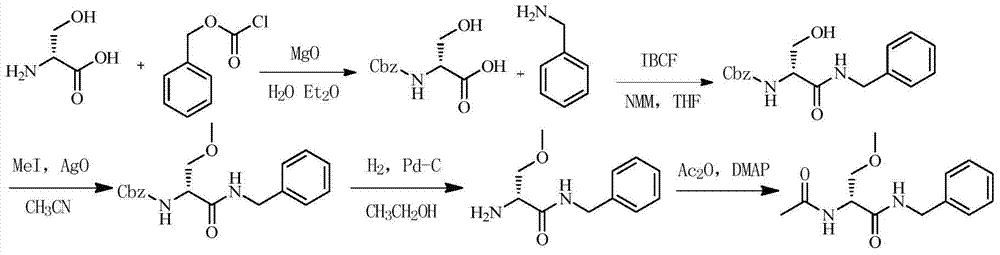
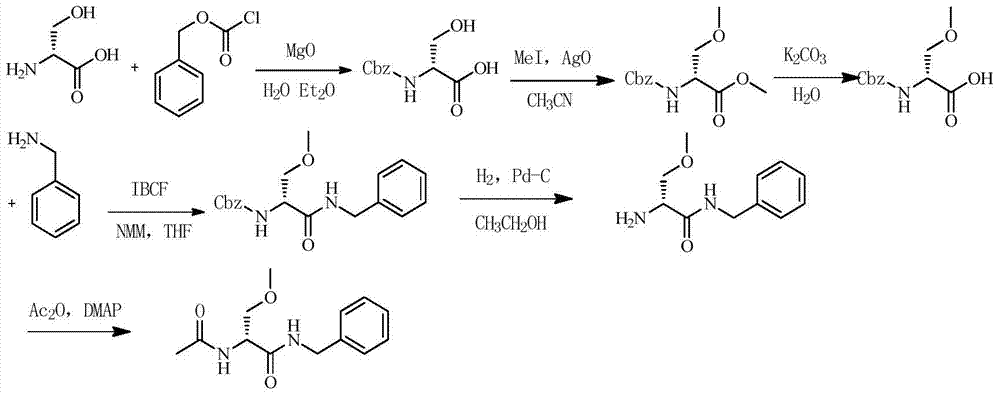
The invention discloses a method for preparing Lacosamide by a one-pot method. The method comprises the steps: subjecting D-serine and an acetylation reagent to a reaction in a manner of taking dichloromethane as a solvent, and adjusting the pH of a reacted substance to 12-13 by an organic base after the reaction ends; then, controlling the temperature to -5 to 0 DEG C, and adding methyl trifluoromethanesulfonate into the reacted substance for a reaction; and cooling a reacted substance to the temperature of -30 to -25 DEG C, adding a dehydrating agent and benzylamine into the reacted substance for a reaction, carrying out depressurized-concentration drying on material liquid after the reaction ends so as to obtain crude Lacosamide, and then, carrying out recrystallization, thereby obtaining pure Lacosamide. The preparation method is simple and easy in operation, and the prepared Lacosamide has the purity of 99.90% or more and the chiral purity of 99.90%.
Owner:QILU PHARMA HAINAN +1
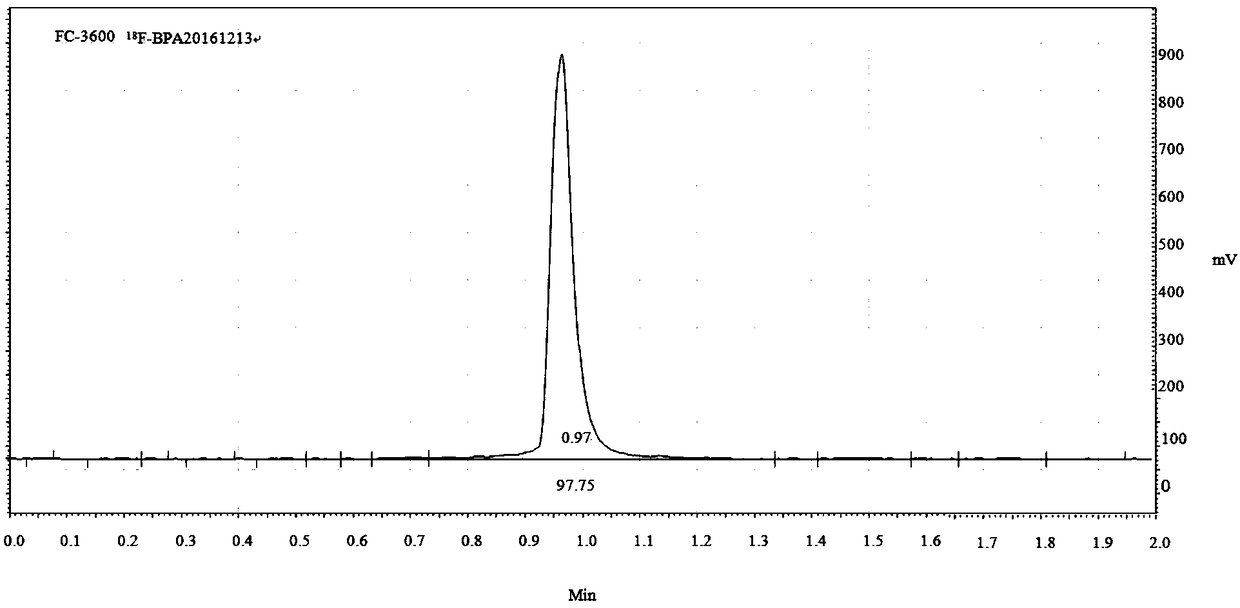
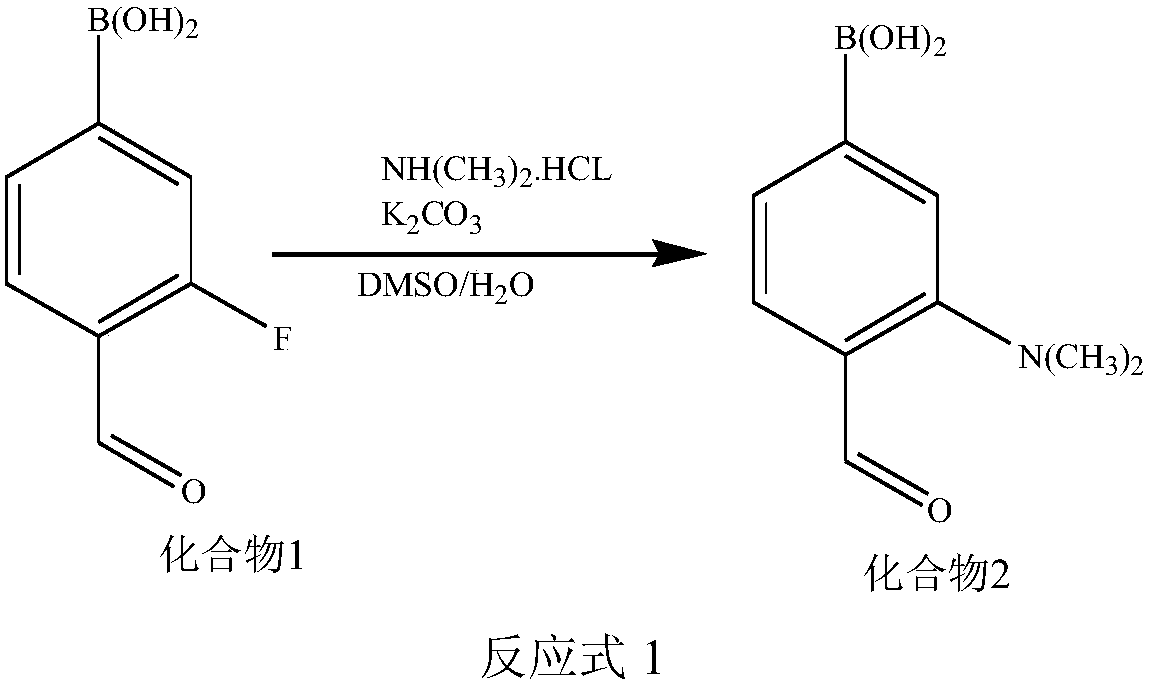
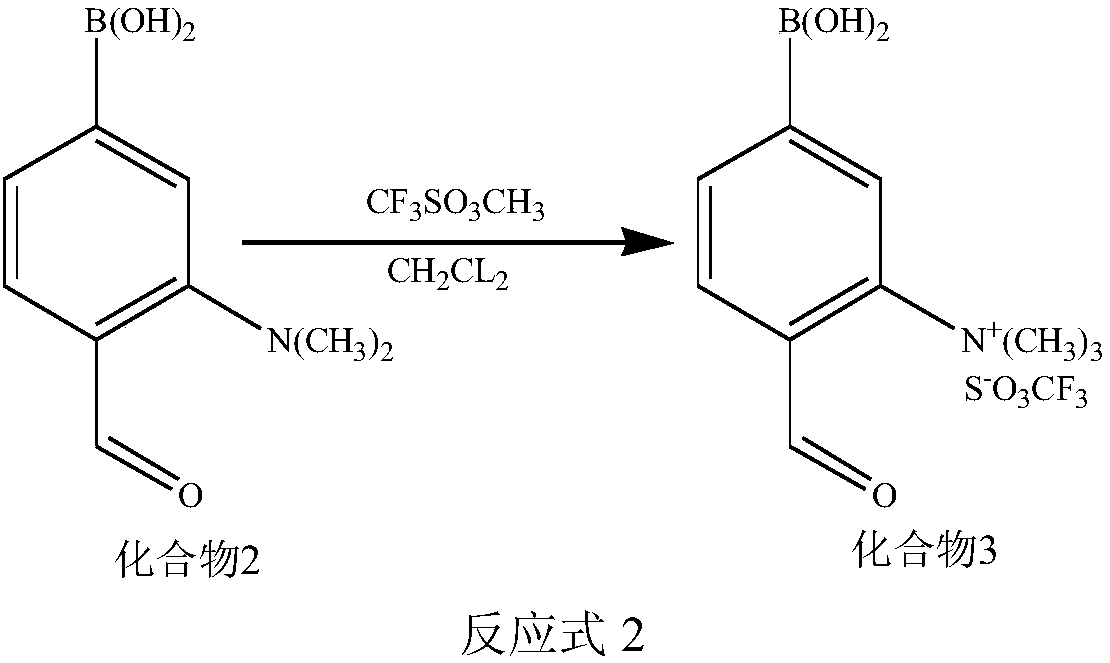
Synthetic methods of F-BPA and F-BPA intermediates, intermediates and application of intermediates
ActiveCN108299482AMeet the requirements of clinical applicationImprove image qualityGroup 3/13 element organic compoundsIsotope introduction to acyclic/carbocyclic compoundsAqueous solutionPhase-transfer catalyst
The invention provides a nucleophilic fluorination based synthetic method of F-BPA, a synthetic method of intermediates, the intermediates and an application of the intermediates. The nucleophilic fluorination based synthetic method of F-BPA comprises the following steps: 1, synthesizing a compound 2 from raw materials including a compound 1, dimethylamine hydrochloride and the like; 2, synthesizing a compound 3 from raw materials including the compound 2, methyl-trifluoromethanesulfonate and the like; 3, synthesizing a compound 4 from raw materials including the compound 3, K2.2.2 and the like; 4, synthesizing a compound 5 from raw materials including the compound 4, an NaBH4 aqueous solution, an HI aqueous solution and the like; 5, synthesizing the target product from raw materials including the compound 5, N-(diphenylmethyl)tert-butyl glycinate and the like under the catalytic action of an Maruoka chiral phase transfer catalyst.
