A-pi-D-pi-A type small molecule solar cell receptor material based on thiophene indenone and fluorene and preparation method of A-pi-D-pi-A type small molecule solar cell receptor material
A solar cell and thienindanone technology, which is applied in the fields of electrical solid-state devices, semiconductor/solid-state device manufacturing, circuits, etc., can solve the problems of insufficient molecular design, synthesis route optimization, low photovoltaic efficiency, single structure, etc., and achieve stable photochemistry properties, high molar extinction coefficient, strong UV absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Synthesis of target molecule FTC1
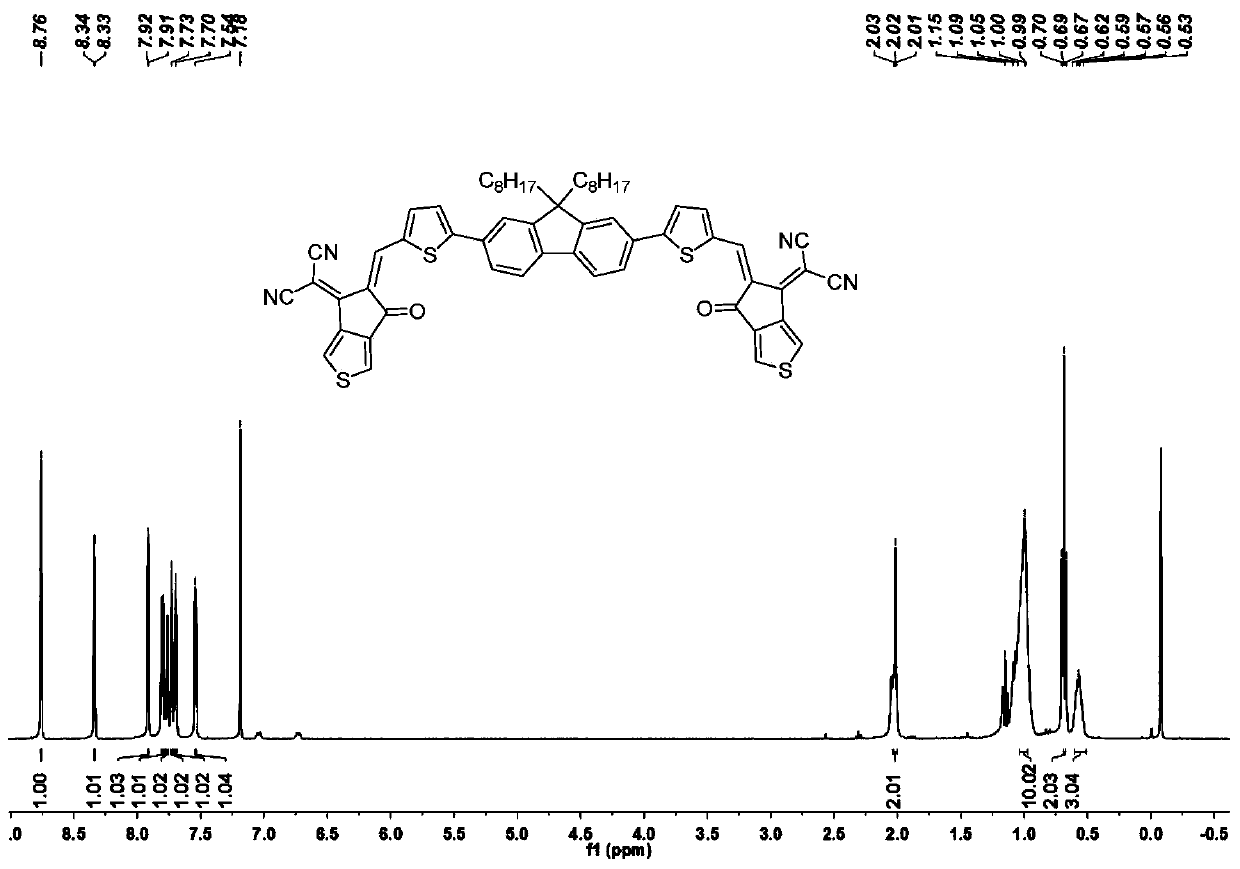
[0063] Add intermediate 2 (152mg, 0.25mmol), thienindanone (200mg, 1mmol), chloroform (20mL) and magnetons into a dry and clean 50mL three-necked flask, stir and dissolve the reactants, and evacuate the system into a vacuum state. And replace with argon, add pyridine (1mL) under argon atmosphere, react at room temperature for 12h, point the plate to monitor until the reaction is complete, add 10mL of water to quench the reaction, let the system cool to room temperature, and extract with an appropriate amount of dichloromethane for 3 -5 times, then washed with an appropriate amount of saturated NaCl solution for 3-5 times, kept the organic phase and added an appropriate amount of anhydrous MgSO 4 The powder was dried, excess solvent was removed, separation and purification by silica gel column chromatography (petroleum ether:dichloromethane:ethyl acetate=12:2:1) gave dark blue solid powder target molecule FTC1 (146mg, 60%). 1 HNMR (40...
