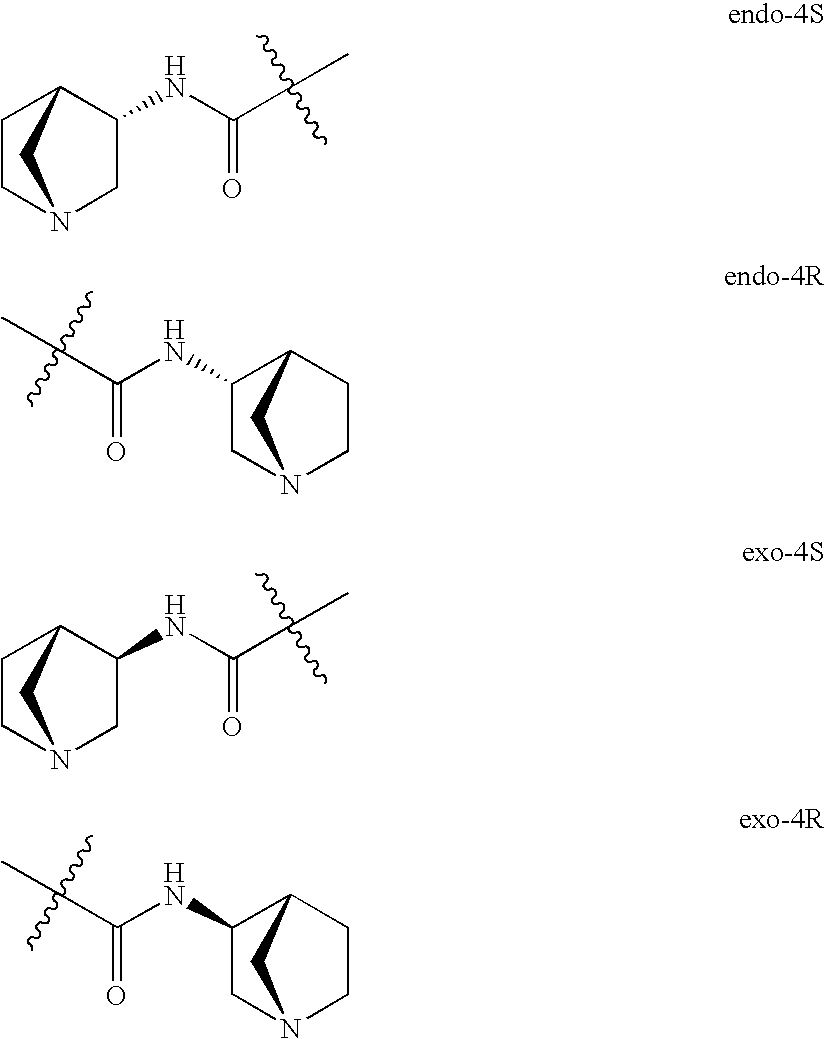
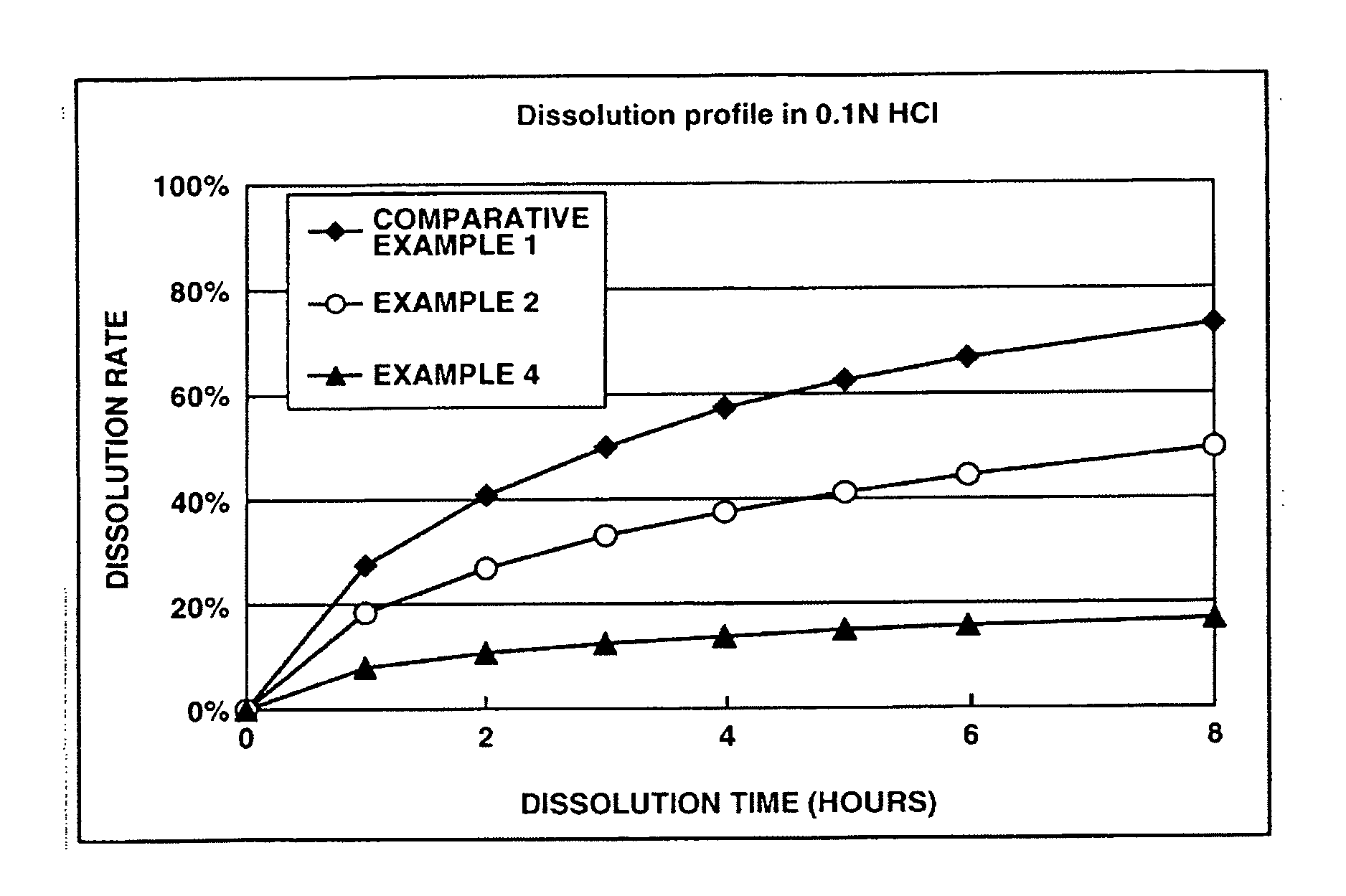
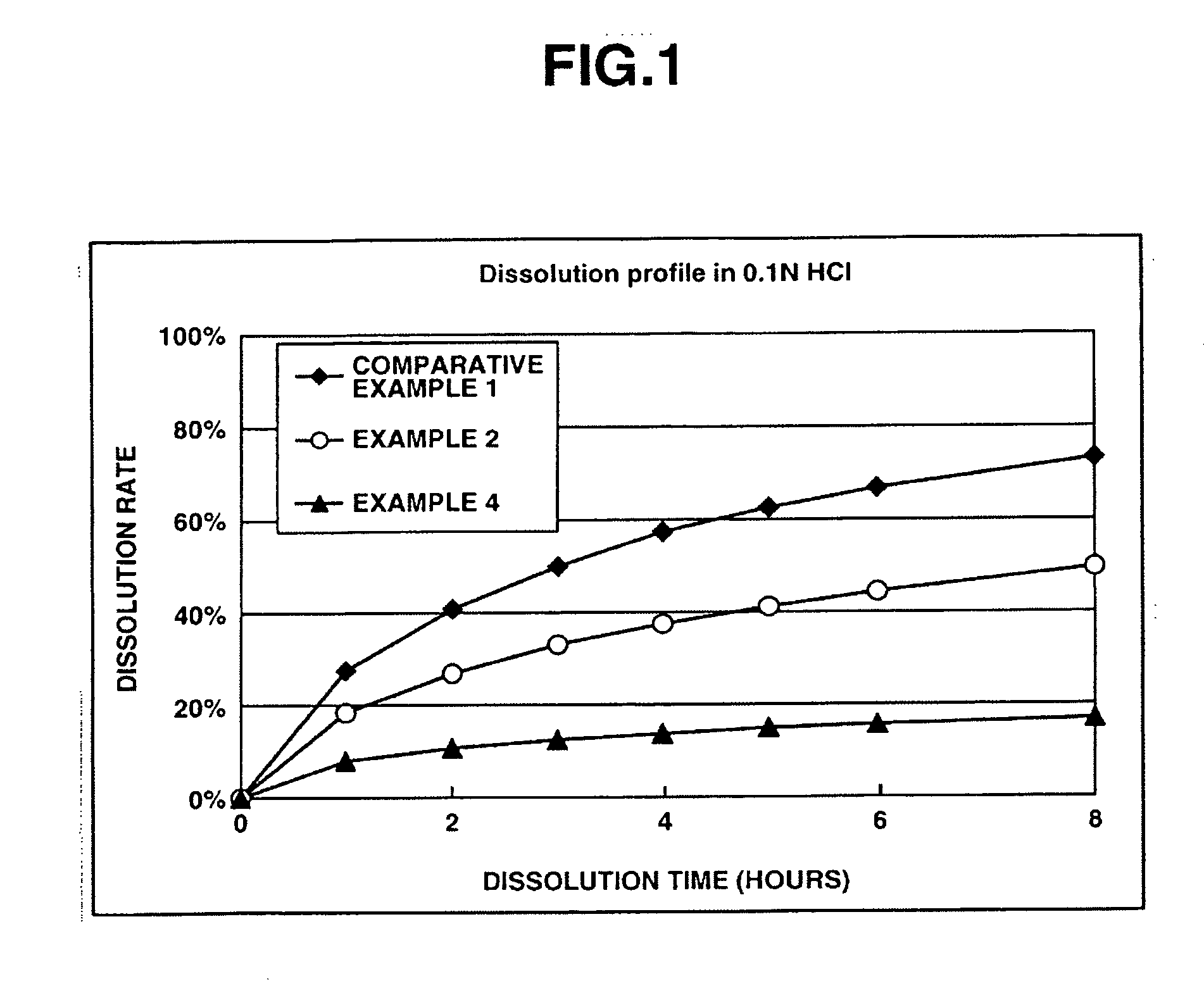
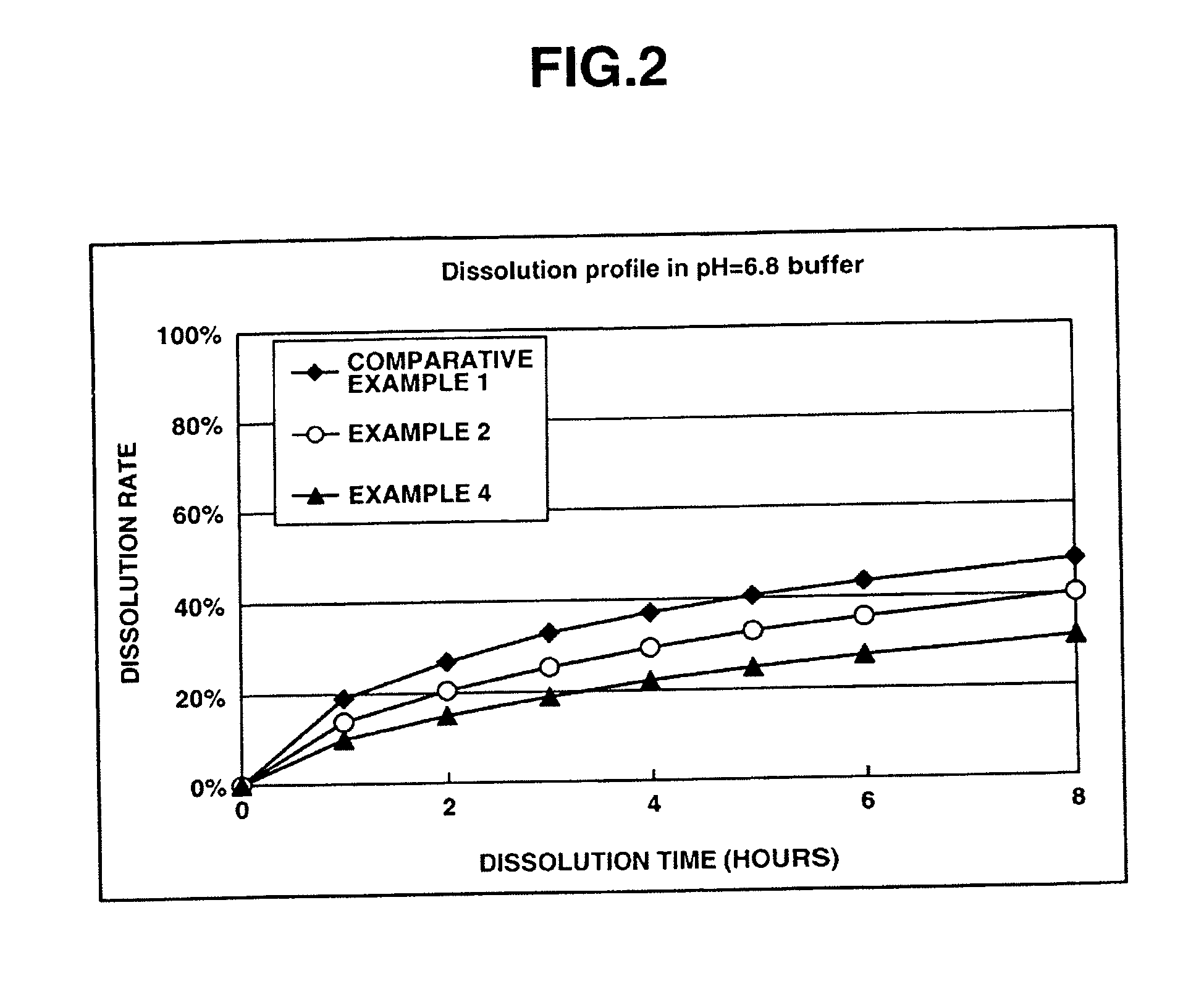
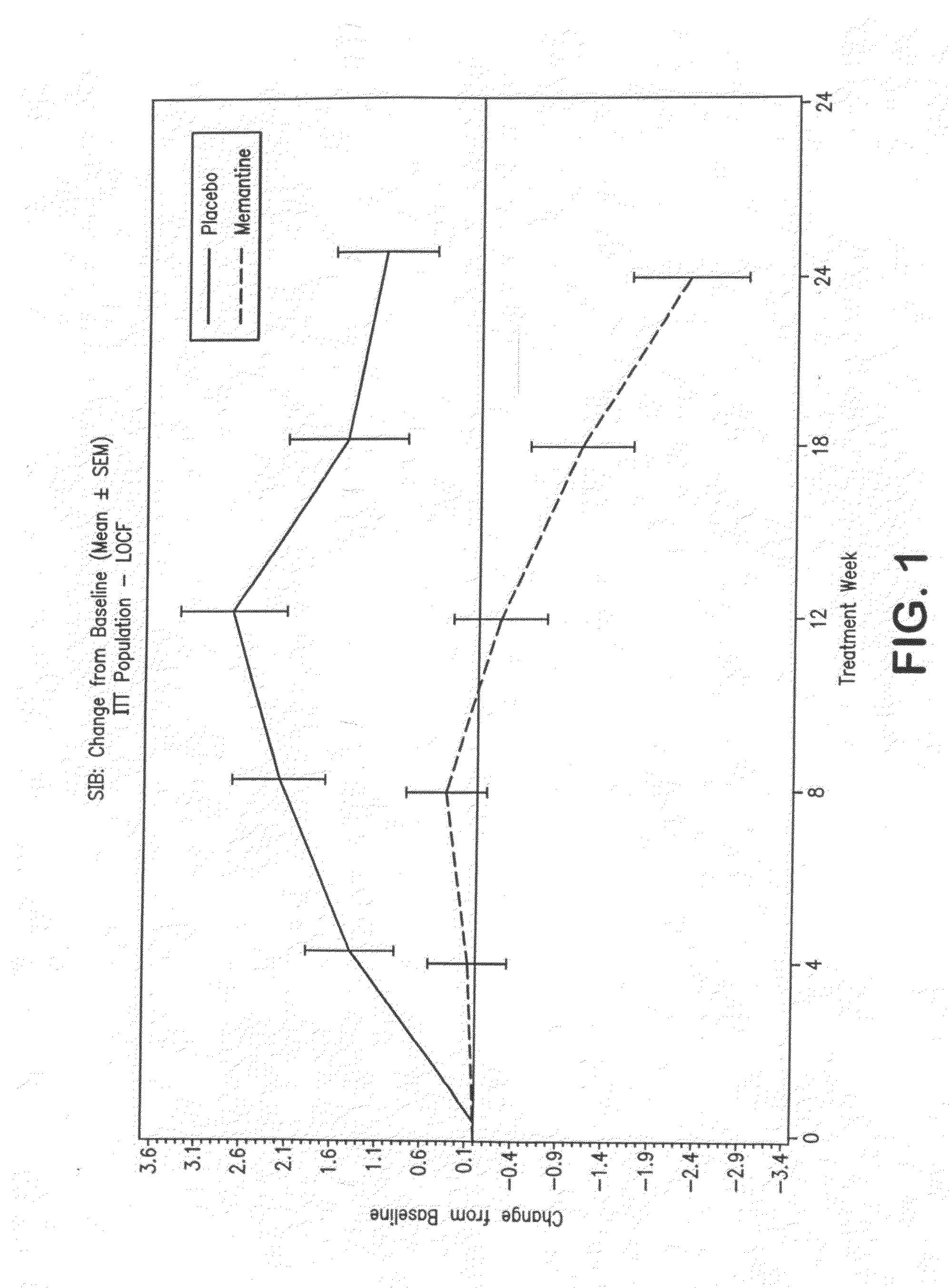
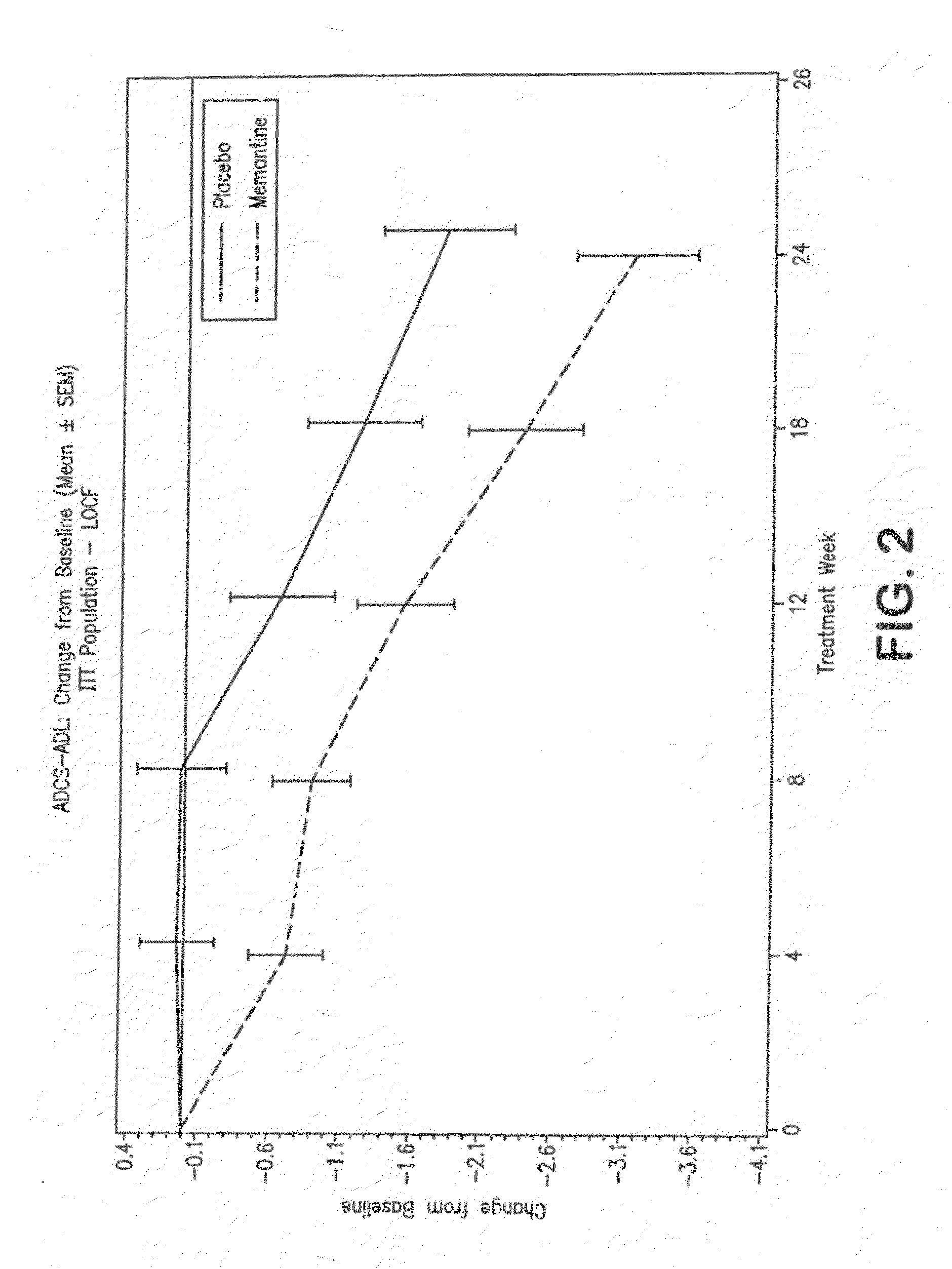
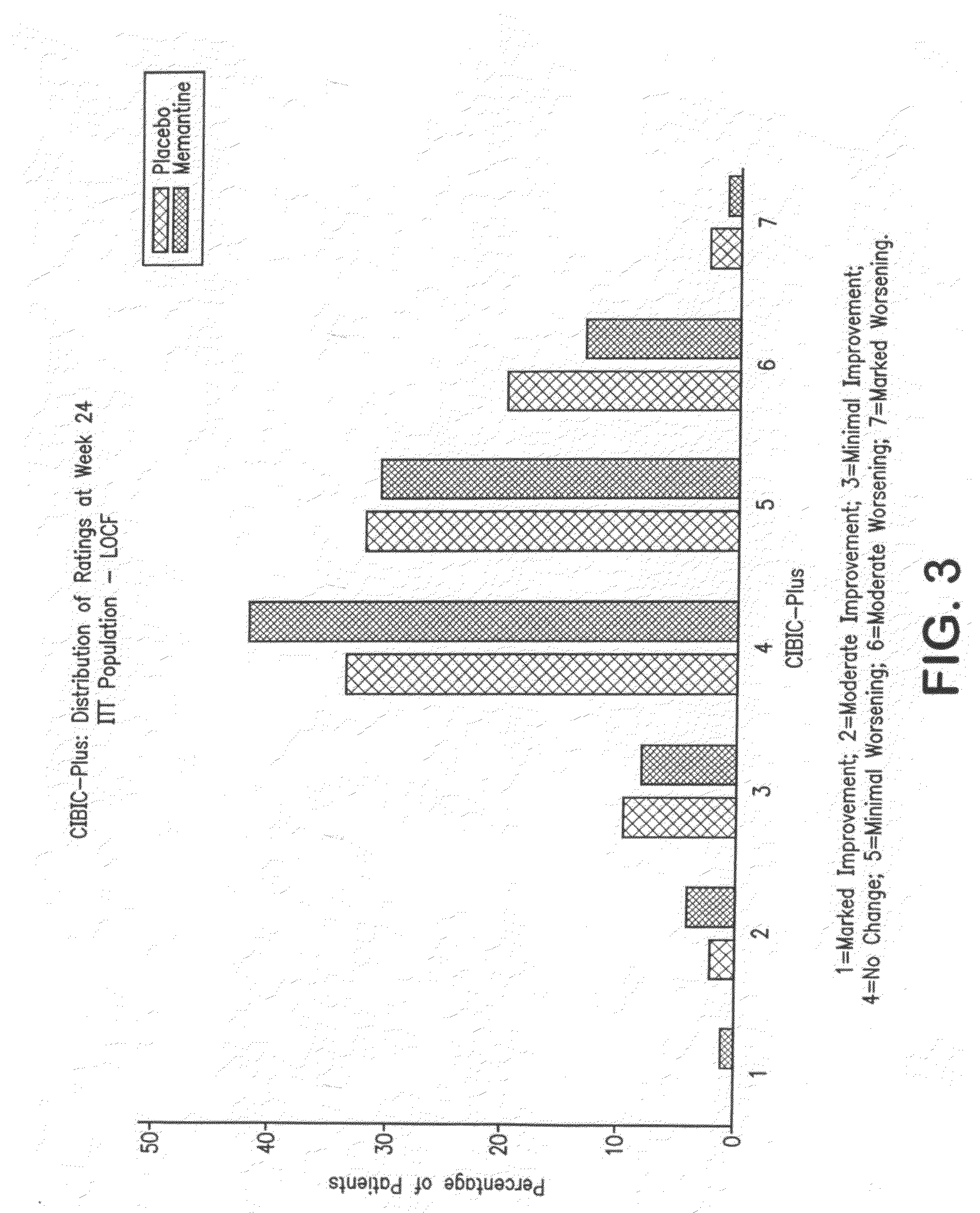
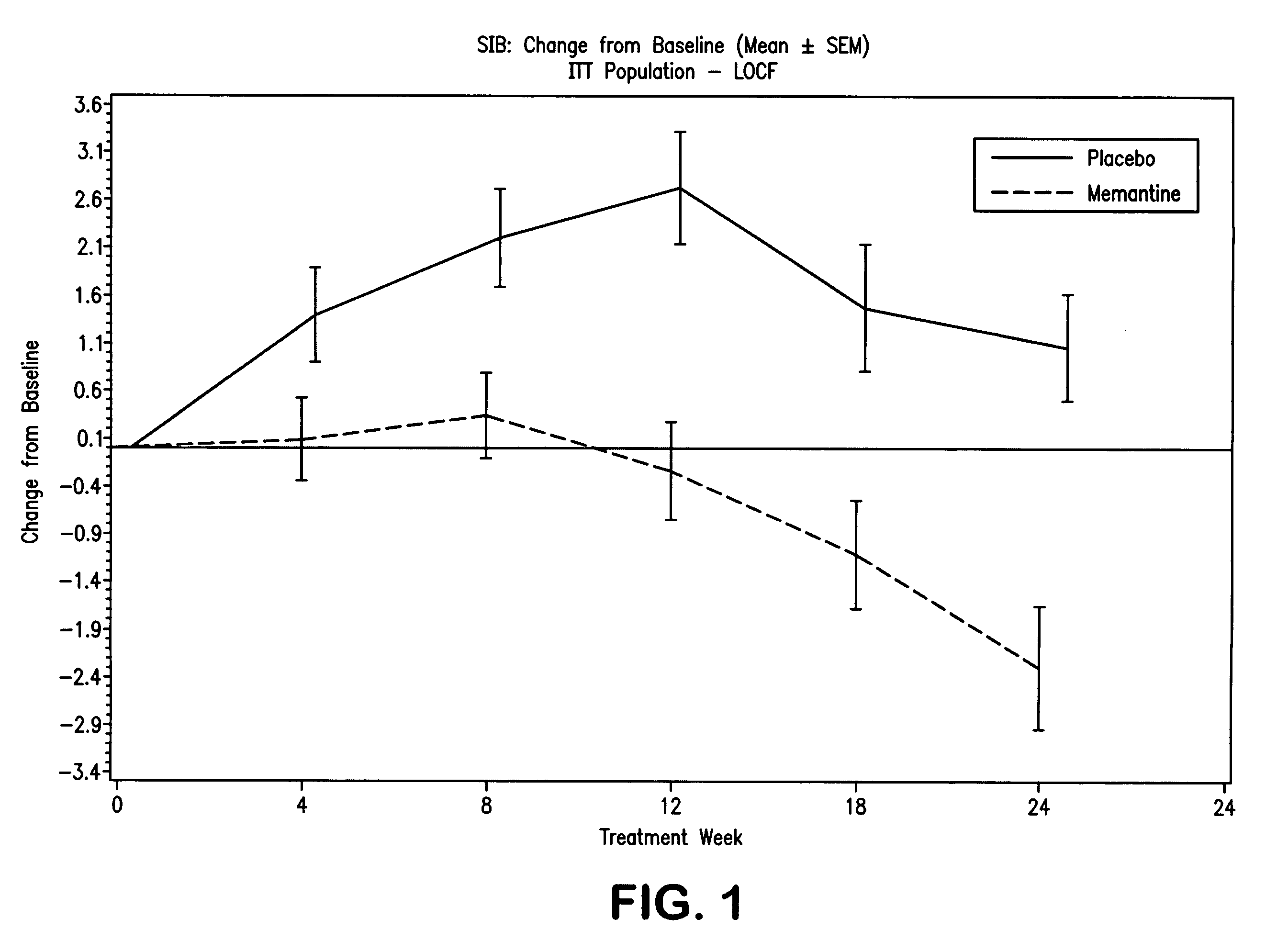
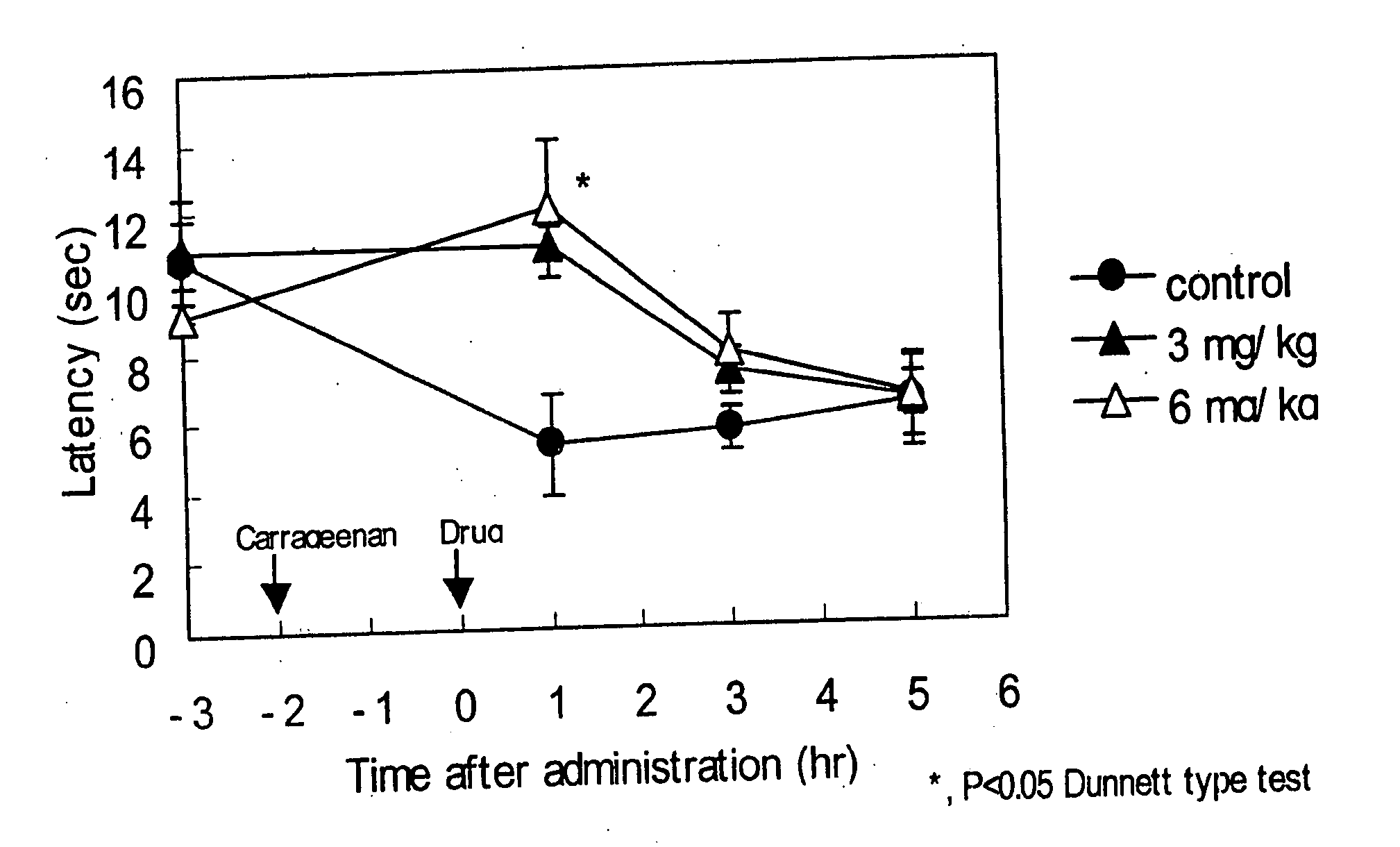
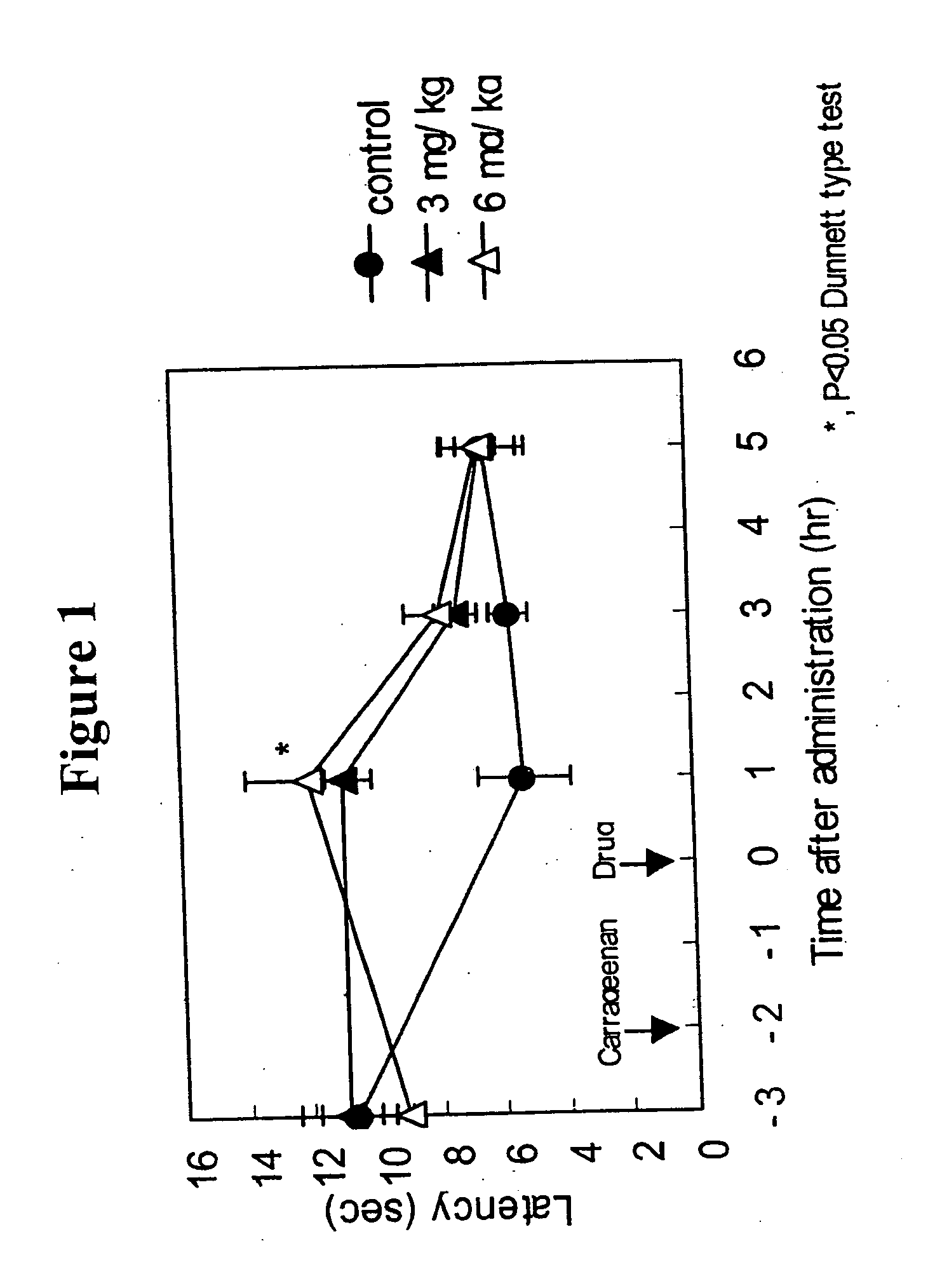
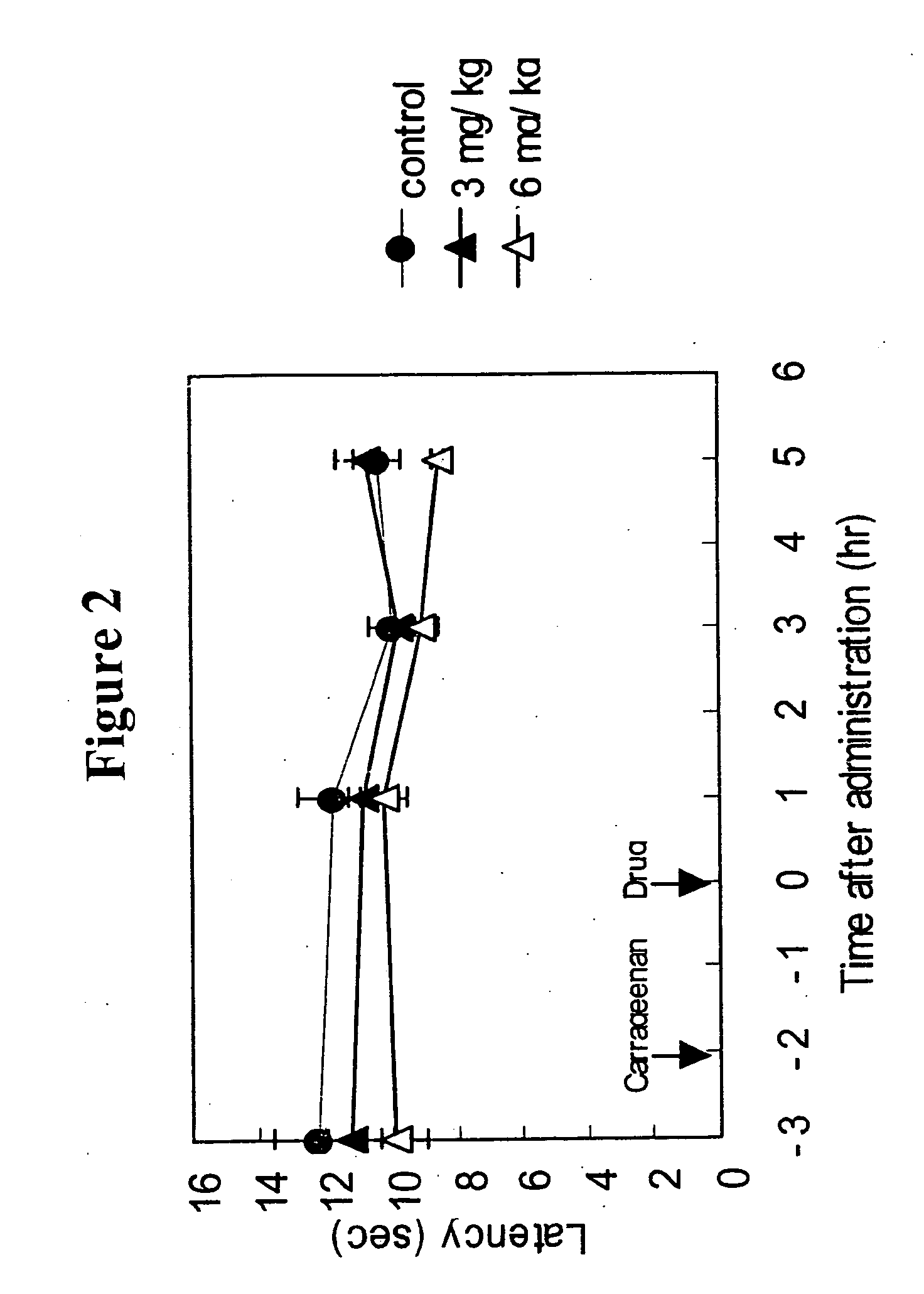
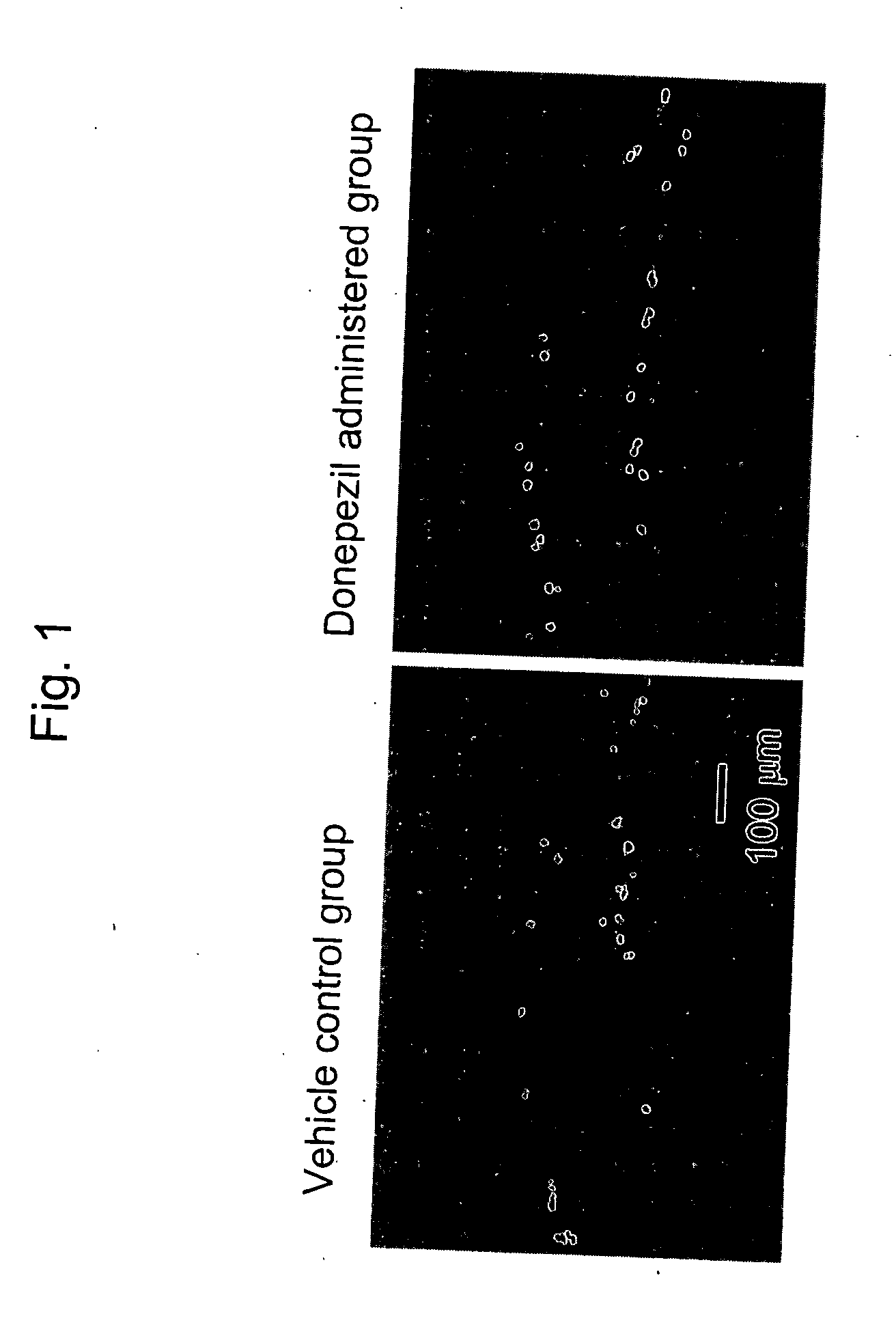
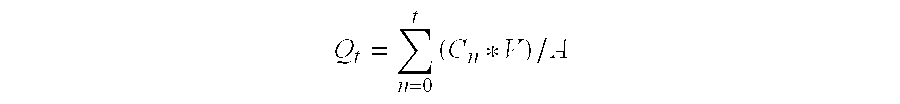Patents
Literature
320 results about "Cholinesterase inhibition" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acetylcholinesterase inhibition. An acetylcholinesterase inhibitor (often abbreviated AChEI) or anti-cholinesterase is a chemical or a drug that inhibits the acetylcholinesterase enzyme from breaking down acetylcholine, thereby increasing both the level and duration of action of the neurotransmitter acetylcholine.
