Cholinergic enhancers with improved blood-brain barrier permeability for the treatment of diseases accompanied by cognitive impairment
a blood-brain barrier and enhancer technology, applied in the field of cholinergic enhancers with improved blood-brain barrier permeability for the treatment of diseases accompanied by cognitive impairment, can solve the problems of limited bioavailability, poor pharmacodynamic effects, and a small amount of galantamine in the brain, and achieve low peripheral side effects and high pharmacodynamic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
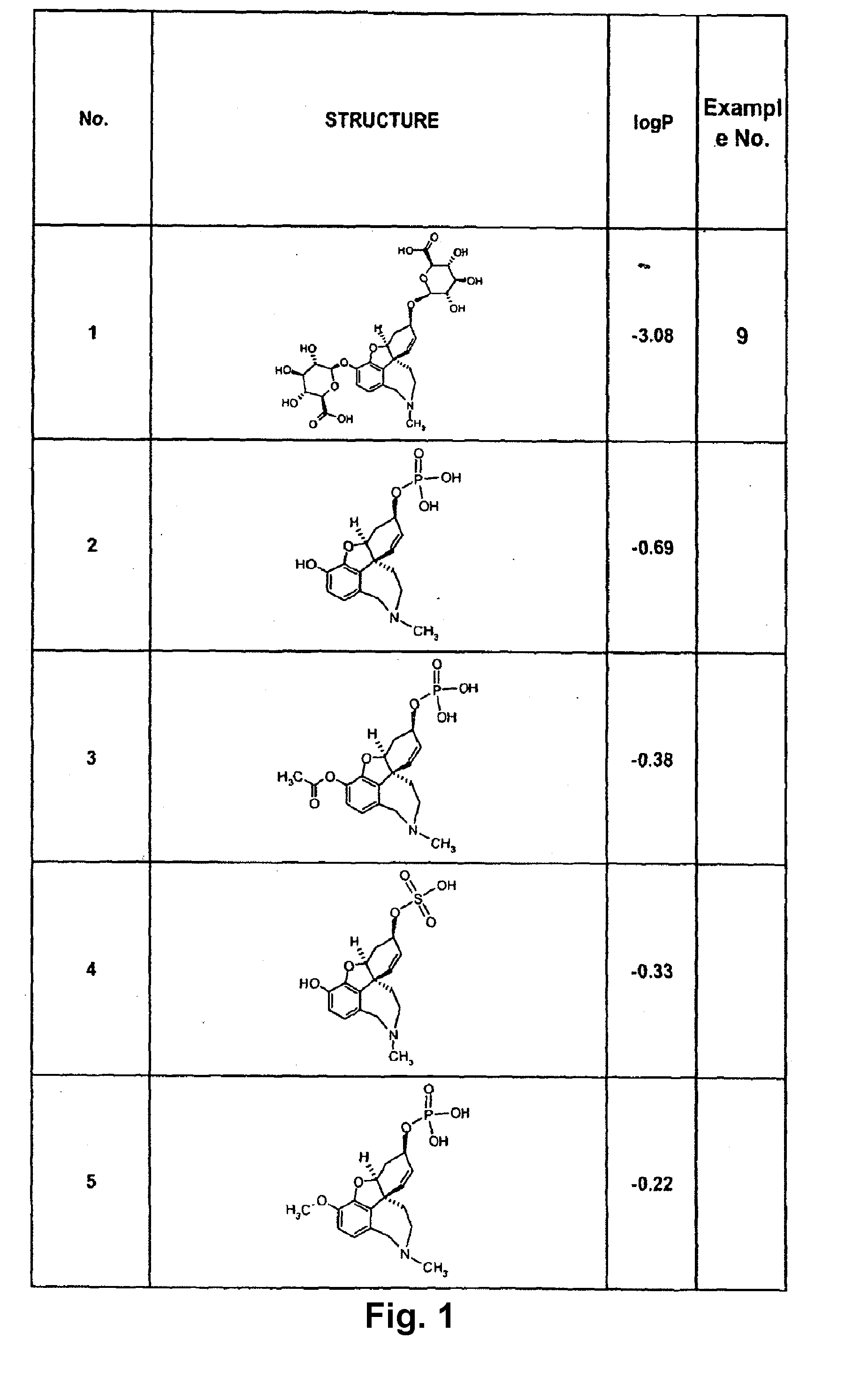
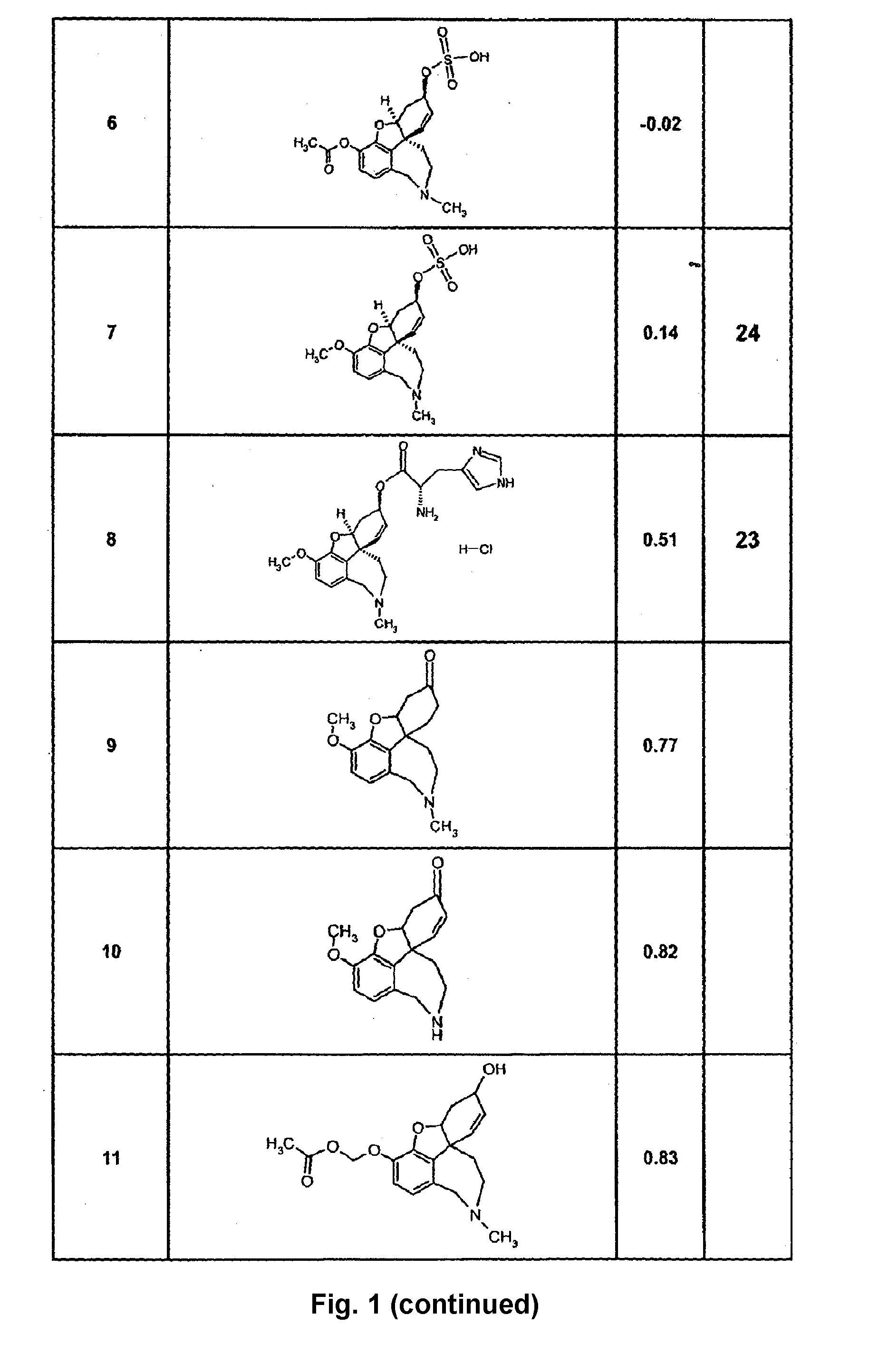
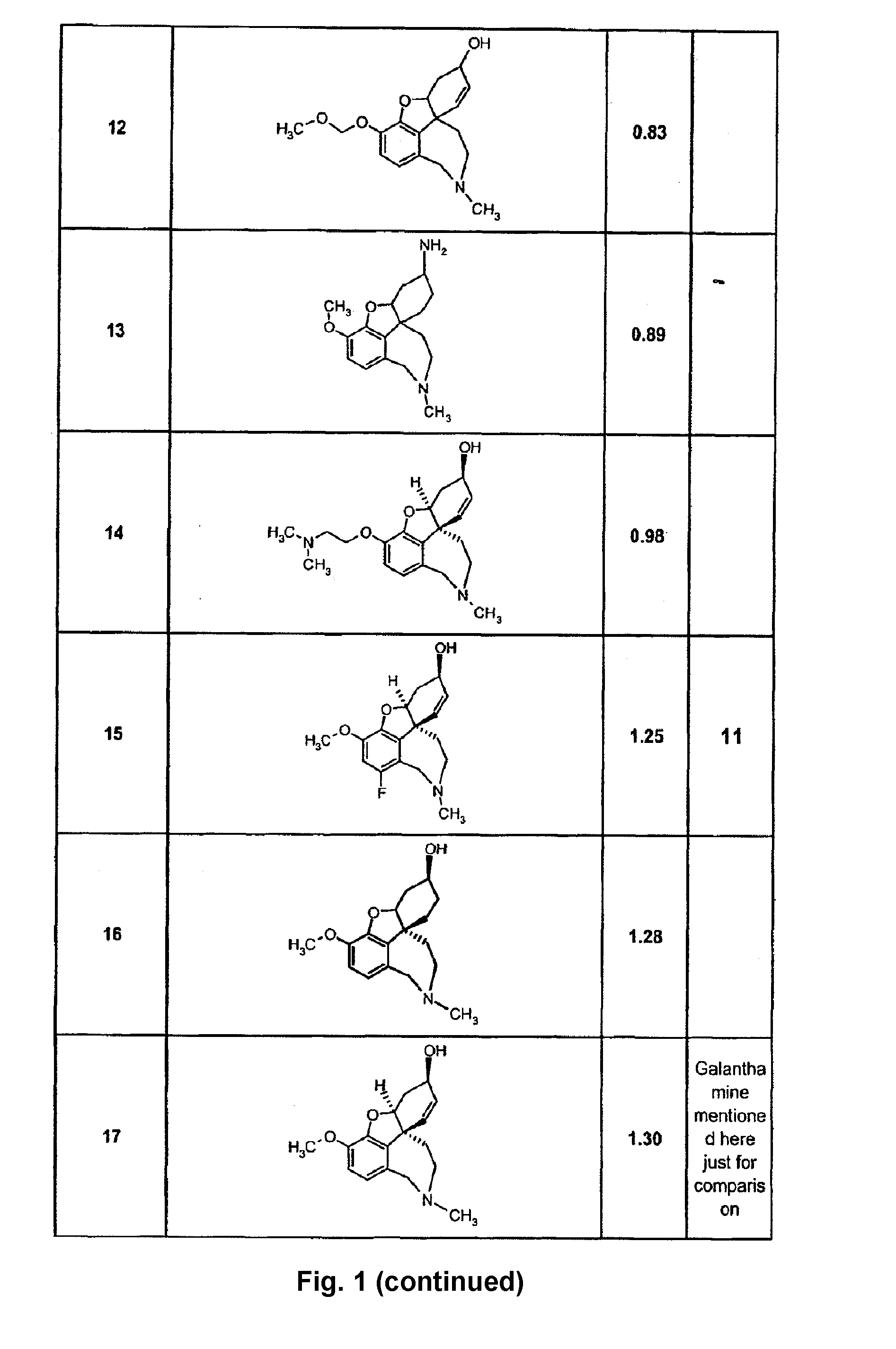
Image
Examples
example 1
N-Methoxymethyl-galanthaminiumchloride(=(4aS,6R,8aS)-4a,5,9,10,11,12-Hexahydro-11-methoxymethyl-11-methyl-6H-6-hydroxy-3-methoxy-benzofuro[3a,3,2-ef][2]benzaze-pinium, chloride)
[0204]N-Methoxymethyl-galanthaminiumchloride is obtained from Galantamine via alkylation using chloromethylmethylether:
