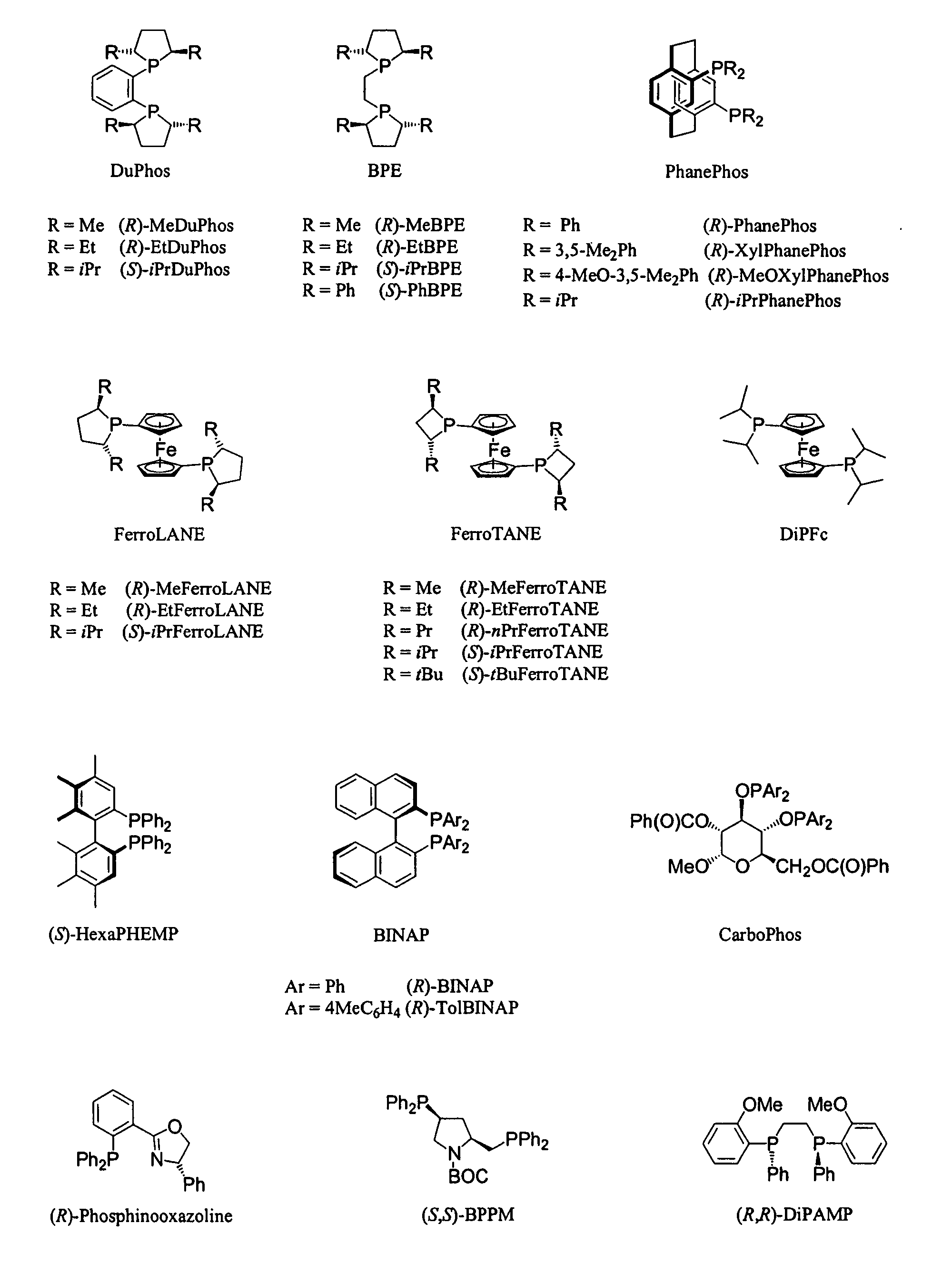
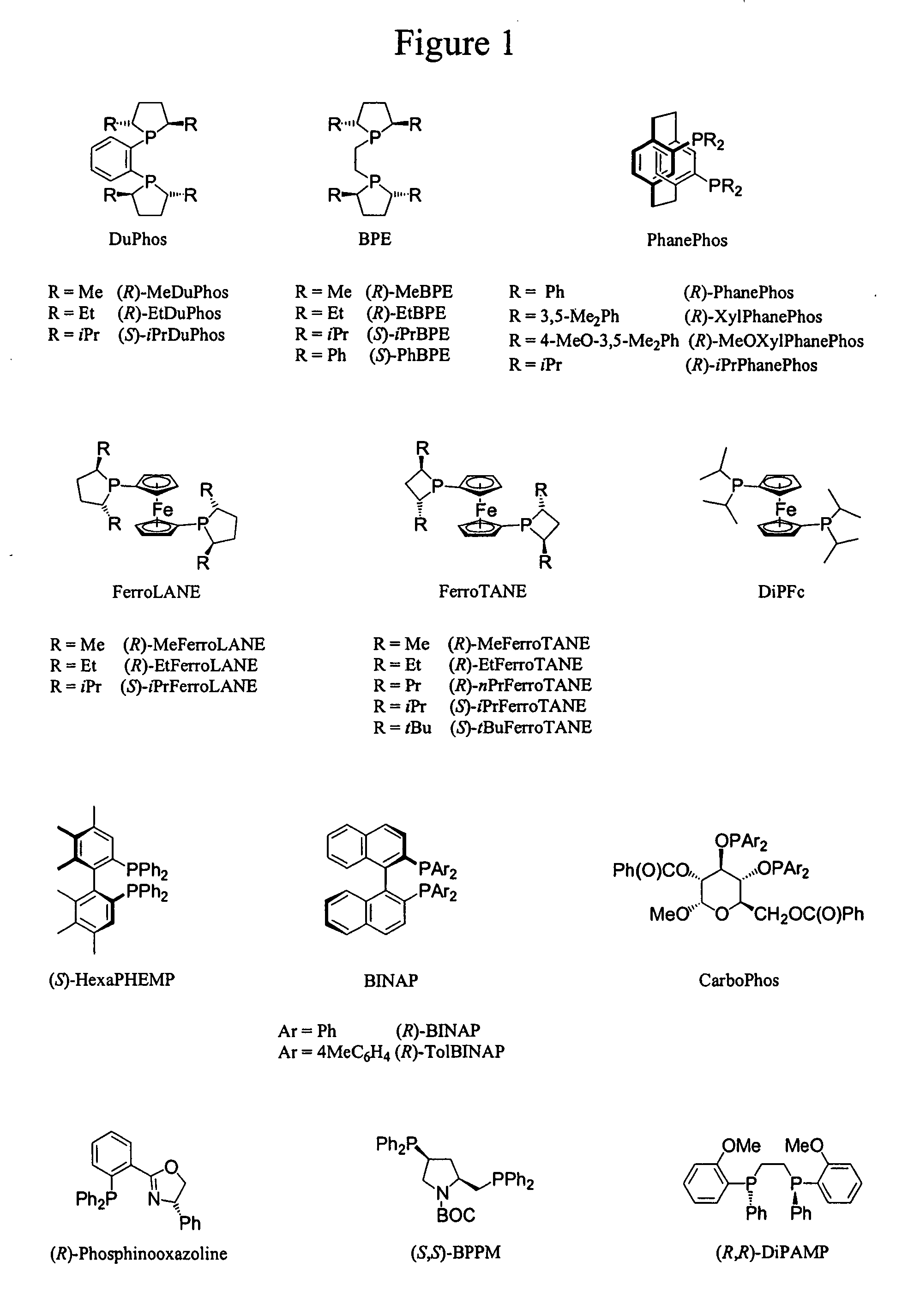
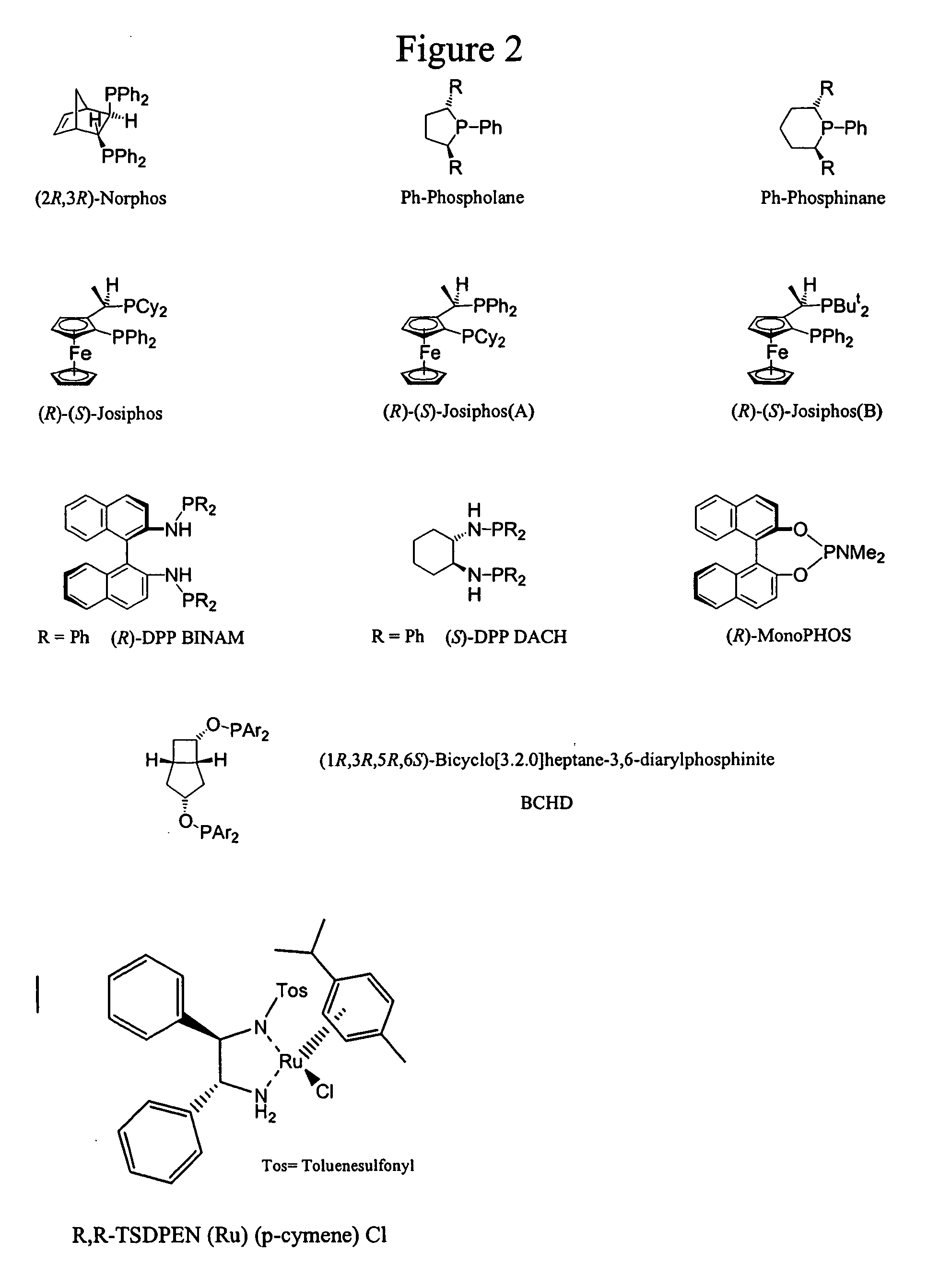
Patents
Literature
54 results about "Indenone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
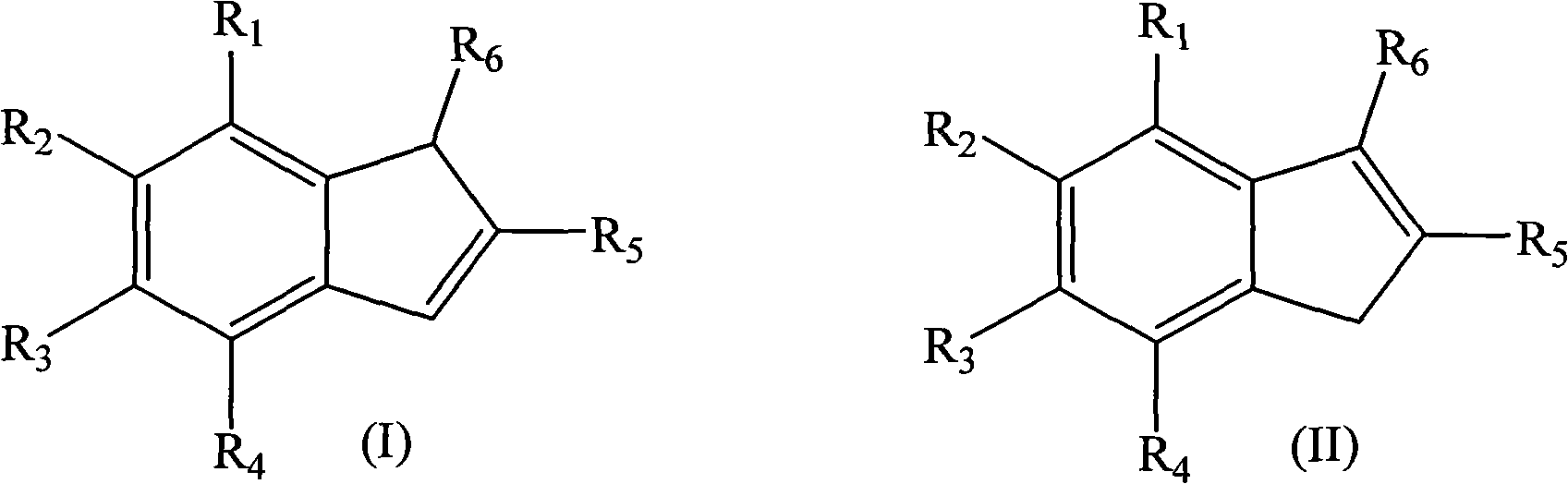
Indenone is a polycyclic ketone with chemical formula C₉H₆O. It is composed of a benzene ring fused with a cyclopentenone ring. Indenones can be used as intermediates in the synthesis of more complex molecules.