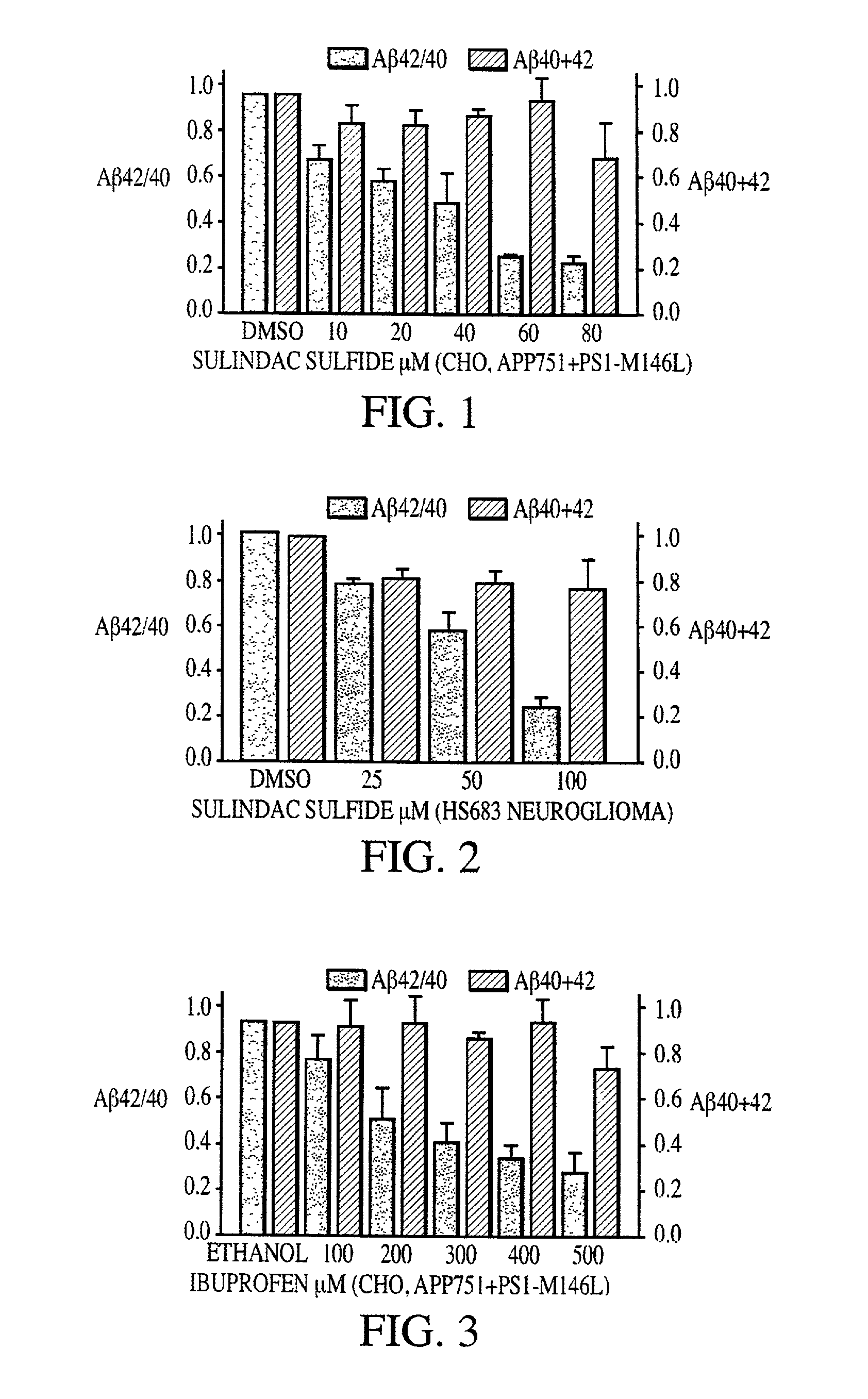
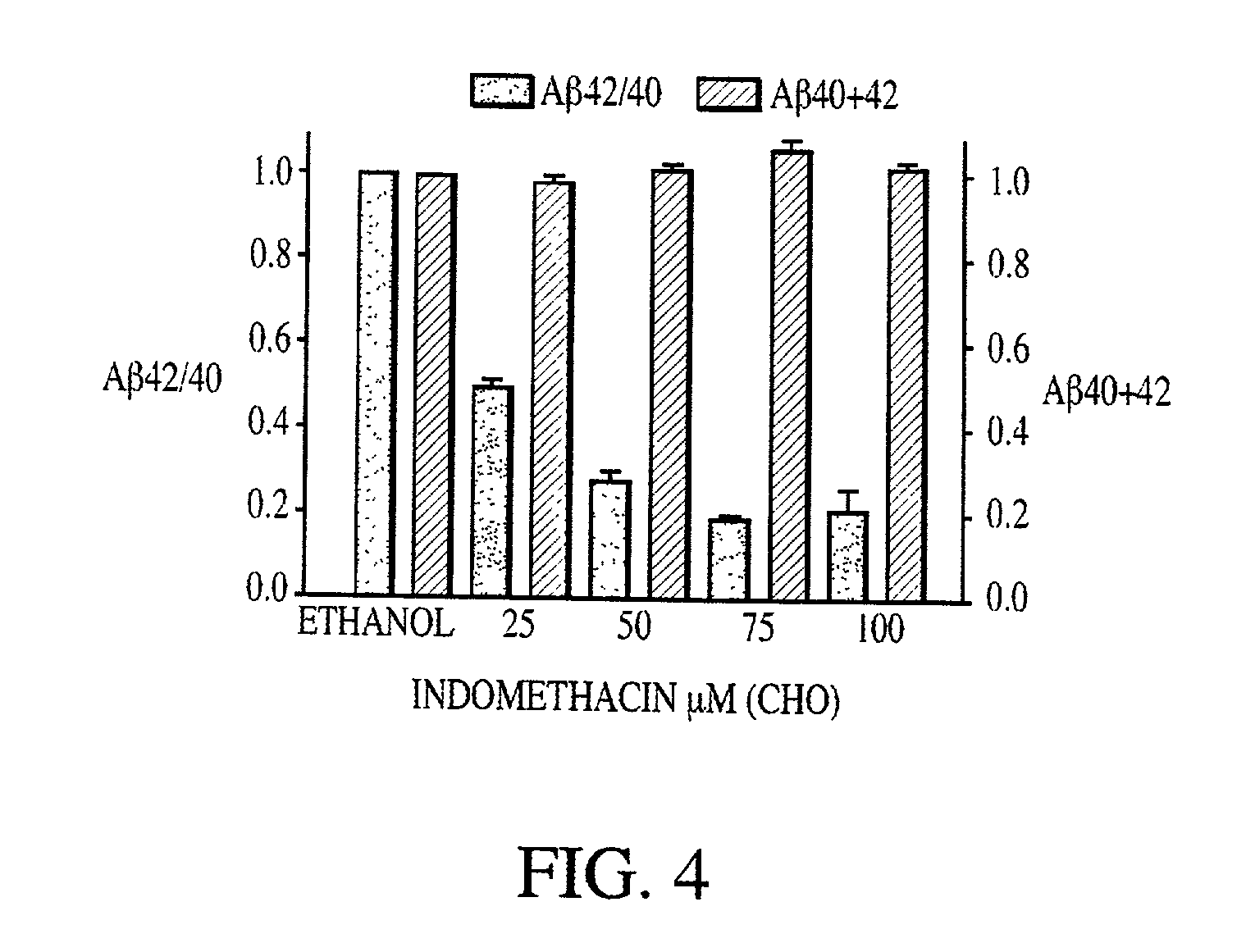
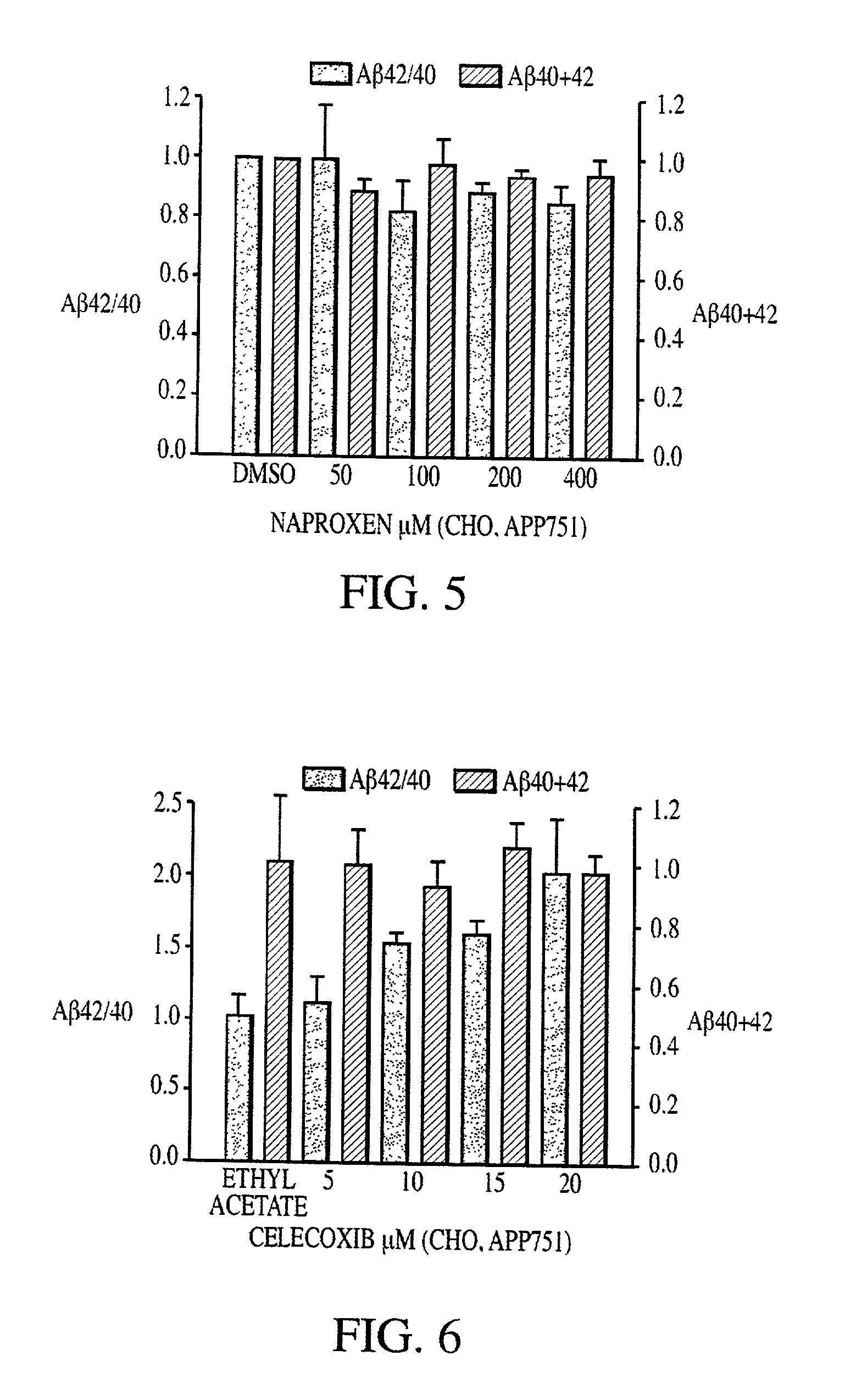
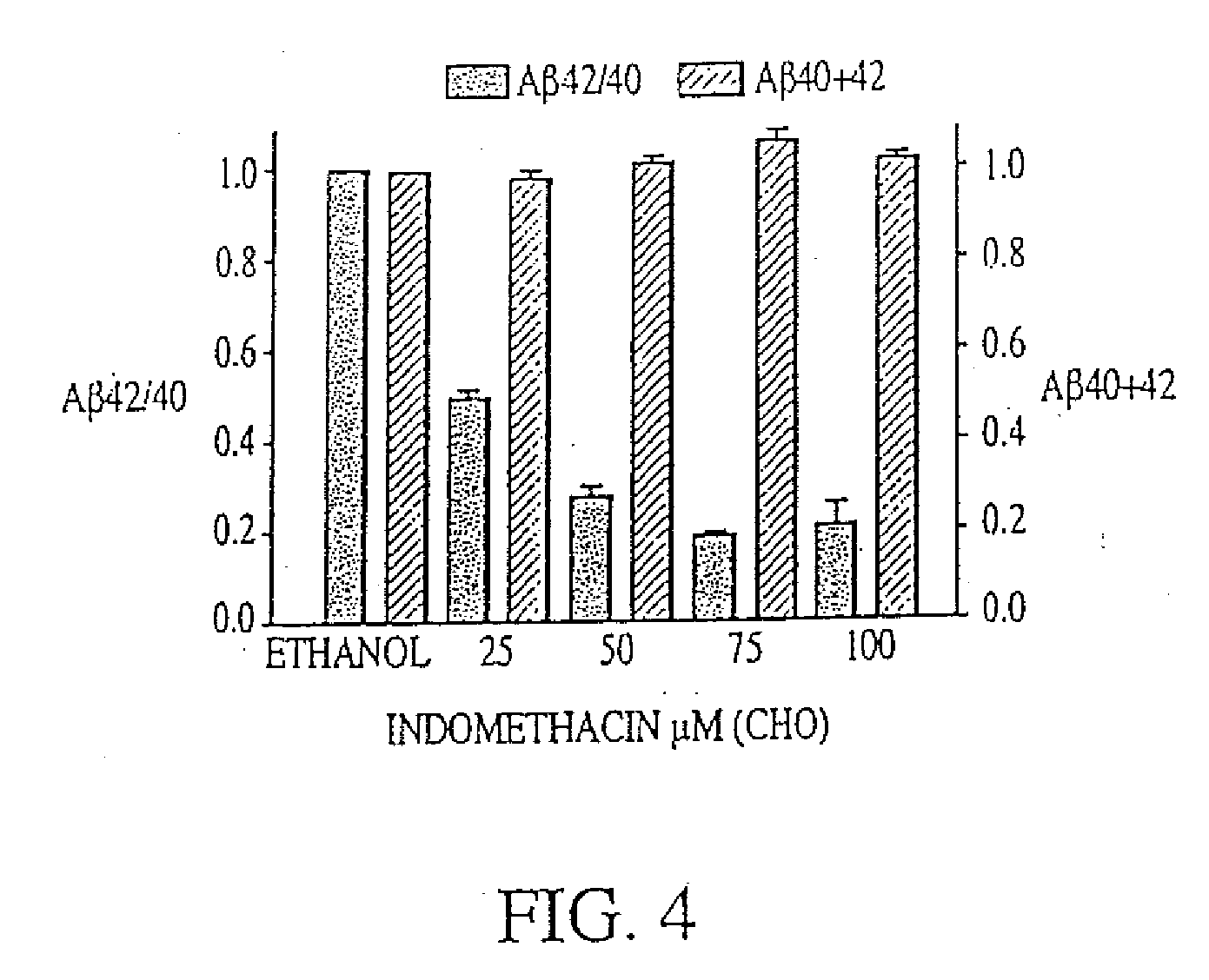
Abeta 42 lowering agents
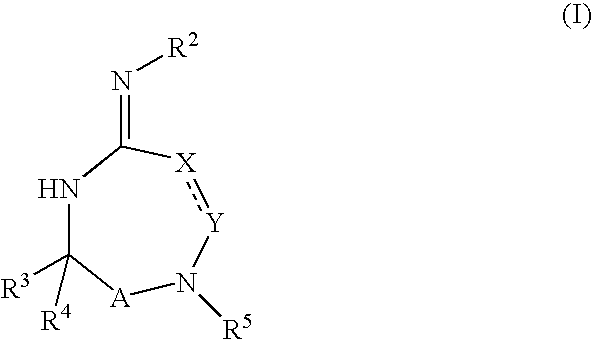
InactiveUS20020128319A1Prevent and delay and reverse progressionLower Level RequirementsCompounds screening/testingCompound screeningRegimenCholinesterase inhibition
The invention provides a method of preventing, delaying, or reversing the progression of Alzheimer's disease by administering an A.beta..sub.42 lowering agent to a mammal under conditions in which levels of A.beta..sub.42 are selectively reduced, levels of A.beta..sub.38 are increased, and levels of A.beta..sub.40 are unchanged. The invention provides methods and materials for developing and identifying A.beta..sub.42 lowering agents. In addition, the invention provides methods for identifying agents that increase the risk of developing, or hasten progression of, Alzheimer's disease. The invention also provides compositions of A.beta..sub.42 lowering agents and antioxidants, A.beta..sub.42 lowering agents and non-selective secretase inhibitors, as well as A.beta..sub.42 lowering agents and acetylcholinesterase inhibitors. The invention also provides kits containing A.beta..sub.42 lowering agents, antioxidants, non-selective secretase inhibitors, and / or acetylcholinesterase inhibitors as well as instructions related to dose regimens for A.beta..sub.42 lowering agents, antioxidants, non-selective secretase inhibitors, and acetylcholinesterase inhibitors.