Owner:CHINA INSTITUTE OF ATOMIC ENERGY
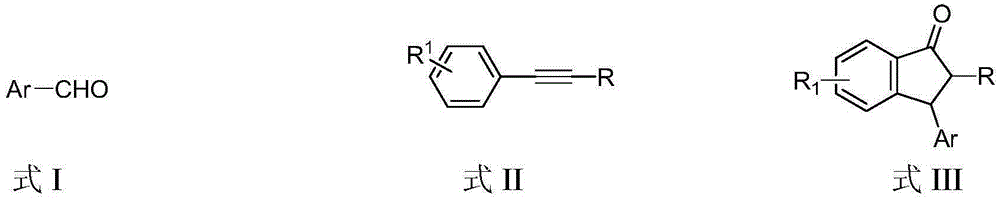
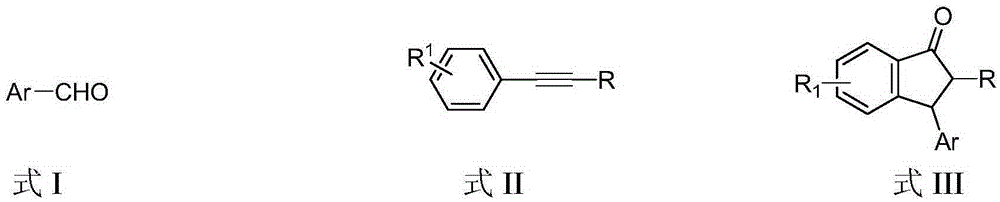
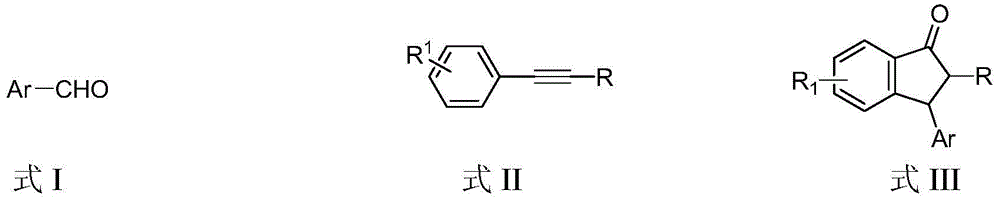
Preparation method of 3-aryl-1-indanone derivate
InactiveCN105348062ARaw materials are easy to getSimple and fast operationCarbonyl compound preparation by condensationArylStructural formula
The invention discloses a preparation method of a 3-aryl-1-indanone derivate. The structural formula of the 3-aryl-1-indanone derivate is shown in the formula III. The preparation method comprises the following steps that a compound shown in the formula I reacts with a compound shown in the formula II in the presence of methyl trifluoromethanesulfonate, and therefore the 3-aryl-1-indanone derivate shown in the formula III is obtained. The preparation method of the 3-aryl-1-indanone derivate is scientific and reasonable, 3-aryl-1-indanone derivates with various substituent groups can be obtained, the raw materials are easy to obtain, no metal participates in the reaction, reaction atoms are economical, and meanwhile the preparation method has the advantages that operation is simple and convenient, the synthesis productive rate is high, the product is easy to purify, and environmental protection is achieved.
Owner:TSINGHUA UNIV
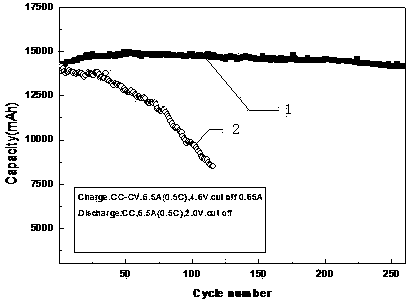
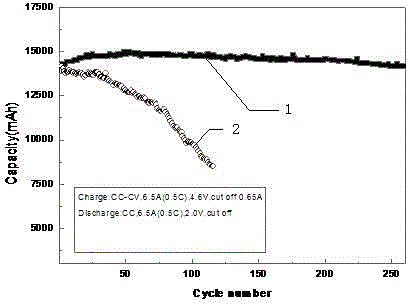
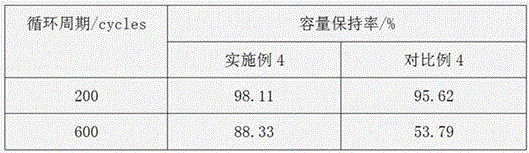
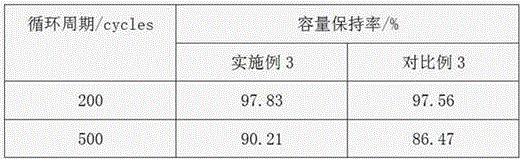
Circulation improvement type lithium and manganese-rich solid solution battery
ActiveCN104282941AReduce production processIncrease capacitySecondary cellsOrganic solventPower performance
The invention relates to a circulation improvement type lithium and manganese-rich solid solution batteryincluding a positive electrode, a negative electrode and an electrolyte, wherein the positive electrode comprises a positive electrode active material, a positive electrode conductive agent and a positive electrode binder, the negative electrode includes a negative electrode active material, a negative electrode binder and a negative electrode conductive agent, and the circulation improvement type lithium and manganese-rich solid solution batteryis characterized in that, the electrolyte includes a lithium salt, an organic solvent, a film forming additive, a stabilizer and auxiliaries; the and the auxiliary are methyl trifluoromethansulfonate and 4, 4 '-sulfonyl diphenylamine. The invention provides a circulation improvement type lithium and manganese-rich solid solution batterywhich has the advantages of low cost and high capacity, and on the premise of satisfying the multiplying power performance and high and low temperature performance of a normal lithium and manganese-rich solid solution lithium ion battery, the circulation life of the battery system is obviously increased.
Owner:WANXIANG 123 CO LTD
Method for preparing methyl hesperidin
InactiveCN108129531AReduce processingReduce dosageSugar derivativesSulfonic acids salts preparationOrganic solventFiltration
The invention provides a method for preparing methyl hesperidin. The method comprises the following steps: enabling hesperidin as a main raw material to react with trifluoro-methyl methanesulfonate inthe presence of an alkali, performing acidification, filtration and desolvation, further washing with an organic solvent, and filtering, thereby obtaining the methyl hesperidin, wherein the purity ofthe methyl hesperidin is 95% or greater, and the yield of the methyl hesperidin is greater than 90%. The byproduct trifluoro-mesylate is a raw material for synthesizing trifluoromethanesulfonic acid.Compared with the prior art, the method for preparing the methyl hesperidin is safe in reaction process, simple and convenient to operate, efficient and environmental-friendly and applicable to industrial production.
