A kind of indenone with trifluoromethylthio group and its derivatives and preparation method thereof
A technology of trifluoromethylthio and derivatives, applied in sulfide preparation, organic chemistry and other directions, can solve the problems of high cost and poor substrate adaptability, and achieve the effects of low cost, short reaction time and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example 1
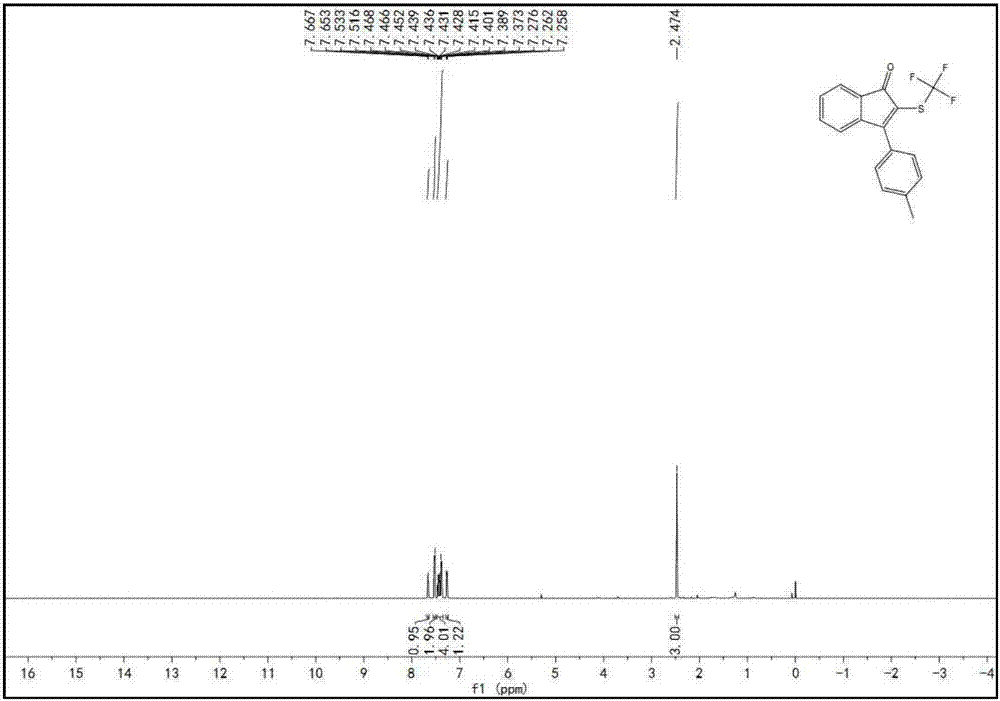
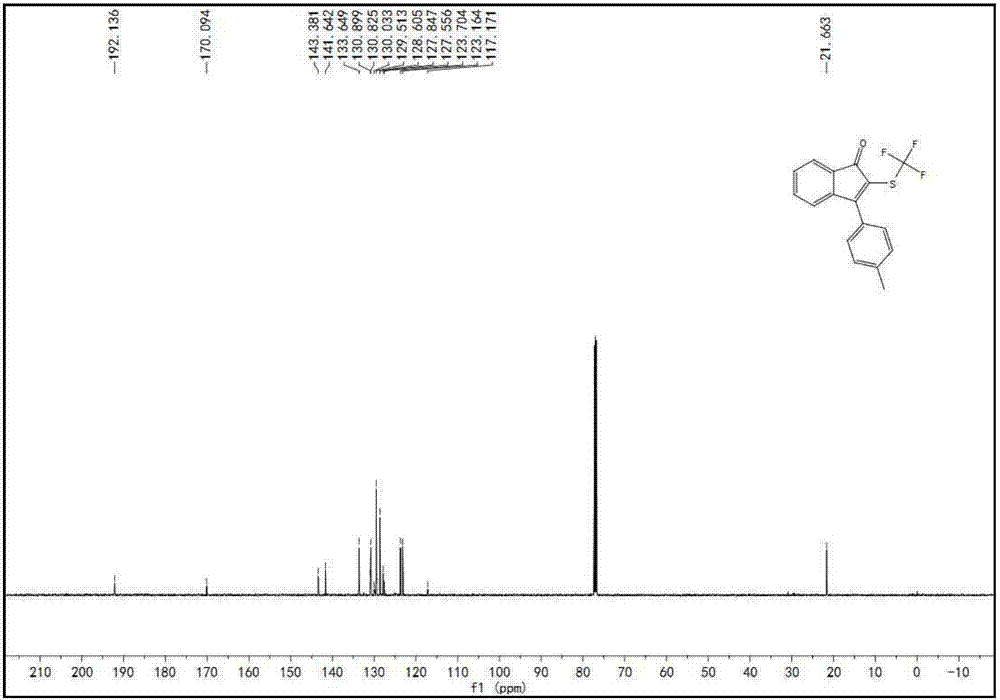
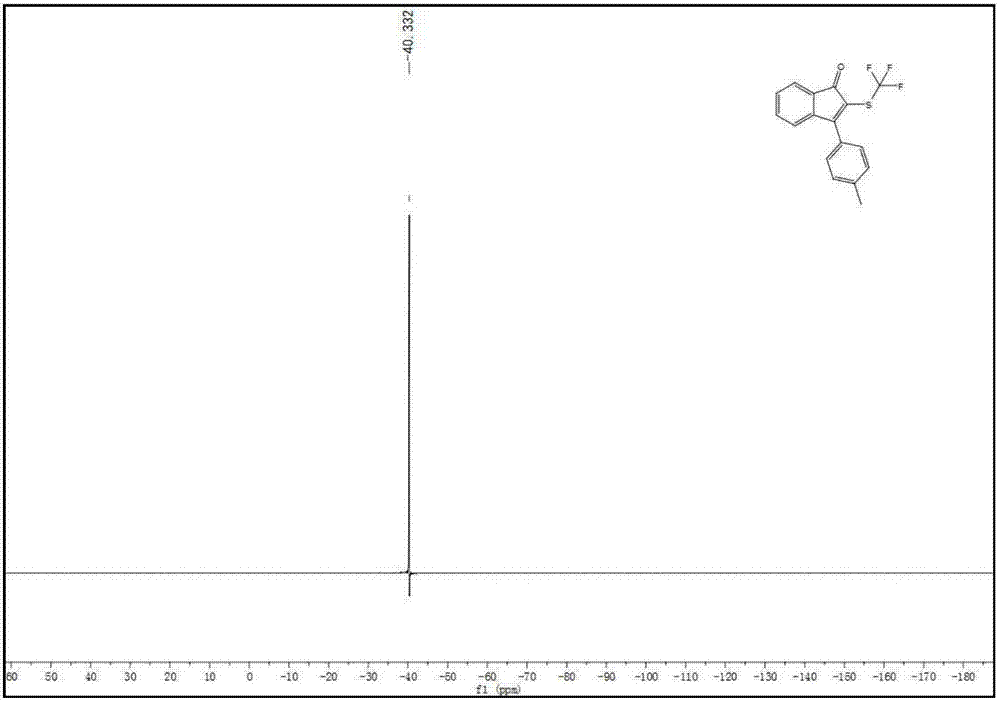
[0084] Specific example one: Synthesis of 3-(4-(methyl)benzene) using 3-(4-(methyl)benzene)-1-phenylprop-2-yn-1-ketone and silver trifluoromethanethiolate -2-((trifluoromethyl)thio)-1H-inden-1-one;
[0085] Weigh raw material A, raw material B, potassium persulfate, and HMPA in a molar ratio of 1:1.5:1:0.1, wherein (A) compound is 0.1 mmol. The reaction solution adopts DMSO, and the reaction system is o The reaction was stirred at C for 12 hours. Cool after the reaction, wash with saturated sodium chloride aqueous solution, remove water with anhydrous sodium sulfate, suction filter through a short silica gel column, rotary evaporate the filtrate, remove potassium persulfate, remove solvent, and use silica gel column chromatography for the residue, petroleum ether Rinse, TLC detection, combine the effluents containing the product, distill off the solvent with a rotary evaporator, and dry in vacuo to obtain the brown-yellow target product with a yield of 35% and a purity of 99...
specific example 2
[0086] Concrete example two: Synthesis of 3-(4-(methyl)benzene) using 3-(4-(methyl)benzene)-1-phenylprop-2-yn-1-ketone and silver trifluoromethanethiolate -2-((trifluoromethyl)thio)-1H-inden-1-one;
