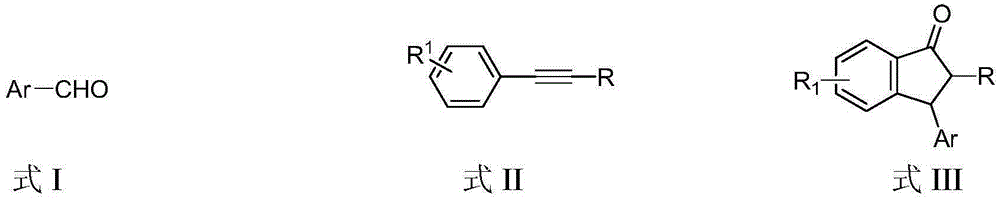
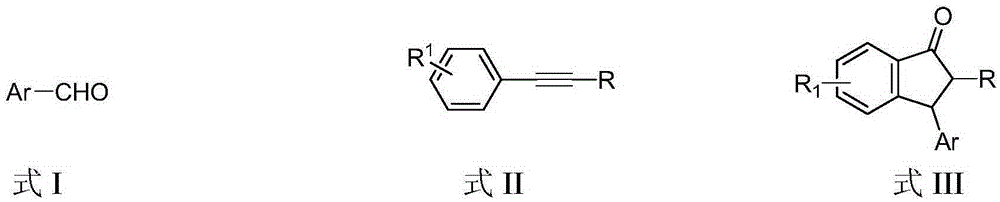
Preparation method of 3-aryl-1-indanone derivate
A technology for indanone and derivatives, which is applied in the field of preparation of 3-aryl-1-indanone derivatives, can solve the problems of difficult purification of drugs or materials in the later stage, achieve reaction atom economy, high synthesis yield, The effect of easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, 2,3-diphenyl-2,3-indanone (in the formula III, R 1 is H, Ar is phenyl, R is phenyl)
[0023] Into a 25 mL reactor, add toluene (0.22 mmol, 39 mg), benzaldehyde (0.2 mmol, 20 μL), methyl trifluoromethanesulfonate (0.04 mmol, 4.6 μL) in sequence, and finally add 0.5 mL of 1,2- Dichloroethane was used as solvent, the reactor was sealed, and reacted at 50°C for 24 hours. After the reaction system was cooled, 15 mL of water and 15 mL of dichloromethane were added and stirred for 30 min, and then extracted three times with 15 mL of dichloromethane, and the organic phases were combined. The organic phase was washed with saturated brine, and finally dried over anhydrous magnesium sulfate for 0.5 h. After filtration, the organic phase was rotary evaporated to obtain a crude product. The crude product is separated by column chromatography using petroleum ether: ethyl acetate = 25:1 (volume ratio) as the eluent, and 200-300 mesh silica gel as the adsorption phase t...
Embodiment 2
[0028] Example 2, 2-phenyl-3-p-methylphenyl-2,3-indanone (in formula III, R 1 is H, Ar is p-methylphenyl, R is phenyl)
[0029] To a 25mL reactor, add toluene (0.22mmol, 39mg), 4-methylbenzaldehyde (0.2mmol, 22μL), methyl trifluoromethanesulfonate (0.04mmol, 4.6μL), and finally add 0.5 Use mL1,2-dichloroethane as solvent, seal the reactor, and react at 50°C for 24 hours. After the reaction system was cooled, 15 mL of water and 15 mL of dichloromethane were added and stirred for 30 min, and then extracted three times with 15 mL of dichloromethane, and the organic phases were combined. The organic phase was washed with saturated brine, and finally dried over anhydrous magnesium sulfate for 0.5 h. After filtration, the organic phase was rotary evaporated to obtain a crude product. The crude product is separated by column chromatography with petroleum ether: ethyl acetate = 25:1 (volume ratio) as the eluent, and 200-300 mesh silica gel as the adsorption phase to obtain a yellow ...
Embodiment 3
[0034] Example 3, 2-phenyl-3-m-methylphenyl-2,3-indanone (in formula III, R 1 is H, Ar is m-methylphenyl, R is phenyl)
[0035]To a 25mL reactor, add toluene (0.22mmol, 39mg), 3-methylbenzaldehyde (0.2mmol, 22μL), methyl trifluoromethanesulfonate (0.04mmol, 4.6μL) in sequence, and finally add 0.5 Use mL1,2-dichloroethane as solvent, seal the reactor, and react at 50°C for 24 hours. After the reaction system was cooled, 15 mL of water and 15 mL of dichloromethane were added and stirred for 30 min, and then extracted three times with 15 mL of dichloromethane, and the organic phases were combined. The organic phase was washed with saturated brine, and finally dried over anhydrous magnesium sulfate for 0.5 h. After filtration, the organic phase was rotary evaporated to obtain a crude product. The crude product is separated by column chromatography with petroleum ether: ethyl acetate = 50:1 (volume ratio) as the eluent, and 200-300 mesh silica gel as the adsorption phase to obtai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com