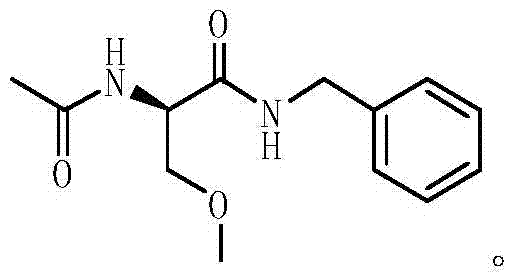
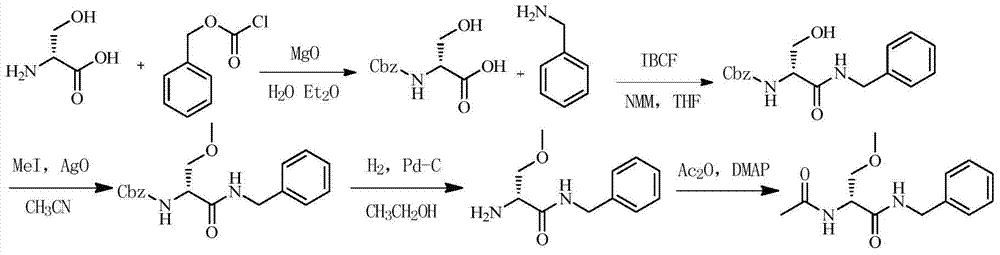
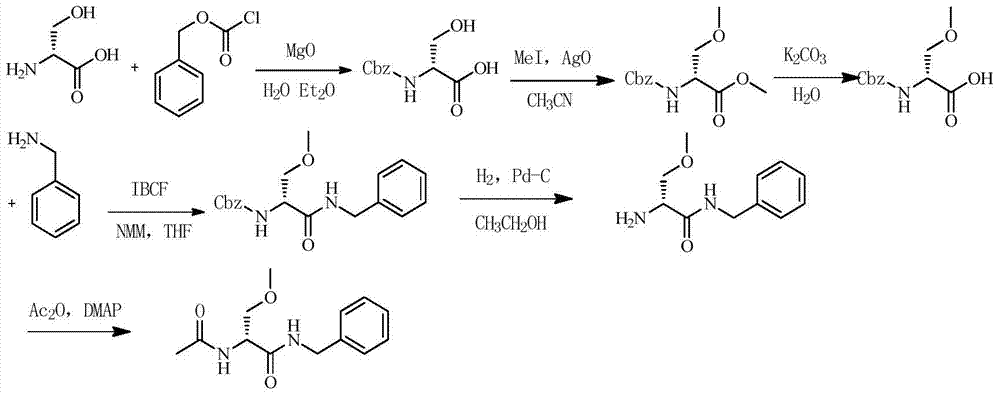
One-pot method for preparing lacosamide
A technology for preparing lacosamide and lacosamide, applied in the field of medicine, can solve the problems of high cost, expensive raw materials, complicated process and the like, and achieve the effects of reducing time cost, reducing production cost and simplifying reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] (1) Add 50g of D-serine to 200ml of dichloromethane, control the reaction temperature at 20-25°C, slowly add 48.6g of acetic anhydride dropwise, and after 2 hours of reaction, adjust the pH to 12-13 with sodium methoxide solution;
[0040] (2) Add 78.1 g of methyl trifluoromethanesulfonate dropwise under temperature control at -5 to 0°C, and after the drop is completed, raise the temperature to room temperature and react for 3 hours;
[0041] (3) Cool down to -30~-25°C, add 91.1g of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC.HCL), 1-hydroxybenzotri Nitriazole (HOBt) 64.75g, 51g of benzylamine was slowly added dropwise, and then reacted for 2 hours; the temperature was controlled at 32-38°C and the dichloromethane was concentrated under reduced pressure to obtain the crude product of lacosamide;
[0042] (4) Dissolve the above solid in 100ml of ethyl acetate, add 100ml of n-heptane dropwise, stir and cool down to 0-10°C for crystallization after dropp...
Embodiment 2
[0044] (1) Add 100 g of D-serine to 350 ml of dichloromethane in turn, control the reaction temperature at 20-25 ° C, slowly add 97.3 g of acetic anhydride dropwise, and react for 2.5 hours, adjust the pH to 12-13 with triethylamine solution;
[0045] (2) Add 156 g of methyl trifluoromethanesulfonate dropwise under temperature control at -5 to 0° C. After the drop is completed, heat up to room temperature and react for 3.5 hours;
[0046] (3) Cool down to -30°C to -25°C, add 182g of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC.HCL), 1-hydroxybenzotri Nitriazole (HOBt) 129g, slowly dropwise add 100g benzylamine to react for 2.5 hours, control temperature at 33-38°C and concentrate to dry dichloromethane under reduced pressure to obtain crude lacosamide;
[0047](4) Dissolve the above solid in 300ml of ethyl acetate, stir and cool down to 0-10°C to crystallize, filter with suction, and rinse the filter cake with 50ml of ethyl acetate. 167.3 g of the product wa...
Embodiment 3
[0049] (1) Add 50g of D-serine to 200ml of dichloromethane, control the reaction temperature at 20-25°C, slowly add 48.7g of acetic anhydride dropwise, after 2.5 hours of reaction, use N,N-diisopropylethylamine solution Adjust the pH to 12-13;
[0050] (2) Add 78.2 g of methyl trifluoromethanesulfonate dropwise under temperature control -5 to 0°C. After the drop is completed, raise the temperature to room temperature and react for 2.5 hours;
[0051] (3) Cool down to -30~-25°C, add 90.5g of 1-ethyl-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC.HCL), 1-hydroxybenzotri Add 64.0 g of nitrogen azole (HOBt), slowly dropwise add 50 g of benzylamine, and then react for 2.5 hours; control the temperature at 32-38°C and concentrate to dry dichloromethane under reduced pressure to obtain the crude product of lacosamide;
[0052] (4) Dissolve the above solid in 100ml of ethyl acetate, add 100ml of n-hexane dropwise, stir and cool down to 0-10°C to crystallize after dropping, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com