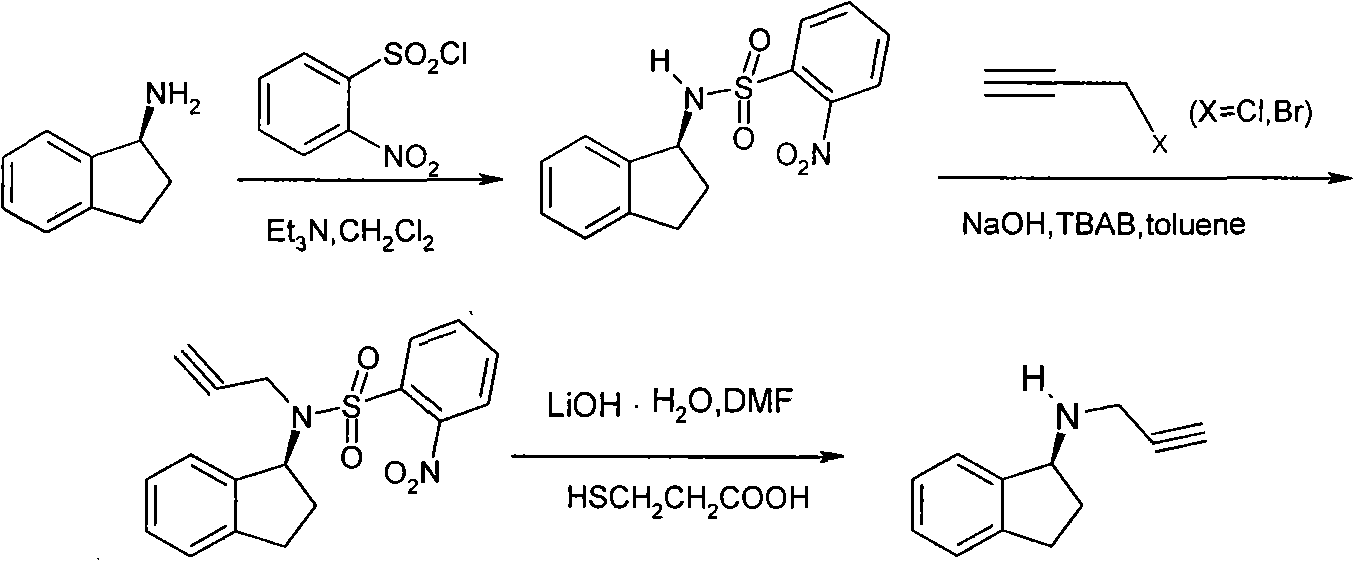
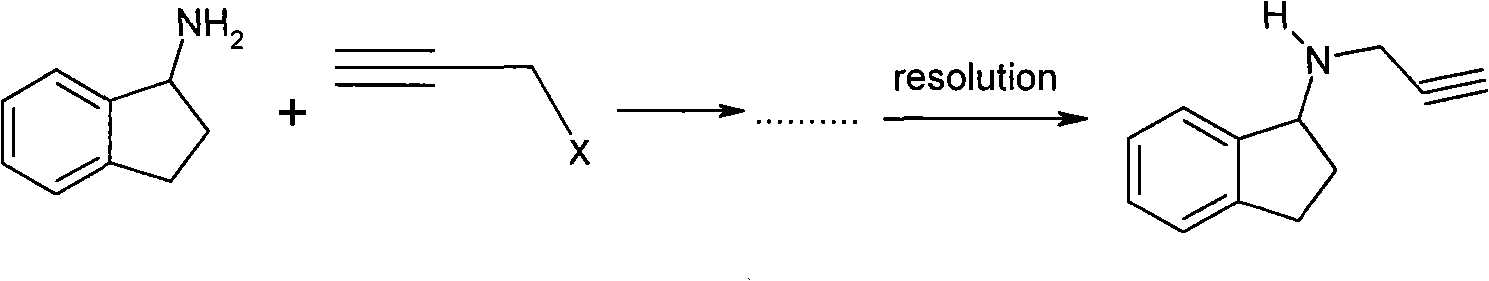
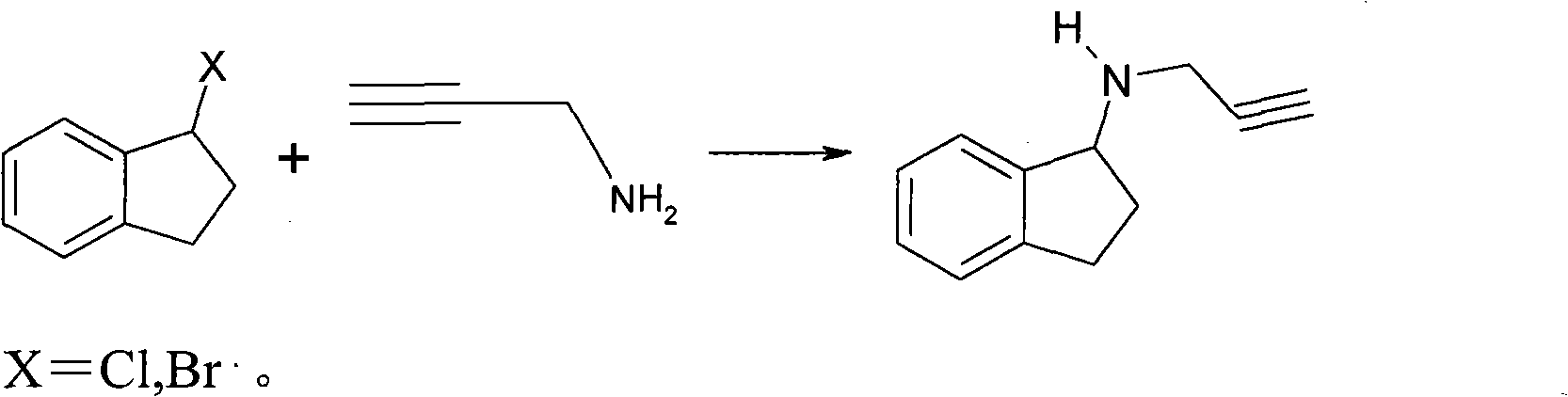
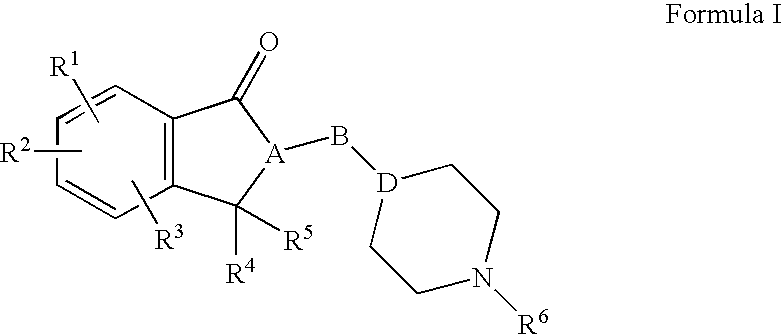
Process for the synthesis of enantiomeric indanylamine derivatives
InactiveUS20060199974A1Reduce processing stepsCarbamic acid derivatives preparationOrganic compound preparationNucleophilic substitutionIndenone
A process for manufacturing (R)-propynylaminoindans, and alternatively, a process for manufacturing (S)-propynylaminoindans. The chiral propynylaminoindans include alkoxy or alkylcarbamates derivatives. The process comprises transfer or pressure hydrogenation in the presence of an optically active catalyst to reduce 1-indanones. The chiral product, either (S)- or (R)-indanols undergo nucleophilic substitution to produce the named product. In an additional aspect, the invention relates to novel intermediates and compounds, namely, substituted indanones, substituted (S)-indanols and substituted (R)-indanols.
Owner:TEVA PHARMA IND LTD
Prepn process of 5-chloro-2,3-dihydro-1-indenone
InactiveCN1403434ALess corrosiveReduce the impactOrganic compound preparationCarbonyl compound preparationBenzeneAluminium chlorohydrate
The present invention relates to the preparation process of one kind of chemical product, and is the preparation process of 5-chloro-2,3-dihydro-1-indenone. The preparation process of 5-chloro-2,3-dihydro-1 indenone includes the reaction between 3-chloropropionyl chloride and benzene chloride to produce 3,4'-dichlorophenyl propanone; and the cyclization of the 3,4'-dichlorophenyl propanone. The present invention has high yield, high product quality and less material corrosion, and its effluent may be used in preparing one kind of water treating agent to reduce environmental pollution.
Owner:王明春
Substituted 2-indolinone as ptk inhibitors containing a zinc binding moiety
InactiveUS20080125478A1Effective for treating diseaseHigh activityBiocideOrganic chemistryDiseasePTK Inhibitors
The present invention relates to substituted 2-indolinone containing zinc-binding moiety based derivatives that have enhanced or unique properties as inhibitors of protein tyrosine kinase (PTK) receptors and their use in the treatment of PTK related diseases and disorders such as cancer. The said derivatives may further act as HDAC inhibitors.
Owner:CURIS INC
Method for preparing indene compounds
ActiveCN101318887AThe synthetic route is simpleLow costOrganic compound preparationCarbonyl compound preparationOrganic acidCompound organic
The invention discloses a method for preparing an indene compound, comprising the following steps that: a raw material of a substituted benzoic ether compound is converted into an indenone compound under the condition of cyclodehydration in the presence of a Lewis acid compounded organic acid or inorganic acid. The indenone compound is reduced to a hydroxyindane compound by a reducer; the hydroxyindane compound is converted to the indene compound through dehydration. The method of the invention uses the substituted benzoic ether compound which is common and easy to prepare to synthesize the indene compound, thereby simplifying the synthesis route and saving cost and allowing for mass production.
Owner:SHANGHAI RES INST OF CHEM IND
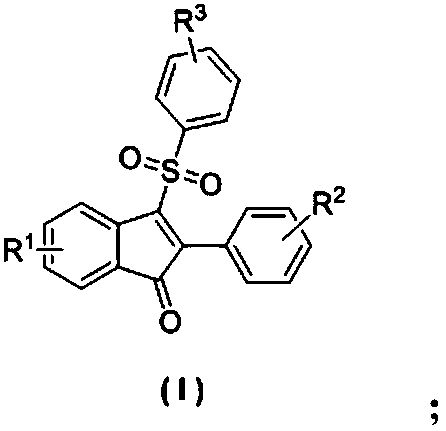
Sigma receptor binding agent containing indanone derivative
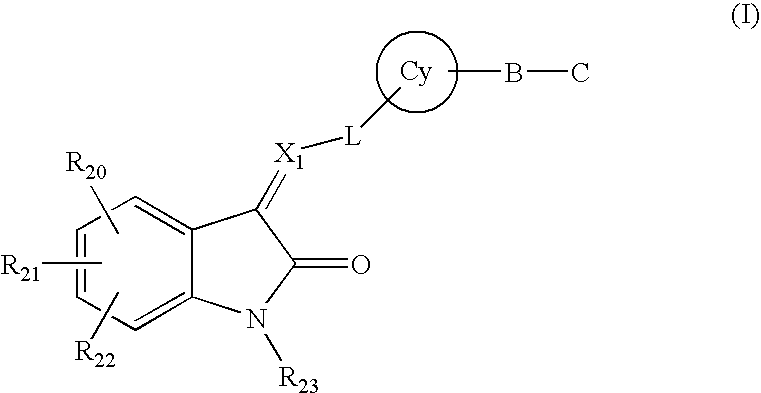
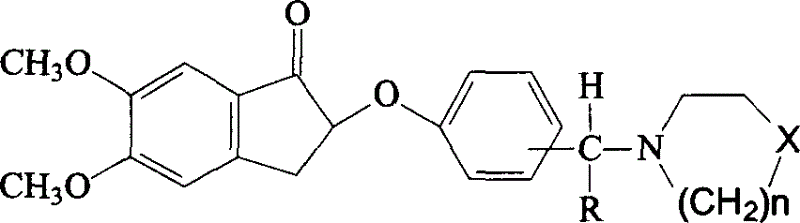
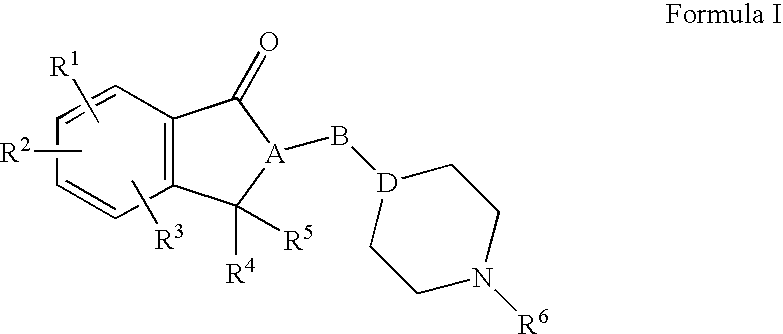
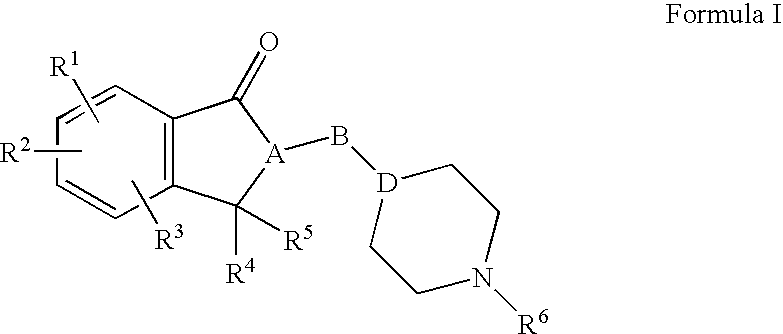
A method for treatment of a mental disorder containing the step of administering a therapeutically effective amount of a sigma receptor binding agent containing an indanone compound represented by the following formula (I), a pharmacologically acceptable salt thereof or a hydrate of them.The variables of formula (I) are recited in the present specification.
