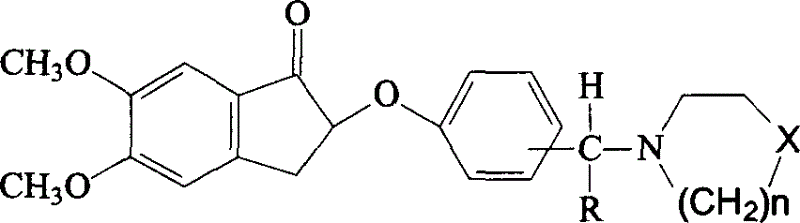
Phenoxy indenone compounds ,process for preparing same and use thereof
A technology for phenoxyindanone and compound, which is applied in the field of synthesis of phenoxyindanone derivatives, can solve the problems of different structures, no structure-activity relationship, etc., and achieves simple preparation, delayed hydrolysis, and wide source of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
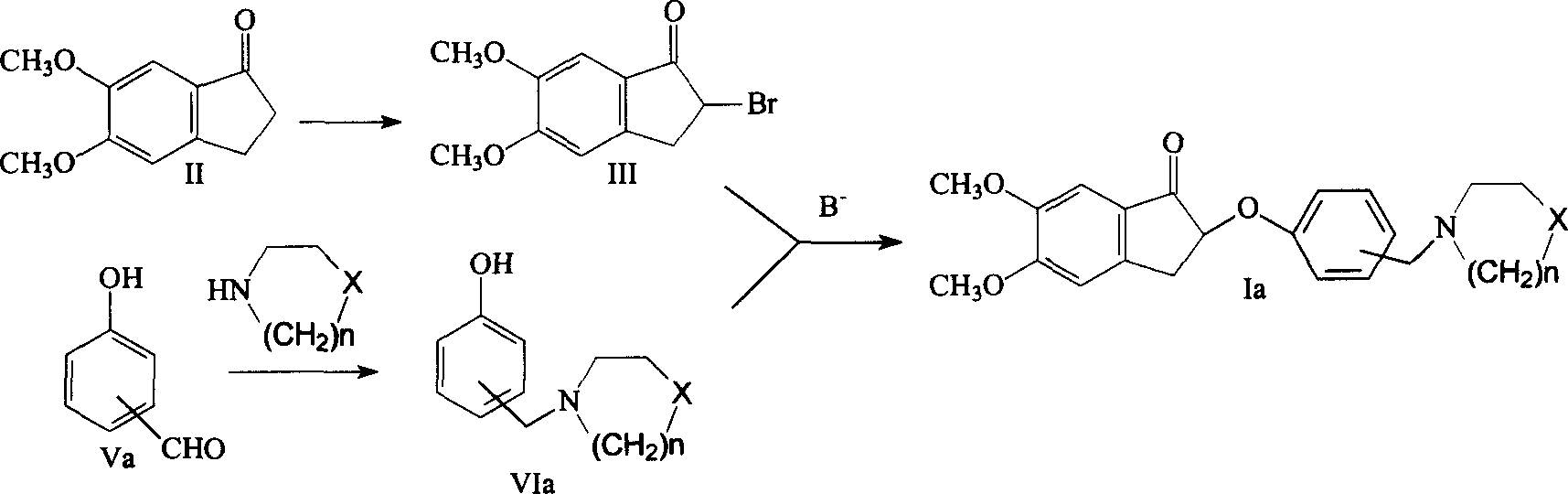
[0024] Example 1: Preparation of 2-bromo-5,6-dimethoxy-1-indanone (III)
[0025] 3.84g (0.02mol) of 5,6-dimethoxyindanone (II) was dissolved in 20mL of chloroform, the temperature was raised to reflux, 4.93g of copper bromide was added in batches, and the reaction was continued for 3 hours. Cool in an ice bath, remove the solid by suction filtration, and wash with 20ml×2 chloroform, combine the filtrates, and successively wash with saturated NaHCO 3 , saturated NaCl solution washing, anhydrous Na 2 SO 4 After drying, the solvent was recovered under reduced pressure to obtain a yellow solid, which was recrystallized from ethanol to obtain 4.62 g of a white solid, m.p.=184-186°C.
Embodiment 2
[0026] Example 2: Preparation of 2-bromo-5,6-dimethoxy-1-indanone (III)
[0027] 3.84g (0.02mol) 5,6-dimethoxyindanone (II) was dissolved in 30ml THF, and 7.68g (0.024mol) pyridine perbromide was added in batches within 3 hours at room temperature, and then the reaction was continued for 2 Hour. Cool in an ice bath, remove the solid by suction filtration, wash with THF 20ml×2, combine the filtrates, recover the solvent under reduced pressure, add 30ml of dichloromethane and 10ml of water to the residue, separate the organic layer, and wash with saturated NaHCO 3 , saturated NaCl solution washing, anhydrous Na 2 SO 4 After drying, the solvent was recovered to obtain a crude product, which was recrystallized from ethanol to obtain 4.36 g of a white solid.
Embodiment 3
[0028] Embodiment 3: the preparation of 1-[(3-hydroxyphenyl) methyl] piperidine
[0029] Dissolve 2.44g (0.02mol) of 3-hydroxybenzaldehyde in 20ml of methanol, add dropwise a mixed solution of 4.4ml of piperidine and 5ml of methanol at room temperature, and continue the reaction for 30 minutes after the addition; Add 0.53g KBH 4 , Continue to react for 1 hour after adding. Cool in an ice bath, adjust the pH to 2 with 2N hydrochloric acid, recover the solvent, add 30ml of 2N hydrochloric acid and 15ml of ethyl acetate to the residue, separate the organic layer and discard it; adjust the pH of the aqueous layer to 10 with concentrated ammonia, add 20ml of ethyl acetate ×3 extraction, combined organic layers, washed with saturated NaCl, anhydrous Na 2 SO 4 After drying, the solvent was recovered to obtain a crude product, which was recrystallized from ethanol-water to obtain 3.42 g of white granular crystals, with a yield of 89.5%, m.p.136-138°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com