Owner:HANDAN ZHAODU FINE CHEM CO LTD
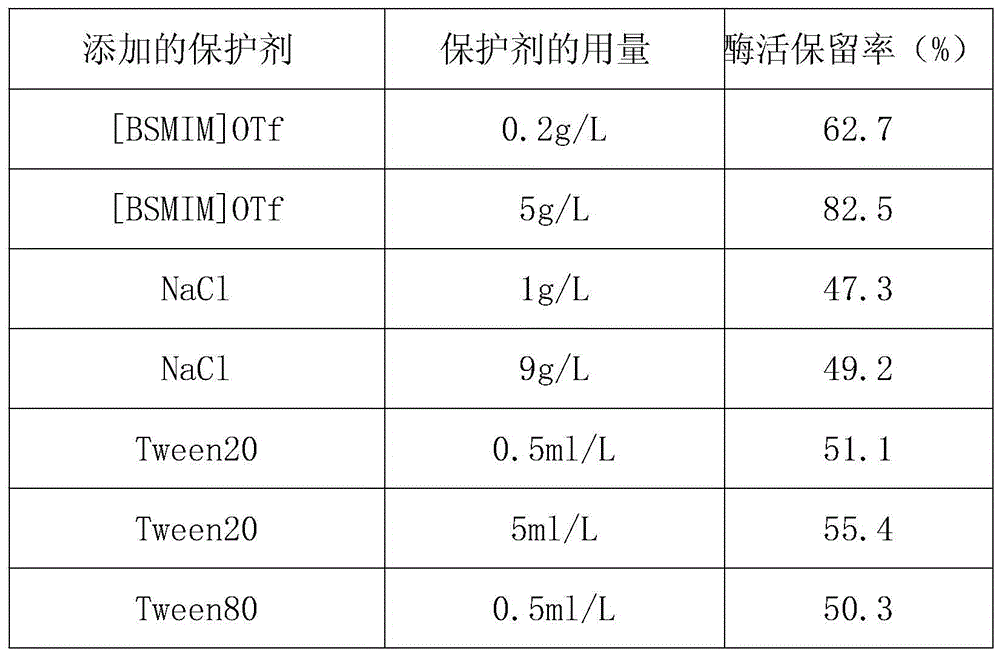
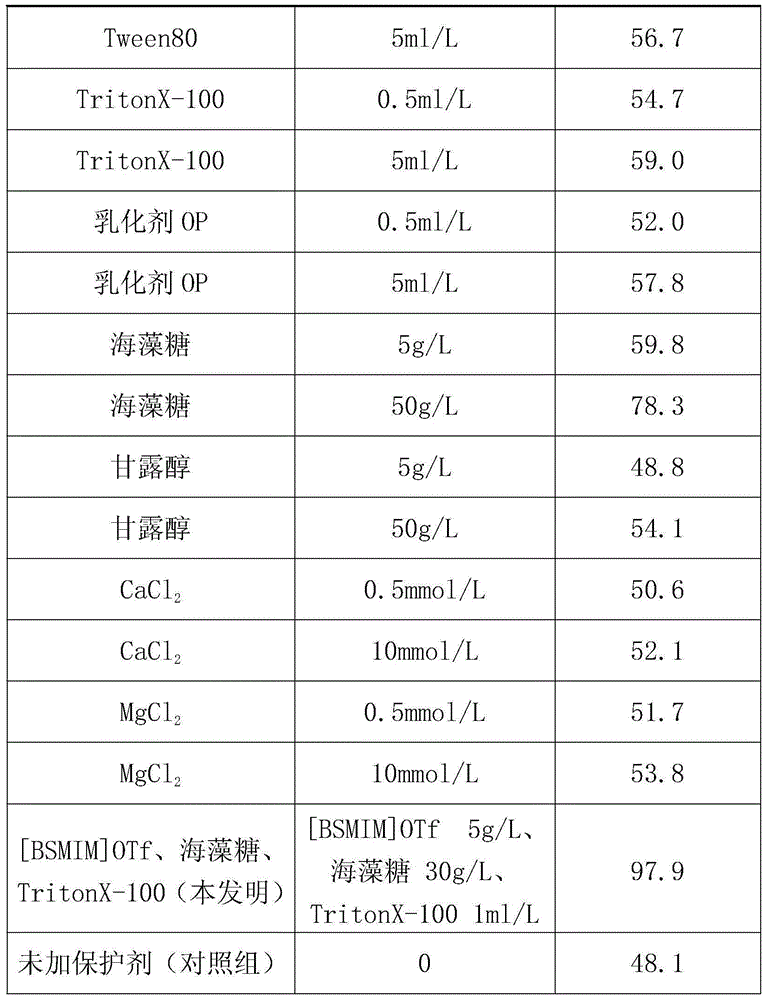
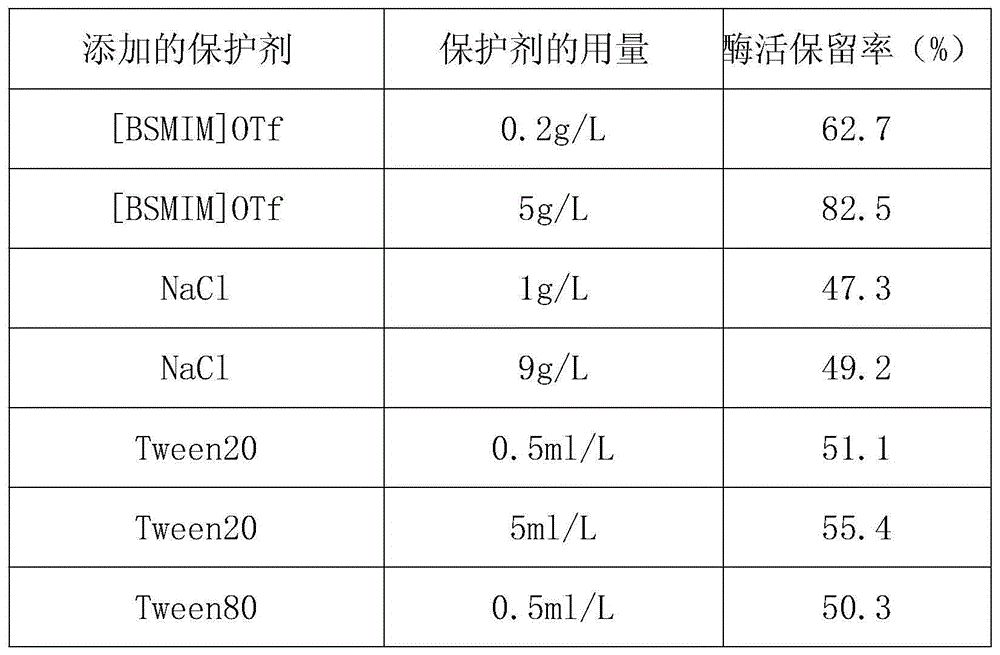
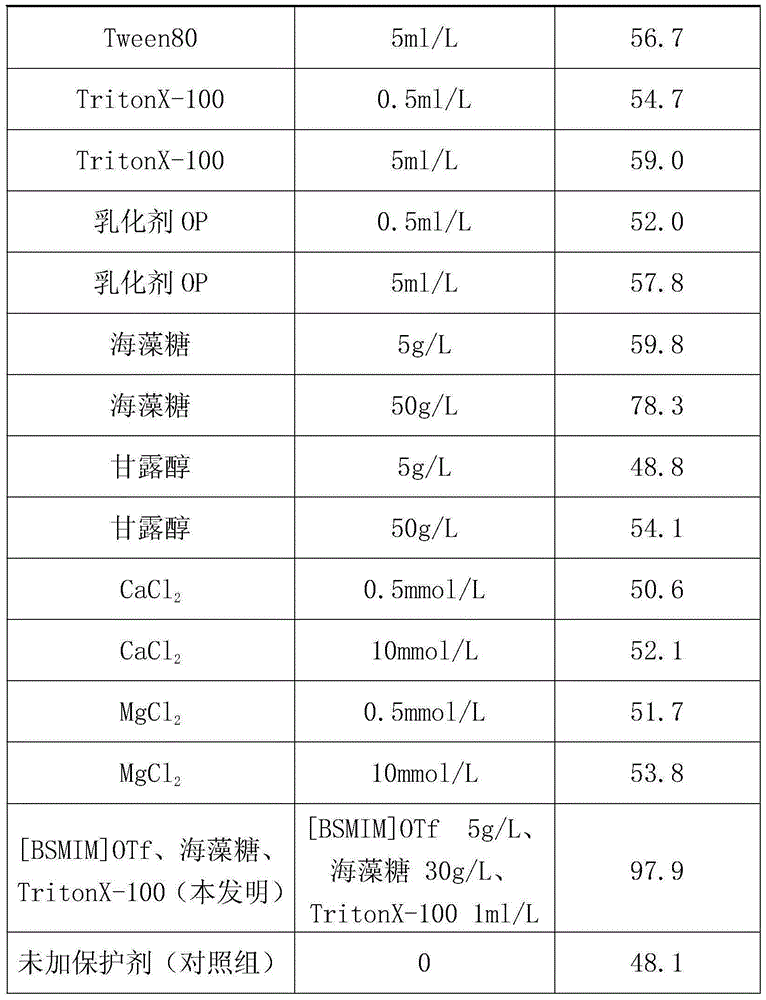
Method for improving thermal stability of liquid fructosaminase
ActiveCN104611319AImprove thermal stabilityImprove stabilityEnzyme stabilisationSodium azideHydroxymethyl
Owner:浙江夸烨生物科技有限公司
Preparation method of sulfonyl azide compounds
ActiveCN103360318AGood water solubilityMaintain stabilitySugar derivativesSugar derivatives preparationSodium azideAzide
The invention relates to a preparation method of sulfonyl azide compounds, which comprises the following steps: (a) reacting compounds disclosed as Formula (II) with methyl trifluoromethanesulfonate to obtain compounds disclosed as Formula (III), wherein the compounds disclosed as Formula (II) are compounds disclosed as Formula (II-1) or Formula (II-2), and the compounds disclosed as Formula (III) are compounds disclosed as Formula (III-1) or Formula (III-2); and (b) reacting the compounds disclosed as Formula (III) obtained in the step (a) with sodium azide to obtain sulfonyl azide compounds disclosed as Formula (I), wherein the sulfonyl azide compounds disclosed as Formula (I) are compounds disclosed as Formula (I-1) or Formula (I-2). The whole technical process has the advantages of mild conditions, high yield and high repetitiveness, is simple to operate, is safe and controllable, and can easily implement industrial production.