[0205]To a solution of (−)-Galantamine (5.00 g, 17.4 mmol) in dry dimethylformamide (12 mL) chloromethylmethylether (1.12 g, 13.9 mmol) is added at −5 bis 0° C. in the course of 15 min and stirred for 4 hrs. at room temperature. The reaction mixture is poured on ethyl acetatet (500 mL) and the precipitate obtained is filtered and washed using ethyl acetate (3×50 mL).
[0206]The crude product (4.20 g, 82%) has a purity of 96% (HPLC). For further purification the crude product is dissolved in dry ethanol, stirred after the addition of activated charcoal, filtered and added to ethyl acetate (500 mL). The precipitate is filtered and washed using ethyl acetate (3×50 mL) and dry diethylether (1×50 mL...
example 2
Tert-Butoxycarbonylamino-acetic acid (N-norgalanthaminyl)-methyl ester
[0211]
[0212]To a solution of N-Boc-glycine chloromethylester (1.0 mmol) and norgalantamine (1.0 mmol) in dry DMF (2.0 mL) triethylamine (3 mmol) was added dropwise and the reaction stirred under nitrogen for 3 days. The triethylammonium chloride formed was filtered and washed with dry ether and the filtrate rotoevaporated to dryness. The residue was redissolved in dry acetone (2 ml) upon heating and left to stand overnight at 4° C. for additional precipitation of the triethylammonium salt. After renewed filtration and rotoevaporation the mixture was chromatographed on silica using ethyl acetate / petrol ether. The target product was isolated as an oil.
[0213]13H NMR (DMSO-d6) δ 28.5, 33.9, 37.9, 42.0, 48.2, 51.2, 56.2, 56.9, 61.9, 79.5, 79.9, 88.8, 111.8, 121.3, 126.6, 129.8, 130.8, 133.6, 145.8, 148.2, 156.3, 169.6.
example 3
2-tert-Butoxycarbonylamino-3-phenylpropionic acetic acid (N-norgalantha-minyl)-methyl ester
[0214]
[0215]This compound was prepared using the procedure of example 2 with N-Boc-phenylalanine chloromethylester.
[0216]13H NMR (DMSO-d6) δ 28.5, 33.9, 36.9, 37.9, 48.2, 51.2, 54.6, 56.2, 56.9, 61.9, 79.5, 80.2, 88.8, 111.8, 121.3, 126.0, 126.6, 127.8, 128.7, 129.8, 130.8, 133.6, 139.5, 145.8, 148.2, 4156.0, 171.6.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com