Owner:RGT UNIV OF CALIFORNIA
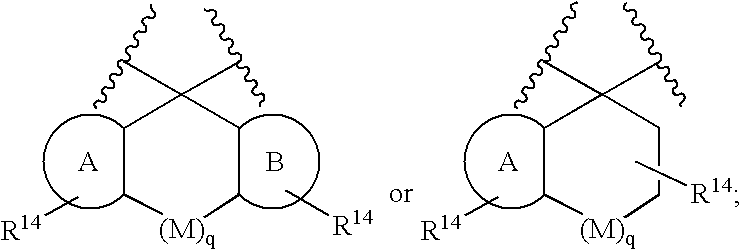
Heterocyclic aspartyl protease inhibitors
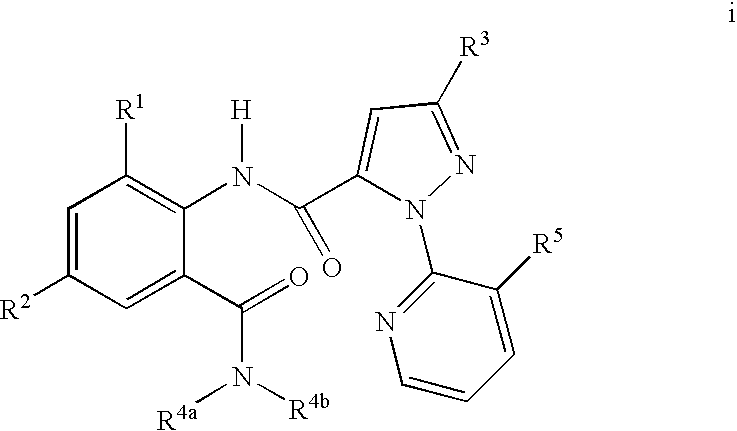
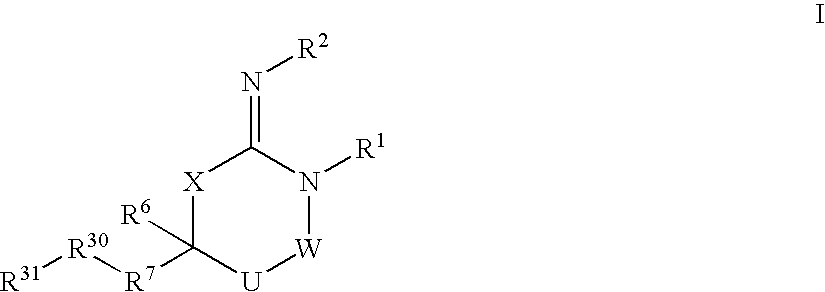
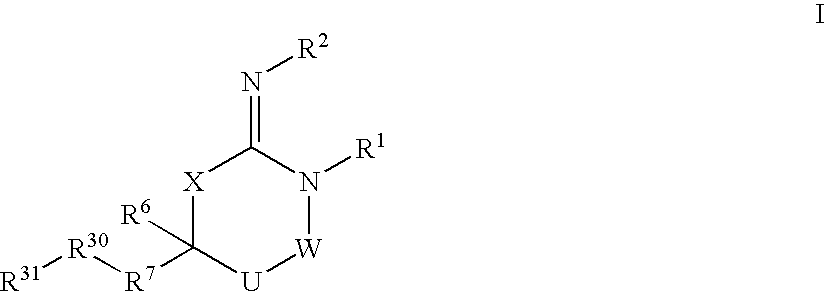
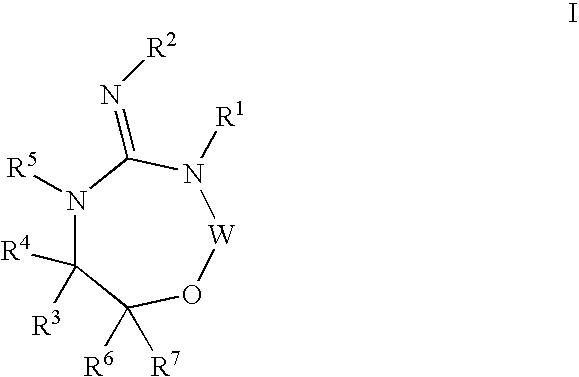
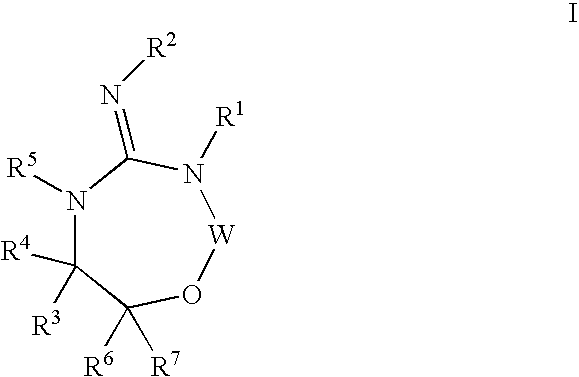
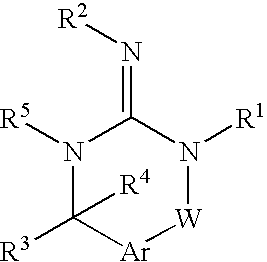
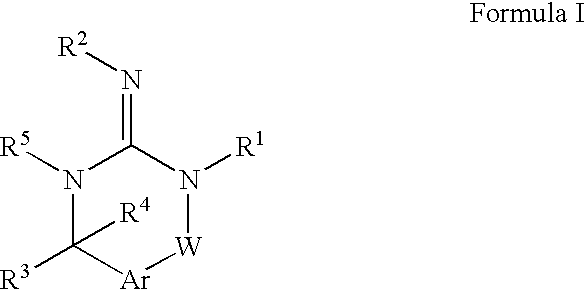
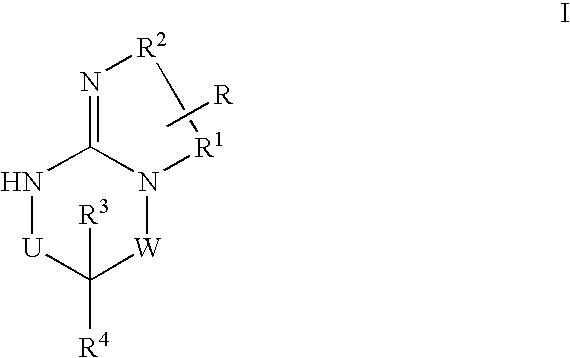
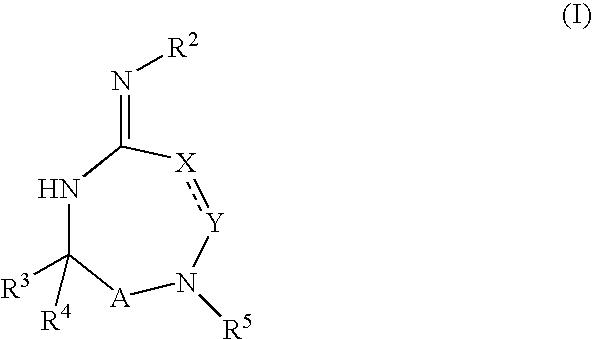
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein W is a bond, —C(═S)—, —S(O)—, —S(O)2—, —C(═O)—, —O—, —C(R6)(R7)—, —N(R5)— or —C(═N(R5))—; X is —O—, —N(R5)— or —C(R6)(R7)—; provided that when X is —O—, U is not —O—, —S(O)—, —S(O)2—, —C(═O)— or —C(═NR5)—; U is a bond, —S(O)—, —S(O)2—, —C(O)—, —O—, —P(O)(OR15)—, —C(═NR5)—, —(C(R6)(R7))b— or —N(R5)—; wherein b is 1 or 2; provided that when W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, U is not —S(O)—, —S(O)2—, —O—, or —N(R5)—; provided that when X is —N(R5)— and W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, then U is not a bond; and R1, R2, R3, R4, R5, R6, and R7 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:PHARMACOPEIA DRUG DISCOVERY +1
Synergistic Mixtures of Anthranilamide Invertebrate Pest Control Agents
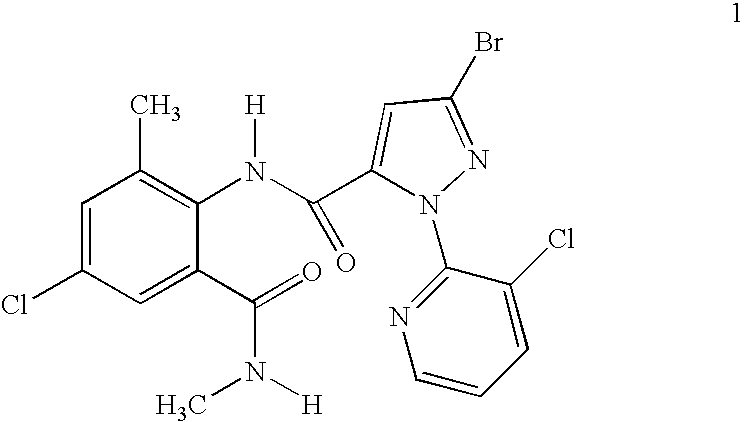
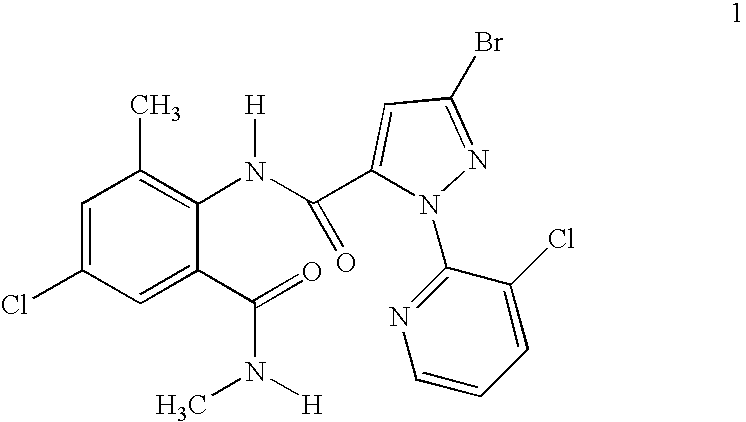
Disclosed are mixtures and compositions for controlling invertebrate pests relating to combinations comprising (a) 3-bromo-N-[4-chloro-2-methyl-6-[(methylamino)carbonyl]phenyl]-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxamide, and its N-oxides, and suitable salts thereof and a component (b) wherein the component (b) is at least one compound or agent selected from neonicotinoids, cholinesterase inhibitors, sodium channel modulators, chitin synthesis inhibitors, ecdysone agonists, lipid biosynthesis inhibitors, macrocyclic lactones, GABA-regulated chloride channel blockers, juvenile hormone mimics, ryanodine receptor ligands, octopamine receptor ligands, mitochondrial electron transport inhibitors, nereistoxin analogs, pyridalyl, flonicamid, pymetrozine, dieldrin, metaflumizone, biological agents, and suitable salts of the foregoing. Also disclosed are methods for controlling an invertebrate pest comprising contacting the invertebrate pest or its environment with a biologically effective amount of a mixture or composition of the invention.
Owner:FMC CORP
Aspartyl protease inhibitors
Disclosed are compounds of formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, U, W, X, R1, R2, R6, R7, R30 and R31 are as described above in the specification. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:MERCK SHARP & DOHME LLC
Aspartyl protease inhibitors
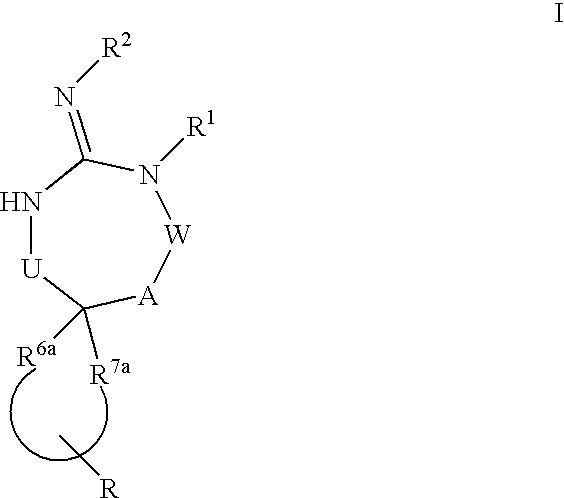
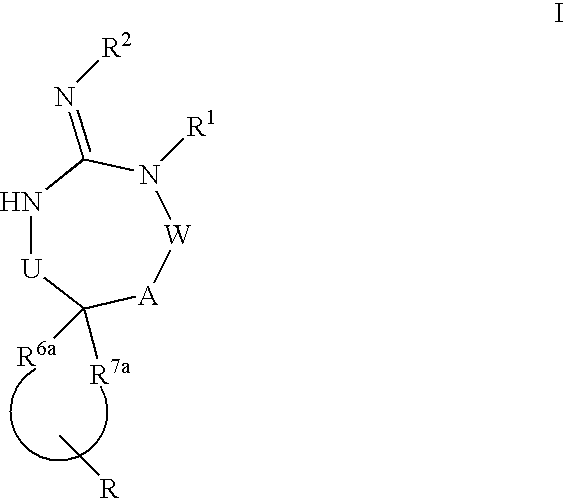
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein U, W, A, R, R1, R2, R6a and R7, are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:MERCK SHARP & DOHME LLC +1
Aspartyl protease inhibitors
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein W, R1, R2, R3, R4, R5, R6, and R7 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic antagonist.
Owner:SCHERING CORP
Heterocyclic aspartyl protease inhibitors
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein Ar, R1, R2, R3, R4 and R5 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:MERCK SHARP & DOHME LLC
Aspartyl protease inhibitors
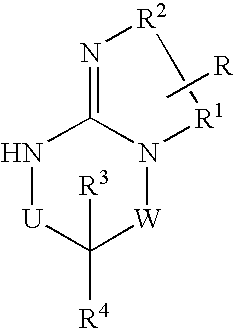
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein U, W, R, R1, R2, R3 and R4 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:MERCK SHARP & DOHME LLC
Treatment of diseases with combinations of alpha 7 Nicotinic Acetylcholine Receptor agonists and other compounds
The present invention relates to compositions and methods to treat diseases or condition with an α7 nAChR full agonist and an inhibitor of cholinesterase, and or beta secretase and or gamma secretase.
Owner:CORBETT JEFFREY W +1
Sustained release formulations
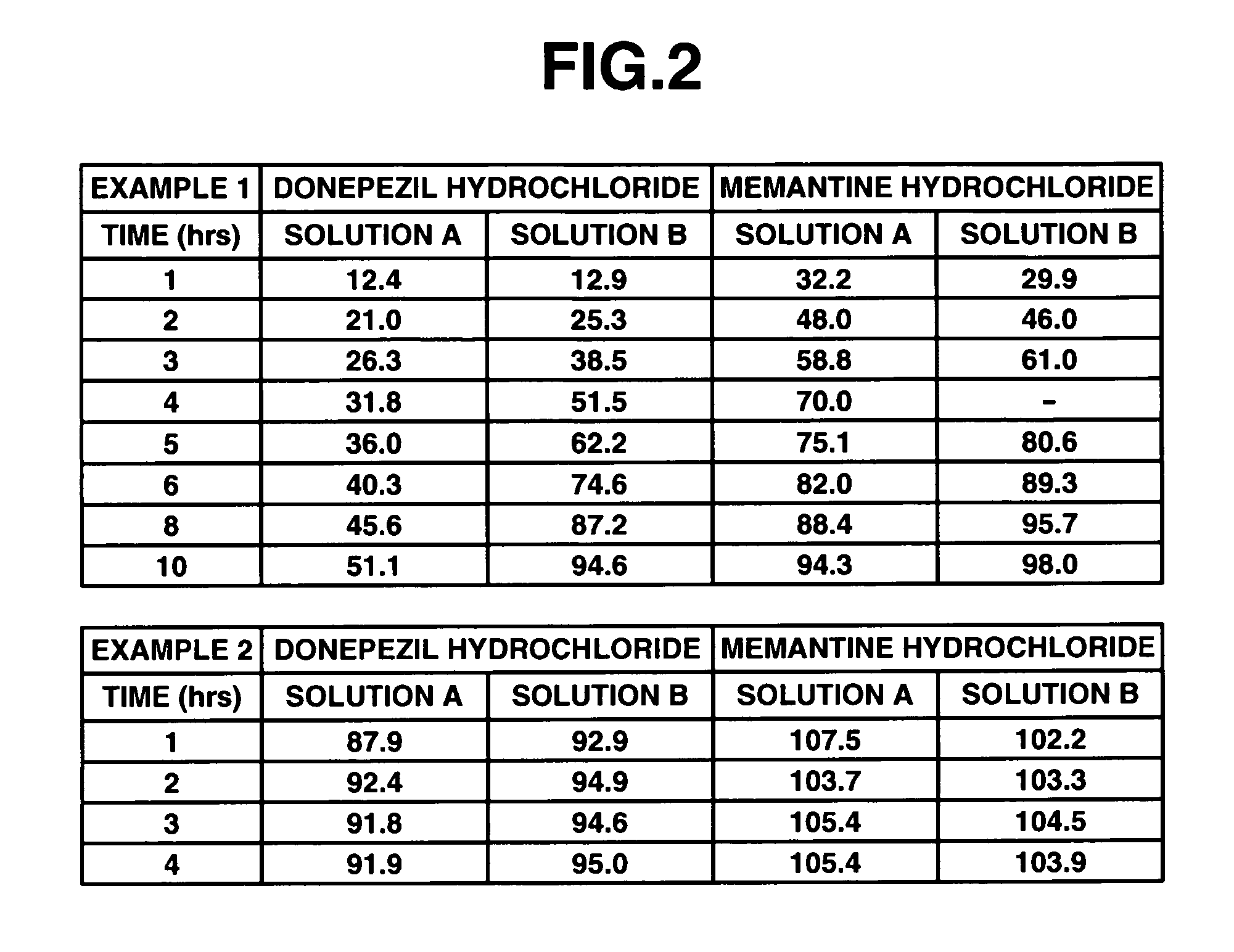
InactiveUS20060280789A1Reduce in quantityImprove complianceBiocidePill deliveryDonepezilCholinesterase
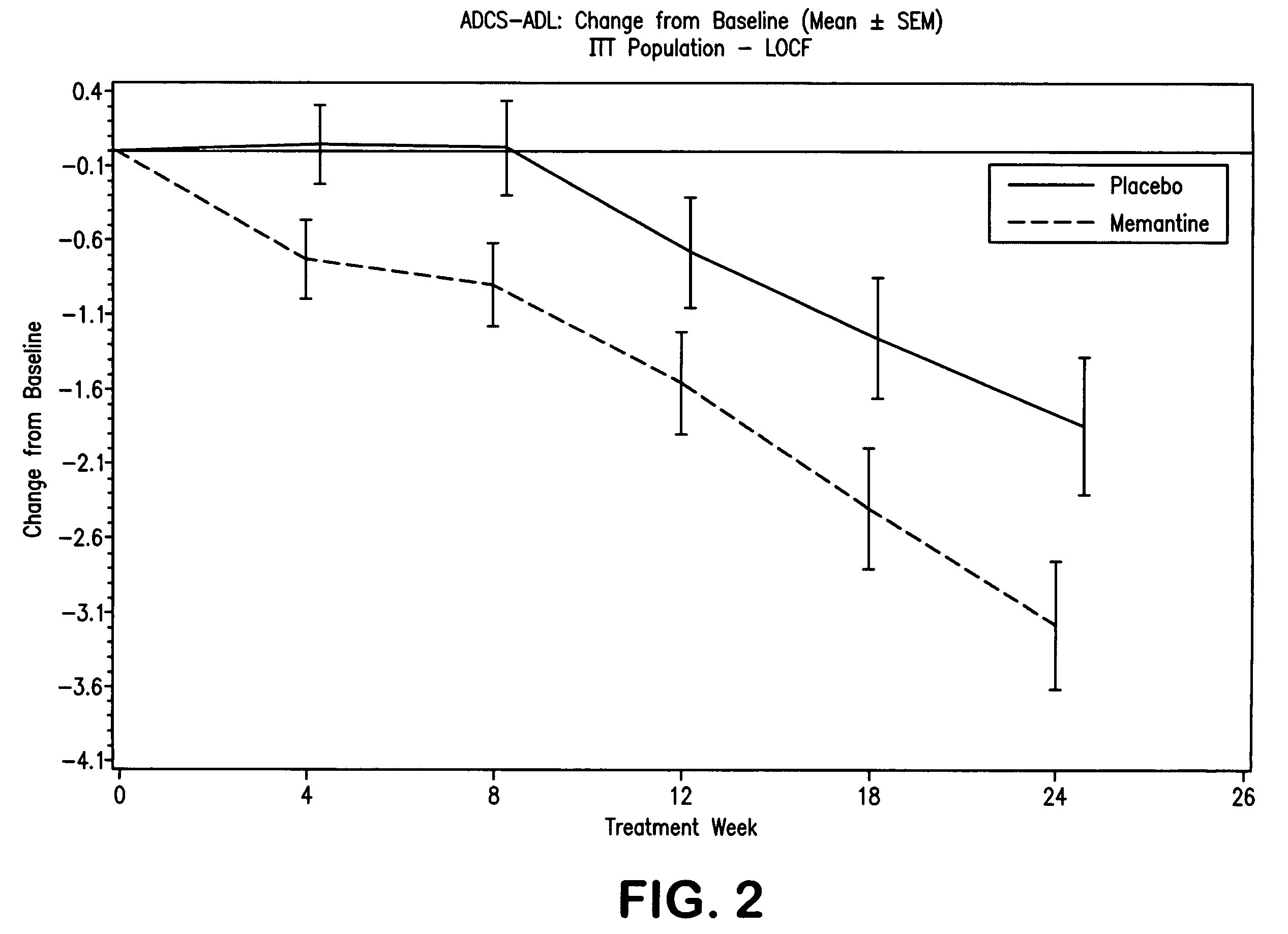
The invention provides sustained release formulations of basic drugs, stereoisomers of basic drugs, pharmaceutically acceptable salts of basic drugs, and pharmaceutically acceptable salts of stereoisomers of basic drugs. The basic drugs may be anti-dementia drugs, such as cholinesterase inhibitors or memantine. In one embodiment, the cholinesterase inhibitor is donepezil.
Owner:EISAI CO LTD
Combination therapy using 1-aminocyclohexane derivatives and acetylcholinesterase inhibitors
InactiveUS20100227852A1Avoid damageReduce riskBiocideNervous disorderCholinesterase inhibitionRivastigmine
Owner:MERZ PHARMA GMBH & CO KGAA
Combination therapy using 1-aminocyclohexane derivatives and acetylcholinesterase and inhibitors
InactiveUS20090124659A1Avoid damageReduce riskBiocideNervous disorderCholinesterase inhibitionDepressant
The invention relates to a novel drug combination therapy useful in the treatment of dementia comprising administering an 1-aminocyclohexane derivative such as memantine or neramexane and an acetylcholinesterase inhibitor (AChEI) such as galantamine, tacrine, donepezil, or rivastigmine.