Owner:IIMURA YOICHI +2
Indanone and tetralone compounds for inhibiting cell proliferation
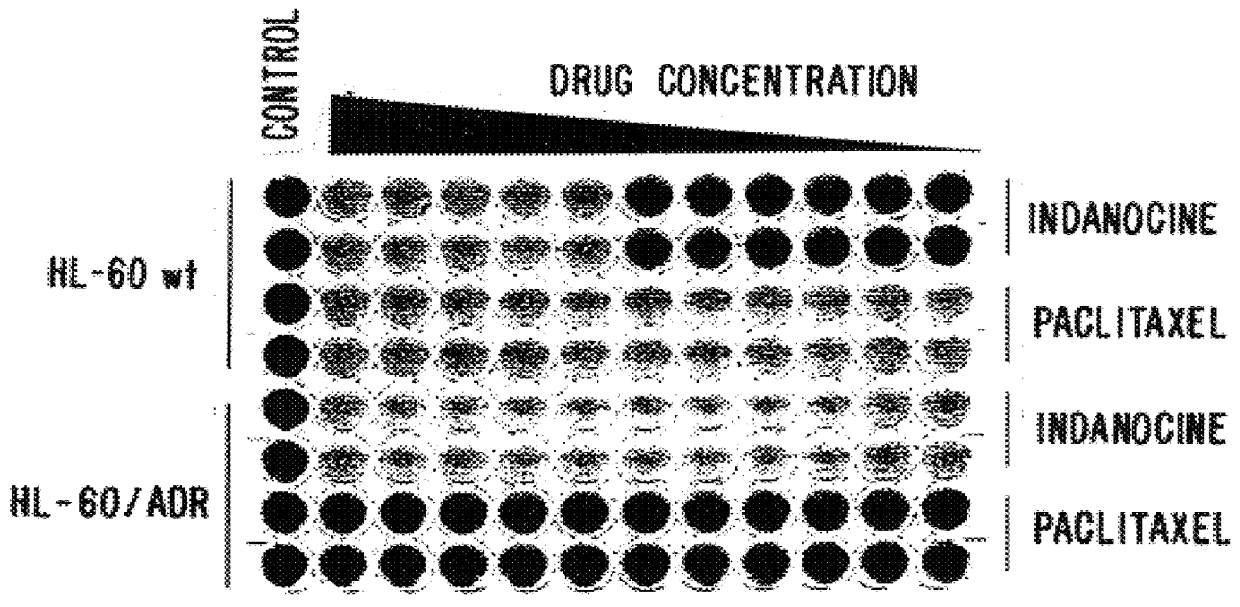
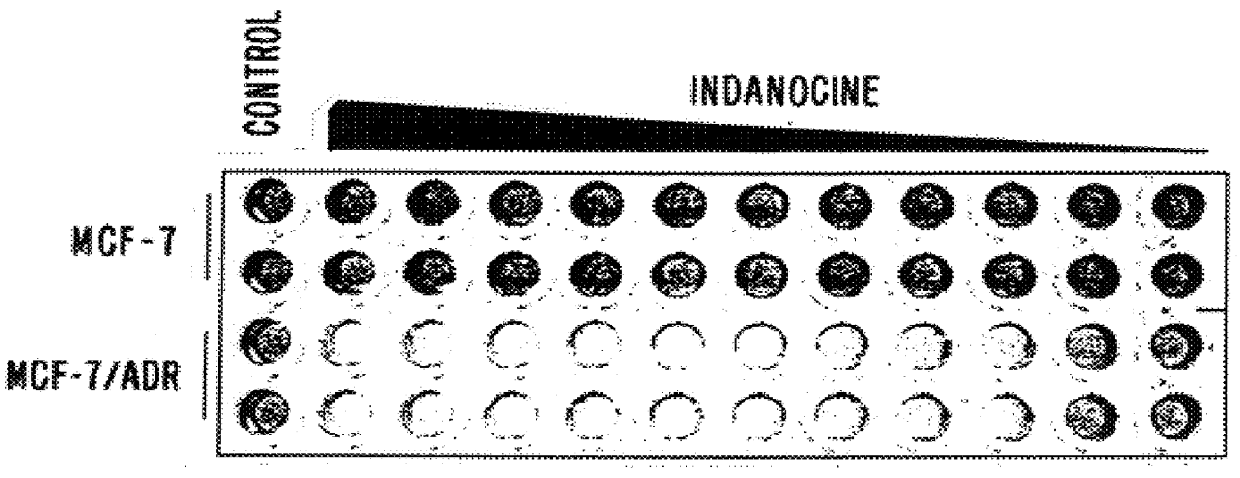
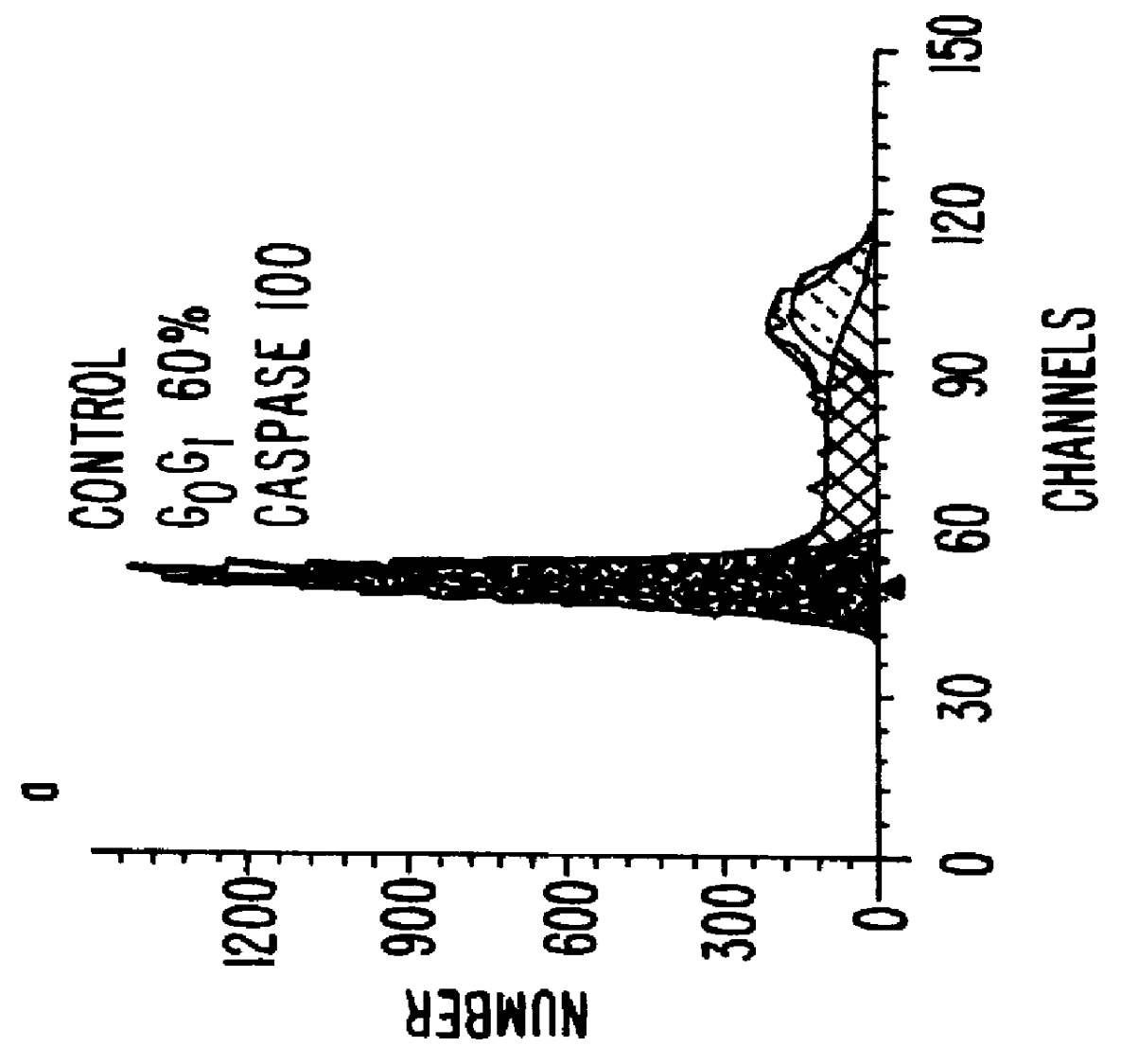
A new family of indanone and tetralone tubulin-binding compounds (TBs) is disclosed. Unlike classical TBs, which inhibit mitosis among affected dividing cells, the TBs of the invention possess two unique properties: (1) they induce apoptosis among stationary phase (non-dividing) malignant cells, yet do not impair the viability of normal nonproliferating cells; and, (2) they affect cells which have acquired MDR more powerfully than they affect cells without MDR. Thus, the TBs of the invention provide means to target malignant cells for chemotherapy, even after previous therapies have failed, without affecting normal cells and tissues in the host.
Owner:RGT UNIV OF CALIFORNIA
Phenoxy indenone compounds ,process for preparing same and use thereof
InactiveCN1557811AEasy to makeWide variety of sourcesOrganic active ingredientsNervous disorderCholinesterase inhibitionHydrolysis
The present invention provides one kind of phenoxy indenone compound. Pharmacological experiment shows that the compound may be used as acetylcholinesterase inhibitor and can competitively inhibit the activity of acetylcholine, delay the hydrolysis of acetylcholine and thus raise the synapse effect of acetylcholine. The compound may be used in preparing medicine for treating presenile dementia. The present invention has easy to obtain material, has mild reaction condition, high yield, simple operation and low production cost, and is suitable for industrial production.
Owner:ZHEJIANG UNIV
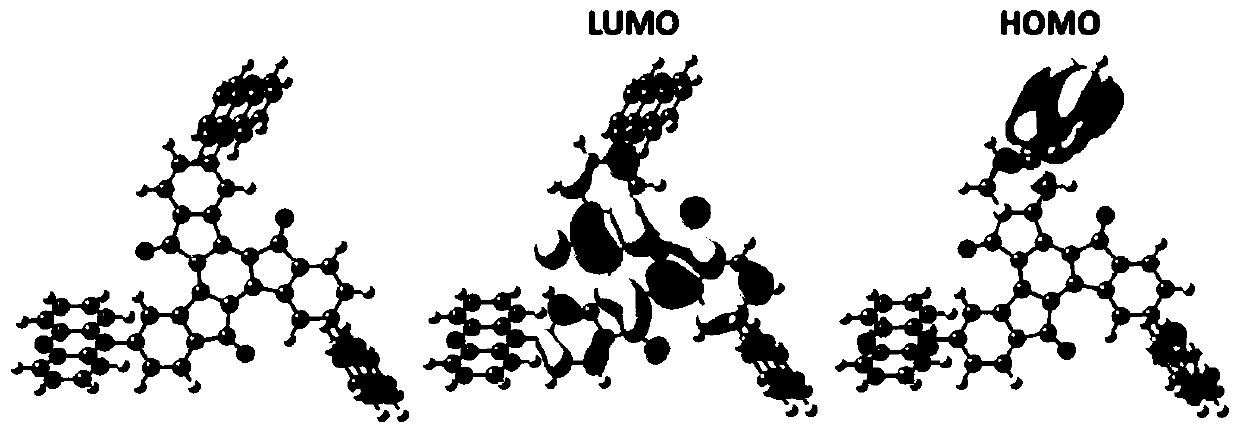
Electroluminescent materials using truxenone as electron acceptor and application of electroluminescent materials
ActiveCN110590642AStrong absorption capacityOrganic chemistrySolid-state devicesElectron donorFluorescence
The invention provides electroluminescent materials using truxenone as an electron acceptor and an application of the electroluminescent materials, and belongs to the field of organic electroluminescence. The derivatives use the truxenone as the electron acceptor, and carbazole, 1,3,6,9-tetramethylcarbazole, 3,6-di-tert-butylcarbazole, 3-carbazolylcarbazole, 9,9-dimethylacridine, phenoxazine and phenothiazine as electron donors. A synthetic method of the series of compounds comprises the following steps: performing a coupling reaction by using 5-bromo-1-indenone as a raw material, performing an oxidation reaction, and performing a Buchwald-Hartwig coupling reaction to obtain the truxenone substituted by different electron donor groups. The series of compounds have strong absorption in theultraviolet-visible light regions, a dilute solution of the compounds emits strong fluorescence, the emitting color is orange-red or red, and the compounds can be used as light-emitting materials to be applied to organic light-emitting diodes.
