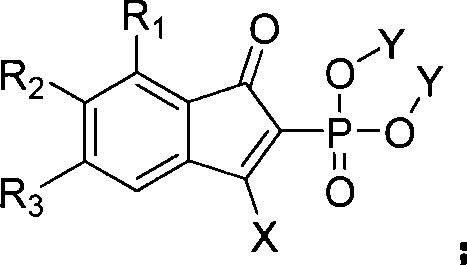
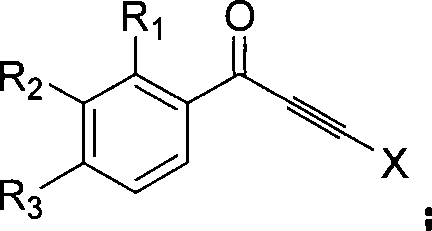
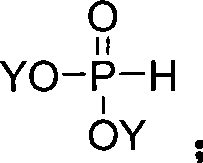Method for synthesizing indenone-2-phosphonate derivative
A technology of phosphonate and derivatives, which is applied in the field of phosphonate preparation, can solve the problems of demand, limited range of substrate selection, and expensive catalyst, and achieve the effects of wide application range, low price, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This example is the synthesis of 3-phenylindanone-2-phosphonic acid methyl ester. Using 1,3-diphenylpropynyl ketone and dimethyl phosphite as raw materials, the reaction formula is as follows:
[0042]
[0043] Experimental steps:
[0044] (1) Put 0.206 g (1 mmol) of 1,3-diphenylpropynyl ketone and 0.81 g (3 mmol) of manganese acetate in a round bottom flask, add 20 mL of acetic acid, heat to 80 ° C, and then add dimethyl phosphite 0.220 grams (2mmol), TLC tracking reaction until the end;
[0045] (2) Rotary evaporation removes acetic acid, adds acetone, filters;
[0046] (3) The residue after the filtrate was concentrated was separated by chromatographic column to obtain the target product (yield 74%).
[0047] Product NMR data: 1 H NMR (400MHz, CDCl 3 ): δ 3.65 (d, 6H, 3 J PH =11.4Hz, 2CH 3 ), 7.15-7.17 (m, 1H, ArH), 7.41-7.44 (m, 2H, ArH), 7.53-7.54 (m, 3H, ArH), 7.60-7.63 (m, 3H, ArH). 13C NMR (100MHz, CDCl 3 ): δ 53.1(d, 2 J P-C =5.7Hz), 121.5(d, 1 ...
Embodiment 2
[0049] This example is the synthesis of 3-phenyl-6-nitroindanone-2-phosphonic acid methyl ester. With 1-(3-nitrophenyl)-3-phenylpropynone and dimethyl phosphite as raw materials, the reaction formula is as follows:
[0050]
[0051] Experimental steps:
[0052] (1) Put 1mmol of 1-(3-nitrophenyl)-3-phenylpropynone and 2mmol of manganese acetate in a round bottom flask, add 20mL of acetonitrile, heat to 90°C, then add dimethyl phosphite 1.5mmol, TLC tracking reaction until the end;
[0053] (2) Rotary evaporation removes acetonitrile, adds acetone, filters;
[0054] (3) The residue after the filtrate was concentrated was separated by chromatographic column to obtain the target product (yield 68%).
Embodiment 3
[0056] This example is the synthesis of 3-phenyl-7-bromoindanone-2-phosphonic acid methyl ester. With 1-(2-bromophenyl)-3-phenylpropynone and dimethyl phosphite as raw materials, the reaction formula is as follows:
[0057]
[0058] Experimental steps:
[0059] (1) Put 1mmol of 1-(2-bromophenyl)-3-phenylpropynone and 2.5mmol of manganese acetate in a round bottom flask, add 20mL of ethanol, heat to 30°C, and then add dimethyl phosphite 2mmol, TLC tracking reaction until the end;
[0060] (2) rotary evaporation removes ethanol, adds acetone, filters;
[0061] (3) The residue after the filtrate was concentrated was separated by chromatographic column to obtain the target product (yield 64%).
[0062] Product NMR data: 1 H NMR (400MHz, CDCl 3 ): δ 3.67 (d, 6H, 3 J PH =11.4Hz, 2CH 3 ), 7.10-7.29 (m, 2H, ArH), 7.53-7.58 (m, 6H, ArH); 13 C NMR (100MHz, CDCl 3 ): δ 53.2(d, 2 J P-C =5.9Hz), 119.8, 122.5(d, 1 J P-C =201.9Hz), 123.1, 128.1(d, 3 J P-C = 10.9Hz), 128.5,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com