[0087] Weigh raw material A, raw material B, potassium persulfate, and HMPA at a molar ratio of 1:1.5:2:0.1, wherein (A) compound is 0.1 mmol. The reaction solution adopts DMSO, and the reaction system is o The reaction was stirred at C for 12 hours. Cool after the reaction, wash with saturated sodium chloride aqueous solution, remove water with anhydrous sodium sulfate, suction filter through a short silica gel column, rotary evaporate the filtrate, remove potassium persulfate, remove solvent, and use silica gel column chromatography for the residue, petroleum ether Rinse, TLC detection, combine the effluents containing the product, distill off the solvent with a rotary evaporator, and dry in vacuo to obtain the brown-yellow target product with a yield of 65% and a purity of 99...
specific example 3
[0088] Specific example three: Synthesis of 3-(4-(methyl)benzene) using 3-(4-(methyl)benzene)-1-phenylprop-2-yn-1-ketone and silver trifluoromethanethiolate -2-((trifluoromethyl)thio)-1H-inden-1-one;
[0089] Weigh raw material A, raw material B, potassium persulfate, and HMPA at a molar ratio of 1:1.5:3:0.1, wherein the compound (A) is 0.1 mmol. The reaction solution adopts DMSO, and the reaction system is o The reaction was stirred at C for 12 hours. Cool after the reaction, wash with saturated sodium chloride aqueous solution, remove water with anhydrous sodium sulfate, suction filter through a short silica gel column, rotary evaporate the filtrate, remove potassium persulfate, remove solvent, and use silica gel column chromatography for the residue, petroleum ether Rinse, TLC detection, combine the effluents containing the product, distill off the solvent with a rotary evaporator, and dry in vacuo to obtain the brown-yellow target product with a yield of 84% and a purity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com