Owner:DALIAN UNIV OF TECH
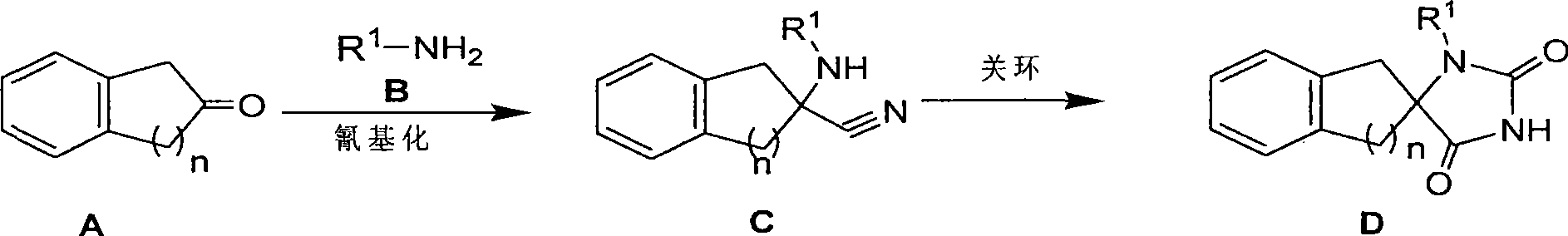
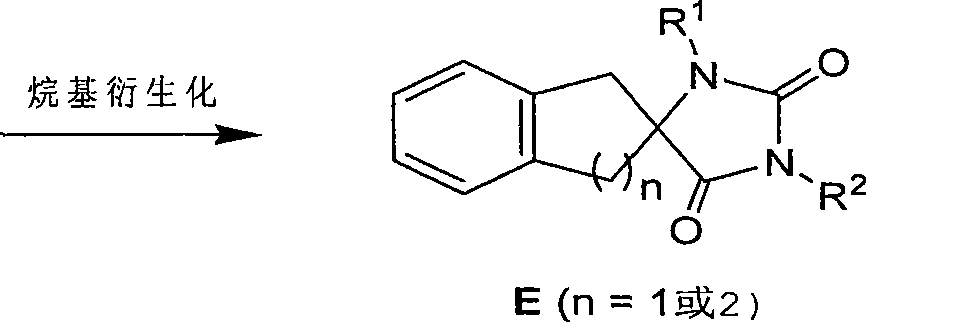
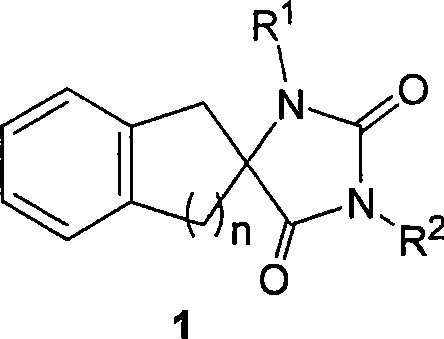
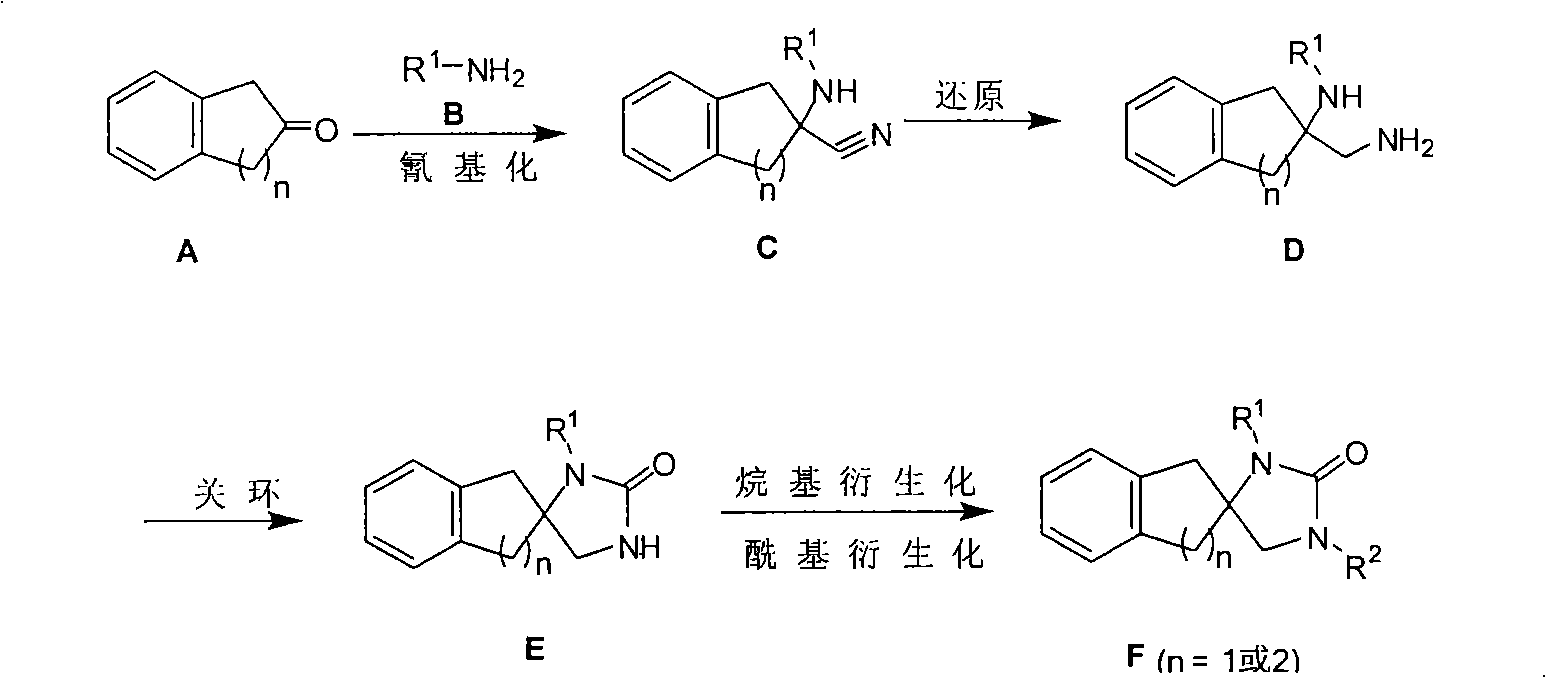
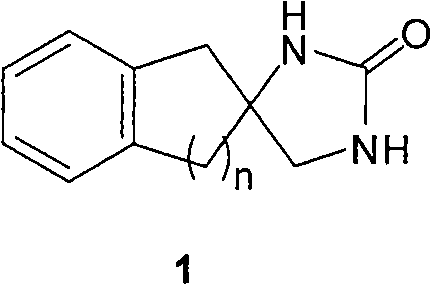
Synthetic method for aromatic ring bisamide spiro drug template
The invention relates to a synthetic method for an aromatic ring bisamide spiro drug template, which solves the technical problems that the original synthetic method has more reaction steps and low efficiency and is not beneficial to synthesis on a large scale. The technique comprises the following steps of: firstly carrying out cyanation and amination on 2-indenone (n=1) or 2-tetralone (n=2) compound A by a one-pot mode; then utilizing isocyanate sulfonic chloride and hydrochloric acid to close the ring directly and obtaining the substituted aromatic ring bisamide spiro drug template with 3 bit N; and then easily obtaining the substituted aromatic ring bisamide spiro drug template with 1 bit N and 3 bit N doubly by alkylation reaction of 1 bit N. The method has less reaction steps and is a synthetic method for an aromatic ring bisamide spiro drug template with large-scale preparation.
Owner:WUXI APPTEC SUZHOU
3-phenyl glutaric acid compound, preparation method and purpose thereof
InactiveCN102381961ARaw materials are cheap and easy to getFew reaction stepsOxygen-containing compound preparationOrganic compound preparationAcetic acidGlutaric acid
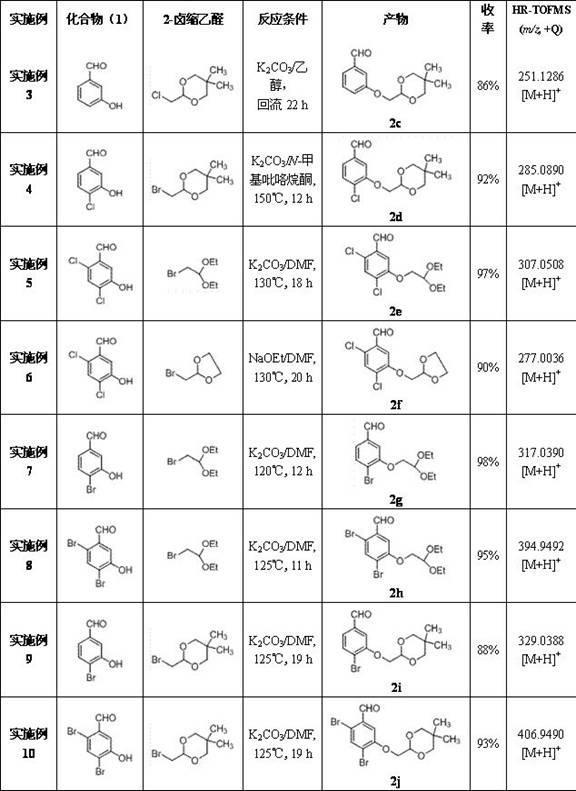
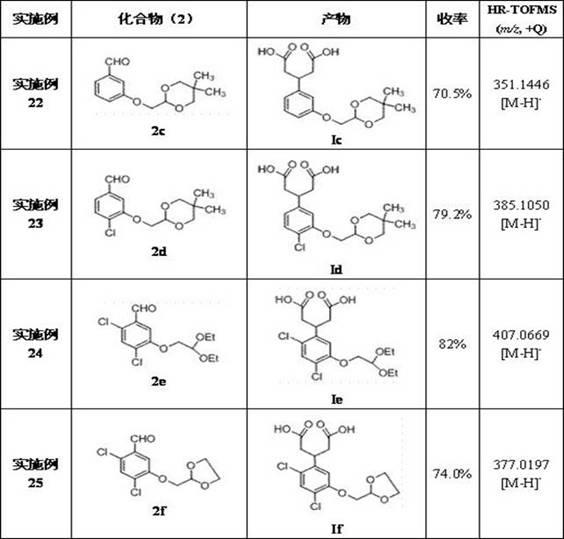
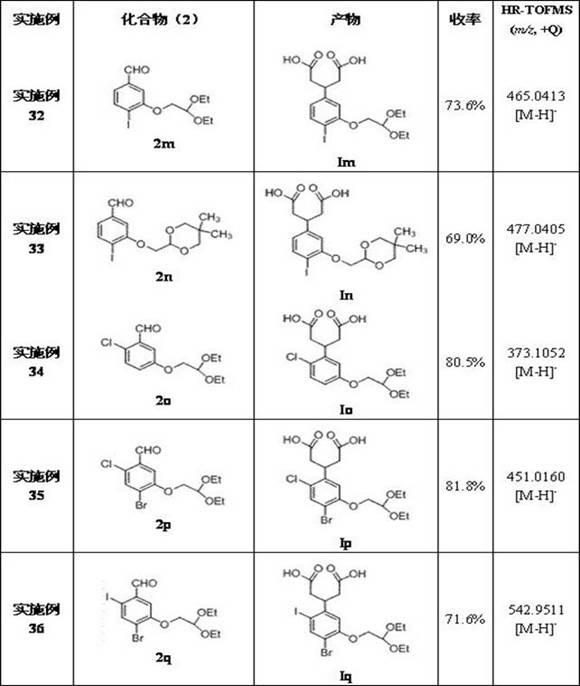
The invention discloses a 3-phenyl glutaric acid compound (I), and a preparation method thereof and application thereof to preparation of 1- indenone-3acetate compounds. In the formula, R1 and R2 respectively represent H, Cl, Br and I; R1 and R2 can be the same or not; R3 represents CH(OR4OR5)2; R4 and R5 represent C1-12 alkyl, or R4 and R5 are connected to form a ring.
