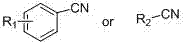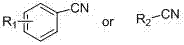Application of tetrazole compounds to anti-epileptic medicines
A technology of antiepileptic drugs and compounds, applied in the direction of active ingredients of heterocyclic compounds, organic chemistry, drug combination, etc., can solve the problems of side effects and tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0020] Taking 5-(o-tolyl)-1H-tetrazole (compound I4) as an example to introduce the general synthesis method of compound I
[0021] In a round bottom flask, add sodium azide (3mmol), triethylamine hydrochloride (3mmol), toluene (5mL) and compound III4 (1mmol), heat at 120ºC, react for about 12h, follow the reaction by TLC, after the reaction is complete, drop Add 6N hydrochloric acid, filter with suction, wash with water, wash with dichloromethane and recrystallize with ethanol to obtain the pure compound I4 as light yellow solid. The melting point is 153-155°C, and the yield is 51.5%. 1 H-NMR (DMSO-d 6 , 600 MHz, δ ppm): 7.69 (d, 1H, ArH, J=7.8 Hz), 7.49 (td, 1H, ArH, J=1.2, 7.2 Hz), 7.44 (d, 1H, ArH, J=7.8 Hz ), 7.40 (td, 1H, ArH, J=1.2, 7.2 Hz), 2.48 (s, 3H, CH 3 ).
Embodiment A
[0022] Embodiment A: anticonvulsant pharmacological experiment and neurotoxicity experiment
[0023] Taking 5-(o-tolyl)-1H-tetrazole (compound I4) as an example, the maximum electroshock test (MES) was used in pharmacological experiments to evaluate the anticonvulsant activity of the compound. Get 9 Kunming mice (male and female) and divide them into 3 groups, 3 in each group, respectively intraperitoneally inject 30mg / kg, compound I4 of 100mg / kg dose, carry out electrical stimulation to the tested mice after 0.5 hours, observe Protection of compounds against electrical stimulation-induced convulsions. It is valid if the hind limbs are not stiff. For compounds that are 100% effective at a dose of 100 mg / kg, the half effective dose is further evaluated by Bliss method. The half effective dose of compound I4 was determined to be 9.6 mg / kg. The mouse rotarod ataxia test is used to evaluate the neurotoxicity of the compound. After intraperitoneal injection for 0.5 hours, the mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com