Owner:MERZ PHARMA GMBH & CO KGAA
Neural tourniquet
ActiveUS8729129B2BiocideNervous disorderCholinesterase inhibitionCholinergic anti-inflammatory pathway
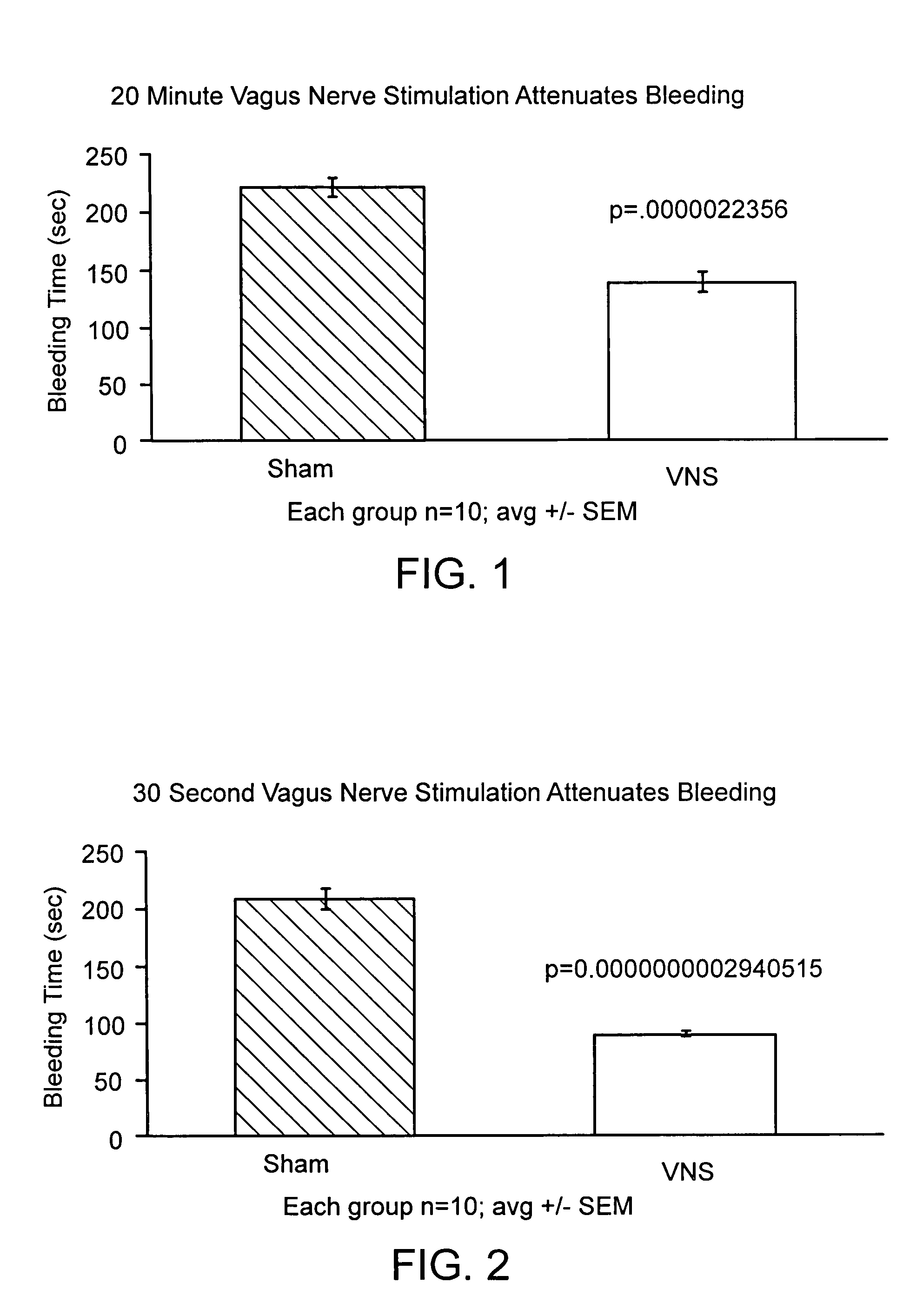
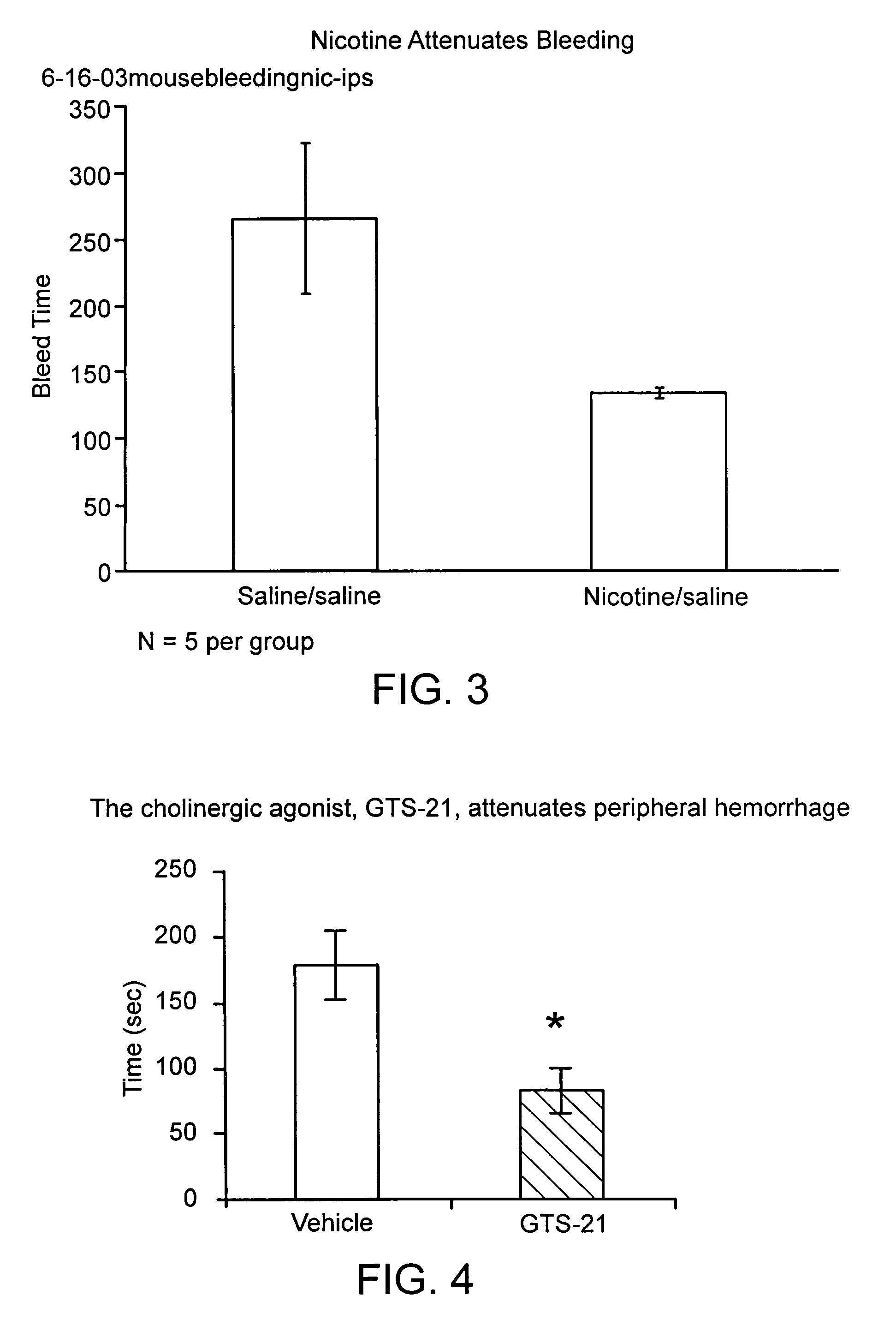
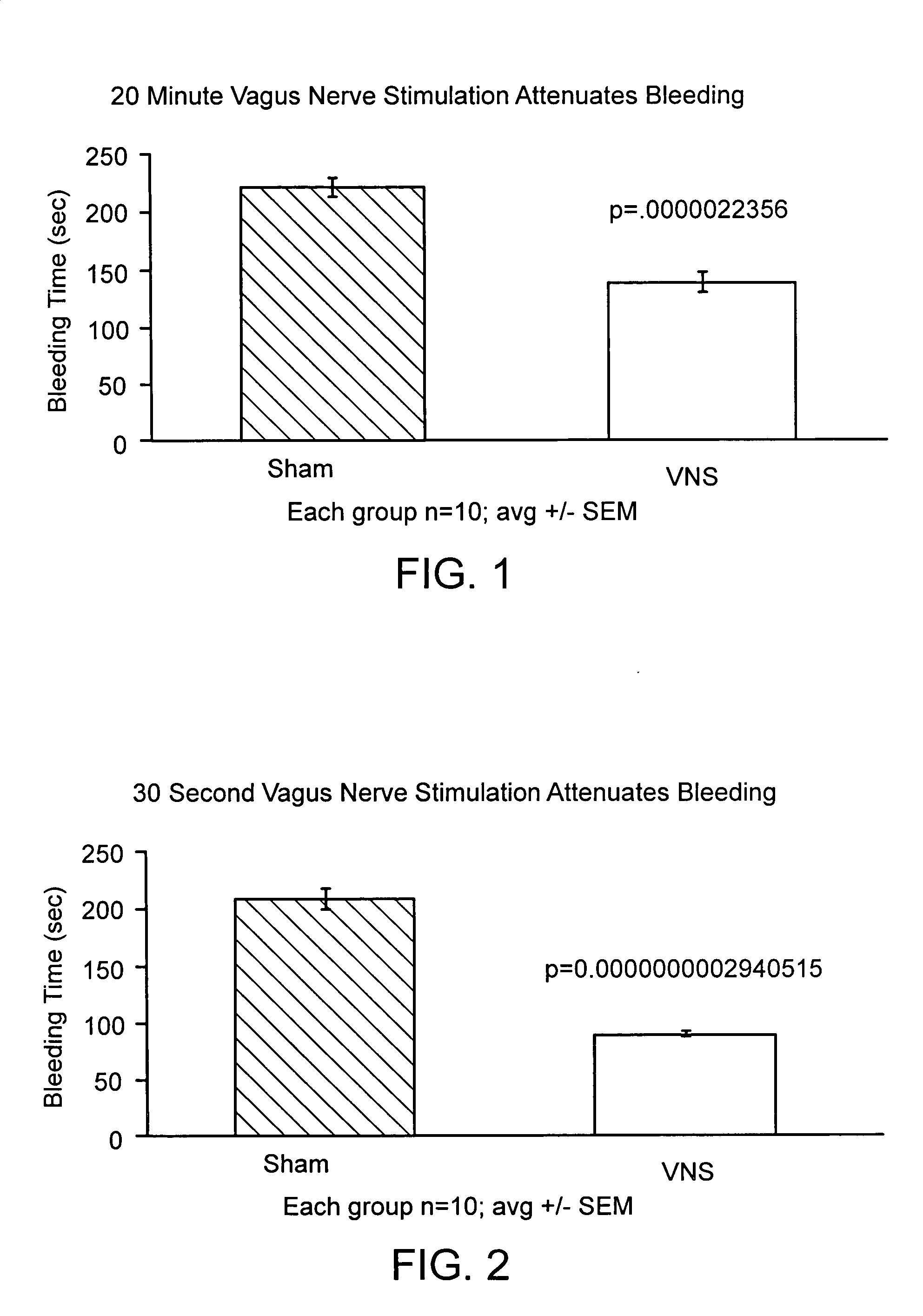
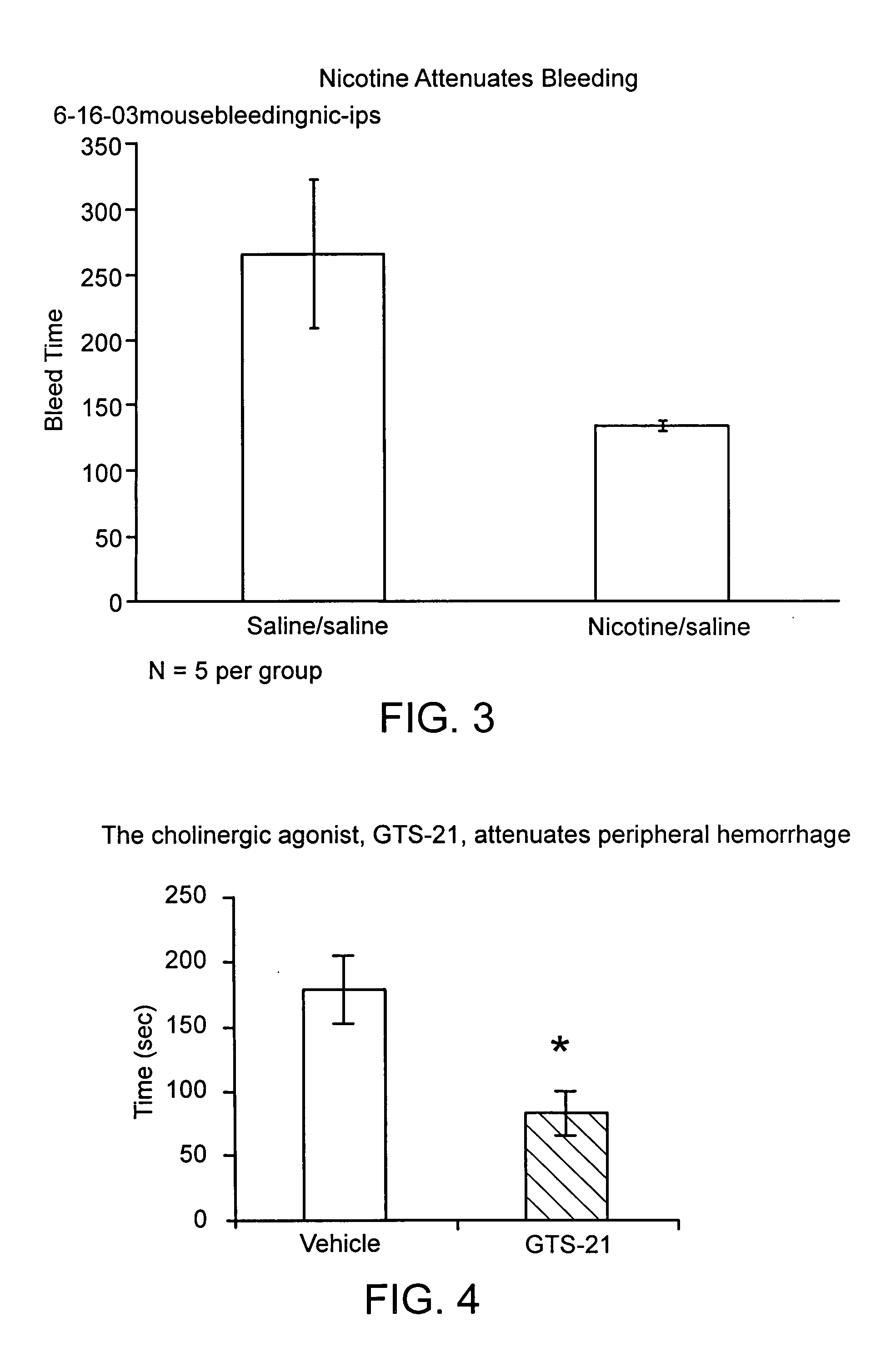
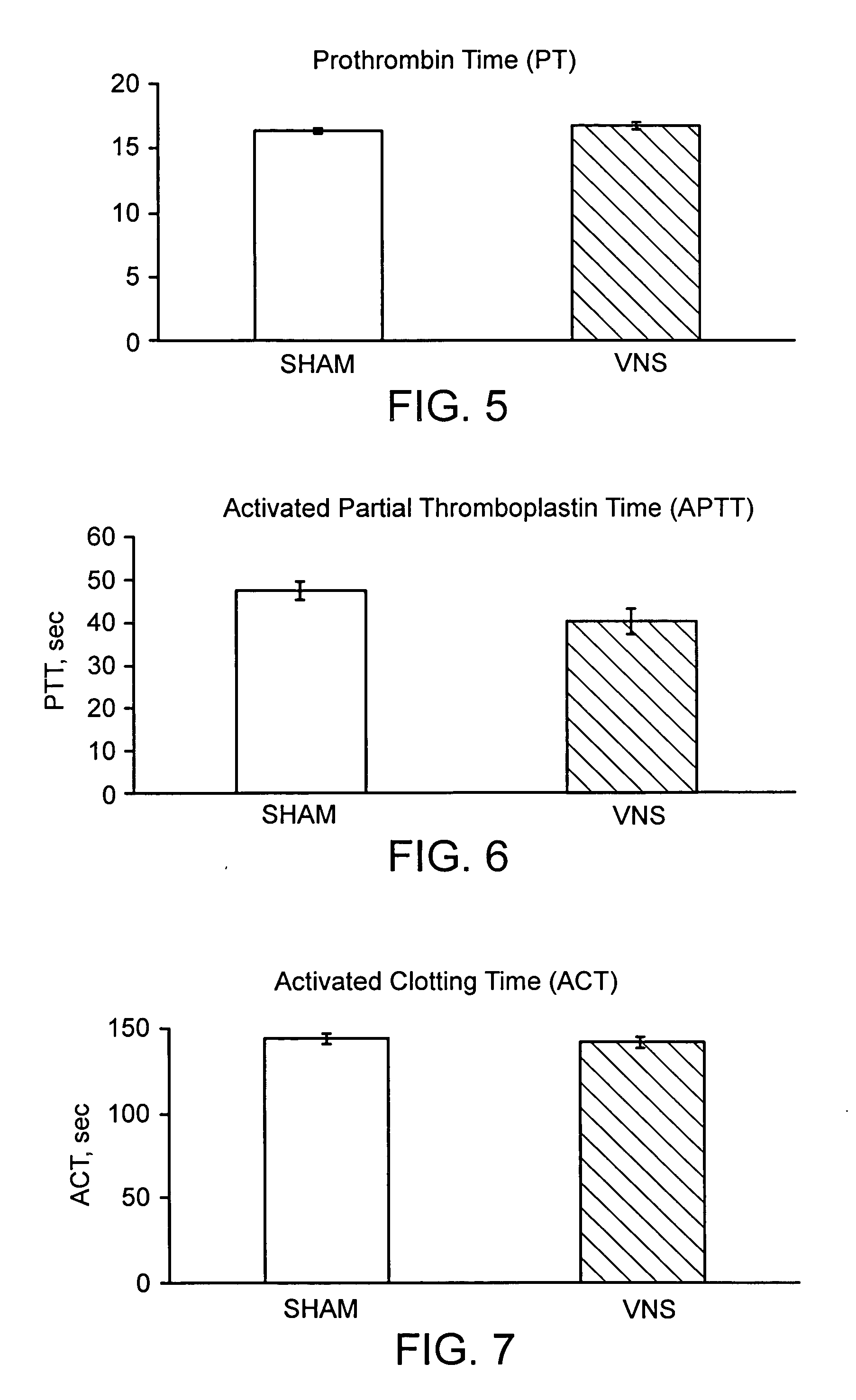
Disclosed is a method of reducing bleed time in a subject by activation of the cholinergic anti-inflammatory pathway in said subject. The cholinergic anti-inflammatory pathway can be activated by direct or indirect stimulation of the vagus nerve. The cholinergic anti-inflammatory pathway can also be activated by administering an effective amount of cholinergic agonist or acetylcholinesterase inhibitor to the subject.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
New uses for quaternary ammonium anticholinergic muscarinic receptor antagonists in patients being treated for cognitive impairment or acute delirium
ActiveUS20080114014A1Maximizing beneficial effectMaximize the effectBiocideNervous disorderSolubilityFecal incontinence
A method for treating the adverse effects of acetyl-cholinesterase inhibitors used in the treatment of cognitive disorders such as acute delirium and cognitive impairment in elderly human patients. The administration of a clinically effective amount of a quaternary ammonium anti-cholinergic muscarinic receptor antagonist having very low lipid solubility substantially eliminates the adverse effects of urinary and / or fecal incontinence, nausea, bradycardia, bronchorrhea or brochospasm caused by the acetyl-cholinesterase inhibitors, without affecting the beneficial activity of the acetyl-cholinesterase inhibitors. This permits the administration of the optimum effective dosing of acetyl-cholinesterase inhibitors to provide maximum benefit to the patient with the added benefit of reducing or eliminating the unwanted side effects of fecal and urinary incontinence. Further, the combination of rivastigmine and glycopyrrolate has been effective in significantly improving cognitive function in patients suffering from acute dementia or cognitive impairment.
