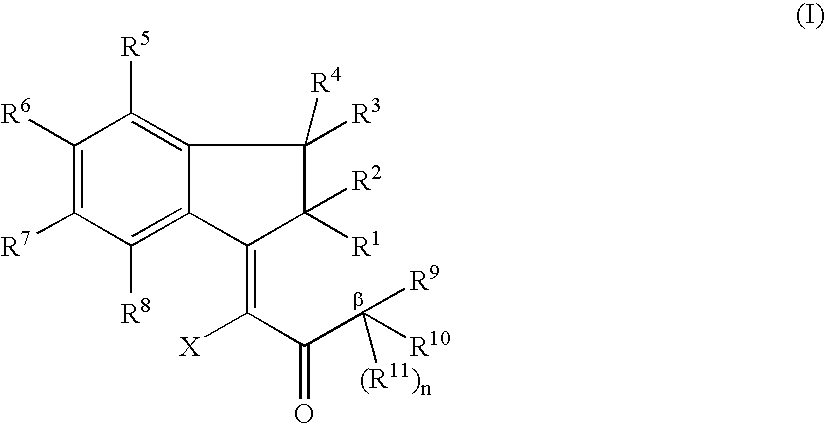
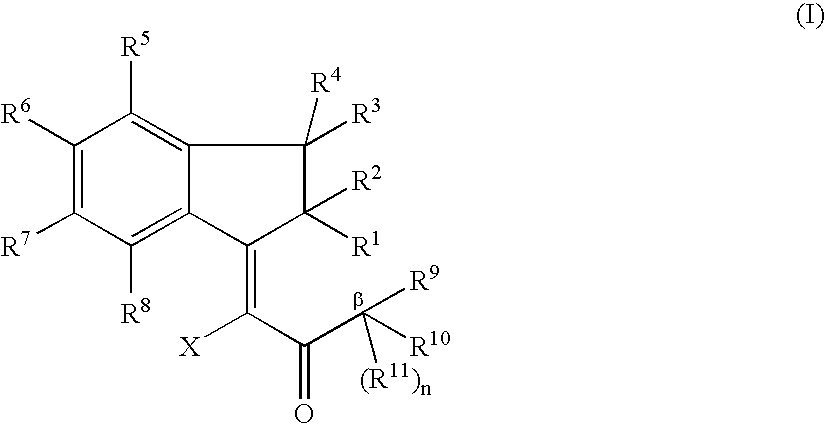
Novel Indanone Compounds
a technology of indanylidene and compounds, which is applied in the field of new indanylidene compounds, can solve the problems of too low photostability for application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
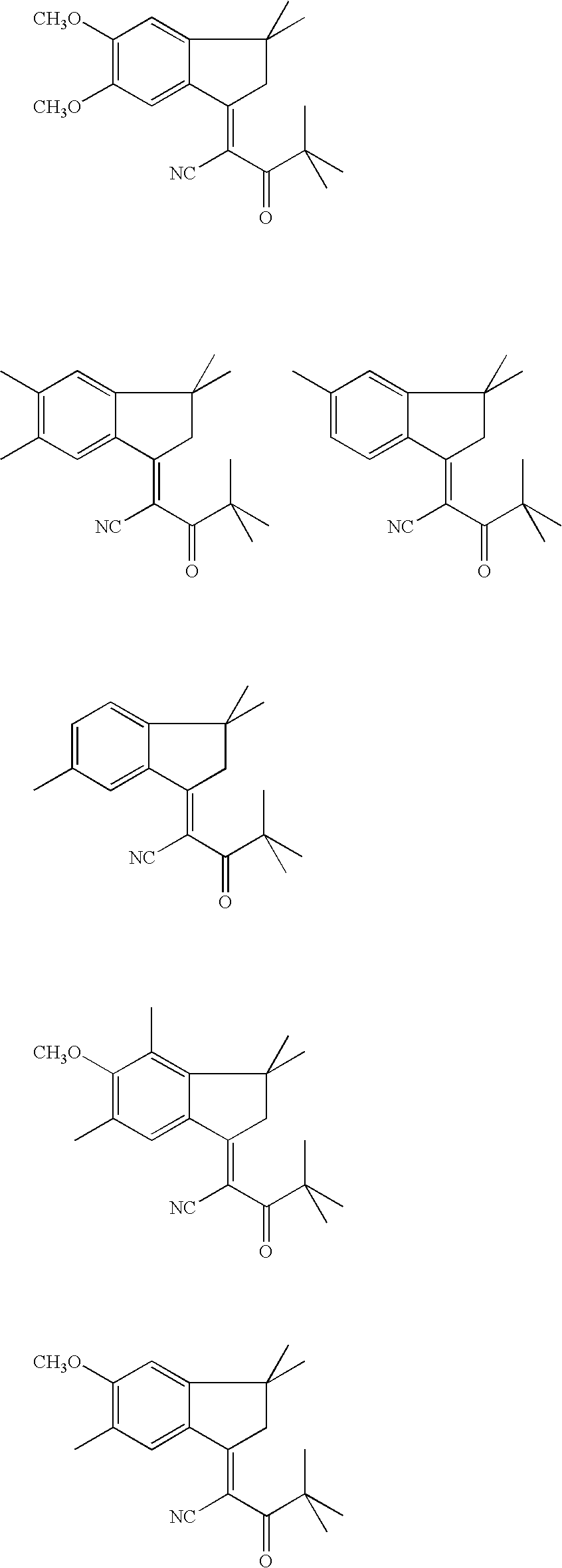
example 1
2-(5,6-Dimethoxy-3,3-dimethyl-1-indanylidene)-4,4-dimethyl-3-oxo-pentanonitrile
[0196]
44 g (0.2 mol) of 5,6-dimethoxy-3,3-dimethyl-1-indanone, 25 g (0.2 mol) of pivaloylacetonitrile, 32 g of propionic acid and 17 g of ammonium acetate are mixed in 80 g of xylene and heated at 120° C. for 7 hours. After the system has been cooled to room temperature and the organic phase has been washed, the xylene is distilled off, and the crude product which remains is recrystallized in methanol. Yield: 50% theory; E1 / 1 730 (λmax 373 nm).
example 2
2-(5-Methoxy-3,3,4,6-tetramethyl-1-indanylidene)-4,4-dimethyl-3-oxo-pentanonitrile
[0197]
[0198] The procedure was analogous to that in Example 1 starting from 5-methoxy-3,3,4,6-tetramethyl-1-indanone. Yield: 50% of theory; E1 / 1 588 (λmax 340 nm).
example 3
2-(3,3,5,6-tetramethyl-1-indanylidene-4,4-dimethyl-3-oxo-pentanonitrile
[0199]
[0200] The procedure was analogous to that in Example 1 starting from 3,3,5,6-tetramethyl-1-indanone. Yield: 55% of theory; E1 / 1 630 (λmax 342 nm).
PUM
| Property | Measurement | Unit |
|---|---|---|
| critical wavelength λamt | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com