Owner:SICHUAN UNIV
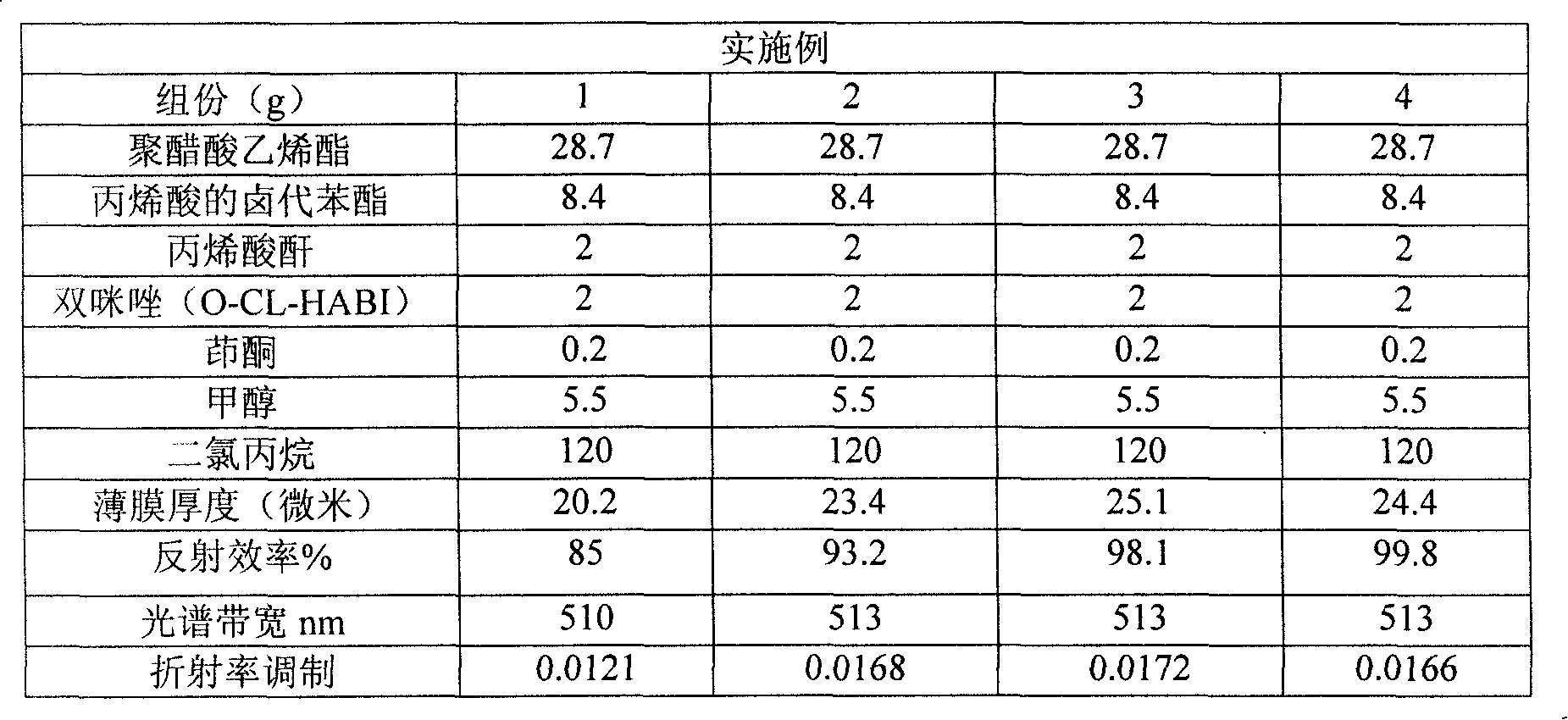
Preparation of hologram reflection-type photo-induced polyalcohol dry film
InactiveCN101231362APolyhalogenated compound compositionsPhotographic processesDichloropropanePolymer science
The invention relates to a holographic reflection type photopolymer film composition and preparation method thereof. The preparation method comprises the following steps of dissolving indenone, bis-imidazole (O-CL-HABI), polyvinyl acetate and acrylic anhydride in a dichloropropane / methanol mixed solution under red light condition, coating the obtained solution on transparent PETG (poly(ethylene terephthlate-co-cyclohexanedimethyllene terephthalate), and thermosetting in a convection thermostat. The film of the invention has very high diffraction efficiency and good light transmission performance, overcomes the disadvantages of the material prepared by the prior art including poor storage durability and easy fading away, and effectively solves problems of raw material including oversensitivity to ambient humidity and high volatility.
Owner:李妤 +1
Novel Indanone Compounds
Novel indanylidene compounds can be used as UV-A filters in cosmetic compositions for protecting skin and hair and for technical applications.
Owner:SYMRISE GMBH & CO KG
New method for preparing rasagiline mesylate
ActiveCN102010353AGood choiceHigh yieldOrganic compound preparationSulfonic acids salts preparationRasagiline MesylateSolvent
The invention relates to a new preparation method for rasagiline mesylate for a Parkinson's disease resistant medicament, and belongs to the technical field of rasagiline mesylate preparation. The method comprises the following steps of: introducing a chiral auxiliary agent into 1-indenone (II) serving as a raw material, and performing reduction reaction to obtain a compound (III); then reacting the obtained compound (III) and 3-propiolic halide to obtain a compound (IV); and finally, dripping methylsulfonic acid into the obtained compound (IV) at a certain temperature in a certain solvent toobtain rasagiline mesylate (I). The method has the advantages of chiral synthesis, unique reaction route, good selectivity, high yield and a few reaction steps, and is simple and convenient to operate. The raw materials used for the reaction route are cheap and easily obtained, the cost is low, the optical purity is high, and the method is suitable for industrialized production. The method solvesthe problems of high cost due to the adoption of a key intermediate R-1-indamine, low reaction yield, long reaction steps and disadvantage on industrialized production in the conventional rasagiline mesylate synthesis process.
Owner:NENTER & CO
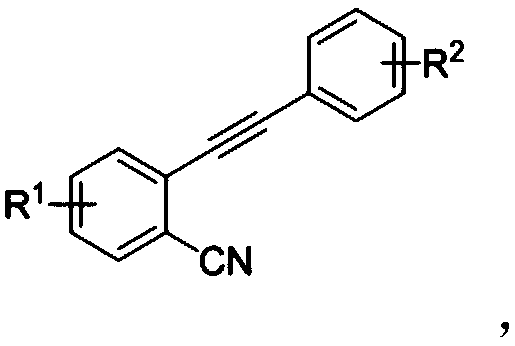
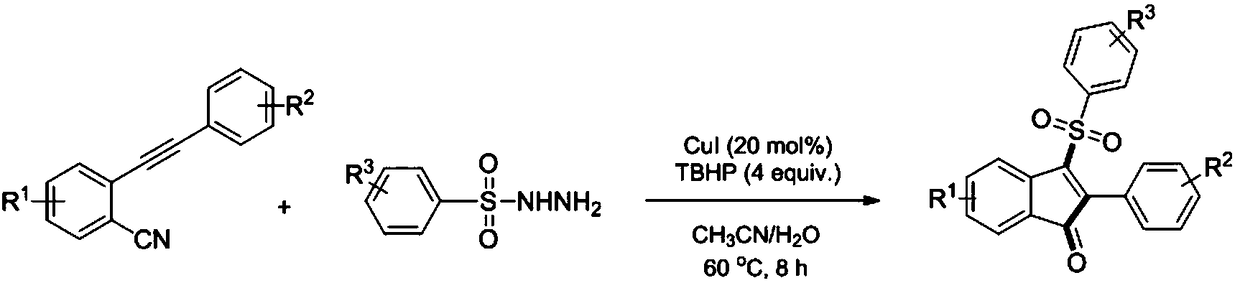
3-sulfonylation-indenone compound and preparation method thereof
ActiveCN108689892AMild reaction conditionsHigh regional selectivityOrganic chemistryOrganic compound preparationChemical industryAryl
The invention discloses a 3-sulfonylation-indenone compound and a preparation method thereof. The preparation method is characterized in that the 3-sulfonylation-indenone compound is synthesized fromortho-aryne benzonitrile and aryl-sulfuryl hydrazine under the actions of copper iodide and tert-butyl hydroperoxide serving as catalysts. The 3-sulfonylation-indenone compound has the advantages of adoption of readily-available raw materials, mild reaction conditions, easiness and convenience in operation, high synthesis yield and contribution to industrial production. The derivative has potential application in the fields of chemical industry and medicine. A method is provided for the synthesis of the 3-sulfonylation-indenone compound for the first time.
Owner:ZHENGZHOU UNIV
Process for preparing indan derivatives
PCT No. PCT / JP97 / 00040 Sec. 371 Date Jul. 9, 1998 Sec. 102(e) Date Jul. 9, 1998 PCT Filed Jan. 10, 1997 PCT Pub. No. WO97 / 25436 PCT Pub. Date Jul. 17, 1997This invention relates to a process for preparing indan derivatives and comprises a process for preparing cis-1-amino-2-indanol by treating (+ / -)indan-1,2-diol and / or its 2-formate derivative with specific microbes to give optically active 2-hydroxy-1-indanone, converting the optically active 2-hydroxy-1-indanone to its oxime, and treating the oxime with hydrogen or a hydrogen donor in the presence of a heterogeneous hydrogenation catalyst, a process for preparing optically active 2-hydroxy-1-indanone and / or optically active indan-1,2-diol by treating (+ / -)indan-1,2-diol and / or its 2-formate derivative with specific microbes, and a process for preparing cis-1-amino-2-indanol by treating the oxime of 2-hydroxy-1-indanone with hydrogen or a hydrogen donor in the presence of a heterogeneous hydrogenation catalyst.
Owner:NIPPON STEEL CHEMICAL CO LTD
Process for the synthesis of indanylamine or aminotetralin derivatives and novel intermediates
InactiveUS20060052639A1Easy to handleOrganic compound preparationCarboxylic acid amides preparationTetraloneHydrogen
A process for preparing indanylamine and aminotetralin derivatives from indanone or tetralone oximes by acylating the oximes with an organic anhydride, followed by catalytic hydrogenation in the presence of an organic anhydride with subsequent hydrolysis is described. The process is commercially feasible providing indanylamine and aminotetralin derivatives in high yield that are useful as intermediates in the production of therapeutically active compounds. Also described are novel intermediates, 1-indanone O-acetyl oximes and 1-tetralone O-acetyl oximes.
Owner:TEVA PHARMA IND LTD
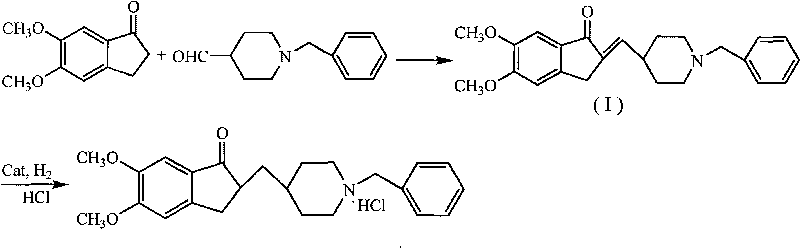
Catalyst for hydrogenation reaction of donepezil hydrochloride key intermediate and application thereof
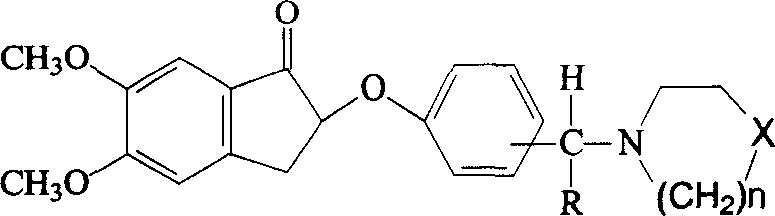
ActiveCN101693195ALow costHigh activityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsHydrogenation reactionActive component
The invention discloses a catalyst for hydrogenation reaction of a donepezil hydrochloride key intermediate and application thereof. The key intermediate is 2-(1-benzyl-4-piperidine methylene)-5,6-dimethoxy-1-indenone. The catalyst takes Al2O3 as a carrier and comprises composite active components selected from one or several simple substances or the simple substances and oxides of Pd, Cu, Co, Ni, Zn, Fe and Cr, wherein the mass content of each active component is 1-15 percent of the total mass of the catalyst and the total content of the active components is 5-30 percent of the total mass of the catalyst. The catalyst is prepared by utilizing a conventional catalyst preparation method and has the characteristics of low cost, stable activity and cycle use. The hydrogenation reaction by applying the catalyst has the characteristics of easy control of operation conditions, good catalyst performance and high product yield.