Owner:QAAM PHARMA LLC
Compositions and methods for bowel care in individuals with chronic intestinal pseudo-obstruction
ActiveUS7635709B2Shorten the construction periodDifficult to administerBiocideAmine active ingredientsBowel careSide effect
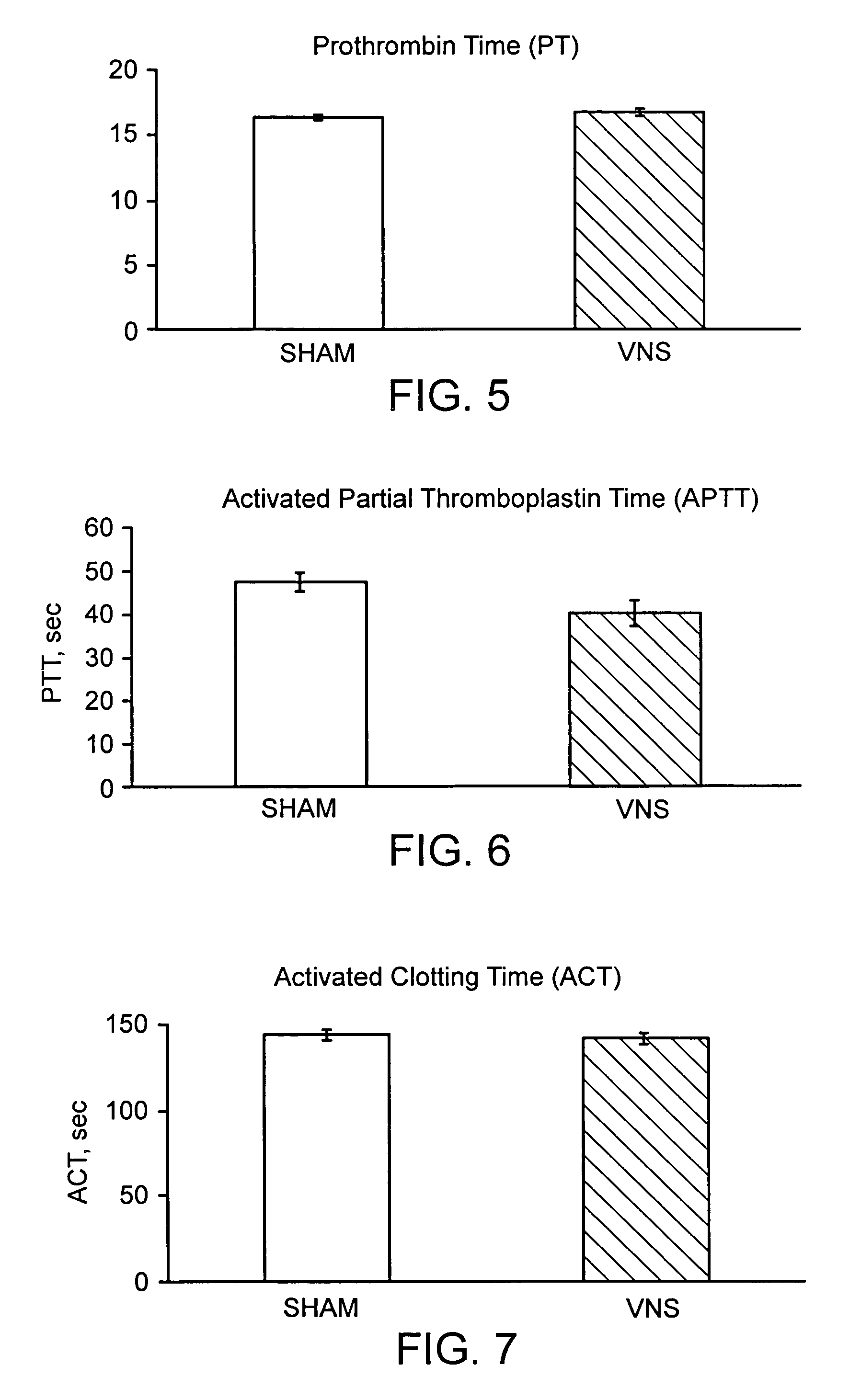
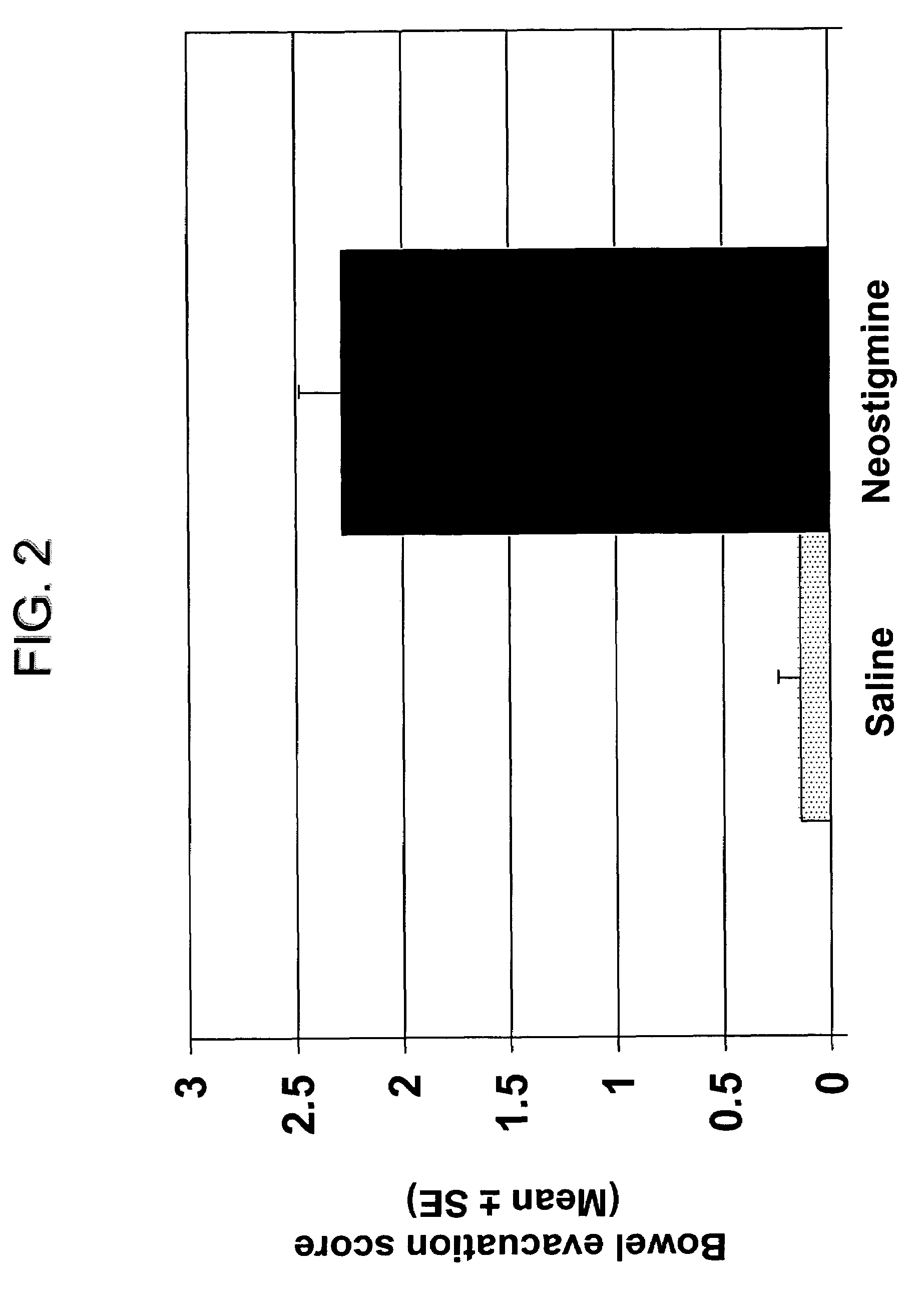
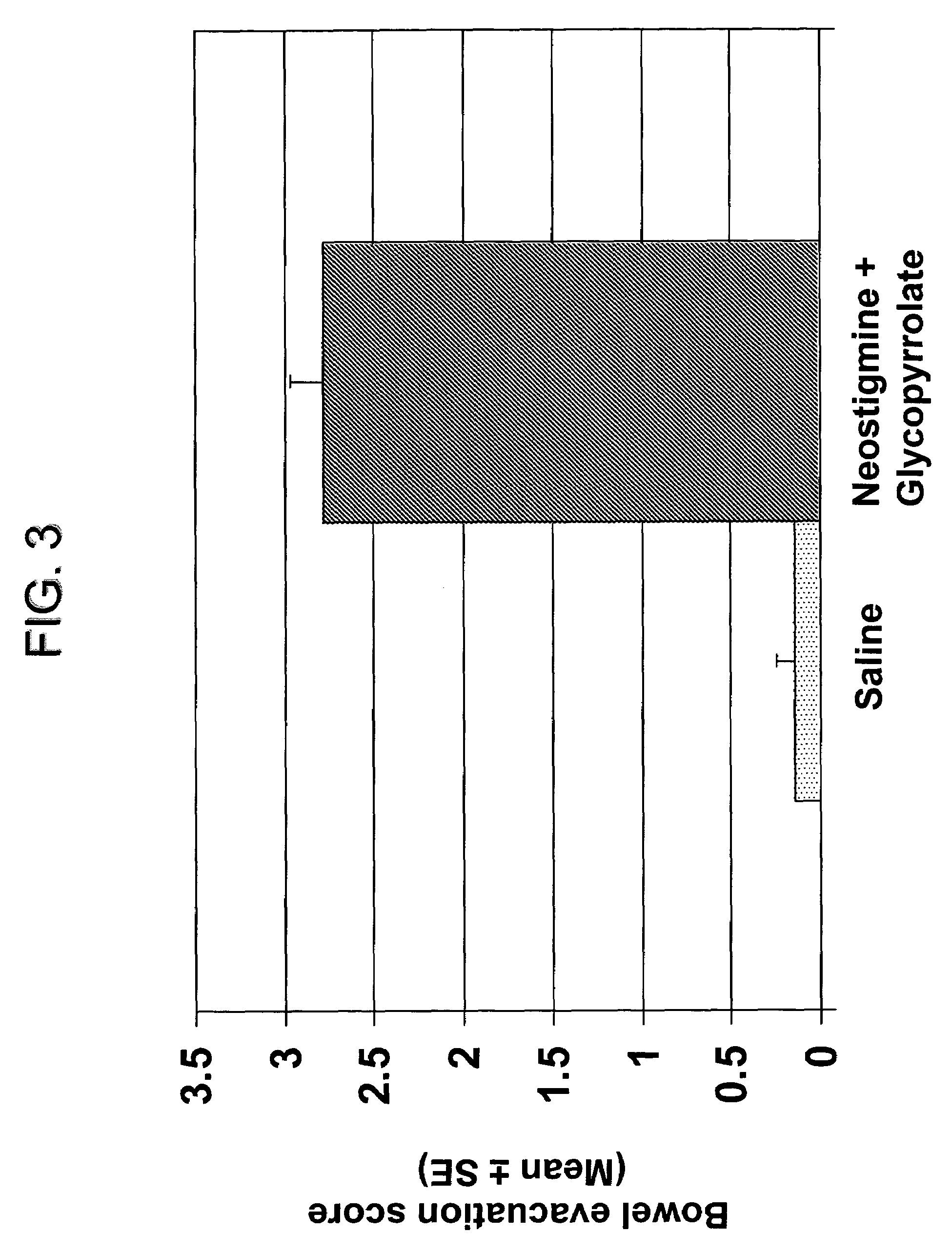
The present disclosure provides compositions and methods for on-going bowel care for persons with chronic intestinal pseudo-obstruction. The compositions and methods can be administered in a non-clinical setting. The compositions comprise acetylcholinesterase inhibitors for stimulating motility of the bowel in combination with anti-cholinergic agents to counteract the potentially dangerous cardiac side effects of the acetylcholinesterase inhibitor. In some examples, the acetylcholinesterase inhibitor, neostigmine, and the anti-cholinergic agent, glycopyrrolate, are combined in a pharmaceutical composition. Certain examples also provide the frequency and duration of administration of the disclosed drug combinations.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS
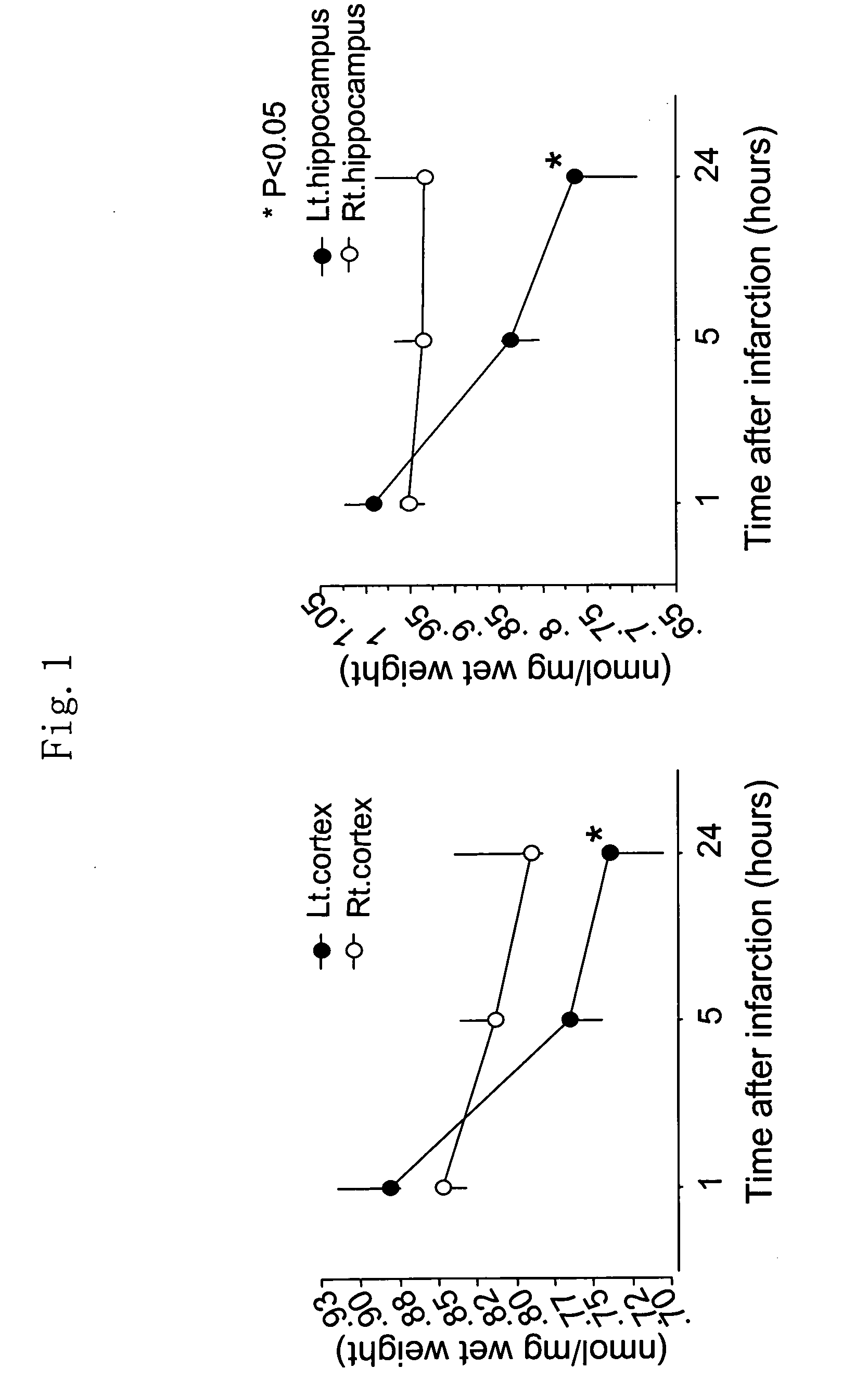
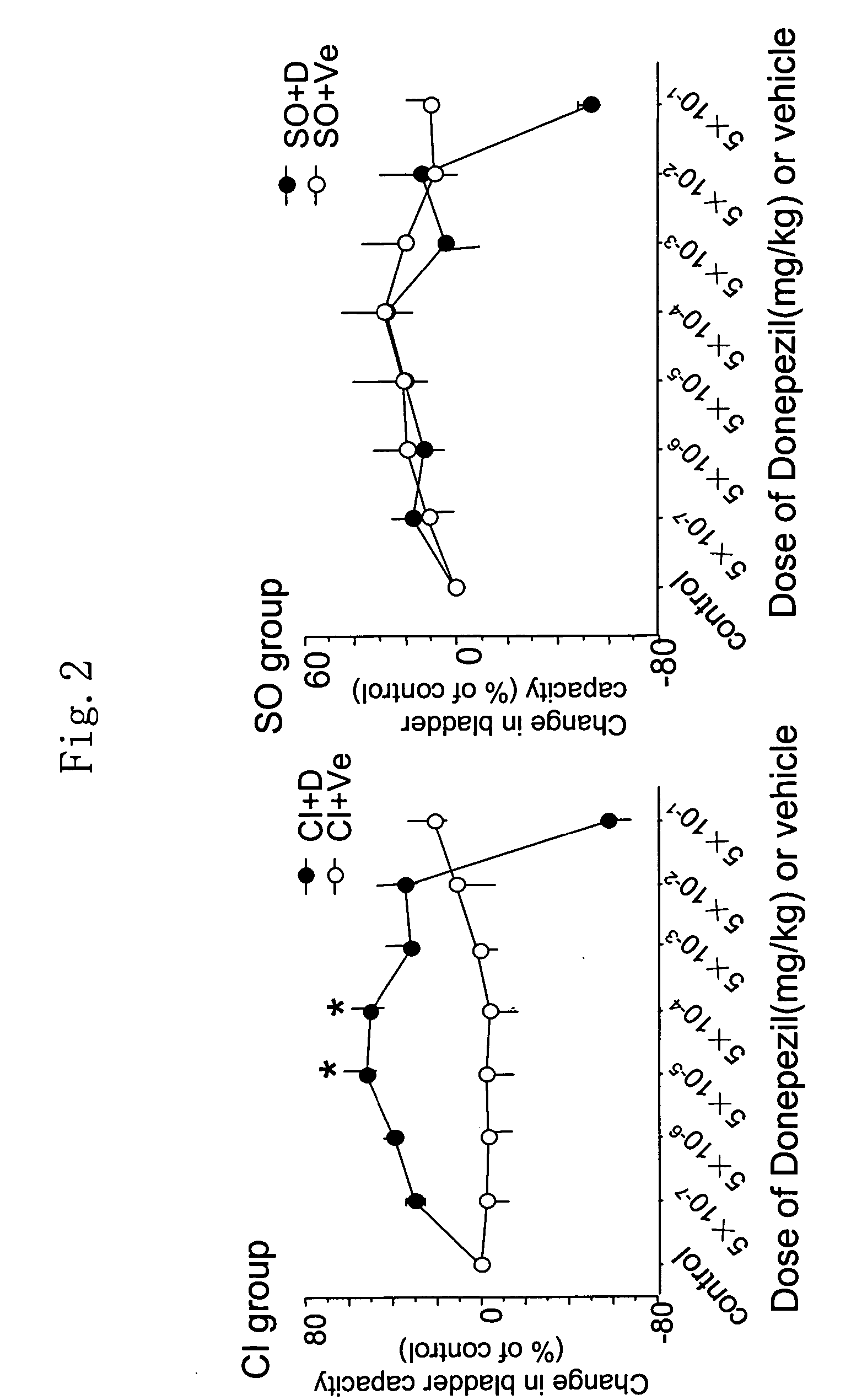
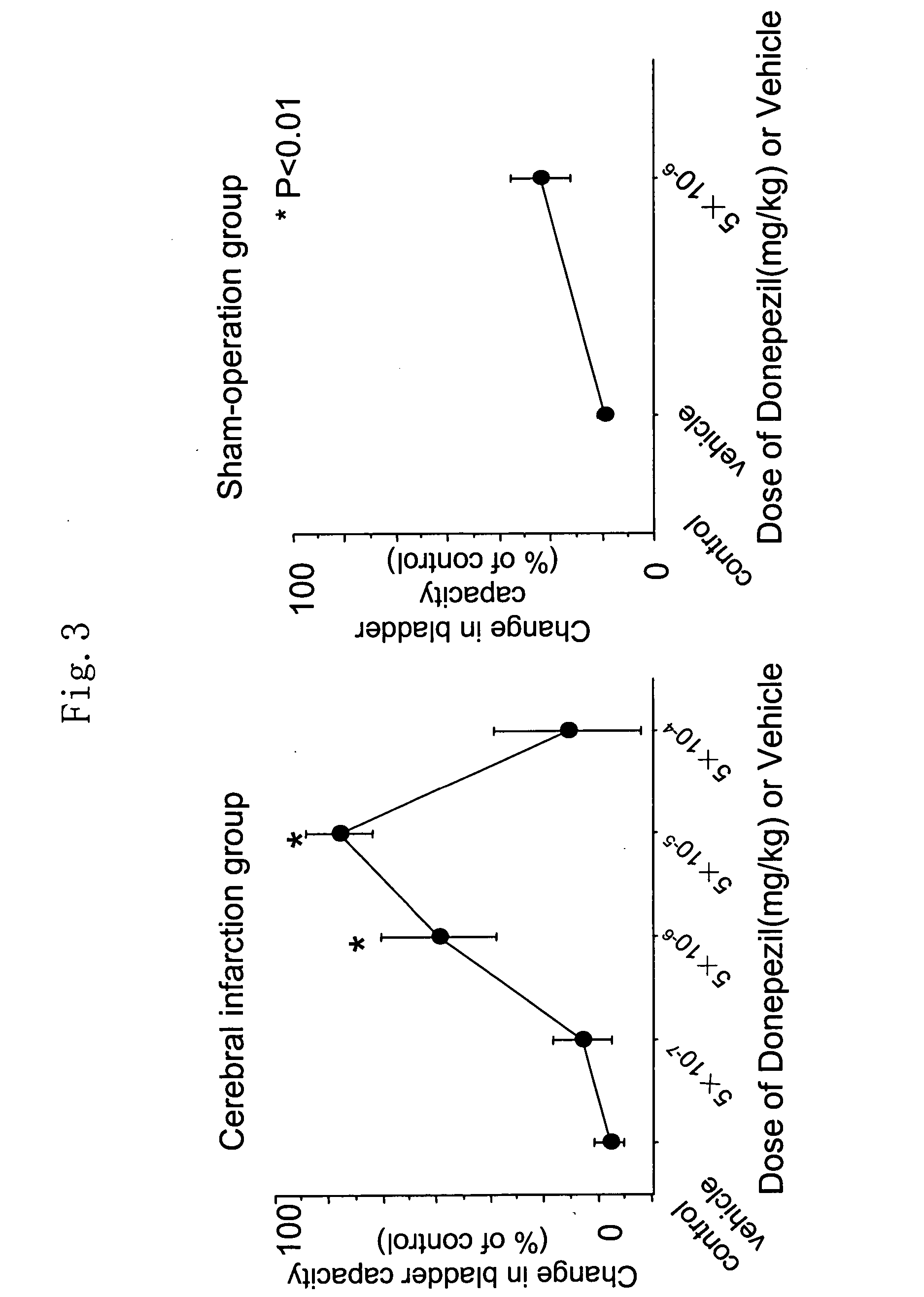
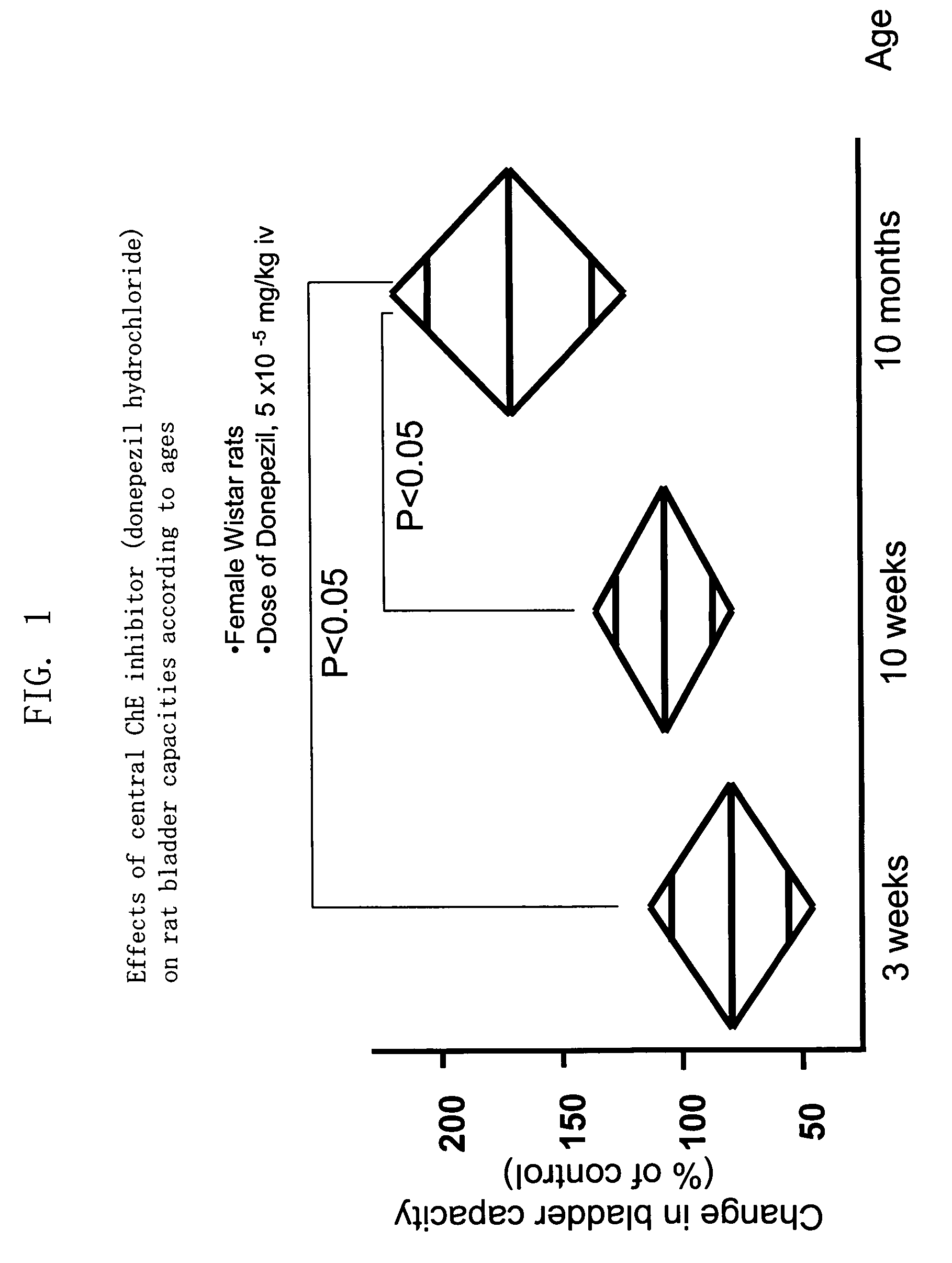
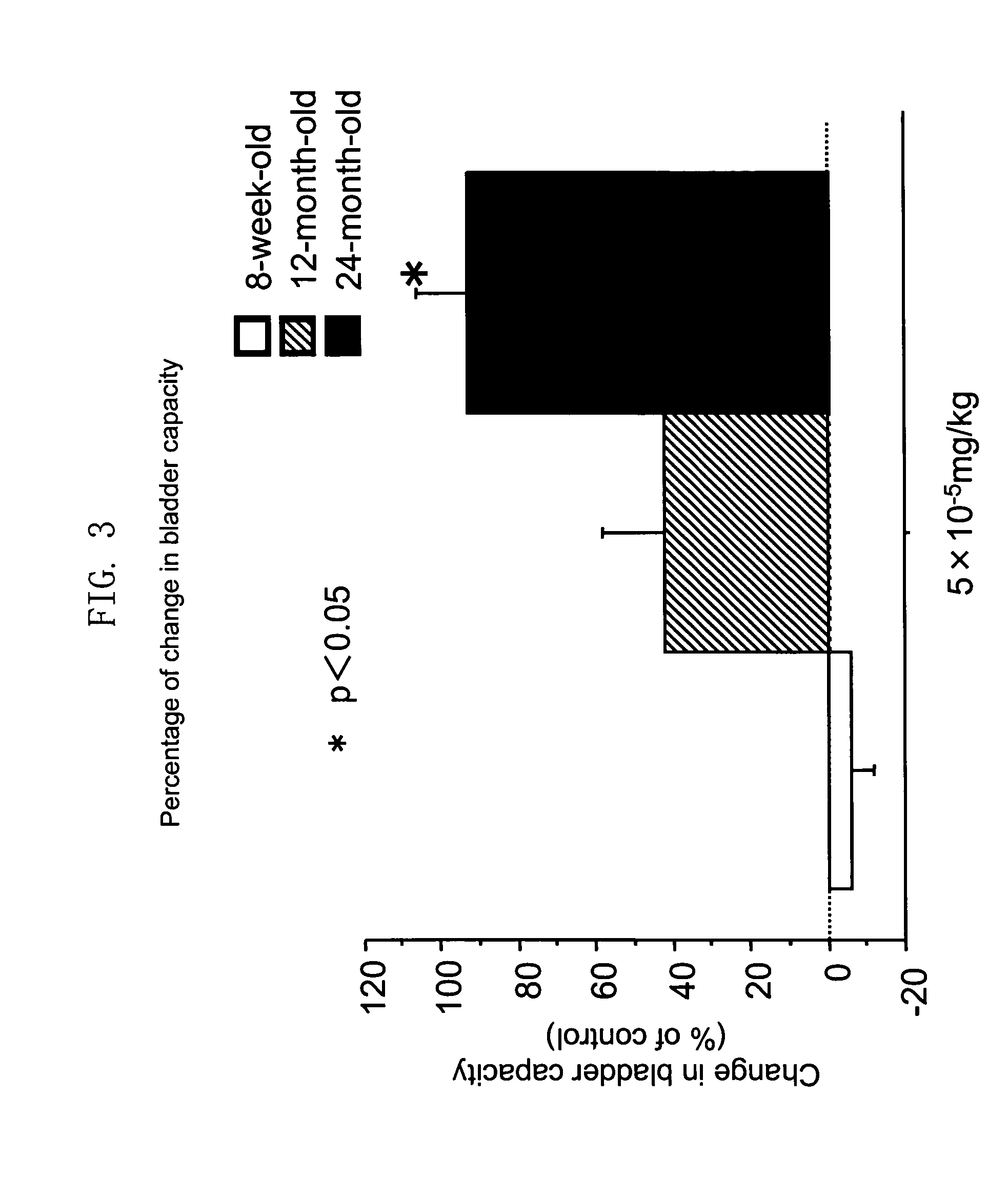
Therapeutic agent for overactive bladder resulting from cerebral infarction
An agent for treating overactive bladder resulting from cerebral infarction, comprising administrating a compound having a cholinesterase inhibitory activity or a pharmacologically acceptable salt thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
Preparation and use of compounds as aspartyl protease inhibitors
ActiveUS20070010667A1Nervous disorderMetabolism disorderImmunodeficiency virusNeuro-degenerative disease
Disclosed are compounds of the formula I or a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, wherein A is a bond, —C(O)—, or —C(R3′)(R4′)—; X is —N(R1)— or —C(R6)(R7)—; Y is —S(O)2—, —C(═O)—, —PO(OR9) or —C(R6′R7′)—; is a single or double bond and R, R1, R2, R3, R4, R3′, R4′, R5, R6, R6′, R7 and R7′ are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I. Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes. Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic antagonist.
Owner:MERCK SHARP & DOHME LLC
Heterocyclic aspartyl protease inhibitors
Disclosed are compounds of the formula Ior a stereoisomer, tautomer, or pharmaceutically acceptable salt or solvate thereof, whereinW is a bond, —C(═S)—, —S(O)—, —S(O)2—, —C(═O)—, —O—, —C(R6)(R7)—, —N(R5)— or —C(═N(R5))—;X is —O—, —N(R5)— or —C(R6)(R7)—; provided that when X is —O—, U is not —O—, —S(O)—, —S(O)2—, —C(═O)— or —C(═NR5)—;U is a bond, —S(O)—, —S(O)2—, —C(O)—, —O—, —P(O)(OR15)—, —C(═NR5)—, —(C(R6)(R7))b— or —N(R5)—; wherein b is 1 or 2; provided that when W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, U is not —S(O)—, —S(O)2—, —O—, or —N(R5)—; provided that when X is —N(R5)— and W is —S(O)—, —S(O)2—, —O—, or —N(R5)—, then U is not a bond;and R1, R2, R3, R4, R5, R6, and R7 are as defined in the specification; and pharmaceutical compositions comprising the compounds of formula I.Also disclosed is the method of inhibiting aspartyl protease, and in particular, the methods of treating cardiovascular diseases, cognitive and neurodegenerative diseases, and the methods of inhibiting of Human Immunodeficiency Virus, plasmepins, cathepsin D and protozoal enzymes.Also disclosed are methods of treating cognitive or neurodegenerative diseases using the compounds of formula I in combination with a cholinesterase inhibitor or a muscarinic m1 agonist or m2 antagonist.
Owner:PHARMACOPEIA INC +1
Neural tourniquet
ActiveUS20050282906A1BiocideNervous disorderCholinesterase inhibitionCholinergic anti-inflammatory pathway
Disclosed is a method of reducing bleed time in a subject by activation of the cholinergic anti-inflammatory pathway in said subject. The cholinergic anti-inflammatory pathway can be activated by direct or indirect stimulation of the vagus nerve. The cholinergic anti-inflammatory pathway can also be activated by administering an effective amount of cholinergic agonist or acetylcholinesterase inhibitor to the subject.
Owner:THE FEINSTEIN INST FOR MEDICAL RES
Composition containing anti-dementia drug
InactiveUS20060246003A1Improve complianceReduce the burden onIn-vivo radioactive preparationsPill deliveryCholinesteraseCholinesterase inhibition
An object of the present invention is to provide, for the case of implementing a therapeutic method in which at least two kinds of anti-dementia drugs are used together, a composition that has a good therapeutic effect on dementia, and also gives excellent compliance. Another object of the present invention is to provide a composition containing at least two kinds of anti-dementia drugs, in which release of the anti-dementia drugs from the composition is controlled, whereby a combined effect of the anti-dementia drugs can be achieved well. Still another object of the present invention is to provide a composition for which the frequency of administration and the amount taken are reduced and hence compliance can be improved, and a method of manufacturing such a composition. According to the present invention, there is provided a composition containing at least two kinds of anti-dementia drugs; such a composition containing at least one sustained-release portion containing an anti-dementia drug; and such a composition containing at least one cholinesterase inhibitor, and at least one N-methyl-D-aspartate receptor antagonist.