Embodiment 2
[0065] Synthesis of target molecule FTC2
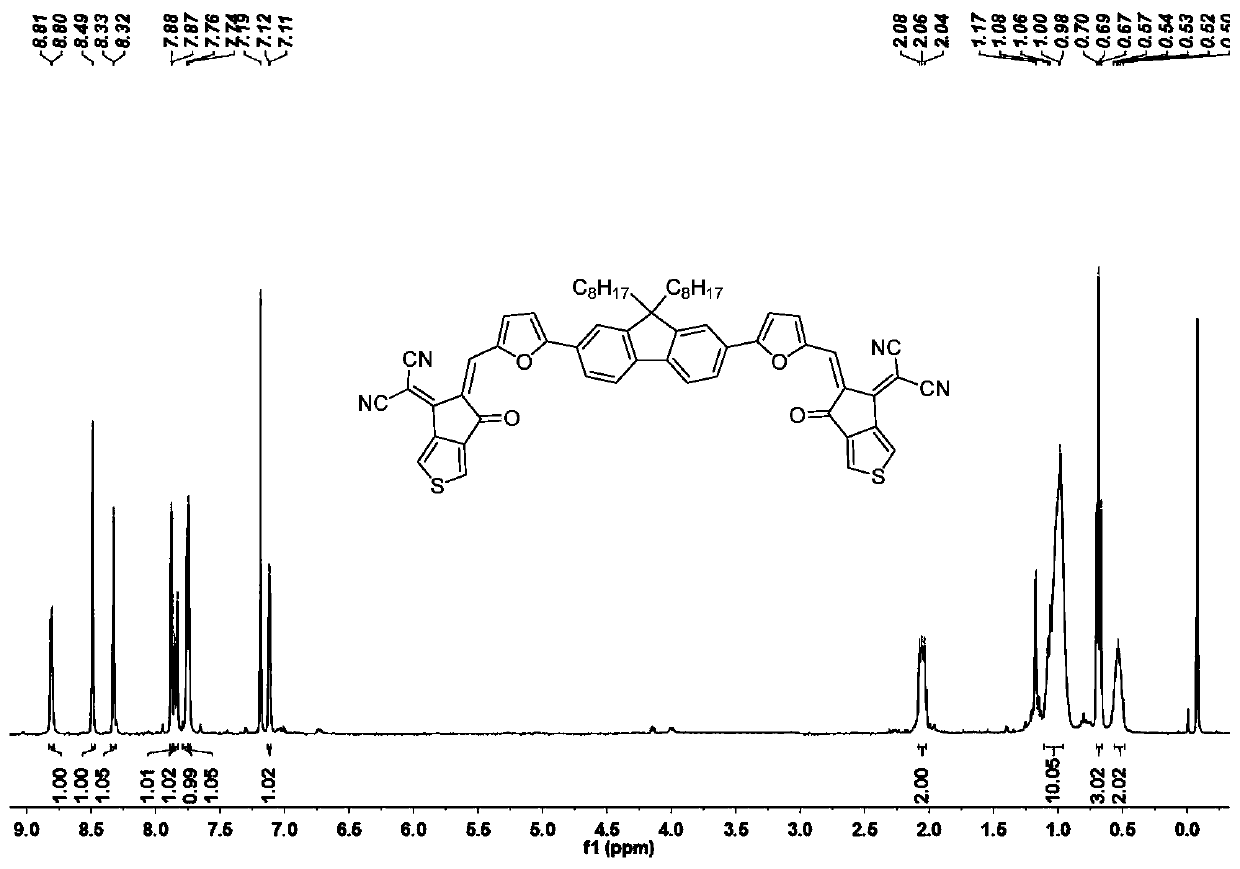
[0066] The synthesis method of the target molecule FTC2 is similar to that of the target molecule FTC1. Intermediate 3 (145 mg, 0.25 mmol) and thienindanone (200 mg, 1 mmol) were used as reactants to separate and purify the target molecule FTC2 (148 mg, 63 %). 1 H NMR (400MHz, CDCl 3 )δ: 8.81(d, J=3.9Hz, 1H), 8.49(s, 1H), 8.33(d, J=2.2Hz, 1H), 7.88(d, J=2.2Hz, 1H), 7.84(d, J=8.8Hz,1H),7.76(s,1H),7.74(s,1H),7.12(d,J=4.0Hz,1H),2.08–2.02(m,2H),1.03(dd,J=32.5 ,5.7Hz,10H),0.69(t,J=7.0Hz,3H),0.52(dd,J=13.1,6.6Hz,2H). 13 C NMR (101MHz, CDCl 3 )δ:162.88,156.63,152.58,150.87,143.58–142.08,131.92,130.16,129.16,127.93,127.67,125.48,121.18,119.88,115.12,114.15,112.51,107.98,77.21,76.86,76.73,70.02–68.05,67.87 , 55.86, 40.21, 32.89–32.10, 31.76, 29.51, 23.84, 22.58, 14.07. MALDI-TOF-MS, m / z: calcd for C 59 h 50 N 4 o 4 S 2 [M] + :942.327; found 943.525.