Owner:CANGZHOU SENARY CHEM SCI TEC
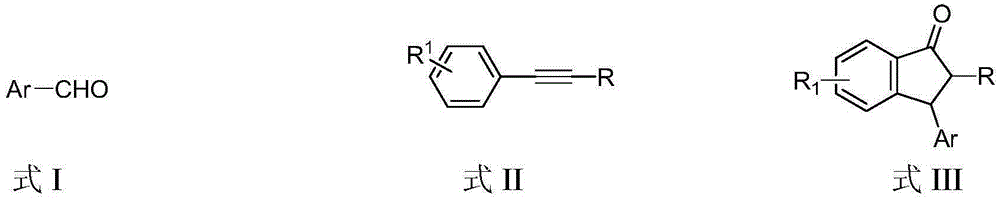
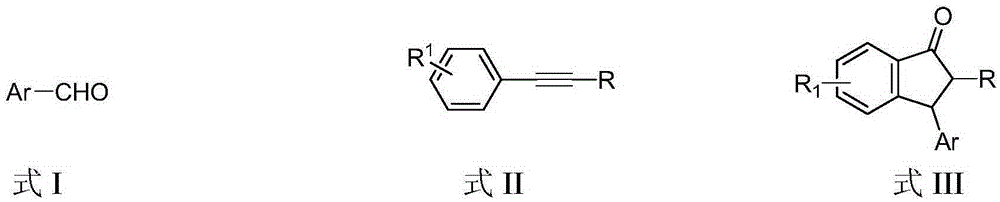
Preparation method of 3-aryl-1-indanone derivate
InactiveCN105348062ARaw materials are easy to getSimple and fast operationCarbonyl compound preparation by condensationArylStructural formula
The invention discloses a preparation method of a 3-aryl-1-indanone derivate. The structural formula of the 3-aryl-1-indanone derivate is shown in the formula III. The preparation method comprises the following steps that a compound shown in the formula I reacts with a compound shown in the formula II in the presence of methyl trifluoromethanesulfonate, and therefore the 3-aryl-1-indanone derivate shown in the formula III is obtained. The preparation method of the 3-aryl-1-indanone derivate is scientific and reasonable, 3-aryl-1-indanone derivates with various substituent groups can be obtained, the raw materials are easy to obtain, no metal participates in the reaction, reaction atoms are economical, and meanwhile the preparation method has the advantages that operation is simple and convenient, the synthesis productive rate is high, the product is easy to purify, and environmental protection is achieved.
Owner:TSINGHUA UNIV
Synthesis method of 5- hydroxide radical-1-indenone
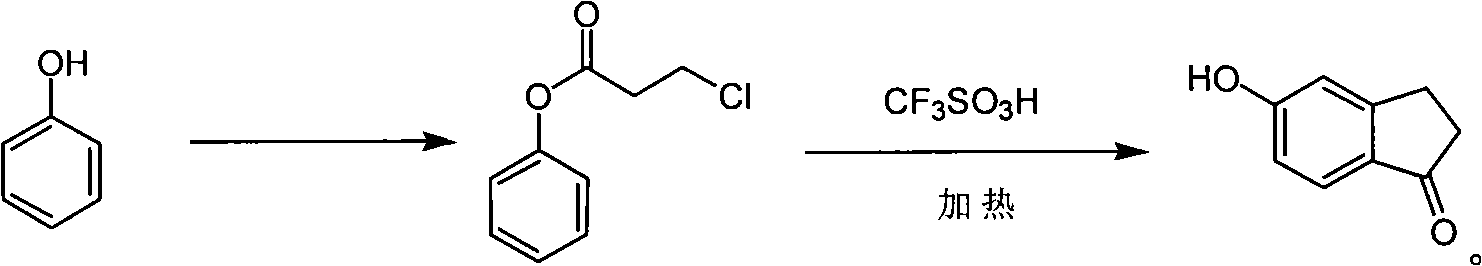
ActiveCN101585758AFast preparationEasy to operateOrganic compound preparationCarbonyl compound preparationOrganic synthesisTrifluoroacetic acid
The invention relates to a new synthesis method of 5- hydroxide radical-1-indenone, which mainly solves the technical problems that reaction conditions are demanding, post-processing is difficult and raw materials are not available of the existing synthesis method. The invention utilizes phenol to be reacted with 3-chlorpromazine chloride to obtain corresponding 3- chloride acid ester of phenol, closing ring to obtain the 5- hydroxide radical-1-indenone under the action of trifluoroacetic acid. The reaction formula is shown as follow. The obtained 5- hydroxide radical-1-indenone is used for medicinal chemistry and organic synthesis.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
Method for preparing multi-substituted cyclopentadiene and substituted indene
InactiveCN101993330AImprove conversion rateRaise the ratioHydrocarbon from oxygen organic compoundsHydrogen atomAlcohol
The invention relates to a method for synthesizing multi-substituted cyclopentadiene and substituted indene. The method comprises the following steps of: reacting (substituted) cyclopentenone or indanone with a Grignard reagent; and then carrying out hydrolysis reaction treated with an active hydrogen atom-containing reagent. Through the method, multi-substituted cyclopentenone and substituted indene can be widely prepared. The active hydrogen atom-containing reagent is water, alcohol or phenol. Because of the improvement of the active hydrogen atom-containing reagent, the ratio of double bond products in a ring is greatly improved and the reaction controllability is greatly enhanced.
Owner:CHINA PETROLEUM & CHEM CORP +1
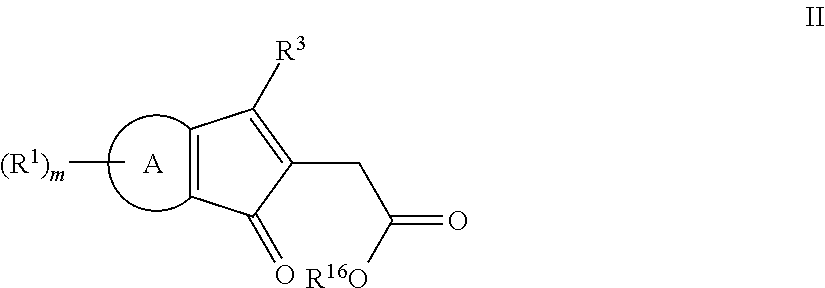
Methods of making fused ring compounds
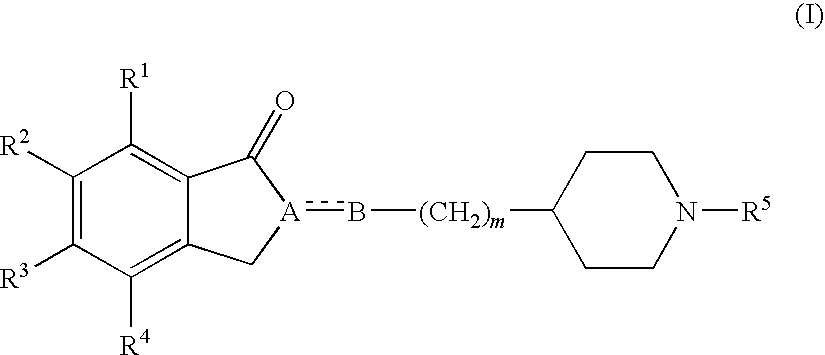
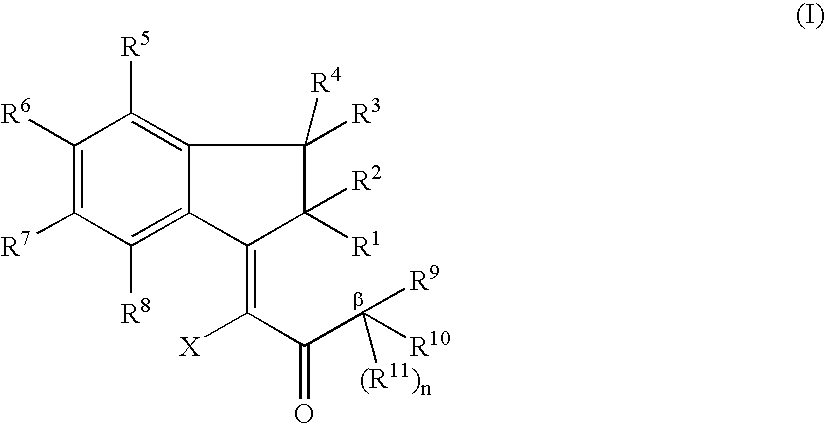
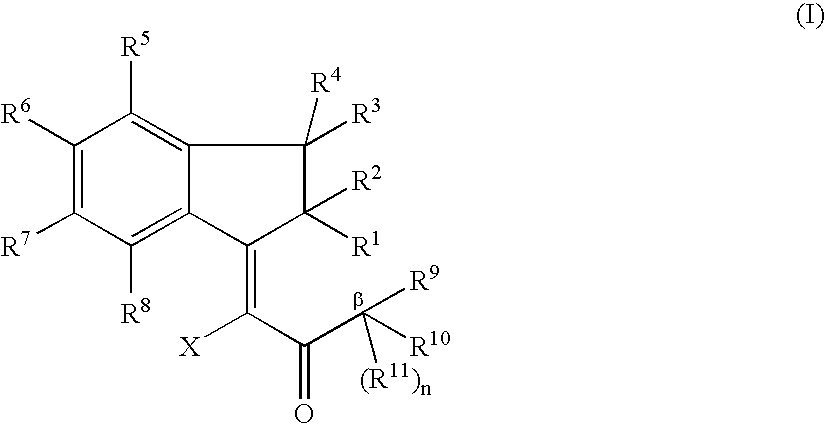
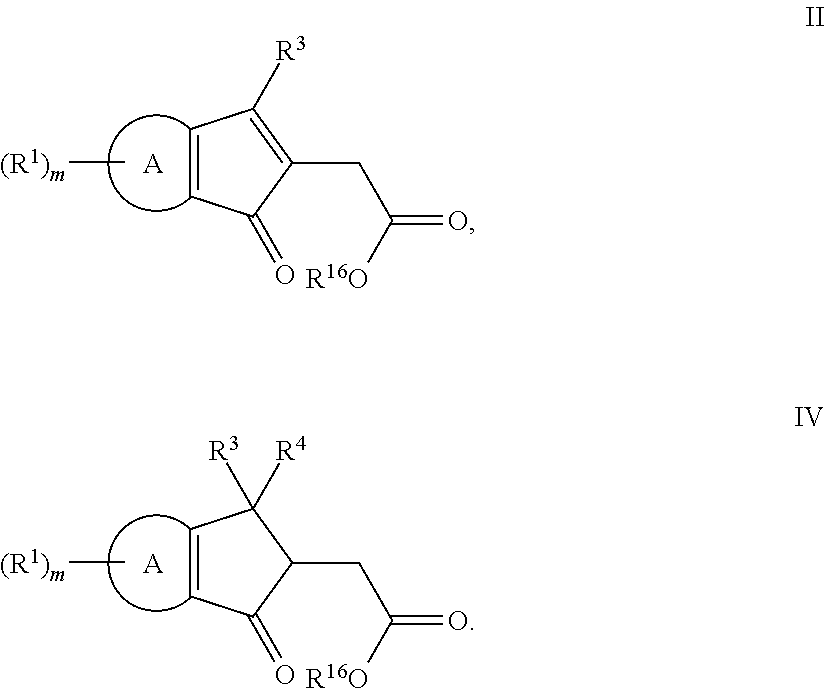
The present invention relates to methods of making fused ring compounds, such as indeno-fused naphthols, and fused ring indenopyran compounds, such as indeno-fused naphthopyrans, that each employ an unsaturated compound represented by the following Formula II.Referring to the unsaturated compound of Formula II: Ring-A can be selected from optionally substituted aryl (e.g., phenyl); m can be, for example, from 0 to 4; R1 for each m can be selected from optionally substituted hydrocarbyl (e.g., C1-C6 alkyl) optionally interrupted with at least one linking group (e.g., —O—); and R3 and R16 can each be independently selected from, for example, hydrogen or optionally substituted hydrocarbyl, such as C1-C8 alkyl. When Ring-A is a phenyl group, the unsaturated compound represented by Formula II can be referred to as an unsaturated indanone acid / ester compound, or an indenone acid / ester compound (depending on whether R16 is hydrogen, or an optionally substituted hydrocarbyl group).
Owner:TRANSITIONS OPTICAL INC
Synthesizing method of aromatic ring ureas loop-coil medicament template
InactiveCN101555230AImprove biological activityReduce usageOrganic chemistryAlkyl transfer2-Tetralone
The invention relates to a synthesizing method of an aromatic ring ureas loop-coil medicament template, which mainly solves the technical problems of massive reaction steps, low efficiency and no favorability for large-scale synthesis in prior methods. In the synthesizing method, a 2-indenone (n=1) type or 2-tetralone (n=2) type compound undergoes cyanation and amination by means of one-pot boiling; then, cyano-group is reduced into amino-group, and finally N-N'carbonyl diimidazole is used for direct ring closing so as to obtain an aromatic ring ureas loop-coil medicament template with substitution on 3-position N; and then, an aromatic ring ureas loop-coil medicament template with double-substitution on 1,3-position N can be easily obtained by means of alkylation or acylation reaction on 1-position N. The synthesizing method is capable of the large-scale preparation of the aromatic ring ureas loop-coil medicament template.
Owner:WUXI APPTEC (TIANJIN) CO LTD +1
Compositions, synthesis, and methods of using indanone based cholinesterase inhibitors
InactiveUS8247563B2Strong inhibitory activityReduce adverse side effectsBiocideSenses disorderCholinesteraseIndenone
The present invention provides novel indanone derivatives which can be advantageously used for treating and / or preventing of a medical condition for which inhibition of a cholinesterase is desired.