Owner:EISIA R&D MANAGEMENT CO LTD
Transdermal administration of huperzine
The present invention provides a composition of transdermally administered huperzine for improving memory and cognitive function. In one aspect, huperzine is delivered in a sufficient amount to achieve and maintain a blood plasma Huperzine level of about 0.5 ng / mL to about 30 ng / mL. Huperzine may be delivered by itself, or in combination with other elements, such as additional cholinesterase inhibitors, drugs or treatment agents, or positive health promoting substances. Various formulations for the transdermal delivery of huperzine are disclosed, and may include selected penetration enhancers.
Owner:XEL HERBACEUTICALS INC
Composition containing anti-dementia drug
InactiveUS20060160852A1Improve complianceSimple and convenient manufacturing methodBiocidePill deliveryCholinesteraseCholinesterase inhibition
An object of the present invention is to provide, for the case of implementing a therapeutic method in which at least two kinds of anti-dementia drugs are used together, a composition that has a good therapeutic effect on the dementia, and also gives excellent compliance. Another object of the present invention is to provide a composition containing at least two kinds of the anti-dementia drugs, for which release of the anti-dementia drugs from the composition is controlled, whereby a combined effect of the anti-dementia drugs can be achieved well. Still another object of the present invention is to provide: a composition, for which the frequency of administration and the amount taken are reduced, and hence compliance can be improved; and a method of manufacturing such a composition. According to the present invention, there are provided a composition containing at least two kinds of the anti-dementia drugs; such a composition containing at least one sustained release portion containing an anti-dementia drug; and such a composition containing at least one cholinesterase inhibitor, and at least one N-methyl-D-aspartate receptor antagonist.
Owner:EISIA R&D MANAGEMENT CO LTD
Method of reducing abeta42 and treating diseases
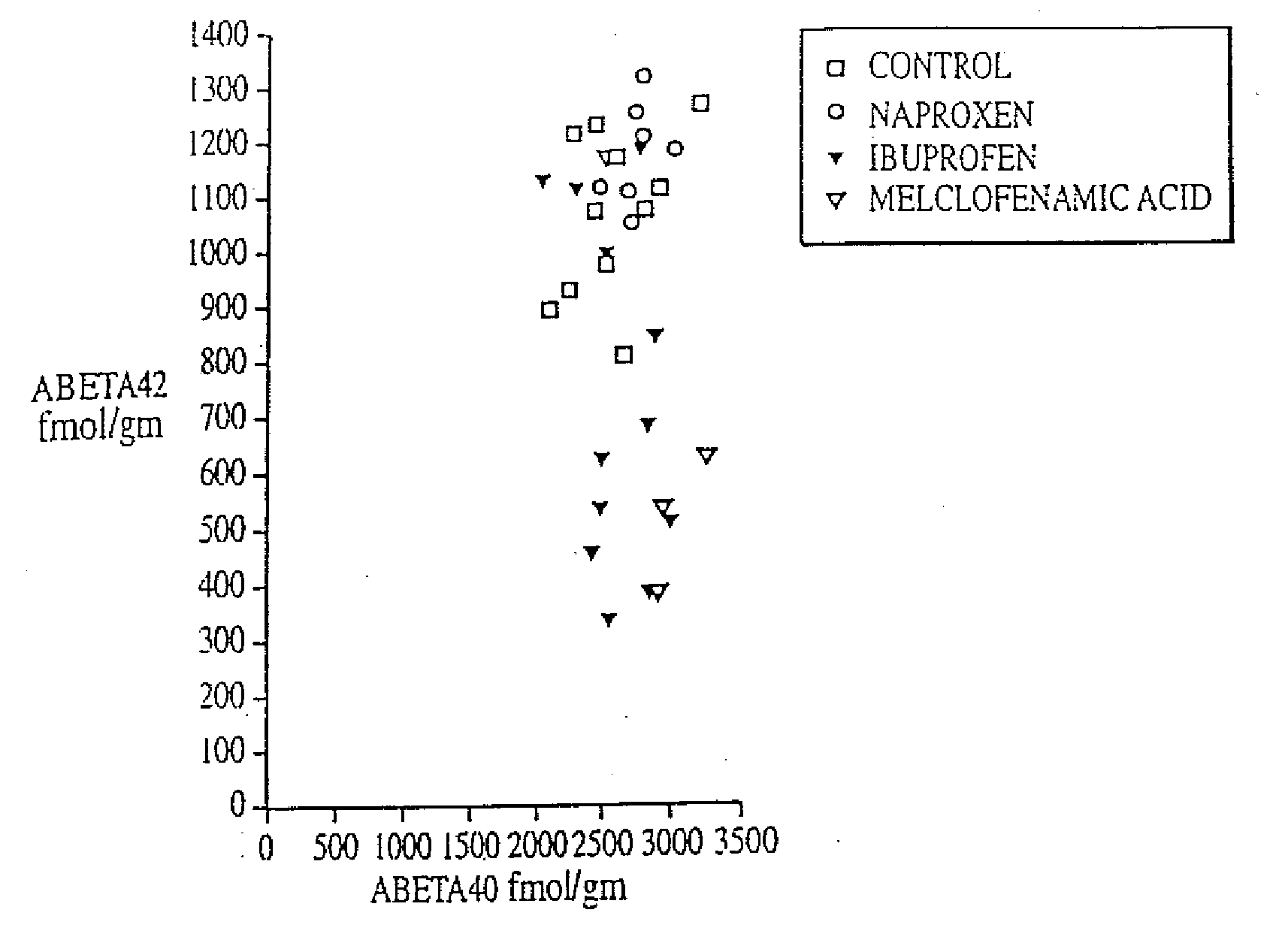
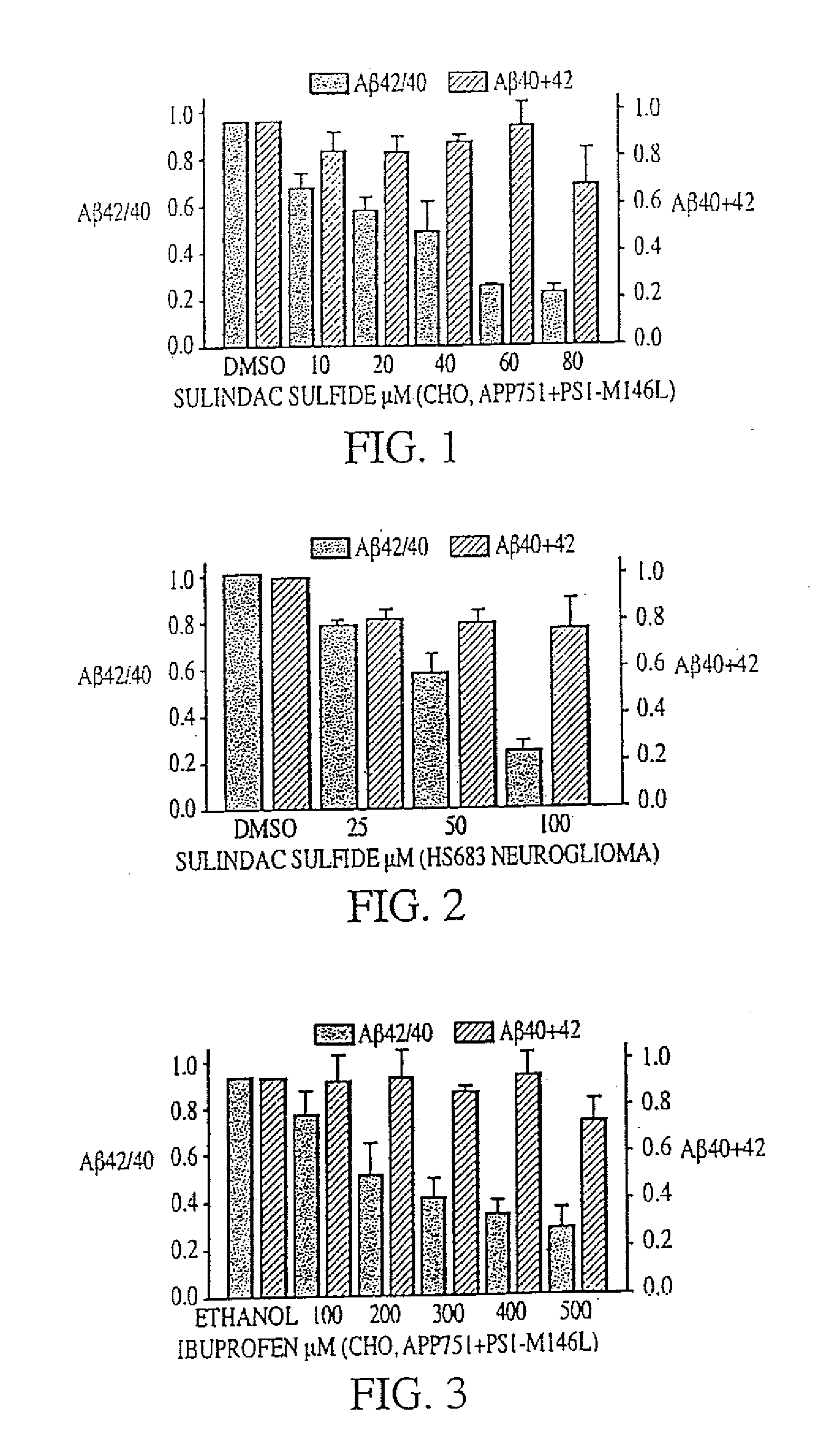
InactiveUS20080021085A1Increased risk of developingIncreased riskBiocideNervous disorderCholinesterase inhibitionRegimen
The invention provides a method of preventing, delaying, or reversing the progression of Alzheimer's disease by administering an Aβ42 lowering agent to a mammal under conditions in which levels of Aβ42 are selectively reduced, levels of Aβ38 are increased, and levels of Aβ40 are unchanged. The invention provides methods and materials for developing and identifying Aβ42 lowering agents. In addition, the invention provides methods for identifying agents that increase the risk of developing, or hasten progression of, Alzheimer's disease. The invention also provides compositions of Aβ42 lowering agents and antioxidants, Aβ42 lowering agents and non-selective secretase inhibitors, as well as Aβ42 lowering agents and acetylcholinesterase inhibitors. The invention also provides kits containing Aβ42 lowering agents, antioxidants, non-selective secretase inhibitors, and / or acetylcholinesterase inhibitors as well as instructions related to dose regimens for Aβ42 lowering agents, antioxidants, non-selective secretase inhibitors, and acetylcholinesterase inhibitors.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES +1
Cholinergic enhancers with improved blood-brain barrier permeability for the treatment of diseases accompanied by cognitive impairment
InactiveUS20090253654A1Improve efficacyLow peripheral side effectBiocideGroup 5/15 element organic compoundsChemical structureChemical synthesis
The present invention refers to compounds that, in addition to enhancing the sensitivity to acetylcholine and choline, and their exogenous agonists, of neuronal cholinergic receptors and / or acting as cholinesterase inhibitors and / or neuroprotective agents, have enhanced blood-brain barrier permeability in comparison to their parent compounds. The compounds are derived (either formally by their chemical structure or directly by chemical synthesis) from natural compounds belonging to the class of amaryllidaceae alkaloids e.g., galantamine, narwedine and lycoramine, or from metabolites of said compounds. The compounds of the present invention can either interact as such with their target molecules, or they can act as “pro-drugs”, in the sense that after reaching their target regions in the body they are converted by hydrolysis or enzymatic attack to the original parent compound and react as such with their target molecules, or both. The compounds of this invention may be used as medicaments.