Owner:TIANJIN CHASE SUN PHARM CO LTD
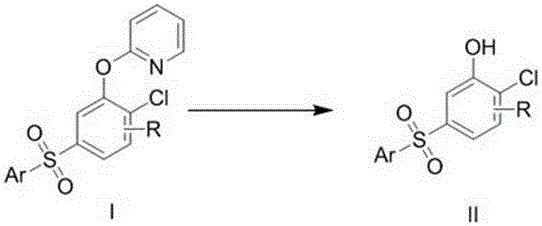
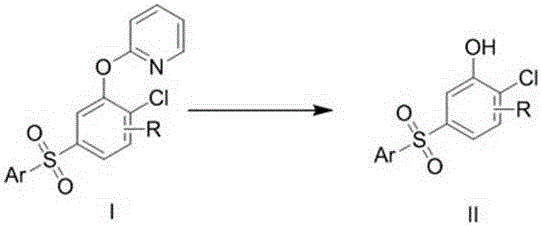
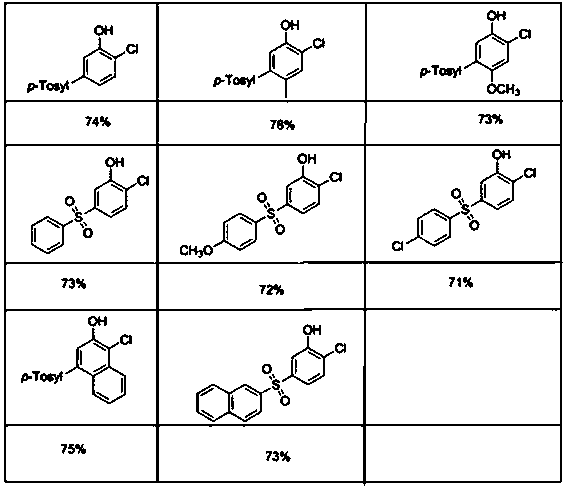
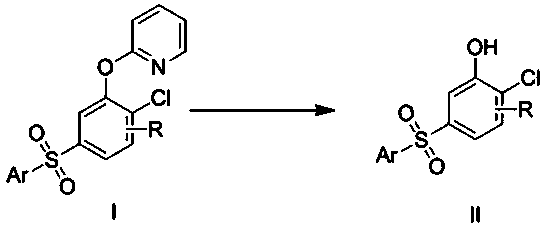
Preparation method of 5-arylsulfonyl-2-chlorophenol compound
InactiveCN106478492AEasy to prepareMild reaction conditionsOrganic chemistryOrganic compound preparation2-ChlorophenolPyridine
The invention discloses a preparation method of a 5-arylsulfonyl-2-chlorophenol compound, which is prepared from a 2-(5-arylsulfonyl-2-chlorophenoxy) pyridine compound as a raw material by the following steps: A: directly adding the 2-(5-arylsulfonyl-2-chlorophenoxy) pyridine compound, methyl trifluoromethansulfonate and toluene into a reacting device, stirring for reacting for 24 hours at 100 DEG C, and then separating a crude product; and B: adding the crude product obtained in the step A to methanol, adding sodium, refluxing for 15 minutes, and separating to obtain the 5-arylsulfonyl-2-chlorophenol compound. The preparation method is simple and mild in reaction conditions, is finished in one step, and can be well applied to preparation of the 5-arylsulfonyl-2-chlorophenol compound.
Owner:ANYANG NORMAL UNIV
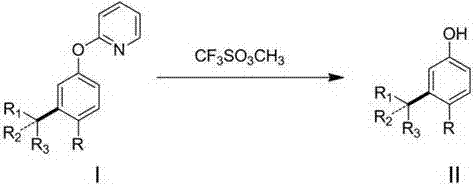
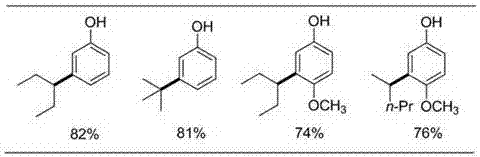
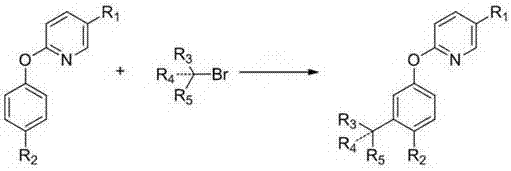
Meta-position alkylphenol synthesizing method
InactiveCN107032960AOvercoming Difficult Synthesis ProblemsEasy to synthesizeOrganic chemistryOrganic compound preparationAlkylphenolNitrogen gas
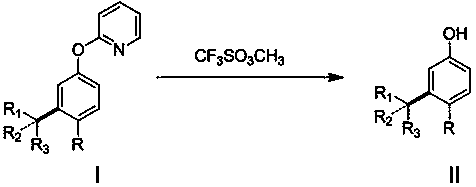
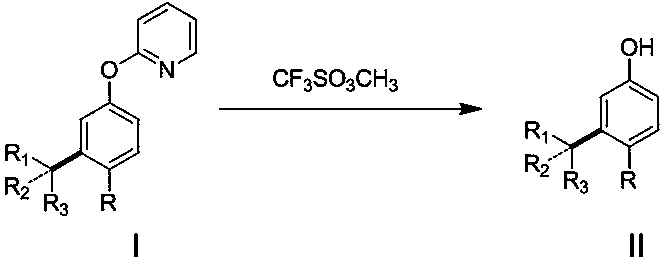
Disclosed is a meta-position alkylphenol synthesizing method. The meta-position alkylphenol synthesizing method is obtained through direct reaction of 2-(3-alkyl-phenoxy) pyridine and methyl trifluoromethansulfonate. The method comprises a reaction process of directly adding the 2-(3-alkyl-phenoxy) pyridine, the methyl trifluoromethansulfonate and methylbenzene into a reaction device; in a nitrogen atmosphere, performing stirring at 100 DEG C for reaction for 24 hours, and after the reaction is completed, separating out coarse products; adding the coarse products and sodium into methyl alcohol for refluxing for 15 minutes, and then performing separation to obtain meta-position alkylphenol compounds. The meta-position alkylphenol synthesizing method is reliable in raw material source and simple in reaction and is an economic and efficient meta-position alkylphenol preparing method.
Owner:ANYANG NORMAL UNIV
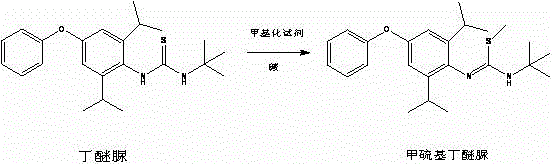
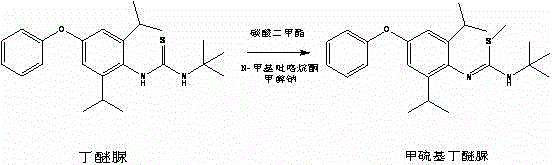
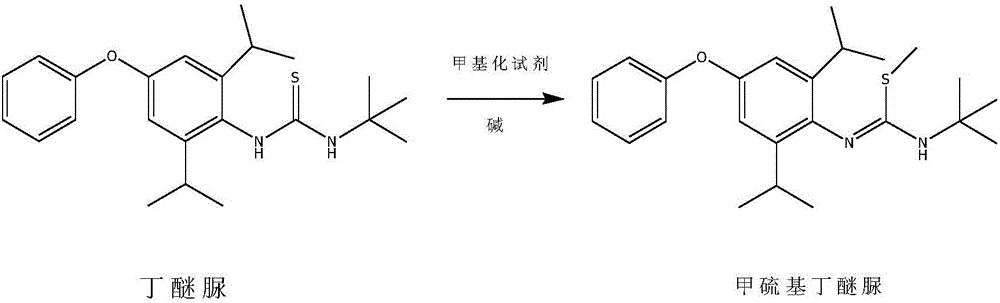
Method for preparing methylmercaptodiafenthiuron
The invention discloses a method for preparing methylmercaptodiafenthiuron. The method comprises the following reaction formula described in the specification, wherein in the formula, a methylation reagent is selected from methyl chloride, methyl bromide, methyl benzenesulfonate, methyl trifluoromethansulfonate, methyl p-toluenesulfonate, dimethyl carbonate, trimethyl phosphate, dimethyl sulfate, diazomethane, methyl trichloroethanimidate, and formaldehyde+formic acid; an alkali is selected from M2CO3, NCO3, MHCO3, MXR1, N(XR1)2, MH, NH2 or NO; M is selected from Li, Na or K; N is selected from Mg or Ca; X is selected from O or S; R1 is selected from H or C1-C6 alkyl. The compound shown in the reaction formula undergoes a reaction with the methylation reagent and the alkali in a proper solvent to prepare the methylmercaptodiafenthiuron. According to the method, the methylation reagent with low price is used, so the product cost is reduced; and the method is mild in reaction condition, simple, convenient and feasible in post-treatment method, economic and effective, and prone to industrialized production.
Owner:HAILIR PESTICIDES & CHEM GRP
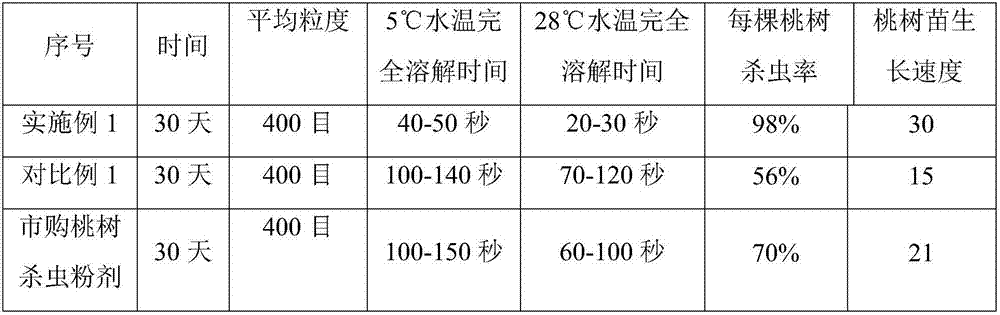
Quick-preparation type plant alkaloid insecticidal powder for peach trees and preparation method of quick-preparation type plant alkaloid insecticidal powder
InactiveCN107318898AReduce formulation costsExcellent dissolution efficiencyBiocideAnimal corpse fertilisersN dimethylformamideCalla
The invention discloses quick-preparation type plant alkaloid insecticidal powder for peach trees and a preparation method of the quick-preparation type plant alkaloid insecticidal powder. the plant alkaloid insecticidal powder is prepared from the following components in parts by weight: 4.534-8.655 parts of 1,2-dicarboxy-propyl carborane, 3.982-9.376 parts of N-bromosuccinimide, 9.456-14.269 parts of cod-liver oil extracts, 4.257-8.657 parts of methyl methacrylate, 1.354-4.156 parts of biscyclopentadienyl iron, 5.698-7.156 parts of acetic p-toluenesulfonic anhydride, 4.598-7.325 parts of divinyltetramethyldisiloxane, 18.657-20.723 parts of alliin, 10.687-15.893 parts of divinyltetramethyldisiloxane, 12.586-17.951 parts of calla extract liquor, 7.635-12.675 parts of methyl trifluoromethanesulfonate, 1.589-7.896 parts of N, N-dimethylformamide, 10.893-16.753 parts of 2-oxo-1-imidazolidinecarbonyl chloride, 8.394-12.756 parts of 3-acetyl-1-chlorocarbonyl-2-imidazolidone, 14.659-18.546 parts of 3-chlorocarbonyl-1-methanesulfonyl-2-imidazolidinone, 4.893-8.885 parts of 1-(2-hydroxyethyl)-2-imidazolidinone, 6.786-10.563 parts of 1-methylsulphonyl-2-imidazolidone, 15.689-22.486 parts of 1,3-dimethyl-2-imidazolidinone, 17.986-22.258 parts of 1-acetyl-2-imidazolidinone and the like and 5.698-8.467 parts of pistia stratiotes extract liquor.