Owner:REVIVA PHARMA INC
Method for synthesizing indenone-2-diphenyl sulfide derivatives
The invention belongs to the field of organic synthesis, and relates to a preparation method of indenone, particularly a method for synthesizing indenone-2-diphenyl sulfide derivatives. The method comprises the following steps: using 1,3-disubstituted allylene ketone and thiophenol as the reactants, dissolving the reactants in a solvent at 20-120 DEG C, and reacting under the action of manganese acetate to prepare the indenone-2-diphenyl sulfide derivatives, wherein the mol ratio of 1,3-disubstituted allylene ketone to thiophenol is less than or equal to 1:2, and the mol ratio of 1,3-disubstituted allylene ketone to manganese acetate is less than or equal to 1:2. The invention uses the cheap, accessible and recyclable manganese acetate to replace noble metal, thereby reducing the cost; and the 1,3-disubstituted allylene ketone and thiophenol can smoothly react to produce the indenone-2-diphenyl sulfide derivatives. In addition, the substrates for 1,3-disubstituted allylene ketone and thiophenol have wide sources.
Owner:SUZHOU UNIV
Preparation method of indanone intermediate
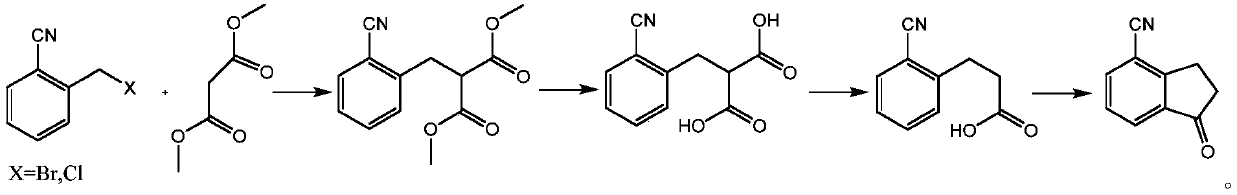
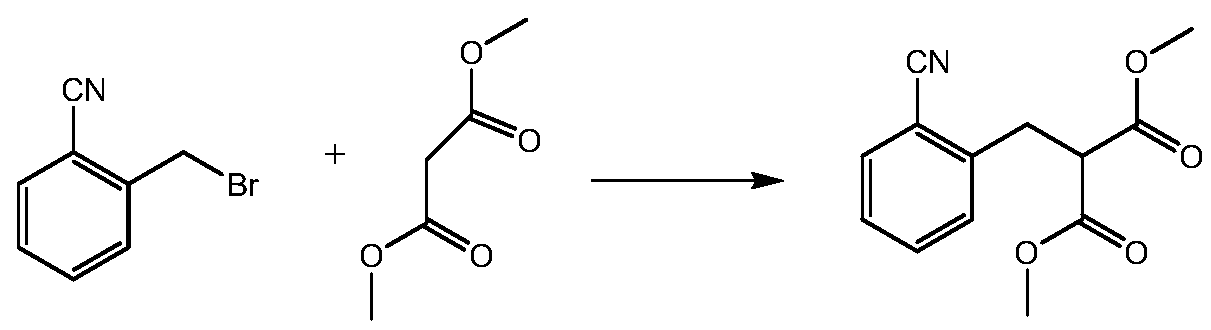
InactiveCN110627683ACarboxylic acid nitrile preparationOrganic compound preparationPropanoic acidOrganic solvent
The invention provides a preparation method of an indanone intermediate, and belongs to the field of pharmaceutical chemicals. The prepration method comprises the following steps: carrying out condensation reaction, hydrolysis and decarboxylation of 2-cyanobenzyl bromide or 2-cyanobenzyl chloride and dimethyl malonate in an organic solvent to obtain 2-cyanophenylpropionic acid; and reacting the obtained 2-cyanobenzene propionic acid with aluminum trichloride and an assistant agent to obtain 4-cyano-1-indanone. According to the invention, no highly toxic cyaniding reagent is needed, and the method has the advantages of high product purity, good safety, high yield, simple operation and the like. The method provided by the invention can effectively prepare the indanone intermediate, and provides favorable conditions for further preparation of ozanimod.
Owner:SUNSHINE LAKE PHARM CO LTD
Compositions, synthesis, and methods of using indanone based cholinesterase inhibitors
InactiveUS20080153878A1Strong inhibitory activityReduce adverse side effectsBiocideSenses disorderCholinesteraseIndenone
The present invention provides novel indanone derivatives which can be advantageously used for treating and / or preventing of a medical condition for which inhibition of a cholinesterase is desired.
Owner:REVIVA PHARMA INC
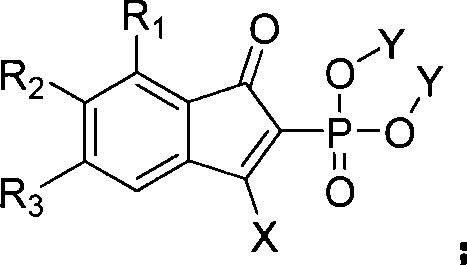
Method for synthesizing indenone-2-phosphonate derivative
InactiveCN101497628ATake advantage of cheapTake advantage of the priceGroup 5/15 element organic compoundsMANGANESE ACETATEKetone
The invention discloses a method for preparing indenone-2-phosphonate derivatives. One of 1, 3-disubstituted propiolic ketone and phosphite ester are taken as reactants, are dissolved into a solvent, and react at a temperature of between 20 and 120 DEG C under the action of manganese acetate to obtain the indenone-2-phosphonate derivatives. The method has the advantages that the method has wide range of applicable substrates, simple and convenient operation, mild conditions, and low production cost, and is suitable for mass production.
Owner:SUZHOU UNIV +1
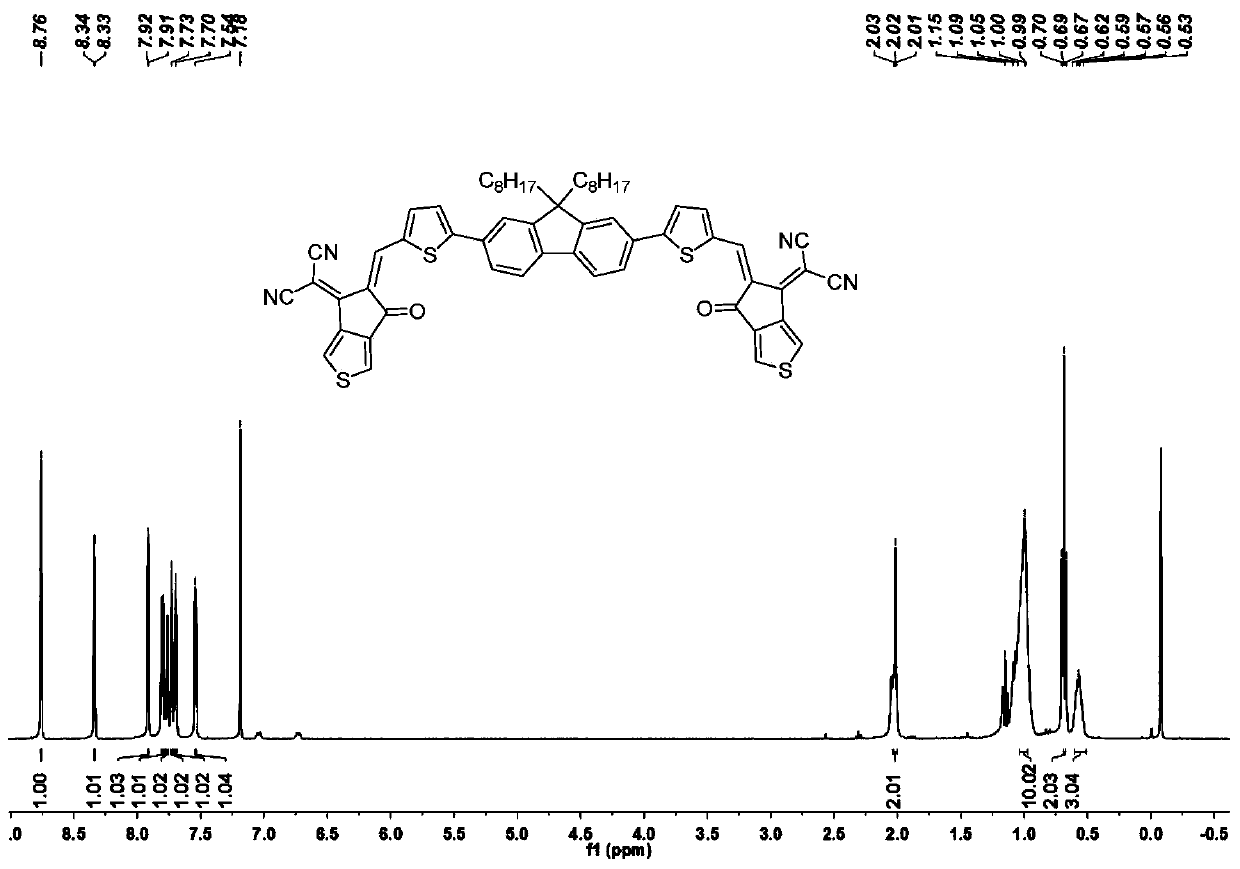