Embodiment 3
[0068] Synthesis of target molecule FTC3
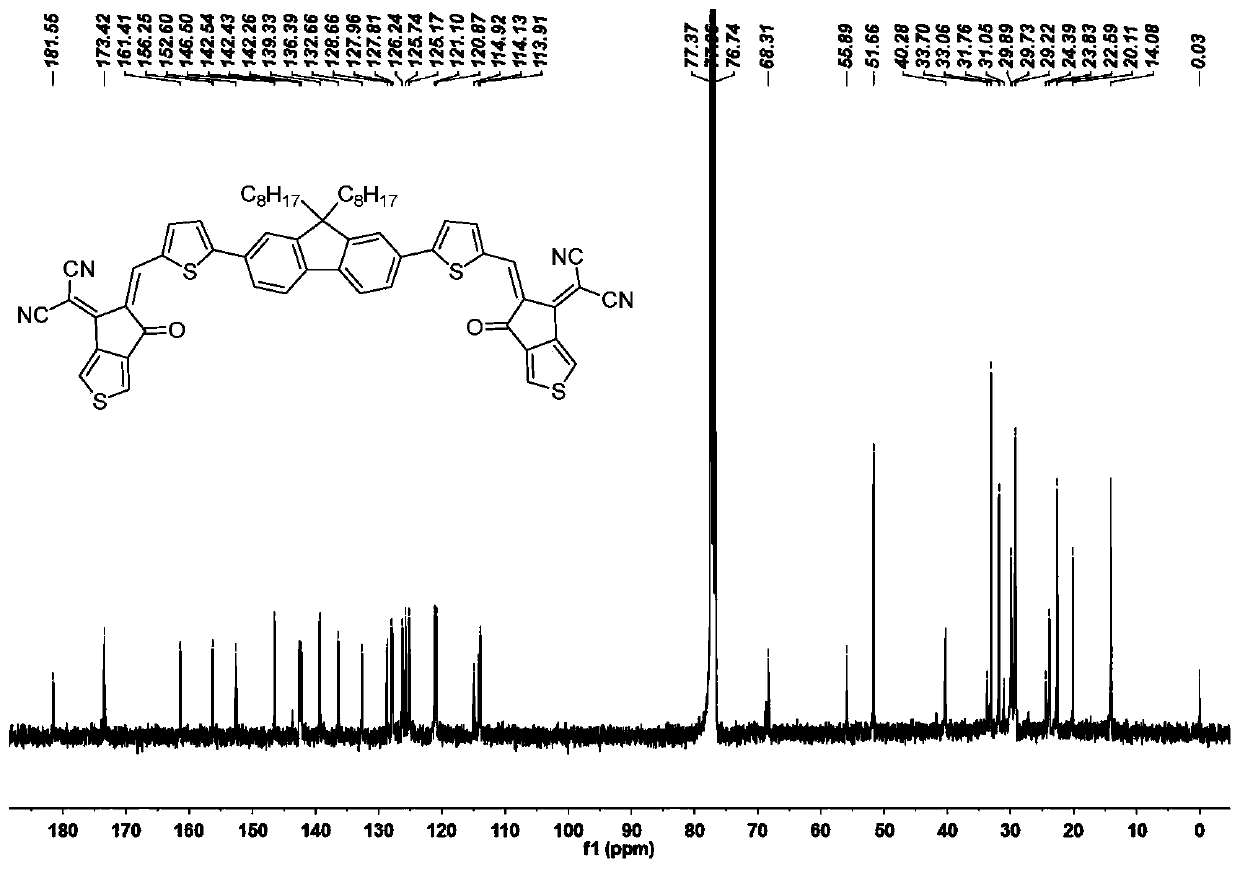
[0069] The synthesis method of the target molecule FTC3 is similar to that of the target molecule FTC1, with intermediate 4 (149 mg, 0.25 mmol) and thienindanone (200 mg, 1 mmol) as reactants, separated and purified to obtain a deep red solid powder target molecule FTC3 (120 mg, 50 %). 1 H NMR (400MHz, CDCl 3 )δ:8.84(s,1H),8.42(s,1H),7.99(s,1H),7.88(d,J=4.0Hz,1H),7.87(dd,J=13.8,6.0Hz,2H), 7.84–7.77(m, 2H), 7.77(s, 1H), 7.62(d, J=4.1Hz, 1H), 2.10(d, J=7.8Hz, 2H), 1.11(d, J=29.8Hz, 11H ),0.77(t,J=6.7Hz,4H),0.65(s,2H). 13 CNMR (101MHz, CDCl 3)δ:181.51,173.41,161.40,156.23,152.61,146.46,142.73–142.11,139.29,136.40,132.66,128.67,127.94,126.24,125.73,125.16,121.10,120.88,114.91,114.03,77.36,77.04,76.73,68.33 ,55.89,51.63,40.27,33.06,31.75,29.89,29.21,23.85,22.59,20.11,14.06.MALDI-TOF-MS,m / z: calcd for C 63 h 54 N 4 o 2 S 2 [M] + :962.369; found 963.428.
PUM
| Property | Measurement | Unit |
|---|---|---|
| band gap | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com