Owner:徐州市贾汪区信守农业发展有限公司
Preparation method of sulfonyl azide compounds
ActiveCN103360318BGood water solubilityMaintain stabilitySugar derivativesSugar derivatives preparationSodium azideMedicinal chemistry
The invention relates to a preparation method of sulfonyl azide compounds, which comprises the following steps: (a) reacting compounds disclosed as Formula (II) with methyl trifluoromethanesulfonate to obtain compounds disclosed as Formula (III), wherein the compounds disclosed as Formula (II) are compounds disclosed as Formula (II-1) or Formula (II-2), and the compounds disclosed as Formula (III) are compounds disclosed as Formula (III-1) or Formula (III-2); and (b) reacting the compounds disclosed as Formula (III) obtained in the step (a) with sodium azide to obtain sulfonyl azide compounds disclosed as Formula (I), wherein the sulfonyl azide compounds disclosed as Formula (I) are compounds disclosed as Formula (I-1) or Formula (I-2). The whole technical process has the advantages of mild conditions, high yield and high repetitiveness, is simple to operate, is safe and controllable, and can easily implement industrial production.
Owner:TIANJIN CHASE SUN PHARM CO LTD
The preparation method of 3-aryl-1-indanone derivatives
InactiveCN105348062BRaw materials are easy to getSimple and fast operationCarbonyl compound preparation by condensationArylMetallole
The invention discloses a preparation method of a 3-aryl-1-indanone derivate. The structural formula of the 3-aryl-1-indanone derivate is shown in the formula III. The preparation method comprises the following steps that a compound shown in the formula I reacts with a compound shown in the formula II in the presence of methyl trifluoromethanesulfonate, and therefore the 3-aryl-1-indanone derivate shown in the formula III is obtained. The preparation method of the 3-aryl-1-indanone derivate is scientific and reasonable, 3-aryl-1-indanone derivates with various substituent groups can be obtained, the raw materials are easy to obtain, no metal participates in the reaction, reaction atoms are economical, and meanwhile the preparation method has the advantages that operation is simple and convenient, the synthesis productive rate is high, the product is easy to purify, and environmental protection is achieved.
Owner:TSINGHUA UNIV
Preparation method of ortho-alkylphenol
InactiveCN109081770AEasy to routeEasy to implementOrganic chemistryOrganic compound preparationOrtho positionAlkylphenol
The invention relates to a preparation method of ortho-alkylphenol. A 2-(2-alkylphenoxy) pyridine derivative is used as a raw material to prepare the ortho-alkylphenol. The reaction process includes:directly adding the 2-(2-alkylphenoxy) pyridine derivative, methyl trifluoromethansulfonate and methylbenzene in a reaction device; reacting for 24 h; separating a coarse product; then adding the coarse product in methanol; adding sodium; refluxing for 15 minutes; and separating to obtain the ortho-alkylphenol. The preparation method is simple in route and easy to implement, and side reactions areless in a preparation process.
Owner:ANYANG NORMAL UNIV
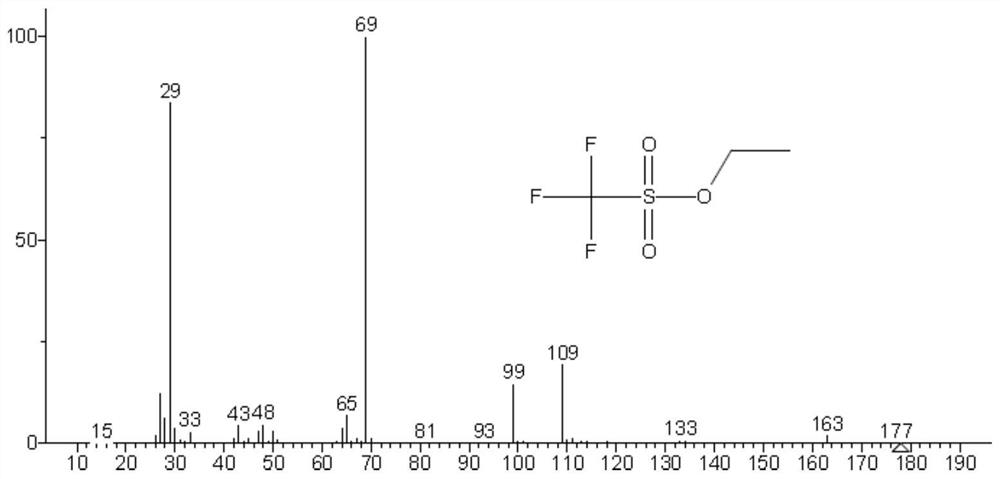
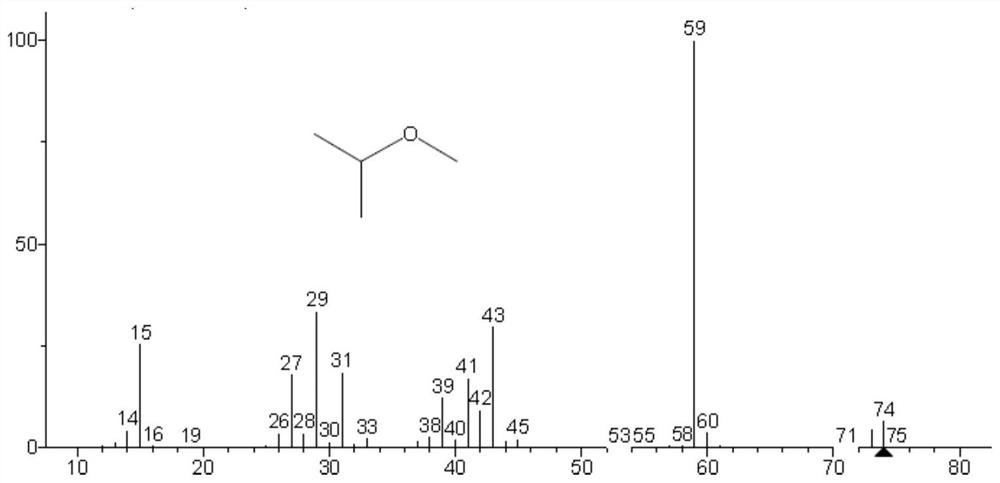
Method for simultaneously detecting methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate in tubulin inhibitor bulk drug
ActiveCN112730642AQuality is easy to controlImprove securityComponent separationBulk chemical productionGas liquid chromatographicPolyethylene glycol
The invention discloses a method for simultaneously detecting genotoxic impurities including methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate in tubulin inhibitor bulk drugs, and the bulk drugs are (3Z, 6Z)-3-[(E)-3-(5-tert-butyl)-1H-imidazolyl-4-yl) methylene]-6-((E)-3-(3-fluorophenyl)-2-propylene subunit)piperazine-2, 5-diketone. The method comprises the following steps: (1) taking a test sample of the raw material medicine, adding isopropanol, uniformly shaking, carrying out ultrasonic treatment, filtering, taking the subsequent filtrate, and introducing a sample to determine trifluoromethanesulfonic acid methyl ester and trifluoromethanesulfonic acid ethyl ester; wherein methyl trifluoromethanesulfonate reacts in isopropanol to generate methyl isopropyl ether, and ethyl trifluoromethanesulfonate reacts in isopropanol to generate ethyl isopropyl ether; and (2) detection conditions: taking polyethylene glycol modified by nitro terephthalic acid as a capillary column of a stationary phase, and determining by adopting a gas chromatograph-mass spectrometer. According to the method, the methyl trifluoromethanesulfonate and the ethyl trifluoromethanesulfonate in the tubulin inhibitor bulk drug can be qualitatively and quantitatively detected simply, quickly and efficiently at the same time.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
A kind of synthetic method of m-position alkylphenol
InactiveCN107032960BOvercoming Difficult Synthesis ProblemsEasy to synthesizeOrganic chemistryOrganic compound preparationAlcoholAlkylphenol
Owner:ANYANG NORMAL UNIV
Preparation method of flame-retardant anti-static cable material
InactiveCN108517089ARaw materials are easy to getSimple processPlastic/resin/waxes insulatorsMANGANESE ACETATEPolyvinyl chloride
The invention discloses a preparation method of a flame-retardant anti-static cable material. According to the process, the steps of high-temperature banburying, magnetic stirring, mold casting, stillstanding cooling, water cooling drawing, solid solution aging and the like are respectively performed on active adhesives (prepared from raw materials of vinyltriethoxysilane, polymethylphenyl silicon oil, sodium pyrosulfite, cyclosiloxane fatty ester, dimethyl phthalate, manganese acetate, sodium chromate, hexamine and the like) and raw materials of polyvinyl chloride, polyethylene wax, polyethylene glycol terephthalate, diethylene glycol dibenzoate, ditexanol, dimethoxymethane, sodium p-tolylsulfinate, tricaprylyl citrate, 1-phenyl-1-cyclopentanecarbonitrile, dipropylene aldehyde pentaerythritol, organic phosphite ester, methyl trifluoromethanesulfonate, diammonium phosphate, ammonium chloride and the like to obtain the flame-retardant anti-static cable material. The prepared flame-retardant anti-static cable material has the advantages of good electric chemical performance, anti-static and flame-retardant performance; various user requirements can be met.
