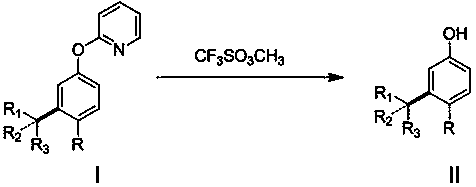
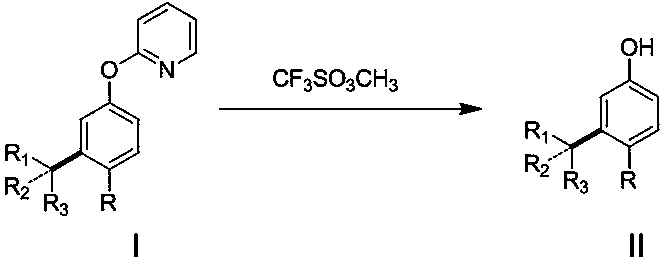
A kind of synthetic method of m-position alkylphenol
A technology for an alkyl phenol and a synthesis method, applied in the field of chemistry, can solve the problems of difficult separation, many by-products, complicated and lengthy synthesis steps of meta-alkyl phenol, etc., and achieves the effects of simple reaction and reliable source
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
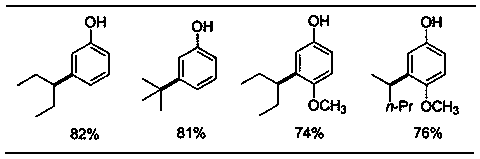
[0013] Add 34mg (0.2mmol) 2-phenoxypyridine, 90mg (0.6 mmol) 3-bromopentane, 6mg (0.01mmol) dichlorobis(4-methylisopropylphenyl) into a 20mL pressure-resistant reaction tube Ruthenium, 55mg (0.4mmol) potassium carbonate, 11mg (0.06mmol) 1-adamantanic acid, 1.5mL benzene, sealed under nitrogen, heated to 120°C for reaction, stirred for 24 hours, after reaction, separated by column chromatography to obtain the target product 2-(3-(3-Pentyl)phenoxy) 35 mg, yield 73%.
preparation example 2
[0015] Add 40mg (0.2mmol) 2-(4-methoxyphenoxy)pyridine, 90mg (0.6mmol) 2-bromopentane, 6mg (0.01mmol) dichlorobis(4-methyl) into a 20mL pressure-resistant reaction tube Isopropylphenyl) ruthenium, 55mg (0.4mmol) potassium carbonate, 11mg (0.06mmol) 1-adamantanic acid, 1.5mL benzene, sealed under nitrogen, heated to 120°C for reaction, stirred for 24 hours, after the reaction, After separation by column chromatography, 44 mg of the target product 2-(2-pentyl-4-methoxyphenoxy)pyridine was obtained with a yield of 81%.
preparation example 3
[0017] Add 34 mg (0.2 mmol) of phenoxypyridine, 82 mg (0.6 mmol) of tert-butane bromide, 6 mg (0.01 mmol) of dichlorobis(4-methylisopropylphenyl) ruthenium into a 20 mL pressure-resistant reaction tube, 55mg (0.4mmol) of potassium carbonate, 11mg (0.06mmol) of 1-adamantanic acid, 1.5mL of benzene, sealed under nitrogen, heated to 120°C for reaction, stirred for 24 hours, after reaction, separated by column chromatography to obtain the target product 2- (3-tert-butylphenoxy)pyridine 25 mg, yield 56%.
[0018] The application's m-position alkylphenol preparation embodiment:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com