Owner:GALANTOS PHARMA
Method for the treatment of cognitive dysfunction
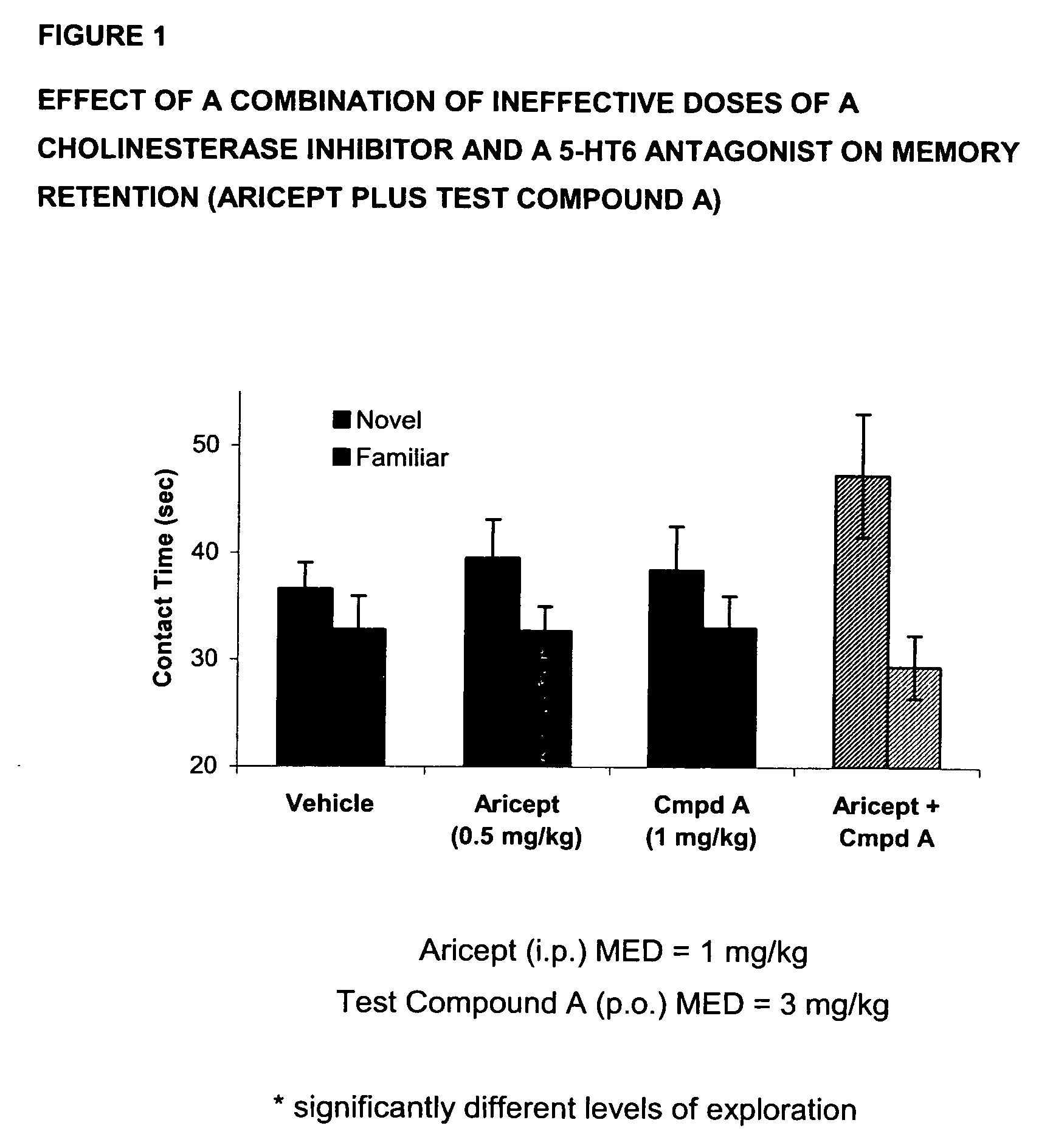
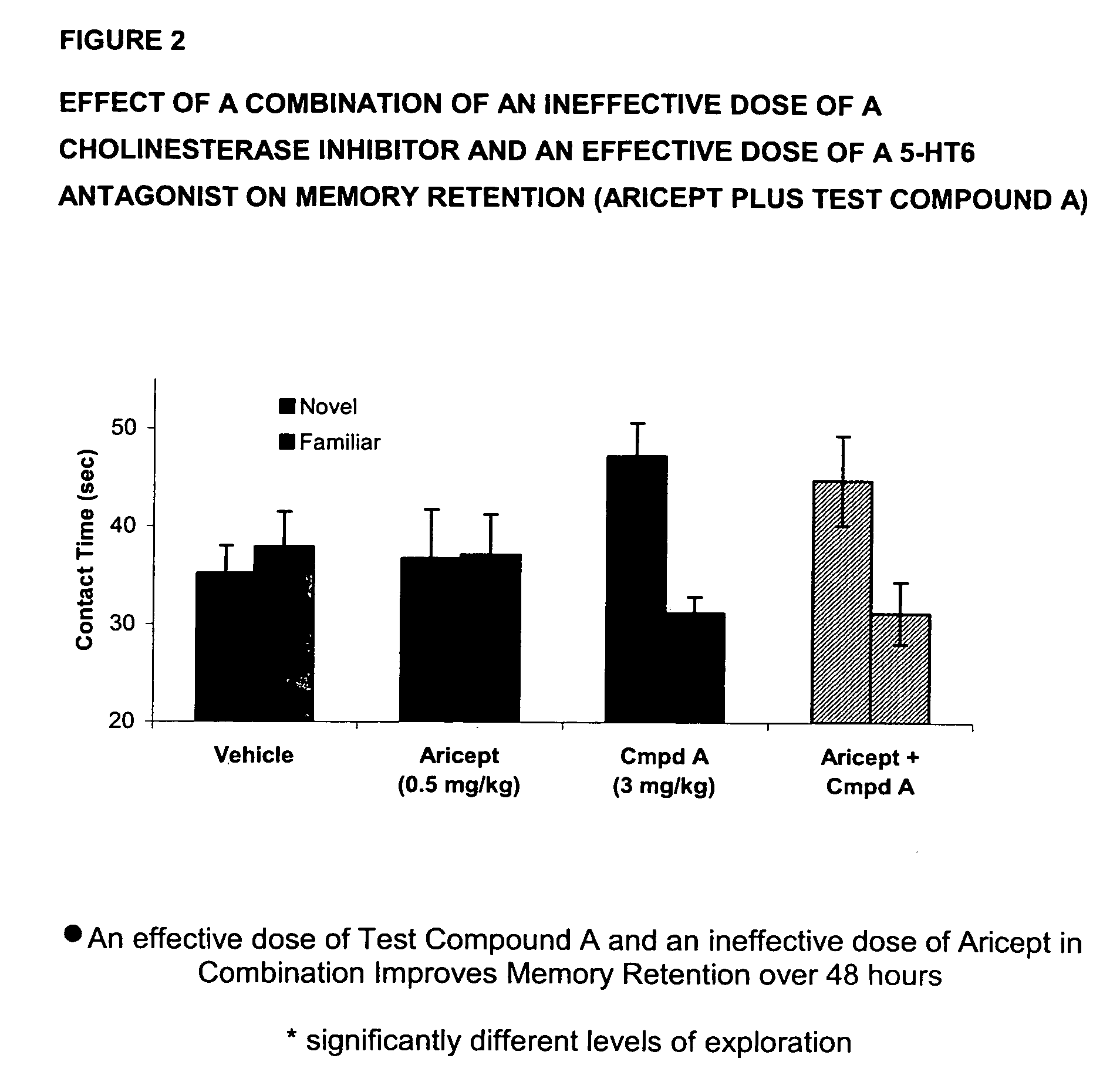
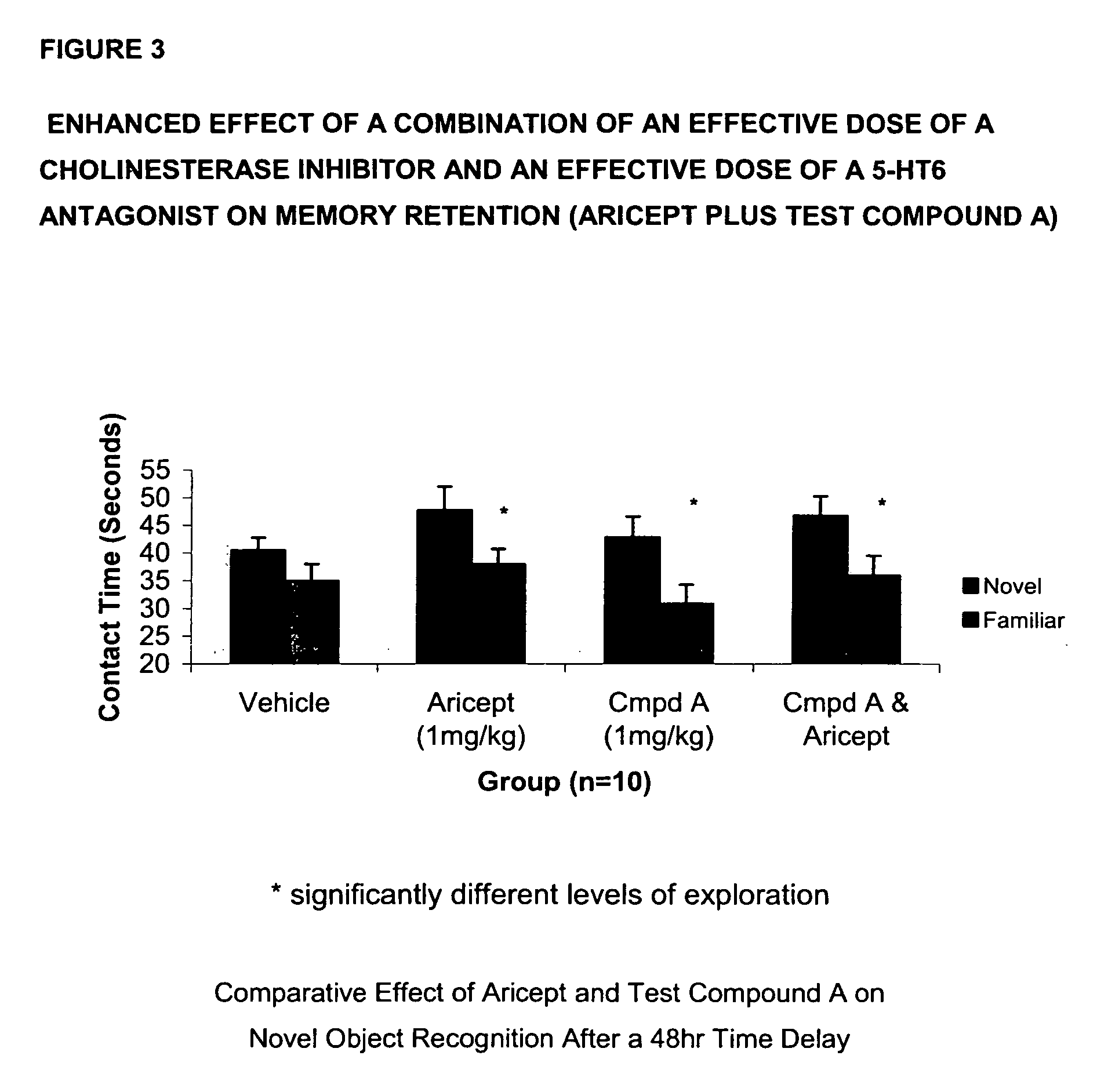
The present invention provides a method for the treatment of a cognitive disorder such as Alzheimer's disease in a patient in need thereof which comprises providing to said patient a therapeutically effective amount of a combination of an acetylcholinesterase inhibitor and a 5-hydroxytryptamine-6 antagonist.
Owner:WYETH LLC
Combined Acetylcholinesterase Inhibitor and Quaternary Ammonium Antimuscarinic Therapy to Alter Progression of Cognitive Diseases
ActiveUS20130172398A1Prevents or substantially ameliorates the undesired side effects of acetyl-cholinesteraseMaximize the effectBiocideAmine active ingredientsDementia with Lewy bodiesPsychiatry
A method administers quaternary ammonium anti-cholinergic muscarinic receptor antagonists in combination with acetyl-cholinesterase inhibitors to treat either cognitive impairment or acute delirium. This therapy results in a modification of a cognitive disorder or disease, namely a slow down in the disease progression. In one preferred embodiment, the disease is dementia with Lewy Bodies. New formulations for quaternary ammonium anti-cholinergic muscarinic receptor antagonists are also disclosed.
Owner:QAAM PHARMA LLC
Methods of treating alzheimer's disease and pharmaceutical compositions thereof
ActiveUS20140073681A1Good effectImprove and augment effectBiocideNervous disorderCholinesterase inhibitionBenzylamine
The present invention describes methods of treating dementia comprising administering an effective daily dose of N-(2-(6-fluoro-1H-indol-3-yl)ethyl-(2,2,3,3-tetrafluoropropoxy)benzylamine to improve or augment the effect of an acetylcholinesterase inhibitor.
Owner:H LUNDBECK AS
Therapeutic agent for overactive bladder involved in aging
A method for treating overactive bladder involved in aging, comprising administrating a compound having a cholinesterase inhibitory activity, a pharmacologically acceptable salt or a solvate thereof to a patient with the overactive bladder involved in aging.
Owner:EISIA R&D MANAGEMENT CO LTD
Dihydropyridine compounds and compositions for headaches
The invention provides methods for treating and / or preventing headaches by administering to patients therapeutically effective amounts of 1,2-dihydropyridine compounds, and, optionally, cholinesterase inhibitors and / or anti-migraine agents. The headaches may be primary headaches, such as migraines, or secondary headaches. The invention also provides combinations, commercial packages, and pharmaceutical compositions comprising therapeutically effective amounts of 1,2-dihydropyridine compounds and, optionally, cholinesterase inhibitors and / or anti-migraine agents. The 1,2-dihydropyridine compound may be, for example, 3-(2-cyanophenyl)-5-(2-pyridyl)-1-phenyl-1,2-dihydropyridin-2-one. The cholinesterase inhibitor may be, for example, 1-benzyl-4-((5,6-dimethoxy-1-indanon)-2-yl)methylpiperidine.
Owner:EISIA R&D MANAGEMENT CO LTD
Nerve Regeneration Stimulator
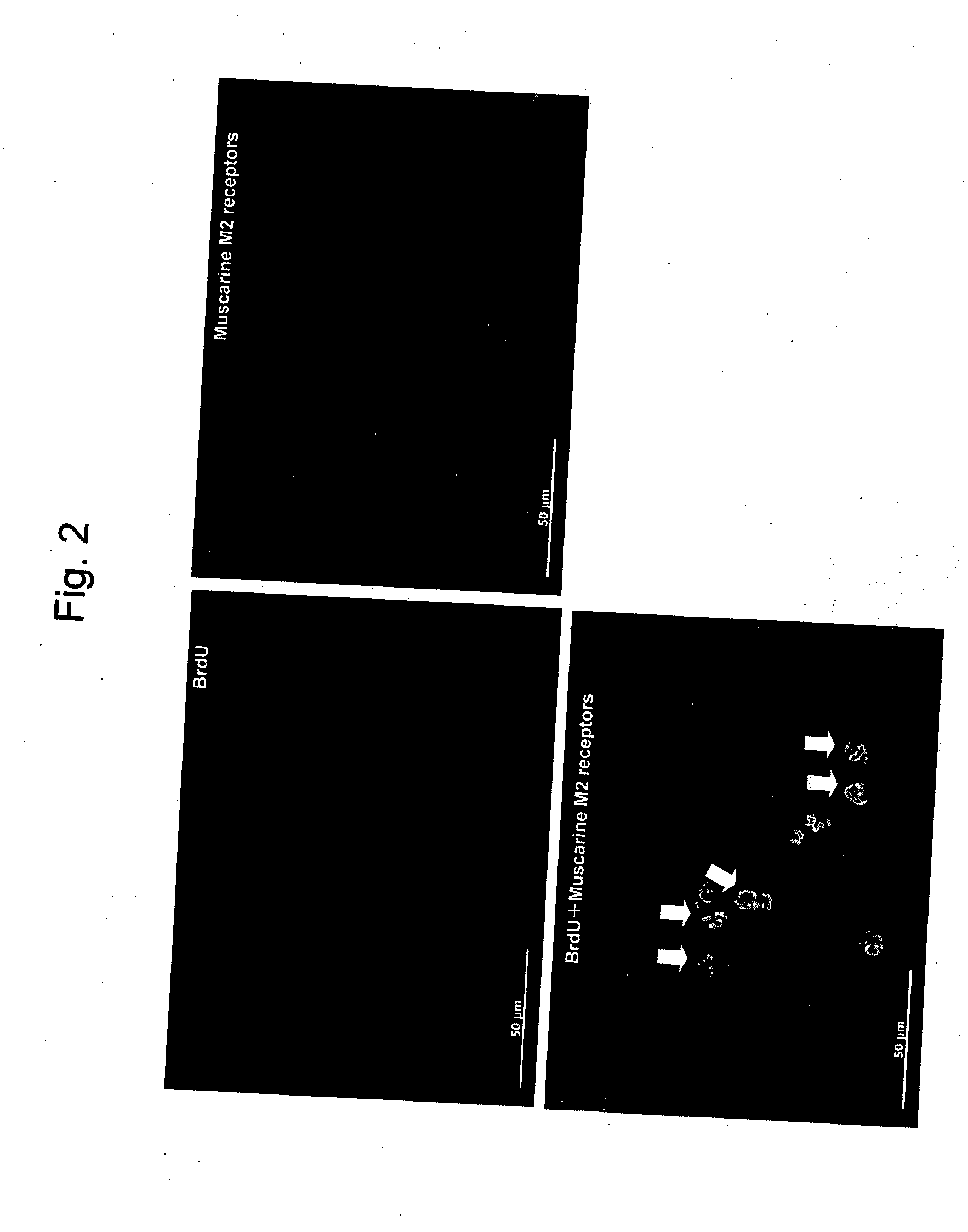
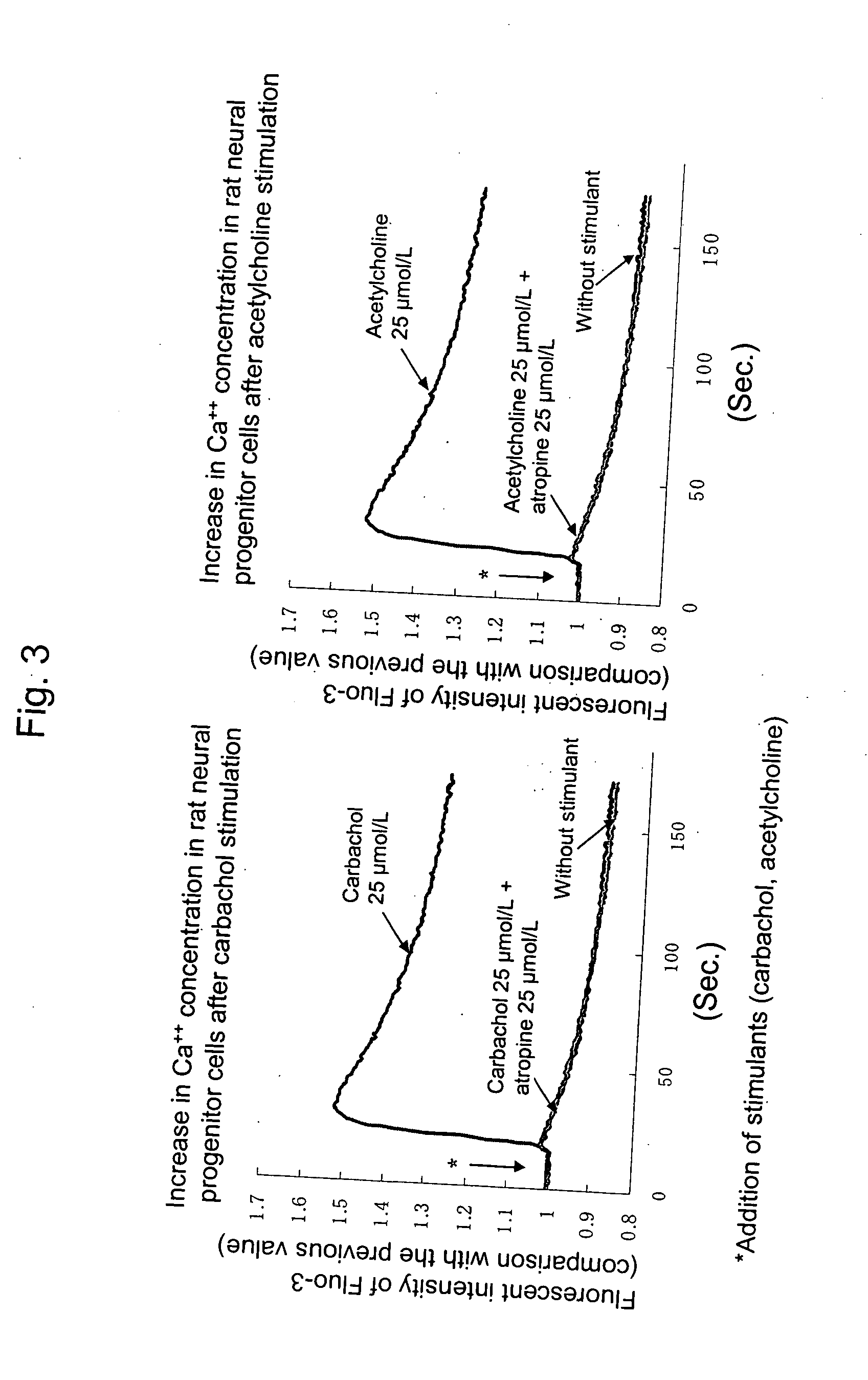
InactiveUS20080045500A1Efficiently nerve regenerationPromote nerve regenerationBiocideCompound screeningCholinesteraseCholinesterase inhibition
A nerve regeneration stimulator comprising a compound having a cholinesterase inhibitory activity, a pharmacologically acceptable salt thereof or a solvate thereof.
Owner:EISIA R&D MANAGEMENT CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com