Owner:苏州耐思特塑胶有限公司
A kind of preparation method of 5-arylsulfonyl-2-chlorophenol compound
InactiveCN106478492BEasy to prepareMild reaction conditionsOrganic chemistryOrganic compound preparation2-ChlorophenolPyridine
Owner:ANYANG NORMAL UNIV
Method for detecting methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate residues
ActiveCN113252809AHigh selectivityHigh sensitivityComponent separationMethyl methanesulfonateTriflic acid
The invention is applicable to the technical field of analytical chemistry, and provides a method for detecting methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate residues. The method comprises the steps of dissolving a sample to be detected with alcohol, and fixing the volume to obtain a sample solution; dissolving methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate reference substances with alcohol, and carrying out volume metering to acquire a plurality of reference substance solutions with concentration gradients; sequentially injecting the reference substance solutions and the sample solution according to chromatographic conditions and mass spectrum conditions for analysis to acquire spectrum data; and drawing a linear correlation working curve according to the spectrum data, and calculating the residues of methyl trifluoromethanesulfonate and ethyl trifluoromethanesulfonate in the sample to be detected. The sample treatment process is simple and convenient, alcohol serves as a solvent and a derivatization reagent, the derivatization reaction is mild, rapid and complete, interference of a complex derivatization reagent on detection is avoided, the detection specificity, precision and accuracy are improved, and the method has an important research value in the aspects of bulk drug quality research, impurity analysis and control research and the like.
Owner:英格尔检测技术服务(上海)有限公司
A cycle-improved lithium-rich manganese solid solution battery
Owner:WANXIANG 123 CO LTD
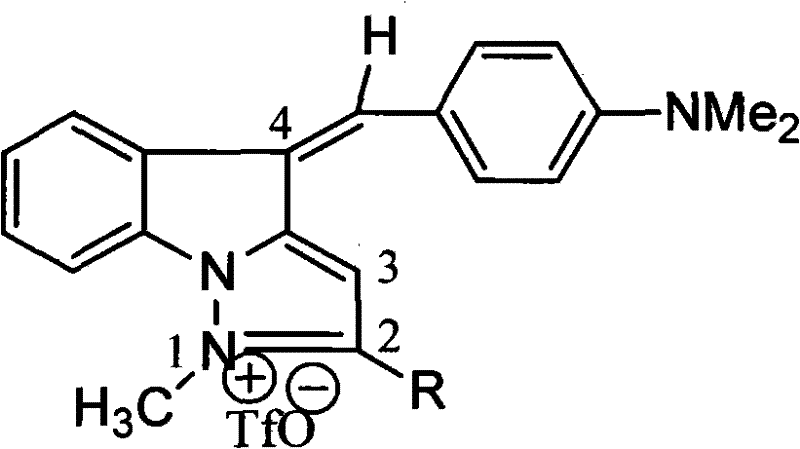
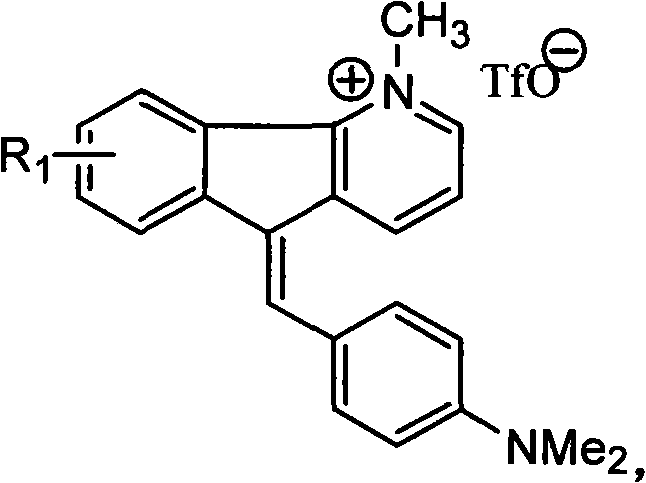
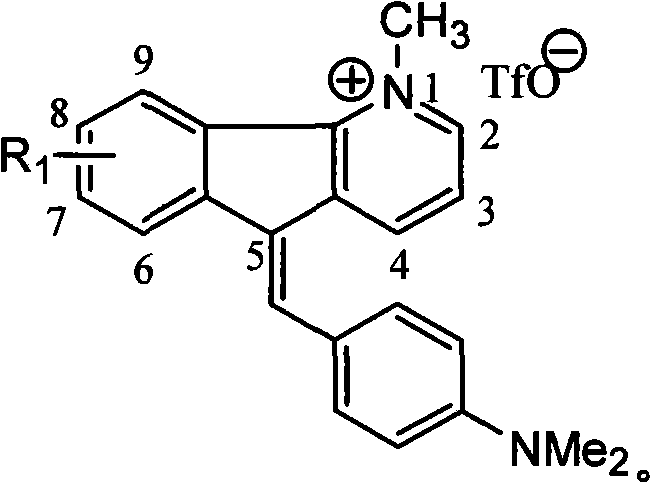
1-methyl-7h-indene[1, 2-b]quinolinetrifluoromesylate-7-(4-dimethylamino) benzyl alkene derivant and preparation thereof
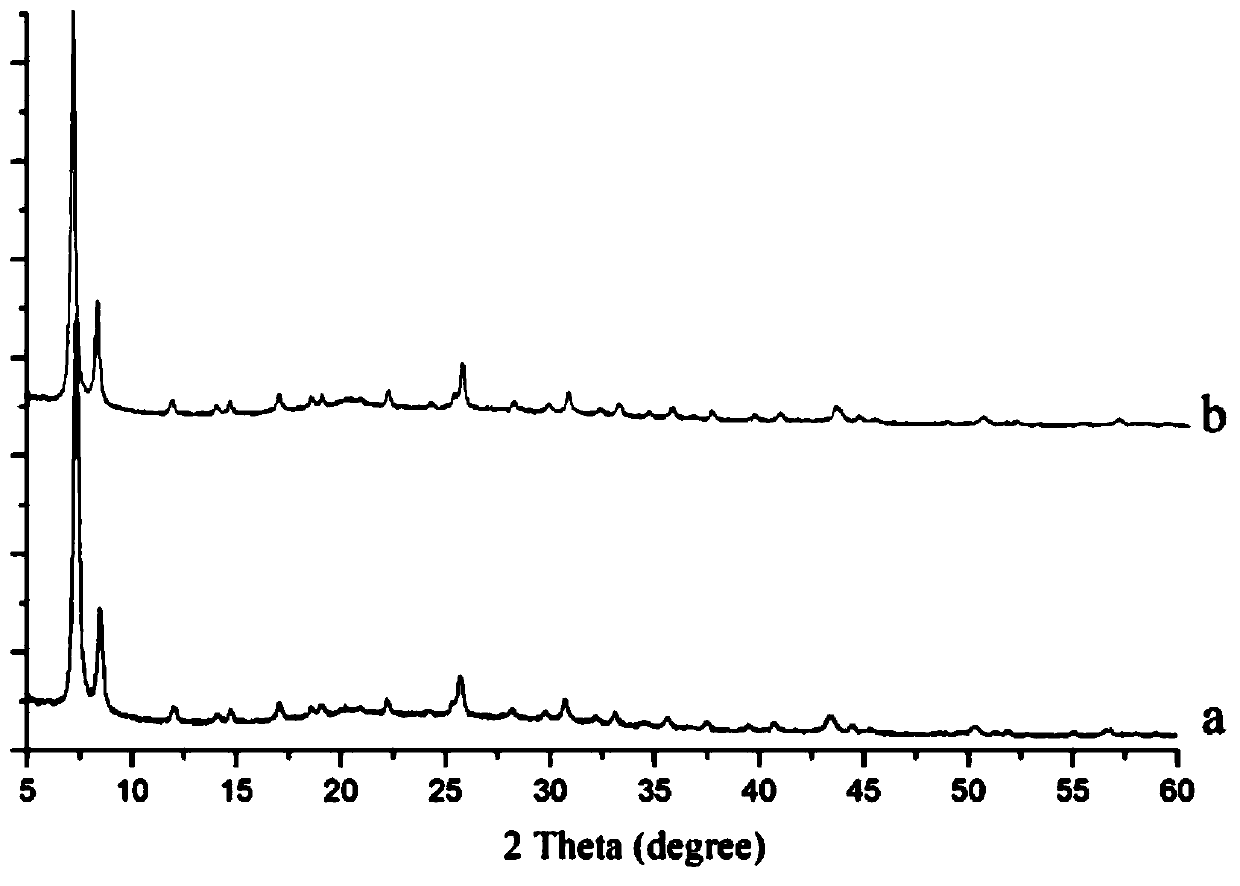
InactiveCN101948429BEnhanced inhibitory effectSimple processOrganic active ingredientsOrganic chemistryTriflic acidNitrogen gas
Owner:SUZHOU UNIV
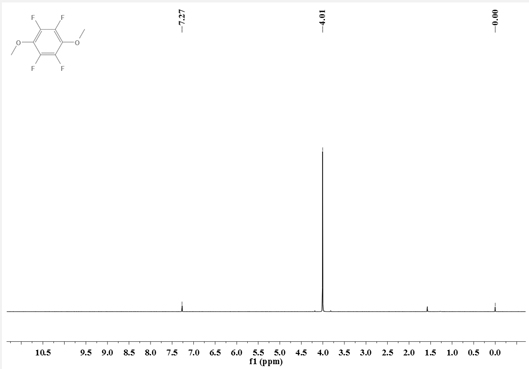
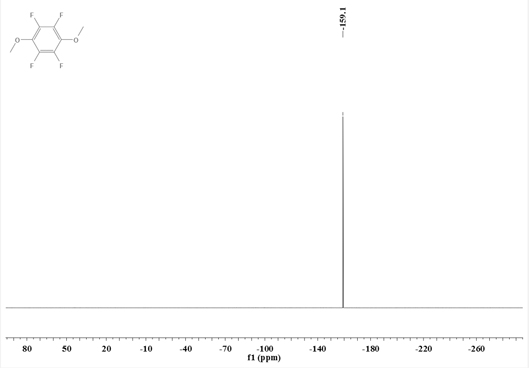
Synthesis method of 1, 4-dimethoxy tetrafluorobenzene
ActiveCN113087600AImprove anti-overcharge performanceMild reaction conditionsOrganic compound preparationEther preparationSodium methoxidePentafluoroaniline
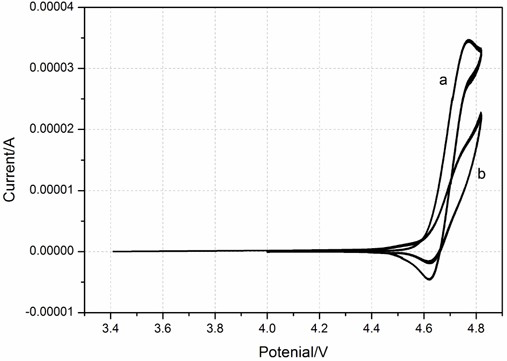
The invention discloses a synthesis method of 1, 4-dimethoxy tetrafluorobenzene, the synthesis method comprises the following steps: (1) reacting N, N-dimethyl pentafluoroaniline with sodium methoxide to obtain an intermediate I; (2) mixing the intermediate I with a first organic solvent, and then reacting with methyl trifluoromethanesulfonate to obtain an intermediate II; and (3) mixing the intermediate II with a second organic solvent, and reacting with methanol in alkali and protective gas atmosphere to obtain the 1, 4-dimethoxy tetrafluorobenzene. The synthesis method of the 1, 4-dimethoxy tetrafluorobenzene has the advantages of mild reaction conditions, no need of harsh conditions, high controllability, simple post-treatment, easiness in operation, capability of effectively improving the overcharge resistance of the lithium ion battery and the like.