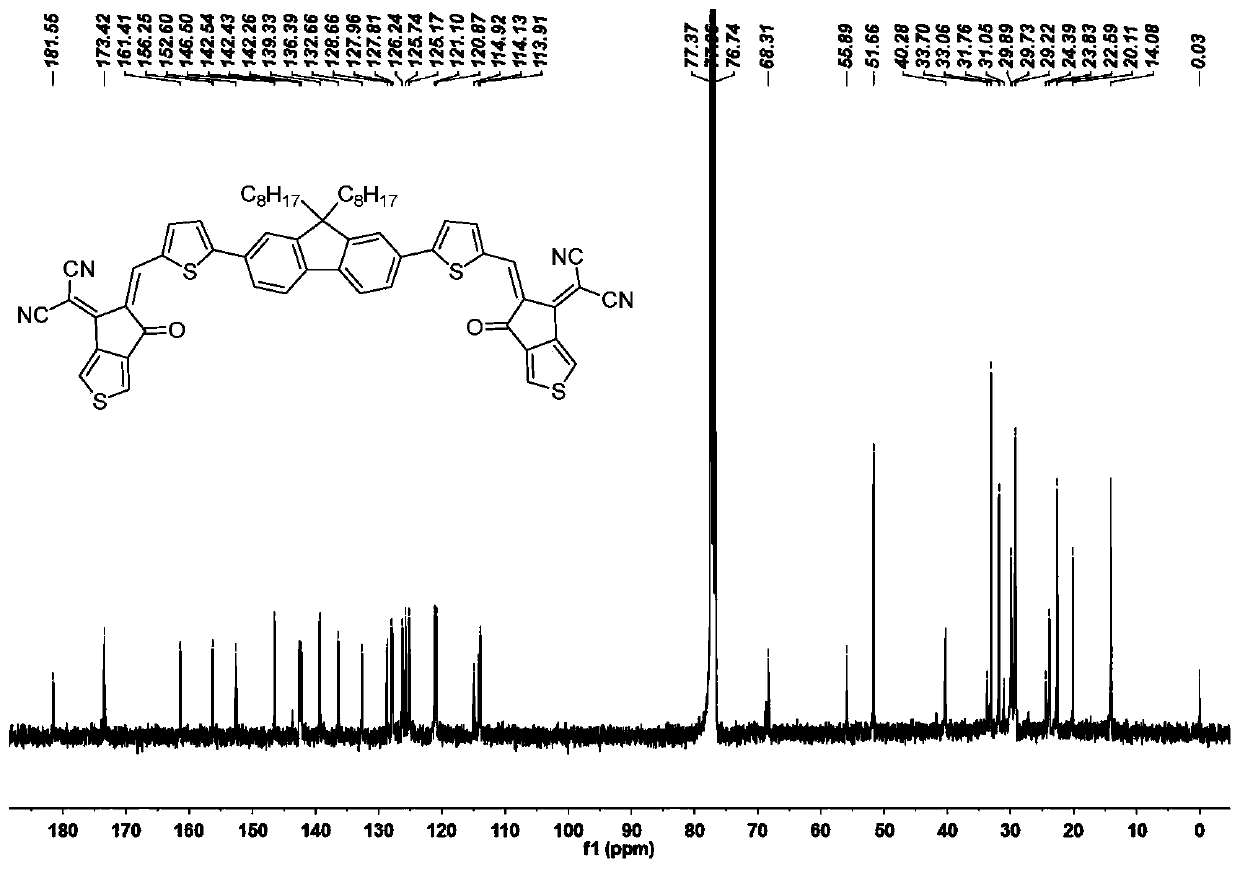
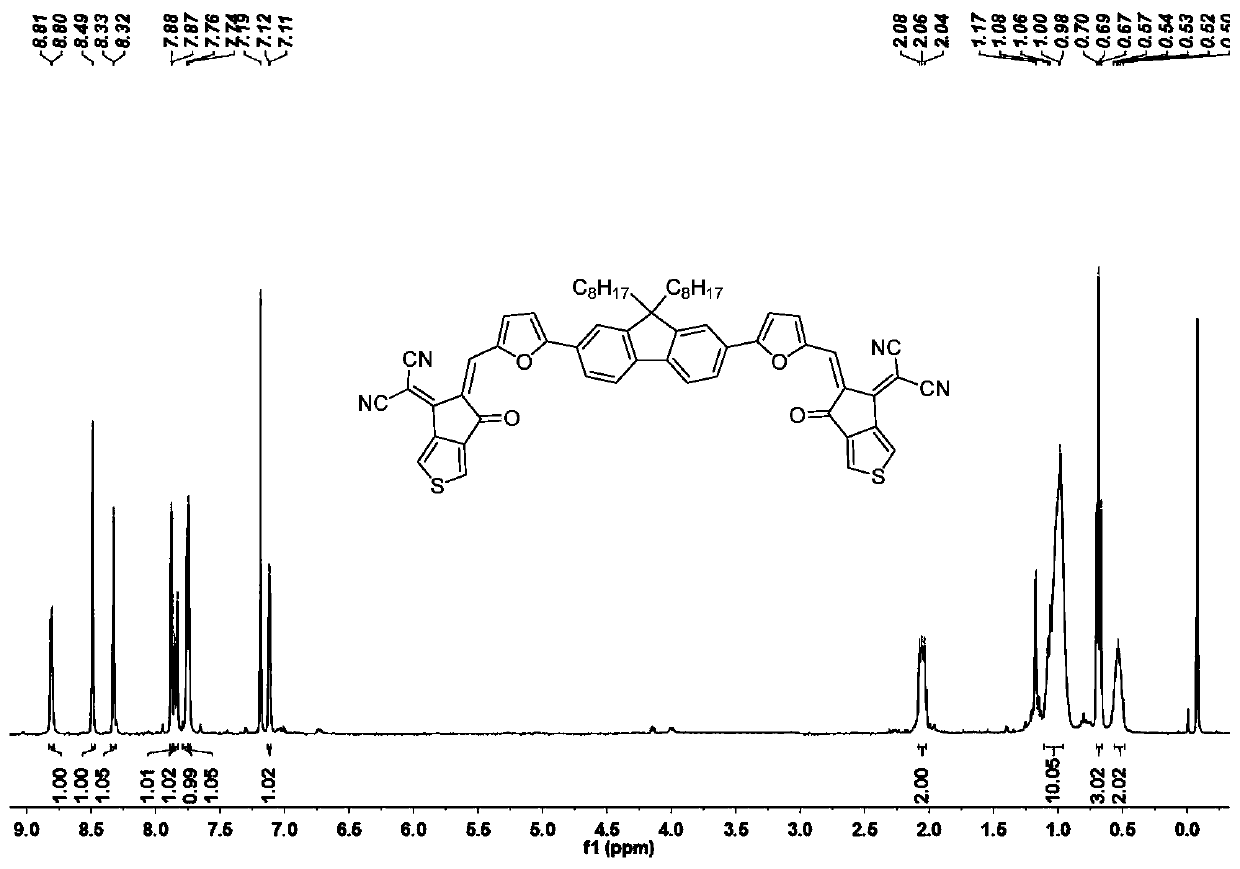
A-pi-D-pi-A type small molecule solar cell receptor material based on thiophene indenone and fluorene and preparation method of A-pi-D-pi-A type small molecule solar cell receptor material
InactiveCN110790743AStrong donating abilityStrong UV Absorbing PropertiesOrganic chemistrySolid-state devicesSolar cellIndenone
The invention discloses an A-pi-D-pi-A type small molecule solar cell receptor material based on thiophene indenone and fluorene and a preparation method of the A-pi-D-pi-A type small molecule solar cell receptor material. According to the receptor material, fluorine is adopted as a central core structure, groups such as thiophene, furan and benzene are adopted as pi bridges, and aldehyde groups at both ends are subjected to a condensation reaction with active sites of an electrondrawing group thiophene indanone to obtain symmetric target molecules at normal temperature and normal pressure. The A-pi-D-pi-A type small molecule solar cell receptor material based on thiophene indenone and fluorine is simple in synthesis method, easy in reaction condition control, high in yield, good in universal applicability and possible in efficient synthesis, can be widely applied to fields such as energy, life, analysis, material science, and the like, and is particularly suitable for being used as organic small molecule solar cell receptor materials, and the like.
Owner:DONGGUAN UNIV OF TECH
Application of tetrazole compounds to anti-epileptic medicines
The invention relates to compounds serving as anti-epileptic medicines and as shown in a general formula I and a general formula II, wherein R1 is selected from substituted groups and polysubstituted group derivatives such as 2-chlorine; 3-chlorine; 4-chlorine; 2-methyl; 3-methyl; 4-methyl; 2-fluorine; 3-fluorine; 4-fluorine; 2-methoxyl; 3-methoxyl; 4-methoxyl; 2-nitryl; 3-nitryl; 4-nitryl; 2-bromine; 3-bromine; 4-bromine; 2-tertiary butyl; 3-tertiary butyl; 4-tertiary butyl; 2-amino; 3-amino; and 4-amino. R2 is selected from: cyclopropyl; 3-pyrazolyl; 4-oxazolyl; 2-pyridyl; 2-oxocyclopentane; 5-(2-methyl pyridyl); 4-thiazolyl; 7-indolyl; 5-(1H-benzimidazolyl); 1-(2,3-dihydro-1H-indenyl); 6-(1H-indazolyl); 4-isoquinolyl; and 7-(1-indenone).
Owner:王增涛
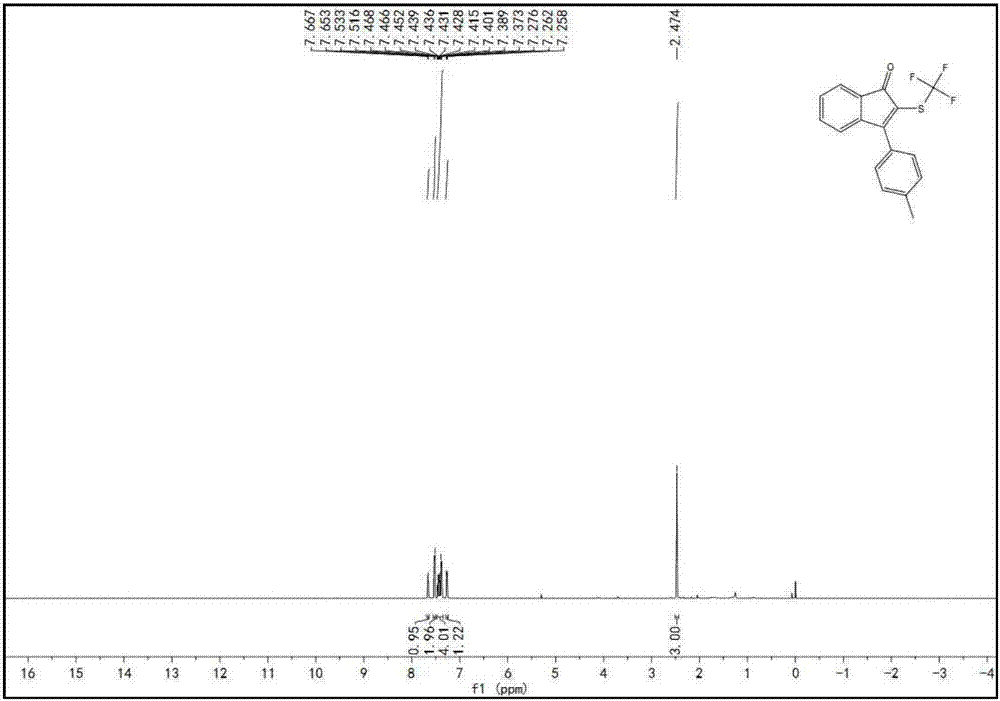
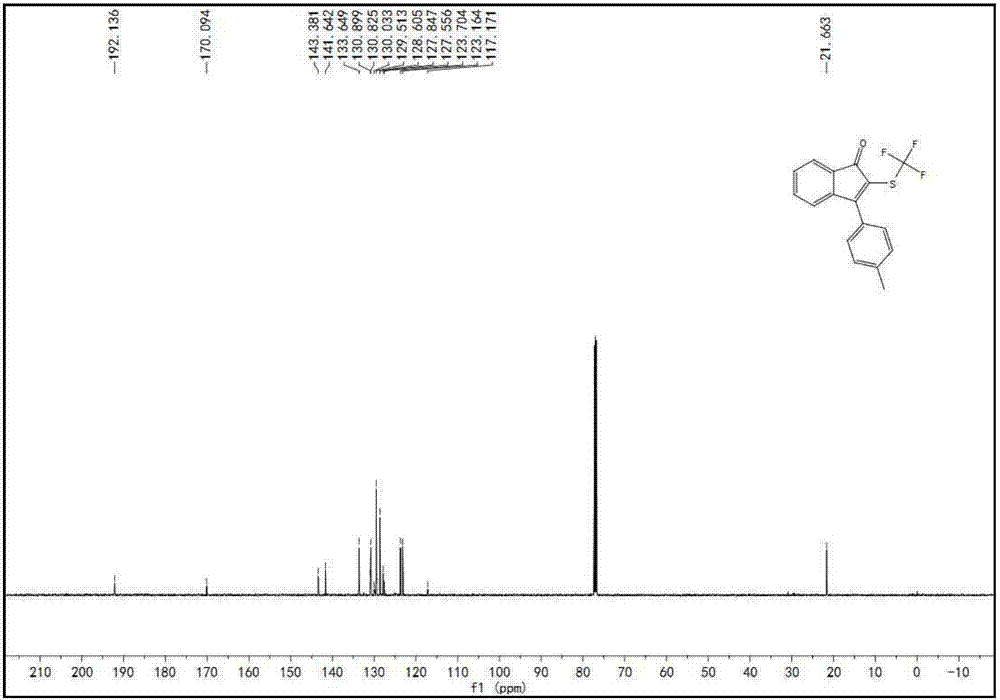
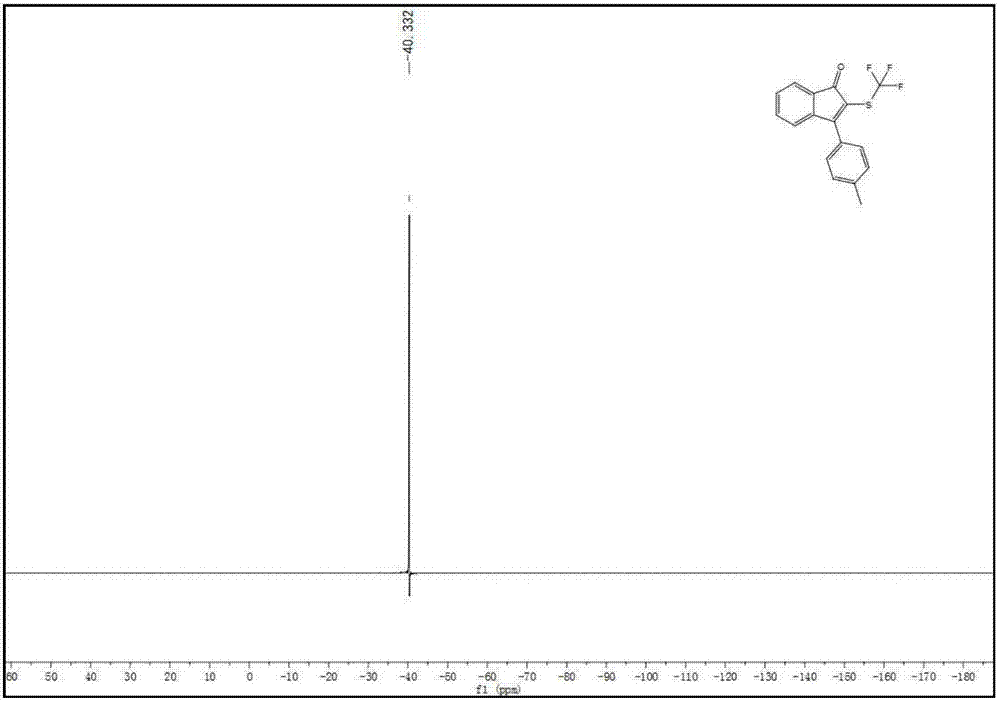
A kind of indenone with trifluoromethylthio group and its derivatives and preparation method thereof
The invention discloses a preparation method of indanone with trifluoromethylthio group and its derivatives. In the method, acetylene compounds and silver trifluoromethanethiolate compounds are selected as raw materials, and a persulfate reagent is used as an oxidizing agent. Using hexamethylphosphoric triamide as a stabilizer, the reaction was carried out in a reaction solvent at a reaction temperature of 80 oC for 12 hours. After the reaction was completed, indanone compounds were obtained through post-processing. This preparation method solved the problem of solving the problem of amides It is a technical problem to directly generate indanones by combining heterocyclic rings, and the preparation method has many advantages such as economical and cost-effective, high stability, mild reaction conditions, short reaction time, cheap and easy-to-obtain raw materials, and high product yield and purity.
Owner:瑞安宝源化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com