Owner:ZHANGJIAGANG GUOTAI HUARONG NEW CHEM MATERIALS CO LTD
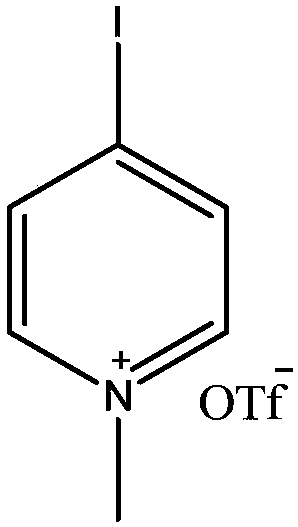
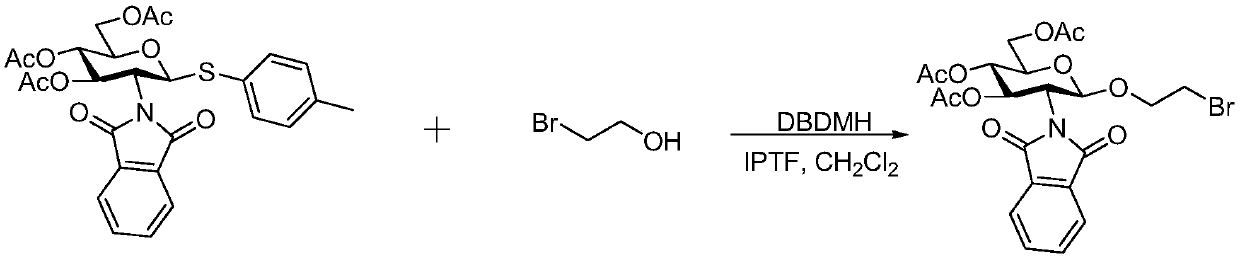
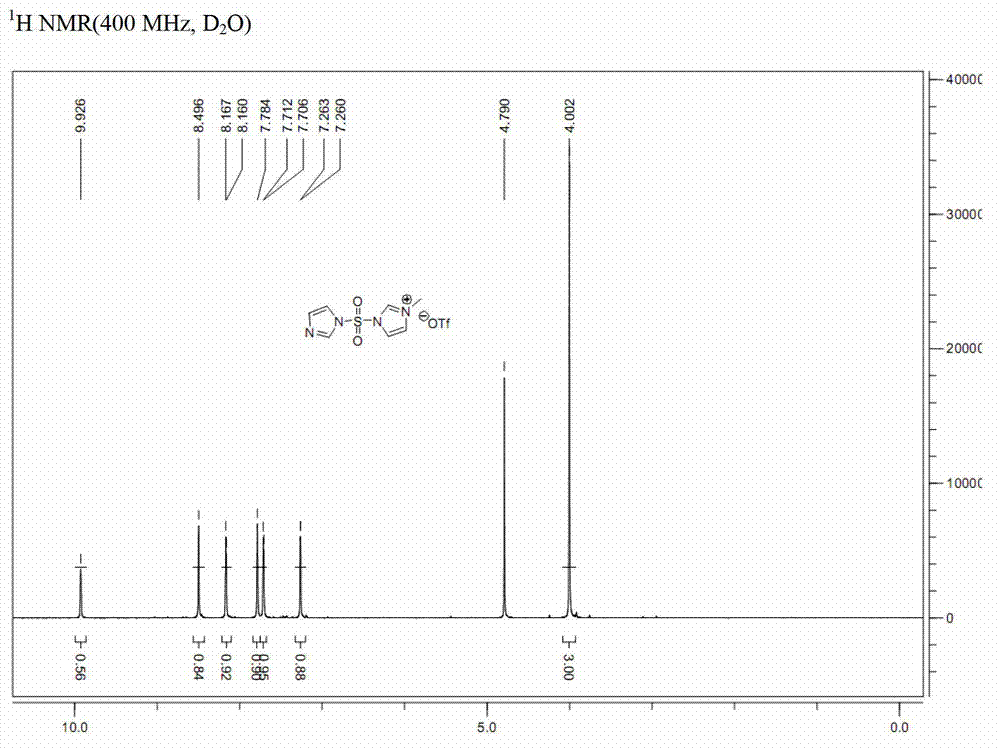
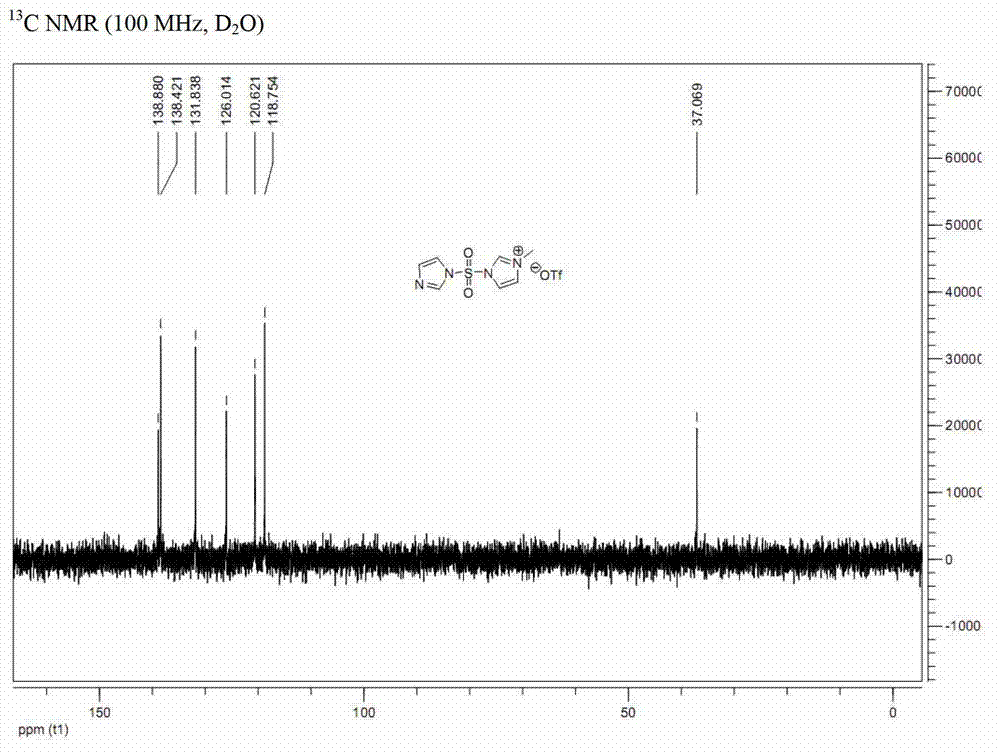
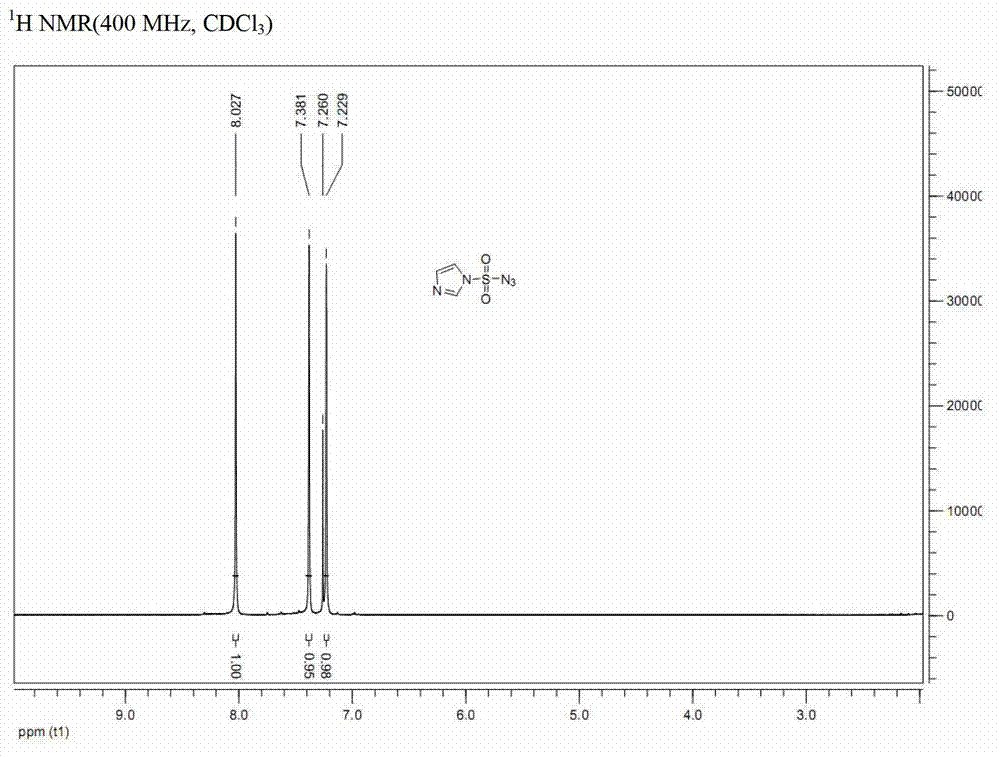
Preparation method for synthesizing O-glycoside based on catalytic activation of thioglycoside by 4-iodopyridin-N-methyltrifluoromethanesulfonate
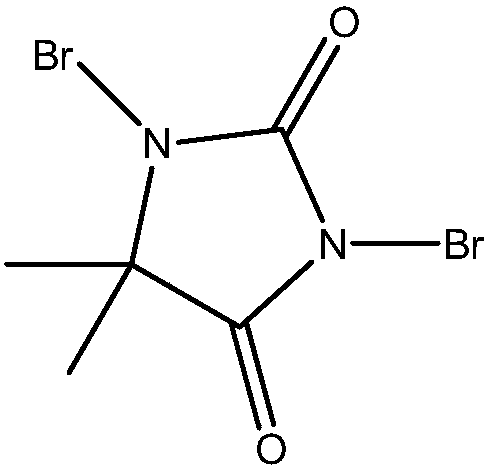
The invention discloses a synthetic method of O-glycoside. The technical solution is that alcohol is glycosylated in an anhydrous organic solvent by using a reagent combination of 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) and a catalytic equivalent of 4-iodopyridin-N-methyl trifluoromethanesulfonate (IPTF) as activator and thioglycoside as a glycosyl donor to generate the O-glycoside. The method is simple and reliable, and is suitable for large-scale industrial production.
Owner:LANZHOU UNIVERSITY
1-methyl-5h-indene (1,2-b) pyridine trifluoromethanesulfonic salt-5-(4-dimethylamino group) benzylidene derivative and preparation method thereof
InactiveCN101948428BEnhanced inhibitory effectSimple processOrganic active ingredientsOrganic chemistryA-DNANitrogen gas
The invention belongs to the field of anti-tumor medicine preparation and in particularly relates to a 1-methyl-5H-indene (1,2-b) pyridine trifluoromethanesulfonic salt-5-(4-dimethylamino group) benzylidene derivative used as a DNA topoisomerase inhibitor and a preparation method thereof. The preparation method comprises the following steps of: (1) salifying reaction: stirring and reacting 5H-indene (1,2-b) pyridine and trifluoromethanesulfonic methyl ester in the molar ratio of 1:2-3 under the nitrogen protection at room temperature to obtain 1-methyl-5H-indene (1,2-b) pyridine trifluoromethanesulfonic salt; and (2) coupling reaction: distilling the 1-methyl-5H-indene (1,2-b) pyridine trifluoromethanesulfonic salt obtained in the step (1) with 4-dimethylaminobenzaldehyde in the molar ratio of 1.0:1.4-1.6 in glacial acetic acid to obtain the 1-methyl-5H-indene (1,2-b) pyridine trifluoromethanesulfonic salt-5-(4-dimethylamino group) benzylidene derivative. The obtained compound has strong function for inhibiting leukemia cell and human laryngeal squamous carcinoma. Besides, the preparation method has simple process and good productivity as high as about 65%.
Owner:SUZHOU UNIV
A method for improving the thermal stability of liquid fructose amino acid oxidase
ActiveCN104611319BImprove thermal stabilityImprove stabilityEnzyme stabilisationSodium azideTrehalose
The invention discloses a method for improving thermal stability of liquid fructosaminase. The method comprises the following steps: 1) preparing a tris(hydroxymethyl) aminomethane-hydrochloric acid buffer solution comprising fructosaminase and sodium azide and having a pH value of 7.5-8.5; 2) under a low-temperature stirring condition, adding 1-butyl sulfonate-3-methyl trifluoromethanesulfonate, trehalose and TritonX-100 into the buffering solution prepared in the step (1). With adoption of the method disclosed by the invention, the thermal stability of the fructosaminase is obviously improved, and the fructosaminase can be applied to develop a determination reagent for glycated albumin determined by an FAOD (fructosaminase) clearance method; the method for improving thermal stability of liquid fructosaminase cannot interfere GA content determination by the FAOD clearance method, can obviously improve the thermal stability of the liquid for fructosaminase, enables the liquid fructosaminase to be still enough in activity 12 months after preservation at the temperature of 4 DEG C.
Owner:浙江夸烨生物科技有限公司
One-pot method for preparing lacosamide
ActiveCN105523957BReduce usageReduce generationOrganic compound preparationCarboxylic acid amides preparationOrganic baseAcetylation
The invention discloses a method for preparing Lacosamide by a one-pot method. The method comprises the steps: subjecting D-serine and an acetylation reagent to a reaction in a manner of taking dichloromethane as a solvent, and adjusting the pH of a reacted substance to 12-13 by an organic base after the reaction ends; then, controlling the temperature to -5 to 0 DEG C, and adding methyl trifluoromethanesulfonate into the reacted substance for a reaction; and cooling a reacted substance to the temperature of -30 to -25 DEG C, adding a dehydrating agent and benzylamine into the reacted substance for a reaction, carrying out depressurized-concentration drying on material liquid after the reaction ends so as to obtain crude Lacosamide, and then, carrying out recrystallization, thereby obtaining pure Lacosamide. The preparation method is simple and easy in operation, and the prepared Lacosamide has the purity of 99.90% or more and the chiral purity of 99.90%.
Owner:QILU PHARMA HAINAN +1
Trimethylsilyl trifluoromethanesulfonate preparation method
InactiveCN108373481ASuitable for mass productionSilicon organic compoundsTrimethylsilanolTrimethylsilyl trifluoromethanesulfonate
The invention provides a trimethylsilyl trifluoromethanesulfonate preparation method. A reaction route is shown as CF3SO2X+(CH3)3SiOH->CF3SO2OSI(CH3)3+XH, wherein X=OCH3,OC2H5. The preparation methodincludes the production steps: adding methyl trifluoromethanesulfonate or ethyl trifluoromethanesulfonate in a mole ratio of 1:(1.2-4) into trimethyl silanol, adding a small quantity of a catalyst, performing stirring reaction for 1-10 hours at the reaction temperature ranging from -20 DEG C to 100 DEG C in a dry environment, evaporating out newly generated methanol or ethanol during reaction, rectifying a final product, and collecting a fraction at 135-145 DEG C to obtain a product. By adoption of the method, purity reaches 99.5% or above, the raw material trimethyl silanol can be recycled, the newly generated byproduct methanol or ethanol can be used, and environmental friendliness and suitableness for large-scale production are realized.
Owner:JIANGXI GUOHUA IND CO LTD
Synthetic method for improving yield of methamidophos intermediate
PendingCN111233919AHigh purityRaise quality standardsGroup 5/15 element organic compoundsAlkaneMeth-
The invention discloses a synthetic method for improving the yield of a methamidophos intermediate, and belongs to the technical field of fine chemical production. The method comprises the following steps: reacting O,O-dimethyl phosphoramidothioate with a catalyst and dimethyl sulfate at 25-60 DEG C to obtain methamidophos; wherein a solvent is halogenated alkane, and the catalyst comprises one ofmethyl iodide, methyl methanesulfonate, methyl trifluoromethanesulfonate, trimethylsilyl trifluoromethanesulfonate or isopropyl titanate. The method has the advantages that the process is simple, thereaction time is short, the obtained reaction yield is 90% or above, and the target product purity is high.
Owner:安道麦股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
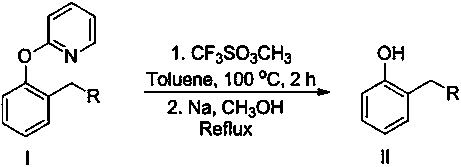
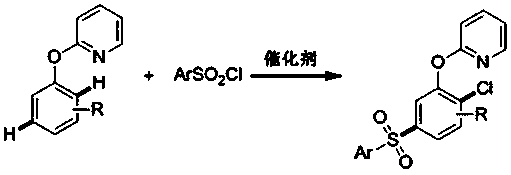
![1-methyl-7h-indene[1, 2-b]quinolinetrifluoromesylate-7-(4-dimethylamino) benzyl alkene derivant and preparation thereof 1-methyl-7h-indene[1, 2-b]quinolinetrifluoromesylate-7-(4-dimethylamino) benzyl alkene derivant and preparation thereof](https://images-eureka.patsnap.com/patent_img/3f14bb44-39a0-41bb-9bf0-a608f214beeb/BSA00000183622800011.PNG)
![1-methyl-7h-indene[1, 2-b]quinolinetrifluoromesylate-7-(4-dimethylamino) benzyl alkene derivant and preparation thereof 1-methyl-7h-indene[1, 2-b]quinolinetrifluoromesylate-7-(4-dimethylamino) benzyl alkene derivant and preparation thereof](https://images-eureka.patsnap.com/patent_img/3f14bb44-39a0-41bb-9bf0-a608f214beeb/BSA00000183622800031.PNG)
![1-methyl-7h-indene[1, 2-b]quinolinetrifluoromesylate-7-(4-dimethylamino) benzyl alkene derivant and preparation thereof 1-methyl-7h-indene[1, 2-b]quinolinetrifluoromesylate-7-(4-dimethylamino) benzyl alkene derivant and preparation thereof](https://images-eureka.patsnap.com/patent_img/3f14bb44-39a0-41bb-9bf0-a608f214beeb/BSA00000183622800032.PNG)